Abstract
Introduction:
Difficulties with cognition are extremely common among breast cancer survivors and can significantly impact quality of life, daily functioning, and ability to return to work. One promising intervention is increasing physical activity, as it has been effective in improving cognition in non-cancer populations. Few physical activity intervention trials with cognition outcomes have included cancer survivors. This project builds upon our previous work indicating that increased physical activity can improve objectively measured processing speed and self-reported cognition among breast cancer survivors.
Methods:
The I Can! study will examine whether a physical activity intervention improves cognition among 250 post-treatment breast cancer survivors (Stages I-III, <5 years post-treatment) who are reporting cognitive difficulties. This 2-arm randomized controlled trial comparing a 6-month physical activity intervention (Exercise Group) to a health & wellness attention-comparison condition (Health & Wellness Group) will examine intervention effects on cognition (at 3 and 6 months) and maintenance of effects at 12 months. The primary aim is to investigate the impact of exercise on objectively measured processing speed and self-reported cognition. Secondary aims are to investigate maintenance of cognitive changes and examine candidate biological mechanisms and psychological mediators.
Conclusion:
The I Can! study will contribute to the scientific, public health, and survivorship intervention literature by providing new information on the impact of physical activity for cognitive impairment in breast cancer survivors. Findings from this study will inform guidelines for physical activity to improve the lives of millions of breast cancer survivors.
Keywords: Survivorship, Exercise, Neurocognitive, DNA methylation, Depression, Anxiety
1. Introduction
Breast cancer is the most common cancer diagnosis [1] with more than 3.5 million survivors in the US [2]. Cognitive impairment is a troubling, disruptive, and persistent symptom reported by up to 75% of breast cancer survivors [3–9]. Cognitive impairments can negatively impact quality of life, daily functioning, and ability to return to work [10–15].
Many treatments for breast cancer are associated with long-term cognitive problems [8,16–20]. No known effective pharmacologic treatments for cognitive impairment in cancer survivors exist [21,22], motivating the search for lifestyle strategies to improve cognition. One promising intervention is physical activity. The Institute of Medicine’s report on Cognitive Aging recommends physical activity to reduce age-related cognitive decline, particularly for women [23,24]. Cognitive impairment in cancer survivors may be a symptom of accelerated aging caused by chemotherapy, endocrine therapy, and/or psychological stress [25–27]. Aerobic physical activity interventions in non-cancer populations have been effective in improving processing speed [24,28–31] and self-reported cognition [32–35]. While the mechanisms are unknown, one potential hypothesis is that exercise can impact cellular aging which could reduce accelerated aging and improve cognition in cancer survivors.[126–129]Therefore, a potential strategy to improve cognition and thus quality of life among breast cancer survivors is physical activity. Breast cancer survivors often decrease their activity levels during and after cancer treatment [36] and overall, have very low levels of physical activity [37]. The low amount of physical activity in breast cancer survivors and the strong association between physical activity and improvements in cognition among both healthy [24,32] and cognitively-impaired [33,38,39] populations suggests that physical activity may be an impactful target for cancer survivors.
In a randomized controlled trial (RCT) pilot study, we previously observed that increasing physical activity over 12 weeks improved objectively measured processing speed and self-reported cognition in breast cancer survivors (n = 87) [40–42]. Consistent with this finding, a 24-week RCT (n = 19) showed greater improvements to information processing speed in the aerobic physical activity group compared to the control group [41,43]. Processing speed, the ability to take in information and use it quickly and appropriately, is commonly impaired in breast cancer survivors [11,28,44–46]. Information processing speed is central to overall cognition and can impact memory, procedural learning, and overall cognitive performance [28,29]. Given the importance of processing speed to overall cognition, it is conceivable that sustained processing speed improvement, in longer intervention trials, could have secondary beneficial effects in other cognitive domains [28,29].
In addition to objectively measured cognitive deficits, cancer survivors frequently self-report concerns about cognitive changes. Objective and self-report measures are generally not highly correlated, but may both provide clinically relevant information [47,48]. A literature review [49] found only two trials that tested the impact of physical activity on self-reported cognitive impairments in cancer survivors [50,51]. In a 6-week RCT (n = 479), increased physical activity significantly improved self-reported cognition [51], but a smaller 12-week RCT (n = 41) found no such changes [50]. Physical activity has promise for improving objective and subjective cognition in breast cancer survivors, but additional studies are warranted. Furthermore, because breast cancer survivors face long-term challenges with cognition, assessing maintenance is important to understanding the impact of exercise on cognition.
Building upon our 3-month pilot RCT, this paper describes the protocol of I Can! Improving Cognition After Cancer, a RCT in breast cancer survivors designed to compare changes in objectively measured and self-reported cognition between a home-based physical activity intervention and a Health & Wellness attention-comparison condition.
2. Methods
2.1. Overview
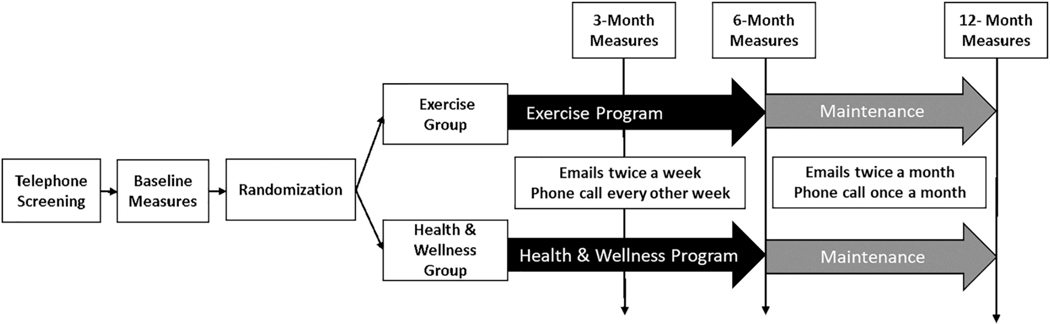
The I Can! study examines the effects of a 6-month physical activity intervention on changes in processing speed and self-reported cognitive concerns among 250 breast cancer survivors at 3 and 6 months (middle and end of intervention) and 12 months (maintenance). Participants are randomly assigned to receive (1) a home-based physical activity intervention grounded in Control Theory and Social Cognitive Theory (Exercise group), or (2) a health & wellness attention-comparison condition (Health & Wellness group). At baseline, 3, 6, and 12 months, participants complete neurocognitive testing, questionnaires assessing self-reported cognition, a battery of psychosocial questionnaires, a non-fasting blood draw, the 3-min Step Test, and wear an ActiGraph GT3X+ accelerometer for 7 days. See Fig. 1 for study flow and Table 1 for measurement and intervention schedule.
Fig. 1.
Flow of I Can! study visits and intervention contacts.
Table 1.
Measurement and intervention schedule.
| Week | Description of I Can! study activities | |
|---|---|---|
| −1 | Initial phone screening for breast cancer eligibility, physical activity level, cognition, etc. | |
| 0 | Orientation/Measurement | |
| Written informed consent | ||
| Objective & self-report cognition measures, questionnaires, blood draw, height & weight, medical chart abstraction, given an ActiGraph to wear on the hip for the next 7 days | ||
| 1 | Randomization | |
| Return ActiGraph, step test, sit-to-stand test, & randomized to Exercise group or Health & Wellness group | ||
| Physical Activity Intervention | Health & Wellness Condition | |
| 45 min in-person intro to the Exercise group: | 45 min in-person intro to Health & Wellness group: | |
| • Taught how to use and sync Fitbit • Guided 10 min walk at moderate intensity • Personalized Weekly Goal • Discuss topic of the week: Self-monitoring, Exercising safely, & Exercise & resources |
• Review menu of topics to be covered • Participants chooses topics of interest and receives handouts on each topic (sample topics below) • Discuss topic of the week: Brain Health |
|
| 1–24 | Emails 1–2 times a week with reminders to be active & use Fitbit and information & resources for exercising | Emails 1–2 times a week on Health & Wellness topics |
| 3, 5, 7, 9, 11 | 20 in phone counseling: | 20 min phone counseling: |
| • Review Fitbit data & revise goal and action plan • Discuss topic (Week 3: Building motivation; Week 5: Setting goals; Week 7: Social support; Week 9: Slips; Week 11: Benefits of exercise for cancer survivors) |
• Discuss any questions from the previous call • Discuss topic (Week 3: Social connections; Week 5: Healthy eating; Week 7: Stress management; Week 9: Balance exercises; Week 11: Time management) |
|
| 11 | Start wearing ActiGraph for 3 Month measurement | |
| 12 | 3 Month Measurement | |
| Objective & self-report cognition measures, questionnaires, blood draw, weight, step test, sit-to-stand test, return ActiGraph | ||
| 13, 15, 17, 19, 21, 23 | 20 min phone counseling: | 20 min phone counseling: |
| • Review Fitbit data and progress towards goal • Revise goal and action plan • Discuss topic (Week 13: Problem solving barriers; Week 15: Time management; Week 17: Rewards; Week 19: Talking back to negative thoughts; Week 21: Strength and core exercises; Week 23: Maintenance & Holidays/vacations) |
• Discuss any questions from the previous call • Discuss topic (Week 13: Keep your mind active; Week 15: Sleep; Week 17: Building healthy recipes; Week 19: Talking back to negative thoughts; Week 21: Nutrition labels; Week 23: Maintenance of healthy habits) |
|
| 23 | Start wearing ActiGraph for 6 Month measurement | |
| 24 | 6 Month Measurement | |
| Objective & self-report cognition measures, questionnaires, blood draw, weight, step test, sit-to-stand test, return ActiGraph, satisfaction survey | ||
| 28, 32, 36, 40, 44, 48 | Maintenance Period: | Maintenance Period: |
| • Continue to wear and sync Fitbit • Monthly check-in phone calls • Emails every other week with reminders to be active & use Fitbit, information and information & resources for exercisingsd |
• Monthly check-in phone calls • Emails every other week on Health & Wellness topics |
|
| 51 | Start wearing ActiGraph for 12 Month measurement | |
| 52 | 12 Month Measurement | |
| Objective & self-report cognition measures, questionnaires, blood draw, weight, step test, | ||
2.2. Specific aims
2.2.1. Primary aim
Investigate the impact of the physical activity intervention on changes in processing speed and self-reported cognition compared to the Health & Wellness attention-comparison condition. Hypothesis: The Exercise group will show greater improvements in processing speed, assessed by neurocognitive testing, and self-reported cognition, during the 6-month intervention compared to the Health & Wellness group.
2.2.2. Secondary aims
Investigate the impact of the Exercise group on maintenance of changes in cognition compared to the Health & Wellness group. Hypothesis: At 12 months, the Exercise group will show greater improvements in processing speed, assessed by neurocognitive testing, and self-reported cognition compared to the Health & Wellness group.
Examine candidate biological mechanisms and psychological mediators of intervention-related changes in cognition. Hypothesis: The Exercise group will have greater improvements in aging-related DNA methylation profile as well as reduced anxiety and depression compared to the Health & Wellness group, which will partially mediate the relationship between the intervention effect and cognition outcomes.
2.2.3. Exploratory aims
Explore a dose-response relationship between changes in physical activity and fitness, objectively measured by ActiGraph accelerometer, 3-min step test, and 30-s sit-to-stand test with changes in processing speed and self-reported cognition.
Explore changes to other cognitive domains (memory, executive function, and attention), objectively measured with neurocognitive testing, in the Exercise group compared to the Health & Wellness group.
2.3. Eligibility criteria
Eligible participants are female breast cancer survivors, age 40 years or older, who completed active treatment (e.g., chemotherapy, radiotherapy) at least 6 months prior to enrollment, are within 5 years of diagnosis of stage 1, 2, or 3 breast cancer, and meet the following inclusion/exclusion criteria:
Additional inclusion criteria: (1) sedentary, defined as self-reporting less than 60 min of moderate to vigorous physical activity (MVPA), accumulated in 10-min bouts, each week, (2) self-reporting difficulties with cognition with a score of 4 or higher on a 0–10 scale [52] (3) have a Fitbit compatible device (e.g., smartphone, computer) with Internet access, and (4) received chemotherapy and/or hormonal therapy (i.e., aromatase inhibitors, tamoxifen).
Exclusion criteria: (1) medical condition that could make it unsafe to participate in unsupervised physical activity, as determined by the Physical Activity Readiness Questionnaire (PAR-Q) [53], including self-reported neuropathy that interferes with ambulation, or unable to walk unassisted (e.g. uses a cane), (2) currently taking tamoxifen or an aromatase inhibitor that will be stopped in the next 6 months, or (3) unable to commit to a 12-month study.
2.4. Recruitment
The primary recruitment method is through registry lists of breast cancer survivors from the California Cancer Registry and the UC San Diego Epic electronic medical record. Women are mailed a letter and flyer about the I Can! study and informed that the study team will reach out to them by phone or email, if they do not opt out by contacting the study office. In addition, study information is provided to oncologists at the UC San Diego Moores Cancer Center and surrounding area, UC San Diego clinics, and local cancer support and philanthropy groups so they can refer breast cancer survivors to the study [40,54,55]. All methods and procedures have been approved by the UC San Diego institutional review board.
2.5. Telephone screening
Potential participants are first screened for eligibility over the phone. To reduce the likelihood of adverse events, participants are screened for factors that may increase risk of injury. Screener questions include the 10-item Physical Activity Readiness Questionnaire (PAR-Q) [53] and questions adapted from our previous trials [41,56]. The study physician advises on any medical questions that arise when screening for eligibility. Interested and eligible participants are then scheduled for an in-person orientation/measurement visit.
2.5.1. Orientation/Measurement (Visit 1)
At Visit 1, the first in-person visit (approximately 2–2.5 h), study requirements are discussed and written informed consent is obtained. Participants then complete baseline measures including anthropometric measures, blood draw, neurocognitive testing, and psychosocial questionnaires (see Measures section). Participants are fitted with a hip worn accelerometer, an ActiGraph GT3X+ (ActiGraph, LLC), and instructed to wear it during waking hours for the next 7 days. Using protocols successfully employed in previous studies to ensure adequate ActiGraph wear, participants receive two reminder calls over the next 7 days. After wearing the ActiGraph for 7 days for at least 10 h per day, participants return the device to the clinic at their randomization visit.
2.5.2. Randomization (Visit 2)
At Visit 2 (approximately 1.5–2 h), participants’ ActiGraph data is first screened for adequate wear time. If participants have sufficient wear time, they complete the final baseline measures, a 3-min step test to assess cardiopulmonary fitness and a 30-s sit-to-stand test to assess lower-body strength. Participants receive $20 for completing the baseline measures.
Once all baseline measures are completed, participants are randomized using a 1:1 allocation ratio of Exercise group to Health & Wellness group. Randomization uses a permuted-block design that includes strata for treatment with chemotherapy (Yes or No) and treatment with hormone therapy (Yes or No). Randomization lists are generated by the study statistician. Measurement staff are blinded to group assignments. After randomization, participants meet with a health coach to review the expectations and requirements for their randomly assigned group.
2.5.3. 3, 6, and 12 Month Measurements (Visit 3, 4, & 5)
Participants in both groups are mailed an ActiGraph and asked to wear it during the 7 days prior to each follow-up measurement visit, using the same protocols specified in Visit 1 to ensure adequate wear. At each measurement visit (approximately 2–2.5 h), participants repeat the measures that were completed at baseline including neurocognitive testing, self-reported cognition, psychosocial measures, blood draw, anthropometric measures, step test, and sit-to-stand test (See Measures section). Participants receive $50 for completing each visit and a $50 bonus for completing all three follow-up visits (3, 6, and 12 months).
2.6. Treatment conditions
2.6.1. Exercise group
The exercise intervention is based on Control Theory [57–59] and Social Cognitive Theory (targeted theoretical constructs italicized below) [60]. Participants start with an in-person meeting with their health coach where they receive a Fitbit, an electronic activity tracker for self-monitoring physical activity (see details below). To promote accountability, participants are informed that their health coach can see the physical activity data collected by the Fitbit. Fitbit data is used to support performance feedback and reviewing goals during contacts with their health coach. Using motivational interviewing techniques to increase self-efficacy and promote intention formation, a goal with a specific plan for meeting that goal is set. Participants discuss with their health coach ways to gradually increase their exercise over time to meet the study goal of at least 150 min of MVPA per week [61]. To ensure that participants know what moderate intensity activity feels like and to increase behavioral capability, participants are taken on a 10-min walk at 50 to 70% of their age-based maximum heart rate (based on CDC guidelines). To ensure that participants are increasing their activity level safely, the health coach covers key safety information including the importance of gradually increasing activity, stretching, and what to do when you are sick. Participants set a specific, personalized goal. They can choose any activity of interest that increases their heart rate to their target heart rate goal and that can be sustained for at least 10 min at a time. Based on our previous studies, we anticipate the most common activity to be walking, with other activities including exercise classes, hiking, biking, and gym equipment (e.g., elliptical, treadmill).
Brief phone calls (15–20 min) are scheduled every other week during the first 6 months to review Fitbit data, discuss progress towards goal, revise goal, and provide support. Each call also covers a specific topic to support behavior change. To support the calls, the health coach accesses participants’ Fitbit data through a web-based database, Fitabase (Small Steps Labs, San Diego, CA). Through Fitabase, the health coach can see graphs of daily light, moderate, and vigorous physical activity, date of last Fitbit sync, and Fitbit battery level. The Fitbit data is used to support the health coach calls by identifying days with low activity where activity could be added, and days with high activity to reinforce what is working well. In addition, the health coach checks Fitabase once a week so that participants can be proactively reached out to by their health coach as needed. In general, participants receive a text or email from their health coach if: 1) their active minutes on their Fitbit are 20% lower than their personal goal; 2) their active minutes are 20% higher than their personal goal; or 3) they have met their goal when they had not previously been achieving their goal (i.e., 4 weeks without achieving goal). This method of proactively reaching out to participants, rather than waiting for scheduled phone calls, was highly successful and extremely well-liked in our previous study [41]. Participants are encouraged to reach the 150 min/week of MVPA by 3 months (consistent with the American College of Sports Medicine guidelines for physical activity for cancer survivors), and then maintain that level of activity for the next 3 months.
Participants also receive static emails twice a week throughout the 6-month intervention. The emails further target components of Control Theory and Social Cognitive Theory with content including: 1) self-monitoring physical activity; 2) how to get social support for being active; 3) online resources, including how to find places to be active and exercise videos; and 4) tips on how to change outcome expectations and use one’s thoughts for exercise motivation. Each week, participants receive one in-depth email addressing these components and one brief tip of the week email.
During the maintenance period (months 6–12) participants are asked to continue wearing and syncing their Fitbit. Emails with theory-based content and reminders to wear and sync their Fitbit are sent every other week. They also receive brief check-in calls (10–15 min) once a month from their health coach to support continued progress towards their personal exercise goal. The health coach uses motivational interviewing techniques to guide the content of each
2.6.2. Health & wellness group
Participants start with an in-person meeting with their health coach to discuss breast cancer and cognition, general brain health, and age-related brain changes. They are then presented with the list of topics that are covered in the Health & Wellness group to identify topics of most interest that the health coach will focus on during the phone calls. Participants in the Health & Wellness group receive brief phone calls (15–20 min) and emails on the same schedule as the Exercise group. Calls and emails consist of a variety of topics to support overall wellness, such as brain health, stress management, nutrition, and sleep/insomnia. Participants are encouraged to set a general goal related to the topic of the week (e.g., try a stress management technique discussed during the call). If a participant expresses concerns and challenges to meeting their goal, the health coach will reach out by text or email to check in between calls, attempting to keep contact time similar between the two treatment groups. Each week, participants receive one in-depth email addressing a health topic and one brief tip of the week email.
During the maintenance period (months 6–12), participants receive monthly check-in calls (10–15 min) and emails every other week from their health coach to discuss health topics from the emails, maintain connection to the study, and increase retention at 12 month measurement.
2.7. COVID-19 considerations
Due to the COVID-19 pandemic, from March 19, 2020 through May 31, 2020, all in-person assessment and randomization visits were stopped in guidance with UC San Diego research policies and California state orders. Since intervention contacts for both study groups are by phone, intervention delivery was able to continue without interruption. Additional participant resources and information regarding COVID-19 that are appropriate for each study group were added to the respective intervention calls. To minimize data loss during the pause of in-person visits, enrolled participants who become due for their 3 month assessment visit during office closures were asked to complete measures remotely. NIH Toolbox measures were obtained through Zoom video conferencing using the NIH Toolbox recommendations for remote delivery [62]. Participants were mailed the ActiGraph to objectively measure physical activity per protocol on the due date. Survey measures were emailed to participants on the due date through REDCap, a secure, web-based platform designed to support data capture for research studies [63]. For the 6 month assessment, only the ActiGraph and surveys were completed remotely. Eleven participants who came due for their 6 month visit during the closure had their active intervention period extended (calls every 2 weeks continued for both study groups), and were brought in for an in-person visit as soon as the clinic re-opened June 1, 2020. Participants whose in-person visit occurred more than 3 weeks after their last ActiGraph and surveys were remotely completed were asked to repeat the ActiGraph and surveys at the time of the in-person visit. No 12 month assessments were due during the COVID-19 closure.
Minor protocol changes were enacted to resume in-person visits. All questionnaires are being completed at home through REDCap surveys to reduce the amount of time in clinic. In addition, the step-test has been paused until it is safe and allowable to conduct visits without masking. All masking, physical distance, and sanitation protocols required by UC San Diego are being followed. The study protocols may continue to shift to support remote delivery, as well as home visits and in-home phlebotomy, to respond to the changing requirements related to the COVID-19 pandemic to maintain safety for participants and study staff.
2.8. Measures
The primary outcomes are objectively measured processing speed and self-reported cognition, which are assessed at baseline, 3, 6, and 12 months. Processing speed is measured with the Oral Symbol Digit test from the NIH Toolbox Cognition Domain (nihtoolbox.org) [64,65], which was developed by the NIH’s Blueprint for Neuroscience Research to create a standard instrument to measure cognition in prevention and intervention trials. The measure is a computer-based version of the Wechsler Adult Intelligence Scale Digit-Symbol-Coding test and has been validated and normed in individuals age 3 to 85. The primary self-reported cognition measure is the Patient Reported Outcomes Measurement Information System (PROMIS) Cognitive Function scale that uses Computer Adaptive Testing to assess individuals’ perceptions of deficits in the areas of mental acuity, concentration, verbal and nonverbal memory, and verbal fluency, as well as perceived changes in these cognitive functions [66].
Secondary outcomes focus on the psychological and biological mechanisms. Proposed psychological mechanisms of anxiety, depression, fatigue, and physical functioning are measured at baseline, 3, 6, and 12 months. The PROMIS-Cancer scales for anxiety, depression, fatigue, and physical functioning, developed for cancer survivors, are administered using Computer Adaptive Testing [67]. The proposed biological mechanism, aging-related DNA methylation, is also measured at baseline, 3, 6, and 12 months. Non-fasting blood is drawn by certified phlebotomists at UC San Diego Moores Cancer Center. Serum, EDTA plasma, and buffy coat are prepared from the collected blood; an aliquot of whole EDTA-treated blood is also saved. All biospecimens are stored at −80 °C at the Moores Cancer Center. We will use state-of-the-art Next Generation Sequencing techniques to conduct targeted, aging-associated DNA methylation profiling, including bi-sulfite sequencing and Illumina MiSeq. [68]. DNA isolation from whole blood and methylation profiling will be conducted in participant-matched batches, i.e., all samples from a given participant will always be treated together. Methylation profiling will target validated loci whose level of methylation is associated with chronological age, which can be accelerated in cancer, and cancer risk and which are impacted by lifestyle behaviors [68–72]. The target loci are located in or near genes that regulate mechanisms of aging and cancer [68–72].
Additional measures from the ActiGraph, 3-min step test, and sit-to-stand test will be used to assess a dose-response relationship between changes in physical activity/fitness and changes in processing speed and self-reported cognition. The ActiGraph GT3X+ is used to measure minutes of moderate to vigorous physical activity at baseline, 3, 6, and 12 months. For 7 days at each assessment point, participants wear the ActiGraph on the hip during waking hours. The ActiGraph GT3X+ provides second-by-second estimates of activity that can be categorized into minutes spent in sedentary, light, moderate, and vigorous activity using calibration thresholds [73]. Its ability to measure physical activity with fidelity has been validated against heart rate telemetry and total energy expenditure [74,75]. Sufficient wear-time is defined as 5 days with ≥600 min of wear time or 3000 min (50 h) across 4 days. Time spent in sedentary, light, moderate and vigorous physical activity is derived using published, standardized cut-points [73]. We are using the ActiGraph to measure change in physical activity and not the Fitbit because: 1) participants cannot be blinded to Fitbit data, which would likely impact the baseline assessment; 2) Fitbit uses a proprietary scoring method to classify physical activity as light, moderate, and vigorous that is frequently modified, which would impact measurement consistency over the course of the intervention; 3) ActiGraphs are the gold-standard objective measure of free-living physical activity, which will aid in comparison of results to other studies; and 4) the Health & Wellness Group does not receive Fitbits. The 3-min step test is used to assess cardiopulmonary fitness. Participants are asked to step up and down on a 12-in. step at a constant pace (24 steps per minute) for 3 consecutive minutes. After a 5-s recovery period from stepping, the participant’s heart rate is measured for 1 min. This test of post-exercise heart rate has high reliability and sensitivity to change [76]. Lower-body strength is estimated via the 30-s sit-to-stand test. Participants are asked to stand up from a seated position as many times as they are able in a 30-s time period. This measure has good validity for measuring lower body functional muscle power and has been shown to be sensitive to intervention effects [77].
Since cancer treatments can impact memory, executive function, and attention, these cognitive domains are also measured at baseline, 3, 6, and 12 months. Tests from the NIH toolbox cognition domain and the recommended measures by the International Cognitive and Cancer Taskforce (ICCT) [78] are used. For each domain, the individual test scores will be transformed into z-scores and combined to create a combined score. The National Adult Reading Test is administered at baseline as a measure of crystalized ability and will be used as a covariate in analyses. See Table 2 for full list of measures.
Table 2.
I Can! study measures. All measures are collected at baseline, 3, 6, and 12 months, excepted where noted.
| Primary aims | |
| Objective Processing Speed | Oral Symbol Digit Test from the NIH Toolbox Cognition measures [64,65] |
| Self-reported Cognition | PROMIS Cognitive Function Measure [66] |
| Secondary Aims | |
| Psychological Mechanisms | PROMIS Anxiety, Depression, Fatigue, and Physical Functioning Measures for Cancer Survivors [67] |
| DNA methylation age | Aging-associated DNA methylation profiling, including bi-sulfite sequencing and Illumina Mi-Seq [68] |
| Dose-Response | |
| Physical Activity & Fitness | 7-day, waking hours, hip worn ActiGraph GT3X+ [74,75], 3-min step-test [76], 30-s sit-to-stand test [77] |
| Other relevant measures | |
| Height & weight | Digital scale & stadiometer (height baseline only) |
| Demographics (baseline only) | Age, education, income, race, and marital status with standard surveys |
| Clinical | Chart reviews for date of diagnosis, stage at diagnosis, cancer treatments, date of end of active treatment, hormonal therapy, ER, PR, and HER2 status, and current medications. (baseline only) |
| Self-report of medical history (baseline and 12 months only) | |
| Memory domain | Hopkins Verbal Learning Test-Revised [79], NIH Toolbox Picture Sequence Memory Test & List Sorting Working Memory Test [65] |
| Executive Functioning domain | Trail Making Test [80] & NIH Toolbox Dimensional Change Card Sort Test [65] |
| Attention domain | NIH Toolbox Flanker Inhibitory Control and Attention Test [65] & Conners’ Continuous Performance Test 3 (CPT-3) [81] |
| Crystalized cognitive ability | National Adult Reading Test [82] (baseline only) |
| Quality of Life | PROMIS Sleep-related Impairment [83] and General Self-efficacy [84]; Cognitive and Affective Mindfulness Scale-Revised [85] |
2.9. Data analysis
We will summarize baseline characteristics and assess if randomization achieved balance by comparing distributions of key baseline covariates (demographics, baseline physical activity, baseline objective and self-report cognitive outcomes, cancer treatment, and related variables) between treatment groups (Exercise group vs. Health & Wellness group) using graphical methods, non-parametric and parametric tests as appropriate (e.g., Wilcoxon tests for non-Gaussian data, chi-square tests for categorical variables). Given the multiple cognitive domains, psychological factors, and biomarkers to be tested, we will use a Holm’s sequential rejection test to adjust for multiple comparisons in all analyses [86]. We note that in our sample size estimates, we used a Bonferroni correction, which is likely conservative since the outcomes will be correlated.
To test efficacy of the treatment we will use a mixed model paradigm. For the primary aim, cognition scores at 3- and 6-months will be the repeated measures dependent variable; fixed effects will include baseline cognition, treatment group, time. A subject-specific intercept will be included to account for within-subject correlations. A statistically significant coefficient for treatment will indicate that follow-up cognition scores differed between arms. We will also examine group differences in change scores, by incorporating baseline scores in the repeated measures outcome, and including a group*time interaction term. This analysis will be conducted for objective processing speed and self-report cognition (Table 2). We will explore potential moderating effects of key variables (e.g., age, time since cancer diagnosis, cancer treatment) on the association between treatment and objective and self-reported cognitive outcomes, by including the potential moderator, as well as 2- and 3-way interactions between the moderator, treatment and time. To test if intervention effects are sustained at 12 months (Secondary Aim 1), we will include baseline, 3, 6, and 12 month cognition scores, as the dependent variable and repeat the above analysis. By using appropriate contrasts, we will compare changes in cognition from baseline to 12 months (sustained treatment effect) or 6- to 12 months (maintenance of treatment effect) within- and between- groups. This mixed model approach will also be used for the additional cognitive domains, i.e., memory, executive function, attention (Exploratory Aim 2).
For Secondary Aim 2, we will test if the psychological and biological variables (M) mediate the association between treatment (X) and cognition outcomes (Y) using multilevel multiple mediator models [87–89] [90]. Total, direct and mediated effects of X (and M) on Y can be derived from these models. To assess the robustness, we will apply bootstrap methods [91] to resample and refit mediation models to evaluate consistency of results. This analysis will be conducted for objective and self-report cognition.
Finally, to estimate a dose-response relationship between change in physical activity (via ActiGraph and/or 3-min step test) and cognition (Exploratory Aim 1), we will use generalized additive mixed models (GAMMs), which can accommodate a variety of distributions (e.g., positively-skewed physical activity data), account for dependency in error terms due to clustering (repeated measures), and estimate complex, dose-response relationships of unknown form [92].
To assess COVID-19-related protocol adjustments, we will conduct a sensitivity analysis by adding a binary variable (and its interactions with treatment, time) to models, to indicate whether an assessment was in-person vs remote. Although due to our RCT design, we expect that both arms will be similarly affected by these protocol changes, we believe it is a valuable opportunity to quantify the impact of mode of assessment on study results.
2.10. Sample size and power
We estimated the needed sample size for the Primary Aim, i.e., to compare differences in neuropsychological outcomes between the treatment and control groups over 6 months. We assumed a 2-sided test with a significance level (alpha) of 0.05/2 to account for two outcomes, i.e., objective processing speed, and self-reported cognition. We used means and standard deviations from randomized controlled trials including our pilot trial with breast cancer survivors as well as other studies with older adults [93–96] to guide anticipated effect sizes for the proposed intervention. Also, correlations on repeated measures of the cognitive tests ranged from 0.5 to 0.9 in our previous study; we used a conservative value of 0.5 in the present power calculations. Under these assumptions, with 110 participants per group, there is 80% power to detect a 0.3 standardized mean group difference in changes (i.e., effect size) in post-treatment cognition scores (at 3 and 6 months), adjusted for baseline scores (i.e., ANCOVA model). Using SD estimates from our previous work, this effect size corresponds to intervention-related mean group differences of 3.3 units in processing speed, and 2.5 units in self-reported cognitive abilities. If we also included the 12-month time-point (Secondary Aim 1), we have 80% power to detect a smaller 0.27 effect size corresponding to mean treatment differences of 3 units in processing speed, and 2.3 units in self-reported cognitive abilities over 12-months.
Allowing for a 10% attrition rate at 12 months (6% attrition achieved in previous 6-month trial with 333 breast cancer survivors), we aim to recruit a total of 250 participants to our study.
2.11. Missing data
Our analytic approach using mixed models [96] will yield unbiased results even if some observations are missing, as long as the data are “missing at random,” i.e., the reasons for missingness can be predicted from observed measurement [97,98]. This assumption has been tenable in our previous studies, and we expect this to be the case in this study. However, we will conduct sensitivity analyses to test for informative drop-outs [98] and develop alternate approaches, e.g., pattern-mixture models, if needed [99–101].
3. Discussion
The main purpose of the I Can! study is to examine whether increasing physical activity among breast cancer survivors can improve objectively measured processing speed and self-reported cognition, compared to health and wellness attention-comparison. Physical activity is a promising intervention for cognitive impairments to improve the quality of life of breast cancer survivors. This study will provide a rich data set that includes neurocognitive testing, self-reported cognition, objective measures of physical activity, psychological measures, and key biological measures, enabling a novel and innovative investigation of the relationships between physical activity and cognition in breast cancer survivors.
To enhance the efficacy of interventions to improve cognitive impairments, it is important to understand the potential mechanisms linking physical activity with objective and self-reported cognition. Cognitive impairments are associated with greater anxiety and depression; increasing physical activity can decrease anxiety and depression [102–104]. Determining the mechanisms through which physical activity can improve cognitive impairment is an important step in understanding how to intervene on factors that impact breast cancer survivors’ quality of life. To our knowledge, our 12-week RCT in 87 breast cancer survivors is the only published study to test whether anxiety and depression were potential mechanisms linking physical activity and improved cognition [105]. Results from that study provided preliminary evidence that reductions in anxiety mediated improvements in self-reported cognitive abilities, but there was no evidence of mediation for objectively measured processing speed or other measures of psychological function [105]. The current larger and longer I Can! RCT will expand the knowledge base regarding psychological mechanisms of physical activity-induced changes in cognition among breast cancer survivors.
It is unclear how physical activity and cognition are causally linked. One provocative hypothesis is that exercise can slow, or even reverse, cellular aging and thereby improve cognitive performance at the cellular level. This hypothesis is particularly relevant for breast cancer survivors, who may experience accelerated aging caused by chemotherapy and psychological stress [25–27]. The accelerated aging hypothesis proposes that cancer and its treatment lead to steeper and earlier declines in cognitive function compared to non-cancer populations [27,106,107]. Cellular aging can be estimated using targeted profiling of DNA methylation, which is dynamic and varies chronologically, with disease (e.g., metabolic syndrome, cancer), and lifestyle (e.g., stress, diet, exercise) [108–110]. Results of this study will allow us to explore the relationship of cellular aging with cognition, physical activity, and cancer characteristics (e.g., cancer stage, treatments), whether the rate of cellular aging can be slowed by the physical activity intervention, and if cellular aging appears to be on the causal pathway between physical activity and cognition among breast cancer survivors.
To date, the few published trials testing whether increasing physical activity can improve cognition in cancer survivors have been conducted with small sample sizes [41,43]. There are several larger ongoing and recently completed trials [111,112]. Of the four largest studies, three are using a clinic-based, in-person physical activity intervention with one focusing on aromatase inhibitor-associated cognitive changes (n = 254; NCT02793921) [112], and another’s intervention is only 5 weeks long (n = 152; NCT02934880). The fourth is a 6-week trial with colorectal cancer survivors (n = 116) focusing on the role of inflammatory responses from ibuprofen and exercise (NCT01238120). Therefore, the I Can! study provides a unique contribution to the field as it is a fully powered trial, uses a home-based physical activity intervention supporting free-living activity, and examines maintenance of the intervention effects. Home-based trials are particularly important as cancer survivors report many barriers to engaging in clinic-based exercise programs related to scheduling and lack of time [113]. Home-based, technology supported approaches that do not require in-person attendance offer several advantages over traditional lifestyle interventions, including the potential to be more cost-effective, accessible, and convenient [114–116]. They also have the potential to support maintenance of physical activity which may support continued benefits for cognition and quality of life. The need for testing the impact of home-based physical activity is further highlighted by the current COVID-19 pandemic, during which frequent clinic visits are not possible.
Another strength of this study is the use of objective measures of physical activity to assess for a dose-response relationship between cognition and changes in physical activity. Our previous pilot trial showed a significant dose-response between total minutes of ActiGraph measured physical activity, comprised of MVPA and light activity, and improved neurocognitive function and improved self-reported cognition within the Exercise group [41]. This preliminary finding suggests that many types of activity, not just those conducted at moderate intensity, could benefit cognition. The I Can! RCT will build on our pilot results through a more rigorous design. Performing high levels of MVPA can be difficult for many people, underscoring the need to determine the necessary minutes of different activity intensities to improve cognition. In addition to the ActiGraph data collected at four time points to assess physical activity across both arms, we will be able to examine patterns of physical activity in the Exercise Group. Passive collection of activity data via Fitbit throughout the intervention and maintenance periods further expands upon previous studies by allowing us to identify the precise timing and volume of changes in physical activity over the entire 12-month study period. Overall, study findings will inform guidelines for physical activity and dose needed to improve cognition in cancer survivors.
The I Can! trial has some limitations. The study focuses on breast cancer survivors who have completed treatment at least six months prior to enrollment. Some studies report pre-treatment impairments in objectively measured cognitive function [117,118]. This trial’s focus on cancer survivors who have already completed treatment does not enable us to consider the impact of exercise on pre-treatment cognitive impairment or measure improvements from pre- to post-treatment. We did not limit eligibility to any specific type of breast cancer so that the results will be generalizable to a larger group of breast cancer survivors. The focus on older breast cancer survivors will also limit the generalizability of findings to younger survivors or survivors of other types of cancer. Since the limited number of trials published to date are generally less than 6 months in duration, it is not known whether the goal of reaching 150 min of MPVA at 3 months and then maintaining activity until 6-months is long enough to support change in cognition. However, the 12-month follow-up measures will allow us to detect maintenance of intervention effects and any potential benefits to cognition with a longer exercise regimen. The intervention utilizes a Fitbit requiring participants to have a Fitbit compatible device with internet. Smartphone usage continues to increase across all races and ethnicities; however, relying on a technology-based intervention may perpetuate the digital divide contributing to cancer disparities. While there is some evidence to suggest that strength training may have benefits for cognition in cancer survivors [119,120], this study focuses on aerobic exercise. Further, this study is not examining the interaction between physical activity and other important behavioral factors that may impact cognition such as sleep and nutrition [121–123]. Lastly, the study is being conducted in San Diego, California where we aim to recruit a representative sample of Hispanics, but due to the racial make-up of the area, we may not have enough diversity for results to be generalizable based on race.
4. Conclusion
This is one of the first fully powered trials using a home-based physical activity intervention supporting free-living activity and assessing both objective and self-reported cognition. Findings will inform future research not only in breast cancer survivors, but also in other cancer populations that experience cognitive deficits such as colorectal cancer [124] and prostate cancer [125]. This study will contribute to the scientific, public health, and intervention literature that is needed to identify interventions to support cognition in cancer survivors. Results of this innovative trial have strong potential to impact survivorship care planning, improving the lives of the growing population of breast cancer survivors.
Acknowledgments
Funding
Research reported in this publication is supported by the National Cancer Institute (NCI) of the National Institutes of Health (USA) under award number R01CA2257456. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Reference
- [1].American Cancer Society, American Cancer Society: Cancer Facts and Figures 2018, American Cancer Society, Atlanta, GA, 2018. [Google Scholar]
- [2].Miller KD, Nogueira L, Mariotto AB, et al. , Cancer treatment and survivorship statistics, 2019, CA Cancer J. Clin. 0 (0) (2019). [DOI] [PubMed] [Google Scholar]
- [3].Jim HS, Phillips KM, Chait S, et al. , Meta-analysis of cognitive functioning in breast cancer survivors previously treated with standard-dose chemotherapy, J. Clin. Oncol. 30 (29) (2012) 3578–3587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Falleti MG, Sanfilippo A, Maruff P, Weih L, Phillips K-A, The nature and severity of cognitive impairment associated with adjuvant chemotherapy in women with breast cancer: a meta-analysis of the current literature, Brain Cogn. 59 (2005). [DOI] [PubMed] [Google Scholar]
- [5].Anderson-Hanley C, Sherman ML, Riggs R, Agocha VB, Compas BE, Neuropsychological effects of treatments for adults with cancer: a meta-analysis and review of the literature, J. Int. Neuropsychol. Soc. 9 (7) (2003) 967–982. [DOI] [PubMed] [Google Scholar]
- [6].Jansen CE, Miaskowski C, Dodd M, Dowling G, Kramer J, A metaanalysis of studies of the effects of cancer chemotherapy on various domains of cognitive function, Cancer. 104 (10) (2005) 2222–2233. [DOI] [PubMed] [Google Scholar]
- [7].Ono M, Ogilvie JM, Wilson JS, et al. , A meta-analysis of cognitive impairment and decline associated with adjuvant chemotherapy in women with breast cancer, Front. Oncol. 5 (2015) 59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Janelsins MC, Kesler SR, Ahles TA, Morrow GR, Prevalence, mechanisms, and management of cancer-related cognitive impairment, Int. Rev. Psychiatry. 26 (1) (2014) 102–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Hardy SJ, Krull KR, Wefel JS, Janelsins M, Cognitive changes in cancer survivors, Am. Soc. Clin. Oncol. Educ. Book. 38 (2018) 795–806. [DOI] [PubMed] [Google Scholar]
- [10].Reid-Arndt SA, Yee A, Perry MC, Hsieh C, Cognitive and psychological factors associated with early posttreatment functional outcomes in breast cancer survivors, J. Psychosoc. Oncol. 27 (4) (2009) 415–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Wefel JS, Lenzi R, Theriault RL, Davis RN, Meyers CA, The cognitive sequelae of standard-dose adjuvant chemotherapy in women with breast carcinoma: results of a prospective, randomized, longitudinal trial, Cancer. 100 (11) (2004) 2292–2299. [DOI] [PubMed] [Google Scholar]
- [12].Duijts SF, van Egmond MP, Spelten E, van Muijen P, Anema JR, van der Beek AJ, Physical and psychosocial problems in cancer survivors beyond return to work: a systematic review, Psychooncology. 23 (5) (2014) 481–492. [DOI] [PubMed] [Google Scholar]
- [13].Jagsi R, Hawley ST, Abrahamse P, et al. , Impact of adjuvant chemotherapy on long-term employment of survivors of early-stage breast cancer, Cancer. 120 (12) (2014) 1854–1862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Lauzier S, Maunsell E, Drolet M, et al. , Wage losses in the year after breast cancer: extent and determinants among Canadian women, J. Natl. Cancer Inst. 100 (5) (2008) 321–332. [DOI] [PubMed] [Google Scholar]
- [15].Steiner JF, Cavender TA, Main DS, Bradley CJ, Assessing the impact of cancer on work outcomes: what are the research needs? Cancer. 101 (8) (2004) 1703–1711. [DOI] [PubMed] [Google Scholar]
- [16].Bernstein LJ, McCreath GA, Komeylian Z, Rich JB, Cognitive impairment in breast cancer survivors treated with chemotherapy depends on control group type and cognitive domains assessed: a multilevel meta-analysis, Neurosci. Biobehav. Rev. 83 (2017) 417–428. [DOI] [PubMed] [Google Scholar]
- [17].Kohli S, Griggs JJ, Roscoe JA, et al. , Self-reported cognitive impairment in patients with cancer, J. Oncol. Pract. 3 (2) (2007) 54–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Janelsins MC, Heckler CE, Peppone LJ, et al. , Cognitive complaints in survivors of breast cancer after chemotherapy compared with age-matched controls: an analysis from a nationwide, multicenter, prospective longitudinal study, J. Clin. Oncol. 35 (5) (2017) 506–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Rosenfeld CS, Shay DA, Vieira-Potter VJ, Cognitive effects of aromatase and possible role in memory disorders, Front. Endocrinol. (Lausanne) 9 (2018) 610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Gervais NJ, Remage-Healey L, Starrett JR, Pollak DJ, Mong JA, Lacreuse A, Adverse effects of aromatase inhibition on the brain and behavior in a nonhuman primate, J. Neurosci. 39 (5) (2019) 918–928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Vance DE, Frank JS, Bail J, et al. , Interventions for cognitive deficits in breast Cancer survivors treated with chemotherapy, Cancer Nurs. 40 (1) (2017) E11–E27. [DOI] [PubMed] [Google Scholar]
- [22].National Comprehensive Cancer Network, National Comprehensive Cancer Network Practice Guidelines in Oncology, Fort Washington, PA, National Comprehensive Cancer Network, 2018. [Google Scholar]
- [23].Institute of Medicine, Cognitive Aging: Progess in Understanding and Opportunities for Action, The National Academies Press, Washington DC, 2015. [PubMed] [Google Scholar]
- [24].Colcombe S, Kramer AF, Fitness effects on the cognitive function of older adults: a meta-analytic study, Psychol. Sci. 14 (2) (2003) 125–130. [DOI] [PubMed] [Google Scholar]
- [25].Epel ES, Psychological and metabolic stress: a recipe for accelerated cellular aging? Hormones (Athens, Greece) 8 (1) (2009) 7–22. [DOI] [PubMed] [Google Scholar]
- [26].Mandelblatt JS, Hurria A, McDonald BC, et al. , Cognitive effects of cancer and its treatments at the intersection of aging: what do we know; what do we need to know? Semin. Oncol. 40 (6) (2013) 709–725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Ahles TA, Root JC, Ryan EL, Cancer- and cancer treatment-associated cognitive change: an update on the state of the science, J. Clin. Oncol. 30 (30) (2012) 3675–3686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Ball K, Edwards JD, Ross LA, The impact of speed of processing training on cognitive and everyday functions, J. Gerontol B Psychol. Sci. Soc. Sci. 62 (1) (2007) 19–31. [DOI] [PubMed] [Google Scholar]
- [29].Salthouse TA, The processing-speed theory of adult age differences in cognition, Psychol. Rev. 103 (3) (1996) 403–428. [DOI] [PubMed] [Google Scholar]
- [30].Brown AD, McMorris CA, Longman RS, et al. , Effects of cardiorespiratory fitness and cerebral blood flow on cognitive outcomes in older women, Neurobiol. Aging 31 (12) (2010) 2047–2057. [DOI] [PubMed] [Google Scholar]
- [31].Netz Y, Dwolatzky T, Zinker Y, Argov E, Agmon R, Aerobic fitness and multidomain cognitive function in advanced age, Int. Psychogeriatr. 23 (1) (2011) 114–124. [DOI] [PubMed] [Google Scholar]
- [32].Angevaren M, Aufdemkampe G, Verhaar HJ, Aleman A, Vanhees L, Physical activity and enhanced fitness to improve cognitive function in older people without known cognitive impairment, Cochrane Database Syst. Rev. 3 (2008), CD005381. [DOI] [PubMed] [Google Scholar]
- [33].Foster PP, Rosenblatt KP, Kuljis RO, Exercise-induced cognitive plasticity, implications for mild cognitive impairment and Alzheimer’s disease, Front. Neurol. 2 (2011) 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Rolland Y, Abellan van Kan G, Vellas B. healthy brain aging: role of exercise and physical activity, Clin. Geriatr. Med. 26 (1) (2010) 75–87. [DOI] [PubMed] [Google Scholar]
- [35].Smith PJ, Blumenthal JA, Hoffman BM, et al. , Aerobic exercise and neurocognitive performance: a meta-analytic review of randomized controlled trials, Psychosom. Med. 72 (3) (2010) 239–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Irwin ML, Crumley D, McTiernan A, et al. , Physical activity levels before and after a diagnosis of breast carcinoma: the health, eating, activity, and lifestyle (HEAL) study, Cancer. 97 (7) (2003) 1746–1757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Lynch BM, Dunstan DW, Healy GN, Winkler E, Eakin E, Owen N, Objectively measured physical activity and sedentary time of breast cancer survivors, and associations with adiposity: findings from NHANES (2003–2006), Cancer Causes Control 21 (2) (2010) 283–288. [DOI] [PubMed] [Google Scholar]
- [38].Ahlskog JE, Geda YE, Graff-Radford NR, Petersen RC, Physical exercise as a preventive or disease-modifying treatment of dementia and brain aging, Mayo Clin. Proc. 86 (9) (2011) 876–884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Strohle A, Schmidt DK, Schultz F, et al. , Drug and exercise treatment of¨ Alzheimer disease and mild cognitive impairment: a systematic review and meta-analysis of effects on cognition in randomized controlled trials, Am. J. Geriatr. Psychiatry 23 (12) (2015) 1234–1249. [DOI] [PubMed] [Google Scholar]
- [40].Hartman SJ, Natarajan L, Palmer BW, Parker B, Patterson RE, Sears DD, Impact of increasing physical activity on cognitive functioning in breast cancer survivors: Rationale and study design of Memory & Motion, Contemp. Clin. Trials 45 (Pt B) (2015) 371–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Hartman SJ, Nelson SH, Myers E, et al. , Randomized controlled trial of increasing physical activity on objectively measured and self-reported cognitive functioning among breast cancer survivors: the memory & motion study, Cancer. 124 (1) (2018) 192–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Hartman SJ, Nelson SH, Weiner LS, Patterns of fitbit use and activity levels throughout a physical activity intervention: exploratory analysis from a randomized controlled trial, JMIR Mhealth Uhealth. 6 (2) (2018), e29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Campbell KL, Kam JW, Neil-Sztramko SE, et al. , Effect of aerobic exercise on cancer-associated cognitive impairment: a proof-of-concept RCT, Psychooncology. 27 (1) (2017) 53–60. [DOI] [PubMed] [Google Scholar]
- [44].Deary IJ, Corley J, Gow AJ, et al. , Age-associated cognitive decline, Br. Med. Bull. 92 (2009) 135–152. [DOI] [PubMed] [Google Scholar]
- [45].Correa DD, Ahles TA, Neurocognitive changes in cancer survivors, Cancer J. 14 (6) (2008) 396–400. [DOI] [PubMed] [Google Scholar]
- [46].Ahles TA, Saykin AJ, McDonald BC, et al. , Longitudinal assessment of cognitive changes associated with adjuvant treatment for breast cancer: impact of age and cognitive reserve, J. Clin. Oncol. 28 (29) (2010) 4434–4440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Hutchinson AD, Hosking JR, Kichenadasse G, Mattiske JK, Wilson C, Objective and subjective cognitive impairment following chemotherapy for cancer: a systematic review, Cancer Treat. Rev. 38 (7) (2012) 926–934. [DOI] [PubMed] [Google Scholar]
- [48].Pullens MJ, De Vries J, Roukema JA, Subjective cognitive dysfunction in breast cancer patients: a systematic review, Psychooncology. 19 (11) (2010) 1127–1138. [DOI] [PubMed] [Google Scholar]
- [49].Zimmer P, Baumann FT, Oberste M, et al. , Effects of exercise interventions and physical activity behavior on cancer related cognitive impairments: a systematic review, Biomed. Res. Int. 2016 (2016) 1820954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Rogers LQ, Hopkins-Price P, Vicari S, et al. , A randomized trial to increase physical activity in breast cancer survivors, Med. Sci. Sports Exerc. 41 (4) (2009) 935–946. [DOI] [PubMed] [Google Scholar]
- [51].Mustian K, Janelsins M, Peppone L, Kamen C, Guido J, Heckler C, EXCAP exercise effects on cognitive impairment and inflammation: A URCC NCORP RCT in 479 cancer patients, J. Clin. Oncol. 33 (suppl) (2015) (abstr 9504). [Google Scholar]
- [52].Williams AM, Shah R, Shayne M, et al. , Associations between inflammatory markers and cognitive function in breast cancer patients receiving chemotherapy, J. Neuroimmunol. 314 (2018) 17–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Thomas S, Reading J, Shephard RJ , Revision of the physical activity readiness questionnaire (PAR-Q), Can. J. Sport Sci. 17 (4) (1992) 338–345. [PubMed] [Google Scholar]
- [54].Patterson RE, Marinac CR, Natarajan L, et al. , Recruitment strategies, design, and participant characteristics in a trial of weight-loss and metformin in breast cancer survivors, Contemp. Clin. Trials. 47 (2016) 64–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Hartman SJ, Marinac CR, Natarajan L, Patterson RE, Lifestyle factors associated with cognitive functioning in breast cancer survivors, Psychooncology. 24 (6) (2015) 669–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Hartman SJ, Dunsiger SI, Bock BC, et al. , Physical activity maintenance among Spanish-speaking Latinas in a randomized controlled trial of an internet-based intervention, J. Behav. Med. 40 (3) (2016) 392–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Carver CS, Scheier MF , Control theory: a useful conceptual framework for personality-social, clinical, and health psychology, Psychol. Bull. 92 (1) (1982) 111–135. [PubMed] [Google Scholar]
- [58].Carver CS, Scheier M, On the self-regulation of behavior. Cambridge, UK, Cambridge University Press, New York, NY, USA, 1998. [Google Scholar]
- [59].Michie S, Abraham C, Whittington C, McAteer J, Gupta S, Effective techniques in healthy eating and physical activity interventions: a meta-regression, Health Psychol. 28 (6) (2009) 690–701. [DOI] [PubMed] [Google Scholar]
- [60].Bandura A, Social Foundations of Thought and Action, Prentice-Hall, Englewood Cliffs, NJ, 1986. [Google Scholar]
- [61].Schmitz KH, Courneya KS, Matthews C, et al. , American College of Sports Medicine roundtable on exercise guidelines for cancer survivors, Med. Sci. Sports Exerc. 42 (7) (2010) 1409–1426. [DOI] [PubMed] [Google Scholar]
- [62].NIH Toolbox, Remote Administration Guidelines for the NIH Toolbox®: Response to COVID-19. https://nihtoolbox.my.salesforce.com/sfc/p/#2E000001H4ee/a/2E000000UcbY/wsdEYpor8yh7OGc8wq4rg1auQ.06sJ9flossedn16Mw, 2020. (Accessed 5/22/20).
- [63].Harris PA, Taylor R, Minor BL, et al. , The REDCap consortium: Building an international community of software platform partners, J. Biomed. Inform. 95 (2019) 103208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Heaton RK, Akshoomoff N, Tulsky D, et al. , Reliability and validity of composite scores from the NIH Toolbox cognition battery in adults, J. Int. Neuropsychol. Soc. 20 (6) (2014) 588–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Weintraub S, Dikmen SS, Heaton RK, et al. , The cognition battery of the NIH toolbox for assessment of neurological and behavioral function: validation in an adult sample, J. Int. Neuropsychol. Soc. 20 (6) (2014) 567–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Lai JS, Wagner LI, Jacobsen PB, Cella D, Self-reported cognitive concerns and abilities: two sides of one coin? Psychooncology. 23 (10) (2014) 1133–1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Yost KJ, Eton DT, Garcia SF, Cella D, Minimally important differences were estimated for six patient-reported outcomes measurement information system-cancer scales in advanced-stage cancer patients, J. Clin. Epidemiol. 64 (5) (2011) 507–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Hannum G, Guinney J, Zhao L, et al. , Genome-wide methylation profiles reveal quantitative views of human aging rates, Mol. Cell 49 (2) (2013) 359–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Durso DF, Bacalini MG, Sala C, et al. , Acceleration of leukocytes’ epigenetic age as an early tumor and sex-specific marker of breast and colorectal cancer, Oncotarget. 8 (14) (2017) 23237–23245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Perna L, Zhang Y, Mons U, Holleczek B, Saum KU, Brenner H, Epigenetic age acceleration predicts cancer, cardiovascular, and all-cause mortality in a German case cohort, Clin. Epigenetics 8 (2016) 64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Zheng Y, Joyce BT, Colicino E, et al. , Blood epigenetic age may predict cancer incidence and mortality, EBioMedicine. 5 (2016) 68–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Raina A, Zhao X, Grove ML, et al. , Cerebral white matter hyperintensities on MRI and acceleration of epigenetic aging: the atherosclerosis risk in communities study, Clin. Epigenetics 9 (2017) 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Freedson PS, Melanson E, Sirard J, Calibration of the computer science applications, Inc. accelerometer, Med. Sci. Sports Exerc. 30 (1998) 777–781. [DOI] [PubMed] [Google Scholar]
- [74].Plasqui G, Westerterp KR, Physical activity assessment with accelerometers: an evaluation against doubly labeled water, Obesity. 15 (10) (2007) 2371–2379. [DOI] [PubMed] [Google Scholar]
- [75].Melanson EL, Freedson PS, Validity of the computer science and applications, Inc. (CSA) activity monitor, Med. Sci. Sports Exerc. 27 (1995). [PubMed] [Google Scholar]
- [76].McArdle W, Katch F, Katch V, Exercise Physiology: Energy, Nutrition, and Human Performance, 6th ed., Lippincott Williams & Wilkins, Philadelphia, 2007. [Google Scholar]
- [77].Jones CJ, Rikli RE, Beam WC, A 30-s chair-stand test as a measure of lower body strength in community-residing older adults, Res. Q. Exerc. Sport 70 (2) (1999) 113–119. [DOI] [PubMed] [Google Scholar]
- [78].Wefel JS, Vardy J, Ahles T, Schagen SB, International cognition and Cancer task force recommendations to harmonise studies of cognitive function in patients with cancer, Lancet Oncol. 12 (7) (2011) 703–708. [DOI] [PubMed] [Google Scholar]
- [79].Benedict RHB, Schretlen D, Groninger L, Brandt J, Hopkins verbal learning test – revised: normative data and analysis of inter-form and test-retest reliability, Clin. Neuropsychol. 12 (1) (1998) 43–55. [Google Scholar]
- [80].Reitan Reitan R, Validity of the trail making test as an Indicator of organic brain damage, Percept. Mot. Skills 8 (3) (1958) 271–276. [Google Scholar]
- [81].Conners CK, Staff M, Connelly V, Campbell S, MacLean M, Barnes J, Conners’ continuous performance test II (CPT II v. 5), Multi-Health Syst. Inc. 29 (2000) 175–196. [Google Scholar]
- [82].Nelson HE, Willison J, National Adult Reading Test (NART), Nfer-Nelson Windsor, 1991. [Google Scholar]
- [83].Buysse DJ, Yu L, Moul DE, et al. , Development and validation of patient-reported outcome measures for sleep disturbance and sleep-related impairments, Sleep. 33 (6) (2010) 781–792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Gruber-Baldini AL, Velozo C, Romero S, Shulman LM, Validation of the PROMIS® measures of self-efficacy for managing chronic conditions, Qual. Life Res. 26 (7) (2017) 1915–1924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Feldman G, Hayes A, Kumar S, Greeson J, Laurenceau J-P, Mindfulness and emotion regulation: the development and initial validation of the cognitive and affective mindfulness scale-revised (CAMS-R), J. Psychopathol. Behav. Assess. 29 (3) (2007) 177. [Google Scholar]
- [86].Holm S, A simple sequentially rejective multiple test procedure, Scand. J. Stat. 6 (2) (1979) 65–70. [Google Scholar]
- [87].Baron RM, Kenny DA, The moderator-mediator variable distinction in social psychological research: conceptual, strategic, and statistical considerations, J. Pers. Soc. Psychol. 51 (6) (1986) 1173–1182. [DOI] [PubMed] [Google Scholar]
- [88].Kraemer HC, Stice E, Kazdin A, Offord D, Kupfer D, How do risk factors work together? Mediators, moderators, and independent, overlapping, and proxy risk factors, Am. J. Psychiatry 158 (6) (2001) 848–856. [DOI] [PubMed] [Google Scholar]
- [89].MacKinnon DP, Introduction to Statistical Mediation Analysis, Lawrence Erlbaum Associates, New York, 2008. [Google Scholar]
- [90].Bauer DJ, Preacher KJ, Gil KM, Conceptualizing and testing random indirect effects and moderated mediation in multilevel models: new procedures and recommendations, Psychol. Methods 11 (2) (2006) 142–163. [DOI] [PubMed] [Google Scholar]
- [91].Efron B, Tibshirani R, An Introduction to the Bootstrap, Chapman & Hall, New York, 1993. [Google Scholar]
- [92].Wood SN, Generalized additive models : an introduction with R. Second Edition. Ed, CRC Press, Boca Raton, 2017. [Google Scholar]
- [93].Baker LD, Frank LL, Foster-Schubert K, et al. , Effects of aerobic exercise on mild cognitive impairment: a controlled trial, Arch. Neurol. 67 (1) (2010) 71–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Hooghiemstra AM, Eggermont LH, Scheltens P, et al. , Study protocol: EXERcise and cognition in sedentary adults with early-ONset dementia (EXERCISE-ON), BMC Neurol. 12 (2012) 75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Nouchi R, Taki Y, Takeuchi H, et al. , Beneficial effects of short-term combination exercise training on diverse cognitive functions in healthy older people: study protocol for a randomized controlled trial, Trials. 13 (2012) 200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Diggle P, Analysis of Longitudinal Data, Second Paperback Edition, Oxford University Press, Oxford, 2013. [Google Scholar]
- [97].Schafer JL, Graham JW, Missing data: our view of the state of the art, Psychol. Methods 7 (2) (2002) 147–177. [PubMed] [Google Scholar]
- [98].Little RJA, Modeling the drop-out mechanism in repeated-measures studies, J. Am. Stat. Assoc. 90 (431) (1995) 1112–1121. [Google Scholar]
- [99].Hedeker D, Gibbons RD, Application of random-effects pattern-mixture models for missing data in longitudinal studies, Psychol. Methods 2 (1) (1997) 64–78. [Google Scholar]
- [100].Park T, Lee SY, Simple pattern-mixture models for longitudinal data with missing observations: analysis of urinary incontinence data, Stat. Med. 18 (21) (1999) 2933–2941. [DOI] [PubMed] [Google Scholar]
- [101].Daniels MJ, Hogan JW, Reparameterizing the pattern mixture model for sensitivity analyses under informative dropout, Biometrics. 56 (4) (2000) 1241–1248. [DOI] [PubMed] [Google Scholar]
- [102].Courneya KS, Mackey JR, Bell GJ, Jones LW, Field CJ, Fairey AS, Randomized controlled trial of exercise training in postmenopausal breast cancer survivors: cardiopulmonary and quality of life outcomes, J. Clin. Oncol. 21 (9) (2003) 1660–1668. [DOI] [PubMed] [Google Scholar]
- [103].Segar ML, Katch VL, Roth RS, et al. , The effect of aerobic exercise on self-esteem and depressive and anxiety symptoms among breast cancer survivors, Oncol. Nurs. Forum 25 (1) (1998) 107–113. [PubMed] [Google Scholar]
- [104].Fong DY, Ho JW, Hui BP, et al. , Physical activity for cancer survivors: meta-analysis of randomised controlled trials, BMJ. 344 (2012), e70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Hartman SJ, Weiner LS, Nelson SH, et al. , Mediators of a physical activity intervention on cognition in breast cancer survivors: evidence from a randomized controlled trial, JMIR Cancer. 5 (2) (2019), e13150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Mandelblatt JS, Stern RA, Luta G, et al. , Cognitive impairment in older patients with breast cancer before systemic therapy: is there an interaction between cancer and comorbidity? J. Clin. Oncol. 32 (18) (2014) 1909–1918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Merriman JD, Von Ah D, Miaskowski C, Aouizerat BE, Proposed mechanisms for cancer- and treatment-related cognitive, Semin. Oncol. Nurs. 29 (4) (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Yara S, Lavoie JC, Levy E, Oxidative stress and DNA methylation regulation in the metabolic syndrome, Epigenomics. 7 (2) (2015) 283–300. [DOI] [PubMed] [Google Scholar]
- [109].Delgado-Cruzata L, Zhang W, McDonald JA, et al. , Dietary modifications, weight loss, and changes in metabolic markers affect global DNA methylation in Hispanic, African American, and afro-Caribbean breast cancer survivors, J. Nutr. 145 (4) (2015) 783–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Arner P, Sinha I, Thorell A, Ryden M, Dahlman-Wright K, Dahlman I, The epigenetic signature of subcutaneous fat cells is linked to altered expression of genes implicated in lipid metabolism in obese women, Clin. Epigenetics 7 (2015) 93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Witlox L, Schagen SB, de Ruiter MB, et al. , Effect of physical exercise on cognitive function and brain measures after chemotherapy in patients with breast cancer (PAM study): protocol of a randomised controlled trial, BMJ Open 9 (6) (2019), e028117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Gentry AL, Erickson KI, Sereika SM, et al. , Protocol for exercise program in cancer and cognition (EPICC): a randomized controlled trial of the effects of aerobic exercise on cognitive function in postmenopausal women with breast cancer receiving aromatase inhibitor therapy, Contemp. Clin. Trials. 67 (2018) 109–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Pekmezi D, Martin MY, Kvale E, Meneses K, Demark-Wahnefried W, Enhancing exercise adherence for the breast cancer survivors, ACSMs Health Fit J 16 (4) (2012) 8–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Lewis BA, Napolitano MA, Buman MP, Williams DM, Nigg CR, Future directions in physical activity intervention research: expanding our focus to sedentary behaviors, technology, and dissemination, J. Behav. Med. 40 (1) (2016) 112–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Thomas JG, Bond DS, Review of innovations in digital health technology to promote weight control, Curr. Diabetes Rep. 14 (5) (2014) 485. [DOI] [PubMed] [Google Scholar]
- [116].Archer E, Groessl EJ, Sui X, et al. , An economic analysis of traditional and technology-based approaches to weight loss, Am. J. Prev. Med. 43 (2) (2012) 176–182. [DOI] [PubMed] [Google Scholar]
- [117].Jansen CE, Cooper BA, Dodd MJ, Miaskowski CA, A prospective longitudinal study of chemotherapy-induced cognitive changes in breast cancer patients, Support Care Cancer 19 (10) (2011) 1647–1656. [DOI] [PubMed] [Google Scholar]
- [118].Ahles TA, Saykin AJ, McDonald BC, et al. , Cognitive function in breast cancer patients prior to adjuvant treatment, Breast Cancer Res. Treat. 110 (1) (2008) 143–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Baumann FT, Drosselmeyer N, Leskaroski A, et al. , 12-week resistance training with breast cancer patients during chemotherapy: effects on cognitive abilities, Breast Care. 6 (2) (2011) 142–143. [Google Scholar]
- [120].Schmidt ME, Wiskemann J, Armbrust P, Schneeweiss A, Ulrich CM, Steindorf K, Effects of resistance exercise on fatigue and quality of life in breast cancer patients undergoing adjuvant chemotherapy: a randomized controlled trial, Int. J. Cancer 137 (2) (2015) 471–480. [DOI] [PubMed] [Google Scholar]
- [121].Liou KT, Ahles TA, Garland SN, et al. , The relationship between insomnia and cognitive impairment in breast cancer survivors, JNCI Cancer Spectr. 3 (3) (2019) (pkz041). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122].Carroll JE, Small BJ, Tometich DB, et al. , Sleep disturbance and neurocognitive outcomes in older patients with breast cancer: interaction with genotype, Cancer. 125 (24) (2019) 4516–4524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Coro D, Hutchinson A, Dahlenburg S, Banks S, Coates A, The relationship between diet and cognitive function in adult cancer survivors: a systematic review, J. Cancer Surviv. 13 (5) (2019) 773–791. [DOI] [PubMed] [Google Scholar]
- [124].Vardy J, Dhillon HM, Pond GR, et al. , Cognitive function and fatigue after diagnosis of colorectal cancer, Ann. Oncol. 25 (12) (2014) 2404–2412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].McGinty HL, Phillips KM, Jim HS, et al. , Cognitive functioning in men receiving androgen deprivation therapy for prostate cancer: a systematic review and meta-analysis, Support Care Cancer 22 (8) (2014) 2271–2280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].Enroth S, Enroth SB, Johansson Å, Gyllensten U, Protein profiling reveals consequences of lifestyle choices on predicted biological aging, Scientific Reports 511 (1) (2015), 17282, 10.1038/srep17282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [127].Ferrari L, Vicenzi M, Tarantini L, et al. , Effects of Physical Exercise on Endothelial Function and DNA Methylation, International Journal of Environmental Research and Public Health 16 (14) (2019), 2530, 10.3390/ijerph16142530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [128].Mason AE, Hecht FM, Daubenmier JJ, et al. , Weight Loss Maintenance and Cellular Aging in the Supporting Health Through Nutrition and Exercise Study, Psychosom Med 80 (7) (2018) 609–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [129].Putterman E, Weiss J, Lin J, et al. , Aerobic exercise lengthens telomeres and reduces stress in family caregivers: A randomized controlled trial - Curt Richter Award Paper 2018, Psychoneuroendocrinology 98 (2018) 245–252, 10.1016/j.psyneuen.2018.08.002. [DOI] [PubMed] [Google Scholar]