Abstract
Reactivation of Human Endogenous Retrovirus K (HERV-K), subtype HML-2, has been associated with pathophysiology of amyotrophic lateral sclerosis (ALS). We aimed to assess the efficacy of antiretroviral therapy in inhibiting HML-2 in patients with ALS and a possible association between the change in HML-2 levels and clinical outcomes. We studied the effect of 24-weeks antiretroviral combination therapy with abacavir, lamivudine, and dolutegravir on HML-2 levels in 29 ALS patients. HML-2 levels decreased progressively over 24 weeks (P=0.001) and rebounded within a week of stopping medications (P=0.02). The majority of participants (82%), defined as “responders”, experienced a decrease in HML-2 at week 24 of treatment compared to the pre-treatment levels. Differences in the evolution of some of the clinical outcomes could be seen between responders and non-responders: FVC decreased 23.69% (SE=11.34) in non-responders and 12.71% (SE=8.28) in responders. NPI score decreased 91.95% (SE=6.32) in non-responders and 53.05% (SE=10.06) in responders (P =0.01). Thus, participants with a virological response to treatment showed a trend for slower progression of the illness. These findings further support the possible involvement of HML-2 in the clinical course of the disease.
Keywords: Amyotrophic lateral sclerosis, ALS, HERV-K, HML-2, Endogenous retrovirus, Antiretroviral
1. INTRODUCTION
Human endogenous retroviruses (HERVs) and, in particular HERV-K subtype HML-2 are thought to play a role in the pathophysiology of amyotrophic lateral sclerosis (ALS)1. Reverse transcriptase activity has been detected in the blood and cerebrospinal fluid of ALS patients2–6.
Increased retrotransposon expression has been found in 20% of ALS patients, using transcriptome stratification7. Although two groups did not find a difference between HML-2 expression in ALS patients and controls 8, 9, we found that levels of HML-2 polymerase transcripts and protein are increased in the brain of ALS patients and there is a specific pattern of expression of HML-2 polymerase loci that includes a locus exclusively expressed in ALS patients10, 11. Moreover, HML-2 envelope causes neurotoxicity in transfected neuronal cultures and triggers an ALS-like syndrome in transgenic mice12. Recently, a conotoxin-like protein (CTXLP) has been described to be encoded by HML-2. It is expressed in the motor cortex of ALS patients and it is linked with inflammatory pathways and necroptosis13. Increased levels of antibodies against HML-2 have been found in ALS patients and they inversely correlate with clinical measures of disease severity14.
Several antiretroviral drugs approved for treatment of HIV infection are also effective against HML-2 in vitro15. The Lighthouse study was an open-label safety study conducted in Australia using an antiretroviral combination therapy with abacavir, lamivudine, and dolutegravir (Triumeq; ViiV Healthcare. North Carolina) in ALS patients16. Preliminary observations suggested a decrease in HML-2 levels with the treatment. We now present a comprehensive study of HML-2 levels in samples from this cohort of participants that were obtained at multiple timepoints. With this study we intend to assess the efficacy of antiretroviral therapy in inhibiting Human Endogenous Retrovirus K (HERV-K), subtype HML-2, in patients with amyotrophic lateral sclerosis (ALS) and to assess a possible association between the change in HML-2 levels and clinical outcomes.
2. METHODS
2.1. Study design
The Lighthouse study was an open-label phase 2a trial conducted in patients with ALS at four centers across Sydney and Melbourne, Australia. In this study, patients were observed during a 10-week lead-in period before receiving antiretroviral combination therapy with abacavir, lamivudine, and dolutegravir for 24 weeks16. Patients gave informed consent and the ethics committees at each site approved the protocol. The study was registered on clinicaltrials.gov (NCT02868580) and was conducted in accordance with Good Clinical Practice (GCP) and the Declaration of Helsinki (2000).
2.2. Patient selection criteria
Patients were aged 18–75 years and were diagnosed with El Escorial criteria17 for possible, probable, or definite diagnosis of ALS. Diagnosis was within 24 months of screening; all had no family history of ALS and FVC of at least 60% predicted. All patients provided written informed consent. For complete selection criteria see Gold et al16.
2.3. Clinical samples
Serum samples from ALS patients participating in the Lighthouse study16 were shipped from three participating centers and analyzed at the National Institute of Neurological Disorders and Stroke (USA) for HML-2 levels. Demographic characteristics and baseline clinical data are described in Table 1.
Table 1.
Demographics and clinical characteristics at baseline of the patients analyzed.*
| Total (n = 29) | Responders (n = 18) | Non-responders (n = 4) | |
|---|---|---|---|
| Age | 55 (10.97) | 53.65 (11.68) | 55.16 (9.35) |
| Gender | |||
| Males | 18 | 10 | 2 |
| Females | 11 | 8 | 2 |
| Disease duration (days) | 325 (230) | 302.4 (219) | 281 (207) |
| Weight (SD) | 80.25 (16.99) | 76.95 (18.9) | 89.55 (13.12) |
| ALSFRS-R total score | 39.51 (5.31) | 40.06 (5.12) | 40.00 (8.7) |
| % Predicted FVC | 80.46 (16.00) | 76.50 (24.37) | 80.50 (14.31) |
| NPI | 1.69 (1.49) | 2.05 (1.58) | 0.87 (0.45) |
None of the parameters were statistically different between Responders and Non-responders (P > 0.5).
2.4. Outcomes
Outcomes included HML-2 levels, neurophysiological index (NPI), ALS Functional Rating Scale Revised (ALSFRS-R) and forced vital capacity (FVC). HML-2 levels for the pre-treatment period were averaged from the screening visit (week 0, lead-in period) and baseline visit (week 10, lead-in period). Following initiation of treatment, HML-2 levels were measured at weeks 4, 8, 16, 20 and 24. Another sample was analyzed in the post-treatment period (week 25). We defined responders as those showing a reduction in HML-2 levels on treatment when pre-treatment levels were compared to week 24. Conversely, non-responders showed either no change or an increase in HML-2 at week 24 when compared to pretreatment levels.
2.5. Analysis of HML-2 levels in serum
Investigators who performed the laboratory analysis of HML-2 levels were blinded to all sample collection details including the participant identification and the timepoint when the sample was collected. The methodology for HML-2 detection in serum was performed as previously published16 and it is based on studies showing that HML-2 has genome flexibility, as viral particles can contain both DNA and RNA18–20. Briefly, total nucleic acids were extracted from 400 μl of serum with an EZ1 Advance XL device (Qiagen) and the EZ1 Virus Mini Kit v2.0 (Qiagen), following manufacturer’s instructions. The droplet digital PCR (ddPCR) reaction was set in an AutoDG Droplet Digital PCR System (Bio-Rad) in duplicate with two sets of primers and probe: one designed to detect HML-2 env (forward primer: 5’ ATTTGGTGCCAGGAACTGAG 3’; reverse primer: 5’ GCTGTCTCTTCGGAGCTGTT 3’ and probe 5’ 6-FAM-AGGAGTTGCTGATGGCCTCG-Iowa Black FQ 3’) and another designed to detect genomic copies of the single copy gene RPP30. RPP30 copy number was used as a measure of cellular DNA in the serum sample. The master mix was composed of 12.5 μl of ddPCR Supermix (no dUTP) (Bio-Rad), 1.25 μl of an HML-2 assay including primers and probe (Bio-Rad), 1.25 μl of RPP30 assay (Bio-Rad), 2.5 μl of cDNA and 7.5 μl of RNAse-free water. The PCR was performed with the following cycling conditions: 95°C for 10 minutes, 40 cycles at 95°C for 30 seconds followed by 60°C for one minute, and 95°C for 10 minutes. To determine the absolute number of copies per reaction, plates were analyzed in a QX200 Digital PCR reader (Bio-Rad). Results were expressed as a ratio of number of copies of HML-2 to the number of copies of RPP30 but is hereby termed, HML-2 ratio. Sensitivity of the HML-2 assay is 2 copies per reaction and the linear range is between 10000 copies/ul and 10 copies/μl.
2.6. Statistical analysis
Cohort characteristics were described overall and by responder group. Differences between responder groups in these characteristics were tested using a Student’s t-test for continuous variables and a chi-square test for categorical variables. To analyze the effect of the drug on the HML-2 levels, the average of the pre-treatment visits was calculated (screening and baseline) and the difference between each treatment visit and the average pre-treatment HML-2 level was calculated with a linear mixed effects model with a Sidak’s multiple comparisons post-hoc test. Patients who experienced a decrease in the HML-2 ratio at week 24 compared to the baseline value (average of screening and baseline visits) were classified as “responders” and patients with no change or increase in HML-2 ratio over that same time period were classified as “non-responders”. Percent change in clinical scores from baseline (average screening and baseline visits) to week 24 were also calculated for each participant and summarized by responder group, and t-tests were used to find differences between groups. Analyses were performed using R (version 4.0.0) and GraphPad Prism 8. Statistical significance was set at P <0.05.
2.7. Standard protocol approvals, registrations, and patient consents
This study was approved by the ethics committee of each participating center. General consent was given by all participant patients. The Lighthouse study was registered on clinicaltrials.gov (NCT02868580) and was conducted in accordance with Good Clinical Practice (GCP) and the Declaration of Helsinki (2000).
2.8. Data availability
Anonymized data will be shared at the request of any qualified investigator.
3. RESULTS
3.1. Study participants
Serum samples of ALS patients enrolled in the Lighthouse trial were analyzed retrospectively for HML-2 levels. Samples from 29 patients were received. Twenty-two patients had both pre-treatment and week 24 (end of treatment) samples (Figure 1) and were classified according to their virological response to treatment. Eighteen patients were considered responders as defined by a drop in HML-2 levels at week 24 compared to pre-treatment levels. The remaining four patients were non-responders. Baseline and demographic characteristics of the study participants are listed in Table 1.
Fig. 1.
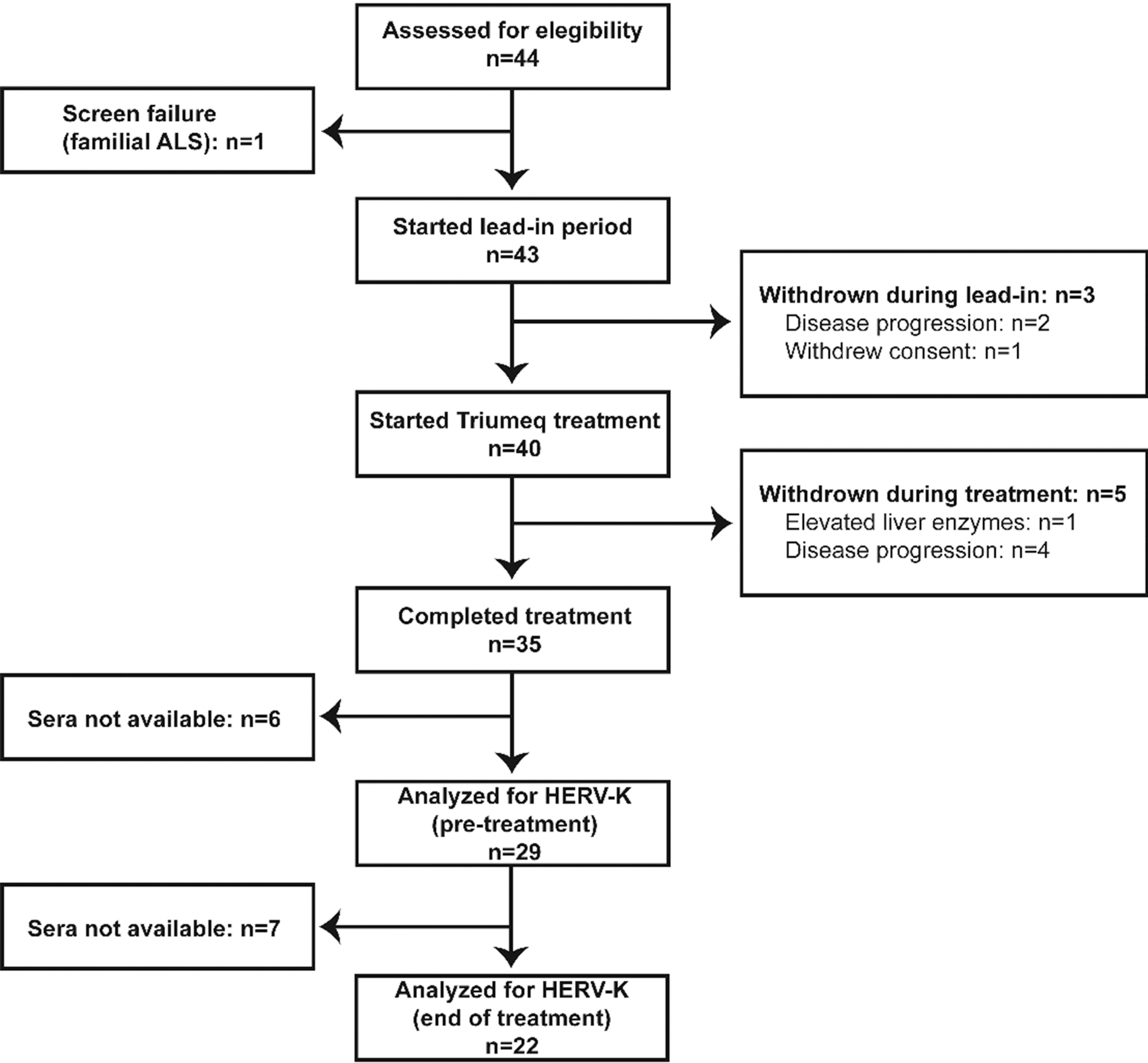
Flow diagram of participant enrollment in the Lighthouse trial and HML-2 analysis.
3.2. Antiretroviral therapy decreased HML-2 levels
Compared to the average HML-2 ratio of the pre-treatment visits (15.29, SE=1.39) (n=29), the HML-2 ratio was significatively lower at week 4 (11.84, SE=1.33; P = 0.008) (n=26), at week 8 (12.10, SE=1.27; P = 0.02) (n=24), and decreased further at week 24 of treatment (9.20, SE=1.12; P = 0.001) (n=22) (Figure 2A). Interestingly, the HML-2 ratio measured at the end of study visit, which was conducted only one week after the discontinuation of the drug, was higher than at week 24 (last visit under treatment) (15.72, SE=2.87; P = 0.02). This rebound resulted in values of HML-2 that were similar to those prior to initiation of treatment (Figure 2A). The majority of patients (82%) experienced a decrease in HML-2 levels at week 24 of treatment compared to the pre-treatment levels and were considered responders; in only 4 patients (18%), HML-2 levels did not change or increased at week 24 compared to the pre-treatment levels (non-responders) (Figure 2B).
Figure 2: Antiretroviral combination therapy decreases HML-2 levels, which correlates with a deceleration in clinical progression.
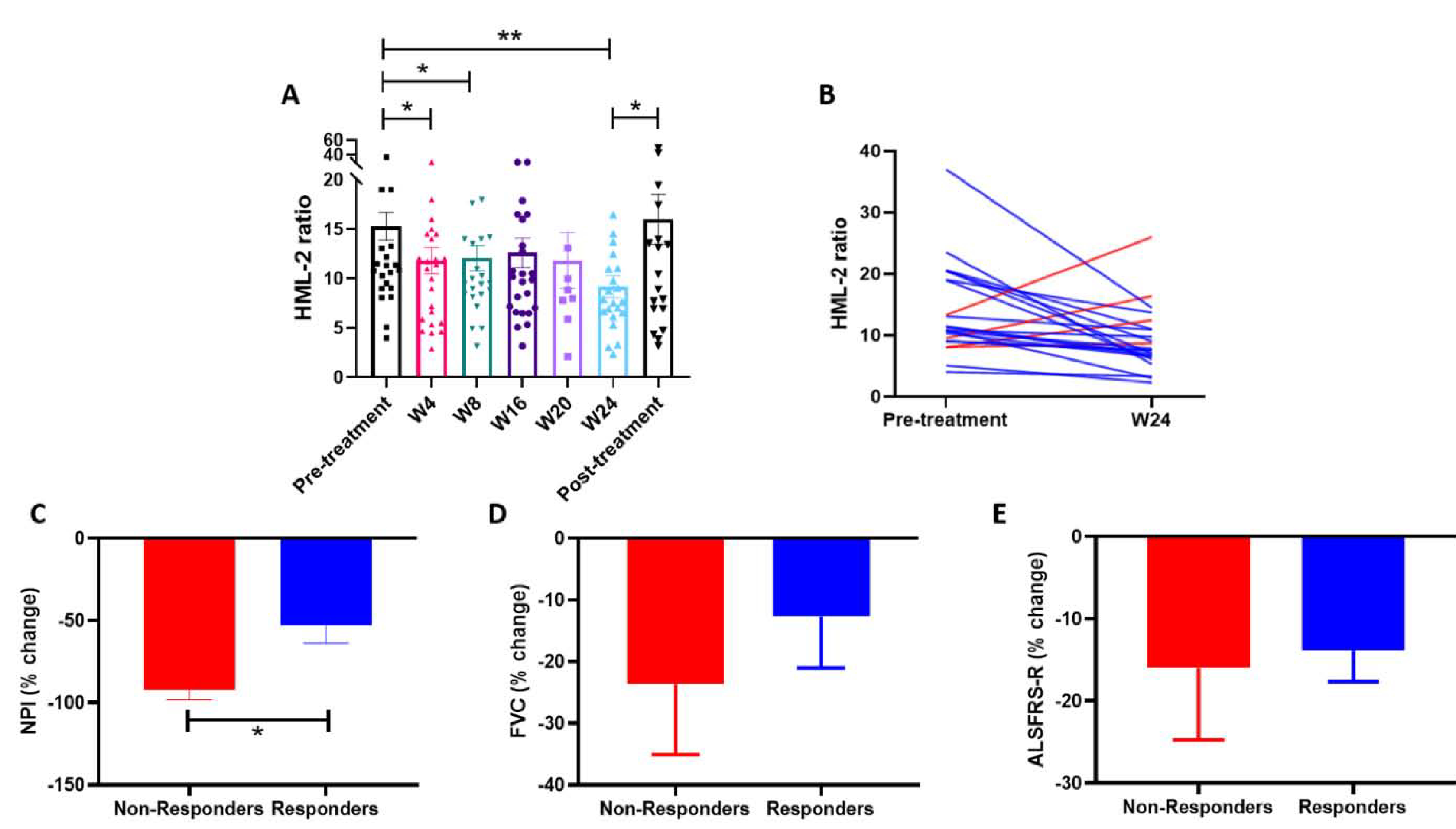
A) Mean HML-2 ratio as measured by ddPCR at baseline/screening (Pre-treatment) (n=29), at week 4 (n=26), week 8 (n=24), week 16 (n=26), week 20 (n=9) and week 24 of treatment (n=22), and one week after stopping treatment (Post-treatment) (n=23). B) HML-2 ratio at baseline/screening and at week 24. Blue lines represent responders and red lines represent non-responders. C, D and E) Comparison of the percent change in clinical scores from pre-treatment (mean baseline/screening) to week 24 between responders and non-responders. C) Percent change in NPI in responders and non-responders (*P=0.01). D) Percent change in FVC (% predicted) in responders and non-responders. E) Percent change in ALSFRS-R in responders and non-responders.
3.3. HML-2 levels and clinical progression
There were no statistically significant correlations between the change in the three clinical outcomes and the change in HML-2 levels, although for the three variables correlation coefficients were negative, i.e. the greater the decrease in HML-2 level, the slower clinical progression. For the change in ALSFRS-R the correlation coefficient was −0.28 (95% CI: −0.6307062 – 0.1554349) (Spearman test; P=0.2), for the change in FVC the correlation coefficient was −0.16 (95% CI: −0.547414 – 0.277168) (Spearman test; P=0.47) and for NPI, it was −0.17 (95% CI: −0.5994514 – 0.3411155) (Spearman test; P=0.52).
When the participants were divided according to their virological response to treatment, the NPI score showed a significantly slower rate of progression in those who had a decrease in HML-2 ratio (responders) compared to the non-responders. Non-responders had a −91.95% (SE=6.32) decline in NPI at week 24 of treatment compared to the pre-treatment period (average screening and baseline visits), while responders had a −53.05% (SE=10.63) decline (P =0.01; T-test) (Figure 2C). Although not statistically significant, a slightly more favorable evolution in the FVC could also be seen in those patients that experienced a reduction in HML-2: non-responders had a −23.69 % (SE=11.34) decline in FVC, while responders had a −12.71% (SE=8.28) decline (P=0.56) (Figure 2D). ALSFRS-R declined −15.95% (SE=8.79) in non-responders and 13.82% (SE=3.84) in responders at the end of the treatment compared to the pre-treatment period (Figure 2E).
4. DISCUSSION
Our results show that a combination antiretroviral therapy regimen of abacavir, dolutegravir, and lamivudine is associated with a decrease in HERV-K, subtype HML-2, levels and the decrease is sustained during treatment. The rapid increase of the HML-2 levels observed upon discontinuation of treatment support the direct effect of the drug on HML-2.
Previously, in vitro studies had shown that antiretroviral drugs originally designed to treat HIV infection are effective against HML-2. Triumeq is a combination drug of two nucleoside analogue reverse transcriptase inhibitors (NRTIs), lamivudine and abacavir, and the integrase inhibitor dolutegravir. Both lamivudine and abacavir have been shown to inhibit HML-2 in vitro15, 21. While most drugs are not as potent against HML-2 as they are against HIV, abacavir is one exception which is more potent against HML-215. Both lamivudine22 and abacavir23 have good penetration across the blood brain barrier. Although the effect of dolutegravir has not been studied against HML-2 in vitro, other integrase inhibitors, such as raltegravir, have shown efficacy against this endogenous retrovirus15. In a previous study indinavir, which is a HIV-protease inhibitor, was tested in a double-blind placebo-controlled trial24. Four of 23 participants receiving indinavir developed nephrolithiasis, and only 12 patients in the treatment group completed the 9-month trial. In the intention-to-treat analysis, there was a faster rate of decline in the ALSFRS-R score in the treatment group compared to the placebo group. In another study, zidovudine, which is an NRTI, was used to treat 12 patients with ALS. Only interim results were published with patients completing between 2–10 months of therapy. The authors did not report a significant change in the disease course with zidovudine since 5/12 patients died at the time of publication. They noted that zidovudine was well tolerated among the patients and that serum CK levels decreased during treatment although no other outcome measures were reported25. Interferon-beta, which is a treatment for multiple sclerosis and has antiviral activity26–28, has been also used to treat ALS patients in a randomized placebo-controlled study. No significant differences were found between the patients treated with interferon-beta 1a (n=31) for six months and the control group (n=30) for any of the measures of disease progression and disability29.
We observed a progressive decline in HML-2 levels over a period of 24 weeks of treatment. Due to the absence of an untreated control group, it could represent the natural history of the illness. However, the rebound of the virus after discontinuation of the drugs, provides strong evidence for a direct antiviral effect. To further determine if there might be an effect of HML-2 inhibition on clinical progression of ALS, we analyzed the differences between those patients that experienced a decrease in HML-2 levels due to the treatment and those that did not. It is interesting that the best response was noted for the NPI score, in the patients with a decrease in HML-2 during the treatment. NPI is a composite score of the electrical activity of the motor nerves in the extremities. Hence, one explanation might be that this specific antiretroviral combination therapy is more effective in the lower motor neurons. It is important to highlight that in most patients HML-2 levels were responsive to the drug. Due to this almost optimal general response, the number of individuals in the “non-responders” group is limited.
In a previous study, we treated five patients with antiretroviral drugs who had HIV infection and presented with an ALS-like syndrome. Two patients who received treatment within six months of onset of symptoms had complete recovery and the others had slow progression. These patients received different combinations of antiretroviral drugs, including lamivudine, abacavir and dolutegravir and had a decrease in HML-2 levels following the treatment30. However, it was unclear if the drugs had a direct effect on HML-2 since HIV-Tat protein is known to transactivate HML-2 and thus the effects of the drugs could have been mediated by its effects on HIV.
Our study has several limitations. It was an open label study with a small sample size that was designed as a safety study. Samples from all patients were not available for analysis. In the Lighthouse study concentrations of neurofilament light and phosphorylated heavy chain in serum remained stable during the lead-in and the treatment period16 thus, the study might have benefited from the inclusion of other prognosis biomarkers of ALS, such as levels of total tau and phosphotau in CSF31. Other validated outcome measures, such as the mean change in handheld dynamometry megascore32, could have been also included. For future studies, it would be important to select patients with higher HML-2 levels and try to achieve better control with antiretroviral therapy. Our laboratory is currently performing a study to measure blood levels of HML-2 in controls and in ALS patients. The results would be useful in identifying patients with abnormally higher levels of HML-2 for future studies.
5. CONCLUSIONS
This study is the first clear demonstration of an antiviral effect of combination antiretroviral therapy abacavir, dolutegravir and lamivudine on HML-2 in vivo. A drop in HML-2 and a rebound after drug discontinuation was seen in the majority of the study participants. The improved clinical response observed in those patients where the drug showed an antiviral effect against HML-2 further supports the possible involvement of this endogenous retrovirus in the pathogenesis of the disease. Future placebo-controlled studies with increased sample size, more biomarkers, additional outcome measures and with other CNS penetrating antiretroviral drugs or drugs specifically designed against HML-2 are necessary to determine if this approach can have a greater impact on the progression of the illness.
Highlights.
Antiretroviral combination therapy with abacavir, lamivudine, and dolutegravir for 24 weeks decreased HERV-K (HML-2) levels in most patients with ALS.
A rebound in HML-2 levels occurred after discontinuation of the antiretroviral drugs.
Participants that had an antiviral effect against HML-2 showed a trend for slower progression in several clinical parameters.
6. ACKNOWLEDGEMENTS
We would like to thank all the patients who participated in the study. This research was supported by the Intramural Research Program of the National Institute of Neurological Disorders and Stroke at NIH (NS 003130) and the ALS Association (20-SI-559). This is in part an EU Joint Programme - Neurodegenerative Disease Research (JPND) project. The project is supported through the following funding organizations under the aegis of JPND - www.jpnd.eu (United Kingdom, Medical Research Council (MR/L501529/1; MR/R024804/1)) and through the Motor Neuron Disease Association. This study represents independent research part funded by the National Institute for Health Research (NIHR) Biomedical Research Centre at South London and Maudsley NHS Foundation Trust and King’s College London. The Lighthouse trial in Australia was supported by FightMND and the MND Research Institute of Australia.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DISCLOSURES
Authors declare no conflict of interests.
REFERENCES
- 1.Garcia-Montojo M, Doucet-O’Hare T, Henderson L, Nath A. Human endogenous retrovirus-K (HML-2): a comprehensive review. Crit Rev Microbiol 2018;44:715–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McCormick AL, Brown RH Jr., Cudkowicz ME, Al-Chalabi A Garson JA. Quantification of reverse transcriptase in ALS and elimination of a novel retroviral candidate. Neurology 2008;70:278–283. [DOI] [PubMed] [Google Scholar]
- 3.Steele AJ, Al-Chalabi A, Ferrante K, Cudkowicz ME, Brown RH Jr., Garson JA. Detection of serum reverse transcriptase activity in patients with ALS and unaffected blood relatives. Neurology 2005;64:454–458. [DOI] [PubMed] [Google Scholar]
- 4.Viola MV, Frazier M, White L, Brody J, Spiegelman S. RNA-instructed DNA polymerase activity in a cytoplasmic particulate fraction in brains from Guamanian patients. J Exp Med 1975;142:483–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Andrews WD, Tuke PW, Al-Chalabi A, et al. Detection of reverse transcriptase activity in the serum of patients with motor neurone disease. J Med Virol 2000;61:527–532. [DOI] [PubMed] [Google Scholar]
- 6.MacGowan DJ, Scelsa SN, Imperato TE, Liu KN, Baron P, Polsky B. A controlled study of reverse transcriptase in serum and CSF of HIV-negative patients with ALS. Neurology 2007;68:1944–1946. [DOI] [PubMed] [Google Scholar]
- 7.Tam OH, Rozhkov NV, Shaw R, et al. Postmortem Cortex Samples Identify Distinct Molecular Subtypes of ALS: Retrotransposon Activation, Oxidative Stress, and Activated Glia. Cell Rep 2019;29:1164–1177 e1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mayer J, Harz C, Sanchez L, et al. Transcriptional profiling of HERV-K(HML-2) in amyotrophic lateral sclerosis and potential implications for expression of HML-2 proteins. Mol Neurodegener 2018;13:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Garson JA, Usher L, Al-Chalabi A, Huggett J, Day EF, McCormick AL. Quantitative analysis of human endogenous retrovirus-K transcripts in postmortem premotor cortex fails to confirm elevated expression of HERV-K RNA in amyotrophic lateral sclerosis. Acta Neuropathol Commun 2019;7:45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Douville R, Liu J, Rothstein J, Nath A. Identification of active loci of a human endogenous retrovirus in neurons of patients with amyotrophic lateral sclerosis. Ann Neurol 2011;69:141–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Garcia-Montojo M, Li W, Nath A. Technical considerations in detection of HERV-K in amyotrophic lateral sclerosis: selection of controls and the perils of qPCR. Acta Neuropathol Commun 2019;7:101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li W, Lee MH, Henderson L, et al. Human endogenous retrovirus-K contributes to motor neuron disease. Science Translational Medicine 2015;7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Curzio DD, Gurm M, Turnbull M, et al. Pro-Inflammatory Signaling Upregulates a Neurotoxic Conotoxin-Like Protein Encrypted Within Human Endogenous Retrovirus-K. Cells 2020;9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Arru G, Mameli G, Deiana GA, et al. Humoral immunity response to human endogenous retroviruses K/W differentiates between amyotrophic lateral sclerosis and other neurological diseases. Eur J Neurol 2018;25:1076–e1084. [DOI] [PubMed] [Google Scholar]
- 15.Tyagi R, Li W, Parades D, Bianchet MA, Nath A. Inhibition of human endogenous retrovirus-K by antiretroviral drugs. Retrovirology 2017;14:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gold J, Rowe DB, Kiernan MC, et al. Safety and tolerability of Triumeq in amyotrophic lateral sclerosis: the Lighthouse trial. Amyotroph Lateral Scler Frontotemporal Degener 2019;20:595–604. [DOI] [PubMed] [Google Scholar]
- 17.Ludolph A, Drory V, Hardiman O, et al. A revision of the El Escorial criteria - 2015. Amyotroph Lateral Scler Frontotemporal Degener 2015;16:291–292. [DOI] [PubMed] [Google Scholar]
- 18.Laderoute MP, Larocque LJ, Giulivi A, Diaz-Mitoma F. Further Evidence that Human Endogenous Retrovirus K102 is a Replication Competent Foamy Virus that may Antagonize HIV-1 Replication. Open AIDS J 2015;9:112–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Laderoute MP, Giulivi A, Larocque L, et al. The replicative activity of human endogenous retrovirus K102 (HERV-K102) with HIV viremia. AIDS 2007;21:2417–2424. [DOI] [PubMed] [Google Scholar]
- 20.Dube D, Contreras-Galindo R, He S, et al. Genomic flexibility of human endogenous retrovirus type K. J Virol 2014;88:9673–9682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Contreras-Galindo RA, Dube D, Fujinaga K, Kaplan MH, Markovitz DM. Susceptibility of Human Endogenous Retrovirus Type-K to Reverse Transcriptase Inhibitors. J Virol 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gibbs JE, Rashid T, Thomas SA. Effect of transport inhibitors and additional anti-HIV drugs on the movement of lamivudine (3TC) across the guinea pig brain barriers. J Pharmacol Exp Ther 2003;306:1035–1041. [DOI] [PubMed] [Google Scholar]
- 23.Capparelli EV, Letendre SL, Ellis RJ, Patel P, Holland D, McCutchan JA. Population pharmacokinetics of abacavir in plasma and cerebrospinal fluid. Antimicrob Agents Chemother 2005;49:2504–2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Scelsa SN, MacGowan DJ, Mitsumoto H, et al. A pilot, double-blind, placebo-controlled trial of indinavir in patients with ALS. Neurology 2005;64:1298–1300. [DOI] [PubMed] [Google Scholar]
- 25.Westarp ME, Bartmann P, Rossler J, et al. Antiretroviral therapy in sporadic adult amyotrophic lateral sclerosis. Neuroreport 1993;4:819–822. [DOI] [PubMed] [Google Scholar]
- 26.Garcia-Montojo M, De Las Heras V, Bartolome M, Arroyo R, Alvarez-Lafuente R. Interferon beta treatment: bioavailability and antiviral activity in multiple sclerosis patients. J Neurovirol 2007;13:504–512. [DOI] [PubMed] [Google Scholar]
- 27.Dominguez-Mozo MI, Garcia-Montojo M, De Las Heras V, et al. MHC2TA mRNA levels and human herpesvirus 6 in multiple sclerosis patients treated with interferon beta along two-year follow-up. BMC Neurol 2012;12:107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gerlach N, Schimmer S, Weiss S, Kalinke U, Dittmer U. Effects of type I interferons on friend retrovirus infection. J Virol 2006;80:3438–3444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Beghi E, Chio A, Inghilleri M, et al. A randomized controlled trial of recombinant interferon beta-1a in ALS. Italian Amyotrophic Lateral Sclerosis Study Group. Neurology 2000;54:469–474. [DOI] [PubMed] [Google Scholar]
- 30.Bowen LN, Tyagi R, Li W, et al. HIV-associated motor neuron disease: HERV-K activation and response to antiretroviral therapy. Neurology 2016;87:1756–1762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lanznaster D, Hergesheimer RC, Bakkouche SE, et al. A beta 1–42 and Tau as Potential Biomarkers for Diagnosis and Prognosis of Amyotrophic Lateral Sclerosis. Int J Mol Sci 2020;21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Miller T, Cudkowicz M, Shaw PJ, et al. Phase 1–2 Trial of Antisense Oligonucleotide Tofersen for SOD1 ALS. N Engl J Med 2020;383:109–119. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Anonymized data will be shared at the request of any qualified investigator.


