Abstract
BACKGROUND:
New guidelines for managing cervical precancer among women in the United States use risk directly to guide clinical actions for individuals who are being screened. These risk-based management guidelines have previously only been based on risks from a large integrated healthcare system. We present here data representative of women of low income without continuous insurance coverage to inform the 2019 guidelines and ensure applicability.
OBJECTIVE:
We examined the risks of high-grade precancer after human papillomavirus and cytology tests in underserved women and assessed the applicability of the 2019 guidelines to this population.
STUDY DESIGN:
We examined cervical cancer screening and follow-up data among 363,546 women enrolled in the Centers for Disease Control and Prevention’s National Breast and Cervical Cancer Early Detection Program from 2009 to 2017. We estimated the immediate (prevalent) risks of cervical intraepithelial lesion grade 3 or cancer by using prevalence-incidence mixture models. Risks were estimated for each combination of human papillomavirus and cytology result and were stratified by screening history. We compared these risks with published estimates used in new risk-based management guidelines.
RESULTS:
Women who were up-to-date with their screening, defined as being screened with cytology within the past 5 years, had immediate risks of cervical intraepithelial neoplasia grade 3 or higher similar to that of women at Kaiser Permanente Northern California, whose data were used to develop the management guidelines. However, women in the Centers for Disease Control and Prevention’s National Breast and Cervical Cancer Early Detection Program had greater immediate risks if they were never screened or not up-to-date with their screening.
CONCLUSION:
New cervical risk–based management guidelines are applicable for underinsured and uninsured women with a low income in the United States who are up-to-date with their screening. The increased risk observed here among women who received human papillomavirus–positive, high-grade cytology results, who were never screened, or who were not up-to-date with their cervical cancer screening, led to a recommendation in the management guidelines for immediate treatment among these women.
Keywords: cervical cancer screening, HPV and cytology cotesting, low-income women, risk-based management guidelines
Introduction
The 2012 and the recent 2019 consensus risk-based guidelines, sponsored by the American Society for Colposcopy and Cervical Pathology (ASCCP), used the principle of equal management for equal risk to determine the management after an abnormal cervical cancer screening result.1,2 This principle states that if 2 individuals have the same risk of cervical intraepithelial neoplasia (CIN) grade 3 or cancer (CIN3+), irrespective of patient characteristics and test results, their recommended clinical management should be the same. Under the new management guidelines, risk is directly used to determine whether an individual should undergo the following: immediate treatment (immediate CIN3+ risk of ≥60%); colposcopy with or without biopsy (immediate CIN3+ risk of ≥4%); or, if no clinical action is needed, whether an individual should return for retesting in 1, 3, or 5 years (5-year CIN3+ risk of ≥0.5%, 0.15%–0.5%, and <0.15%, respectively). These guidelines were largely based on calculating the risk of cervical precancer among 1.5 million women in a large integrated healthcare delivery system (Kaiser Permanente Northern California [KPNC]).3 Our aim was to understand whether these risks, which were estimated from the KPNC data, represent populations at an increased risk of cervical cancer, such as women of low income without continuous insurance coverage.
To ensure applicability to other populations, the 2019 guidelines compared the risks among several groups.3 Included in their assessment were some of the pre-publication results that we present here for a high-risk population: women served by the Centers for Disease Control and Prevention’s (CDC) National Breast and Cervical Cancer Early Detection Program (NBCCEDP). Here, we present the full results of our analysis. Since 1991, NBCCEDP established policies determining eligibility and reimbursement criteria for women with a low income (defined as family income of ≤250% of the federal poverty level) to gain access to screening and diagnostic exams for breast and cervical cancer.4 The program currently funds 70 recipients representing health departments in all 50 states, the District of Columbia, 6 US territories, and 13 tribal organizations. Women at the age of 21 to 64 years are eligible for cervical cancer services, prioritizing those who were rarely or never screened (ie, last cervical screening of >5 years ago). The program provides cervical cancer screening services for women at average risk on the basis of the United States Preventive Services Task Force (USPSTF) guidelines5 and management of abnormal screening results, which are based on the ASCCP guidelines.1 For women who are diagnosed as having precancers or cancers in the NBCCEDP, treatment is provided through the Centers for Medicare and Medicaid Services (CMS) state Medicaid program or through other means at local or state levels.
The NBCCEDP reimbursement policy evolved over time from USPSTF and ASCCP guidelines.4 Conventional cytology was used in the NBCCEDP until 2005 when the program also began reimbursing for liquid-based cytology. The NBCCEDP began reimbursing providers for human papillomavirus (HPV) tests to triage atypical squamous cells of undetermined significance (ASC-US) cytology results in 2005 following the 2002 ASCCP consensus guidelines.6 However, HPV test data were not systematically reported to the CDC until the NBCCEDP data collection was expanded in 2009. Screening with both HPV and cytology (cotesting) was reimbursed and collected as part of the program policy starting in late 2012 following the USPSTF guidelines recommendation update. The program began reimbursing for primary HPV testing in 2019. The program reimbursement rate is based on CMS Medicare fee schedules and American Medical Association current procedural terminology codes.7
We estimated the immediate (prevalent at the time of the first visit) risk for diagnosing CIN3+ among women in the CDC’s NBCCEDP. We further examined the differences in risk estimates by screening history, age, and race or ethnicity. These risks were compared with risks estimated in the KPNC population to determine whether the 2019 risk-based management guidelines could be applied to underinsured and uninsured women of low income in the United States.
Materials and Methods
Since the NBCCEDP’s inception in 1991, the CDC has collected a set of standardized data items called minimum data elements for each service provided by the program. Local providers collect and maintain data on screening, diagnosis, and treatment offered to women. These data are reported to the funded recipients, who then report the data to the CDC as a standardized record on every screening provided. The CDC uses these data to ensure that high-quality screening, diagnostic follow-up, and treatment services are provided. Demographic characteristics and previous history of screening are collected at enrollment. Providers report dates and results of screening tests and any diagnostic procedures, outcomes, and the date of treatment initiation, if applicable. This study used data reported during January 2009 to December 2017 from 50 states, the District of Columbia, 12 tribal organizations, and 5 United States territories. This study was approved by CDC’s Institutional Review Board.
We identified women aged 30 to 64 years in the program and eligible for screening, who had an HPV test from January 1, 2009, to December 31, 2017. Until 2019, data were not specifically collected to differentiate among HPV results originating from cotesting or from HPV triage of ASC-US results. Women were excluded from the analysis if they had received a diagnosis for CIN2+ or a hysterectomy before the first HPV-based test.
For women receiving screening services, screening history is determined initially during the patient intake process into the NBCCEDP by either self-report or physician reporting and subsequently updated by using the program’s database. The exact wording on the patient intake forms varies from program to program, but generally asks the woman if she has ever been screened for cervical cancer and, if so, what type and when was her last screening test. In this analysis, we stratified women into those who reported being up-to-date with their cervical cancer screening (ie, screened during the past 5 years) and those who were rarely or never screened (ie, not having a screening test during the past 5 years or never having been screened). Of note, our definition of up-to-date screening was screening within the past 5 years rather than strictly adhering to the 3-year retesting interval recommended by current guidelines after a negative cytology result.5 This allows including as up-to-date individuals who are retested late owing to scheduling difficulties rather than grouping them with women who had previously never been screened. We presented the rarely or never screened category both separately and combined with women having an unknown screening history.
Cytology test results were classified according to the Bethesda terminology as follows: negative for intraepithelial lesion or malignancy (NILM); ASC-US; low-grade squamous intraepithelial lesion (LSIL); atypical glandular cells (AGC); atypical squamous cells, cannot exclude high-grade lesion (ASC-H); high-grade squamous intraepithelial lesion (HSIL); and squamous cell carcinoma (SCC). The designation HSIL+ is used to refer to HSIL and SCC together. Cytology results of ASC-H, AGC, and HSIL+ are also referred to as high-grade cytology. HPV results were classified as positive or negative for any of the 13 high-risk HPV types (16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, and 68). The program did not record the type of HPV test used. Histopathology results were classified in order of severity as normal; CIN grades 1, 2, or 3; or cancer.
Risk assessment
In the NBCCEDP, immediate (prevalent at the initial visit) risks for CIN2+, CIN3+, and cancer were estimated among women and stratified by screening history (screened within 5 years, rarely or never screened, or unknown screening history) and for each combination of cytology (unsatisfactory results were excluded) and HPV results. We further examined differences in absolute risk by age (30–44 and 45–64 years) and race or ethnicity.
In our risk estimates, we use the same definitions and statistical methods used to develop risk estimates for the ASCCP guidelines.3 These methods and definitions account for differential follow-up with colposcopy or retesting by test results because of disease management recommendations. In brief, we defined precancer or cancer as prevalent at the time of the initial visit if it was detected after a colposcopy referral. We defined incident precancer or cancer as occurring after the initial visit; the exact time of the onset of incident precancer or cancer is known only to occur (eg, interval-censored using statistical terminology) between the last disease-free visit and the time of the precancer or cancer diagnosis. Finally, women without precancer or cancer diagnoses were considered right censored at the time of their last disease-free visit. Women were considered to be CIN2+ and CIN3+ outcome free at a visit only if they had a normal and CIN1 result in colposcopy, a negative cotest, or an HPV-negative with ASC-US follow-up test result. To avoid verification bias (ie, by assuming CIN2+ or CIN3+ outcome free) from positive test results (eg, HPV positive, LSIL, or greater cytology) that were missing colposcopy results, women were considered to have missing disease status at that time. Women whose disease status was never confirmed (eg, positive test results at the initial visit who never underwent colposcopy and never had a subsequent negative cotest or HPV-negative with ASC-US follow-up test result) were considered uninformative for risk estimation.
Prevalence-incidence mixture models were used to estimate risks.8 These joint models combine a logistic regression model for prevalent disease and a proportional hazard model for interval-censored incident disease, accounting for missing prevalent disease status among those women with positive results at the initial visit who did not undergo immediate colposcopy. The models were stratified by hybrid capture 2 positivity because of nonproportionality of the hazards; cytology results followed the proportionality of hazards. Because most of the women in the cohort only contributed 1 screening cycle to the data, we present only the stable immediate risk estimates and not the incident or cumulative risk estimates. SAS software version 9 (SAS Institute, Cary, NC) was used for data cleaning and processing; R software (The R Foundation, Vienna, Austria) was used for all statistical analyses.
Results
A total of 363,546 women who are at the age of 30-64 years in the NBCCEDP had at least 1 HPV and cytology test since 2009 (Table 1). Most women (75%) were at the age of 40 to 59 years, which is well within the recommended age range for cervical cancer screening.4 The NBCCEDP population was racially and ethnically diverse with 40.2% Hispanic, 34.7% non-Hispanic White, 13.1% non-Hispanic Black, 5.7% Asian or Pacific Islander, and 4.5% American Indian or Alaskan Native. A large proportion of these women (31.1%) had not been screened during the past 5 years or had never been screened (unknown history, 11.5%). Results from the initial screening test indicated 13.5% of women were HPV positive; 80.4% of cytology results were normal (7.0% HPV+) followed by 15.8 % ASC-US (33.1% HPV+), 2.4% LSIL (72.4% HPV+), and approximately 0.5% each of ASC-H (66.5% HPV+), HSIL (89.4% HPV+), and AGC (29.7% HPV+). Because our analysis population consists of women with at least 1 HPV and cytology test, without differentiating among the modes of screening (eg, primary cytology with HPV triage vs HPV and cytology cotesting), the proportion of women with ASC-US was higher than would normally be expected in settings conducting cotesting because of the inclusion of women undergoing triage of primary ASC-US cytology.
TABLE 1.
Demographic characteristics of women in the CDC’s NBCCEDP cohort, 2009 to 2017
| n | % | |
|---|---|---|
| Total | 363,546 | ~ |
| Age, y | ||
| 30–39 | 52,746 | 14.5 |
| 40–49 | 146,340 | 40.3 |
| 50–59 | 129,224 | 35.6 |
| 60–64 | 35,236 | 9.7 |
| Race and ethnicity | ||
| Non-Hispanic White | 125,990 | 34.7 |
| Non-Hispanic Black | 47,482 | 13.1 |
| Hispanic | 146,073 | 40.2 |
| Asian or Pacific Islander | 20,788 | 5.7 |
| American Indian/Alaskan Native | 16,432 | 4.5 |
| Multiracial | 2407 | 0.7 |
| Unknown | 4374 | 1.2 |
| Screening status | ||
| Screened within 5 y | 208,658 | 57.4 |
| Never or rarely screened | 113,126 | 31.1 |
| Unknown screening history | 41,762 | 11.5 |
| HPV result | ||
| Positive | 48,979 | 13.5 |
| Negative | 314,567 | 86.5 |
| Cytology by HPV result | ||
| NILM | 292,296 | 80.4 |
| HPV negative | 271,862 | 93 |
| HPV positive | 20,434 | 7 |
| ASC-US | 57,356 | 15.8 |
| HPV negative | 38,382 | 66.9 |
| HPV positive | 18,974 | 33.1 |
| LSIL | 8883 | 2.4 |
| HPV negative | 2455 | 27.6 |
| HPV positive | 6428 | 72.4 |
| ASC-H | 1479 | 0.4 |
| HPV negative | 496 | 33.5 |
| HPV positive | 983 | 66.5 |
| HSIL+ | 1863 | 0.5 |
| HPV negative | 198 | 10.6 |
| HPV positive | 1665 | 89.4 |
| AGC | 1667 | 0.5 |
| HPV negative | 1172 | 70.3 |
| HPV positive | 495 | 29.7 |
| Unsatisfactory | 2 | 0 |
| HPV negative | 2 | 100 |
| HPV positive | 0 | 0 |
AGC, atypical glandular cell; ASC-H, atypical squamous cells; ASC-US, atypical squamous cells of uncertain significance; CDC, Centers for Disease Control and Prevention; HPV, human papillomavirus; HSIL, high-grade squamous intraepithelial lesion; cannot exclude HSIL; LSIL, low-grade squamous intraepithelial lesion; NBCCEDP, National Breast and Cervical Cancer Early Detection Program; NILM, negative for intraepithelial lesion or malignancy.
The distributions of HPV and cytology testing results stratified by screening history are presented in Table 2. For each combination of test result, we reported the number of women who were diagnosed as having CIN2+, CIN3+, or cancer. Compared with women who were up-to-date with screening, women who were rarely or never screened were more likely to have high-grade cytology (1.6% vs 1.2%; P<.001), which accounted for 56% of CIN3+ detected in rarely or never screened women compared with 40% of CIN3+ detected in women who were up-to-date with screening. There were slight differences in the proportion that were HPV-positive women (13.0% vs 13.6%). The level of follow-up with colposcopy or retesting differed by test results because of disease management recommendations, but it did not differ by screening history. Most of the women in the cohort were screened only once within the NBCCEDP. Most of those with abnormal results of HPV-positive ASC-US or higher received a colposcopy follow-up, allowing risk estimation of the immediate CIN3+ risks of these results. Women whose test results had a recommended management of a 1-year retesting had lower follow-up with colposcopy or retesting within the program (approximately 50% for HPV negative with LSIL cytology; approximately 25% for HPV positive with normal cytology), although numbers were sufficient for risk estimation. Few women with a combination of test results that recommended retesting in 3 or 5 years (ASC-US/HPV− or NILM/HPV−) returned to the program for their next screening exam, so risk was not presented for these categories.
TABLE 2.
Distribution of HPV and cytology results of the CDC’s NBCCEDP cohort from 2009 to 2017
| Test results | N | % | CIN2+ | CIN3+ | Cancer | Follow-up time, mo | % with colposcopy or follow-up |
||
|---|---|---|---|---|---|---|---|---|---|
| HPV | Cytology | Median | IQR | ||||||
| Screened in the past 5 y | |||||||||
| Total | 208,656 | 100.0 | 2873 | 1495 | 236 | 25 | 14–42 | ||
| HPV Positive | NILM | 11,315 | 5.4 | 196 | 115 | 7 | 16 | 13–27 | 31 |
| ASC-US | 11,754 | 5.6 | 1215 | 545 | 22 | 18 | 13–33 | 87 | |
| LSIL | 3728 | 1.8 | 410 | 159 | 6 | 18 | 13–30 | 85 | |
| ASC-H | 567 | 0.3 | 200 | 138 | 10 | 16 | 12–27 | 92 | |
| AGC | 244 | 0.1 | 92 | 69 | 15 | 17 | 14–29 | 91 | |
| HSIL+ | 747 | 0.4 | 486 | 337 | 37 | 15 | 12–26 | 89 | |
| HPV Negative | NILM | 152,606 | 73.1 | 44 | 19 | 4 | 34 | 17–50 | |
| ASC-US | 25,186 | 12.1 | 104 | 45 | 7 | 26 | 14–46 | ||
| LSIL | 1461 | 0.7 | 31 | 11 | 0 | 15 | 13–26 | 57 | |
| ASC-H | 293 | 0.1 | 29 | 14 | 0 | 17 | 14–31 | 90 | |
| AGC | 669 | 0.3 | 33 | 19 | 12 | 21.5 | 14–38 | 91 | |
| HSIL+ | 86 | 0.0 | 33 | 24 | 3 | 11 | 8–17 | 87 | |
| Rarely or never screened | |||||||||
| Total | 113,126 | 100.0 | 1827 | 1124 | 165 | 22 | 14–38 | ||
| HPV Positive | NILM | 6554 | 5.8 | 112 | 68 | 11 | 15 | 13–25 | 23 |
| ASC-US | 5130 | 4.5 | 636 | 315 | 30 | 16 | 13–29 | 84 | |
| LSIL | 1862 | 1.7 | 215 | 87 | 1 | 16 | 13–27 | 81 | |
| ASC-H | 293 | 0.3 | 121 | 88 | 15 | 14 | 10–19 | 85 | |
| AGC | 175 | 0.2 | 80 | 67 | 20 | 16 | 10–32 | 85 | |
| HSIL+ | 667 | 0.6 | 504 | 402 | 58 | 17 | 13–27 | 91 | |
| HPV Negative | NILM | 87,187 | 77.1 | 19 | 13 | 6 | 29 | 16–43.25 | |
| ASC-US | 9926 | 8.8 | 31 | 10 | 2 | 27 | 14–45 | ||
| LSIL | 701 | 0.6 | 14 | 3 | 0 | 15 | 13–23 | 51 | |
| ASC-H | 160 | 0.1 | 16 | 7 | 2 | 16.5 | 13–33.25 | 86 | |
| AGC | 386 | 0.3 | 31 | 25 | 17 | 15 | 13–25.75 | 85 | |
| HSIL+ | 85 | 0.1 | 48 | 39 | 3 | 14 | 10–26 | 89 | |
| Unknown screening history | |||||||||
| Total | 41,762 | 100.0 | 721 | 414 | 71 | 28 | 15-47 | ||
| HPV Positive | NILM | 2565 | 6.1 | 42 | 21 | 4 | 15 | 13–27 | 24 |
| ASC-US | 2090 | 5.0 | 276 | 126 | 15 | 19 | 13–30 | 83 | |
| LSIL | 838 | 2.0 | 109 | 59 | 9 | 17 | 13–28 | 77 | |
| ASC-H | 123 | 0.3 | 40 | 23 | 3 | 34 | 19.75–39 | 79 | |
| AGC | 76 | 0.2 | 28 | 22 | 7 | 31 | 13.5–49.75 | 87 | |
| HSIL+ | 251 | 0.6 | 183 | 136 | 22 | 22 | 14–36 | 89 | |
| HPV Negative | NILM | 32,069 | 76.8 | 2 | 2 | 1 | 37 | 21–58 | |
| ASC-US | 3270 | 7.8 | 9 | 5 | 2 | 27 | 14–47 | ||
| LSIL | 293 | 0.7 | 9 | 3 | 2 | 15 | 13-19.5 | 52 | |
| ASC-H | 43 | 0.1 | 1 | 1 | 0 | 16 | 13–6 | 79 | |
| AGC | 117 | 0.3 | 7 | 5 | 5 | 17 | 13–25 | 84 | |
| HSIL+ | 27 | 0.1 | 15 | 11 | 1 | 13.5 | 13–20.75 | 85 | |
AGC, atypical glandular cell; ASC-H, atypical squamous cells-cannot exclude HSIL; ASC-US, atypical squamous cells of uncertain significance; CDC, Centers for Disease Control and Prevention; CIN2+, cervical intraepiethial neoplasia grade II or greater; CIN3, cervical intraepiethial neoplasia grade III or greater; HPV, human papillomavirus; HSIL, high-grade squamous intraepithelial lesion; IQR, interquartile range; LSIL, low-grade squamous intraepithelial lesion; NBCCEDP, National Breast and Cervical Cancer Early Detection Program; NILM, negative for intraepithelial lesion or malignancy.
The immediate CIN3+ risks among NBCCEDP women are stratified by screening history and presented in Table 3. For nearly every combination of HPV and cytology test result, the immediate risk of CIN3+ was higher among women who were rarely or never screened or whose screening history was unknown than among women screened during the past 5 years. Risks were further stratified by age and race or ethnicity and are presented in Supplemental Table 1 and Supplemental Table 2, respectively. Among women with HPV-positive results, older age was associated with lower risk among women screened during the past 5 years (odds ratio [OR], 0.79; 95% confidence interval [CI], 0.69–0.89) but not significantly among women who were rarely or never screened or with unknown screening (OR, 1.09; 95% CI, 0.95–1.24). When stratified by test results and screening history, the risk of CIN3+ did not differ by race or ethnicity. For example, the prevalent risks of HPV-positive and ASC-US results were between 4.4% and 4.7% for non-Hispanic whites, non-Hispanic blacks, and Hispanics who were up-to-date with screening and increased to 5.6% to 7.5% among women who were rarely or never screened. Risk estimates for CIN2+ and cancer are reported in Supplemental Table 3 and Supplemental Table 4, respectively.
TABLE 3.
| Testing results |
CDC’s NBCCEDPa |
KPNCb |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Screened within the past 5 y |
Rarely, never, or unknown screening (combined) |
Rarely or never screened |
Unknown screening |
||||||||
| HPV | Cytology | Immediate CIN3+ risk (%) |
95% CI (%) | Immediate CIN3+ risk (%) |
95% CI (%) | Immediate CIN3+ risk (%) |
95% CI (%) | Immediate CIN3+ risk (%) |
95% CI (%) | Immediate CIN3+ risk (%) |
95% CI (%) |
| Positive | HSIL+ | 49.6 | (45.8–53.4) | 64.1 | (60.8–67.3) | 65.4 | (61.6–69.2) | 60.4 | (54.1–66.8) | 48.9 | (47.2–50.6) |
| AGC | 29.4 | (23.4–35.4) | 41.6 | (35.0–48.2) | 45.3 | (37.3–53.3) | 33.3 | (22.0–44.7) | 26.3 | (23.3–29.3) | |
| ASC-H | 25.2 | (21.4–28.9) | 30.6 | (25.7–35.4) | 33.8 | (28.0–39.7) | 22.2 | (13.9–30.5) | 25.7 | (24.2–27.3) | |
| LSIL | 4.1 | (3.4–4.8) | 6.2 | (5.1–7.2) | 5.2 | (4.1–6.3) | 8.4 | (6.3–10.6) | 4.3 | (4.0–4.6) | |
| ASC-US | 4.5 | (4.1–4.9) | 6.6 | (6.0–7.2) | 6.7 | (5.9–7.4) | 6.5 | (5.4–7.7) | 4.4 | (4.2–4.7) | |
| NILM | 2.4 | (1.8–2.9) | 3.5 | (2.7–4.4) | 3.6 | (2.5–4.6) | 3.3 | (1.9–4.7) | 2.1 | (2.0–2.3) | |
| Negative | HSIL+ | 32.0 | (21.4–42.6) | 47.8 | (38.0–57.7) | 50.0 | (38.8–61.2) | 41.0 | (21.0–60.9) | 25.2 | (18.5–31.9) |
| AGC | 3.0 | (1.6–4.3) | 7.0 | (4.6–9.5) | 7.6 | (4.8–10.5) | 5.1 | (0.8–9.5) | 1.1 | (0.6–1.5) | |
| ASC-H | 4.9 | (2.3–7.5) | 4.7 | (1.5–7.8) | 5.1 | (1.4–8.8) | 2.9 | (0.0–8.6) | 3.4 | (2.1– 4.8) | |
| LSIL | 1.0 | (0.3–1.7) | 1.2 | (0.2–2.1) | 0.8 | (0.0–1.8) | 2.0 | (0.0–4.2) | 1.1 | (0.6–1.5) | |
| ASC-US | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | 0.0 | (0.0–0.1) | |
| NILM | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | 0.0 | (0.0–0.0) | |
AGC, atypical glandular cell; ASC-H, atypical squamous cells-cannot exclude; ASC-US, atypical squamous cells of uncertain significance; CDC, Centers for Disease Control and Prevention; CI, confidence interval; CIN3, cervical intraepiethial neoplasia grade III or greater; HSIL, high-grade squamous intraepithelial lesion; HSIL HPV, human papillomavirus; LSIL, low-grade squamous intraepithelial lesion; KPNC, Kaiser Permanente Northern California; LSIL, low-grade squamous intraepithelial lesion; N/A, not available; NBCCEDP, National Breast and Cervical Cancer Early Detection Program; NILM, negative for intraepithelial lesion or malignancy.
CDC population is at the age of 30 to 64 years who have an HPV test result, which includes primary Papanicolaou test results of ASC-US triaged by HPV
KPNC population is at the age of 25 to 65 years who underwent HPV cotesting. Risk estimates are from Egemen et al.9
Data comparing the risks estimated in KPNC9 with those in the NBCCEDP are presented in Table 3 and the Figure. Risks estimated among women in the KPNC population were similar to those among women in the NBCCEDP who were screened within the past 5 years but were significantly lower (P<.001 for most results) than risks among women in the NBCCEDP who were rarely or never screened or whose screening history was unknown.
FIGURE. Immediate CIN3+ risk in CDC vs. KPNC by (A) low-risk and (B) high-risk categories.
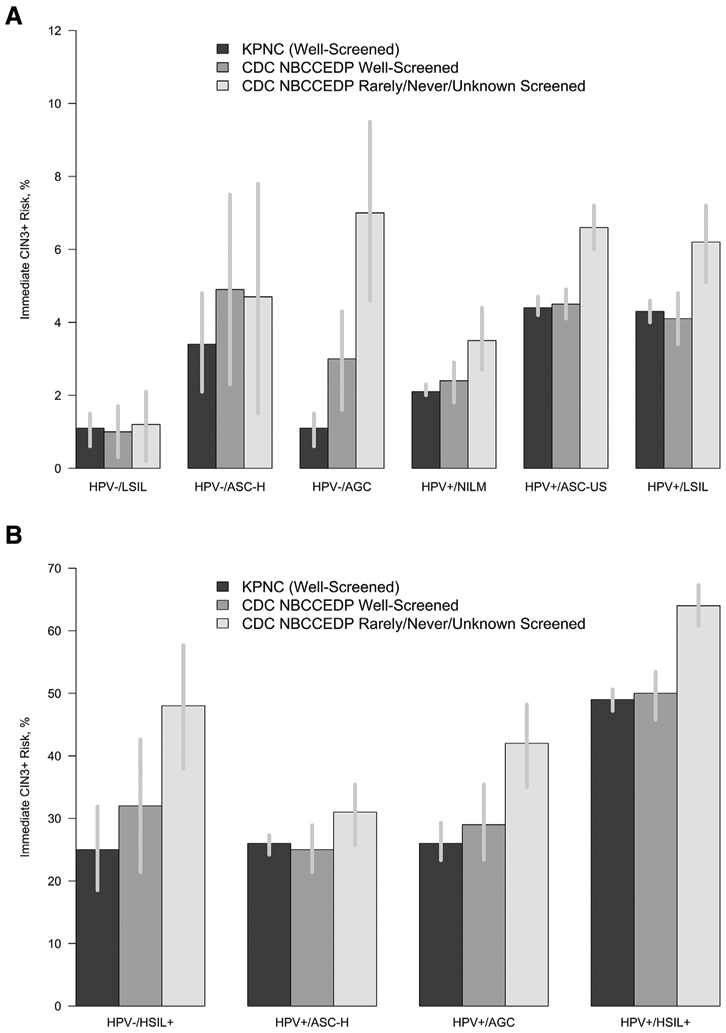
Immediate risk of CIN3 is the percentage of risk of having prevalent CIN3+ at the initial visit.
AGC, atypical glandular cells; ASC-H, atypical squamous cells where HSIL cannot be excluded; ASC-US, atypical squamous cells of uncertain significance; CDC, Centers for Disease Control and Prevention; CIN3+, cervical intraepithelial lesion grade 3 or higher; HPV, human papillomavirus; HSIL, high-grade squamous intraepithelial lesions; KPNC, Kaiser Permanente Northern California; LSIL, low-grade squamous intraepithelial lesions; NBCCEDP, National Breast and Cervical Cancer Early Detection Program; NILM, negative for intraepithelial lesion or malignancy.
Comment
Principal findings
The immediate risk of CIN3+ in underserved women in the NBCCEDP are similar to that of women from KPNC, provided that they are up-to-date on their cervical screening. Women who were rarely or never screened had elevated immediate risks of CIN3+ compared with women who were screened during the past 5 years. The 2019 ASCCP risk-based management consensus guidelines incorporate these data and recommend a different management for women who are not up-to-date with screening. In particular, expedited excisional treatment (ie, proceeding directly to treatment without requiring colposcopic biopsy) is preferred for underscreened women with results of HPV-positive HSIL. This more aggressive management is recommended because the immediate risk of CIN3+ is 64.5%, which exceeds the expedited treatment threshold of 60%. The risk of CIN3+ did not differ by race or ethnicity after accounting for screening history.
Results
The development of risk-based management guidelines for cervical cancer has relied on, as the underlying base population, women from a large integrated health delivery system at KPNC. The KPNC cervical screening program has a uniquely large number of participants (approximately 1.5 million participants) and long-term experience (15 years) with HPV-based screening, permitting precise estimation of precancer risk even for many unusual screening results.3 However, such a population may not be representative of underinsured and uninsured women of low income. In this study, we report the immediate risks of CIN3+, stratified by HPV and cytology results, in the NBCCEDP, which serves women of low income without continuous insurance coverage.
Clinical implications
Risks among women in the CDC’s NBCCEDP screened during the past 5 years were similar to those among women at KPNC, supporting evidence for the applicability of the 2019 ASCCP risk-based management consensus guidelines to diverse settings in the United States.2,3 Further analysis determined that the increased CIN3+ risk found among rarely or never screened women was not large enough to warrant recommendation of different management guidelines for these women. The exception is for the small but important percentage of rarely or never screened women who had a positive result for HPV with HSIL or SCC cytology results. Citing these results, the 2019 ASCCP guidelines recommend these women undergo expedited treatment because of their uniquely high risk, which exceeds the 60% immediate CIN3+ risk threshold for expedited treatment set by the new guidelines.2
The exceptional racial or ethnic diversity of the NBCCEDP allowed for a thorough examination of immediate CIN3+ risks by race and ethnicity. Our analysis found that once stratified by screening history and current test results, risks did not differ by race or ethnicity and should not be a consideration for clinical management. However, the proportions of never or rarely screened differed by race or ethnicity, indicating that risks without adjustment for screening histories could differ.
Research implications
Although our study was able to look at immediate risks, it will be important to conduct long-term follow-up among underserved women to investigate whether women who were rarely or never screened have increased risks in subsequent testing rounds or in postcolposcopy or posttreatment surveillance.
Strengths and limitations
The NBCCEDP is one of the few national programs for underserved women that collects information on cervical cancer screening. In addition, programmatic data were used to inform clinical management guidelines for cervical cancer.
Because the NBCCEDP was not constructed for research purposes, some information on results, such as whether women were undergoing cotesting vs HPV triage for ASC-US cytology, was not collected. As such, we could not infer the distribution of test results under a cotesting scenario.
In this analysis, we were not able to present the risk of the most common low-grade abnormalities, such as HPV-negative results with NILM or ASC-US cytology, because there was insufficient follow-up to estimate the 5-year risk among this cohort of women. Considering the increased risk among women who were never or rarely screened, the lack of follow-up visits among women in the study may need to be further investigated. It is very likely that the follow-up could have been done outside of the program, even with the same provider, yet were not captured in these data. Follow-up of abnormal results remains a current and future focus of the program to ensure that underinsured and uninsured women receive adequate screening and management.
Conclusions
The results from this study indicate that new cervical risk–based management guidelines can be applied to underinsured and uninsured women of low income in the United States who are up-to-date with their screening. These results also highlight the need for clinicians to collect screening history to help ensure that women who are not up-to-date with their screening can receive management appropriate to their increased risks.
Supplementary Material
AJOG at a Glance.
Why was this study conducted?
New risk-based management guidelines for cervical cancer are primarily based on data from a large integrated healthcare delivery system. This study presents the risk from underserved women with a low income in the United States and assess the applicability of the guidelines to such populations.
Key findings
Screening test results in underserved women who were up-to-date with screening imply the same risk as women from a large integrated healthcare delivery system. Not being screened in the past 5 years implies a greater risk for the same test result.
What does this add to what is known?
New management guidelines are largely applicable to underserved populations, but women with human papillomavirus–positive high-grade squamous intraepithelial lesion and who were not screened in the past 5 years should undergo expedited treatment.
Acknowledgments
We would like to thank Information Management Services, Inc, for their assistance in processing the data.
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Footnotes
This analysis was funded in part by the Intramural Research Program of the US National Institutes of Health (NIH)/National Cancer Institute.
The authors report no conflict of interest.
Contributor Information
Mona Saraiya, Centers for Disease Control and Prevention, Division of Cancer Prevention and Control, Atlanta, GA.
Li C. Cheung, National Cancer Institute, Division of Cancer Epidemiology and Genetics, Rockville, MD.
Ashwini Soman, Centers for Disease Control and Prevention, Division of Cancer Prevention and Control, Atlanta, GA.
Jacqueline Mix, Centers for Disease Control and Prevention, Division of Cancer Prevention and Control, Atlanta, GA.
Kristy Kenney, Centers for Disease Control and Prevention, Division of Cancer Prevention and Control, Atlanta, GA.
Xiaojian Chen, National Cancer Institute, Division of Cancer Epidemiology and Genetics, Rockville, MD.
Rebecca B. Perkins, Boston University School of Medicine, Boston, MA.
Mark Schiffman, National Cancer Institute, Division of Cancer Epidemiology and Genetics, Rockville, MD.
Nicolas Wentzensen, National Cancer Institute, Division of Cancer Epidemiology and Genetics, Rockville, MD.
Jacqueline Miller, Centers for Disease Control and Prevention, Division of Cancer Prevention and Control, Atlanta, GA.
References
- 1.Massad LS, Einstein MH, Huh WK, et al. 2012 updated consensus guidelines for the management of abnormal cervical cancer screening tests and cancer precursors. Obstet Gynecol 2013;121:829–46. [DOI] [PubMed] [Google Scholar]
- 2.Perkins RB, Guido RS, Castle PE, et al. 2019 ASCCP risk-based management consensus guidelines for abnormal cervical cancer screening tests and cancer precursors. J Low Genit Tract Dis 2020;24:102–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cheung LC, Egemen D, Chen X, et al. 2019 ASCCP risk-based management consensus guidelines: methods for risk estimation, recommended management, and validation. J Low Genit Tract Dis 2020;24:90–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee NC, Wong FL, Jamison PM, et al. Implementation of the National Breast and Cervical Cancer Early Detection Program: the beginning. Cancer 2014;120(Suppl16):2540–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.US Preventive Services Task Force, Curry SJ, Krist AH, et al. Screening for cervical cancer: US Preventive Services Task Force Recommendation Statement. JAMA 2018;320:674–86. [DOI] [PubMed] [Google Scholar]
- 6.Wright TC Jr, Cox JT, Massad LS, Twiggs LB, Wilkinson EJ. ASCCP-Sponsored Consensus Conference. 2001 Consensus Guidelines for the management of women with cervical cytological abnormalities. JAMA 2002;287:2120–9. [DOI] [PubMed] [Google Scholar]
- 7.Centers for Medicare and Medicaid Services. Physician fee schedule. 2020. Available at: https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/PhysicianFeeSched. Accessed May 13, 2020.
- 8.Cheung LC, Pan Q, Hyun N, et al. Mixture models for undiagnosed prevalent disease and interval-censored incident disease: applications to a cohort assembled from electronic health records. Stat Med 2017;36:3583–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Egemen D, Cheung LC, Chen X, et al. Risk estimates supporting the 2019 ASCCP risk-based management consensus guidelines. J Low Genit Tract Dis 2020;24:132–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


