Abstract
Importance
Congenital diaphragmatic hernia (CDH), a severe birth defect characterized by a diaphragmatic malformation allowing herniation by abdominal organs into the thorax, is associated with high mortality.
Objective
The purpose of our study was to examine (1) the overall CDH prevalence and (2) mortality and survival trends of infants with CDH using data collected by hospital- and population-based birth defects surveillance programs from multiple countries affiliated with the International Clearinghouse for Birth Defects Surveillance and Research (ICBDSR).
Design, Setting, and Participants Methods
Twenty-five hospital- and population-based surveillance programs in 19 countries from members of the ICBDSR provided birth defects mortality data between 1974 and 2015. Prevalence estimates and mortality rates from 2001 to 2012, a period in which the majority of the programs had the most complete data, were further examined. Included were CDH cases involving live births, stillbirths, or elective termination of pregnancy for fetal anomalies.
Main Outcomes and Measures
Prevalence and 95% confidence intervals (CI) from Poisson regression and cumulative mortality rates and 95% CI from the Kaplan-Meier Product-Limit method were calculated for each country and registry type. Joinpoint regression analyses were conducted to assess time trends.
Results
Overall, the prevalence of CDH from all countries combined was 2.6 per 10,000 total births (95% CI: 2.5–2.7), slightly increasing between 2001 and 2012 (average annual percent change [AAPC]=0.47%). The overall percent mortality of CDH was 37.7%, with hospital-based registries having more deaths involving live births than population-based registries (45.1% compared to 33.8%). Mortality rates decreased over time (AAPC=−2.43%). Infants with multiple congenital anomalies and syndromes had higher 1-week mortality rates (45.2% and 40.8%) than those with isolated defects (28.6%) overall. Most deaths due to CDH occurred among 2- to 6-day-old infants for both registry types (36.3%, hospital-based; 12.1%, population-based).
Conclusions and Relevance
The prevalence of CDH has increased over time; although the mortality rate has slightly decreased, it remains high especially during the first week of life and varied by registry type. Further research is needed to inform development of measures and interventions to decrease deaths among infants with CDH.
INTRODUCTION
Congenital diaphragmatic hernia (CDH) is a severe birth defect characterized by a diaphragmatic malformation allowing protrusion of lower abdominal organs into the thoracic cavity.1 Worldwide, CDH occurs in approximately 1 in every 3,000 live births.2 Respiratory failure, due to pulmonary hypertension and pulmonary hypoplasia, is the leading cause of CDH-related mortality.3,4 Approximately 64% of CDH cases are isolated and 36% have multiple anomalies.1 Infants with CDH have significant morbidity and mortality, with a mortality rate between 30% and 60% or as high as 89% when additional chromosomal or structural anomalies are present.2,5–8 Approximately 30% of infants with CDH have additional anomalies, which leads to a higher morbidity rate compared to infants with CDH only.9
The pre- and postnatal diagnosis, clinical management, and treatment of infants with CDH has significantly improved in recent years.10–12 Despite these advances, the overall mortality rate has remained high over the last three decades.13–16 Many studies have examined specific treatments and their associated mortality rates in single tertiary centers but have shown little to no significant improvements in survival rates.17,18 Additionally, estimates of mortality may vary among registries and single institutions due to differences in case ascertainment and reporting.19
Worldwide, CDH mortality and survival trends are not well studied; this study provides the opportunity to use aggregated data from multiple countries to further explore these topics. The purpose of our study was to examine (1) the overall CDH prevalence and (2) mortality and survival of infants with CDH using data collected by population- and hospital-based birth defects surveillance programs from countries affiliated with the International Clearinghouse for Birth Defects Surveillance and Research (ICBDSR). We examined the total prevalence, survival probabilities, time trends, and mortality among birth outcomes and clinical presentation.
METHODS
Study Design and Setting
The ICBDSR, affiliated with the World Health Organization, is a voluntary, non-profit organization established in 1974 (http://www.icbdsr.org/), the aim of which is to prevent birth defects and reduce the related burden of their consequences by assembling birth defect surveillance and research programs from around the world. Currently, 42 surveillance programs with birth defects registries (either hospital- or population-based) from 36 countries are members, with 27 contributing data annually. Each registry provides the ICBDSR with aggregated data on children and fetuses affected with any of 39 different birth defects for surveillance purposes. Data are collected on the total annual number of live births and stillbirths for each of the surveillance years to assist in the prevalence estimation. Summaries of these data can be found at http://www.icbdsr.org/wp-content/annual_report/Report2014.pdf.
The study period for this analysis was birth years 1974 to 2015. We further examined the prevalence estimates and mortality rates from 2001 to 2012, a period in which the majority of the programs had the most complete data. We used data from 25 ICBDSR member programs, representing 19 countries in the Middle East, Europe, North America, Central America, and South America (Appendix Table 1). We included programs that collected data on both CDH and associated mortality. We examined the type of surveillance method (hospital-based vs. population-based registries), year that surveillance began, surveillance period for CDH, criteria used to define a stillbirth, national legislation pertaining to elective termination of pregnancy for fetal anomalies (ETOPFA), and prenatal screening service availability (Table 1a).
Table 1a.
Description of birth defects registries included in the congenital diaphragmatic hernia mortality study by type of registry: surveillance period, coverage, ascertainment period, stillbirth definition, ETOPFA allowed, and availability of prenatal screening services.
| Country-Registry | Surveillance Period (n) | Coverage | Ascertainment Period | Stillbirth Definition | ETOPFA Allowed | Prenatal Screening Services |
|---|---|---|---|---|---|---|
| Hospital-Based Registries | ||||||
| Argentina-RENAC | 2009–2014 (6) | N | hospital discharge | >500 g | No | Yes, no official program |
| Colombia-Bogotá | 2000–2014 (15) | R | 1st day | >500 g | Yes, since 2006 | Yes |
| Colombia-Cali | 2011–2014 (4) | R | 1st day | >500 g | Yes, since 2006 | Yes |
| South America-ECLAMC | 1995–2015 (21) | R1 | hospital discharge | >500 g | No5 | Yes |
| Spain-ECEMC | 1986–2013 (28) | R2 | 3 days | 24 weeks or 500 g4 | Yes, since 1985 | Yes |
| Mexico-RYVEMCE | 1978–2013 (36) | R | 3 days | ≥20 gestational weeks or ≥500 g | No | No |
| Iran-TROCA | 2004–2012 (9) | R | 1 year | 20 weeks | Yes, restrictions since 2013 | Yes |
| Israel-SMC | 2000–2014 (15) | R3 | hospital discharge | Not included | Yes, but not registered | Yes |
| Population-Based Registries | ||||||
| Costa Rica-CREC | 2000–2014 (15) | N | 1 year | 20 weeks or >500 g | No | Yes, only high risk pregnancies |
| Czech Republic | 1993–2014 (21) | N | 15 years | 22 weeks or >500 g | Yes | Yes |
| France-Paris | 1981–2014 (34) | R | 28 days | 22 weeks | Yes | Yes |
| Germany-Saxony Anhalt | 1980–2014 (35) | R | 1 year | >500 g | Yes | Yes, since 1990 |
| Italy-Lombardy | 2003–2012 (10) | R | 6 years | 23 weeks | Yes | Yes |
| Italy-Tuscany | 1992–2014 (22) | R | 1 year | 20 weeks | Yes | yes |
| Malta-MCAR | 1995–2013 (19) | N | 1 year | 22 weeks | No | Yes, gradually introduced |
| Netherlands-Northern | 1981–2014 (34) | R | 10 years | 24 weeks | Yes | Yes, since 2007 |
| Slovak Republic | 2001–2013 (14) | N | hospital discharge | >500 g | Yes | Yes |
| Sweden | 1987–2014 (28) | N | before ‘87 1 month, after ‘87 1 year | until 2006: 28 weeks, 2007 and after: 22 weeks | Yes, registration since 1999 | Yes, since early 1980’s |
| Ukraine-OMNI-Net | 2000–2013 (14) | R | 1 year | until 2006: 28 weeks/>1000 g 2007 and after: 22 weeks/>500 g | Yes | Yes |
| United Kingdom-Wales | 1998–2014 (17) | R | 18 years | 24 weeks | Yes | Yes, since 2003 |
| Mexico-Nuevo León | 2011–2015 (5) | R | 6 days | Not included | No | Yes, only US |
| USA-Arkansas | 1993–2012 (20) | S | 2 years | 20 weeks | Yes, until 20 weeks | Yes |
| USA-Atlanta | 1974–2012 (39) | R | 6 years | 20 weeks | Yes6 | Yes |
| USA-Texas | 1996–2012 (17) | S | 1 year | 20 weeks | Yes, until 20 weeks | Yes |
| USA-Utah | 1999–2012 (14) | S | 2 years | 20 weeks | Yes | Yes |
Several regions in SA
several regions in Spain currently covering around 18% of total births
referral area of one hospital
if gestational age of death is not determined (since 1980)
except for anencephaly
Elective terminations were ascertained from prenatal diagnostic sites beginning in 1994, prior to that they were only rarely ascertained from hospital records.
n=Total number of years; N=National, R=Regional, S=Statewide; CREC=Costa Rican Birth Defect Registry; ECEMC=Spanish Collaborative Study of Congenital Malformations; ECLAMC=Latin American Collaborative Study of Congenital Malformations; ETOPFA=Elective Termination of Pregnancy for Fetal Anomalies; MCAR=Malta Congenital Anomalies Registry; OMNI-Net=Ukraine Birth Defects Prevention Program; RENAC=National Network of Congenital Anomalies of Argentina; RYVEMCE=Mexican Registry and Epidemiological Surveillance of External Congenital Malformations; TROCA=Tabriz Registry of Congenital Anomalies; SMC=Soroka Medical Center; USA=United States of America.
Congenital Diaphragmatic Hernia Case Definition
ICBDSR defines CDH as “a congenital malformation characterized by herniation into the thorax of abdominal contents through a defect of the diaphragm. Includes: total absence of the diaphragm. Excludes: hiatus hernia, eventration of the diaphragm, and phrenic palsy.” CDH corresponds to ICD-10 code “Q79” and ICD-9 code “756.6”. Each program provided information on the number of CDH cases and the pregnancy outcomes (live birth, stillbirth, or ETOPFA) per year. Each case was also classified based on clinical presentation for 18 programs (72%). Isolated cases were defined as infants or fetuses with CDH, but no other unrelated major birth defects. Cases with multiple congenital anomalies (MCA) were defined as infants or fetuses having two or more unrelated major anomalies. Syndromic cases were defined as having CDH as part of a recognized syndrome or a genetic disorder.
Mortality
Table 1b presents the methods of each program for follow-up of live born cases. Each program provided information on mortality based on their follow-up methods. The different methods included follow-up until discharge from the maternity hospital (20 of 25 programs), follow-up by a clinician or registry staff (9 of 25 programs), or follow-up by linkage with death certificates (12 of 25 programs). Mortality was examined by age at death using six categories: < 1 day, 2–6 days, 7–27 days, 28–364 days, 1–4 years, and ≥ 5 years.
Table 1b.
Description of program follow-up method for live births by registry type.
| Country-Registry | Follow-up until discharge from the maternity hospital | Follow-up by a clinician or registry staff | Linkage with death certificates | Maximum follow-up period reported in study |
|---|---|---|---|---|
| Hospital-Based Registries | ||||
| Argentina-RENAC | Yes | Yes | No | 2–6 days |
| Colombia-Bogotá | Yes | Yes | No | 1 day |
| Colombia-Cali | Yes | Yes | No | No mortality reported for livebirths |
| South America-ECLAMC | Yes | Yes | No | 28–364 days |
| Spain-ECEMC | Yes1 | No | No | 2–6 days |
| Mexico-RYVEMCE | Yes | No | No | 2–6 days |
| Iran-TROCA | Yes | Yes3 | No | 2–6 days |
| Israel-SMC | Yes | No | Yes, up to 2014 | 28–364 days |
| Population-Based Registries | ||||
| Costa Rica-CREC | No | No | Yes5 | ≥5 years |
| Czech Republic | No | No | Yes | ≥5 years |
| France-Paris | Yes | Yes | No | 7–27 days |
| Germany-Saxony Anhalt | Yes | Yes4 | No | ≥5 years |
| Italy-Lombardy | No | No | Yes, up to 2015 | 7–27 days |
| Italy-Tuscany | No | No | Yes, up to 2015 | 28–364 days |
| Malta-MCAR | Yes2 | No | Yes6 | ≥5 years |
| Netherlands-Northern | Yes | Yes | No | ≥5 years |
| Slovak Republic | Yes | No | No | 7–27 days |
| Sweden | No | No | Yes, up to April 2016 | ≥5 years |
| Ukraine-OMNI-Net | Yes | Yes | No | 28–364 days |
| United Kingdom-Wales | Yes | No | Yes, to GP system, till 18 years | ≥ 5 years |
| Mexico-Nuevo León | Yes | No | No | ≥5 years |
| USA-Arkansas | Yes | No | Yes, up to 2015 | ≥5 years |
| USA-Atlanta | Yes | No | Yes, up to 2008 | ≥5 years |
| USA-Texas | Yes | No | Yes, up to 2013 | ≥5 years |
| USA-Utah | Yes | No | Yes, until age 2 | ≥5 years |
the participating physicians in the program are especially focused on the ascertainment of birth defects
babies are followed up until discharge and their hospital files are again seen at 1 year of age, linkage with mortality data continues indefinitely
children in university hospital(s)
until 18 years
just for reported cases
continuous linkage with mortality register, for this study data has linkage up to 2015.
CREC=Costa Rican Birth Defect Registry; ECEMC=Spanish Collaborative Study of Congenital Malformations; ECLAMC=Latin American Collaborative Study of Congenital Malformations; GP=General Practitioner; MCAR=Malta Congenital Anomalies Registry; OMNI-Net=Ukraine Birth Defects Prevention Program; RENAC=National Network of Congenital Anomalies of Argentina; RYVEMCE=Mexican Registry and Epidemiological Surveillance of External Congenital Malformations; TROCA=Tabriz Registry of Congenital Anomalies; SMC=Soroka Medical Center; USA=United States of America
Statistical Analysis
The total CDH prevalence was calculated for each program and registry type (hospital- vs. population-based). Prevalence was calculated as the total number of CDH cases (live births + stillbirths + ETOPFA) divided by the total number of births (live births + stillbirths). ETOPFA was not included in the denominator of the prevalence formula because of the lack of information on the total number of terminations for each program. A Poisson approximation of the binomial distribution was used for prevalence estimation and associated 95% confidence intervals (CI). The proportion and 95% CI of CDH resulting in a live birth, stillbirth, or ETOPFA was also calculated.
Age-specific mortality was calculated for each of the six age at death categories as the number of deaths among the live born cases divided by the total number of live born CDH cases. The cumulative percent mortality and corresponding CIs were calculated using a Kaplan-Meier Product-Limit method for each program, registry type, and the total to account for censoring. Mortality was examined by clinical presentation (isolated, MCA, syndromic) when available.
Three-year rolling averages of the total prevalence were calculated and graphed for each registry type and geographic region of the participating programs from 2001 to 2012. Joinpoint regression analysis was used to identify statistically significant temporal trends in CDH prevalence and mortality by registry type. Iran-TROCA was excluded from the Joinpoint regression analysis since its prevalence rates over time were outliers compared to the other registries. Survival probability of the live births was calculated and graphed for North American and European programs, which had the highest number of participating programs and a follow-up period of 5 years or more. Survival probability was calculated as the cumulative proportion of cases that died at different time periods after birth subtracted from the total number of live births with CDH.
Each program has locally approved ethics procedures, and because this study was conducted using aggregated data, no additional ethics committee approval was required.
RESULTS
Of the 25 ICBDSR member programs we obtained data from (Appendix Table 1), 8 were hospital-based and 17 were population-based. Most population-based programs had regional coverage (n=9) (national coverage [n=5] and state coverage [n=3]). The ascertainment period and criteria to define stillbirth varied among programs. Six of the 25 countries or regions did not allow ETOPFA. Most healthcare programs in the regions included in the registries offered prenatal screening services in recent years (Table 1a).
Prevalence
Supplementary Table 1 presents the overall CDH prevalence for all registries from 1974 to 2015. A total of 28,701,270 births and 7,581 total CDH cases were reported by all programs combined, resulting in an overall CDH prevalence of 2.6 per 10,000 births (95% CI: 2.5–2.7).
The program specific CDH prevalence (per 10,000 births) and types of pregnancy outcomes (live births, stillbirths, and ETOPFA) by registry type for the years 2001–2012, when the majority of programs had the most complete data, are presented in Table 2. Overall, from 2001–2012, the average CDH prevalence was 2.8 per 10,000 births (95% CI: 2.7–2.9). Hospital-based registries had an average CDH prevalence of 2.8 per 10,000 births (95% CI: 2.6–2.9), similar to population-based registries (2.8 per 10,000 births; 95% CI: 2.7–2.9). Iran-TROCA and Malta-MCAR had the highest CDH prevalence (5.7 and 5.4 per 10,000 births, respectively), whereas the programs with the lowest CDH prevalence were hospital-based registries (Spain-ECEMC, Mexico-RYVEMCE [1.1 and 1.1 per 10,000 births, respectively]). The average proportion of stillbirths for all registries was 3.7% (95% CI: 3.2–4.3), similar to the proportion of stillbirths among population-based registries (3.0% [95% CI: 2.5–3.6]), whereas hospital-based registries had a higher proportion of stillbirths (5.6% [95% CI: 4.4–7.0]). Ukraine-OMNI-Net and Italy-Lombardy, had the highest proportion of stillbirths (16.2% for both). Population-based registries were more often from countries that allowed ETOPFA and, therefore, had a higher proportion of ETOPFA (10.2%) compared to only two hospital-based registries in regions where ETOPFA is allowed (2.8%). France-Paris (30.5%) and Sweden (28.7%) had the highest proportion of ETOPFA among all the programs.
Table 2.
Total number of births, total number of CDH cases, prevalence per 10,000 births, live birth proportion, stillbirth proportion, and ETOPFA proportion by registry type for surveillance period 2001–2012.
| Country-Registry | Surveillance Period | Total Births | Total Cases of CDH | Total Prevalence per 10,000 total births (95% CI) | Live Birth % (95% CI) | Stillbirth % (95% CI) | ETOPFA % (95% CI) |
|---|---|---|---|---|---|---|---|
| Hospital-Based Registries | |||||||
| Argentina-RENAC1 | 2009–2012 | 422,173 | 139 | 3.3 (2.7, 3.9) | 95.7 (90.8, 98.4) | 4.3 (1.6, 9.2) | - |
| Colombia-Bogotá2 | 2001–2012 | 356,454 | 72 | 2.0 (1.6, 2.5) | 97.2 (90.3, 99.7) | 2.8 (0.3, 9.7) | - |
| South America-ECLAMC1 | 2001–2012 | 1,847,181 | 716 | 3.8 (3.4, 4.2) | 92.6 (90.4, 94.4) | 7.4 (5.6, 9.6) | - |
| Spain-ECEMC | 2001–2012 | 1,195,025 | 130 | 1.1 (0.9, 1.3) | 72.3 (63.8, 79.8) | 1.3 (0.5, 6.6) | 25.4 (18.2, 33.8) |
| Mexico-RYVEMCE1 | 2001–2012 | 264,306 | 30 | 1.1 (0.8, 1.6) | 90.0 (73.5, 97.9) | 10 (2.1, 26.5) | - |
| Iran-TROCA | 2004–2012 | 160,755 | 92 | 5.7 (4.6, 7.0) | 97.8 (92.4, 99.7) | 1.1 (0.0, 5.9) | 1.1 (0.0, 5.9) |
| Israel-SMC3 | 2001–2012 | 157,544 | 39 | 2.5 (1.8, 3.4) | 100.0 (91.0, 100.0) | 0.0 (0.0, 9.0) | - |
| TOTAL | 2001–2012 | 4,403,438 | 1,218 | 2.8 (2.6, 2.9) | 91.6 (89.9, 93.1) | 5.6 (4.4, 7.0) | 2.8 (1.9, 3.9) |
| Population-Based Registries | |||||||
| Costa Rica-CREC1 | 2001–2012 | 876,607 | 137 | 1.6 (1.3, 1.8) | 98.5 (94.8, 99.8) | 1.5 (0.2, 5.2) | - |
| Czech Republic | 2001–2012 | 1,273,386 | 326 | 2.6 (2.3, 2.9) | 81.3 (76.6, 85.4) | 0.0 (0.0, 1.1) | 18.7 (14.6, 23.4) |
| France-Paris | 2001–2012 | 319,636 | 85 | 2.7 (2.1, 3.3) | 67.1 (56.0, 76.9) | 2.4 (0.3, 8.2) | 30.5 (21.1, 41.5) |
| Germany-Saxony Anhalt | 2001–2012 | 208,108 | 58 | 2.8 (2.1, 3.6) | 75.9 (62.8, 86.1) | 3.4 (0.4, 11.9) | 20.7 (11.2, 33.4) |
| Italy-Lombardy | 2003–2012 | 133,182 | 37 | 2.8 (2.0, 3.8) | 67.6 (50.2, 82.0) | 16.2 (6.2, 32.0) | 16.2 (6.2, 32.0) |
| Italy-Tuscany | 2001–2012 | 352,844 | 76 | 2.2 (1.7, 2.7) | 78.9 (68.1, 87.5) | 1.3 (0.0, 7.1) | 19.8 (11.5, 30.5) |
| Malta-MCAR1 | 2001–2012 | 48,202 | 26 | 5.4 (3.5, 7.9) | 92.3 (74.9, 99.0) | 7.7 (0.9, 25.1) | - |
| Netherlands-Northern | 2001–2012 | 221,846 | 60 | 2.7 (2.1, 3.5) | 75.0 (62.1, 85.3) | 10.0 (3.8, 20.5) | 15.0 (7.1, 26.6) |
| Slovak Republic | 2001–2012 | 667,992 | 119 | 1.8 (1.5, 2.1) | 97.5 (92.8, 99.5) | 1.7 (0.2, 5.9) | 0.8 (0.0, 4.6) |
| Sweden | 2001–2012 | 1,230,002 | 397 | 3.2 (2.9, 3.6) | 70.0 (65.3, 74.5) | 1.3 (0.4, 2.9) | 28.7 (24.3, 33.4) |
| Ukraine-OMNI-Net4 | 2001–2012 | 347,418 | 105 | 3.0 (2.5, 3.7) | 59.0 (49.0, 68.6) | 16.2 (9.7, 24.7) | 21.9 (14.4, 31.0) |
| United Kingdom-Wales | 2001–2012 | 404,385 | 160 | 4.0 (3.4, 4.6) | 72.5 (64.9, 79.3) | 0.6 (0.0, 3.4) | 26.9 (20.2, 34.5) |
| USA-Arkansas4 | 2001–2012 | 470,593 | 144 | 3.1 (2.6, 3.6) | 96.5 (92.1, 98.9) | 2.8 (0.8, 7.0) | 0.0 (0.0, 2.5) |
| USA-Atlanta4 | 2001–2012 | 609,837 | 208 | 3.4 (3.0, 3.9) | 77.4 (71.1, 82.9) | 3.8 (1.7, 7.4) | 9.1 (5.6, 13.9) |
| USA-Texas | 2001–2012 | 4,668,071 | 1,289 | 2.8 (2.6, 2.9) | 96.2 (95.0, 97.2) | 2.6 (1.8, 3.6) | 1.2 (0.7, 2.0) |
| USA-Utah | 2001–2012 | 624,990 | 217 | 3.5 (3.0, 4.0) | 91.2 (86.7, 94.7) | 6.0 (3.2, 10.0) | 2.8 (1.0, 5.9) |
| TOTAL | 2001–2012 | 12,457,099 | 3,444 | 2.8 (2.7, 2.9) | 86.1 (84.9, 87.2) | 3.0 (2.5, 3.6) | 10.2 (9.2, 11.3) |
| ALL REGISTRIES | 2001–2012 | 16,860,537 | 4,662 | 2.8 (2.7, 2.9) | 87.5 (86.6, 88.5) | 3.7 (3.2, 4.3) | 8.3 (7.5, 9.1) |
ETOPFA not allowed
ETOPFA not registered
data on live born children with congenital diaphragmatic hernia from one hospital
percentages of live birth, stillbirth, and ETOFA do not add up to 100% due to unknown pregnancy outcome of some cases.
CI=Confidence Interval; CREC=Costa Rican Birth Defect Registry; ECEMC=Spanish Collaborative Study of Congenital Malformations; ECLAMC=Latin American Collaborative Study of Congenital Malformations; ETOPFA=Elective Termination of Pregnancy for Fetal Anomalies; MCAR=Malta Congenital Anomalies Registry; OMNI-Net=Ukraine Birth Defects Prevention Program; RENAC=National Network of Congenital Anomalies of Argentina; RYVEMCE=Mexican Registry and Epidemiological Surveillance of External Congenital Malformations; TROCA=Tabriz Registry of Congenital Anomalies; SMC=Soroka Medical Center; USA=United States of America.
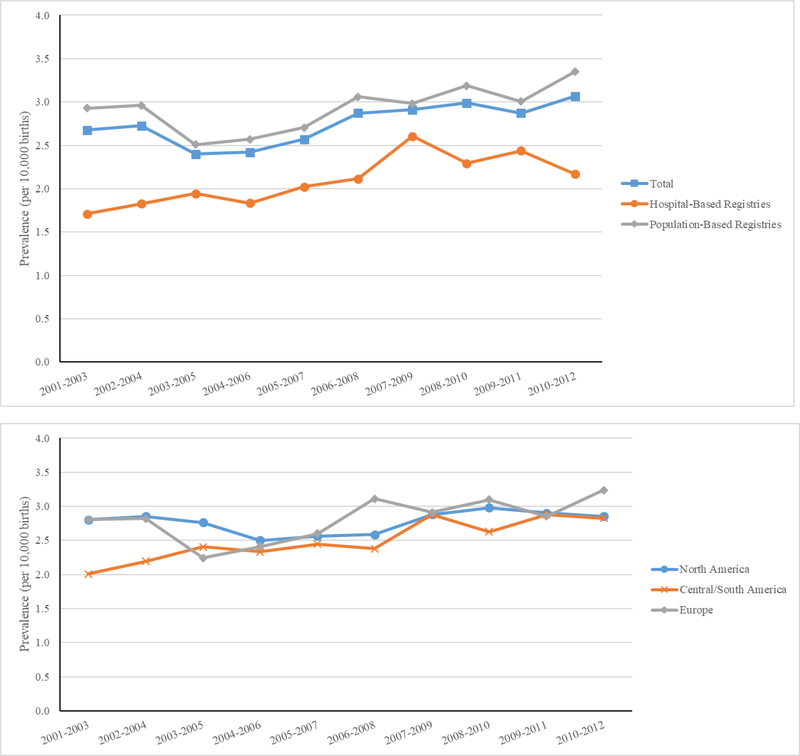
Figure 1 displays the three-year rolling averages of total CDH prevalence by type of registry and region from 2001 to 2012. Population-based registries had the highest averages, hospital-based programs had the lowest, with the total average in the middle. Among the regions, Central and South America showed an increase in the three-year rolling average prevalence. Joinpoint regression showed an increasing linear trend in prevalence between 2001 and 2012, with an average annual percent change (AAPC) of 0.47% (data not shown). Time trends also differed by registry type. Population-based registries had a greater AAPC during this period than hospital-based registries (0.91% vs −0.17%) (data not shown).
Figure 1. Three-year rolling averages of congenital diaphragmatic hernia prevalence by registry type and continent, 25 surveillance systems in 19 countries, 2001–2012.1.
1 Iran-TROCA and Israel-SMC are not included in these graphs.
Data on birth defects co-occurring with CDH were provided by 18 programs (72%) (Table 3). The percentages of isolated cases of CDH were similar between hospital-based and population-based programs. Overall 63.8% of CDH cases were isolated. For CDH cases that were determined to be MCA or syndromic, the differences between hospital-based and population-based programs were larger. Hospital-based registries had higher percentages of CDH cases with MCA compared to population-based registries (32.2% and 27.9%, respectively), whereas proportions of syndromic cases were higher among population-based registries (10.0%) compared to hospital-based registries (2.1%). The highest percentage of stillbirth cases among all total stillbirths were MCA and syndromic cases identified from hospital-based registries (13.5% and 13.0%, respectively).
Table 3.
Pregnancy outcome of infants affected with CDH according to clinical presentation 2001–2012.
| Country- Registry | Isolated CDH | MCA CDH | Syndromic CDH | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total Cases | Pregnancy Outcome | Total Cases | Pregnancy Outcome | Total Cases | Pregnancy Outcome | ||||||||||||
| N | % | LB % | SB % | ETOPFA % | N | % | LB % | SB % | ETOPFA % | N | % | LB % | SB % | ETOPFA % | |||
| Hospital-Based Registries | |||||||||||||||||
| Argentina-RENAC1 | 100 | 71.9% | 99.0% | 1.0% | 0.0% | 35 | 25.2% | 91.4% | 8.6% | 0.0% | 4 | 2.9% | 50.0% | 50.0% | 0.0% | ||
| Colombia-Bogotá2 | 58 | 80.6% | 98.3% | 1.7% | 0.0% | 12 | 16.7% | 91.7% | 8.3% | 0.0% | 2 | 2.7% | 100.0% | 0.0% | 0.0% | ||
| SA-ECLAMC1,3 | 443 | 61.9% | 91.5% | 2.5% | 0.0% | 273 | 38.1% | 84.6% | 15.4% | 0.0% | - | - | - | - | - | ||
| Spain-ECEMC | 84 | 64.6% | 75.0% | 0.0% | 25.0% | 32 | 24.6% | 68.8% | 6.2% | 25.0% | 14 | 10.8% | 64.3% | 7.1% | 28.6% | ||
| Mexico-RYVEMCE1 | 19 | 63.3% | 89.5% | 10.5% | 0.0% | 8 | 26.7% | 87.5% | 12.5% | 0.0% | 3 | 10.0% | 100.0% | 0.0% | 0.0% | ||
| Israel-SMC4 | 36 | 92.7% | 100.0% | 0.0% | 0.0% | 3 | 7.3% | 100.0% | 0.0% | 0.0% | 0 | 0.0% | 0.0% | 0.0% | 0.0% | ||
| TOTAL | 740 | 65.7% | 95.1% | 2.0% | 2.9% | 363 | 32.2% | 84.3% | 13.5% | 2.2% | 23 | 2.1% | 69.6% | 13.0% | 17.4% | ||
| Population-Based Registries | |||||||||||||||||
| Costa Rica-CREC1,5 | 95 | 69.3% | 100.0% | 0.0% | 0.0% | - | - | - | - | - | - | - | - | - | - | ||
| Czech Republic6 | - | - | - | - | - | - | - | - | - | - | 7 | 2.1% | 57.1% | 0.0% | 42.9% | ||
| France-Paris | 53 | 62.4% | 86.8% | 1.9% | 11.3% | 18 | 21.2% | 50.0% | 0.0% | 50% | 14 | 16.4% | 14.3% | 7.1% | 78.6% | ||
| Germany-Saxony Anhalt | 37 | 63.8% | 89.2% | 2.7% | 8.1% | 14 | 24.1% | 50.0% | 0.0% | 50% | 7 | 12.1% | 57.1% | 14.3% | 28.6% | ||
| Italy-Lombardy | 16 | 43.2% | 81.3% | 12.5% | 6.2% | 21 | 52.5% | 57.1% | 19.0% | 23.8% | 3 | 7.5% | 33.3% | 0.0% | 66.7% | ||
| Malta-MCAR1 | 17 | 65.4% | 94.1% | 5.9% | 0.0% | 6 | 23.1% | 100.0% | 0.0% | 0.0% | 3 | 11.5% | 66.7% | 33.3% | 0.0% | ||
| Netherlands-Northern | 41 | 68.3% | 82.9% | 7.3% | 9.8% | 8 | 13.3% | 62.5% | 12.5% | 25% | 11 | 18.4% | 54.5% | 18.2% | 27.3% | ||
| Slovak Republic3 | 81 | 68.1% | 97.6% | 1.2% | 1.2% | 38 | 31.9% | 97.4% | 2.6% | 0.0% | - | - | - | - | - | ||
| Sweden | 224 | 56.4% | 83.5% | 0.9% | 15.6% | 137 | 34.5% | 62.0% | 1.5% | 36.5% | 36 | 9.1% | 16.6% | 2.8% | 80.6% | ||
| Ukraine-OMNI-Net | 68 | 64.8% | 64.7% | 13.2% | 20.6% | 33 | 31.4% | 45.4% | 24.2% | 24.2% | 4 | 3.8% | 75.0% | 0.0% | 25.0% | ||
| United Kingdom-Wales | 86 | 54.0% | 82.5% | 1.2% | 16.3% | 47 | 29.6% | 72.3% | 0.0% | 27.7% | 26 | 16.4% | 50.0% | 0.0% | 50.0% | ||
| USA-Utah | 132 | 60.8% | 96.2% | 2.3% | 1.5% | 59 | 27.2% | 88.1% | 10.2% | 1.7% | 26 | 12.0% | 73.1% | 15.4% | 11.5% | ||
| TOTAL | 850 | 62.1% | 87.6% | 2.8% | 9.4% | 381 | 27.9% | 68.8% | 5.8% | 24.9% | 137 | 10.0% | 43.8% | 7.3% | 48.9% | ||
| ALL REGISTRIES | 1,590 | 63.8% | 91.1% | 2.5% | 6.4% | 744 | 29.8% | 76.3% | 9.5% | 13.8% | 160 | 6.4% | 47.5% | 8.1% | 44.4% | ||
ETOPFA not allowed
ETOPFA not registered
data only provided for isolated and MCA cases
data on live born children with congenital diaphragmatic hernia from one hospital
data only provided for isolated cases
data only provided for syndromic cases.
CREC=Costa Rican Birth Defect Registry; ECEMC=Spanish Collaborative Study of Congenital Malformations; ECLAMC=Latin American Collaborative Study of Congenital Malformations; ETOPFA=Elective Termination of Pregnancy for Fetal Anomalies; LB=Live Birth; MCA=Multiple Congenital Anomalies; MCAR=Malta Congenital Anomalies Registry; OMNI-Net=Ukraine Birth Defects Prevention Program; RENAC=National Network of Congenital Anomalies of Argentina; RYVEMCE=Mexican Registry and Epidemiological Surveillance of External Congenital Malformations; SA=South America; SB=Stillbirth; SMC=Soroka Medical Center; TROCA=Tabriz Registry of Congenital Anomalies; USA=United States of America.
Mortality
Table 4 displays mortality among live births with CDH by age of death. About 37.7% of live births with CDH resulted in death among all registries between 2001 and 2012. Hospital-based registries had a higher cumulative percent mortality (45.1%) compared to population-based registries (33.8%). The programs with the highest cumulative percent mortality were South America-ECLAMC (56.7%), Costa Rica-CREC (54.8%), and Israel-SMC (53.8%), with the lowest being Iran-TROCA (2.2%). Time trend analyses showed that overall mortality rates during 2001–2012 decreased linearly by a statistically significant AAPC of −2.43% (data not shown). However, time trends in mortality rates varied by registry type. For population-based registries, mortality rates decreased almost imperceptibly with an AAPC of −0.34%, while hospital-based registries had a higher decrease in mortality with an AAPC of −0.73% (data not shown).
Table 4.
Mortality in CDH affected live births for surveillance period 2001–2012.
| Country-Registry | Surveillance Period | Live Births with CDH | Age at Death | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Day 1 | Day 2-Day 6 | Day 7-Day 27 | Day 28-Day 364 | Year 1–4 | Year 5 and Above | Kaplan-Meier Mortality Estimate (95% CI) | |||
| Hospital-Based Registries | |||||||||
| Argentina-RENAC1 | 2009–2012 | 133 | 48.9%4 | - | - | - | - | 48.9% (40.4, 57.4) | |
| Colombia-Bogotá2 | 2001–2012 | 70 | 20.0% | - | - | - | - | - | 20.0% (10.6, 29.4) |
| South America-ECLAMC1 | 2001–2012 | 663 | 0.2% | 50.1% | 5.6% | 0.9% | - | - | 56.7% (52.9, 60.5) |
| Spain-ECEMC | 2001–2012 | 94 | 7.4% | 4.3% | - | - | - | - | 11.7% (5.2, 18.2) |
| Mexico-RYVEMCE1 | 2001–2012 | 27 | 3.7% | 3.7% | - | - | - | - | 7.4% (0.0, 17.3)5 |
| Iran-TROCA | 2004–2012 | 90 | 2.2% | 0.0% | - | - | - | - | 2.2% (0.0, 5.3)5 |
| Israel-SMC3 | 2001–2012 | 39 | 23.1% | 7.7% | 17.9% | 5.1% | - | - | 53.8% (38.2, 69.5) |
| TOTAL | 1,116 | 3.0% | 36.3% | 3.9% | 0.7% | - | - | 45.1% (42.0, 48.1) | |
| Population-Based Registries | |||||||||
| Costa Rica-CREC1 | 2001–2012 | 135 | 6.7% | 37.0% | 6.7% | 2.2% | 2.2% | 0.0% | 54.8% (46.4, 63.2) |
| Czech Republic | 2001–2013 | 265 | 8.7% | 12.8% | 2.3% | 3.4% | 1.1% | 0.0% | 28.3% (22.9, 33.7) |
| France-Paris2 | 2001–2012 | 57 | 5.3% | 22.8% | 5.3% | - | - | - | 33.4% (21.1, 45.6) |
| Germany-Saxony Anhalt | 2001–2012 | 44 | 15.9% | 4.5% | 2.3% | 4.5% | 0.0% | 0.0% | 27.2% (14.1, 40.4) |
| Italy-Lombardy | 2003–2012 | 25 | 0.0% | 16.0% | 8.0% | - | - | - | 24.0% (7.2, 40.7) |
| Italy-Tuscany | 2001–2012 | 60 | 6.7% | 8.3% | 8.3% | 0.0% | - | - | 23.3% (12.6, 34.0) |
| Malta1 | 2001–2012 | 24 | 33.3% | 4.2% | 0.0% | 0.0% | 0.0% | 0.0% | 37.5% (18.1, 56.9) |
| Netherlands-Northern | 2001–2012 | 45 | 11.1% | 4.4% | 2.2% | 15.6% | 0.0% | 0.0% | 33.3% (19.5, 47.1) |
| Slovak Republic | 2001–2012 | 116 | 0.0% | 41.4% | 3.4% | - | - | - | 44.8% (35.8, 53.9) |
| Sweden | 2001–2012 | 278 | 10.4% | 3.6% | 2.9% | 5.4% | 1.1% | 0.4% | 23.8% (18.7, 28.7) |
| Ukraine-OMNI-Net | 2001–2012 | 62 | 16.1% | 21.0% | 0.0% | 6.5% | - | - | 43.6% (31.2, 55.9) |
| United Kingdom-Wales | 2001–2012 | 116 | 8.6% | 17.2% | 3.4% | 5.2% | 1.7% | 0.0% | 36.1% (27.5, 44.9) |
| USA-Arkansas | 2001–2012 | 139 | 14.4% | 10.8% | 4.3% | 2.9% | 2.2% | 0.0% | 34.6% (26.6, 42.4) |
| USA-Atlanta | 2001–2012 | 161 | 8.7% | 6.8% | 6.8% | 1.9% | 0.0% | 0.0% | 24.2% (17.6, 30.8) |
| USA-Texas | 2001–2012 | 1,240 | 8.3% | 9.4% | 8.5% | 7.7% | 1.4% | 0.1% | 35.4% (32.7, 38.0) |
| USA-Utah | 2001–2012 | 198 | 12.6% | 7.1% | 4.5% | 7.6% | 0.5% | 0.0% | 32.3% (25.8, 38.8) |
| TOTAL | 2,965 | 9.1% | 12.1% | 5.9% | 5.5% | 1.1% | 0.1% | 33.8% (32.1, 35.5) | |
| ALL REGISTRIES | 2001–2012 | 4,081 | 7.4% | 19.0% | 5.4% | 4.9% | 0.9% | 0.1% | 37.7% (36.2, 39.2) |
ETOPFA not allowed
ETOPFA not registered
data on live born children with congenital diaphragmatic hernia from one hospital
Percentage refers to first week mortality
Lower limit confidence intervals fitted to zero.
CI=Confidence Interval; CREC=Costa Rican Birth Defect Registry; ECEMC=Spanish Collaborative Study of Congenital Malformations; ECLAMC=Latin American Collaborative Study of Congenital Malformations; MCAR=Malta Congenital Anomalies Registry; OMNI-Net=Ukraine Birth Defects Prevention Program; RENAC=National Network of Congenital Anomalies of Argentina; RYVEMCE=Mexican Registry and Epidemiological Surveillance of External Congenital Malformations; TROCA=Tabriz Registry of Congenital Anomalies; SMC=Soroka Medical Center; USA=United States of America.
The overall mortality for the first 24 hours of life was 7.4% and for the first week of life was 26.4% (data not shown). MCA cases had higher first week mortality than isolated cases in both hospital-based registries (58.8% vs 36.2%) and population-based registries (29.4% vs 21.3%); however, syndromic cases in population-based registries had a higher first week mortality than hospital-based registries (46.7% vs 18.8%) (data not shown). The highest proportion of death occurred among infants aged 2 to 6 days (19.0%) among all the programs, with the hospital-based registries having a higher proportion of death compared to population-based registries (36.3% vs 12.1%). Infants with MCA and syndromes had higher 1-week mortality rates (45.2% and 40.8%) than those with isolated defects (28.6%) overall (data not shown). The overall mortality rate during the 27-day neonatal period (31.8%) was only slightly higher than the overall 26.4% in the first week of life. Registries in countries or regions where ETOPFA was not allowed had higher first week mortality compared to the countries or regions where ETOPFA was allowed. The cumulative 5-year mortality rate was 37.7% overall. The cumulative 5-year mortality rate was 45.1% among hospital-based registries and 33.8% among population-based registries.
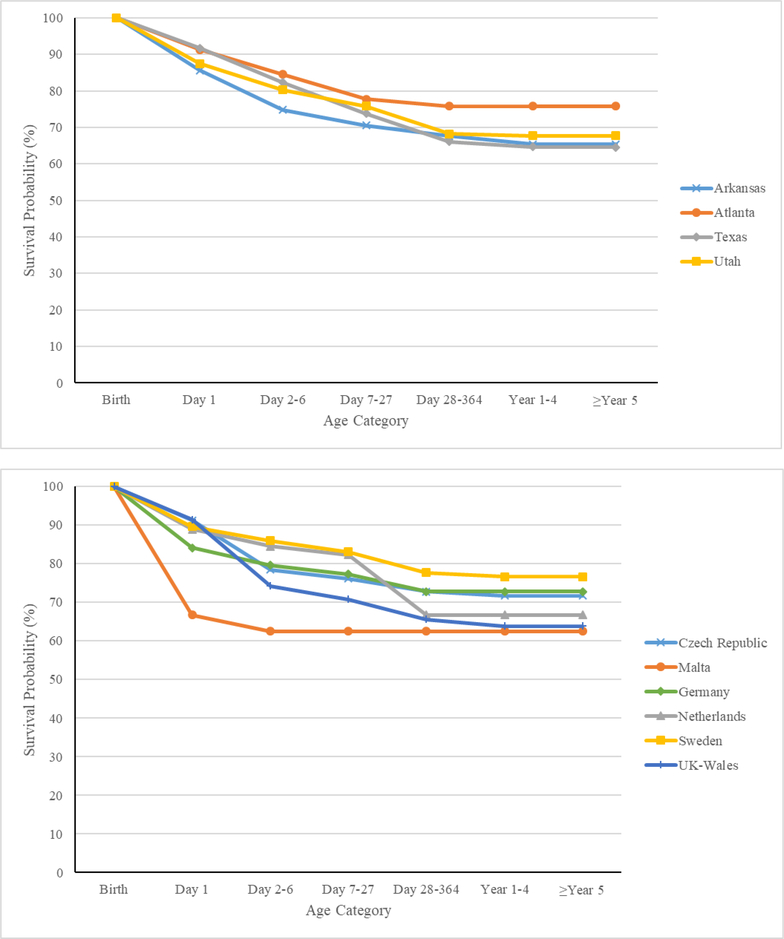
Figure 2 presents survival probabilities from 2001 to 2012 for programs located in North America and in Europe with complete data for all age categories. The survival probabilities in North America ranged from 64.6% to 75.8%, with USA-Atlanta having the highest survival probability and USA-Texas the lowest. In Europe, survival ranged from 63.9% to 76.6%. Sweden had a survival probability of 76.6% at 5 years or older, yet also had the highest percentage of ETOPFA (28.7%) among the European programs.
Figure 2.
Survival by age for children with congenital diaphragmatic hernia in North American and European surveillance systems, 2001–2012.
DISCUSSION
Ours is one of the first studies to examine CDH mortality across multiple countries. The overall CDH prevalence from 1974 to 2015 was 2.6 per 10,000 total births. The majority of CDH cases were isolated (63.8%). We found that CDH-related infant mortality, especially in the first week (26.1%), is a concern in many countries. The average survival probability for children 5 years old or greater with CDH varied between 64% and 77%.
The overall CDH prevalence (2.6 per 10,000 total births from 1974 to 2015) is similar to previously published estimates. In a large population-based study among registries in the European Surveillance of Congenital Anomalies, the overall prevalence was 2.3 per 10,000 births for the period between 1980 and 2009.20 Among other population-based registries outside of Europe, the prevalence ranged from 2.5 to 3.8 per 10,000 births.21,22
Our overall mortality results are similar to previously published studies, which showed CDH-related infant mortality rates ranging from 20% to 50%.23–26 In a United States population-based study, the authors reported a mortality rate of 28% for infants with CDH up to the first week of life, similar to the total mortality rate for the first week of life in our study (26.1%) for surveillance years 2001–2012.27 ‘Hidden mortality’ (unreported CDH cases involving death during gestation, shortly after birth, or before surgery) may exist among hospital-based registries and referral institutions.28 Many of the outcomes derived from population-based studies have shown lower survival than studies from single institutions.21,29,30 Our study contrasts with this concept, with population-based registries showing a lower mortality rate than hospital-based registries. This may be due to the fact that only two of the seven hospital-based registries included ETOPFA, and none of the registries reported treatment type. Additionally, Israel-SMC was the only single-hospital registry. The other hospital-based registries contained from 3 to 70 hospitals in their programs. Many other factors such as geographic regions, socioeconomic status, case ascertainment, and case selection biases need to be studied to examine the differences in mortality among hospital- and population-based registries. Overall prevalence rates were similar among the hospital- and population-based registries; however, hospital-based registries had higher cumulative percent mortality than population-based registries. Both registry types had the highest mortality among infants with CDH aged 2 to 6 days, with hospital-based registries having double the mortality rate of population-based registries. Currently, there is no common protocol in the treatment and management of infants with CDH. The use of early versus delayed surgical correction is not clearly defined for infants with CDH; however, there is a general trend towards delaying repair until after a period of stabilization.31–34 Often, the period of stabilization is supported by an effort to reduce the risk of pulmonary hypertension.17 Gentili et al. found a stabilization interval of 43.9 ± 38.7 hours (range 22–168 hours) before patients underwent surgical correction.35 It is possible that the lack of a standardized treatment protocol before surgical repair might contribute to infant mortality within the first week of life.33,36 Additionally, many of the hospital-based registries are in developing countries. The higher mortality rate during the first week could be explained by fewer resources, underreporting, and less healthcare access of the countries with registries in more resource-constrained settings compared to the higher-income countries that have population-based registries.
We observed higher proportions of ETOPFA among population-based registries and higher proportions of stillbirths among hospital-based registries. This association may be due to the higher number of programs that include ETOPFA belonging to population-based registries, whereas the higher stillbirth rates among the hospital-based registries may be due to the fact that only two programs reported ETOPFA, leading to a relative increase in stillbirths registered. Among the hospital-based registries, Mexico-RYVEMCE had the highest proportion of stillbirths, yet the lowest prevalence of CDH among all the programs. This program was also the only program that did not offer prenatal screening services, which may affect a mother’s decision on the outcome of the pregnancy if CDH is detected early. Most countries or regions that allowed ETOPFA had higher proportions of ETOPFA than stillbirths, especially in the European countries. The proportion of cases resulting in live births, stillbirths, and ETOPFA for population-based registries was similar to McGivern et al.’s study, which found 10.0% of cases resulted in an ETOPFA and 3.6% of cases resulted in a stillbirth (compared to 10.2% and 3.0%, respectively, in our data). Additionally, mortality was higher among the countries or regions that allowed ETOPFA, which may be due to the most severe cases surviving until birth but dying soon after.
In our study, MCA/syndromic cases of CDH had higher 1-week mortality rates than isolated cases. In general, prognosis of isolated CDH cases is better than CDH cases with multiple anomalies.37 This finding is similar to prior studies, which have reported higher mortality rates among MCA/syndromic cases than isolated CDH cases.1,18,38 We found an overall higher survival rate among all registries for isolated cases at 1 week (71.4%; data not shown), similar to the recent finding by McGivern et al. that 72.7% of isolated cases survived the first week of life. CDH cases are more likely to be terminated when other anomalies are present compared to isolated CDH cases.18
A major strength of our study is its large sample size and inclusion of registries from multiple countries. Additionally, it included stillbirths and ETOPFA as well as live births and reported prevalence and mortality rates for each outcome and clinical presentation. Despite these strengths, there are some limitations. First, our study is based on aggregated data and not individual data; therefore, it does not include information on prenatal diagnoses or post-birth treatment and management. In addition, some surveillance programs did not contribute data on clinical presentation and due to differences in surveillance procedures, not all of the programs were able to link to death certificates; therefore, some deaths may be missing due to administrative data linkage limitations. Furthermore, there are limitations with the consistency in data collection for this many registries across multiple countries, leading to variability in the data. However, we describe the characteristics of each registry, and our results are similar to other studies previously published.
Our study provides prevalence and mortality estimates for infants with CDH using registries from 19 countries. The overall mortality rate for CDH remains high, especially during the first week of life, but it has decreased slightly over the study period. Clinical presentation of CDH and its association with other anomalies is a major concern and may indicate a specific etiologic or genetic cause. Further research is needed to examine the differences between population- and hospital-based registries and the ‘hidden mortality’ that might be present. Additional data on treatment procedures and prenatal diagnostic services would be useful to further examine the differences in mortality among the countries and programs. Our study provides data regarding mortality among CDH cases, which can be used to inform development of measures and interventions to decrease deaths among infants with CDH.
Supplementary Material
Key Points.
Question
What are the age-specific mortality rates among infants with congenital diaphragmatic hernia (CDH), based on registries from multiple countries?
Findings
The overall prevalence of CDH was 2.6 per 10,000 births from the time period 1974 to 2015, but varied by registry type (2.5 per 10,000 births for hospital-based and 2.7 per 10,000 births for population-based registries). The 5-year survival probability varies between 64% and 77% from 2001 to 2012.
Meaning
The prevalence of CDH has increased over time, and rates of mortality have decreased; however, mortality remains high especially during the first week of life.
Acknowledgements
We would like to acknowledge each ICBDSR member program’s staff for providing data and information on the characteristics of their program.
Members of ECEMC’s peripheral group are greatly acknowledged.
Funding Source: This study received support from the CDC National Center on Birth Defects and Developmental Disabilities (#5U01DD000491) and the Arkansas Biosciences Institute (#037062).
Abbreviations
- CDH
Congenital Diaphragmatic Hernia
- CI
Confidence Interval
- ETOPFA
Elective Termination of Pregnancy for Fetal Anomalies
- ICBDSR
International Clearinghouse for Birth Defects Surveillance and Research
- MCA
Multiple Congenital Anomalies
Appendix
Appendix Table 1.
Congenital diaphragmatic hernia surveillance period, by country, registry, and type of registry, International Clearinghouse for Birth Defects Surveillance and Research (ICBDSR).
| Country | Registry | Surveillance Years (1974 – 2015) | ||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 74–77 | 78–79 | 80 | 81–85 | 86–90 | 91 | 92 | 93 | 94 | 95 | 96 | 97 | 98 | 99 | 00 | 01 | 02 | 03 | 04–08 | 09 | 10 | 11 | 12 | 13 | 14 | 15 | N3 | ||
| Hospital-Based Registries | ||||||||||||||||||||||||||||
| Argentina | RENAC | 6 | ||||||||||||||||||||||||||
| Colombia | Bogotá | 15 | ||||||||||||||||||||||||||
| Colombia | Cali | 4 | ||||||||||||||||||||||||||
| S. America | ECLAMC | 20 | ||||||||||||||||||||||||||
| Spain | ECEMC1 | 28 | ||||||||||||||||||||||||||
| Mexico | RYVEMCE | 36 | ||||||||||||||||||||||||||
| Iran | TROCA | 9 | ||||||||||||||||||||||||||
| Israel | SMC | 15 | ||||||||||||||||||||||||||
| Population-Based Registries | ||||||||||||||||||||||||||||
| Costa Rica | CREC | 15 | ||||||||||||||||||||||||||
| Czech Rep. | National | 21 | ||||||||||||||||||||||||||
| France | Paris | 34 | ||||||||||||||||||||||||||
| Germany | Saxony Anhalt | 35 | ||||||||||||||||||||||||||
| Italy | Lombardy | 10 | ||||||||||||||||||||||||||
| Italy | Tuscany | 22 | ||||||||||||||||||||||||||
| Malta | MCAR | 19 | ||||||||||||||||||||||||||
| Netherlands | Northern | 34 | ||||||||||||||||||||||||||
| Slovak Rep. | National | 14 | ||||||||||||||||||||||||||
| Sweden | National2 | 28 | ||||||||||||||||||||||||||
| Ukraine | OMNI-Net | 14 | ||||||||||||||||||||||||||
| UK | Wales | 17 | ||||||||||||||||||||||||||
| Mexico | Nuevo León | 5 | ||||||||||||||||||||||||||
| USA | Arkansas | 20 | ||||||||||||||||||||||||||
| USA | Atlanta | 39 | ||||||||||||||||||||||||||
| USA | Texas | 17 | ||||||||||||||||||||||||||
| USA | Utah | 14 | ||||||||||||||||||||||||||
Spain included information on elective termination of pregnancy for fetal anomalies from 1995–2014
Sweden included information on elective terminations of pregnancy for fetal anomalies from 1999–2014
Number of surveillance years.
CREC=Costa Rican Birth Defect Registry; ECEMC=Spanish Collaborative Study of Congenital Malformations; ECLAMC=Latin American Collaborative Study of Congenital Malformations; MCAR=Malta Congenital Anomalies Registry; OMNI-Net=Ukraine Birth Defects Prevention Program; RENAC=National Network of Congenital Anomalies of Argentina; RYVEMCE=Mexican Registry and Epidemiological Surveillance of External Congenital Malformations; TROCA=Tabriz Registry of Congenital Anomalies; SMC=Soroka Medical Center; UK=United Kingdom; USA=United States of America.
Footnotes
Disclaimer: The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
This study has been replicated by Suman Maity, the epidemiologist at the Arkansas Center for Birth Defects Research and Prevention.
References
- 1.Shanmugam H, Brunelli L, Botto LD, Krikov S, Feldkamp ML. Epidemiology and prognosis of congenital diaphragmatic hernia: A population-based cohort study in Utah. Birth Defects Res. 2017;109(18):1451–1459. [DOI] [PubMed] [Google Scholar]
- 2.Stege G, Fenton A, Jaffray B. Nihilism in the 1990s: the true mortality of congenital diaphragmatic hernia. Pediatrics. 2003;112(3 Pt 1):532–535. [DOI] [PubMed] [Google Scholar]
- 3.Leeuwen L, Fitzgerald DA. Congenital diaphragmatic hernia. J Paediatr Child Health. 2014;50(9):667–673. [DOI] [PubMed] [Google Scholar]
- 4.Kesieme EB, Kesieme CN. Congenital diaphragmatic hernia: review of current concept in surgical management. ISRN Surgery. 2011;2011:974041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kaiser JR, Rosenfeld CR. A population-based study of congenital diaphragmatic hernia: impact of associated anomalies and preoperative blood gases on survival. J Pediatr Surg. 1999;34(8):1196–1202. [DOI] [PubMed] [Google Scholar]
- 6.Balayla J, Abenhaim HA. Incidence, predictors and outcomes of congenital diaphragmatic hernia: a population-based study of 32 million births in the United States. J Matern-Fetal Neo M. 2014;27(14):1438–1444. [DOI] [PubMed] [Google Scholar]
- 7.Group CDHS. Does extracorporeal membrane oxygenation improve survival in neonates with congenital diaphragmatic hernia? The Congenital Diaphragmatic Hernia Study Group. J Pediatr Surg. 1999;34(5):720–724; discussion 724–725. [DOI] [PubMed] [Google Scholar]
- 8.Heiss K, Manning P, Oldham KT, et al. Reversal of mortality for congenital diaphragmatic hernia with ECMO. Ann Surg. 1989;209(2):225–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ruano R, Javadian P, Kailin JA, et al. Congenital heart anomaly in newborns with congenital diaphragmatic hernia: a single-center experience. Ultrasound Obst Gyn. 2015;45(6):683–688. [DOI] [PubMed] [Google Scholar]
- 10.Glenn IC, Abdulhai S, McNinch NL, et al. Evaluating the utility of the “late ECMO repair”: a congenital diaphragmatic hernia study group investigation. Pediaatr Surg Int. 2018;34(7):721–726. [DOI] [PubMed] [Google Scholar]
- 11.Harting MT, Hollinger L, Tsao K, et al. Aggressive surgical management of congenital diaphragmatic hernia: Worth the effort?: A multicenter, prospective, cohort study. Ann Surg. 2018;267(5):977–982. [DOI] [PubMed] [Google Scholar]
- 12.Boloker J, Bateman DA, Wung JT, Stolar CJ. Congenital diaphragmatic hernia in 120 infants treated consecutively with permissive hypercapnea/spontaneous respiration/elective repair. J Pediatr Surg. 2002;37(3):357–366. [DOI] [PubMed] [Google Scholar]
- 13.Migliazza L, Bellan C, Alberti D, et al. Retrospective study of 111 cases of congenital diaphragmatic hernia treated with early high-frequency oscillatory ventilation and presurgical stabilization. J Pediatr Surg. 2007;42(9):1526–1532. [DOI] [PubMed] [Google Scholar]
- 14.Schultz CM, DiGeronimo RJ, Yoder BA. Congenital diaphragmatic hernia: a simplified postnatal predictor of outcome. J Pediatr Surg. 2007;42(3):510–516. [DOI] [PubMed] [Google Scholar]
- 15.Brownlee EM, Howatson AG, Davis CF, Sabharwal AJ. The hidden mortality of congenital diaphragmatic hernia: a 20-year review. J Pediatr Surg. 2009;44(2):317–320. [DOI] [PubMed] [Google Scholar]
- 16.Mah VK, Zamakhshary M, Mah DY, et al. Absolute vs relative improvements in congenital diaphragmatic hernia survival: what happened to “hidden mortality”. J Pediatr Surg. 2009;44(5):877–882. [DOI] [PubMed] [Google Scholar]
- 17.Garriboli M, Duess JW, Ruttenstock E, et al. Trends in the treatment and outcome of congenital diaphragmatic hernia over the last decade. Pediatr Surg Int. 2012;28(12):1177–1181. [DOI] [PubMed] [Google Scholar]
- 18.Samangaya RA, Choudhri S, Murphy F, Zaidi T, Gillham JC, Morabito A. Outcomes of congenital diaphragmatic hernia: a 12-year experience. Prenatal Diag. 2012;32(6):523–529. [DOI] [PubMed] [Google Scholar]
- 19.Mah VK, Chiu P, Kim PC. Are we making a real difference? Update on ‘hidden mortality’ in the management of congenital diaphragmatic hernia. Fetal Diagn Ther. 2011;29(1):40–45. [DOI] [PubMed] [Google Scholar]
- 20.McGivern MR, Best KE, Rankin J, et al. Epidemiology of congenital diaphragmatic hernia in Europe: a register-based study. Arch Dis Child-Fetal. 2015;100(2):F137–144. [DOI] [PubMed] [Google Scholar]
- 21.Colvin J, Bower C, Dickinson JE, Sokol J. Outcomes of congenital diaphragmatic hernia: a population-based study in Western Australia. Pediatrics. 2005;116(3):e356–363. [DOI] [PubMed] [Google Scholar]
- 22.Yang W, Carmichael SL, Harris JA, Shaw GM. Epidemiologic characteristics of congenital diaphragmatic hernia among 2.5 million california births, 1989–1997. Birth Defects Res A Clin Mol Teratol. 2006;76(3):170–174. [DOI] [PubMed] [Google Scholar]
- 23.Beresford MW, Shaw NJ. Outcome of congenital diaphragmatic hernia. Pediatr Pulmonol. 2000;30(3):249–256. [DOI] [PubMed] [Google Scholar]
- 24.Group CDHS. Estimating disease severity of congenital diaphragmatic hernia in the first 5 minutes of life. J Pediatr Surg. 2001;36(1):141–145. [DOI] [PubMed] [Google Scholar]
- 25.Javid PJ, Jaksic T, Skarsgard ED, Lee S. Survival rate in congenital diaphragmatic hernia: the experience of the Canadian Neonatal Network. J Pediatr Surg. 2004;39(5):657–660. [DOI] [PubMed] [Google Scholar]
- 26.Zalla JM, Stoddard GJ, Yoder BA. Improved mortality rate for congenital diaphragmatic hernia in the modern era of management: 15 year experience in a single institution. J Pediatr Surg. 2015;50(4):524–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang Y, Hu J, Druschel CM, Kirby RS. Twenty-five-year survival of children with birth defects in New York State: a population-based study. Birth Defects Res A Clin Mol Teratol. 2011;91(12):995–1003. [DOI] [PubMed] [Google Scholar]
- 28.Harrison MR, Bjordal RI, Langmark F, Knutrud O. Congenital diaphragmatic hernia: the hidden mortality. J Pediatr Surg. 1978;13(3):227–230. [DOI] [PubMed] [Google Scholar]
- 29.Downard CD, Wilson JM. Current therapy of infants with congenital diaphragmatic hernia. Semin Neonatol. 2003;8(3):215–221. [DOI] [PubMed] [Google Scholar]
- 30.Al-Shanafey S, Giacomantonio M, Henteleff H. Congenital diaphragmatic hernia: experience without extracoporeal membrane oxygenation. Pediatr Surg Int. 2002;18(1):28–31. [DOI] [PubMed] [Google Scholar]
- 31.Nio M, Haase G, Kennaugh J, Bui K, Atkinson JB. A prospective randomized trial of delayed versus immediate repair of congenital diaphragmatic hernia. J Pediatr Surg. 1994;29(5):618–621. [DOI] [PubMed] [Google Scholar]
- 32.Langer JC, Filler RM, Bohn DJ, et al. Timing of surgery for congenital diaphragmatic hernia: is emergency operation necessary? J Pediatr Surg. 1988;23(8):731–734. [DOI] [PubMed] [Google Scholar]
- 33.Moyer V, Moya F, Tibboel R, Losty P, Nagaya M, Lally KP. Late versus early surgical correction for congenital diaphragmatic hernia in newborn infants. Cochrane DB Syst Rev. 2002(3):Cd001695. [DOI] [PubMed] [Google Scholar]
- 34.Chiu P, Hedrick HL. Postnatal management and long-term outcome for survivors with congenital diaphragmatic hernia. Prenatal Diag. 2008;28(7):592–603. [DOI] [PubMed] [Google Scholar]
- 35.Gentili A, De Rose R, Iannella E, Bacchi Reggiani ML, Lima M, Baroncini S. Is the time necessary to obtain preoperative stabilization a predictive index of outcome in neonatal congenital diaphragmatic hernia? Int J Pediatr. 2012;2012:402170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dao DT, Burgos CM, Harting MT, et al. Surgical repair of congenital diaphragmatic hernia after extracorporeal membrane oxygenation cannulation: Early repair improves survival. Ann Surg. 2019; Epub Ahead of Print. [DOI] [PubMed] [Google Scholar]
- 37.Chandrasekharan PK, Rawat M, Madappa R, Rothstein DH, Lakshminrusimha S. Congenital Diaphragmatic hernia - a review. Matern Health Neonatol Perinatol. 2017;3:6.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tennant PW, Pearce MS, Bythell M, Rankin J. 20-year survival of children born with congenital anomalies: a population-based study. Lancet. 2010;375(9715):649–656. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.