Abstract
Background:
Myelin loss is a central feature of several neurodegenerative diseases, including Alzheimer’s disease (AD). In animal studies, a link has been established between obesity and impairment of oligodendrocyte maturation, the cells that produce and maintain myelin. Although clinical magnetic resonance imaging (MRI) studies have revealed microstructural alterations of cerebral white matter tissue in subjects with obesity, no specific myelin vs. obesity correlation studies have been performed in humans using a direct myelin content metric.
Objectives:
To assess the association between obesity and myelin integrity in cerebral white matter using advanced MRI methodology for myelin content imaging.
Methods:
Studies were performed in the clinical unit of the National Institute on Aging on a cohort of 119 cognitively unimpaired adults. Using advanced MRI methodology, we measured whole-brain myelin water fraction (MWF), a marker of myelin content. Automated brain mapping algorithms and statistical models were used to evaluate the relationships between MWF and obesity, measured using the body mass index (BMI) or waist circumference (WC), in various white matter brain regions.
Results:
MWF was negatively associated with BMI or WC in all brain regions evaluated. These associations, adjusted for sex, ethnicity, and age, were statistically significant in most brain regions examined (p < 0.05), with higher BMI or WC corresponding to lower myelin content. Finally, in agreement with previous work, MWF exhibited a quadratic, inverted U-shaped, association with age; this is attributed to the process of myelination from youth through middle age, followed by demyelination afterward.
Conclusions:
These findings suggest that obesity was significantly associated with white matter integrity, and in particular myelin content. We expect that this work will lay the foundation for further investigations to clarify the nature of myelin damage in neurodegeneration, including AD, and the effect of lifestyle factors such as diet and physical activity on myelination.
1. INTRODUCTION
People suffering from obesity are at higher risk for a myriad of diseases and health conditions, including hypertension, type 2 diabetes, stroke, elevated cholesterol, and sleep apnea. Furthermore, epidemiological evidence has established a link between obesity and central nervous system (CNS) degeneration (1–3). This may reflect neuronal and synaptic degeneration secondary to obesity-induced metabolic disturbances; in turn, these may be contributors to the onset and progression of neurodegenerative diseases including Alzheimer’s and Parkinson’s diseases (1, 4–7). Moreover, growing evidence indicates that lifestyle factors such as diet and physical activity improve brain structure and function, suggesting novel therapeutic strategies against neurodegenerative disorders (8–10).
Microstructural changes in the gray and white matter of the brain secondary to obesity have been documented by conventional quantitative magnetic resonance imaging (MRI) (11–14). Using diffusion tensor imaging (DTI), magnetization transfer (MT), MR relaxation time mapping, and morphometry, it has been shown that body mass index (BMI) is negatively correlated with tissue integrity in several brain structures (11–13, 15, 16). However, as recognized by these authors, it is very difficult to interpret these findings in terms of underlying histopathologic changes. Indeed, while sensitive to changes in brain tissue microstructure, conventional MRI techniques are not specific; in addition to axonal degeneration and demyelination, these parameters may also reflect other tissue properties such as macromolecular content, flow, and fiber architecture. Similar comments apply to the non-specificity of MR spectroscopy studies of the correlation between obesity and myelination (17). Thus, to the best of our knowledge, the specific association between obesity and myelination remains to be established. Furthermore, these previous MRI investigations were conducted on cohorts of limited size and limited age range, both limiting the statistical power of the analysis and providing results that may not be reflective of a wide adult age range.
Several multicomponent MRI relaxometry methods have been introduced for myelin content mapping through measurement of the myelin water fraction (MWF) (18), including BMC-mcDESPOT (19–21) which provides rapid whole-brain MWF maps (19–22). BMC-mcDESPOT has been extensively used in several MWF-based studies to provide quantitative evidence of myelin loss in mild cognitive impairment and dementia (23), to investigate myelination patterns with normative aging (24–26), and to demonstrate an association between myelin content and cerebral blood flow (27).
Production and maintenance of myelin through oligodendrocyte metabolism is critical for saltatory conduction and normal axonal function. Growing evidence is establishing a direct relationship between myelin loss and a number of functional neurological disorders (18, 23, 28), suggesting that the breakdown of the myelin sheath could represent an important feature of early neurodegeneration, including mild cognitive impairment and Alzheimer’s disease (AD) (23, 29, 30). This notion is further supported by animal studies showing that a high fat diet can trigger myelin damage while obesity inhibits the maturation of the oligodendrocyte cells (9, 31, 32).
In this work, we examined the association between myelin content and obesity in 119 cognitively unimpaired subjects with healthy weight, overweight, or obesity spanning a wide age-range (22 to 94 years). Our main goal was to characterize the association between regional MWF, as a measure of myelin content, and BMI and waist circumference (WC), as measures of obesity, and to develop new insights into the specific effect of obesity on regional myelin integrity.
2. MATERIALS AND METHODS
2.1. Participants
One hundred thirty-one participants were recruited from the Genetic and Epigenetic Signatures of Translational Aging Laboratory Testing (GESTALT) study and the Baltimore Longitudinal Study of Aging (BLSA). Conducted by the National Institute on Aging, the goal of these studies is to evaluate multiple aging biomarkers. The criteria for exclusion and inclusion are identical for the GESTALT and BLSA studies. Participants were excluded if they had significant medical or neurologic conditions. Participants had undergone the Mini Mental State Examination (MMSE). Nine participants with cognitive impairment and three participants with MRI data corrupted by motion artifacts were excluded. The final cohort therefore consisted of 119 cognitively unimpaired volunteers (mean ± standard deviation MMSE = 28.7 ± 1.5) ranging in age from 22 to 94 years (56.3 ± 20.9 years) of whom 63 were men (58.1 ± 22 years) and 56 were women (54.4 ± 19.7 years). The number of participants per age-decade was: 13 (6 males) in the range of 20-29 years, 16 (10 males) within 30-39 years, 31 (13 males ) within 40-49 years, 8 (5 males) within 50-59 years, 9 (3 males) within 60-69 years, 17 (10 males) within 70-79 years, 22 (13 males) within 80-89 years, and 3 (3 males) within 90-99 years. Age did not differ significantly between men and women. Following established BMI cutoff points (33), the cohort consisted of 51 lean participants (BMI < 25), 52 overweight participants (25 ≤ BMI < 30), and 16 participants with obesity (BMI ≥ 30), while following the National Institutes of Health cutoff points for WC, the cohort consisted of 67 lean participants (WC < 94 cm for men and WC < 80 cm for women), 33 overweight participants (94 ≤ WC < 102 for men and 80 ≤ WC < 88 for women), and 19 participants with obesity (WC ≥ 102 for men and WC ≥ 88 for women). The cohort included 85 Caucasians participants, 26 African American participants, and 8 Asians or Pacific Islanders. Participants provided written informed consent and all experimental procedures were performed in compliance with our local Institutional Review Board.
2.2. Data acquisition
All MRI images were acquired on a 3T Philips MRI system (Achieva, Best, The Netherlands). We used the quadrature body coil for radiofrequency (RF) signal transmission and an eight-channel phased-array head coil for signal reception. Each participant was studied using the following BMC-mcDESPOT imaging protocol for MWF mapping:
Ten 3D spoiled gradient recalled echo (SPGR) images were obtained with flip angles (FAs) incremented linearly from 2° to 20°, with echo time (TE)/repetition time (TR) = 1.37/5 ms. The acquisition matrix for all images was 150 × 130 × 94, while the acquisition voxel size was 1.6 mm × 1.6 mm × 1.6 mm. The total acquisition time was ~5 min.
Ten 3D balanced steady state free precession (bSSFP) images were obtained with FAs of 2°, 7°, 11°, 16°, 24°, 32°, 40°, and 60°, with TE/TR = 2.8/5.8 ms. To account for the off-resonance effects (34), all bSSFP images were acquired with RF excitation phase increments of 0° or 180°. The acquisition matrix for all images was 150 × 130 × 94, while the acquisition voxel size was 1.6 mm × 1.6 mm × 1.6 mm. The total acquisition time was ~12 min
To correct for excitation RF inhomogeneity, we used the double-angle method (DAM). For this, two fast spin-echo images were acquired with FAs of 45° and 90°, TE = 102 ms, TR= 3000 ms, and acquisition voxel size of 2.6 mm × 2.6 mm × 4 mm. The total acquisition time was ~4 min.
All SPGR, bSSFP, and DAM images were acquired with field-of-view of 240 mm × 208 mm × 150 mm, and reconstructed to a voxel size of 1 mm × 1 mm × 1 mm.
2.3. Data processing
The scalp tissue was discarded using the FMRIB Software Library (FSL) with the SPGR image averaged over all 10 FAs used as the input image (35). Then, the SPGR, bSSFP, and DAM images were linearly registered to the averaged SPGR image. Next, a whole-brain MWF map was generated for the parenchymal regions using BMC-mcDESPOT from the registered SPGR, bSSFP, and DAM images (19–21, 23). Finally, the derived MWF map was nonlinearly registered to the Montreal Neurological Institute (MNI) standard space.
Twenty-one white matter regions of interest (ROIs) were defined from the MNI structural atlas corresponding to the whole brain (WB) white matter, frontal lobes (FL), parietal lobes (PL), temporal lobes (TL), occipital lobes (OL), cerebellum (CRB), body of corpus callosum (BCC), genu of corpus callosum (GCC), splenium of corpus callosum (SCC), internal capsules (IC), cerebral peduncle (CP), anterior corona radiata (ACR), posterior corona radiata (PCR), anterior thalamic radiation (ATR), posterior thalamic radiation (PTR), inferior fronto-occipital fasciculus (IFOF), superior fronto-occipital fasciculus (SFOF), inferior longitudinal fasciculus (ILF), superior longitudinal fasciculus (SLF), forceps minor (Fm), and forceps major (FM) (Fig. 1). ROIs were eroded to avoid partial volume contamination from adjacent structures. Within each ROI, the mean MWF was calculated.
Figure 1.
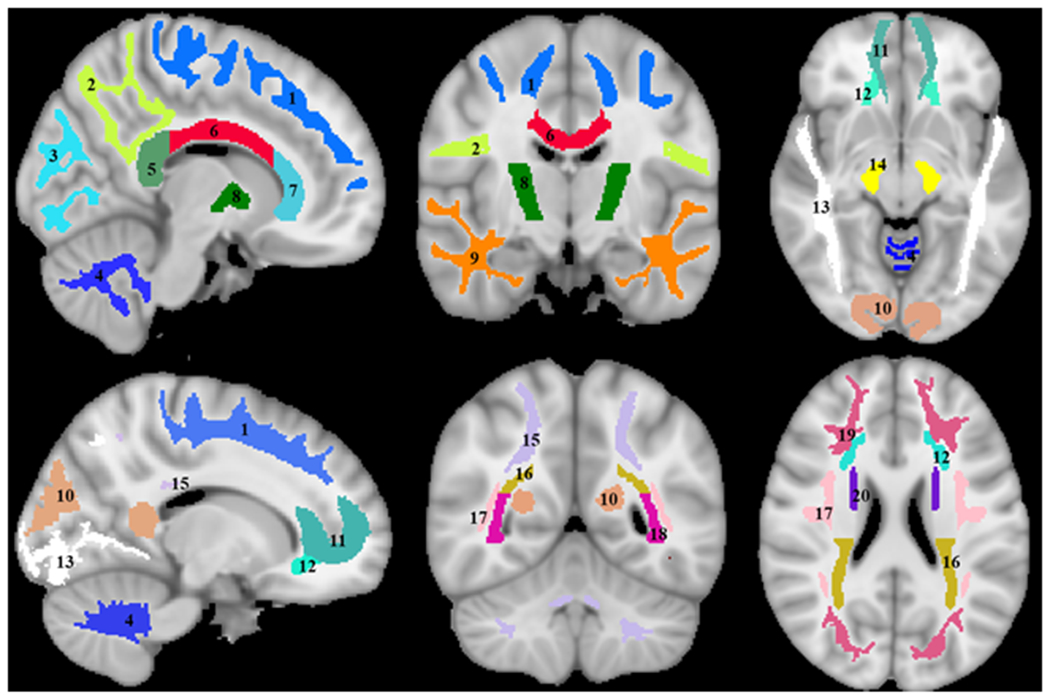
Visualization of the white matter ROIs investigated. 1) Frontal lobes, 2) Parietal lobes, 3) Occipital lobes, 4) Cerebellum, 5) Splenium of corpus callosum, 6) Body of corpus callosum, 7) Genu of corpus callosum, 8) Internal capsule, 9) Temporal lobes, 10) Forceps major, 11) Forceps minor, 12) Anterior corona radiata, 13) Inferior longitudinal fasciculus, 14) Cerebral peduncles, 15) Anterior thalamic radiation, 16) Posterior corona radiata, 17) Superior longitudinal fasciculus, 18) Posterior thalamic radiation, 19) Inferior fronto-occipital fasciculus, and 20) Superior fronto-occipital fasciculus.
2.4. Statistical analysis
For each ROI, the correlation between BMI or WC and MWF was investigated using multiple linear regression with the mean MWF within the ROI as the dependent variable and BMI or WC, sex, ethnicity, age, and age2 as independent variables, after mean age centering. The inclusion of age2 as an independent variable is based on our and others’ recent observations that myelination follows a quadratic relationship with age (25, 36).
To investigate the associations between BMI or WC and MWF between the groups of subjects with healthy weight (lean), overweight, and obesity, we performed between-group ANCOVA analyses for each ROI. These included i) obese vs. lean groups, ii) overweight vs. lean groups, and iii) obese vs. overweight groups. All between-group comparisons controlled for sex, ethnicity, age and age2.
The threshold for statistical significance was set to p < 0.05 after correction for multiple ROI comparisons using the false discovery rate (FDR) method for all statistical analyses. Calculations were performed with MATLAB (MathWorks, Natick, MA, USA). All MATLAB codes are available upon request from the corresponding author.
3. RESULTS
Figure 2 shows regression relationships between MWF and BMI, after adjusting for sex, ethnicity, age, and age2, for the indicated 21 cerebral white matter regions. Visual inspection indicates that larger BMI corresponds to lower MWF in all examined ROIs, with the best-fit lines displaying regional variation in this relationship. Further, the multiple regression analysis indicates that this negative correlation between MWF and BMI was statistically significant (pBMI < 0.05) or close to significance (pBMI < 0.1) in most brain regions investigated (Table 1). In addition, our results indicate that the steepest negative slopes in MWF versus BMI were found in the corona radiata and thalamic radiation regions, while the smallest slopes were found in the forceps minor and cerebral peduncle regions. Comparison of each of the steepest and smallest slopes indicated statistically significantly different slopes between the posterior corona radiata and the forceps minor or cerebral peduncle (p < 0.05; Z-test computed as the difference between the two slopes divided by the square root of the sum of the squared standard error of the slopes (37)). Furthermore, as expected, significant age effects were found for all brain regions evaluated (Table 1). Similarly, the quadratic effect of age, age2, was statistically significant or close to significance in most brain regions (Table 1).
Figure 2.
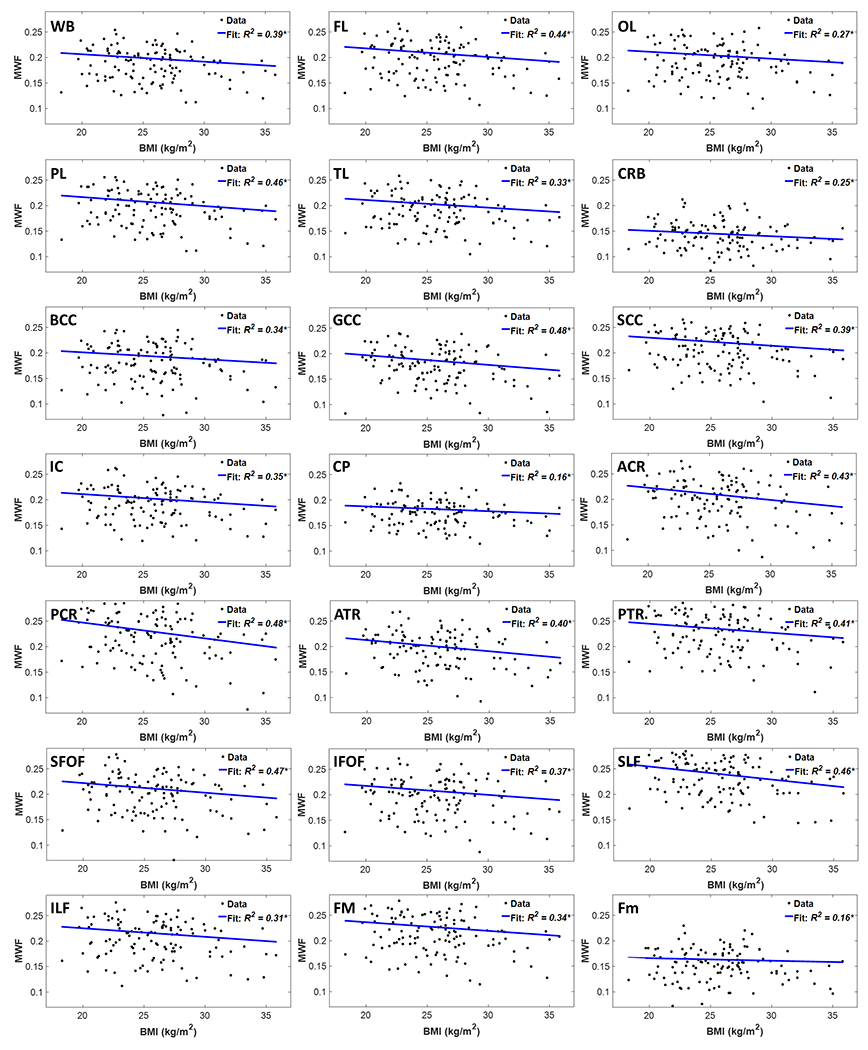
Regression results for the relationship between MWF and BMI adjusted for age, age2, sex, and ethnicity (N = 119). Results are shown for 21 brain structures/ROIs. For each ROI, the coefficient of determination, R2, of the multiple linear regression model is reported with the symbol * indicating significance at p < 0.01. All ROIs exhibited significant negative correlations between MWF and BMI. WB: whole brain white matter, FL: frontal lobes, PL: parietal lobes, TL: temporal lobes, OL: occipital lobes, CRB: cerebellum, BCC: body of corpus callosum, GCC: genu of corpus callosum, SCC: splenium of corpus callosum, IC: internal capsules, CP: cerebral peduncle, ACR: anterior corona radiata, PCR: posterior corona radiata, ATR: anterior thalamic radiation, PTR: posterior thalamic radiation, IFOF: inferior fronto-occipital fasciculus, SFOF: superior fronto-occipital fasciculus, ILF: inferior longitudinal fasciculus, SLF: superior longitudinal fasciculus, Fm: forceps minor, FM: forceps major.
Table 1.
Slope, β, and significance, p, of the regression terms incorporated in the multiple linear regression given by: MWF ~ β0 + βBMI × BMI + βage × age + βage2 ×age2 + βsex × sex + βethnicity × ethnicity. Sex and ethnicity results are not shown as they exhibited non-significant associations with MWF in all ROIs. All p-values presented are obtained after FDR correction.
| MWF | ||||||
|---|---|---|---|---|---|---|
| BMI | Age | Age2 | ||||
| βBMI (× 103) | pBMI | βage (× 104) | page | βage2 (× 105) | page2 | |
| WB | −1.46 | 0.053 | −7.19 | 0.000 | −2.06 | 0.012 |
| FL | −1.68 | 0.049 | −8.56 | 0.000 | −2.29 | 0.012 |
| OL | −1.37 | 0.099 | −5.73 | 0.000 | −2.29 | 0.019 |
| PL | −1.45 | 0.036 | −6.34 | 0.000 | −2.08 | 0.013 |
| TL | −1.75 | 0.029 | −8.68 | 0.000 | −2.16 | 0.012 |
| CRB | −1.06 | 0.108 | −4.52 | 0.000 | −1.11 | 0.104 |
| BCC | −1.36 | 0.100 | −7.53 | 0.000 | −1.79 | 0.038 |
| GCC | −1.90 | 0.019 | −9.51 | 0.000 | −1.55 | 0.034 |
| SCC | −1.58 | 0.036 | −6.92 | 0.000 | −2.51 | 0.012 |
| IC | −1.53 | 0.053 | −7.17 | 0.000 | −1.74 | 0.026 |
| CP | −0.93 | 0.137 | −3.15 | 0.000 | −0.91 | 0.156 |
| ACR | −2.38 | 0.017 | −10.30 | 0.000 | −1.93 | 0.030 |
| PCR | −3.09 | 0.005 | −11.15 | 0.000 | −2.59 | 0.012 |
| ATR | −2.18 | 0.017 | −8.82 | 0.000 | −1.49 | 0.056 |
| PTR | −1.76 | 0.050 | −9.60 | 0.000 | −2.10 | 0.021 |
| SFOF | −1.90 | 0.036 | −10.42 | 0.000 | −2.30 | 0.012 |
| IFOF | −1.76 | 0.053 | −9.10 | 0.000 | −2.10 | 0.024 |
| SLF | −2.54 | 0.005 | −8.55 | 0.000 | −2.11 | 0.012 |
| ILF | −1.68 | 0.005 | −7.57 | 0.000 | −2.17 | 0.023 |
| Fm | −1.70 | 0.055 | −7.25 | 0.000 | −2.39 | 0.012 |
| FM | −0.50 | 0.508 | −3.81 | 0.001 | −1.65 | 0.044 |
MWF: myelin water fraction, BMI: body mass index, WB: whole brain, FL: frontal lobes, PL: parietal lobes, TL: temporal lobes, OL: occipital lobes, CRB: cerebellum, BCC: body of corpus callosum, GCC: genu of corpus callosum, SCC: splenium of corpus callosum, IC: internal capsules, CP: cerebral peduncle, ACR: anterior corona radiata, PCR: posterior corona radiata, ATR: anterior thalamic radiation, PTR: posterior thalamic radiation, IFOF: inferior fronto-occipital fasciculus, SFOF: superior fronto-occipital fasciculus, ILF: inferior longitudinal fasciculus, SLF: superior longitudinal fasciculus, Fm: forceps minor, FM: forceps major.
Figure 3 shows regression relationships between MWF and WC, after adjusting for sex, ethnicity, age, and age2, for the indicated 21 cerebral white matter regions. Visual inspection indicates that larger WC corresponds to lower MWF in all examined ROIs, with the best-fit lines displaying regional variation. Further, the multiple regression analysis indicates that this negative correlation between MWF and WC was statistically significant (pWC < 0.05) or close to significance (pWC < 0.1) in most brain regions investigated (Table 2). In addition, our results indicate that the steepest negative slopes in MWF with WC were found in the corona radiata, longitudinal fasciculus, and thalamic radiation regions, while the smallest slopes were found in the forceps minor and cerebral peduncle regions. Comparison of each of the steepest and smallest slopes indicated statistically significantly different slopes between the posterior corona radiata or posterior longitudinal fasciculus and the forceps minor or cerebral peduncle. Furthermore, as expected, significant age effects were found for all brain regions evaluated (Table 2). Similarly, the quadratic effect of age, age2, was statistically significant in most brain regions (Table 2).
Figure 3.
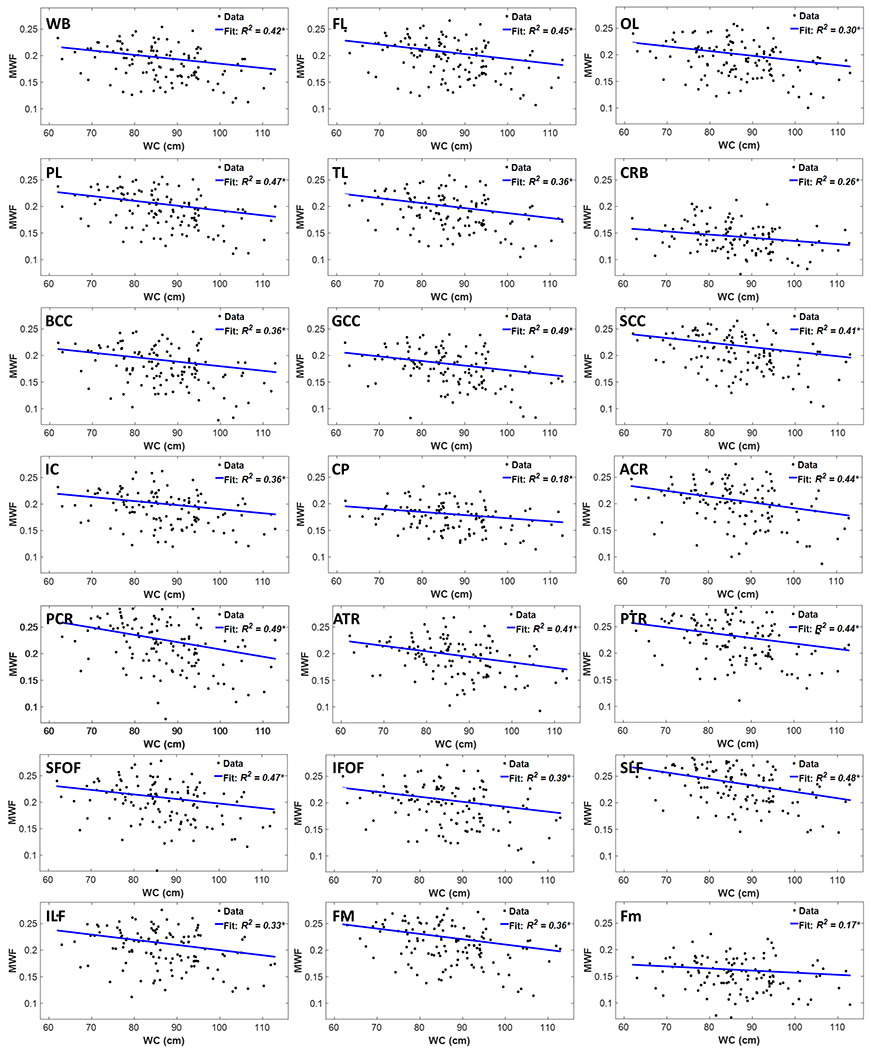
Regression results for the relationship between MWF and WC adjusted for age, age2, sex, and ethnicity (N = 119). Results are shown for 21 brain structures/ROIs. For each ROI, the coefficient of determination, R2, of the multiple linear regression model is reported with the symbol * indicating significance at p < 0.01. All ROIs exhibited significant negative correlations between MWF and WC. WB: whole brain white matter, FL: frontal lobes, PL: parietal lobes, TL: temporal lobes, OL: occipital lobes, CRB: cerebellum, BCC: body of corpus callosum, GCC: genu of corpus callosum, SCC: splenium of corpus callosum, IC: internal capsules, CP: cerebral peduncle, ACR: anterior corona radiata, PCR: posterior corona radiata, ATR: anterior thalamic radiation, PTR: posterior thalamic radiation, IFOF: inferior fronto-occipital fasciculus, SFOF: superior fronto-occipital fasciculus, ILF: inferior longitudinal fasciculus, SLF: superior longitudinal fasciculus, Fm: forceps minor, FM: forceps major.
Table 2.
Slope, β, and significance, p, of the regression terms incorporated in the multiple linear regression given by: MWF ~ β0 + βWC × WC + βage × age + βage2 ×age2 + βsex × sex + βethnicity × ethnicity. The results of sex and ethnicity are not shown as they exhibited non-significant associations with MWF in all ROIs. All p-values presented are obtained after FDR correction.
| MWF | ||||||
|---|---|---|---|---|---|---|
| WC | Age | Age2 | ||||
| βWC (× 104) | pWC | βage (× 104) | page | βage2 (× 105) | page2 | |
| WB | −8.28 | 0.005 | −6.23 | 0.000 | −2.30 | 0.003 |
| FL | −9.02 | 0.005 | −7.52 | 0.000 | −2.56 | 0.003 |
| OL | −8.83 | 0.008 | −4.67 | 0.000 | −2.56 | 0.008 |
| PL | −9.08 | 0.005 | −5.30 | 0.000 | −2.34 | 0.003 |
| TL | −9.36 | 0.005 | −7.57 | 0.000 | −2.44 | 0.008 |
| CRB | −5.98 | 0.026 | −3.83 | 0.000 | −1.29 | 0.058 |
| BCC | −8.51 | 0.011 | −6.52 | 0.000 | −2.05 | 0.016 |
| GCC | −8.69 | 0.005 | −8.55 | 0.000 | −1.79 | 0.014 |
| SCC | −8.57 | 0.007 | −5.93 | 0.000 | −2.76 | 0.003 |
| IC | −7.61 | 0.011 | −6.31 | 0.000 | −1.95 | 0.011 |
| CP | −5.89 | 0.023 | −2.45 | 0.000 | −1.10 | 0.089 |
| ACR | −10.90 | 0.005 | −9.10 | 0.000 | −2.23 | 0.011 |
| PCR | −13.66 | 0.002 | −9.65 | 0.000 | −2.96 | 0.003 |
| ATR | −10.35 | 0.005 | −7.67 | 0.000 | −1.78 | 0.023 |
| PTR | −10.16 | 0.005 | −8.41 | 0.000 | −2.40 | 0.006 |
| SFOF | −8.59 | 0.008 | −9.48 | 0.000 | −2.54 | 0.003 |
| IFOF | −9.34 | 0.008 | −8.02 | 0.000 | −2.38 | 0.009 |
| SLF | −12.08 | 0.000 | −7.20 | 0.000 | −2.44 | 0.003 |
| ILF | −9.71 | 0.007 | −6.43 | 0.000 | −2.30 | 0.008 |
| Fm | −10.01 | 0.005 | −6.072 | 0.000 | −2.56 | 0.003 |
| FM | −3.95 | 0.210 | −3.32 | 0.003 | −2.56 | 0.029 |
MWF: myelin water fraction, WC: waist circumference, WB: whole brain, FL: frontal lobes, PL: parietal lobes, TL: temporal lobes, OL: occipital lobes, CRB: cerebellum, BCC: body of corpus callosum, GCC: genu of corpus callosum, SCC: splenium of corpus callosum, IC: internal capsules, CP: cerebral peduncle, ACR: anterior corona radiata, PCR: posterior corona radiata, ATR: anterior thalamic radiation, PTR: posterior thalamic radiation, IFOF: inferior fronto-occipital fasciculus, SFOF: superior fronto-occipital fasciculus, ILF: inferior longitudinal fasciculus, SLF: superior longitudinal fasciculus, Fm: forceps minor, FM: forceps major.
We further note that with a null hypothesis of no relationship between MWF and BMI or WC, the probability of a given regression relationship exhibiting a slope greater than zero, Pr = 0.5, equals the probability of it exhibiting a slope less than zero. The probability then of all these 21 regional relationships exhibiting a negative slope, as we found (Fig. 2), is Pr = 0.521 < 10−6. Though not regionally specific, this provides further strong statistical support to the hypothesized inverse relationship between BMI or WC and myelin content.
Table 3 summarizes the results of the between-group ANCOVA analyses of the associations between BMI or WC and MWF. In comparing subjects with obesity to lean subjects, we found significantly lower MWF in the group of subjects with obesity in all ROIs despite controlling for age, age2, sex, and ethnicity. Moreover, controlling for these same covariates, while comparison of overweight to lean subjects as defined by BMI did not show statistically significant differences in MWF, we found significantly lower MWF in the overweight group as compared to the lean group as defined by WC, in various ROIs before or after FDR correction. Further, in comparing subjects with obesity to overweight subjects as defined by BMI, we found significantly lower MWF in the group of subjects with obesity in most ROI; however, this finding as restricted to only a few ROIs, before FDR correction, when the groups were defined by WC.
Table 3.
Significance, p-value, of the between-group ANCOVA analyses of the effect of BMI or WC on MWF in all ROIs. All between-group comparisons were controlled for age, age2, sex, and ethnicity. All p-values presented are obtained after FDR correction.
| Obese vs. Lean | Overweight vs. Lean | Obese vs. Overweight | ||||
|---|---|---|---|---|---|---|
| BMI | WC | BMI | WC | BMI | WC | |
| WB | 0.033 | 0.002 | 0.865 | 0.165* | 0.0156 | 0.045 |
| FL | 0.033 | 0.002 | 0.967 | 0.165* | 0.0150 | 0.040 |
| OL | 0.046 | 0.003 | 0.936 | 0.165 | 0.038 | 0.528 |
| PL | 0.030 | 0.000 | 0.927 | 0.100 | 0.0156 | 0.040 |
| TL | 0.033 | 0.002 | 0.754 | 0.166* | 0.145 | 0.043 |
| CRB | 0.063 | 0.004 | 0.608 | 0.131 | 0.238 | 0.726 |
| BCC | 0.045 | 0.002 | 0.979 | 0.180* | 0.086 | 0.528 |
| GCC | 0.030 | 0.006 | 0.795 | 0.165 | 0.038 | 0.400 |
| SCC | 0.030 | 0.002 | 0.763 | 0.147 | 0.015 | 0.528 |
| IC | 0.054 | 0.011 | 0.984 | 0.294 | 0.034 | 0.400* |
| CP | 0.126 | 0.016 | 0.722 | 0.180 | 0.213 | 0.522 |
| ACR | 0.030 | 0.003 | 0.706 | 0.215 | 0.038 | 0.528* |
| PCR | 0.003 | 0.000 | 0.288 | 0.012 | 0.015 | 0.528 |
| ATR | 0.046 | 0.008 | 0.538 | 0.165* | 0.102* | 0.462 |
| PTR | 0.030 | 0.002 | 0.712 | 0.037 | 0.038 | 0.528 |
| SFOF | 0.037 | 0.003 | 0.281 | 0.215 | 0.086 | 0.405 |
| IFOF | 0.064 | 0.007 | 0.735 | 0.355 | 0.039 | 0.400* |
| SLF | 0.003 | 0.000 | 0.312 | 0.091 | 0.015 | 0.401* |
| ILF | 0.050 | 0.004 | 0.722 | 0.309 | 0.015 | 0.401* |
| Fm | 0.033 | 0.002 | 0.827 | 0.031 | 0.038 | 0.533 |
| FM | 0.096 | 0.236 | 0.584* | 0.674 | 0.010 | 0.528 |
MWF: myelin water fraction, BMI: body mass index, WB: whole brain, FL: frontal lobes, PL: parietal lobes, TL: temporal lobes, OL: occipital lobes, CRB: cerebellum, BCC: body of corpus callosum, GCC: genu of corpus callosum, SCC: splenium of corpus callosum, IC: internal capsules, CP: cerebral peduncle, ACR: anterior corona radiata, PCR: posterior corona radiata, ATR: anterior thalamic radiation, PTR: posterior thalamic radiation, IFOF: inferior fronto-occipital fasciculus, SFOF: superior fronto-occipital fasciculus, ILF: inferior longitudinal fasciculus, SLF: superior longitudinal fasciculus, Fm: forceps minor, FM: forceps major.
indicates significance or close to significance before FDR correction (but lost significance after FDR correction).
4. DISCUSSION
Using advanced MR methodology for myelin content quantification, in this study, we provided the first demonstration of associations between obesity and MWF. We found a strong quantitative relationship between obesity, measured either by BMI or WC, and lower myelin content. These associations were observed in a large cohort of cognitively unimpaired subjects spanning a wide age range and were statistically significant in several critical brain regions, even after adjusting for age, ethnicity, and sex. These results do not prove, but strongly suggest, a causal link between obesity and white matter integrity, especially myelin content, also consistent with basic science and animal data (9, 11–13, 15–17, 31, 32).
We found that the brain regions investigated exhibit similar trends but different slopes for obesity, as measured by WC or BMI, vs. myelination (Fig. 2). Interestingly, these negative slopes were the largest in magnitude for the corona radiata, longitudinal fasciculus, and thalamic radiation structures (Tables 1–2). These fiber pathways carry most of the neural traffic to and from the cerebral cortex and have been shown to be susceptible to a number of pathologies, including leukoencephalopathy and multiple sclerosis (38, 39); these conditions are associated with deficits in intellectual, social, and emotional functioning (40, 41). Furthermore, epidemiological studies have showed that these regions are particularly prone to atrophy and microstructural changes with obesity (42–46); these structural changes could be partially explained by the lower myelin content observed here. Moreover, our results indicated that the anterior lobes, including the frontal and temporal lobes, exhibited more rapid decrease in MWF with age, BMI, or WC as compared to the other lobes (Tables 1–2). This pattern is consistent with the retrogenesis hypothesis, in which posterior brain regions are spared from degeneration as compared to anterior brain regions (47–50). However, longitudinal and histological investigations are required to elucidate the mechanisms underlying this vulnerability and concomitant rapid decline in structural integrity.
Our results indicated that overweight subjects have lower myelin content than lean subjects in various cerebral white matter structures (Table 3). The difference was generally lower in magnitude compared to the obese vs. normal results. Our results agree with Raji and colleagues’ observations of association between higher BMI and lower brain WM volumes in subjects with obesity and, to a lesser extent, in overweight subjects (42). Accelerated demyelination in WM structures observed here, and subsequent axonal loss, could explain these consistent observations of WM atrophy with obesity (42, 51–53). Interestingly, unlike BMI, WC was sensitive enough to capture differences in myelin content between overweight and lean subjects. Besides differences in the number of subjects per group due to the BMI or WC stratifications, the BMI is not a robust surrogate for body fat mass, while high BMI does not necessarily result in a higher mortality (54, 55). In contrast, WC provides higher predictive power of disease risk than does BMI (56, 57). Indeed, increased WC indicates increased susceptibility to insulin resistance, cancer, and dyslipidemia due to its strong association with visceral fat (33, 58, 59).
Our results indicate a quadratic association between myelin content and age in all white matter regions investigated (Tables 1–2), in agreement with previous studies (25, 36). This association is attributed to the process of myelination from young adulthood through middle age, followed by demyelination afterward (36, 60); this agrees with postmortem studies and with MRI studies based on myelin-sensitive methods such as relaxation times and diffusion tensor imaging.
Obesity may represent a modifiable risk factor for disruption of white matter integrity, and therefore an important therapeutic target. Our results indicate the possibility of a direct link between obesity and myelin breakdown, and therefore provides a foundation for further investigation of, for example, the effect of diet and physical activity on myelination and cognitive function.
Our work, while conducted on a large cohort, has several limitations. Our dataset is cross-sectional, and longitudinal and intervention studies will be required to establish a causal relationship between MWF and BMI or WC. Such work is underway. Moreover, errors may have arisen from imperfect registration and segmentation. Finally, MWF estimation could be biased by several experimental and physiological parameters that are not incorporated into the BMC-mcDESPOT formalism. These include, but not limited to, differential water diffusion in the underlying compartments, exchange between water pools, and iron content.
In conclusion, in this first study examining the association between obesity and cerebral myelin content in a large cohort and across a wide age range of cognitively normal subjects, we showed that lower myelin content is associated with obesity in most cerebral white matter structures.
5. AKNOWLEDGEMENTS
This work was supported by the Intramural Research Program of the National Institute on Aging of the National Institutes of Health. We gratefully acknowledge Christopher M. Bergeron, Denise Melvin, and Linda Zukley for their assistance with data acquisition, participant recruitment, and logistics.
Footnotes
6. COMPETING INTERESTS
The authors declare no competing financial interests.
6. REFERENCES
- 1.Mazon JN, de Mello AH, Ferreira GK, Rezin GT. The impact of obesity on neurodegenerative diseases. Life sciences. 2017;182:22–8. [DOI] [PubMed] [Google Scholar]
- 2.Stillman CM, Weinstein AM, Marsland AL, Gianaros PJ, Erickson KI. Body-Brain Connections: The Effects of Obesity and Behavioral Interventions on Neurocognitive Aging. Frontiers in aging neuroscience. 2017;9:115-. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jagust W, Harvey D, Mungas D, Haan M. Central Obesity and the Aging Brain. Archives of neurology. 2005;62(10):1545–8. [DOI] [PubMed] [Google Scholar]
- 4.Uranga RM, Keller JN. The Complex Interactions Between Obesity, Metabolism and the Brain. Frontiers in neuroscience. 2019;13:513-. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bhat ZF, Morton JD, Mason S, Bekhit AEA, Bhat HF. Obesity and neurological disorders: Dietary perspective of a global menace. Critical reviews in food science and nutrition. 2019;59(8):1294–310. [DOI] [PubMed] [Google Scholar]
- 6.Alford S, Patel D, Perakakis N, Mantzoros CS. Obesity as a risk factor for Alzheimer’s disease: weighing the evidence. Obesity reviews : an official journal of the International Association for the Study of Obesity. 2018;19(2):269–80. [DOI] [PubMed] [Google Scholar]
- 7.Chen H, Zhang SM, Schwarzschild MA, Hernan MA, Willett WC, Ascherio A. Obesity and the risk of Parkinson’s disease. American journal of epidemiology. 2004;159(6):547–55. [DOI] [PubMed] [Google Scholar]
- 8.Ashrafian H, Harling L, Darzi A, Athanasiou T. Neurodegenerative disease and obesity: what is the role of weight loss and bariatric interventions? Metabolic brain disease. 2013;28(3):341–53. [DOI] [PubMed] [Google Scholar]
- 9.Graham LC, Grabowska WA, Chun Y, Risacher SL, Philip VM, Saykin AJ, et al. Exercise prevents obesity-induced cognitive decline and white matter damage in mice. Neurobiology of aging. 2019;80:154–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Erickson KI, Weinstein AM, Lopez OL. Physical activity, brain plasticity, and Alzheimer’s disease. Arch Med Res. 2012;43(8):615–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kullmann S, Callaghan MF, Heni M, Weiskopf N, Scheffler K, Häring H-U, et al. Specific white matter tissue microstructure changes associated with obesity. NeuroImage. 2016;125:36–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mueller K, Anwander A, Möller HE, Horstmann A, Lepsien J, Busse F, et al. Sex-dependent influences of obesity on cerebral white matter investigated by diffusion-tensor imaging. PloS one. 2011;6(4):e18544–e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schall M, Iordanishvili E, Mauler J, Oros-Peusquens AM, Shah NJ. Increasing body mass index in an elderly cohort: Effects on the quantitative MR parameters of the brain. Journal of magnetic resonance imaging : JMRI. 2019. [DOI] [PubMed] [Google Scholar]
- 14.Birdsill AC, Oleson S, Kaur S, Pasha E, Ireton A, Tanaka H, et al. Abdominal obesity and white matter microstructure in midlife. Human brain mapping. 2017;38(7):3337–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xu J, Li Y, Lin H, Sinha R, Potenza MN. Body mass index correlates negatively with white matter integrity in the fornix and corpus callosum: a diffusion tensor imaging study. Human brain mapping. 2013;34(5):1044–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Raji CA, Ho AJ, Parikshak NN, Becker JT, Lopez OL, Kuller LH, et al. Brain structure and obesity. Human brain mapping. 2010;31(3):353–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gazdzinski S, Kornak J, Weiner MW, Meyerhoff DJ. Body mass index and magnetic resonance markers of brain integrity in adults. Annals of neurology. 2008;63(5):652–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.MacKay AL, Laule C. Magnetic Resonance of Myelin Water: An in vivo Marker for Myelin. Brain Plasticity. 2016;2(1):71–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bouhrara M, Spencer RG. Incorporation of nonzero echo times in the SPGR and bSSFP signal models used in mcDESPOT. Magnetic resonance in medicine. 2015;74(5):1227–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bouhrara M, Spencer RG. Improved determination of the myelin water fraction in human brain using magnetic resonance imaging through Bayesian analysis of mcDESPOT. NeuroImage. 2016;127:456–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bouhrara M, Spencer RG. Rapid simultaneous high-resolution mapping of myelin water fraction and relaxation times in human brain using BMC-mcDESPOT. NeuroImage. 2017;147:800–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bouhrara M, Reiter DA, Celik H, Fishbein KW, Kijowski R, Spencer RG. Analysis of mcDESPOT- and CPMG-derived parameter estimates for two-component nonexchanging systems. Magnetic resonance in medicine. 2016;75(6):2406–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bouhrara M, Reiter D, Bergeron C, Zukley L, Ferrucci L, Resnick S, et al. Evidence of demyelination in mild cognitive impairment and dementia using a direct and specific magnetic resonance imaging measure of myelin content. Alzheimer’s & Dementia. 2018;14(8):998–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bouhrara M, Cortina LE, Rejimon AC, Khattar N, Bergeron C, Bergeron J, et al. Quantitative age-dependent differences in human brainstem myelination assessed using high-resolution magnetic resonance mapping. NeuroImage. 2020;206:116307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bouhrara M, Rejimon AC, Cortina LE, Khattar N, Bergeron CM, Ferrucci L, et al. Adult brain aging investigated using BMC-mcDESPOT based myelin water fraction imaging. Neurobiology of aging. 2020;85:131–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Qian W, Khattar N, Cortina LE, Spencer RG, Bouhrara M. Nonlinear associations of neurite density and myelin content with age revealed using multicomponent diffusion and relaxometry magnetic resonance imaging. NeuroImage. 2020;223:117369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bouhrara M, Alisch J, Nikkita N, Kim R, Rejimon A, Cortina L, et al. Association of cerebral blood flow with myelin content in cognitively unimpaired adults BMJ Neurology Open. 2020;2:e000053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dean DC III, Sojkova J, Hurley S, Kecskemeti S, Okonkwo O, Bendlin BB, et al. Alterations of Myelin Content in Parkinson’s Disease: A Cross-Sectional Neuroimaging Study. PloS one. 2016;11(10):e0163774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bartzokis G Age-related myelin breakdown: a developmental model of cognitive decline and Alzheimer’s disease. Neurobiology of aging. 2004;25(1):5–18; author reply 49–62. [DOI] [PubMed] [Google Scholar]
- 30.Bartzokis G Alzheimer’s disease as homeostatic responses to age-related myelin breakdown. Neurobiology of aging. 2011;32(8):1341–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Huang H-T, Tsai S-F, Wu H-T, Huang H-Y, Hsieh H-H, Kuo Y-M, et al. Chronic exposure to high fat diet triggers myelin disruption and interleukin-33 upregulation in hypothalamus. BMC neuroscience. 2019;20(1):33-. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xiao G, Burguet J, Kawaguchi R, Havton LA, Hinman JD. Obesity restricts oligodendrocyte maturation and impedes repair after white matter stroke. bioRxiv. 2018:283184. [Google Scholar]
- 33.Janssen I, Katzmarzyk PT, Ross R. Body mass index, waist circumference, and health risk: evidence in support of current National Institutes of Health guidelines. Arch Intern Med. 2002;162(18):2074–9. [DOI] [PubMed] [Google Scholar]
- 34.Deoni SC. Correction of main and transmit magnetic field (B0 and B1) inhomogeneity effects in multicomponent-driven equilibrium single-pulse observation of T1 and T2. Magnetic resonance in medicine. 2011;65(4):1021–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jenkinson M, Beckmann CF, Behrens TE, Woolrich MW, Smith SM. FSL. NeuroImage 2012;62(2):782–90. [DOI] [PubMed] [Google Scholar]
- 36.Arshad M, Stanley JA, Raz N. Adult age differences in subcortical myelin content are consistent with protracted myelination and unrelated to diffusion tensor imaging indices. NeuroImage. 2016;143:26–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.PATERNOSTER R, BRAME R, MAZEROLLE P, PIQUERO A. USING THE CORRECT STATISTICAL TEST FOR THE EQUALITY OF REGRESSION COEFFICIENTS. Criminology. 1998;36(4):859–66. [Google Scholar]
- 38.Rocca MA, Preziosa P, Mesaros S, Pagani E, Dackovic J, Stosic-Opincal T, et al. Clinically Isolated Syndrome Suggestive of Multiple Sclerosis: Dynamic Patterns of Gray and White Matter Changes-A 2-year MR Imaging Study. Radiology. 2016;278(3):841–53. [DOI] [PubMed] [Google Scholar]
- 39.Sabin ND, Cheung YT, Reddick WE, Bhojwani D, Liu W, Glass JO, et al. The Impact of Persistent Leukoencephalopathy on Brain White Matter Microstructure in Long-Term Survivors of Acute Lymphoblastic Leukemia Treated with Chemotherapy Only. AJNR American journal of neuroradiology. 2018;39(10):1919–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Karababa IF, Bayazıt H, Kılıçaslan N, Celik M, Cece H, Karakas E, et al. Microstructural Changes of Anterior Corona Radiata in Bipolar Depression. Psychiatry Investig. 2015;12(3):367–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sanjuan PM, Thoma R, Claus ED, Mays N, Caprihan A. Reduced white matter integrity in the cingulum and anterior corona radiata in posttraumatic stress disorder in male combat veterans: a diffusion tensor imaging study. Psychiatry research. 2013;214(3):260–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Raji CA, Ho AJ, Parikshak NN, Becker JT, Lopez OL, Kuller LH, et al. Brain structure and obesity. Human brain mapping. 2010;31(3):353–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kullmann S, Schweizer F, Veit R, Fritsche A, Preissl H. Compromised white matter integrity in obesity. Obesity reviews : an official journal of the International Association for the Study of Obesity. 2015;16(4):273–81. [DOI] [PubMed] [Google Scholar]
- 44.Spangaro M, Mazza E, Poletti S, Cavallaro R, Benedetti F. Obesity influences white matter integrity in schizophrenia. Psychoneuroendocrinology. 2018;97:135–42. [DOI] [PubMed] [Google Scholar]
- 45.Alarcon G, Ray S, Nagel BJ. Lower Working Memory Performance in Overweight and Obese Adolescents Is Mediated by White Matter Microstructure. Journal of the International Neuropsychological Society : JINS. 2016;22(3):281–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Olivo G, Latini F, Wiemerslage L, Larsson E-M, Schiöth HB. Disruption of Accumbens and Thalamic White Matter Connectivity Revealed by Diffusion Tensor Tractography in Young Men with Genetic Risk for Obesity. Frontiers in human neuroscience. 2018;12:75-. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Stricker NH, Schweinsburg BC, Delano-Wood L, Wierenga CE, Bangen KJ, Haaland KY, et al. Decreased white matter integrity in late-myelinating fiber pathways in Alzheimer’s disease supports retrogenesis. NeuroImage. 2009;45(1):10–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Brickman AM, Meier IB, Korgaonkar MS, Provenzano FA, Grieve SM, Siedlecki KL, et al. Testing the white matter retrogenesis hypothesis of cognitive aging. Neurobiology of aging. 2012;33(8):1699–715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Raz N Aging of the brain and its impact on cognitive performance: Integration of structural and functional findings. The handbook of aging and cognition, 2nd ed. Mahwah, NJ, US: Lawrence Erlbaum Associates Publishers; 2000. p. 1–90. [Google Scholar]
- 50.Bender AR, Völkle MC, Raz N. Differential aging of cerebral white matter in middle-aged and older adults: A seven-year follow-up. NeuroImage. 2016;125:74–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Repple J, Opel N, Meinert S, Redlich R, Hahn T, Winter NR, et al. Elevated body-mass index is associated with reduced white matter integrity in two large independent cohorts. Psychoneuroendocrinology. 2018;91:179–85. [DOI] [PubMed] [Google Scholar]
- 52.Karlsson HK, Tuulari JJ, Hirvonen J, Lepomaki V, Parkkola R, Hiltunen J, et al. Obesity is associated with white matter atrophy: a combined diffusion tensor imaging and voxel-based morphometric study. Obesity (Silver Spring). 2013;21(12):2530–7. [DOI] [PubMed] [Google Scholar]
- 53.Dekkers IA, Jansen PR, Lamb HJ. Obesity, Brain Volume, and White Matter Microstructure at MRI: A Cross-sectional UK Biobank Study. Radiology. 2019;291(3):763–71. [DOI] [PubMed] [Google Scholar]
- 54.Flegal KM, Kit BK, Orpana H, Graubard BI. Association of all-cause mortality with overweight and obesity using standard body mass index categories: a systematic review and meta-analysis. Jama. 2013;309(1):71–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Winter JE, MacInnis RJ, Wattanapenpaiboon N, Nowson CA. BMI and all-cause mortality in older adults: a meta-analysis. The American journal of clinical nutrition. 2014;99(4):875–90. [DOI] [PubMed] [Google Scholar]
- 56.Staiano AE, Reeder BA, Elliott S, Joffres MR, Pahwa P, Kirkland SA, et al. Body mass index versus waist circumference as predictors of mortality in Canadian adults. Int J Obes (Lond). 2012;36(11):1450–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Janssen I, Katzmarzyk PT, Ross R. Waist circumference and not body mass index explains obesity-related health risk. The American journal of clinical nutrition. 2004;79(3):379–84. [DOI] [PubMed] [Google Scholar]
- 58.Kuk JL, Katzmarzyk PT, Nichaman MZ, Church TS, Blair SN, Ross R. Visceral fat is an independent predictor of all-cause mortality in men. Obesity (Silver Spring). 2006;14(2):336–41. [DOI] [PubMed] [Google Scholar]
- 59.Calle EE, Kaaks R. Overweight, obesity and cancer: epidemiological evidence and proposed mechanisms. Nature Reviews Cancer. 2004;4(8):579–91. [DOI] [PubMed] [Google Scholar]
- 60.Bartzokis G, Lu PH, Tingus K, Mendez MF, Richard A, Peters DG, et al. Lifespan trajectory of myelin integrity and maximum motor speed. Neurobiology of aging. 2010;31(9):1554–62. [DOI] [PMC free article] [PubMed] [Google Scholar]


