ABSTRACT
Early-life nutrition interventions can have lifelong cardiometabolic benefits. Most evidence on this topic is derived from observational studies. We evaluated the association of randomized controlled nutritional trials in early life and long-term cardiometabolic outcomes. Through literature search of PubMed, CABI Global Health, Embase, and Cochrane, with manual reference check and weekly alert from PubMed, we identified 8312 records, and included 53 records from 40 cohorts in 21 countries. The total number of participants was 33,551. Interventions were initiated as early as conception, and the longest until 7 y (except 1 study from infancy to 20 y). The cohorts were followed up for between 3 and 73 y. We identified 7 types of interventions (protein-energy supplements, long-chain PUFAs, single micronutrient, multiple micronutrients, infant and young child feeding, dietary counseling, and other) and 4 categories of cardiometabolic outcomes (biomarkers, cardiovascular, body size and composition, and subclinical/clinical outcomes). Most findings were null. Fasting glucose concentration was 0.04 mmol/L lower (95% CI: −0.05, −0.02 mmol/L; I2 = 0%) in the intervention groups than in the control groups (15 studies). BMI (kg/m2) was 0.20 higher (95% CI: 0.12, 0.28; I2 = 54%) in the intervention groups than control groups (14 studies). No significant effect was observed for total cholesterol (12 studies) or blood pressure (17 studies). Ongoing and personalized dietary counseling was associated with lower glucose and cholesterol, better endothelial function, and reduced risk of metabolic syndrome. The timing of intervention mattered, with earlier initiation conferring greater benefit (improved lipid profile and marginally lower glucose concentration) based on 2 studies. In sum, glucose concentration was lower following early-life nutrition interventions, but there is a risk of unintended consequences, including higher BMI. Maternal and child nutrition interventions must be evidence-based and tailored to each population to promote long-term cardiometabolic health.
Keywords: maternal and child nutrition, nutrition intervention, randomized controlled trials, early life, DOHaD, life course, cardiometabolic diseases, noncommunicable diseases, longitudinal studies, cohort studies
This review summarized the most updated information on (7 categories of) randomized controlled nutritional trials in early life and their impact on long-term cardiometabolic outcomes.
Introduction
There is much interest in the role of nutrition in early life, often conceptualized as the “first 1000 days” from conception to the second birthday, on child growth and development (1). The potential for nutrition in early life to impact also on adult outcomes has been explored. Early studies by Ravelli et al. (2) and a large body of work by Barker and colleagues (3, 4) led to the formulation of the developmental origins of health and disease paradigm, positing that insults in fetal and early postnatal periods alter the child's growth and development and affect the risk of later cardiometabolic disease (2–4).
Epidemiological evidence from observational studies is abundant, suggesting associations between various early-life nutritional exposures and long-term health consequences (5–7). For instance, ≥6 mo of breastfeeding, compared with shorter duration, is associated with lower odds for diabetes and obesity (8). A recent systematic review reported that low vitamin D status during pregnancy was associated with greater weight in the offspring at 9 mo of age (9). Famine studies are generally considered pseudoexperimental, drawing lessons from unfortunate “natural experiments” to explore the consequences of severe nutritional deprivation in early life (5). Famine studies have identified early gestation as a critical window of development, as well as numerous long-term morbidity and mortality consequences of severe nutritional deprivation in early life (10–13).
Populations that were malnourished in childhood and subsequently exposed to an obesogenic environment are particularly susceptible to cardiometabolic disturbances. This cycle of early-life malnutrition and increased risk in adulthood can predispose future generations to higher risks (7). In a world of aging populations and an increasingly heavy burden of noncommunicable diseases, particularly cardiometabolic diseases, we consider it urgent and critical to investigate the potentials of early-life nutrition investments in preventing long-term illnesses (14).
There is limited evidence from experimental studies, despite considering this type of study to be more indicative of causal associations (15). Nutrition interventions in early life are usually designed to provide short-term benefits, such as promoting infant growth and preventing childhood diseases (16, 17), and randomized trials conducted in pregnant women and young children have been designed to address these shorter-term outcomes. We therefore aimed at summarizing the evidence on the association between early-life nutrition interventions in the form of randomized controlled trials and their long-term influences on cardiometabolic diseases and associated risk factors.
Methods
Electronic literature search
We developed search terms based on 3 main domains, including “early life,” “nutrition interventions,” and “cardiometabolic outcomes.” We included additional qualifiers to specify the concept of “early life” and the duration of follow-up. In the first screening phase, we did not restrict the type of trial, the category of cardiometabolic outcomes, the language, or the publication date. We searched the following databases: PubMed, CABI Global Health, Embase, and Cochrane. We also set up a PubMed email alert to screen new studies published after the initial search (completed on February 12, 2019). We received ongoing, weekly alerts of new publications, and added new records from the weekly list if they met the inclusion criteria.
Screening process
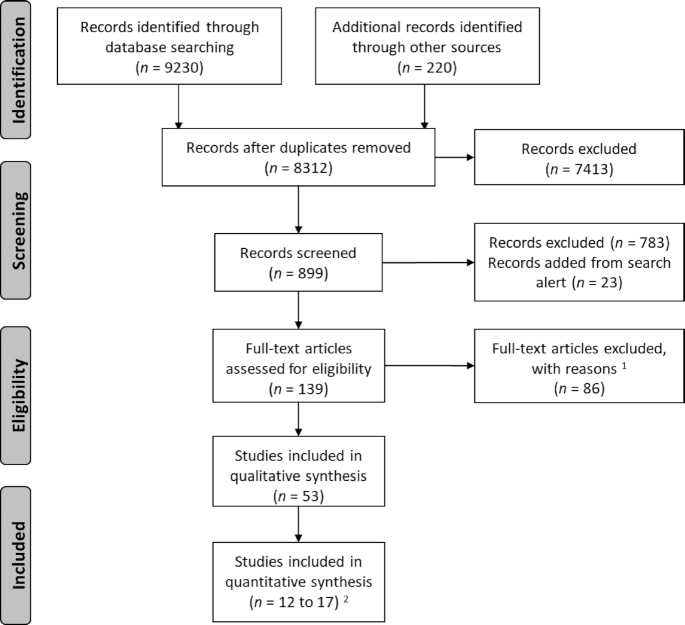
We obtained 9230 records through the electronic literature search process, and an additional 220 records were added through other sources, including searches within known longitudinal studies, manual reference checks, and a search of the gray literature. After removing duplicates, we retained 8312 for first-pass title screening. After removing 7413 records through this initial screening process, we conducted a second round of title and abstract screening of the remaining 899 records, and eventually identified 139 records for full-text review and data extraction.
The inclusion criteria for the articles to be included in the final analysis were: nutrition interventions, randomized controlled trial (acceptable if randomization by cluster or block), intervention conducted in early life (preconception, prenatal, perinatal, postnatal, and during infancy and early childhood), with ≥3 y of follow-up, and outcome(s) relevant to cardiometabolic diseases and risk factors.
Exclusion criteria included: not original research articles (e.g., review, trial protocol, summary of outcomes in a single cohort across decades) (n = 19); observational studies (e.g., cross-sectional analysis) nested within cohorts that had an original randomized controlled trial (n = 6); interventions related to early life development and childcare, but that were not nutritional in nature (n = 1); lacking strictly randomized assignment for the exposure variable (n = 8); no follow-up with the cohort for ≥3 y, or the children were not yet born after maternal intervention (n = 13); no primary outcome of interest (e.g., reported neurodevelopment and dietary pattern) (n = 5); reported maternal outcome but not outcome in the children (n = 1); previously unidentified duplicates (n = 3); reported the same outcomes from the same population as another included study, but from an earlier time point (n = 6); insufficient number of unit in cluster randomization trials (<20 clusters randomized) and without appropriate statistical methods to adjust for this insufficiency (e.g., small-sample corrections or variance-weighted cluster-level analyses) (n = 2) (18); or the analysis not based on original assignment, but used difference-in-difference modeling strategy (n = 2). Famine studies were excluded due to the quasi-experimental exposure assignment and the inability to isolate the nutritional aspects of the famine from other stressors (n = 20). Figure 1 provides the Preferred Reporting Items for Systematic Reviews and Meta-Analyses flow chart. After the screening process, a total of 53 records were retained for data extraction and synthesis of results.
FIGURE 1.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses flow chart study inclusion/exclusion. 1Reasons for exclusion: see Methods. 2For each category of outcome, the number of included studies varied in meta-analysis: 15 included for fasting glucose concentration (21–35); 12 for total cholesterol concentration (21–24,27, 28, 31, 34–38); 17 for blood pressure, same for systolic and diastolic blood pressure (21–23, 27, 31,34, 37, 39–41, 42–45, 46–48); and 14 for BMI (21–23, 25–27,31, 34, 39, 42, 46, 47, 49, 50).
Qualitative and quantitative analysis
We extracted information on basic description of the study, funding and conflict of interest, details of methods, participant profile, and both qualitative and quantitative results. We used the most recent World Bank classification to determine the income levels of the countries where these studies were situated (19).
We first described the publication year, country, cohort, sample size, intervention age, duration of follow-up, summary of the intervention, and outcome category. We evaluated risk of bias in 6 domains based on the Quality in Prognostic Studies tool: study participants, study attrition, prognostic factor measurement, outcome measurement, study confounding, and statistical reporting (20). The research group that developed this tool advise against assigning an overall bias score across all domains, therefore we reported bias assessment by each domain for the studies in a qualitative manner. We also provided a summary of key findings and outcome measurements. To facilitate interpretation of the results, we used bolded and capitalized words to indicate the key message, for instance, “NULL,” “HIGHER,” or “LOWER” value. In this review, the comparison was always the intervention group minus the control group, regardless of how the original article presented the results.
For quantitative synthesis, we conducted meta-analysis using the packages “meta,” “metafor,” “dmetar,” and “esc” in R version 3.6.1 (R Core Team, Foundation for Statistical Computing) (51–53). We included 4 outcomes in meta-analysis: fasting glucose concentration, total cholesterol concentration, blood pressure (systolic and diastolic), and BMI. These outcomes were included based on 2 additional criteria: ≥3 studies reported this outcome; and the reporting format was consistent (e.g., no transformation of the raw data such as z-score or logarithm in some studies but not others). We calculated raw mean differences (MDs) for each study, and used mixed effects models to generate subgroup (based on the types of interventions) and overall effect sizes for each outcome with between-study heterogeneity test, including Higgins and Thompson I2 (percentage of variability). We then plotted the meta-analysis results as forest plots. For each of the selected outcomes, we also provide the corresponding funnel plot to assess publication bias. When a study has >2 arms (e.g., 2 intervention groups and 1 control group), we selected the intervention group based on the value it contributes to this review (maximizing the effect size). We have noted the selection in the figure legends, whenever applicable.
To verify the main findings using standardized measures, we precalculated effect size for each outcome (glucose, total cholesterol, blood pressure, and BMI) by converting either unstandardized regression coefficient or mean and SD to effect size Hedges g, which is bias-corrected standardized mean difference (SMD). The SMD is a suitable measure for this sensitivity analysis because it is more generalizable than MD: the results are standardized regardless of the actual units. Because the findings based on SMD were consistent with the main findings based on MD, we do not present the SMD results.
Results
Summary of study characteristics
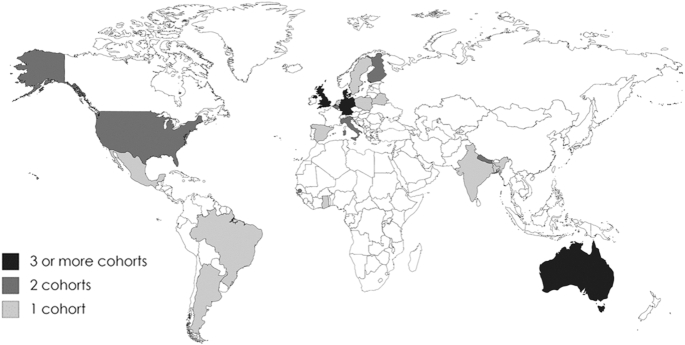
The 53 publications were from 40 cohort studies in 21 countries, including 12 high-income and 9 low- or middle-income countries (Table 1, Figure 2). The total number of participants was 33,551 (counting each cohort once). Publication dates ranged between 1997 and 2020 (Table 1). Interventions were conducted as early as conception, and the longest continued until 7 y of age, with the exception of 1 study—the Special Turku Coronary Risk Factor Intervention Project for Children (STRIP), which was an ongoing dietary counseling intervention that lasted until age 20 y. The cohorts were followed up for between 3 and 73 y (Table 1).
TABLE 1.
Description of studies included in this systematic review
| Type of study | Study (n = 53)1 | Country (n = 21) | Cohort2 (n = 40) | Sample size (% female)3 | Intervention age range (participants) | Duration of follow-up (max) | Description of the intervention (individual or cluster randomization)4 | Cardiometabolic outcomes |
|---|---|---|---|---|---|---|---|---|
| Protein-energy supplementation | ||||||||
| 1 | Hawkesworth et al. 2011 (Trial 1)5 (21) | Gambia | (U) Gambia (2 trials) | Trial 1: 1317 (47.9%) (Trial 2 see single micronutrient) | 20-wk gestation to delivery (mothers) | 17 y | (Cluster: NINT = 16, NCON = 16)Intervention group: Predelivery (20-wk gestation until delivery) provision of protein-energy biscuit (2 biscuits/d, max 1015 kcal energy, 22 g protein, 56 g fat, 47 mg calcium, and 1.8 mg iron)Control group: Postdelivery (delivery until 20 wk postpartum) provision of the same protein-energy biscuit | Biomarkers; Cardiovascular physiology; Body size and composition |
| 2 | Hawkesworth et al. 2009 (54) | Gambia | (U) Gambia (1 trial) | 1317 (47.9%) | 20-wk gestation to delivery (mothers) | 17 y | Same as Hawkesworth et al. (21) | Cardiovascular physiology |
| 3 | Hawkesworth et al. 2008 (22) | Gambia | (U) Gambia (1 trial) | 1317 (47.9%) | 20-wk gestation to delivery (mothers) | 17 y | Same as Hawkesworth et al. (21) | Body size and composition |
| 4 | Kinra et al. 2008 (55) | India | (U) ICDS | 1131 (46.1%) | In utero to 6 y (mothers, infants, and young children) | 18 y | (Cluster: NINT = 15, NCON = 14)Intervention group: Daily protein-calorie supplement “upma,” a local preparation providing 2.51 MJ and 20 g protein to the women and half this amount to the children. No other nutrients. Plus ICDSControl group: Control group had similar ICDS services equivalent to intervention group (uptake was lower) | Biomarkers; Cardiovascular physiology; Body size and composition |
| 5 | Macleod et al. 2013 (23) | United Kingdom | Sorrento Study | 65 (53.5%) | In utero (mothers) | 23 y | (Individual: NINT = 44, NCON = 21)Intervention group 1: Protein, energy, and vitamins: vitamins plus 1810 kJ daily, 90% of energy as carbohydrate (glucose syrup) and 10% as proteinIntervention group 2: Energy and vitamins: vitamins plus 1810 kJ daily of carbohydrateControl group: Vitamins only (vitamin A 0.75 mg, thiamine 1.4 mg, riboflavin 1.7 mg, pyridoxine 2.0 mg, nicotinamide 18 mg, ascorbic acid 60 mg, calciferol 2.5 μg daily delivered in sachets for dissolving in water) | Biomarkers; Cardiovascular physiology; Body size and composition |
| Long-chain PUFA supplementation | ||||||||
| 1 | Asserhøj et al. 2009 (39) | Denmark | (U) Maternal lactation fish oil | 98 (44.9%) | 0–4 mo postpartum (during lactation) (mothers) | 7 y | (Individual: NINT = 64, NCON = 34)Intervention group: Fish-oil supplement (0.6 g/d EPA and 0.8 g/d DHA)Control group: Olive oil supplement/d | Cardiovascular physiology; Body size and composition |
| 2 | Brei et al. 2016 (56) | Germany | INFAT | 114 (NA) | In utero (mothers) | 5 y | (Individual: NINT = 58, NCON = 56)Intervention group: Daily, 1200 mg LCPUFAs (1020 mg DHA + 180 mg EPA + 9 mg vitamin E) as fish oil capsules. Plus individualized dietary counseling aimed at reducing n–6:n–3 ratioControl group: General recommendations regarding healthy nutrition during pregnancy | Body size and composition |
| 3 | Foster et al. 2017 (57) | United States | (U) Obese pregnancy | 63 (41.3%) | 26.6-wk gestation to delivery (mothers) | 4 y | (Individual: NINT = 34, NCON = 29)Intervention group: DHA (800 mg/d) supplementation, from 25- to 29-wk gestation (mean 26.6 wk) until end of pregnancyControl group: Placebo (corn/soy oil), same timing and interval | Body size and composition |
| 4 | Gutierrez-Gomez et al. 2017 (24) | Mexico | POSGRAD | 524 (45.8%) | 18–22-wk gestation to delivery (mothers) | 4 y | (Individual: NINT = 276, NCON = 248)Intervention group: 400 mg/d DHA (LCPUFA) in capsulesControl group:Placebo (a mixture of corn and soy oils) | Biomarkers |
| 5 | Kerling et al. 2019 (40) | United States | KUDOS | 171 (51.5%) | Mean enrollment gestational age 14.5 ± 3.7 wk to delivery (mothers) | 6 y | (Individual: NINT = 770, NCON = 761)Intervention group: Three 600-mg capsules DHA-containing algal oil/dControl group: Three 600-mg capsules with equal mixture of soybean and corn oil (no DHA)/d | Cardiovascular physiology |
| 6 | Muhlhausler et al. 2016 (25) | Australia | DOMInO | 1531 (50.0%) | In utero (mothers) | 5 y | (Individual: NINT = 770, NCON = 761)Intervention group: Three 500-mg capsules DHA-rich fish oil/d (800 mg DHA/d and 100 mg EPA/d)Control group: Three 500-mg vegetable-oil capsules (without DHA)/d | Biomarkers; Body size and composition |
| 7 | Rytter et al. 2012 (41) | Denmark | (U) Maternal pregnancy fish oil | 180 (54.4%) | 30-wk gestation to delivery (mothers) | 19 y | (Individual: NINT = 108, NCON = 72)Intervention group 1: Fish oil capsules (2.7 g n–3 LCPUFA/d)“Intervention” group 2: No capsuleControl group: Olive oil capsules/d | Cardiovascular physiology |
| 8 | Rytter et al. 2011a (26) | Denmark | (U) Maternal pregnancy fish oil | 135 (54.8%) | 30-wk gestation to delivery (mothers) | 19 y | Same as Rytter et al. (41) | Biomarkers; Body size and composition |
| 9 | Rytter et al. 2011b (36) | Denmark | (U) Maternal pregnancy fish oil | 180 (53.9%) | 30-wk gestation to delivery (mothers) | 19 y | Same as Rytter et al. (41) | Biomarkers |
| 10 | See et al. 2018 (27) | Australia | (U) Infant fish oil | 322 (51.6%) | 0–6 mo postpartum (infants) | 5 y | (Individual: NINT = 165, NCON = 157)Intervention group: Daily 650 mg encapsulated n–3 LCPUFA in the form of ethyl esters (280 mg DHA and 110 mg EPA)Control group: Daily olive oil (66.6% ω-9 oleic acid) | Biomarkers; Cardiovascular physiology; Body size and composition |
| 11 | Vinding et al. 2018 (58) | Denmark | COPSAC2010 | 523 (49.0%) | 24-wk gestation to 1-wk postpartum (mothers) | 6 y | (Individual: NINT = 263, NCON = 260)Intervention group: Fish oil: 2.4 g n–3 LCPUFA/dControl group: Placebo were lookalike control supplementation capsules of olive oil (72% n–9 oleic acid and 12% n–6 linoleic acid/d | Body size and composition |
| Single-micronutrient supplementation | ||||||||
| 1 | Belizan et al. 1997 (42) | Argentina | (U) Maternal calcium | 518 (46.2%) | 20-wk gestation to delivery | 7 y | (Individual: NINT = 257, NCON = 261)Intervention group: 2 g/d elemental calciumControl group: Placebo/d | Cardiovascular physiology; Body size and composition |
| 2 | Hawkesworth et al. 2011 (Trial 2)5 (21) | Gambia | (U) Gambia (2 trials) | Trial 2: 389 (NA) (Trial 1 see protein-energy) | 20-wk gestation to delivery (mothers) | 17 y | (Individual: NINT = 193, NCON = 196)Intervention group: Calcium supplementation (1500 mg/d elemental calcium as 3750 mg calcium carbonate)Control group: Placebo/d | Cardiovascular physiology; Body size and composition |
| 3 | Hiller et al. 2007 (43) | Australia | ACT | 179 (NA) | Less than 24-wk gestation to delivery (mothers) | 8 y | (Individual: NINT = 91, NCON = 88)Intervention group: 1.8g/d calciumControl group: Placebo/d | Cardiovascular physiology |
| 4 | Palmer et al. 2020 (59) | Nepal | NNIPS-2 | 290 (47.9%) | In utero until lactation (mothers) | 13 y | (Cluster: NINT = 180, NCON = 90)Intervention group 1: Vitamin A (700 μg retinol equivalent/d)Intervention group 2: β-Carotene (42 mg/d)Control group: Placebo/d | Biomarkers |
| 5 | Taylor et al. 2015 (60) | United Kingdom | Aberdeen Folic Acid Supplementation Trial | 2928 (NA) | In utero (mothers) | 73 y | (Individual: NINT = 951, NCON = 1977)Intervention group 1: 0.2 mg folic acid/dIntervention group 2: 5 mg folic acid/dControl group: Placebo/d | Clinical and subclinical outcomes |
| Multiple-micronutrient supplementation | ||||||||
| 1 | Ekström et al. 2016 (Trial 1)6 (28) | Bangladesh | MINIMat | Trial 1: 1335 (47.0%) (Trial 2 see other intervention) | In utero (mothers) | 4.5 y | (Individual: NINT = 435, NCON = 900)Intervention group: MMS/dControl group: Iron (60 mg/d) and folic acid (400 μg/d) (IFA) | Biomarkers |
| 2 | Kumordzie et al. 2019 (61) | Ghana | iLiNS-DYAD Ghana Trial | 961 (51.9%) | 20-wk gestation to 18 mo postpartum (mothers and infants) | 6 y | (Individual: NINT = 338, NCON = 623)Intervention group: 20 g (118 kcal)/d LNS during pregnancy and the first 6 mo postpartum; followed by 20 g/d LNS 6–18 mo for infantsControl group: Combining 2 groups: 1) daily IFA tablets during pregnancy, 200 mg/d calcium during the first 6 mo postpartum, and no infant supplementation; 2) daily MMN tablets (1–2 RDA) of 18 micronutrients during pregnancy and the first 6 mo postpartum, no infant supplementation | Body size and composition |
| 3 | Mannan et al. 2016 (62) | Bangladesh | MINIMat | 540 (42.0%) | In utero (mothers) | 9 y | (Individual: NINT = 167, NCON = 373)Intervention group: MMS: 60 mg iron, 400 μg folic acid, plus 15 micronutrients/dControl group: Iron (60 mg) and folic acid (400 μg) (IFA)/d | Biomarkers; Body size and composition |
| 4 | Stewart et al. 2011 (63) | Nepal | (U) Antenatal MMS | 545 (NA) | In utero (mothers) | 8 y | (Cluster: 426 total units for 5 arms)Intervention group 1: Folic acid (400 μg/d)Intervention group 2: Folic acid + iron (60 mg ferrous fumarate/d)Intervention group 3: Folic acid + iron + zinc (30 mg zinc sulfate/d)Intervention group 4: Daily folic acid + iron + zinc + 11 vitamins and minerals (10 mg vitamin D as cholecalciferol, 10 mg vitamin E as d-α tocopherol, 1.6 mg thiamine, 1.8 mg | Biomarkers |
| riboflavin, 20 mg niacin, 2.2 mg vitamin B-6, 2.6 mg vitamin B-12, 100 mg vitamin C, 65 mg vitamin K as phylloquinone, 2.0 mg Cu, 100 mg MgControl group: Vitamin A (retinyl palmitate) 1000 μg RE of preformed vitamin A/d | ||||||||
| 5 | Stewart et al. 2009 (64) | Nepal | (U) Antenatal MMS | 3524 (NA) | In utero (mothers) | 8 y | Same as Stewart et al. (63) | Clinical and subclinical outcomes |
| Infant and young child feeding, and milk supplementation | ||||||||
| 1 | de Jong et al. 2011 (44) | the Netherlands | The Groningen LCPUFA study | 341 (48.1%) | 0–8 mo postpartum (infants) | 9 y | (Individual: NINT = 91, NCON = 250)Intervention group: Breastfeeding (BF)Intervention/control group: LCPUFA-supplemented formula group (LF): the LCPUFAs were provided as mix of phospholipids (15%) and triglycerides (85%) to mimic the composition of breast milk.Control group: Standard formula group (CF): standard formula consisted of Nutrilon Premium | Cardiovascular physiology; Body size and composition |
| 2 | Forsyth et al. 2003 (45) | Four European countries: United Kingdom, Italy, Belgium, and Germany | (U) LCPUFA infant formula | 147 (46.9%) | 0–4 mo postpartum (infants) | 5 y | (Individual: NINT = 71, NCON = 76)Intervention group: LCPUFA-supplemented infant formulaControl group: Unsupplemented infant formula | Cardiovascular physiology |
| 3 | Gruszfeld et al. 2016 (65) | Five European countries: Belgium, Germany, Italy, Poland, and Spain | CHOP | 183 (48.6%) | 0–12 mo postpartum (infants) | 5 y | (Individual: NINT = 86, NCON = 97)Intervention group: High-protein formula (2.05 g/dL for infants, and 3.2 g/dL as follow-up formula) (HP)Control group: Low-protein formula (1.25 g/dL for infants, and 1.6 g/dL as follow-up formula), equal energy as HP groupAlso has breastfeeding as observational group | Body size and composition;Clinical and subclinical outcomes |
| 4 | Gruszfeld et al. 2015 (37) | Five European countries: Belgium, Germany, Italy, Poland, and Spain | CHOP | 183 (48.6%) | 0–12 mo postpartum (infants) | 5 y | (Individual: NINT = 92, NCON = 91)Same as Gruszfeld et al. (65) | Biomarkers; Cardiovascular physiology; Body size and composition |
| 5 | Kennedy et al. 2010 (46) | United Kingdom | (U) UK preterm LCPUFA | 107 (47.7%) | 0–9 mo postpartum (preterm infants) | 10 y | (Individual: NINT = 50, NCON = 57)Intervention group: LCPUFA-supplemented infant formulaControl group: Unsupplemented infant formula | Cardiovascular physiology; Body size and composition |
| 6 | Kramer et al. 2007 (66) | Belarus | PROBIT | 13,889 (48.3%) | During postpartum stay in hospitals (mothers) | 6.5 y | (Cluster: NINT = 16, NCON = 16)Intervention group: Based on the Baby-Friendly Hospital Initiative to promote and support breastfeeding, particularly among mothers who have chosen to initiate breastfeedingControl group: The control maternity hospitals and polyclinics continued the practices and policies in effect at the time of randomization | Cardiovascular physiology; Body size and composition |
| 7 | Martin et al. 2017 (47) | Belarus | PROBIT | 13,557 (48.5%) | During postpartum stay in hospitals (mothers) | 16 y | Same as Kramer et al. (66) | Cardiovascular physiology; Body size and composition |
| 8 | Martin et al. 2014 (29) | Belarus | PROBIT | 13,616 (48.5%) | During postpartum stay in hospitals (mothers) | 11.5 y | Same as Kramer et al. (66) | Biomarkers; Cardiovascular physiology; Clinical and subclinical outcomes |
| 9 | Martin et al. 2013 (67) | Belarus | PROBIT | 13,879 (48.5%) | During postpartum stay in hospitals (mothers) | 11.5 y | Same as Kramer et al. (66) | Body size and composition |
| 10 | Singhal et al. 2010 (49) | United Kingdom | (U) SGA cohort | 243 (55.2%) | Trial 1: 0–9 mo postpartumTrial 2: 0–6 mo postpartum (small-for-gestational-age infants) | 8 y | Trial 1: (Individual: NINT = 70, NCON = 83)Intervention group 1: Nutrient-enriched formula, 28% more protein and 6% more energy than control formula, plus more micronutrientsControl group 1: Standard term formulaTrial 2: (Individual: NINT = 41, NCON = 49)Intervention group 2: Nutrient-enriched formula, 43% more protein and 12% more energy than control formula, plus more micronutrientsControl group 2: Standard term formulaAlso has breastfeeding group as an observational reference | Body size and composition |
| 11 | Singhal et al. 2004 (38) | United Kingdom | (U) Preterm cohort | 216 (50.5%) | Postpartum until weight reached 2000 g or discharge home (median 4 wk) (preterm infants) | 16 y | Trial 1: (Individual: NINT = 66, NCON = 64)Intervention group 1: Banked breastmilk from donationControl group 1: Nutrient-enriched preterm formula, enriched in protein (20 g) and fat (45 g) but not carbohydrate (70 g/L)Trial 2: (Individual: NINT = 44, NCON = 42)Intervention group 2: Standard term formula (15 g protein, 38 g fat, 70 g/L carbohydrate)Control group 2: Nutrient-enriched preterm formula, enriched in protein (20 g) and fat (45 g) but not carbohydrate (70 g/L) | Biomarkers |
| 12 | Singhal et al. 2003 (30) | United Kingdom | (U) Preterm cohort | 216 (50.5%) | Postpartum until weight reached 2000 g or discharge home (median 4 wk) (preterm infants) | 16 y | Same as Singhal et al. (38) | Biomarkers |
| 13 | Singhal et al. 2002 (68) | United Kingdom | (U) Preterm cohort | 216 (50.5%) | Postpartum until weight reached 2000 g or discharge home (median 4 wk) (preterm infants) | 16 y | Same as Singhal et al. (38) | Biomarkers |
| 14 | Singhal et al. 2001 (48) | United Kingdom | (U) Preterm cohort | 216 (50.5%) | Postpartum until weight reached 2000 g or discharge home (median 4 wk) | 16 y | Same as Singhal et al. (38) | Cardiovascular physiology |
| 15 | Toftlund et al. 2018 (31) | Denmark | (U) Preterm cohort | 235 (48.5%) | 0–4 mo postpartum (preterm infants) | 6 y | (Individual: NINT = 71, NCON = 164)Intervention group: Preterm formula (68 kcal, 2 g protein, 7.4 g carbohydrate, 3.5 g fat/100 mL)Control group: Breastmilk | Biomarkers; Cardiovascular physiology; Body size and composition |
| 16 | Totzauer et al. 2018 (69) | Five European countries: Belgium, Germany, Italy, Poland, and Spain | CHOP | 440 (47.6%) | 0–12 mo postpartum (infants) | 6 y | Same as Gruszfeld et al. (65) | Body size and composition |
| 17 | Weber et al. 2014 (50) | Five European countries: Belgium, Germany, Italy, Poland, and Spain | CHOP | 518 (NA) | 0–12 mo postpartum (infants) | 6 y | Same as Gruszfeld et al. (65) | Body size and composition; Clinical and subclinical outcomes |
| 18 | Williams et al. 2012 (32) | United Kingdom | BCG | 569 (45.4%) | 0–5 y (mothers and infants) | 27 y | (Individual: NINT = 531, NCON = 38)Intervention group: Free (cow) milk supplements [through the provision of tokens equating to half-pint (284 mL) milk/d] for pregnant women and their infants up to 5 yControl group: Breastfeeding only | Biomarkers |
| Dietary counseling | ||||||||
| 1 | Costa et al. 2017 (33) | Brazil | (U) São Leopoldo dietary counseling | 305 (43.6%) | 0–1 y (mothers) | 8 y | (Individual: NINT = 126, NCON = 179)Intervention group: During each home visit, mothers received dietary advice in accordance with the baby's ageControl group: Standard care | Biomarkers |
| 2 | Hakanen et al. 2006 (70) | Finland | STRIP | 585 (NA) | 7 mo to 10 y (mother and children) | 10 y | (Individual: original NINT = 540, NCON = 522)Intervention group: Received individualized dietary counseling at 1- to 3-mo intervals until the child was 2 y and biannually thereafter. The main focus was on replacing intake of saturated fat with unsaturated fatControl group: Basic health education routinely given at Finnish well-baby clinics and by school health care. Biannually until 7 y and annually thereafter | Clinical and subclinical outcomes |
| 3 | Lehtovirta et al. 2018 (71) | Finland | STRIP | 450 (48.4%) | 7 mo to 20 y (mother and children) | 20 y | Same as Hakanen et al. (70) | Biomarkers |
| 4 | Nupponen et al. 2015 (72) | Finland | STRIP | 514 (49.0%) | 7 mo to 20 y (mother and children) | 20 y | Same as Hakanen et al. (70) | Clinical and subclinical outcomes |
| 5 | Pahkala et al. 2020 (34) | Finland | STRIP | 551 (56.0%) | 7 mo to 20 y | 26 y | Same as Hakanen et al. (70) | Biomarkers; Cardiovascular physiology; Body size and composition |
| 6 | Pahkala et al. 2013 (73) | Finland | STRIP | 394 (45.2%) | 7 mo to 19 y (mother and children) | 19 y | Same as Hakanen et al. (70) | Clinical and subclinical outcomes |
| 7 | Raitakari et al. 2005 (74) | Finland | STRIP | 369 (50.7%) | 7 mo to 19 y (mother and children) | 19 y | Same as Hakanen et al. (70) | Biomarkers; Cardiovascular physiology; Body size and composition |
| Other interventions | ||||||||
| 1 | Ekström et al. 2016 (Trial 2)6 (28) | Bangladesh | MINIMat | Trial 2: 1335 (47.0%) (Trial 1 see multiple micronutrients) | In utero (mothers) | 4.5 y | (Individual: NINT = 672, NCON = 663)Intervention group: Early timing of invitation (immediately after detection of pregnancy) to food | Biomarkers |
| supplement, 608 kcal/d, 6 d/wkControl group: Usual timing of invitation (∼20 wk gestation) to food supplementation | ||||||||
| 2 | Luoto et al. 2010 (75) | Finland | (U) Probiotics Study | 113 (39.8%) | 4 wk before delivery until 6 mo postpartum (mothers and infants) | 10 y | (Individual: NINT = 54, NCON = 59)Intervention group: Probiotic supplementation: 1×1010 colony-forming units of Lactobacillus rhamnosusin capsules once a day for 4 wk before expected delivery. After delivery, given either to the mothers (if breastfeeding), or to the children mixed in water for 6 moControl group: Microcrystalline cellulose | Body size and composition; Clinical and subclinical outcomes |
| 3 | Videhult et al. 2015a (35) | Sweden | (U) Probiotics Study | 120 (56.6%) | 4–13 mo postpartum (infants) | 8.8 y | (Individual: NINT = 58, NCON = 62)Intervention group: Daily intake of cereals with probiotic LF19 (Lactobacillus paracasei ssp. paracasei strain F19)Control group: Daily intake of cereals without LF19 | Biomarkers; Body size and composition |
| 4 | Videhult et al. 2015b (76) | Sweden | (U) Probiotics Study | 120 (56.6%) | 4–13 mo postpartum (infants) | 8.8 y | Same as Videhult et al. (35) | Biomarkers |
The studies were sorted by the type of intervention, then listed in alphabetical order (A to Z), followed by chronological order (newer to older). Some studies were in >1 intervention categories, but they were only included in 1 category based on the most dominant feature of the study (e.g., infant feeding with LCPUFA-enriched infant formula could be in categories infant formula and macronutrient supplementation, but was only presented as IYCF study). ICDS, integrated childhood development services; IFA, iron and folic acid supplementation; LCPUFA, long-chain polyunsaturated fatty acid; LNS, lipid-based nutrient supplement; MMN, multi-micronutrient; MMS, multiple-micronutrient supplementation; NA, not applicable; RE, regional equivalent; SGA, small-for-gestational age; U, unofficial.
Cohort abbreviations (“U” means unofficial study name for the purpose of this review only; the rest are official cohort study names). ACT, Australian Calcium Trial; BCG, the Barry-Caerphilly Growth Study; CHOP, the European Childhood Obesity Project; COPSAC2010, Mother-child cohort Copenhagen Prospective Studies on Asthma in Childhood 2010; DOMInO: DHA to Optimize Mother Infant Outcome Trial; iLiNS-DYAD, the International Lipid-based Nutrient Supplements-DYAD trial in Ghana; INFAT, Impact of Nutritional Fatty Acids during Pregnancy and Lactation on Early Human Adipose Tissue Development; KUDOS, Kansas University DHA Outcome Study trial; MINIMat, Maternal and Infant Nutrition Interventions in Matlab Trial; NNIPS-2, Nepal Nutrition Intervention Project—Sarlahi; POSGRAD, the Prenatal Omega-3 Fatty Acid Supplementation, Growth, and Development Trial; PROBIT, the Promotion of Breastfeeding Intervention Trial; STRIP, Special Turku Coronary Risk Factor Intervention Project for Children.
Sample size refers to the main cohort whose cardiometabolic outcomes were assessed (e.g., if maternal intervention, it refers to the offspring).
N INT, sample size in intervention group (or number of intervention clusters in cluster randomization); NCON, sample size in control group (or number of control clusters in cluster randomization).
This study reported results of 2 trials within the same population: 1) protein-energy supplementation, and 2) calcium supplementation.
This study has 2 sets of interventions: 1) multiple micronutrient supplement, and 2) a food-based intervention.
FIGURE 2.
Map of the world indicating the countries and cohorts included. Created with www.mapchart.net.
We identified 7 categories of interventions. 1) Protein-energy supplementation (21–23, 54, 55): in this type of intervention, the study participants (children, and/or their mothers) were provided supplements that contain mainly protein and energy; some of these supplements might include micronutrients, but the focus was protein and calories. 2) Long-chain polyunsaturated fatty acid (LCPUFA) supplementation (39, 56, 57, 24, 40, 25, 41, 26, 36, 27, 58): some studies also included micronutrients with the LCPUFAs. 3) Single-micronutrient supplementation (21, 42, 43, 59, 60): only 1 micronutrient was provided in this type of trial, such as calcium supplementation. 4) Multiple-micronutrient supplementation (MMS) (28, 61–64): ≥2 micronutrients were provided as supplements. One study included a small amount of protein (lipid-based nutrient supplements). 5) Infant and young child feeding (IYCF), and milk supplementation (44, 45, 65, 37, 46, 66, 47, 29, 67, 49, 38, 30, 68, 48, 31, 69, 50, 32): all trials related to promoting or practicing IYCF were included in this category. A few studies that could have been included in the previous categories were included as IYCF trials if IYCF was the main purpose (e.g., LCPUFA-supplemented infant formula). 6) Dietary counseling (33,34, 70–74): either the participants or their caretakers were provided dietary counseling. 7) Other interventions (28, 35, 75, 76): in this category we included all other types of trials that did not fit in any of the previous categories, including a food-based intervention and probiotics trials.
We presented the cardiometabolic outcomes in 4 categories: 1) biomarkers (e.g., glucose and insulin concentrations, lipid profile, and inflammation markers); 2) cardiovascular physiology (e.g., blood pressure); 3) body size and body composition (e.g., BMI); and 4) subclinical and clinical cardiometabolic outcomes (e.g., obesity status).
Most of the included studies were in the low-to-moderate bias category across domains of bias assessment, except for Forsyth et al. (45), which has high risk of bias in 4 domains (Table 2). We did not observe publication bias for any of the selected outcomes (Supplemental Figure 1).
TABLE 2.
Risk-of-bias assessment for included studies
| Domains of bias evaluation (low, moderate, or high risk of bias)1 | ||||||
|---|---|---|---|---|---|---|
| Study category and identification | Study participants | Study attrition | Prognostic factor measurement | Outcome measurement | Study confounding | Statistical analysis and reporting |
| Protein-energy supplementation | ||||||
| Hawkesworth 2011 (Trial 1) (21) | Low | Moderate | Low | Low | Low | Low |
| Hawkesworth 2009 (54) | Low | Moderate | Low | Low | Low | Low |
| Hawkesworth 2008 (22) | Low | Moderate | Low | Low | Low | Low |
| Kinra 2008 (55) | Low | Low | Low | Low | Low | Low |
| Macleod 2013 (23) | High | Moderate | Low | Low | Low | Moderate |
| Long-chain polyunsaturated fatty acid supplementation | ||||||
| Asserhøj 2009 (39) | Moderate | Moderate | Low | Low | Low | Low |
| Brei 2016 (56) | Low | Moderate | Low | Low | Moderate | Low |
| Foster 2017 (57) | Moderate | Low | Low | Low | Moderate | Moderate |
| Gutierrez-Gomez 2017 (24) | Low | Moderate | Low | Low | Moderate | Low |
| Kerling 2019 (40) | Low | Moderate | Low | Low | Moderate | Low |
| Muhlhausler 2016 (25) | Low | Low | Low | Low | Low | Low |
| Rytter 2012 (41) | Low | Moderate | Low | Low | Low | Low |
| Rytter 2011a (26) | Low | Moderate | Low | Low | Low | Low |
| Rytter 2011b (36) | Low | Moderate | Low | Low | Low | Low |
| See 2018 (27) | Moderate | Moderate | Low | Low | Moderate | Moderate |
| Vinding et al. 2018 (58) | Low | Low | Low | Low | Low | Low |
| Single-micronutrient supplementation | ||||||
| Belizan 1997 (42) | Low | Moderate | Low | Low | Moderate | Low |
| Hawkesworth 2011 (Trial 2) (21) | Low | Moderate | Low | Low | Low | Low |
| Hiller 2007 (43) | Moderate | High | Low | Low | Moderate | Moderate |
| Palmer 2020 (59) | Low | High | Low | Low | Low | Low |
| Taylor 2015 (60) | Low | Low | Low | Low | Low | Low |
| Multiple-micronutrient supplementation | ||||||
| Ekström 2016 (Trial 1) (28) | Low | Low | Low | Low | Moderate | Moderate |
| Kumordzie 2019 (61) | Low | Low | Low | Low | Low | Low |
| Mannan 2016 (62) | Low | High | Low | Low | Low | Moderate |
| Stewart 2011 (63) | Low | Moderate | Low | Low | Low | Low |
| Stewart 2009 (64) | Low | Low | Low | Low | Low | Low |
| Infant and young child feeding, and milk supplementation | ||||||
| de Jong 2011 (44) | Low | Low | Low | Low | Moderate | Moderate |
| Forsyth 2003 (45) | Low | Moderate | High | High | High | High |
| Gruszfeld 2016 (65) | Low | High | Low | Low | Low | Low |
| Gruszfeld 2015 (37) | Low | Moderate | Low | Low | High | Moderate |
| Kennedy 2010 (46) | Low | Moderate | Low | Low | Moderate | Low |
| Kramer 2007 (66) | Low | Low | Low | Low | Low | Moderate |
| Martin 2017 (47) | Low | Low | Low | Low | Low | Low |
| Martin 2014 (29) | Low | Low | Low | Low | Low | Low |
| Martin 2013 (67) | Low | Low | Low | Low | Low | Low |
| Singhal 2010 (49) | Low | Moderate | Low | Low | Low | Low |
| Singhal 2004 (38) | Moderate | High | Low | Low | Low | Low |
| Singhal 2003 (30) | Moderate | High | Low | Low | Low | Low |
| Singhal 2002 (68) | Moderate | High | Low | Low | Low | Moderate |
| Singhal 2001 (48) | Moderate | High | Low | Low | Low | Low |
| Toftlund 2018 (31) | Moderate | Moderate | Low | Low | Moderate | Moderate |
| Totzauer 2018 (69) | Low | Moderate | Low | Low | Low | Low |
| Weber 2014 (50) | Low | Moderate | Low | Low | Low | Low |
| Williams 2012 (32) | Low | Moderate | Low | Low | Low | Low |
| Dietary counseling | ||||||
| Costa 2017 (33) | Low | Moderate | Low | Low | Low | Low |
| Hakanen 2006 (70) | Low | Moderate | Low | Low | Moderate | Low |
| Lehtovirta 2018 (71) | Low | Moderate | Low | Low | Low | Moderate |
| Nupponen 2015 (72) | Low | Moderate | Low | Low | Moderate | Low |
| Pahkala 2020 (34) | Low | Moderate | Low | Low | Moderate | Low |
| Pahkala 2013 (73) | Low | Moderate | Low | Low | Moderate | Low |
| Raitakari 2005 (74) | Low | Moderate | Low | Low | Moderate | Moderate |
| Other interventions | ||||||
| Ekström 2016 (Trial 2) (28) | Low | Low | Low | Low | Moderate | Moderate |
| Luoto 2010 (75) | Moderate | Low | Low | Low | Moderate | Low |
| Videhult 2015a (35) | Low | Low | Low | Low | Moderate | Moderate |
| Videhult 2015b (76) | Low | Low | Low | Low | Moderate | Low |
Bias assessment followed specific criteria by each domain:
1) Study participants: High bias—the relation between predictor and outcome is very likely to be different for participants and eligible nonparticipants. Moderate bias—the relation could be different. Low bias—the relation is unlikely to be different.
2) Study attrition: High bias—the relation between predictor and outcome is very likely to be different for completing and noncompleting participants. Moderate bias—the relation could be different. Low bias—the relation is unlikely to be different.
3) Prognostic factor measurement: High bias—the measurement of the predictor is very likely to be different for different levels of the outcome of interest. Moderate bias—the measurement could be different. Low bias—the measurement is unlikely to be different.
4) Outcome measurement: High bias—the measurement of the outcome is very likely to be different related to the baseline level of the predictor. Moderate bias—the measurement could be different. Low bias—the measurement is unlikely to be different.
5) Study confounding: High bias—the observed effect of the predictor on the outcome is very likely to be distorted by another factor related to the predictor and outcome. Moderate bias—the effect could be distorted. Low bias—the effect is unlikely to be distorted.
6) Statistical analysis and reporting: High bias—the reported results are very likely to be spurious or biased related to analysis or reporting. Moderate bias–could be spurious or biased. Low bias—unlikely to be spurious or biased.
Outcome category 1: biomarkers
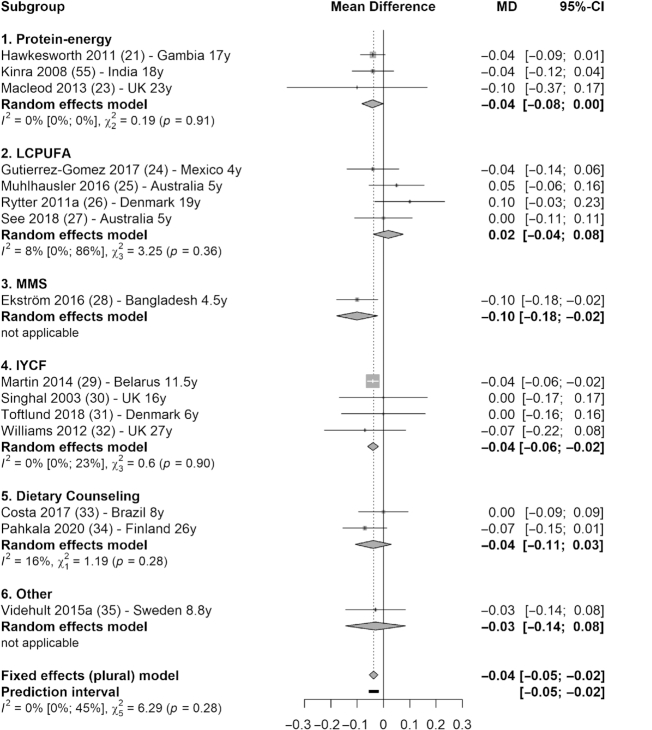
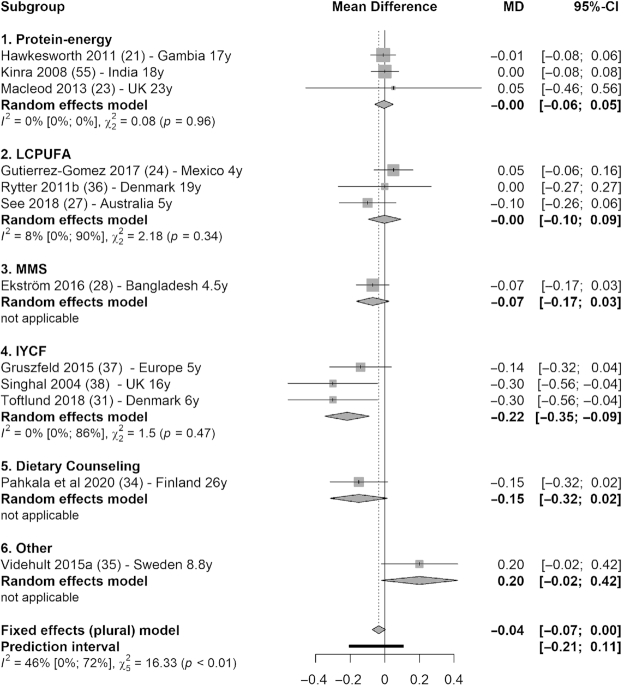
Meta-analysis (15 studies) showed that fasting glucose concentration was significantly lower in intervention groups compared with control groups (MD = −0.04 mmol/L; 95% CI: −0.05, −0.02 mmol/L; I2 = 0%) (Figure 3). Subgroup analysis showed that the reduction in glucose concentration was significant in 3 types of interventions: −0.04 mmol/L (95% CI: −0.08, 0.00 mmol/L; I2 = 0%) in protein-energy category (21–23); −0.10 mmol/L (95% CI: −0.18, −0.02 mmol/L) based on 1 MMS trial (28); and −0.04 mmol/L (95% CI: −0.06, −0.02 mmol/L; I2 = 0%) in IYCF category (29, 30, 31, 32) (Figure 3). There was also a marginal decrease in total cholesterol (12 studies) in the intervention groups compared with control groups (MD = −0.04 mmol/L; 95% CI: −0.07, 0.00 mmol/L; I2 = 46%), which was driven mainly by the 0.22 mmol/L decrease in total cholesterol in the IYCF category (95% CI: −0.35, −0.09 mmol/L; I2 = 0%) (Figure 4).
FIGURE 3.
Forest plot with subgroup analysis of the effect of early-life nutrition interventions on long-term fasting glucose concentration (millimoles per liter). Study identification: last name of the first author and publication year, followed by reference number, country (or region if multisite study), and the duration of follow-up (in years). Different articles by the same author and in the same year are distinguished by a (or b). Hawkesworth et al. (21): the first trial in the article (protein-energy supplementation) was included in this analysis. Macleod et al. (23): the first arm of the intervention was included in this analysis (protein, carbohydrate, and vitamin compared with vitamin only). Ekström et al. (28): the second trial (multiple-micronutrient supplementation compared with iron and folic acid supplementation) was included in this analysis. “Other” denotes other interventions (in this case, probiotics). IYCF, infant and young child feeding; LCPUFA, long-chain polyunsaturated fatty acid supplementation; MD, mean difference; MMS, multiple-micronutrient supplementation.
FIGURE 4.
Forest plot with subgroup analysis of the effect of early-life nutrition interventions on long-term total cholesterol concentration (millimoles per liter). Study identification: last name of the first author and publication year, followed by reference number, country (or region if multisite study), and the duration of follow-up (in years). Different articles by the same author and in the same year are distinguished by a (or b). Hawkesworth et al. (21): the first trial in the article (protein-energy supplementation) was included in this analysis. Macleod et al. (23): the first arm of the intervention was included in this analysis (protein, carbohydrate, and vitamin compared with vitamin only). Ekström et al. (28): the second trial (multiple micronutrient supplementation compared with iron and folic acid supplementation) was included in this analysis. “Other” denotes other interventions (in this case, probiotics). IYCF, infant and young child feeding; LCPUFA, long-chain polyunsaturated fatty acid supplementation; MD, mean difference; MMS, multiple-micronutrient supplementation.
Qualitative evidence showed predominantly a reduction in biomarker concentrations (glycemic markers, lipids, and apolipoproteins, as well as inflammation markers) with a few exceptions (Supplemental Table 1). Two protein-energy trials that presented biomarker outcomes showed marginally lower fasting glucose, and lower insulin and HOMA-IR score (21, 55). One LCPUFA trial reported higher insulin resistance, whereas the other reported lower insulin concentration and insulin resistance (27). Two multiple micronutrient interventions reported lower total cholesterol and lower inflammation markers (62, 77). Most IYCF studies reported null findings, and those studies with significant findings were inconsistent in terms of the direction of effect. Based on the same study in preterm infants, Singhal et al. (78, 79) reported lower C-reactive protein and LDL cholesterol:HDL cholesterol ratio in banked breastmilk compared with preterm formula group, but also higher insulin resistance. They further reported lower leptin concentration (relative to fat mass) in the intervention group (68). In addition, Toftlund et al. (31) reported lower cholesterol concentration in the breastmilk group than in the preterm formula group. Dietary counseling (with evidence coming primarily from the STRIP study) reported lower fasting glucose and insulin resistance, lower circulation fatty acids, higher serum PUFAs, and lower total cholesterol and LDL cholesterol in the intervention groups (71, 34). All other results were null (Supplemental Table 1).
Outcome category 2: cardiovascular physiology
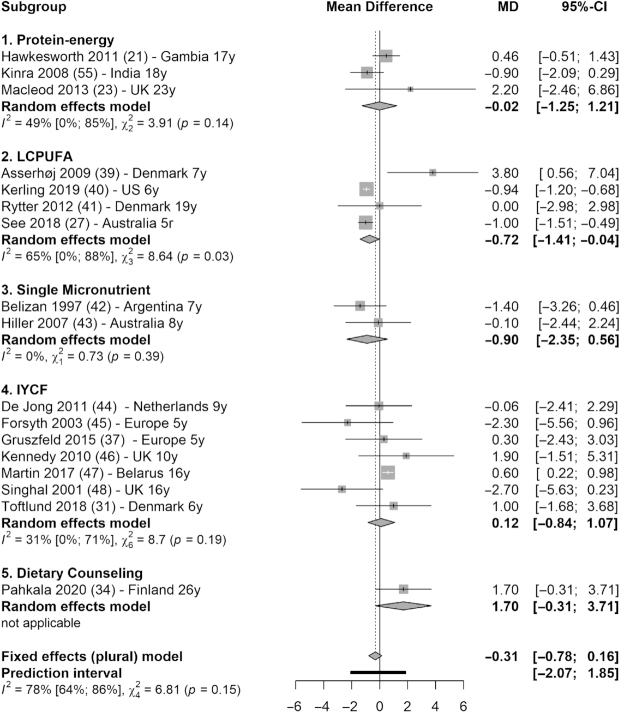
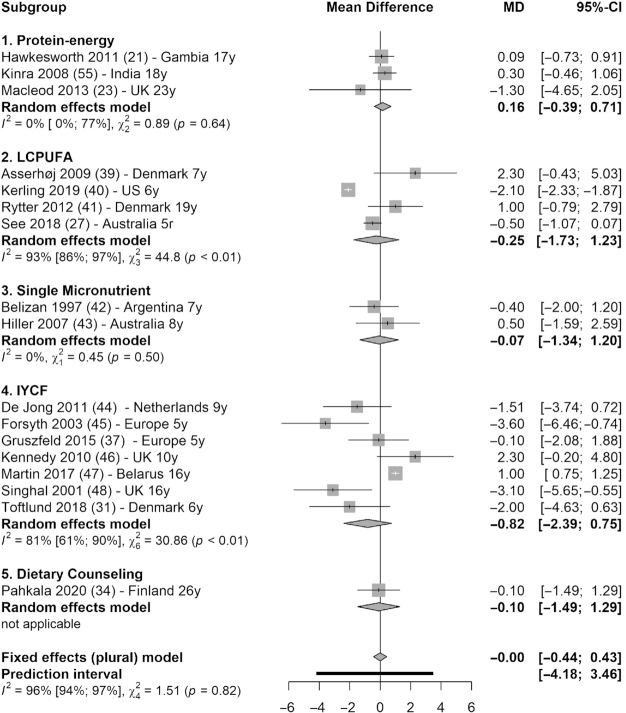
Meta-analysis (17 studies) showed that there was no statistically significant difference between intervention and control groups in blood pressure. Systolic blood pressure reduced by 0.31 mmHg in the intervention groups (95% CI: −0.78, 0.16 mmHg; I2 = 78%), with only LCPUFA interventions showing significant reduction (MD = −0.72 mmHg; 95% CI: −1.41, −0.04 mmHg; I2 = 65%) (39–41, 27) (Figure 5). Diastolic blood pressure was not reduced across interventions (MD = −0.00 mmHg; 95% CI: −0.44, 0.43 mmHg; I2 = 96%) (Figure 6).
FIGURE 5.
Forest plot with subgroup analysis of the effect of early-life nutrition interventions on long-term systolic blood pressure (mmHg). Study identification: last name of the first author and publication year, followed by reference number, country (or region if multisite study), and the duration of follow-up (in years). Hawkesworth et al. (21): the first trial in the article (protein-energy supplementation) was included in this analysis. Macleod et al. (23): the first arm of the intervention was included in this analysis (protein, carbohydrate, and vitamin compared with vitamin only). de Jong et al. (44): the first arm of the intervention was selected (LCPUFA-enriched formula, compared with control formula). IYCF, infant and young child feeding; LCPUFA, long-chain polyunsaturated fatty acid supplementation; MD, mean difference.
FIGURE 6.
Forest plot with subgroup analysis of the effect of early-life nutrition interventions on long-term diastolic blood pressure (mmHg). Study identification: last name of the first author and publication year, followed by reference number, country (or region if multisite study), and the duration of follow-up (in years). Hawkesworth et al. (21): the first trial in the article (protein-energy supplementation) was included in this analysis. Macleod et al. (23): the first arm of the intervention was included in this analysis (protein, carbohydrate, and vitamin compared with vitamin only). de Jong et al. (44): the first arm of the intervention was selected (LCPUFA-enriched formula, compared with control formula). IYCF, infant and young child feeding; LCPUFA, long-chain polyunsaturated fatty acid supplementation; MD, mean difference.
Approximately half (9 of 22) of studies reported significant results in this category (Supplemental Table 1). One protein-energy trial reported lower augmentation index (55). One LCPUFA study found that blood pressure was lower in the intervention group than in the placebo group (40). One single-micronutrient trial reported marginally lower diastolic blood pressure, but only in overweight children (42). Two IYCF trials reported lower blood pressure, and 1 reported marginally lower heart rate (44, 45, 48). Two dietary counseling studies (both from the STRIP study) reported lower blood pressure and better endothelial functions (mainly in boys for the latter) (34, 74). In contrast, 1 LCPUFA study reported higher blood pressure in boys; 1 IYCF trial reported higher blood pressure in the intervention group, but only in girls (39, 46). All other results were null (Supplemental Table 1).
Outcome category 3: body size and body composition
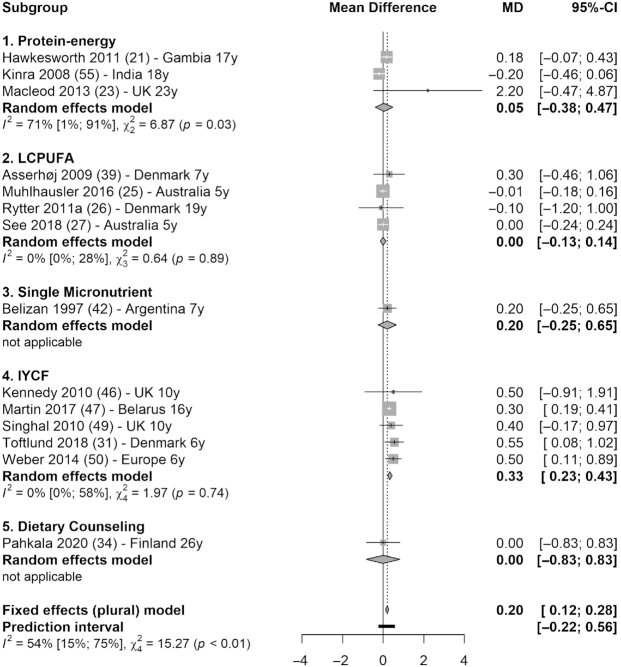
Based on meta-analysis (14 studies), the intervention groups had 0.20 kg/m2 higher BMI than the control groups (95% CI: 0.12, 0.28; I2 = 54%) (Figure 7). The significant increase in BMI was driven by IYCF interventions (MD = 0.33; 95% CI: 0.23, 0.43; I2 = 0%), including LCPUFA-enriched formula compared with unsupplemented formula (46), breastfeeding promoting at the facility level compared with no promotion (47), nutrient-enriched formula compared with standard term formula for short-for-gestational-age infants (49), preterm formula compared with breastmilk (31), and high-protein formula compared with low-protein, equal-caloric formula (50).
FIGURE 7.
Forest plot with subgroup analysis of the effect of early-life nutrition interventions on long-term BMI (kg/m2). Study identification: last name of the first author and publication year, followed by reference number, country (or region if multisite study), and the duration of follow-up (in years). Different articles by the same author and in the same year are distinguished by a (or b). Hawkesworth et al. (21): the first trial in the article (protein-energy supplementation) was included in this analysis. Macleod et al. (23): the first arm of the intervention was included in this analysis (protein, carbohydrate, and vitamin compared with vitamin only). IYCF, infant and young child feeding; LCPUFA, long-chain polyunsaturated fatty acid supplementation; MD, mean difference.
The following studies reported higher weight and associated measurements (such as BMI-for-age z-score, or fat mass percentage), including 1 maternal LCPUFA trial and 6 IYCF trials (high- compared with low-protein formula, milk supplementation compared with no supplementation, baby-friendly hospital compared with control, enriched compared with unenriched formula) (58, 65, 46, 47, 49, 69, 50, 80) (Supplemental Table 1). Two LCPUFA trials reported larger head circumference and lower waist circumference; One multiple-micronutrient trial reported lower BMI-for-age z-score, whereas 1 IYCF trial (breastfeeding compared with formula feeding) reported lower level of early rapid growth (39, 27, 62, 31). All other results were null (Supplemental Table 1).
Outcome category 4: subclinical and clinical outcomes
We did not conduct meta-analysis for any specific outcome in this category because only 8 studies reported subclinical or clinical cardiometabolic outcomes, and the findings were inconsistent (Supplemental Table 1). One IYCF trial (high- compared with low-protein formula) reported higher risk of obesity (50). In contrast, findings from the STRIP study (dietary counseling) reported lower overweight prevalence in girls (70). Both Nupponen et al. (72) (dietary counselling) and Stewart et al. (64) (folic acid plus vitamin A supplementation compared with vitamin A alone) reported lower risk of metabolic syndrome. In addition, Pahkala et al. (73) (dietary counseling) reported lower risk of poor cardiovascular health. The remaining studies reported null findings (Supplemental Table 1).
Timing of intervention
Two studies directly examined the difference in timing of the interventions. Ekström et al. (28) observed that earlier compared with later provision of the same supplemental food item was associated with better lipid profile (Table 1, Supplemental Table 1). Hawkesworth et al. (21) reported that delivering the same maternal protein-energy biscuit pre- compared with post-delivery was associated with marginally lower fasting glucose concentration in the offspring (Table 1, Supplemental Table 1).
Discussion
We conducted this systematic review and meta-analysis in an effort to synthesize up-to-date information regarding the long-term cardiometabolic impact of nutrition interventions (randomized controlled trials) in early life. The primary findings of this review were 4-fold. First, across different types of interventions, most findings were null. However, there was an overall lowering effect on fasting glucose concentration, but BMI was higher in the intervention groups. Second, ongoing and personalized dietary counseling was the only intervention that reported predominantly beneficial cardiometabolic outcomes. Third, among IYCF studies, breastfeeding was more beneficial than formula feeding, although breastfeeding promotion alone did not yield observable long-term benefits. Lastly, timing of intervention mattered within the same study, and earlier exposure to improved nutrition was more beneficial than later, but more studies are needed to replicate this finding.
In this review, we observed mostly null to modest findings in terms of long-term cardiometabolic impacts, unlike the relatively clear associations drawn from famine studies (12, 81, 82). It is possible that the human body is rather resilient: unless undergoing severe energy and nutrient deprivation early in life, metabolic programming either would not occur, or the programming effects could be offset or compensated for in the long term. It is plausible that most cardiometabolic disturbances in early life do not persist into adulthood, although some evidence suggests lifelong tracking of blood pressure level (83). The underlying mechanisms of effect of early-life nutrition are not fully elucidated (5). A few possible mechanisms include in utero growth restriction, ontogenic alterations, metabolic adaptation, and epigenetic modifications (5, 84). Factors in the broader social, economic, and environmental context should be taken into consideration as well (5, 85).
Glycemia levels appeared to benefit from various types of early-life nutrition interventions, the most prominent being protein-energy supplementation in relatively malnourished populations (21, 55). The availability of sufficient protein in early life likely supports the development of essential metabolic organs, especially the pancreas, where insulin, glucagon, and other key glycemic regulators are produced (86). Research showed that protein supplementation promotes brain development, which is a major organ that relies solely on glucose as fuel (87). A few other types of interventions were also associated with lower glucose concentrations later in life. Dietary counseling reduced fasting glucose concentration and insulin resistance. Gestational supplementation with multiple micronutrients compared with only iron and folic acid, as well as LCPUFA supplementation during infancy, also lowered glucose concentration, indicating the importance of a balanced micronutrient profile and improved lipid intake (27, 28).
The effect on BMI and risk of obesity was mainly observed in interventions that provided “enriched” supplements, which provided higher energy or certain macronutrients beyond average needs (69, 50). In well-nourished populations, higher protein intake in infancy can increase the availability of branched-chain amino acids that can enhance the release of insulin-like growth factor I, which is known to stimulate weight gain and body fat deposition (50). One study, however, provided body composition data with short intervals over the follow-up period (birth to 6 y). It reported that despite the increase in BMI, there was a proportional increase in lean, bone, and fat mass in the intervention group (LCPUFA) (58). We therefore suggest that the results for BMI be interpreted with caution, because BMI does not differentiate between lean mass and fat mass (49). It is possible that these interventions stimulated growth proportionally in different types of tissues, without compromising metabolic functions. More research is needed to ascertain this finding and investigate the underlying mechanisms.
The STRIP study of dietary counseling in Finland was the only cohort that reported consistently positive results across all categories of cardiometabolic outcomes, including lower insulin resistance, favorable lipid profile, lower blood pressure, better endothelial function, lower risk of metabolic syndrome, and better indicators for cardiovascular health (70–72, 34, 73). It is possible that only intensive interventions such as STRIP can ensure long-term benefits, especially given that study's behavioral-change nature and personalized design. All other interventions involved changes in 1 or several nutritional components directly provided through the study, without requiring additional lifestyle modifications. Chronic disease is usually a result of cumulative exposures, which might not be sufficiently mitigated through interventions that start and end early in life (88).
Protein-energy supplementation is a common nutrition intervention, especially in low- and middle-income countries, where the need for maternal and child nutrition investments is high (89). For instance, the Institute of Nutrition of Central America and Panama (INCAP) Longitudinal Study reported numerous positive health and human capital outcomes associated with protein-energy supplementation in early life (89, 90). In a recent follow-up study, however, INCAP researchers observed diverging effects of the supplementation on cardiometabolic outcomes, including a protective effect against diabetes but increased risk of obesity (91, 92). In this review, we observed similar conflicting impacts of protein-energy supplementations on cardiometabolic outcomes across studies (21, 55). Similar to the quasi-experimental nature of famine studies (which focus on deprivation), the INCAP study could be viewed as a quasi-experimental trial with a focus on remediating chronic undernutrition. Its various findings are, by nature of the context, different from those obtained in high-income countries. It is important to further investigate the effect of relatively higher protein and energy intake in early life, and to compare the results between malnourished and well-nourished populations.
IYCF was a major category in this review, because we combined behavior change trials and supplementation trials. The 1 IYCF behavior change trial (the Promotion of Breastfeeding Intervention Trial in Belarus), reported mainly null results after implementing policies based on the Baby-Friendly Hospital Initiative (66, 47, 29, 67). Across supplementation trials with IYCF focus, infants who were breastfed, compared with those fed with formula, had lower C-reactive protein, cholesterol, heart rate, and blood pressure later in life, regardless of the baseline characteristics of the cohorts, formula composition, or duration of follow-up (44, 38, 48, 31). When comparing only among formula-fed groups, those fed high-protein or nutrient-enriched (compared with low-protein or standardized) formula had higher level of early rapid growth and higher fat mass during follow-up (65, 49, 69, 50). This is possibly due to the relatively higher percentage of protein and lower percentage of fat in the formula, which differs from breastmilk that typically contains 3–5% fat and 0.8–0.9% protein (93). High protein intake in early life can alter fat distribution in healthy children during developmental processes, including a potentially higher subcutaneous thicker layer (65). We therefore urge public health researchers and practitioners strictly to follow evidence-based programming.
Most studies with single-micronutrient supplementation showed no clear long-term cardiometabolic benefits, except for reduced risk of metabolic syndrome in the folic acid supplementation group reported by Stewart et al. (64). Multiple-micronutrient supplementations seemed to be associated with better lipid profile and glycemic status, lower concentration of inflammation markers, and lower BMI (28, 62). Micronutrients are essential in early-life development, and it has been reported that antenatal micronutrient supplementation increases birth weight, which in itself has long-term implications (64). It is possible that, despite short-term benefits of single-micronutrient trials, the effects are overshadowed by life-long exposure in other aspects. In contrast, multiple-micronutrient supplementations can promote growth and early development in a holistic way for the effects to be long-lasting. Given the relative ease and convenience of micronutrient-centered trials, we should continue investigating their long-term benefits.
Regarding the timing of interventions, we have observed that earlier rather than later enrollment in the trials had more beneficial effects on lipid profile and glucose concentration (21, 54, 22, 28). We did not observe a clear pattern regarding timing in this review due to the limited number of studies with relevant information. There is some evidence in the literature to support the differential impact of nutrition at various time points. The best-studied famine is the Dutch famine, which helped distinguish famine exposure at different trimesters of pregnancy and in infancy for relatively accurate analysis (10–13). Similarly, researchers reported that prenatal exposure (compared with later exposure) to the Chinese famine was associated with significantly higher risk of hyperglycemia in 2 consecutive generations (94–96). Famine research from Bangladesh reported underweight as an outcome following in utero famine exposure, but overweight following postnatal famine exposure (97). Observations based on the Ukraine famine also identified early gestation as a critical window of development (82).
There are a few limitations in this review. We combined different types of interventions in an effort to synthesize early-life nutrition interventions conducted by researchers around the world. This could lead to overgeneralization of the results. The trials in high-income settings can differ from those in low-resource settings, because the populations did not have the same potential to benefit from nutrition interventions. The studies were also conducted at different time points in early life, with varying lengths of follow-up periods. Therefore, the heterogeneity reported in meta-analysis should be interpreted with caution. The sources of heterogeneity can reflect varying degrees of SDs due to sampling differences, or due to actual biological differences. We provided succinct summaries of the studies to help interpret the results. We encourage the readers to refer to the tables and supplemental materials, whenever necessary, for details of study design and outcome. We also caution against unfounded extrapolation of the results. In addition, because of the nature of this review (inclusion criteria regarding the types of study and duration of follow-up), all studies face bias to a certain degree due to loss of follow-up, confounding factors difficult to measure throughout the life course, as well as measurement errors at each follow-up time point. We have attempted to assess bias for each individual study from 6 major domains, but bias undeniably affected the final interpretation of information in this review.
To our knowledge, this review is the first to synthesize information related to early-life nutrition interventions and long-term cardiometabolic impacts with a focus on randomized controlled trials. We emphasized both the type and the timing of interventions in association with different categories of outcomes. In addition, we did not restrict publication year or the language of the articles, hence providing wide coverage of relevant results. In sum, this systematic review and meta-analysis serves both as a reference manual to refine and improve interventions to yield more long-term gains, and as a preventative measure to identify any intervention that might have unintended negative effects.
From a public health programming point of view, it might not be feasible to implement long-term, intensive, and individualized dietary counseling in most settings. However, it is possible to incorporate dietary counseling into other types of study designs, including various types of macro- and micronutrient supplementations. It is also advisable to incorporate individualized dietary and lifestyle counseling into primary healthcare to ensure sustainability. For researchers who are interested in conducting more in-depth reviews on similar topics, we suggest that: First, different timing of the same interventions, ideally within similar contexts, should be prioritized. Second, within each type of intervention, contextual factors should be analyzed at a more granular level, such as socioeconomic status of the communities, seasonality, and underlying dietary patterns.
Supplementary Material
ACKNOWLEDGEMENTS
We thank librarian Hannah Rogers at the Woodruff Health Sciences Center Library for her assistance in developing and refining the search strategy.
The authors' responsibilities were as follows—SH: drafted the protocol, developed the search strategies, conducted the search, carried out the screening and data extraction, conducted data analysis, interpreted the data, and drafted the manuscript; ADS: provided ongoing guidance, and supported the manuscript writing in every iteration; and both authors: read and approved the final manuscript.
Notes
Supported by NIH grant number HD075784. The funder had no role in the systematic review and meta-analysis.
Author disclosures: The authors report no conflicts of interest.
Supplemental Table 1 and Supplemental Figure 1 are available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/advances.
Abbreviations used: INCAP, Institute of Nutrition of Central America and Panama; IYCF, infant and young child feeding; LCPUFA, long-chain polyunsaturated fatty acid; MD, raw mean difference; MMS, multiple micronutrient supplementation; SMD, standardized mean difference; STRIP, Special Turku Coronary Risk Factor Intervention Project for Children.
Contributor Information
Siran He, Nutrition and Health Sciences Program, Laney Graduate School, Emory University, Atlanta, GA, USA.
Aryeh D Stein, Rollins School of Public Health, Emory University, Atlanta, GA, USA.
References
- 1. Martorell R. Improved nutrition in the first 1000 days and adult human capital and health. Am J Hum Biol. 2017;29(2):e22952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ravelli G-P, Stein ZA, Susser MW. Obesity in young men after famine exposure in utero and early infancy. N Engl J Med. 1976;295(7):349–53. [DOI] [PubMed] [Google Scholar]
- 3. Barker DJ. The fetal and infant origins of adult disease. BMJ. 1990;301(6761):1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Barker DJP. The developmental origins of adult disease. Eur J Epidemiol. 2002;18(8):733–6. [DOI] [PubMed] [Google Scholar]
- 5. Hoffman DJ, Reynolds RM, Hardy DB. Developmental origins of health and disease: current knowledge and potential mechanisms. Nutr Rev. 2017;75(12):951–70. [DOI] [PubMed] [Google Scholar]
- 6. Navarro E, Funtikova AN, Fito M, Schroder H. Prenatal nutrition and the risk of adult obesity: long-term effects of nutrition on epigenetic mechanisms regulating gene expression. J Nutr Biochem. 2017;39:1–14. [DOI] [PubMed] [Google Scholar]
- 7. Victora CG, Adair L, Fall C, Hallal PC, Martorell R, Richter L, Sachdev HS;Maternal and Child Undernutrition Study Group . Maternal and child undernutrition: consequences for adult health and human capital. Lancet. 2008;371(9609):340–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Horta BL, Mola CLd, Victora CG. Long-term consequences of breastfeeding on cholesterol, obesity, systolic blood pressure and type 2 diabetes: a systematic review and meta-analysis. Acta Paediatr. 2015;104(s467):30–7. [DOI] [PubMed] [Google Scholar]
- 9. Santamaria C, Bi W, Leduc L, Tabatabaei N, Jantchou P, Luo Z, Audibert F, Nuyt AM, Wei S. Prenatal vitamin D status and offspring's growth, adiposity and metabolic health: a systematic review and meta-analysis. Br J Nutr. 2018;119(3):310–9. [DOI] [PubMed] [Google Scholar]
- 10. Painter RC, Roseboom TJ, Bossuyt PM, Osmond C, Barker DJ, Bleker OP. Adult mortality at age 57 after prenatal exposure to the Dutch famine. Eur J Epidemiol. 2005;20(8):673–6. [DOI] [PubMed] [Google Scholar]
- 11. Painter RC, Osmond C, Gluckman P, Hanson M, Phillips DI, Roseboom TJ. Transgenerational effects of prenatal exposure to the Dutch famine on neonatal adiposity and health in later life. BJOG. 2008;115(10):1243–9. [DOI] [PubMed] [Google Scholar]
- 12. Portrait F, Teeuwiszen E, Deeg D. Early life undernutrition and chronic diseases at older ages: the effects of the Dutch famine on cardiovascular diseases and diabetes. Soc Sci Med. 2011;73(5):711–8. [DOI] [PubMed] [Google Scholar]
- 13. Ravelli GP, Stein ZA, Susser MW. Obesity in young men after famine exposure in utero and early infancy. N Engl J Med. 1976;295(7):349–53. [DOI] [PubMed] [Google Scholar]
- 14. Forouzanfar MH, Afshin A, Alexander LT, Anderson HR, Bhutta ZA, Biryukov S, Brauer M, Burnett R, Cercy K, Charlson FJet al. Global, regional, and national comparative risk assessment of 79 behavioural, environmental and occupational, and metabolic risks or clusters of risks, 1990–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet. 2016;388(10053):1659–724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Collins R, MacMahon S. Reliable assessment of the effects of treatment on mortality and major morbidity, I: clinical trials. Lancet. 2001;357(9253):373–80. [DOI] [PubMed] [Google Scholar]
- 16. Martorell R, Habicht JP, Rivera JA. History and design of the INCAP longitudinal study (1969–77) and its follow-up (1988–89). J Nutr. 1995;125(4 Suppl):1027S–41S. [DOI] [PubMed] [Google Scholar]
- 17. Bisgaard H, Stokholm J, Chawes BL, Vissing NH, Bjarnadottir E, Schoos AM, Wolsk HM, Pedersen TM, Vinding RK, Thorsteinsdottir Set al. Fish oil-derived fatty acids in pregnancy and wheeze and asthma in offspring. N Engl J Med. 2016;375(26):2530–9. [DOI] [PubMed] [Google Scholar]
- 18. Leyrat C, Morgan KE, Leurent B, Kahan BC. Cluster randomized trials with a small number of clusters: which analyses should be used?. Int J Epidemiol. 2018;47(1):321–31. [DOI] [PubMed] [Google Scholar]
- 19. World Bank . World Bank country and lending groups. [Internet]. [cited 2019 Sep 30]. Available from: https://datahelpdesk.worldbank.org/knowledgebase/articles/906519-world-bank-country-and-lending-groups.
- 20. Hayden JA, van der Windt DA, Cartwright JL, Cote P, Bombardier C. Assessing bias in studies of prognostic factors. Ann Intern Med. 2013;158(4):280–6. [DOI] [PubMed] [Google Scholar]
- 21. Hawkesworth S, Walker CG, Sawo Y, Fulford AJ, Jarjou LM, Goldberg GR, Prentice A, Prentice AM, Moore SE. Nutritional supplementation during pregnancy and offspring cardiovascular disease risk in The Gambia. Am J Clin Nutr. 2011;94(6 Suppl):1853S. [DOI] [PubMed] [Google Scholar]
- 22. Hawkesworth S, Prentice AM, Fulford AJC, Moore SE. Dietary supplementation of rural Gambian women during pregnancy does not affect body composition in offspring at 11–17 years of age. J Nutr. 2008;138(12):2468–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Macleod J, Tang L, Hobbs FD, Wharton B, Holder R, Hussain S, Nichols L, Stewart P, Clark P, Luzio Set al. Effects of nutritional supplementation during pregnancy on early adult disease risk: follow up of offspring of participants in a randomised controlled trial investigating effects of supplementation on infant birth weight. PLoS One. 2013;8(12):e83371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Gutierrez-Gomez Y, Stein AD, Ramakrishnan U, Barraza-Villarreal A, Moreno-Macias H, Aguilar-Salinas C, Romieu I, Rivera JA. Prenatal docosahexaenoic acid supplementation does not affect nonfasting serum lipid and glucose concentrations of offspring at 4 years of age in a follow-up of a randomized controlled clinical trial in Mexico. J Nutr. 2017;147(2):242–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Muhlhausler BS, Yelland LN, McDermott R, Tapsell L, McPhee A, Gibson RA, Makrides M. DHA supplementation during pregnancy does not reduce BMI or body fat mass in children: follow-up of the DHA to Optimize Mother Infant Outcome randomized controlled trial. Am J Clin Nutr. 2016;103(6):1489–96. [DOI] [PubMed] [Google Scholar]
- 26. Rytter D, Bech BH, Christensen JH, Schmidt EB, Henriksen TB, Olsen SF. Intake of fish oil during pregnancy and adiposity in 19-y-old offspring: follow-up on a randomized controlled trial. Am J Clin Nutr. 2011;94(3):701–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. See VHL, Mori TA, Prescott SL, Beilin LJ, Burrows S, Huang R. Cardiometabolic risk factors at 5 years after omega-3 fatty acid supplementation in infancy. Pediatrics. 2018;142(1):e20162623. [DOI] [PubMed] [Google Scholar]
- 28. Ekström EC, Lindstrom E, Raqib R, El Arifeen S, Basu S, Brismar K, Selling K, Persson L. Effects of prenatal micronutrient and early food supplementation on metabolic status of the offspring at 4.5 years of age. The MINIMat randomized trial in rural Bangladesh. Int J Epidemiol. 2016;45(5):1656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Martin RM, Patel R, Kramer MS, Vilchuck K, Bogdanovich N, Sergeichick N, Gusina N, Foo Y, Palmer T, Thompson Jet al. Effects of promoting longer-term and exclusive breastfeeding on cardiometabolic risk factors at age 11.5 years: a cluster-randomized, controlled trial. Circulation. 2014;129(3):321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Singhal A, Fewtrell M, Cole TJ, Lucas A. Low nutrient intake and early growth for later insulin resistance in adolescents born preterm. Lancet. 2003;361(9363):1089–97. [DOI] [PubMed] [Google Scholar]
- 31. Toftlund LH, Halken S, Agertoft L, Zachariassen G. Early nutrition and signs of metabolic syndrome at 6 y of age in children born very preterm. Am J Clin Nutr. 2018;107(5):717–24. [DOI] [PubMed] [Google Scholar]
- 32. Williams DM, Martin RM, Davey Smith G, Alberti K, Ben-Shlomo Y, McCarthy A. Associations of infant nutrition with insulin resistance measures in early adulthood: evidence from the Barry-Caerphilly Growth (BCG) study. PLoS One. 2012;7(3):e34161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Costa CS, Campagnolo PD, Lumey LH, Vitolo MR. Effect of maternal dietary counselling during the 1st year of life on glucose profile and insulin resistance at the age of 8 years: a randomised field trial. Br J Nutr. 2017;117(1):134–41. [DOI] [PubMed] [Google Scholar]
- 34. Pahkala K, Laitinen TT, Niinikoski H, Kartiosuo N, Rovio SP, Lagstrom H, Loo BM, Salo P, Jokinen E, Magnussen CGet al. Effects of 20-year infancy-onset dietary counselling on cardiometabolic risk factors in the Special Turku Coronary Risk Factor Intervention Project (STRIP): 6-year post-intervention follow-up. Lancet Child Adolesc Health. 2020;4(5):359–69. [DOI] [PubMed] [Google Scholar]
- 35. Videhult FK, Öhlund I, Stenlund H, Hernell O, West CE. Probiotics during weaning: a follow-up study on effects on body composition and metabolic markers at school age. Eur J Nutr. 2015;54(3):355–63. [DOI] [PubMed] [Google Scholar]
- 36. Rytter D, Schmidt EB, Bech BH, Christensen JH, Henriksen TB, Olsen SF. Fish oil supplementation during late pregnancy does not influence plasma lipids or lipoprotein levels in young adult offspring. Lipids. 2011;46(12):1091–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Gruszfeld D, Weber M, Nowakowska-Rysz M, Janas R, Kozlik-Feldmann R, Xhonneux A, Carlier C, Riva E, Verduci E, Closa-Monasterolo Ret al. Protein intake in infancy and carotid intima media thickness at 5 years—a secondary analysis from a randomized trial. Ann Nutr Metab. 2015;66(1):51–9. [DOI] [PubMed] [Google Scholar]
- 38. Singhal A, Cole TJ, Fewtrell M, Lucas A. Breastmilk feeding and lipoprotein profile in adolescents born preterm: follow-up of a prospective randomised study. Lancet. 2004;363(9421):1571–8. [DOI] [PubMed] [Google Scholar]
- 39. Asserhøj M, Nehammer S, Matthiessen J, Michaelsen KF, Lauritzen L. Maternal fish oil supplementation during lactation may adversely affect long-term blood pressure, energy intake, and physical activity of 7-year-old boys. J Nutr. 2009;139(2):298–304. [DOI] [PubMed] [Google Scholar]
- 40. Kerling EH, Hilton JM, Thodosoff JM, Wick J, Colombo J, Carlson SE. Effect of Prenatal docosahexaenoic acid supplementation on blood pressure in children with overweight condition or obesity: a secondary analysis of a randomized clinical trial. JAMA Netw Open. 2019;2(2):e190088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Rytter D, Christensen JH, Bech BH, Schmidt EB, Henriksen TB, Olsen SF. The effect of maternal fish oil supplementation during the last trimester of pregnancy on blood pressure, heart rate and heart rate variability in the 19-year-old offspring. Br J Nutr. 2012;108(8):1475–83. [DOI] [PubMed] [Google Scholar]
- 42. Belizan JM, Villar J, Bergel E, del Pino A, Di Fulvio S, Galliano SV, Kattan C. Long-term effect of calcium supplementation during pregnancy on the blood pressure of offspring: follow up of a randomised controlled trial. BMJ. 1997;315(7103):281–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Hiller JE, Crowther CA, Moore VA, Willson K, Robinson JS. Calcium supplementation in pregnancy and its impact on blood pressure in children and women: follow up of a randomised controlled trial. Aust N Z J Obstet Gynaecol. 2007;47(2):115–21. [DOI] [PubMed] [Google Scholar]
- 44. de Jong C, Boehm G, Kikkert HK, Hadders-Algra M. The Groningen LCPUFA study: no effect of short-term postnatal long-chain polyunsaturated fatty acids in healthy term infants on cardiovascular and anthropometric development at 9 years. Pediatr Res. 2011;70(4):411–6. [DOI] [PubMed] [Google Scholar]
- 45. Forsyth JS, Willatts P, Agostoni C, Bissenden J, Casaer P, Boehm G. Long chain polyunsaturated fatty acid supplementation in infant formula and blood pressure in later childhood: follow up of a randomised controlled trial. BMJ. 2003;326(7396):953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Kennedy K, Ross S, Isaacs EB, Weaver LT, Singhal A, Lucas A, Fewtrell MS. The 10-year follow-up of a randomised trial of long-chain polyunsaturated fatty acid supplementation in preterm infants: effects on growth and blood pressure. Arch Dis Child. 2010;95(8):588–95. [DOI] [PubMed] [Google Scholar]
- 47. Martin RM, Kramer MS, Patel R, Rifas-Shiman SL, Thompson J, Yang S, Vilchuck K, Bogdanovich N, Hameza M, Tilling Ket al. Effects of promoting long-term, exclusive breastfeeding on adolescent adiposity, blood pressure, and growth trajectories: a secondary analysis of a randomized clinical trial. JAMA Pediatr. 2017;171(7):e170698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Singhal A, Cole TJ, Lucas A. Early nutrition in preterm infants and later blood pressure: two cohorts after randomised trials. Lancet. 2001;357(9254):413–9. [DOI] [PubMed] [Google Scholar]
- 49. Singhal A, Kennedy K, Lanigan J, Fewtrell M, Cole TJ, Stephenson T, Elias-Jones A, Weaver LT, Ibhanesebhor S, MacDonald PDet al. Nutrition in infancy and long-term risk of obesity: evidence from 2 randomized controlled trials. Am J Clin Nutr. 2010;92(5):1133–44. [DOI] [PubMed] [Google Scholar]
- 50. Weber M, Grote V, Closa-Monasterolo R, Escribano J, Langhendries JP, Dain E, Giovannini M, Verduci E, Gruszfeld D, Socha Pet al. Lower protein content in infant formula reduces BMI and obesity risk at school age: follow-up of a randomized trial. Am J Clin Nutr. 2014;99(5):1041. [DOI] [PubMed] [Google Scholar]
- 51. Schwarzer G. meta: an R package for meta-analysis. R News. 2007;7(3):40–5. [Google Scholar]
- 52. Viechtbauer W. Conducting meta-analyses in R with the metafor package. J Stat Soft. 2010;36(3):1–48. [Google Scholar]
- 53. Lüdecke D. esc: Effect Size Computation for Meta Analysis (Version 0.5.1). [Internet]. 2019. [cited March 19, 2020]. doi: 10.5281/zenodo.1249218Available from:, https://CRAN.R-project.org/package=esc. [DOI] [Google Scholar]
- 54. Hawkesworth S. Exploiting dietary supplementation trials to assess the impact of the prenatal environment on CVD risk. Proc Nutr Soc. 2009;68(1):78–88. [DOI] [PubMed] [Google Scholar]
- 55. Kinra S, Rameshwar Sarma KV, Ghafoorunissa, Mendu VV, Ravikumar R, Mohan V, Wilkinson IB, Cockcroft JR, Davey Smith G, Ben-Shlomo Y. Effect of integration of supplemental nutrition with public health programmes in pregnancy and early childhood on cardiovascular risk in rural Indian adolescents: long term follow-up of Hyderabad nutrition trial. BMJ. 2008;337:a605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Brei C, Stecher L, Much D, Karla MT, Amann-Gassner U, Shen J, Ganter C, Karampinos DC, Brunner S, Hauner H. Reduction of the n-6:n-3 long-chain PUFA ratio during pregnancy and lactation on offspring body composition: follow-up results from a randomized controlled trial up to 5 y of age. Am J Clin Nutr. 2016;103(6):1472–81. [DOI] [PubMed] [Google Scholar]
- 57. Foster BA, Escaname E, Powell TL, Larsen B, Siddiqui SK, Menchaca J, Aquino C, Rajam R, Hale DE. Randomized controlled trial of DHA supplementation during pregnancy: child adiposity outcomes. Nutrients. 2017;9(6):566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Vinding RK, Stokholm J, Sevelsted A, Sejersen T, Chawes BL, Bonnelykke K, Thorsen J, Howe LD, Krakauer M, Bisgaard H. Effect of fish oil supplementation in pregnancy on bone, lean, and fat mass at six years: randomised clinical trial. BMJ. [Internet]2018;362:k3312, doi: 10.1136/bmj.k3312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Palmer AC, Schulze KJ, Khatry SK, West KP. Prenatal and childhood exposures are associated with thymulin concentrations in young adolescent children in rural Nepal. J Dev Orig Health Dis. 2020;11:127–35.; [DOI] [PubMed] [Google Scholar]
- 60. Taylor CM, Atkinson C, Penfold C, Bhattacharya S, Campbell D, Davey Smith G, Leary S, Ness A. Folic acid in pregnancy and mortality from cancer and cardiovascular disease: further follow-up of the Aberdeen folic acid supplementation trial. J Epidemiol Community Health. 2015;69(8):789–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Kumordzie SM, Adu-Afarwuah S, Arimond M, Young RR, Adom T, Boatin R, Ocansey ME, Okronipa H, Prado EL, Oaks BMet al. Maternal and infant lipid-based nutritional supplementation increases height of Ghanaian children at 4–6 years only if the mother was not overweight before conception. J Nutr. 2019;149(5):847–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Mannan T, Ahmed S, Akhtar E, Roy AK, Haq MA, Roy A, Kippler M, Ekstrom EC, Wagatsuma Y, Raqib R. Maternal micronutrient supplementation and long term health impact in children in rural Bangladesh. PLoS One. 2016;11(8):e0161294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Stewart CP, Christian P, Schulze KJ, Arguello M, LeClerq SC, Khatry SK, West KP. Low maternal vitamin B-12 status is associated with offspring insulin resistance regardless of antenatal micronutrient supplementation in rural Nepal. J Nutr. 2011;141(10):1912. [DOI] [PubMed] [Google Scholar]
- 64. Stewart CP, Christian P, Schulze KJ, Leclerq SC, West KP Jr, Khatry SK. Antenatal micronutrient supplementation reduces metabolic syndrome in 6- to 8-year-old children in rural Nepal. J Nutr. 2009;139(8):1575–81. [DOI] [PubMed] [Google Scholar]
- 65. Gruszfeld D, Weber M, Gradowska K, Socha P, Grote V, Xhonneux A, Dain E, Verduci E, Riva E, Closa-Monasterolo Ret al. Association of early protein intake and pre-peritoneal fat at five years of age: follow-up of a randomized clinical trial. Nutr Metab Cardiovasc Dis. 2016;26(9):824–32. [DOI] [PubMed] [Google Scholar]
- 66. Kramer MS, Matush L, Vanilovich I, Platt RW, Bogdanovich N, Sevkovskaya Z, Dzikovich I, Shishko G, Collet JP, Martin RMet al. Effects of prolonged and exclusive breastfeeding on child height, weight, adiposity, and blood pressure at age 6.5 y: evidence from a large randomized trial. Am J Clin Nutr. 2007;86(6):1717–21. [DOI] [PubMed] [Google Scholar]
- 67. Martin RM, Patel R, Kramer MS, Guthrie L, Vilchuck K, Bogdanovich N, Sergeichick N, Gusina N, Foo Y, Palmer Tet al. Effects of promoting longer-term and exclusive breastfeeding on adiposity and insulin-like growth factor-I at age 11.5 years: a randomized trial. JAMA. 2013;309(10):1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Singhal A, Farooqi IS, O'Rahilly S, Cole TJ, Fewtrell M, Lucas A. Early nutrition and leptin concentrations in later life. Am J Clin Nutr. 2002;75(6):993–9. [DOI] [PubMed] [Google Scholar]
- 69. Totzauer M, Luque V, Escribano J, Closa-Monasterolo R, Verduci E, ReDionigi A, Hoyos J, Langhendries JP, Gruszfeld D, Socha Pet al. Effect of lower versus higher protein content in infant formula through the first year on body composition from 1 to 6 years: follow-up of a randomized clinical trial. Obesity. 2018;26(7):1203–10. [DOI] [PubMed] [Google Scholar]
- 70. Hakanen M, Lagstrom H, Kaitosaari T, Niinikoski H, Nanto-Salonen K, Jokinen E, Sillanmaki L, Viikari J, Ronnemaa T, Simell O. Development of overweight in an atherosclerosis prevention trial starting in early childhood. The STRIP study. Int J Obes. 2006;30(4):618–26. [DOI] [PubMed] [Google Scholar]
- 71. Lehtovirta M, Pahkala K, Niinikoski H, Kangas AJ, Soininen P, Lagström H, Viikari JSA, Rönnemaa T, Jula A, Ala-Korpela Met al. Effect of dietary counseling on a comprehensive metabolic profile from childhood to adulthood. J Pediatr. 2018;195:190–8.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Nupponen M, Pahkala K, Juonala M, Magnussen CG, Niinikoski H, Rönnemaa T, Viikari JS, Saarinen M, Lagström H, Jula Aet al. Metabolic syndrome from adolescence to early adulthood: effect of infancy-onset dietary counseling of low saturated fat: the Special Turku Coronary Risk Factor Intervention Project (STRIP). Circulation. 2015;131(7):605. [DOI] [PubMed] [Google Scholar]
- 73. Pahkala K, Hietalampi H, Laitinen TT, Viikari JS, Rönnemaa T, Niinikoski H, Lagström H, Talvia S, Jula A, Heinonen OJet al. Ideal cardiovascular health in adolescence: effect of lifestyle intervention and association with vascular intima-media thickness and elasticity (the Special Turku Coronary Risk Factor Intervention Project for Children study). Circulation. 2013;127(21):2088. [DOI] [PubMed] [Google Scholar]
- 74. Raitakari OT, Ronnemaa T, Jarvisalo MJ, Kaitosaari T, Volanen I, Kallio K, Lagstrom H, Jokinen E, Niinikoski H, Viikari JSet al. Endothelial function in healthy 11-year-old children after dietary intervention with onset in infancy: the Special Turku Coronary Risk Factor Intervention Project for children (STRIP). Circulation. 2005;112(24):3786–94. [DOI] [PubMed] [Google Scholar]
- 75. Luoto R, Kalliomaki M, Laitinen K, Isolauri E. The impact of perinatal probiotic intervention on the development of overweight and obesity: follow-up study from birth to 10 years. Int J Obes. 2010;34(10):1531–7. [DOI] [PubMed] [Google Scholar]
- 76. Videhult FK, Andersson Y, Öhlund I, Stenlund H, Hernell O, West CE. Impact of probiotics during weaning on the metabolic and inflammatory profile: follow-up at school age. Int J Food Sci Nutr. 2015;66(6):686–91. [DOI] [PubMed] [Google Scholar]
- 77. Ekström EC, Lindström E, Rubhana R, El-Arifeen S, Basu S, Brismar K, Selling K, Persson LǺ. Effects of prenatal micronutrient and early food supplementation on metabolic status of the offspring at 4.5 years of age. The MINIMat randomized trial in rural Bangladesh. Int J Epidemiol. 2016;45(5):1656–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Singhal A, Cole TJ, Fewtrell M, Lucas A. Breastmilk feeding and lipoprotein profile in adolescents born preterm: follow-up of a prospective randomised study. Lancet. 2004;363(9421):1571–8. [DOI] [PubMed] [Google Scholar]
- 79. Singhal A, Fewtrell M, Cole TJ, Lucas A. Low nutrient intake and early growth for later insulin resistance in adolescents born preterm. Lancet. 2003;361(9363):1089. [DOI] [PubMed] [Google Scholar]
- 80. Ford ND, Behrman JR, Hoddinott JF, Maluccio JA, Martorell R, Ramirez-Zea M, Stein AD. Exposure to improved nutrition from conception to age 2 years and adult cardiometabolic disease risk: a modelling study. Lancet Glob Health. 2018;6(8):e875–e84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Wang J, Li Y, Han X, Liu B, Hu H, Wang F, Li X, Yang K, Yuan J, Yao Pet al. Exposure to the Chinese famine in childhood increases type 2 diabetes risk in adults. J Nutr. 2016;146(11):2289–95. [DOI] [PubMed] [Google Scholar]
- 82. Lumey LH, Khalangot MD, Vaiserman AM. Association between type 2 diabetes and prenatal exposure to the Ukraine famine of 1932–33: a retrospective cohort study. Lancet Diabetes Endocrinol. 2015;3(10):787–94. [DOI] [PubMed] [Google Scholar]
- 83. Whincup P, Cook D, Papacosta O, Walker M. Birth weight and blood pressure: cross sectional and longitudinal relations in childhood. BMJ. 1995;311(7008):773–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Tarry-Adkins JL, Ozanne SE. Mechanisms of early life programming: current knowledge and future directions. Am J Clin Nutr. 2011;94(6 Suppl):1765S. [DOI] [PubMed] [Google Scholar]
- 85. Adair LS, Fall CH, Osmond C, Stein AD, Martorell R, Ramirez-Zea M, Sachdev HS, Dahly DL, Bas I, Norris SAet al. Associations of linear growth and relative weight gain during early life with adult health and human capital in countries of low and middle income: findings from five birth cohort studies. Lancet. 2013;382(9891):525–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Kumar PU, Ramalaxmi BA, Venkiah K, Sesikeran B. Effect of maternal undernutrition on human foetal pancreas morphology in second trimester of pregnancy. Indian J Med Res. 2013;137(2):302–7. [PMC free article] [PubMed] [Google Scholar]
- 87. Goyal MS, Iannotti LL, Raichle ME. Brain nutrition: a life span approach. Annu Rev Nutr. 2018;38:381–99. [DOI] [PubMed] [Google Scholar]
- 88. Ben-Shlomo Y, Kuh D. A life course approach to chronic disease epidemiology: conceptual models, empirical challenges and interdisciplinary perspectives. Int J Epidemiol. 2002;31(2):285–93. [PubMed] [Google Scholar]
- 89. Ramirez-Zea M, Melgar P, Rivera JA. INCAP Oriente longitudinal study: 40 years of history and legacy. J Nutr. 2010;140(2):397–401. [DOI] [PubMed] [Google Scholar]
- 90. Martorell R. Improved nutrition in the first 1000 days and adult human capital and health. Am J Hum Biol. [Internet]2017;29(2). doi: 10.1002/ajhb.22952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Ford ND, Behrman JR, Hoddinott JF, Maluccio JA, Martorell R, Ramirez-Zea M, Stein AD. Exposure to improved nutrition from conception to age 2 years and adult cardiometabolic disease risk: a modelling study. Lancet Glob Health. 2018;6(8):e875–e84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. He S, Le NA, Ramirez-Zea M, Martorell R, Narayan KMV, Stein AD. Leptin partially mediates the association between early-life nutritional supplementation and long-term glycemic status among women in a Guatemalan longitudinal cohort. Am J Clin Nutr. 2020;111(4):804–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Jenness R. The composition of human milk. Semin Perinatol. 1979;3(3):225–39. [PubMed] [Google Scholar]
- 94. Li J, Liu S, Li S, Feng R, Na L, Chu X, Wu X, Niu Y, Sun Z, Han Tet al. Prenatal exposure to famine and the development of hyperglycemia and type 2 diabetes in adulthood across consecutive generations: a population-based cohort study of families in Suihua, China. Am J Clin Nutr. 2017;105(1):221–7. [DOI] [PubMed] [Google Scholar]
- 95. Wang P, Wang J, Lei Y, Xiao L, Luo Z. Impact of fetal and infant exposure to the Chinese great famine on the risk of hypertension in adulthood. PLoS One. 2012;7(11):e49720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Shi Z, Ji L, Ma RCW, Zimmet P. Early life exposure to 1959–1961 Chinese famine exacerbates association between diabetes and cardiovascular disease. J Diabetes. 2020;12:134–41.; [DOI] [PubMed] [Google Scholar]
- 97. Finer S, Iqbal MS, Lowe R, Ogunkolade BW, Pervin S, Mathews C, Smart M, Alam DS, Hitman GA. Is famine exposure during developmental life in rural Bangladesh associated with a metabolic and epigenetic signature in young adulthood? A historical cohort study. BMJ Open. 2016;6(11):e011768. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.