ABSTRACT
Nonalcoholic fatty liver disease (NAFLD) has become the most common cause of liver dysfunction worldwide. Recently, some natural compounds have attracted growing interest in the treatment of NAFLD. In this context, most attention has been paid to natural products derived from fruits, vegetables, and medicinal herbs. Naringenin, a natural flavanone, has been revealed to have pharmacological effects in the treatment of obesity and associated metabolic disorders such as NAFLD. The aim of this study was to examine the therapeutic effects of naringenin and its possible mechanisms of action in the management of NAFLD and related risk factors. The current systematic review was performed according to the guidelines of the 2015 PRISMA (Preferred Reporting Items for Systematic Review and Meta-Analysis) statements. We searched PubMed/Medline, Science Direct, Scopus, ProQuest, and Google Scholar databases up until February 2020. Of 1217 full-text articles assessed, 36 studies met the inclusion criteria. The evidence reviewed in the present study indicates that naringenin modulates several biological processes related to NAFLD including energy balance, lipid and glucose metabolism, inflammation, and oxidative stress by different mechanisms. Overall, the favorable effects of naringenin along with its more potency and efficacy, compared with other antioxidants, indicate that naringenin may be a promising therapeutic approach for the management of NAFLD and associated complications. However, due to the lack of clinical trials, future robust human randomized clinical trials that address the effects of naringenin on NAFLD and other liver-related diseases are crucial. Further careful human pharmacokinetic studies are also needed to establish dosage ranges, as well as addressing preliminary safety and tolerability of naringenin, before proceeding to larger-scale endpoint trials.
Keywords: inflammation, NAFLD, naringenin, obesity, PPARs, oxidative stress
Introduction
Nonalcoholic fatty liver disease (NAFLD) has become the most common cause of liver dysfunction worldwide and is defined by the accumulation of triglyceride (TG) within hepatocytes that exceeds 5% of liver weight (1). NAFLD is initiated by simple steatosis that can progress to nonalcoholic steatohepatitis (NASH), necroinflammation, and fibrosis. The disease can also progress to cirrhosis and finally hepatocellular carcinoma (2, 3). According to a recent meta-analysis with a total number of 8,515,431 patients, the global prevalence of NAFLD is reported to be 25.24%, with the highest prevalence in the Middle East and South America and the lowest in Africa (4). However, the prevalence of NAFLD is significantly higher (43–92%) in patients with obesity and metabolic syndrome (5). In 1998, for the first time, Day and James (6) proposed that NAFLD could be considered as a disease with a “two-hit” process. In the first hit, high-fat diet (HFD), sedentary lifestyle, obesity, and insulin resistance (IR) result in enhanced lipid accumulation in the liver cells (7). In addition, factors that are primarily linked to an increase in oxidative stress (OS) play pivotal roles in NAFLD progression in the second hit (8). The “two-hit” model has been subsequently revised in a “multiple parallel-hit” model (proposed in 2010) in which, in the presence of fat accumulation in the liver and IR, multiple simultaneous alterations result in an imbalance between anti-lipotoxic protection systems in the liver and production of free radicals, leading to decreased mitochondrial content, impaired mitochondrial B-oxidation, endoplasmic stress, OS, and hepatocyte cell death (9). Moreover, disrupted B-oxidation and OS trigger inflammatory signalling pathways, stimulating IR and liver damage (10). There is no approved therapy for NAFLD. However, the Drug Controller General of India has recently approved saroglitazar for the treatment of noncirrhotic NASH (11). The current treatment strategies are focused on protecting the liver from OS, decreasing obesity, and improving diabetes mellitus, dyslipidemia, and hepatic inflammation (12). Although lifestyle interventions including diet-induced weight loss and increased physical activity remain the cornerstone of treatment for NAFLD (13), some natural compounds have recently attracted growing interest in the treatment of NAFLD (14–16). In this context, most attention was paid to natural products derived from fruits, vegetables, and medicinal herbs (17). Increasing evidence indicates that many plant-derived natural products such as anthocyanin, phenolic compounds, glycyrrhizin, wogonin, and those present in coffee, green tea, bitter melon, garlic, fenugreek, and soybean are capable of ameliorating NAFLD (18–20). Among the different classes of natural agents, polyphenols such as quercetin, curcumin, resveratrol, rutin, epicatechins, silymarin, troxerutin, as well as naringenin are considered as bioactive components, which can be effective in the management of NAFLD (20).
Naringenin, 4′,5,7-trihydroxyflavanone, is a natural flavanone that extensively exists in citrus fruits such as grapefruit and orange. Figure 1 shows the biochemical structure of naringenin. Several biological activities of naringenin, including antioxidant, anti-inflammatory, antitumor, antiadipogenic, and cardioprotective effects, have been reported in previous studies (21, 22). Moreover, evidence demonstrates that naringenin can exhibit protective effects on liver damage and improve many metabolic disturbances related to IR including hepatic VLDL overproduction, obesity, and hepatic steatosis (22, 23). Naringenin can also normalize hepatic TG and gene expression of peroxisome proliferator-activated receptor gamma coactivator 1α (PGC1α), carnitine palmitoyltransferase 1α (CPT-1α), and sterol regulatory element-binding transcription factor-1c (SREBF1c). Furthermore, naringenin remarkably increases the expression of peroxisome proliferator-activated receptor (PPAR) α (PPAR-α) and PPARγ in the liver (24). In addition, prevention of OS, transforming growth factor β (TGF-β), one of the main profibrogenic signaling molecules, and the inhibition of the trans-differentiation of hepatic stellate cells, contributing to reduced collagen synthesis, are the main hepatoprotective effects of naringenin (15). The accumulation of total collagen and type I collagen (Col I) associated with liver fibrosis was also inhibited by naringenin treatment (100 mg/kg for 8 wk), which explains its antifibrotic activity (25). Additionally, oral administration of naringenin (50 and 100 mg/kg for 20 d) showed protective effects against necrosis, cholestasis, and membrane permeation in hepatocytes (26). Hermenean et al. (27) demonstrated that treatment with 50 mg/kg of naringenin for 7 d inhibited sinusoidal dilatation and mild fibrosis and also retained the normal bile canaliculi configuration filled with microvilli. Moreover, naringenin treatment (50 mg/kg for 8 wk) improved histopathological abnormalities, including necrosis, hydropic degeneration, and hepatic cord disorganization in the liver of rats (28). A liver sample from a cirrhotic rat, where large collagen deposits are around regeneration nodules, improved via resorption of collagen fibers in the vessel lumen after naringenin administration (100 mg/kg for 4 wk) using an intragastric tube (29). Naringenin could help to lower hepatic marker enzymes (28, 30). In this regard, naringenin-loaded nanoparticles (100 mg/kg for 3 consecutive days by gavage) resulted in decreased concentrations of aspartate transaminase (AST) and alanine transaminase (ALT) (2-fold and 1.5-fold, respectively) in rats with acute liver failure (31). Furthermore, oral administration of naringenin (50 and 100 mg/kg for 20 d) could significantly reduce AST, ALT, alkaline phosphatase (ALP), and lactate dehydrogenase (LDH) concentrations in rats (26).
FIGURE 1.

Structure of naringenin.
All the effects mentioned above indicate that naringenin may have preventive effects against NAFLD and its related complications (32). Accordingly, the aim of this systematic review was to assess the therapeutic effects of naringenin and its possible mechanisms of action in the management of NAFLD and its associated risk factors.
Methods
Search strategy
The current systematic review was performed according to the guidelines of the 2015 PRISMA (Preferred Reporting Items for Systematic Review and Meta-Analysis) statements. We searched PubMed/Medline, Science Direct, Scopus, ProQuest, and Google Scholar as well as Google databases using the following keywords: “naringenin,” “naringenin7-sulfate,” and “non-alcoholic fatty liver disease,” “non-alcoholic steatohepatitis,” “hepatic steatosis,” “fatty liver,” “NAFLD,” “NASH,” “liver fibrosis,” “hepatocellular carcinoma,” “insulin resistance,” “HOMA-IR,” “glucose intolerance,” “diabetes,” “T2DM,” “lipid and glucose metabolism,” “dyslipidemia,” “obesity,” “fat mass,” “BMI,” “inflammation,” and “oxidative stress.”
Eligibility criteria
Relevant studies published in the English language up until February 2020 were eligible in this study. This systematic review included all articles that assessed the effects of naringenin on NAFLD and related disorders. Articles with inadequate information, including letters and comments, were not included in this review. We also excluded all studies that did not directly assess the effects of naringenin on NAFLD and its related risk factors (e.g., studies using a mixture of flavonoids). Additionally, studies that investigated the effects of naringenin on diseases other than NAFLD-associated conditions were ineligible.
Data extraction and quality assessment
Two reviewers first independently retrieved studies by title/abstract for choosing eligible articles; studies were excluded if they could not meet the eligibility criteria. Then, the full texts of the screened articles were critically analyzed for eligibility and extraction of data. In cases of controversy, the articles of debate were discussed by authors, and a final decision was made accordingly.
Results
As shown in Figure 2, our search method initially identified 1217 studies, of which 513 were considered after removal of duplicate articles. Of these, 465 articles were excluded because they did not meet our inclusion criteria. Finally, 48 studies were obtained based on the research topic. After critical analysis, 36 articles were included in the present review. Table 1 shows the main characteristics of the selected studies (23, 24, 33–63). As NAFLD is strongly associated with obesity, information in this table is classified into obesity-associated and liver-associated outcomes, and in each section, the studies are ordered by their design.
FIGURE 2.
Flowchart of study selection.
TABLE 1.
Summary of available literature on possible mechanisms involved in therapeutic/preventive actions of naringenin on NAFLD-related conditions1
| Article type and type of study | Study, year (reference) | Samples | Study design | Main results |
|---|---|---|---|---|
| Obesity-related articles | ||||
| In vitro | Rebello et al., 2019 (40) | hADSC, pWAT | Administration of naringenin: 8 to 10 μM for 7–14 d | Increase in energy expenditure in hADSC; stimulation of expression of key enzymes involved in thermogenesis and insulin sensitivity in hADSC and pWAT; promotion of conversion of human white adipose tissue to a brown/beige phenotype |
| In vitro | Richard et al., 2013 (41) | Murine 3T3-L1 preadipocytes cultured in DMEM | Administration of naringenin: 22, 91, 183 μM for various times | Suppression of adipogenesis in a dose-dependent fashion as judged by examining lipid accumulation and induction of adipocyte marker protein expression; reduction of the ability of insulin to induce insulin receptor substrate 1 tyrosine phosphorylation and substantially inhibiting insulin-stimulated glucose uptake in a dose-dependent manner; prevention of adiponectin protein expression in mature murine 3T3-L1 adipocytes |
| In vitro | Yoshida et al., 2013 (42) | 3T3-L1 cells cultured in DMEM; RAW 264 cells cultured in DMEM | Administration of naringenin: 0, 1, 5, 10, 50, 100 μM for 3 h | Suppression of TLR2 expression during adipocyte differentiation via PPARγ activation; prevention of TNF-α–induced TLR2 expression by inhibiting the activation of NF-κB and JNK pathways; decrease in TLR2 expression in adipose tissue of HFD-fed mice; improvement in hyperglycemia and the suppression of inflammatory mediators, including TNF-α and MCP-1 |
| In vitro | Yoshida et al., 2010 (43) | 3T3-L1 cells maintained in DMEM | 3T3-L1 adipocytes were pretreated with hesperetin (100 μM) or naringenin (100 μM) for 30 min | An inhibition of TNF-α–stimulated FFA secretion from mouse adipocytes; blocking the TNF-α–induced activation of the NF-κB and ERK pathways; prevention of TNF-α from downregulating the transcription of 2 antilipolytic genes, including perilipin, and PDE3B via inhibition of the ERK pathway; and suppression of the transcription of IL-6 with naringenin treatment |
| In vitro | Hirai et al., 2007 (44) | RAW 264 macrophage cell line; 3T3-L1 preadipocytes | RAW 264 macrophage cell line, 3T3-L1 preadipocytes treated with 0, 25, 50, 100, 200 μM naringenin chalcone, naringenin, and rutin for 24 h | Prevention of the production of TNF-α, MCP-1, and NO by LPS-stimulating RAW 264 macrophages in a dose-dependent manner; exhibiting anti-inflammatory properties by inhibition of the production of proinflammatory cytokines in the interaction between adipocytes and macrophages by naringenin |
| In vitro | Harmon et al., 2003 (45) | 3T3-L1 preadipocytes cultured in DMEM | Administration of naringenin: 25, 50, 100 μM for 3 d | Inhibition of insulin-stimulated glucose uptake in 3T3-L1 adipocytes in a dose-dependent manner; exacerbating insulin resistance in susceptible individuals via impaired glucose uptake in adipose tissue |
| Animal | Tsuhako et al., 2019 (33) | Male C57BL/6J STD-fed mice, HFD (60% of calories from fat)-fed mice; 3T3-L1 cells; RAW 264 cells | HFD-fed mice treated with naringenin (100 mg/kg/d for 14 d); cell line and the co-culture system treated with naringenin or vehicle control were 0.6 mL and 1.0 mL, respectively | Suppression of macrophage infiltration into adipose tissue by inhibiting MCP-1 expression in the progression phase to HFD-induced obesity; inhibition of the HFD-induced expression of several chemokines, including MCP-1 and MCP-3, in adipose tissue; suppression of MCP-3 expression in 3T3-L1 adipocytes and a co-culture of 3T3-L1 adipocytes and RAW 264 macrophages |
| Animal | Burke et al., 2019 (34) | Lean, Ldlr-/- mice fed a nonpurified diet | Purified naringenin was continuously supplemented to the purified rodent chow by weight (3%; wt/wt) fed ad libitum for 8 wk | Reduction in body weight and adiposity; decrease in plasma lipids and increase in insulin sensitivity; elevation in energy expenditure |
| Animal | Burke et al., 2018 (35) | Ldlr-/- mice fed a nonpurified diet, HFHC (42% of calories from fat, 0.02% cholesterol)-fed mice, HFHC-fed mice supplemented with naringenin, HFHC-fed mice supplemented with nobiletin, HFHC-fed mice switched to nonpurified diet | HFHC diet–fed mice supplemented with 3% naringenin and 0.3% nobiletin for 12 wk | Prevention of obesity and adipocyte size and number through enhancing energy expenditure and increase in hepatic fatty acid oxidation; improvement in hyperlipidemia, insulin sensitivity, and hepatic steatosis with administration of naringenin |
| Animal | Ke et al., 2017 (36) | Semi-purified HFD (45% calories from fat)–fed mice; murine E0771 mammary tumor cell line | Male mice fed an HFD with 1% wt/wt and 3% wt/wt naringenin for 2 wk | Decrease in body weight, adipose mass, adipocyte size, α-SMA mRNA in mammary adipose tissue, and mRNA of inflammatory cytokines in both mammary and perigonadal adipose tissues |
| Animal | Ke et al., 2016 (37) | Female C57BL/6J mice fed a semi-purified HFD (45% of calories from fat) | Mice fed an HFD with 1% and 3% naringenin for 11 wk | Suppression of weight gain, reduction in hyperglycemia, and decrease in intra-abdominal adiposity; decrease in mRNA level for genes involved in de novo lipogenesis, lipolysis, and TG synthesis/storage |
| Animal | Assini et al., 2015 (23) | C57BL/6J wild-type mice and Fgf21-/- mice fed a standard nonpurified diet, HFD (42% kcal fat, 43% kcal carbohydrate, 0.05% cholesterol)-fed mice, HFD-fed mice supplemented with naringenin, low-fat semisynthetic diet supplemented with naringenin | Mice fed an HFD supplemented with 3% (wt/wt) naringenin, mice fed a low-fat semisynthetic diet supplemented with 3% (wt/wt) naringenin for 16 wk | Prevention of obesity; improving hepatic TG concentrations and normalizing hepatic expression of PGC1α, CPT1a, and SREBF1c; improvement in metabolic parameters |
| Animal | Ke et al., 2015 (38) | Ovariectomized C57BL/6J female mice fed a control diet | Standard diet supplemented with 3% wt/wt naringenin for 11 wk | Decrease in fasting glucose and insulin concentrations with >50% reduction in intra-abdominal and subcutaneous adiposity; decrease in plasma leptin and leptin mRNA in adipose depots; lowering hepatic lipid accumulation with corresponding alterations of hepatic gene expression associated with de novo lipogenesis, fatty acid oxidation, and gluconeogenesis |
| Animal | Yoshida et al., 2014 (39) | Male C57BL/6J STD-fed mice, HFD (60% of calories from fat)-fed mice treated with vehicle control, HFD-fed mice treated with naringenin; 3T3-L1 cells and RAW 264 cells | HFD-fed mice treated with naringenin (100 mg/kg/d) for 1, 7, or 14 d | Suppression of macrophage infiltration into adipose tissue; not demonstrating any differences in HFD-induced changes of serum biochemical parameters; prevention of MCP-1 in adipose tissue via preventing JNK pathway; suppression of MCP-1 expression in adipocytes, macrophages, and a co-culture of adipocytes and macrophages |
| Animal | Cho et al., 2011 (24) | Male Long-Evans hooded rats fed a commercial nonpurified diet | Semipurified, powdered diets were prepared for concentrations of naringenin: 0%, 0.003%, 0.006%, and 0.012% of diet for 6 wk | Reduction in the amount of total TG and cholesterol in plasma and liver; decrease in adiposity and TG contents in parametrial adipose tissue; increase in PPARα protein expression in the liver; enhancement of expression of CPT-1 and UCP2 |
| Liver-related articles | ||||
| In vitro | Goldwasser et al., 2010 (62) | Huh7 cells cultured in DMEM | Administration of naringenin: 0–500 μM for 24 h | Regulation of nuclear receptors PPARα, PPARγ, and LXRα activity; activation of the ligand-binding domain of both PPARα and PPARγ, while inhibition of LXRα in GAL4-fusion reporters; inducing the expression of PGC1α; induction of PPAR-regulated fatty acid oxidation genes such as CYP4A11, ACOX, UCP1 and ApoAI, and inhibition of LXRα-regulated lipogenesis genes, such as FAS, ABCA1, ABCG1, and HMGR; decrease in cholesterol and bile acid production |
| In vitro | Allister et al., 2008 (63) | HepG2 cells cultured in DMEM | HepG2 cells grown in 6-well culture dishes were incubated with either insulin (25 nM or 100 nM), naringenin (25 μM or 100 μM), or the combination of insulin and naringenin at various concentrations up to 60 min | Induction of signaling required the insulin receptor and sensitized the cell to the effects of insulin; upregulation of the LDL receptor, downregulation of microsomal TG transfer protein expression, and inhibition of apoB-100 secretion via activation of both PI3-K and MAPKerk signaling |
| Animal | Ahmed et al., 2019 (46) | STD-fed male Wistar rats | Administration of naringenin: 20 mg/kg by oral gavage for 4 wk | Reduction of the elevated serum AST, ALT, ALP, LDH, and GGT activities as well as total bilirubin and TNF-α concentrations; reduction in the elevated liver lipid peroxidation and enhancement of the liver glutathione content and SOD, GST, and GPx activities; downregulating the elevated hepatic proapoptotic mediator protein 53, Bax, and caspase-3 and upregulating the suppressed antiapoptotic protein Bcl-2 |
| Animal | Rashmi et al., 2018 (61) | Streptozocin-treated male albino mice, liver tissue fixed in 10% buffered formalin | Oral administration of 50–100 mg/kg naringenin for 45 d | Neutralizing 1) hydroxyl radicals, 2) superoxide, 3) hydrogen peroxide, 4) NO radicals, 5) DPPH, and 6) lipid peroxidation; reduction in lipid peroxidation and increase in antioxidant concentrations |
| Animal | Zhao et al., 2018 (47) | STD-fed male ICR mice received CMC-Na, STD-fed mice received alcohol, STD-fed mice received different flavonoids | Mice were supplied orally with 5 kinds of different flavonoids (naringenin, apigenin, quercetin, epigallocatechin gallate, genistein) at an equimolar concentration of 81 mg/kg for each group for 5 wk | Reduction in fibrosis and apoptosis in the liver; attenuation of lipid deposition, partial inflammatory-related factors (NF-κB, COX-2, and IL-6 concentrations), and hepatic histopathological alterations; improvement in serum biochemistry markers, hepatic lipid accumulation, lipid peroxidation, antioxidant capacities, and inflammation by naringenin treatment |
| Animal | Chen et al., 2017 (48) | Male C57BL/6 mice fed STD, mice fed methionine choline–deficient diet | Administration of naringenin: 25, 50, 100 mg/kg for 1 wk; administration of nano-naringenin: 25 mg/kg for 1 wk | Inhibition of the serum ALT and AST concentrations; decrease in lipid accumulation in the mice livers |
| Animal | Wang et al., 2016 (49) | Male specific pathogen-free BALB/c mice fed a standard diet; liver tissue | Administration of naringenin: 50 or 100 mg/kg for 14 d | Decrease in serum concentrations of ALT and AST, liver index, hepatic malondialdehyde content, and increase in hepatic glutathione content and SOD activity; antiapoptotic effects via a connection with the regulation of Bax and Bcl-2 protein expression in hepatic tissue |
| Animal | Sirovina et al., 2016 (50) | Swiss albino inbred mice fed a standard laboratory diet | Naringenin ethanolic solution (0.5% vol/vol) was given to mice intraperitoneally (50 mg/kg/d) for 7 d | Reduction in lipid peroxidation in liver and kidney tissue; decrease in number of vacuolated liver cells and degree of vacuolization |
| Animal | Ozkaya et al., 2016 (51) | Wistar albino male rats divided in 4 groups: control, naringenin, lead acetate, lead acetate + naringenin; liver tissue | Naringenin (50 mg/kg, dissolved in corn oil) was administered by the orogastric gavage during 30 d with intervals of 1 d | Decrease in the grade of necrosis, hydropic degeneration, and hepatic cord disorganization |
| Animal | Chtourou et al., 2015 (52) | Male Wistar rats divided in 4 groups: control, STD-fed mice supplemented with naringenin, HCD (prepared by adding 10 g cholesterol/kg to STD)-fed mice, HCD-fed mice supplemented with naringenin, mice received normal pellet and treated with naringenin | Administration of naringenin: 50 mg/kg for 90 d | A decrease in the plasma fatty acid composition, the hepatic proinflammatory mediators, and the expression of relevant genes including TNF-α, IL-6, IL-1β, iNOS, and MMP-2, 9; reduction in macrophage infiltration, and inhibition of oxidative stress–related biomarker concentrations |
| Animal | Motawi et al., 2014 (54) | Female Wistar rats fed a standard nonpurified diet divided in 4 groups: control, simvastatin, naringenin, simvastatin + naringenin | Administration of naringenin: 20–50 mg/kg/d for 4 wk; administration of simvastatin: 20–40 mg/kg/d for 4 wk | An improvement in liver function, oxidative stress, protein profile, DNA fragmentation, and the histopathological changes by administration of naringenin |
| Animal | Mershiba et al., 2013 (55) | Male rats divided into 4 groups: control, arsenic, naringenin, arsenic + naringenin | Administration of naringenin: 20–50 mg/kg/d for 28 d | Restoring the activities of serum biomarkers and antioxidant enzymes in the tissues in a dose-dependent manner |
| Animal | Jayaraman et al., 2012 (30) | Male Wistar rats fed standard diet divided in 4 groups: groups 1 and 2 received isocaloric glucose, groups 3 and 4 received 20% ethanol, groups 2 and 4 received naringenin | Administration of naringenin: 50 mg/kg/d for 30 d | Decrease in the concentrations/activities/expressions of serum AST, ALT, iron, ferritin, TNF-α, IL-6, NF-κB, COX-2, MIP-2, CD14, and iNOS protein adducts in the liver |
| Animal | Jain et al., 2011 (56) | Male Wistar rats fed standard diet divided into 2 groups: control, received arsenic | Administration of naringenin: 50 mg/kg, orally once daily for 2 wk; administration of silymarine: 50 mg/kg, orally once daily for 2 wk | An increase in glutathione concentrations; improvement in the recovery of altered SOD and CAT activity by naringenin treatment |
| Animal | Jayaraman and Namasivayam, 2011 (57) | Male albino rats fed standard pellet diet divided into 4 groups: groups 1 and 2 received isocaloric glucose, groups 3 and 4 received 20% ethanol, groups 2 and 4 received naringenin | Administration of naringenin: 50 mg/kg for 30 d | A decrease in the levels/activities of bilirubin, ALP, LDH, TBARS, LOOH, CD, and phase I enzymes, and elevation in the activities of ADH, SOD, CAT, and phase II enzymes |
| Animal | Kannappan et al., 2010 (58) | Male Wistar rats received standard pellet diet and water ad libitum divided into 4 groups: control, high-fructose diet (60% fructose, 20% casein)–fed mice, HFD-fed mice supplemented with naringenin, received naringenin | Administration of naringenin: 50 mg/kg/d for 45 d | Inhibition of liver cell leakage, lipid peroxidation, and protein oxidation; improvement in enzymatic antioxidant status; improvement in nonenzymatic antioxidant concentrations |
| Animal | Renugadevi and Prabu, 2010 (59) | Male Wistar rats received commercial standard pelleted diet divided into 5 groups: control, normal naringenin, cadmium, cadmium in combination with naringenin | Administration of naringenin: 25 or 50 mg/kg/d for 4 wk | Reversing the activities of serum hepatic marker enzymes to their near-normal concentrations; reduction in lipid peroxidation and restoring the concentrations of antioxidant defense in the liver |
| Animal | Pari and Gnanasoundari, 2006 (60) | Male Wistar rats fed a pellet diet divided into 5 groups: control, naringenin, oxytetracycline, naringenin, and oxytetracycline | Administration of naringenin: 25 or 50 mg/kg orally for 15 d | Decrease in activities of serum AST, ALT, ALP, and LDH and the concentrations of bilirubin along with reduction in the concentrations of lipid peroxidation markers in the liver; increase in the activities of SOD, CAT, and glutathione peroxidase as well as the concentrations of glutathione, vitamin C, and vitamin E in liver |
ABCA1, ATP binding cassette transporter A-1; ABCG1, ATP binding cassette subfamily G member 1; ACOX, peroxisomal acyl-coenzyme A oxidase; ADH, alcohol dehydrogenase; ALP, alkaline phosphatase; ALT, alanine transaminase; AST, aspartate transaminase; Bax, Bcl-2-associated protein; Bcl-2, B-cell lymphoma 2; CAT, catalase; CD, conjugated dienes; CMC-Na, carboxymethyl cellulose solution; COX, cyclooxygenase; CPT-1, carnitine palmitoyltransferase 1; CYP4A11, cytochrome P450 family 4 subfamilies A member 1; DPPH, diphenyl picrylhydrazyl; ERK, extracellular signal–regulated kinase; FAS, fatty acid synthase; FFA, free fatty acid; Fgf21, fibroblast growth factor 21; GGT, γ-glutamyl transferase; GPx, glutathione peroxidase; hADSC, human white adipocyte cultures; HCD, high-cholesterol diet; HFD, high-fat diet; HFHC, high-fat cholesterol–containing; HMGR, HMG-CoA reductase; iNOS, inducible NO synthase; JNK, Jun N-terminal kinase; LDH, lactate dehydrogenase; LOOH, lipid hydroperoxides; LXRα, liver X-receptor α; LXRE, LXRα response element; MAPKerk, mitogen-activated protein kinase/extracellular-regulated kinase; MCP, monocyte chemoattractant protein; MIP-2, macrophage inflammatory protein 2; MMP, matrix metalloproteinase; NAFLD, nonalcoholic fatty liver disease; PDE3B, phosphodiesterase 3B; PGC1α, peroxisome proliferator-activated receptor γ coactivator 1α; PI3-K, phosphoinositide-3-kinase; PPAR, peroxisome proliferator-activated receptors; pWAT, subcutaneous abdominal adipose tissue; SOD, superoxide dismutase; SREBF1c, sterol regulatory element-binding transcription factor 1c; STD, standard diet; TBARS, thiobarbituric acid reactive substances; TG, triglyceride; TLR2, Toll-like receptor 2; UCP, uncoupling protein; α-SMA, α-smooth muscle actin.
Naringenin, a Multifunctional Flavanone Derived from Citrus Fruits
The antioxidant activity of naringenin
OS is one of the beginning points of many degenerative disease conditions due to an overproduction of free radicals (64). The liver is also one of the main organs attacked by reactive oxygen species (ROS) mostly produced in mitochondria during the respiratory chain activity leading to hepatic steatosis. In addition, the lower activity of antioxidant enzymes is the cause of higher ROS activity (65). In a comparative study, naringenin has been found to exhibit beneficial effects on scavenging ROS and preventing DNA damage via different in vitro assays. Naringenin has also been shown to significantly increase the concentrations of antioxidant enzymes (66). Classically, the antioxidant activity of naringenin is attributed to its hydroxyl substituents (OH), which have high reactivity against ROS and reactive nitrogen species (67). In an experimental study, naringenin administration (50 mg/kg for 90 d) to rats fed a high-cholesterol diet could significantly decrease the concentrations of thiobarbituric acid reactive substances (TBARS), conjugated dienes, and lipid hydroperoxides (LOOH) (P < 0.001), and led to protection against key pathologies of NAFLD, such as NASH and cirrhosis (52). In addition, an in vivo study showed that the activity of antioxidant enzymes such as superoxide dismutase (SOD), catalase (CAT), glutathione peroxidase (GPx), and glutathione reductase (GR) was significantly upregulated (P < 0.05) by naringenin treatment (50 mg/kg) for 30 d (68). All of these enzymes and molecules play a pivotal role in defence against ROS (61). For example, SOD scavenges superoxide anions, and the decrease in its activity results in an increase in the concentration of ROS in cellular compartments, which leads to enhanced lipid peroxidation (67). Moreover, CAT activity was found to be reduced with increasing lipid peroxidation. Lipid peroxidation damages the cell membranes and generates several reactive substances such as TBARS and LOOH (69). It has been shown that naringenin prevents the production of these reactive substances through its antioxidant activity (70).
Pharmacokinetic properties of naringenin
Naringenin indicates merely 15% oral bioavailability after oral administration. Both passive diffusion and an active transport mechanism contribute to naringenin absorption (71). Kanaze et al. (72) studied pharmacokinetic parameters after oral administration of 135 mg naringenin in human subjects. They observed that bioavailability of oral naringenin was merely 5.81% and its elimination half-life was 2.31 h (72). Naringenin binds to albumin in the blood and swiftly flows to highly perfused organs such as the liver, kidney, and heart. Naringenin undergoes an important metabolic step of glucuronidation after absorption, and naringenin-O-B-D-glucuronide, as the most abundant metabolite, is found in plasma. Finally, metabolites of naringenin can be excreted through biliary and urinary pathways (73).
Dietary sources of naringenin
Naringenin is the most plentiful citrus flavanone, with a wide range of biological activities (74). Naringenin and its glycoside are one of the most important flavonoids that are derived from a variety of herbs and fruits such as citrus species (grapefruit, bergamot, lemon, and sour oranges), tart cherries, grapes, cocoa, and vegetables, including tomatoes (75). Compared with healthy subjects, NAFLD patients consume less amounts of fruits and vegetables as sources of dietary fiber, vitamin C, vitamin K, folate, and different kinds of flavonoids such as naringenin (5). After single ingestions of grapefruit juice and orange juice (8 mL/kg) in healthy volunteers, the mean peak plasma concentration (Cmax) of naringenin was 0.7–14.8 μM and 0.1–1.2 μM, respectively (75). Also, after a single, oral supplementation of 135 mg naringenin in healthy subjects, the Cmax value was 4.6–10.2 μM (72). Therefore, due to the relatively acceptable concentrations of naringenin in plasma after the ingestion of the citrus fruits, main health effects can ensue in subjects who consume them regularly (75). It should also be noted that the concentration of flavonoids in a range of 1–10 μM represents physiologically relevant concentrations, whereas concentrations ≥100 μM are considered supraphysiological (76).
Protective Effects of Naringenin in NAFLD and Its Related Complications
The main mechanisms of action of naringenin in the management of NAFLD and its related disorders are discussed in 3 parts, and consist of obesity, metabolic factors, OS, and inflammatory parameters, as explained in the following sections.
Weight-lowering effects of naringenin
The prevalence of NAFLD is elevated as BMI increases (77, 78). Because of the close relation between NAFLD and obesity, medical or dietary supplements that are effective in the treatment of obesity are commonly used for the prevention of NAFLD (79, 80). It is well documented that slow weight loss leads to ameliorated liver enzymes, decreased lipid accumulation in hepatocytes, and reduced liver inflammation and fibrosis (81). Numerous studies have demonstrated that naringenin contributes to the regulation of energy homeostasis and inhibition of adipogenesis (Table 1) (23, 24, 33–45).
Rebello et al. (40) examined the effects of naringenin on energy expenditure in human white adipose tissue. They found that administration of naringenin (8–10 μM for 7–14 d) to a human white adipocyte culture and subcutaneous abdominal adipose tissue significantly resulted in increased energy expenditure, enhanced expression of the key enzymes involved in thermogenesis and insulin sensitivity, and promoted conversion of human white adipose tissue to the brown/beige phenotype. Administration of naringenin (22, 91, and 183 μM) to 3T3-L1 preadipocytes cultured in DMEM inhibited adipogenesis and impaired mature fat cell function (41).
Orally administered naringenin for 14 d contributed to the inhibition of macrophage infiltration into adipose tissue in HFD (60% of calories from fat)-induced obese mice through attenuation of monocyte chemoattractant protein (MCP) 1 (MCP-1) expression in the progression phase to HFD-induced obesity and suppression of HFD-induced expression of several chemokines, including MCP-1 and MCP-3 in adipose tissue (33). Additionally, in another study, Yoshida et al. (43) observed that the administration of naringenin to 3T3-L1 cells for 30 min significantly inhibited Toll-like receptor (TLR) 2 (TLR2) expression during adipocyte differentiation via PPAR-γ activation, prevented TNF-α–induced TLR2 expression by suppressing the activation of NF-κB and Jun N-terminal kinase (JNK) pathways, downregulated inflammatory mediators including TNF-α and MCP-1, and inhibited TNF-α–stimulated free fatty acid (FFA) secretion from mouse adipocytes. In addition, naringenin prevented TNF-α from downregulating the transcription of 2 antilipolytic genes, perilipin, and phosphodiesterase 3B (PDE3B). These effects were mediated through the prevention of the extracellular signal–regulated kinase (ERK) pathway. Naringenin also significantly (P < 0.01) suppressed the transcription of IL-6 inducing FFA secretion in an autocrine manner, and attenuated macrophage infiltration into adipose tissue. These findings may be useful for improving FFA-induced hepatic steatosis (43). RAW 264 macrophage and preadipocytes treated with naringenin (0, 25, 50, 100, and 200 μM for 3 h) exhibited anti-inflammatory effects by attenuating the production of proinflammatory cytokines in the interaction between adipocytes and macrophages (42).
In mice fed a high-fat and high-cholesterol (HFHC) diet including 42% of calories from fat and 0.2% cholesterol for 12 wk, supplementation with 3% naringenin or 0.3% nobiletin for the subsequent 12 wk significantly decreased (∼13%) body-weight gain and adiposity through increased energy expenditure and enhanced hepatic fatty acid oxidation. Furthermore, the mice were fed the HFHC diet 12 wk before the intervention started (35). Compared with HFD-fed mice, body weight, adipose mass, adipocyte size, α-smooth muscle actin (α-SMA) expression in mammary adipose tissue, and mRNA expression levels of MCP-1 in both mammary and perigonadal adipose tissues significantly decreased in ovariectomized mice fed a semi-purified HFD (45% calories from fat for 6 wk) treated with naringenin (1% wt/wt and 3% wt/wt of the diet) for 2 wk. In this study, when cumulative caloric intake was measured prior to starting diets containing naringenin, mice fed an HFD supplemented with naringenin had an 8% reduction in cumulative caloric intake, but this was not significant when compared with mice fed an HFD (36).
In obese mouse models of metabolic dysfunction, an important role of naringenin is the prevention of weight gain, which, in itself, can cause metabolic protection. Thus, Burke et al. (34) examined the effects of naringenin in lean, non-purified diet–fed Ldlr (-/-) mice with isocaloric food consumption. In this study, mice fed 3% naringenin for 8 wk showed lower adiposity-related indices compared with non-purified diet–fed controls. Additionally, naringenin improved circulating lipids and increased insulin sensitivity compared with littermate controls. The results of this study indicated that naringenin can exert beneficial metabolic effects in the absence of obesity or an HFD (34). In another study, ovariectomized mice fed 3% naringenin for 11 wk showed a 50% decrease in intra-abdominal and subcutaneous adiposity compared with a control group (38). Assini et al. (23) reported that treatment with naringenin (3% wt/wt of the diet) for 16 wk prevented obesity by a significant reduction in adipocyte diameter (−104%) in mice fed either an HFD [42% kcal fat, 43% kcal carbohydrate, 0.05% (wt/wt) cholesterol] or a low-fat semisynthetic diet. Administration of naringenin (0, 0.003, 0.006, and 0.012% of diet) to male Long-Evans hooded rats fed a commercial nonpurified diet resulted in a significant decrease in adiposity (26.4%, 14.9%, and 41.1%, respectively) and TG contents in parametrial adipose tissue (24).
Collectively, the above-mentioned findings indicate that naringenin supplementation may have beneficial effects on adiposity and obesity indices. On the whole, it appears that treatment with naringenin ameliorates obesity, which remains an important risk factor for the onset of NAFLD.
Naringenin and metabolic risk factors associated with NAFLD
Lipid profile
The evidence suggests that naringenin participates in many aspects of lipid metabolism through inhibition of 3-hydroxy-3 methylglutaryl-coenzyme A (HMG)-CoA reductase, the key regulatory enzyme for cholesterol synthesis; the reduction in the expression of genes involved in lipid metabolism; and the upregulation of PPARα and PPARγ, which modulate lipid catabolism via activation of cytochrome P450 family 4 subfamily A member 11 (CYPA11), peroxisomal acylcoenzyme A oxidase (ACOX), CPT-1, uncoupling protein 1 (UCP1), and apoA-1 (APOA1). Naringenin also leads to the downregulation of liver X receptor (LXR) and activation of AMP-activated protein kinase (AMPK), which stimulates fatty acid oxidation and prohibits lipogenesis (82). There are numerous experimental studies that have reported the favorable effects of naringenin on lipid profile (Table 1) (24, 34, 35, 37, 38, 47, 63).
Naringenin administration (3% wt/wt of the diet for 8 wk) to mice fed a nonpurified diet also resulted in reduced body weight, adiposity, and plasma lipid concentrations including TG and total cholesterol, elevated energy expenditure, and increased expression of fatty acid metabolism–related genes in the liver (34). Burke et al. (35) found that naringenin or nobiletin, when supplemented to mice fed an HFHC diet (42% of calories from fat and 0.2% cholesterol) for 12 wk, resulted in improvement in hyperlipidemia and hepatic steatosis. In HFD (60% calories from fat)-fed mice treated with naringenin (1–3% wt/wt of the diet for 11 wk), the mRNA expression of genes involved in de novo lipogenesis significantly decreased (by 70%) (37). Furthermore, naringenin treatment (81 mg/kg for 5 wk) led to attenuated hepatic TG concentrations in a mouse model (47). Ovariectomized mice supplemented with naringenin (3% wt/wt of the diet for 11 wk) showed reduced hepatic lipid accumulation due to changes in hepatic gene expression associated with de novo lipogenesis, fatty acid oxidation, and gluconeogenesis (38). Also, naringenin administration (0.003%, 0.006%, and 0.012% of the diet for 6 wk) to rats led to a significant decline in total TG (6%, 42%, and 55%, respectively) and cholesterol in plasma and liver (24). Allister et al. (63) reported that, in HepG2 cells incubated with naringenin (100 μM up to 60 min), LDL receptor was upregulated, microsomal TG transfer protein expression was downregulated, and apoB-100 secretion was inhibited via activation of both phosphoinositide-3-kinase (PI3-K) and mitogen-activated protein kinase/extracellular-regulated kinase (MAPKerk) signaling. Overall, these reports demonstrate an improvement in lipid metabolism, and thereby in lipid profile following naringenin supplementation.
Glycemic parameters
Naringenin has been demonstrated experimentally to enhance glucose uptake in skeletal muscles and to increase insulin sensitivity by boosting tyrosine phosphorylation, indicating its ability to decrease IR (83). Additionally, naringenin may stimulate insulin secretion both in vivo and in vitro by decreasing OS in the pancreatic β cells (84). AMPK is an energy sensor that modulates cellular metabolism, and recent evidence indicates that pharmacological activation of AMPK can lead to the improvement in blood glucose homeostasis (85). Naringenin exhibits antidiabetic effects through upregulation of AMPK and inhibition of gluconeogenesis (86).
Richard et al. (41) demonstrated that administration of naringenin to preadipocytes led to a decrease in the ability of insulin to stimulate insulin receptor substrate-1 (IRS-1) tyrosine phosphorylation and significantly attenuated insulin-induced glucose uptake in a dose-dependent manner. Likewise, naringenin incubation (6 μM for 3 d) in 3T3-L1 preadipocytes reduced insulin-stimulated glucose uptake by ∼20% and also exacerbated IR in susceptible individuals via impairing glucose uptake in adipose tissue (45), while Burke et al. (34) reported that treatment with naringenin (3% wt/wt naringenin for 8 wk) in Ldlr (-/-) mice decreased fasting plasma glucose, the area under the blood glucose curves, and fasting plasma insulin by 37%, 25%, and 59%, respectively, compared with nonpurified diet–fed controls. Immunoblot analysis demonstrated that insulin stimulated maximal phosphorylation of IR and IR substrate-1 after 10 min in HepG2 cells, while naringenin administration (25 μM or 100 μM for up to 60 min) did not affect either at any time point up to 60 min (63). It should be mentioned that, due to discrepancies between results of previous studies, the effect of naringenin on glycemic variables needs to be confirmed in future studies.
OS and inflammation related to NAFLD: protective effect of naringenin
According to the available data, treatments aimed at inhibition of inflammatory signalling pathways are substantial for the management of NAFLD. Previous studies have reported that NAFLD severity was associated with high serum concentrations of inflammatory markers and low serum concentrations of mediators with anti-inflammatory activities (87). Proinflammatory cytokines have been implicated in inducing IR, which is considered to be a major contributor to NAFLD (88). It is well known that inflammation leads to disrupted insulin signalling, which is linked to various metabolic disorders (89). Elevated serum concentrations of TNF-α, one of the major proinflammatory cytokines, are positively related to the intensity of NAFLD (90). TNF-α contributes to the activation of transcription factor NF-κB signalling, which induces the expression of various proinflammatory genes and consists of the JNK, known as the stress-activated protein pathway, leading to the downregulation of the expression of hepatic PPARα target genes (91). Through upregulation of AMPK, naringenin improves proinflammatory responses mediated by TNF-α, IL-6, TLR-4, inducible NO synthase (iNOS), cyclooxygenase-2 (Cox-2), and NADPH oxidase-2 (Nox-2), leading to the prevention of metabolic dysregulations including NAFLD (92). OS appears to be the outcome of an imbalance between pro-oxidant and antioxidant processes in the liver (93). Naringenin, as an antioxidant, could potentially protect hepatocyte structures against ROS (15). Hernandez-Aquino et al. (25) demonstrated that administration of 100 mg/kg naringenin for 8 wk to rats significantly decreased JNK activation and mothers against decapentaplegic homolog (Smad3) phosphorylation in the linker region, reduced α-SMA and Smad3 protein and mRNA levels, and prevented hepatic inflammation, necrosis, fibrosis, and OS markers. On the other hand, naringenin administration (20 mg/kg) by oral gavage for 4 wk increased antinecrotic and antifibrotic effects via preventing NF-κB activation and the subsequent production of IL-1 and IL-10. In male Wistar rats, treatment with naringenin significantly reduced TNF-α levels (−36.46%), downregulated the elevated hepatic proapoptotic mediators including protein 53 (p53), Bax, and caspase-3, and upregulated the suppressed antiapoptotic protein B-cell lymphoma 2 (Bcl-2). The authors concluded that naringenin may exert its hepatoprotective effects through enhancement of the antioxidant defense system and attenuation of inflammation and apoptosis (46).
Oral administration of naringenin (100 mg/kg twice per day for 3 wk) to rats significantly decreased macrophage infiltration, hepatic proinflammatory mediators, and the expression of relevant genes including TNF-α, IL-6, IL-1β, iNOS, and matrix metalloproteinase (MMP) 2 and MMP-9 (29). Jayaraman et al. (68) observed that administration of naringenin (50 mg/kg for 30 d) to rats significantly decreased the concentrations/activities/expressions of serum AST, ALT, iron, ferritin, TNF-α, IL-6, NF-κB, COX-2, macrophage inflammatory protein-2 (MIP-2), conjugated dienes 14 (CD14), and iNOS protein in the liver. Naringenin treatment (50 mg/kg for 30 d) significantly reduced TBARS, S-nitrosothiol, and LOOH concentrations, decreased liver lipid peroxidation, but increased liver glutathione (GSH) content and some hepatic enzyme activities such as SOD, GST, GPx, alcohol dehydrogenase (ADH), and CAT in rats (57). Rashmi et al. (61) found that oral administration of 50 and 100 mg/kg naringenin for 45 d to animals neutralized hydroxyl radicals, superoxide, hydrogen peroxide, NO radicals, diphenyl picrylhydrazyl (DPPH), and lipid peroxidation and increased antioxidant concentrations in mice. Ultimately, according to these findings, naringenin supplementation can improve OS and inflammation status in body organs (Figure 3).
FIGURE 3.
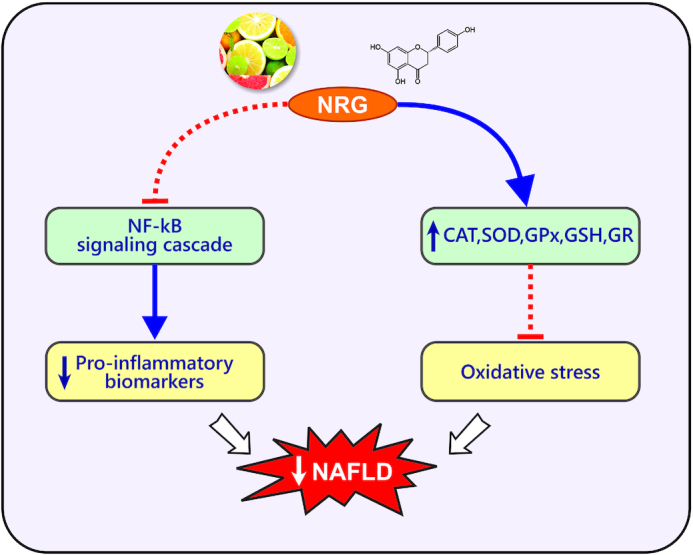
Antioxidant and anti-inflammatory effects of naringenin. CAT, catalase; GPx, glutathione peroxidase; GR, glutathione reductase; GSH, glutathione; NAFLD, nonalcoholic fatty liver disease; NRG, naringenin; SOD, superoxide dismutase.
Future Research Directions and Conclusions
It should be noted that the importance of animal models in NAFLD research has long been appreciated in the prevention and control of the disease in human subjects. Mice and rats are the main experimental models that have been used in understanding NAFLD biology. Although mice and rats fed an HFD (45–75% calories from fat) can replicate the major histopathology and pathogenesis seen in human NAFLD, their relevance to what happens clinically needs to be proved rather than assumed. Overall, because of the beneficial effects of naringenin on OS and inflammatory cascade in the in vitro and in vivo experiments mentioned above, and due to the lack of clinical trials, future robust human randomized clinical trials that address the effects of naringenin on NAFLD, NASH, cirrhosis, and other liver-related diseases are crucial.
Although the doses of naringenin, the aglycone form of the flavonoid naringin, used in many previous in vivo studies were 1% or 3% wt:wt of the diet, there is a significant lack of information regarding clinical studies with pure naringenin. One clinical study demonstrated that the administration of naringin (400 mg/capsule/d) with regular meals for a period of 8 wk lowered plasma total cholesterol by 14% and LDL cholesterol by 17% in hypercholesterolemic subjects. In addition, naringin significantly increased erythrocyte SOD and CAT activities in the hypercholesterolemic group (94). Further careful human pharmacokinetic studies are needed to establish dosage ranges, as well as addressing preliminary safety and tolerability of naringenin, before proceeding to larger-scale endpoint trials. Furthermore, research is needed to confirm the potential therapeutic effects of naringenin by conducting clinical trials. Figure 4 shows the potential mechanisms of action of naringenin in NAFLD and associated metabolic complications.
FIGURE 4.

Potential mechanisms for actions of naringenin in NAFLD and associated metabolic disturbances. ABCA1, ATP binding cassette subfamily A1; ABCG1, ATP binding cassette subfamily G member 1; ACOX, peroxisomal acyl-coenzyme A oxidase; AMPK, AMP-activated protein kinase; APOA1, apoA-1; CPT-1, carnitine-palmitoyltransferase 1; CYP4A11, cytochrome P450 family 4 subfamilies A member 1; HMG COA, 3-hydroxy-3 methylglutaryl–coenzyme A; HO-1, hemeoxygenase 1; LXR, liver X receptor; NAFLD, nonalcoholic fatty liver disease; Nrf2, nuclear factor erythroid 2-related factor 2; NRG, naringenin; PPAR-α, peroxisome proliferator-activated receptor α; UCP1, uncoupling protein 1.
In conclusion, our findings indicate that naringenin may contribute to the prevention of NAFLD and its related risk factors by increasing the expression of PPARα and PPARγ and other PPAR target genes, regulating energy homeostasis, improving fatty acid oxidation, modulating lipid metabolism, reducing hepatic TG accumulation in hepatocytes, and attenuating OS and inflammation by affecting the expression of proinflammatory and profibrotic signalling pathways. On the whole, the favorable effects of naringenin along with its more potency and efficacy, compared with other antioxidants, indicate that naringenin may be a promising therapeutic approach for the management of NAFLD and its associated complications. However, the beneficial effects of naringenin need to be independently confirmed in clinical trials.
ACKNOWLEDGEMENTS
The authors’ responsibilities were as follows—FN and HT: wrote the original manuscript and contributed to the conception of the article; ZN: contributed to data collection; AO and MJH-A: provided advice and consultation; MJH-A: contributed to the final revision of the manuscript; and all authors: read and approved the final manuscript.
Notes
Supported by the Tehran University of Medical Sciences.
Author disclosures: The authors report no conflicts of interest.
The funder had no role in the design, implementation, analysis, or interpretation of the data.
Abbreviations used: ALT, alanine transaminase; AMPK, AMP-activated protein kinase; AST, aspartate transaminase; CAT, catalase; Cmax, peak plasma concentration; Cox-2, cyclooxygenase 2; CPT-1, carnitine palmitoyltransferase 1; FFA, free fatty acid; GPx, glutathione peroxidase; GR, glutathione reductase; HFD, high-fat diet; HFHC, high-fat and high-cholesterol; iNOS, inducible nitric oxide synthase; IR, insulin resistance; JNK, c-Jun N-terminal kinase; LOOH, lipid hydroperoxides; MCP, monocyte chemoattractant protein; MMP, matrix metalloproteinase; NAFLD, nonalcoholic fatty liver disease; NASH, nonalcoholic steatohepatitis; OS, oxidative stress; PPAR, peroxisome proliferator-activated receptor; ROS, reactive oxygen species; Smad3, mothers against decapentaplegic homolog 3; SOD, superoxide dismutase; TBARS, thiobarbituric acid reactive substances; TG, triglyceride; TLR, Toll-like receptor.
Contributor Information
Fatemeh Naeini, Department of Clinical Nutrition, School of Nutritional Sciences and Dietetics, Tehran University of Medical Science, Tehran, Iran.
Zahra Namkhah, Department of Clinical Nutrition, School of Nutritional Sciences and Dietetics, Tehran University of Medical Science, Tehran, Iran.
Alireza Ostadrahimi, Nutrition Research Center, Department of Clinical Nutrition, School of Nutrition and Food Sciences, Tabriz University of Medical Sciences, Tabriz, Iran.
Helda Tutunchi, Nutrition Research Center, Department of Clinical Nutrition, School of Nutrition and Food Sciences, Tabriz University of Medical Sciences, Tabriz, Iran; Student Research Committee, Nutrition Research Center, Department of Clinical Nutrition, School of Nutrition and Food Sciences, Tabriz University of Medical Sciences, Tabriz, Iran.
Mohammad Javad Hosseinzadeh-Attar, Department of Clinical Nutrition, School of Nutritional Sciences and Dietetics, Tehran University of Medical Science, Tehran, Iran.
References
- 1. McCullough AJ. Update on nonalcoholic fatty liver disease. J Clin Gastroenterol. 2002;34(3):255–62. [DOI] [PubMed] [Google Scholar]
- 2. Farrell GC, Larter CZ. Nonalcoholic fatty liver disease: from steatosis to cirrhosis. Hepatology. 2006;43(2 Suppl 1):S99–s112. [DOI] [PubMed] [Google Scholar]
- 3. Tutunchi H, Saghafi-Asl M, Asghari Jafarabadi M, Ostadrahimi A. The relationship between severity of liver steatosis and metabolic parameters in a sample of Iranian adults. BMC Res Notes. 2020;13:218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease—meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64(1):73–84. [DOI] [PubMed] [Google Scholar]
- 5. Perdomo CM, Fruhbeck G, Escalada J. Impact of nutritional changes on nonalcoholic fatty liver disease. Nutrients. 2019;11(3):677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Day CP, James OF.. Steatohepatitis: a tale of two “hits”?. Gastroenterology. 1998;114(4):842–5. [DOI] [PubMed] [Google Scholar]
- 7. Tutunchi H, Ostadrahimi A, Hosseinzadeh-Attar MJ, Miryan M, Mobasseri M, Ebrahimi-Mameghani M. A systematic review of the association of neuregulin 4, a brown fat-enriched secreted factor, with obesity and related metabolic disturbances. Obes Rev. 2020;21(2):e12952. [DOI] [PubMed] [Google Scholar]
- 8. Benedict M, Zhang X. Non-alcoholic fatty liver disease: an expanded review. World J Hepatol. 2017;9(16):715–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Tilg H, Moschen AR.. Evolution of inflammation in nonalcoholic fatty liver disease: the multiple parallel hits hypothesis. Hepatology. 2010;52(5):1836–46. [DOI] [PubMed] [Google Scholar]
- 10. Masarone M, Rosato V, Dallio M, Gravina AG, Aglitti A, Loguercio C, Federico A, Persico M. Role of oxidative stress in pathophysiology of nonalcoholic fatty liver disease. Oxid Med Cell Longev. 2018;2018:9547613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kaul U, Parmar D, Manjunath K, Shah M, Parmar K, Patil KP, Jaiswal A. New dual peroxisome proliferator activated receptor agonist-saroglitazar in diabetic dyslipidemia and non-alcoholic fatty liver disease: integrated analysis of the real world evidence. Cardiovasc Diabetol. 2019;18(1):80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Barb D, Portillo-Sanchez P, Cusi K. Pharmacological management of nonalcoholic fatty liver disease. Metabolism. 2016;65(8):1183–95. [DOI] [PubMed] [Google Scholar]
- 13. Harrison SA, Day CP.. Benefits of lifestyle modification in NAFLD. Gut. 2007;56(12):1760–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zobeiri M, Belwal T, Parvizi F, Naseri R, Farzaei MH, Nabavi SF, Sureda A, Nabavi SM. Naringenin and its nano-formulations for fatty liver: cellular modes of action and clinical perspective. Curr Pharm Biotechnol. 2018;19(3):196–205. [DOI] [PubMed] [Google Scholar]
- 15. Hernandez-Aquino E, Muriel P.. Beneficial effects of naringenin in liver diseases: molecular mechanisms. World J Gastroenterol. 2018;24(16):1679–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Tutunchi H, Ostadrahimi A, Saghafi-Asl M, Maleki V. The effects of oleoylethanolamide, an endogenous PPAR-alpha agonist, on risk factors for NAFLD: a systematic review. Obes Rev. 2019;20(7):1057–69. [DOI] [PubMed] [Google Scholar]
- 17. Ali R, Cusi K.. New diagnostic and treatment approaches in non-alcoholic fatty liver disease (NAFLD). Ann Med. 2009;41(4):265–78. [DOI] [PubMed] [Google Scholar]
- 18. Cicero AFG, Colletti A, Bellentani S. Nutraceutical approach to non-alcoholic fatty liver disease (NAFLD): the available clinical evidence. Nutrients. 2018;10(9):1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Valenti L, Riso P, Mazzocchi A, Porrini M, Fargion S, Agostoni C. Dietary anthocyanins as nutritional therapy for nonalcoholic fatty liver disease. Oxidative Med Cell Longev. 2013;2013:145421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Abenavoli L, Milic N, Luzza F, Boccuto L, De Lorenzo A. Polyphenols treatment in patients with nonalcoholic fatty liver disease. J Transl Int Med. 2017;5(3):144–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Da Pozzo E, Costa B, Cavallini C, Testai L, Martelli A, Calderone V, Martini C. The citrus flavanone naringenin protects myocardial cells against age-associated damage. Oxid Med Cell Longev. 2017;2017:9536148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lin H-J, Ku K-L, Lin IH, Yeh C-C. Naringenin attenuates hepatitis B virus X protein-induced hepatic steatosis. BMC Complement Altern Med. 2017;17(1):505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Assini JM, Mulvihill EE, Burke AC, Sutherland BG, Telford DE, Chhoker SS, Sawyez CG, Drangova M, Adams AC, Kharitonenkov Aet al. Naringenin prevents obesity, hepatic steatosis, and glucose intolerance in male mice independent of fibroblast growth factor 21. Endocrinology. 2015;156(6):2087–102. [DOI] [PubMed] [Google Scholar]
- 24. Cho KW, Kim YO, Andrade JE, Burgess JR, Kim YC. Dietary naringenin increases hepatic peroxisome proliferators-activated receptor alpha protein expression and decreases plasma triglyceride and adiposity in rats. Eur J Nutr. 2011;50(2):81–8. [DOI] [PubMed] [Google Scholar]
- 25. Hernandez-Aquino E, Zarco N, Casas-Grajales S, Ramos-Tovar E, Flores-Beltran RE, Arauz J, Shibayama M, Favari L, Tsutsumi V, Segovia Jet al. Naringenin prevents experimental liver fibrosis by blocking TGFbeta-Smad3 and JNK-Smad3 pathways. World J Gastroenterol. 2017;23(24):4354–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Wali A, Rashid S, Mudasir Rashid S, Ansari M, Khan M, Haq N, Alhareth DY, Ahmad A, Rehman MU. Naringenin regulates doxorubicin-induced liver dysfunction: impact on oxidative stress and inflammation. Plants. 2020;9:550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hermenean A, Ardelean A, Stan M, Hădărugă N, Mihali C-V, Costache M, Dinischiotu A. Antioxidant and hepatoprotective effects of naringenin and its β-cyclodextrin formulation in mice intoxicated with carbon tetrachloride: a comparative study. J Med Food. 2014;17(6):1–8. [DOI] [PubMed] [Google Scholar]
- 28. Wang J, Yang Z, Lin L, Zhao Z, Liu Z, Liu X. Protective effect of naringenin against lead-induced oxidative stress in rats. Biol Trace Elem Res. 2012;146(3):354–9. [DOI] [PubMed] [Google Scholar]
- 29. Hernandez-Aquino E, Quezada-Ramirez MA, Silva-Olivares A, Casas-Grajales S, Ramos-Tovar E, Flores-Beltran RE, Segoviab J, Shibayamac M, Muriel P. Naringenin attenuates the progression of liver fibrosis via inactivation of hepatic stellate cells and profibrogenic pathways. Eur J Pharmacol. 2019;865:172730. [DOI] [PubMed] [Google Scholar]
- 30. Jayaraman J, Jesudoss VA, Menon VP, Namasivayam N. Anti-inflammatory role of naringenin in rats with ethanol induced liver injury. Toxicol Mech Methods. 2012;22(7):568–76. [DOI] [PubMed] [Google Scholar]
- 31. Yen F-L, Wu T-H, Lin L-T, Cham T-M, Lin C-C. Naringenin-loaded nanoparticles improve the physicochemical properties and the hepatoprotective effects of naringenin in orally-administered rats with CCl4-induced acute liver failure. Pharm Res. 2008;26:893–902. [DOI] [PubMed] [Google Scholar]
- 32. Patel K, Singh G, Patel D. A review on pharmacological and analytical aspects of naringenin. Chin J Integr Med. 2014;24:551–60. [DOI] [PubMed] [Google Scholar]
- 33. Tsuhako R, Yoshida H, Sugita C, Kurokawa M. Naringenin suppresses neutrophil infiltration into adipose tissue in high-fat diet-induced obese mice. J Nat Med. 2020;74(1):229–37. [DOI] [PubMed] [Google Scholar]
- 34. Burke AC, Telford DE, Edwards JY, Sutherland BG, Sawyez CG, Huff MW. Naringenin supplementation to a chow diet enhances energy expenditure and fatty acid oxidation, and reduces adiposity in lean, pair-fed Ldlr(-/-) mice. Mol Nutr Food Res. 2019;63(6):e1800833. [DOI] [PubMed] [Google Scholar]
- 35. Burke AC, Sutherland BG, Telford DE, Morrow MR, Sawyez CG, Edwards JY, Drangova M, Huff MW. Intervention with citrus flavonoids reverses obesity and improves metabolic syndrome and atherosclerosis in obese Ldlr(-/-) mice. J Lipid Res. 2018;59(9):1714–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ke JY, Banh T, Hsiao YH, Cole RM, Straka SR, Yee LD, Belury MA. Citrus flavonoid naringenin reduces mammary tumor cell viability, adipose mass, and adipose inflammation in obese ovariectomized mice. Mol Nutr Food Res. 2017;61(9):10. [DOI] [PubMed] [Google Scholar]
- 37. Ke JY, Cole RM, Hamad EM, Hsiao YH, Cotten BM, Powell KA, Belury MA. Citrus flavonoid, naringenin, increases locomotor activity and reduces diacylglycerol accumulation in skeletal muscle of obese ovariectomized mice. Mol Nutr Food Res. 2016;60(2):313–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ke JY, Kliewer KL, Hamad EM, Cole RM, Powell KA, Andridge RR, Straka SR, Yee LD, Belury MA. The flavonoid, naringenin, decreases adipose tissue mass and attenuates ovariectomy-associated metabolic disturbances in mice. Nutr Metab. 2015;12:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Yoshida H, Watanabe H, Ishida A, Watanabe W, Narumi K, Atsumi T, Sugita C, Kurokawa M. Naringenin suppresses macrophage infiltration into adipose tissue in an early phase of high-fat diet-induced obesity. Biochem Biophys Res Commun. 2014;454(1):95–101. [DOI] [PubMed] [Google Scholar]
- 40. Rebello CJ, Greenway FL, Lau FH, Lin Y, Stephens JM, Johnson WD, Coulter AA. Naringenin promotes thermogenic gene expression in human white adipose tissue. Obesity. 2019;27(1):103–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Richard AJ, Amini-Vaughan Z, Ribnicky DM, Stephens JM. Naringenin inhibits adipogenesis and reduces insulin sensitivity and adiponectin expression in adipocytes. Evid Based Complementary Altern Med. 2013;2013:549750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Yoshida H, Watanabe W, Oomagari H, Tsuruta E, Shida M, Kurokawa M. Citrus flavonoid naringenin inhibits TLR2 expression in adipocytes. J Nutr Biochem. 2013;24(7):1276–84. [DOI] [PubMed] [Google Scholar]
- 43. Yoshida H, Takamura N, Shuto T, Ogata K, Tokunaga J, Kawai K, Kai H. The citrus flavonoids hesperetin and naringenin block the lipolytic actions of TNF-alpha in mouse adipocytes. Biochem Biophys Res Commun. 2010;394(3):728–32. [DOI] [PubMed] [Google Scholar]
- 44. Hirai S, Kim YI, Goto T, Kang MS, Yoshimura M, Obata A, Yu R, Kawada T. Inhibitory effect of naringenin chalcone on inflammatory changes in the interaction between adipocytes and macrophages. Life Sci. 2007;81(16):1272–9. [DOI] [PubMed] [Google Scholar]
- 45. Harmon AW, Patel YM.. Naringenin inhibits phosphoinositide 3-kinase activity and glucose uptake in 3T3-L1 adipocytes. Biochem Biophys Res Commun. 2003;305(2):229–34. [DOI] [PubMed] [Google Scholar]
- 46. Ahmed O, Ahmed H, Almuzafar H, Ahmed R, Amin K, El-Nahass E-S, Abdelazeem WH. The preventive effects and the mechanisms of action of navel orange peel hydroethanolic extract, naringin, and naringenin in N-acetyl-p-aminophenol-induced liver injury in Wistar rats. Oxid Med Cell Longev. 2019;2019:1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Zhao L, Zhang N, Yang D, Yang M, Guo X, He J, Wu W, Ji B, Cheng Q, Zhou F. Protective effects of five structurally diverse flavonoid subgroups against chronic alcohol-induced hepatic damage in a mouse model. Nutrients. 2018;10(11):1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Chen C, Jie X, Ou Y, Cao Y, Xu L, Wang Y, Oi R. Nanoliposome improves inhibitory effects of naringenin on nonalcoholic fatty liver disease in mice. Nanomedicine. 2017;12(15):1791–800. [DOI] [PubMed] [Google Scholar]
- 49. Wang C, Fan R-Q, Zhang Y-X, Nie H, Li K. Naringenin protects against isoniazid- and rifampicin-induced apoptosis in hepatic injury. World J Gastroenterol. 2016;22(44):9775–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Sirovina D, Orsolic N, Gregorovic G, Koncic MZ. Naringenin ameliorates pathological changes in liver and kidney of diabetic mice: a preliminary study. Arh Hig Rada Toksikol. 2016;67(1):19–24. [DOI] [PubMed] [Google Scholar]
- 51. Ozkaya A, Sahin Z, Dag U, Ozkaraca M. Effects of naringenin on oxidative stress and histopathological changes in the liver of lead acetate administered rats. J Biochem Mol Toxicol. 2016;30(5):243–8. [DOI] [PubMed] [Google Scholar]
- 52. Chtourou Y, Fetoui H, Jemai R, Ben Slima A, Makni M, Gdoura R. Naringenin reduces cholesterol-induced hepatic inflammation in rats by modulating matrix metalloproteinases-2, 9 via inhibition of nuclear factor kappaB pathway. Eur J Pharmacol. 2015;746:96–105. [DOI] [PubMed] [Google Scholar]
- 53. Esmaeili MA, Alilou M.. Naringenin attenuates CCl4 -induced hepatic inflammation by the activation of an Nrf2-mediated pathway in rats. Clin Exp Pharmacol Physiol. 2014;41(6):416–22. [DOI] [PubMed] [Google Scholar]
- 54. Motawi TK, Teleb ZA, El-Boghdady NA, Ibrahim SA. Effect of simvastatin and naringenin coadministration on rat liver DNA fragmentation and cytochrome P450 activity: an in vivo and in vitro study. J Physiol Biochem. 2014;70(1):225–37. [DOI] [PubMed] [Google Scholar]
- 55. Mershiba SD, Dassprakash MV, Saraswathy SD. Protective effect of naringenin on hepatic and renal dysfunction and oxidative stress in arsenic intoxicated rats. Mol Biol Rep. 2013;40(5):3681–91. [DOI] [PubMed] [Google Scholar]
- 56. Jain A, Yadav A, Bozhkov AI, Padalko VI, Flora SJ. Therapeutic efficacy of silymarin and naringenin in reducing arsenic-induced hepatic damage in young rats. Ecotoxicol Environ Saf. 2011;74(4):607–14. [DOI] [PubMed] [Google Scholar]
- 57. Jayaraman J, Namasivayam N. Naringenin modulates circulatory lipid peroxidation, anti-oxidant status and hepatic alcohol metabolizing enzymes in rats with ethanol induced liver injury. Fundam Clin Pharmacol. 2011;25(6):682–9. [DOI] [PubMed] [Google Scholar]
- 58. Kannappan S, Palanisamy N, Anuradha CV. Suppression of hepatic oxidative events and regulation of eNOS expression in the liver by naringenin in fructose-administered rats. Eur J Pharmacol. 2010;645(1-3):177–84. [DOI] [PubMed] [Google Scholar]
- 59. Renugadevi J, Prabu SM.. Cadmium-induced hepatotoxicity in rats and the protective effect of naringenin. Exp Toxicol Pathol. 2010;62(2):171–81. [DOI] [PubMed] [Google Scholar]
- 60. Pari L, Gnanasoundari M.. Influence of naringenin on oxytetracycline mediated oxidative damage in rat liver. Basic Clin Pharmacol. 2006;98(5):456–61. [DOI] [PubMed] [Google Scholar]
- 61. Rashmi R, Bojan Magesh S, Mohanram Ramkumar K, Suryanarayanan S, Venkata SubbaRao M. Antioxidant potential of naringenin helps to protect liver tissue from streptozotocin-induced damage. Rep Biochem Mol Biol. 2018;7(1):76–84. [PMC free article] [PubMed] [Google Scholar]
- 62. Goldwasser J, Cohen PY, Yang E, Balaguer P, Yarmush ML, Nahmias Y. Transcriptional regulation of human and rat hepatic lipid metabolism by the grapefruit flavonoid naringenin: role of PPARalpha, PPARgamma and LXRalpha. PLoS One. 2010;5(8):e12399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Allister EM, Mulvihill EE, Barrett PHR, Edwards JY, Carter LP, Huff MW. Inhibition of apoB secretion from HepG2 cells by insulin is amplified by naringenin, independent of the insulin receptor. J Lipid Res. 2008;49(10):2218–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Liguori I, Russo G, Curcio F, Bulli G, Aran L, Della-Morte D, Gargiulo G, Testa G, Cacciatore F, Bonaduce Det al. Oxidative stress, aging, and diseases. Clin Interv Aging. 2018;13:757–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Cichoż-Lach H, Michalak A.. Oxidative stress as a crucial factor in liver diseases. World J Gastroenterol. 2014;20(25):8082–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Cavia-Saiz M, Busto MD, Pilar-Izquierdo MC, Ortega N, Perez-Mateos M, Muniz P. Antioxidant properties, radical scavenging activity and biomolecule protection capacity of flavonoid naringenin and its glycoside naringin: a comparative study. J Sci Food Agric. 2010;90(7):1238–44. [DOI] [PubMed] [Google Scholar]
- 67. Zaidun NH, Thent ZC, Latiff AA. Combating oxidative stress disorders with citrus flavonoid: naringenin. Life Sci. 2018;208:111–22. [DOI] [PubMed] [Google Scholar]
- 68. Jayaraman J, Veerappan M, Namasivayam N. Potential beneficial effect of naringenin on lipid peroxidation and antioxidant status in rats with ethanol-induced hepatotoxicity. J Pharm Pharmacol. 2009;61:1383–90. [DOI] [PubMed] [Google Scholar]
- 69. Ayala A, Muñoz M, Argüelles S. Lipid peroxidation: production, metabolism, and signaling mechanisms of malondialdehyde and 4-hydroxy-2-nonenal. Oxid Med Cell Longev. 2014;2014:360438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Hong Y, Yin Y, Tan Y, Hong K, Zhou H. The flavanone, naringenin, modifies antioxidant and steroidogenic enzyme activity in a rat model of letrozole-induced polycystic ovary syndrome. Med Sci Monit. 2019;25:395–401. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 71. Joshi R, Kulkarni YA, Wairkar S. Pharmacokinetic, pharmacodynamic and formulations aspects of naringenin: an update. Life Sci. 2018;215:43–56. [DOI] [PubMed] [Google Scholar]
- 72. Kanaze FI, Bounartzi MI, Georgarakis M, Niopas I. Pharmacokinetics of the citrus flavanone aglycones hesperetin and naringenin after single oral administration in human subjects. Eur J Clin Nutr. 2007;61(4):472–7. [DOI] [PubMed] [Google Scholar]
- 73. Salehi B, Fokou PVT, Sharifi-Rad M, Zucca P, Pezzani R, Martins N, Sharifi-Rad J. The therapeutic potential of naringenin: a review of clinical trials. Pharmaceuticals. 2019;12(1):11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Barreca D, Gattuso G, Bellocco E, Calderaro A, Trombetta D, Smeriglio A, Nabavi SM. Flavanones: citrus phytochemical with health-promoting properties. Biofactors. 2017;43(4):495–506. [DOI] [PubMed] [Google Scholar]
- 75. Erlund I, Meririnne E, Alfthan G, Aro A. Plasma kinetics and urinary excretion of the flavanones naringenin and hesperetin in humans after ingestion of orange juice and grapefruit juice. J Nutr. 2001;131(2):235–41. [DOI] [PubMed] [Google Scholar]
- 76. Warner EF, Zhang Q, Raheem KS, O'Hagan D, O'Connell MA, Kay CD. Common phenolic metabolites of flavonoids, but not their unmetabolized precursors, reduce the secretion of vascular cellular adhesion molecules by human endothelial cells. J Nutr. 2016;146(3):465–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Fabbrini E, Sullivan S, Klein S. Obesity and nonalcoholic fatty liver disease: biochemical, metabolic, and clinical implications. Hepatology. 2010;51(2):679–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Qureshi K, Abrams GA.. Metabolic liver disease of obesity and role of adipose tissue in the pathogenesis of nonalcoholic fatty liver disease. World J Gastroenterol. 2007;13(26):3540–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Eslamparast T, Eghtesad S, Poustchi H, Hekmatdoost A. Recent advances in dietary supplementation, in treating non-alcoholic fatty liver disease. World J Hepatol. 2015;7(2):204–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Tutunchi H, Saghafi-Asl M, Ostadrahimi A. A systematic review of the effects of oleoylethanolamide, a high-affinity endogenous ligand of PPAR-alpha, on the management and prevention of obesity. Clin Exp Pharmacol Physiol. 2020;47(4):543–52. [DOI] [PubMed] [Google Scholar]
- 81. Rafiq N, Younossi ZM.. Effects of weight loss on nonalcoholic fatty liver disease. Semin Liver Dis. 2008;28(4):427–33. [DOI] [PubMed] [Google Scholar]
- 82. Ren B, Qin W, Wu F, Wang S, Pan C, Wang L, Zeng B, Ma S, Liang J. Apigenin and naringenin regulate glucose and lipid metabolism, and ameliorate vascular dysfunction in type 2 diabetic rats. Eur J Pharmacol. 2016;773:13–23. [DOI] [PubMed] [Google Scholar]
- 83. Li S, Zhang Y, Sun Y, Zhang G, Bai J, Guo J, Su X, Du H, Cao H, Yang Jet al. Naringenin improves insulin sensitivity in gestational diabetes mellitus mice through AMPK. Nutr Diabetes. 2019;9(1):28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Rajappa R, Sireesh D, Salai MB, Ramkumar KM, Sarvajayakesavulu S, Madhunapantula SV. Treatment with naringenin elevates the activity of transcription factor Nrf2 to protect pancreatic β-cells from streptozotocin-induced diabetes in vitro and in vivo. Front Pharmacol. 2019;9:1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Long YC, Zierath JR.. AMP-activated protein kinase signaling in metabolic regulation. J Clin Invest. 2006;116(7):1776–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Constantin RP, Constantin RP, Bracht A, Yamamoto NS, Ishii-Iwamoto EL, Constantin J. Molecular mechanisms of citrus flavanones on hepatic gluconeogenesis. Fitoterapia. 2014;92:148–62. [DOI] [PubMed] [Google Scholar]
- 87. Yang J, Fernández-Galilea M, Martínez-Fernández L, González-Muniesa P, Pérez-Chávez A, Martínez JA, Moreno-Aliaga MJ. Oxidative stress and non-alcoholic fatty liver disease: effects of omega-3 fatty acid supplementation. Nutrients. 2019;11(4):872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Stojsavljević S, Gomerčić Palčić M, Virović Jukić L, Smirčić Duvnjak L, Duvnjak M. Adipokines and proinflammatory cytokines, the key mediators in the pathogenesis of nonalcoholic fatty liver disease. World J Gastroenterol. 2014;20(48):18070–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Asrih M, Jornayvaz FR.. Inflammation as a potential link between nonalcoholic fatty liver disease and insulin resistance. J Endocrinol. 2013;218(3):R25–36. [DOI] [PubMed] [Google Scholar]
- 90. Tilg H. The role of cytokines in non-alcoholic fatty liver disease. Dig Dis. 2010;28(1):179–85. [DOI] [PubMed] [Google Scholar]
- 91. Bradley JR. TNF-mediated inflammatory disease. J Pathol. 2008;214(2):149–60. [DOI] [PubMed] [Google Scholar]
- 92. Manchope MF, Casagrande R, Verri WA Jr.. Naringenin: an analgesic and anti-inflammatory citrus flavanone. Oncotarget. 2017;8(3):3766–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Liu W, Baker SS, Baker RD, Zhu L. Antioxidant mechanisms in nonalcoholic fatty liver disease. Curr Drug Targets. 2015;16(12):1301–14. [DOI] [PubMed] [Google Scholar]
- 94. Jung UJ, Lee J, Lee MK, Kim HO, Park EJ, Kim HK, Jeong T, Choi M. Naringin supplementation lowers plasma lipids and enhances erythrocyte antioxidant enzyme activities in hypercholesterolemic subjects. Clin Nutr. 2004;22:561–8. [DOI] [PubMed] [Google Scholar]



