Abstract
Glioma stem cells (GSCs) are thought to underlie glioma initiation, evolution, resistance to therapies, and relapse. They are defined by their capacity to initiate glioma in immunocompromised mice which precludes analysis of their interaction with immune cells. Macrophages dominate the immune cell composition in glioma. We hypothesized that stemness and immune evasion induced by macrophages are closed intertwined in glioma. By using mass cytometry and RNA sequencing, we reveal that in immunocompetent mice, FGL2 promotes the stem-like phenotypes of glioma cells in an expression level–dependent manner. Mechanistically, FGL2-producing glioma cells recruit macrophages into the tumor microenvironment and induce the macrophages to secrete CXCL7 via the CD16/SyK/PI3K/HIF1α pathways. CXCL7, in turn, enhances the stem-like functionality of glioma cells, resulting in an increase in tumor incidence and progression that can be blocked with a neutralizing anti-CXCL7 antibody. Clinically, the FGL2-CXCL7 paracrine loop positively correlated with a higher macrophage signature and poorer prognosis in glioma patients. Thus, glioma cells’ stem-like functionality is regulated by FGL2 in the presence of macrophages, and the FGL2-CXCL7 paracrine signaling axis is critical for regulating this function.
Keywords: FGL2, macrophages, gliomagenesis, CXCL7, CD16
Graphical Abstract

1. Introduction
Glioma stem cells (GSCs), which express stem or precursor cell markers, are key contributors to the development and relapse of primary gliomas and are associated with enhanced resistance to drugs, radiation, and cell stress [1]. Recent single-cell RNA sequencing (RNA-seq) analysis revealed that the growth of cancer depended more on the GSC hierarchy than on genomic heterogeneity and GSCs can be targeted therapeutically [2]. The GSC hierarchy can be bidirectional, such that differentiated cells can 'revert back' to stem-cell-like cells. Cancer stem cells are reported to be regulated by 6 main mechanisms, which include intrinsic factors such as genetics, epigenetics, and metabolism and extrinsic factors such as niche characteristics, the cellular microenvironment, and the host immune system [3]. Single-cell RNA-seq analysis of colon cancers of different clinical grades found no intrinsic differences between clonogenic and non-clonogenic cells, suggesting that the environment rather than the cell’s intrinsic characteristics determines clonogenic capacity and that all malignant cells can function as cancer stem cells [4]. However, in current studies, glioma cells are typically implanted into immunedeficient mice, and limiting dilution assays are used to determine the frequency of GSCs. Such artificial assays test stem-cell potential rather than the stem-like functionality that drives tumor development and progression because the tumor microenvironment in immunodeficient mice is dramatically different from the original tumor niche. Extremely immunodeficient models, which lack immune effector cells, can support tumor growth from most tumor cells, even those that did not contribute to tumor growth in the original niche in patients [5]. Therefore, the effect of the immune system on stem-like functionality and how these effects are mediated remain largely unknown.
Fibrinogen-like protein 2 (FGL2) has been shown to function as a promoter of glioblastoma progression and of the transition from low-grade astrocytoma to high-grade glioblastoma by augmenting immunosuppression [6–8]. Here, we investigated whether and how FGL2 impacts stem-like functionality and the associated cellular and molecular mechanisms. We discovered that glioma cell-secreted FGL2 augments stem-like functionality in an expression level-dependent manner in the presence of an immune response. We identified a subset of macrophages that express CXCL7 after FGL2 treatment and determined that these wired macrophages enhance the stem-like functionality of glioma cells. We also identified the FGL2-CXCL7 paracrine signaling axis as a critical molecular link between glioma-supportive glioma-associated macrophages (GAMs) and stem-like functionality to facilitate malignant development and glioma progression. Our study determined that FGL2 secreted by glioma cells recruits and wires GAMs to support the stem-like functionality of glioma cells and that CXCL7 is a potential target for glioma therapy.
2. Materials and methods
2.1. Animals
C57BL/6J (Cat. No. 000664) and NSG (NOD.Cg-Prkdcscid Il2rgtm1Wjl/SzJ; Cat. No. 005557) mice were obtained from The Jackson Laboratory (Bar Harbor, ME, USA), and Scid (C.B-Igh-1b/IcrTac-Prkdcscid; Cat No. CB17SC) mice from Taconic Biosciences (Rensselaer, NY, USA). FGL2-/- mice were a gift from Dr. Gary Levy (Toronto General Hospital/Research Institute, Toronto, ON, Canada). All mice were aged 6 to 8 weeks when the experimental procedures began. All mice were maintained and treated in accordance with guidelines approved by The University of Texas MD Anderson Cancer Center’s Institutional Animal Care and Use Committee.
2.2. Cells
GL261 mouse glioma cells were obtained from the National Cancer Institute (Rockville, MD, USA). LLC (mouse Lewis lung carcinoma), U-87 MG, RAW264.7, and THP-1 cells were from ATCC (Manassas, VA, USA). The DBT cells (mouse astrocytoma cells) were kindly provided by Dr. Leonid Metelitsa (Baylor College of Medicine, Houston, TX, USA). U-87 MG and RAW264.7 cells were cultured in Dulbecco’s modified Eagle medium (DMEM)/F12, and THP-1 cells were cultured in RPMI 1640 medium supplemented with 10% fetal bovine serum (FBS) (Biochrom, Holliston, MA, USA) and penicillin/streptomycin. FGL2 knockout (FGL2KO) tumor cell lines (GL261-FGL2KO, DBT-FGL2KO, and LLC-FGL2KO) [8] and FGL2-overexpressing (OE) cell lines (GL261-FGL2OE, DBT-FGL2OE, and LLC-FGL2OE) [6] were constructed as described previously. GL261 cells were stained with an anti-FGL2 antibody (homemade by the MD Anderson Monoclonal Antibody Core Facility) and sorted by flow cytometry to get single clones of cells within the top 5% and bottom 5% of FGL2 expression levels. FGL2 expression was confirmed in single-clone cells by Western blotting, and cells were designated as FGL2 KO or low-, mid-, or high-expressing cells. GL261-FGL2low cells were transfected with a lentivirus containing a control pCDH-mRFP vector from System Biosciences (SBI, Mountain View, CA, USA) or mCXCL7 cloned on a pLV-mcherry vector by VectorBuilder, Inc. (Chicago, IL, USA). Cells were spun down at 400×g for 45 minutes with 8 μg/mL polybrene (MilliporeSigma, Burlington, MA, USA) in DMEM in a 24-well plate. Transfected cells were used for in vivo experiments 3 days after transfection. All tumor cells were cultured as described previously [8] [6] and treated with a mycoplasma removal agent (BUF035, BIO-RAD, Hercules, CA, USA) before experiments.
For proliferation assay, 1 × 103 FGL2hi, FGL2mid, FGL2low, and FGL2KO GL261 cells were seeded into a 96-well flat bottom plate and grown in adherent culture. The cell proliferation rates were monitored by IncuCyte® S3 Live-Cell Analysis System. Please refer to Supplementary materials and methods for the detailed information for THP1 and sphere culture.
2.3. Mouse model systems
For the orthotopic glioblastoma mouse models, FGL2hi (GL261-FGL2OE), FGL2mid, FGL2low, and FGL2KO (GL261-FGL2KO) cells were collected while in the logarithmic growth phase, washed twice with phosphate-buffered saline (PBS) solution, and mixed with an equal volume of 3% methylcellulose in PBS. Cells (100, 500, 2000, 1 × 104, and 5 × 104 in a total volume of 5 μL) were injected intracerebrally into wild-type mice, and 2000 FGL2hi or Fgl2KO cells were injected into NSG or Scid mice [6]. For co-implantation experiment, 2000 GL261 glioma cells were mixed with 2× 104 macrophages and then implanted intracerebrally into wild-type mice. For CXCL7-neutralizing experiments, 50 μg anti-CXCL7 or IgG antibodies were injected intracerebrally at day 7 post FGL2hi cells implantation. The mice were observed daily. When the mice showed signs of neurological compromise, they were humanely killed with CO2. Please refer to Supplementary materials and methods for the detailed information for Mass cytometry (CyTOF) assay and Flow Cytometry analysis of brain- infiltrating leukocytes.
2.4. In vitro limiting dilution assays
For in vitro limiting dilution assays, glioma cells were plated in 96-well plates at 5, 10, 20, or 40 cells per well, with 48 replicates for each cell number, and were maintained in serum-free medium supplemented with ITGX (1:100), 20 ng/mL epidermal growth factor, 10 ng/mL basic fibroblast growth factor, 4 ng/mL heparin, and 1% bovine serum albumin (BSA) (stem cell culture medium) at 37 °C. Tumorsphere sizes and sphere numbers were assessed using an Incucyte S3 live-cell analysis system (Essen BioScience, Inc., Ann Arbor, MI, USA). The presence of tumorspheres in each well was determined after 21 days of maintenance.
2.5. Migration assays
4 × 105 THP-1 cells were stimulated with 20 μM PMA (phorbol 12-myristate 13-acetate) for 2 days in the upper inserts of 24-well Transwell plates to become monocyte-derived macrophages as described previously [9]. The medium was then replaced with fresh RPMI 1640 with 0.1% BSA, and the THP-1 cellsin the upper chambers were allowed to migrate for 24 h before fixation and crystal purple staining. The migration assay was performed by adding to the bottom well 600 μL of CM from U-87 cells supplemented with 10 μg/mL of mouse IgG, anti-FGL2, rat IgG, anti-CD16a, or anti-CD32 (Extended Data Table 1). For murine macrophages, 4 × 105 RAW264.7 cells were seeded in the upper chambers of the Transwell plates and allowed to migrate for 24 h before fixation and crystal purple staining. Conditioned medium from GL261-FGL2hi and GL261-FGL2KO cells, supplemented with 10 μg/mL mIgG, anti-FGL2, rIgG, or anti-CD16/32 (Extended Data Table 1), was added to the bottom well to attract RAW264.7 cells. For tumor-cell migration assays, BMDMs were cultured with CM at 4 × 105 cells/well in the bottom of transwell plates, and tumor cells were cultured at 4 × 104 cells/well in the upper inserts. Some bottom wells were supplied with 10 μg/mL rIgG or anti-CXCL7 for 1 h before coculturing. Tumor cells were allowed to migrate for 24 h before migrated cells were detected.
2.6. Bone marrow-derived macrophage culture
Femurs and tibiae from C57BL/6 mice were harvested under sterile conditions, and bone marrow (BM) was collected by flushing. Red blood cells were removed by lysis. BM cells (5 × 105/mL) were cultured in RPMI 1640 medium supplemented with 10% heat-inactivated FBS (Biochrom), penicillin/streptomycin, 50 μM ß-mercaptoethanol, and 20 ng/mL macrophage colony-stimulating factor for 3 days. On day 3, equal volumes of prewarmed fresh medium and conditioned medium (CM) were added, and cultures continued for another 3 days. CM was generated by culturing FGL2KO or FGL2hi (FGL2OE) tumor cells in serum-free medium for 48 h. In some experiments, BM cells were pretreated with 10 μg/mL F1, F2, F3, F4, F5, anti-CD16/32, anti-CCL2, or anti-CXCL7 antibodies, or 100 μM nor-NOHA (Nω-Hydroxy-nor-L-arginine) for 1 h in culture with RPMI 1640 medium. Please refer to Supplementary materials and methods for the detailed information for sphere-formation assays in the presence of BMDMs and THP-1 macrophage culture.
2.7. RNA sequencing
Total RNA from CM-wired BM-derived macrophages (BMDMs) was extracted by using TRIzol according to the manufacturer’s instructions. The mRNA was enriched by oligo (dT) magnetic beads, or the total RNA was depleted of rRNAs with an Arraystar rRNA Removal Kit (Arraystar, Rockville, MD, USA). A KAPA Stranded RNA-Seq Library Prep Kit (Illumina, San Diego, CA, USA) was used for RNA-seq library preparation. The completed libraries were qualified with an Agilent 2100 Bioanalyzer (Agilent, Santa Clara, CA, USA) and quantified by the absolute quantification real-time polymerase chain reaction (qPCR) method. The sequencing was performed using an Illumina NovaSeq 6000 sequencing system. RNA-seq yielded 20 million to 35 million read pairs for each sample. RNA-seq reads were mapped to the genome assembly GRCm38.p6 of Mus musculus by using TopHat2 v. 2.1.1 [10], and then counted per gene by using HTSeq v. 0.11.0 based on GENCODE M19 gene annotations. Differential expression was analyzed using DESeq2 v. 1.24.0, in which raw read counts were input for calculating log fold changes and P values for the Wald test. Gene expression values were normalized by quantile normalization methods. The significantly altered RNAs were identified by ANOVA and the Tukey post hoc test.
2.8. The Cancer Genome Atlas data analysis
The Cancer Genome Atlas mRNA expression data were accessed via the cBioPortal for Cancer Genomics web site (http://www.cbioportal.org/public-portal/). The provisional mRNA expression z-scores (microarray) for genes (e.g., FGL2, PPBP) with matched outcome data (e.g., overall survival [OS], disease-free survival [DFS]) were used. On the basis of a literature review, we created a glioma-associated macrophage (GAM) signature gene set; the genes used to generate the gene signature are listed in Extended Data Table 3 [11]. Hierarchical clustering analysis for mRNA expression of this GAM gene signature was performed using Pearson’s distance metric to identify the distribution pattern of the GAM gene signature. Three different patient groups, GAMlow, GAMmid, and GAMhigh, were identified based on the topology of the clustering dendrogram, as illustrated in the heatmap plots. The hierarchical clustering analysis and heatmap plots were conducted and generated using the packages “ComplexHeatmap” and “stats” for the R environment [12]. FGL2 gene expression levels were compared among the GAM gene signature groups using t-tests, and P values were adjusted using the Bonferroni multiple testing correction. Kaplan-Meier survival analysis and the log-rank test were used to compare OS and DFS among different GAM gene signature groups. The statistical analyses were conducted using the R package (R Development Core Team, Version 3.6.1.2). All tests were 2-sided, and a P value of less than 0.05 was considered statistically significant. Data were accessed from the cBioPortal between October 1, 2019 and March 15, 2020.
2.9. Statistical analysis
All quantitative data are presented as mean ± standard deviation or as indicated. Differences in the experimental means for flow cytometric and cytokine assay values were considered significant if P was less than 0.05 as determined by one-way analysis of variance (ANOVA) on ranks or t-test. Survival curves were analyzed by the Kaplan-Meier method and the log-rank test, and differences were considered significant at P values less than 0.05. For the analysis of the in vitro limiting dilution assay, the tumor-formation efficiency and the sphere-formation efficiency were calculated andcomparedusing Extreme limiting dilution analysis (http://bioinf.wehi.edu.au/software/elda/) based on the likelihood ratio test. All data shown are representative of 3 independent experiments acquired in triplicate (in vitro) or at least 2 independent experiments (in vivo). All statistical analyses were conducted using GraphPad Prism 7 (GraphPad Software, La Jolla, CA, USA). All statistical tests were 2-sided.
3. Results
3.1. FGL2 has no direct effect on stem-like properties of glioma cells in vitro
We have previously shown that FGL2 is mainly expressed in a subpopulation of glioma cells in glioblastoma [8]. In the present study, we hypothesized that FGL2 may regulate tumorigenesis by augmenting stem-like cell populations, thereby contributing to glioma initiation, progression, resistance, and relapse [3]. Single-cell clones with knockout, low, medium, and high FGL2 expression were selected by cell sorting and validated by Western blotting (Fig. 1A). Next, the relationship between FGL2 expression and the stem-cell properties of glioma cells were investigated in vitro. Unexpectedly, the expression level of FGL2 in glioma cells had no impact on the expression levels of key pluripotency transcription factors responsible for stem-cell programming or transition, including Nanog, SOX2, Musashi-1, and OCT4A (Fig. 1A). Regardless of FGL2 expression, all glioma cells expressed CD44 and CD49F expression and either did not express CD133, Nestin, CD171, and A2B5 or expressed them at low levels (Fig. 1B). In vitro limiting dilution assays, another standard stem-cell assay, also showed a lack of association between the expression level of FGL2 and the self-renewal capacity of glioma cells. More specifically, FGL2KO cells had the highest self-renewal ability, whereas FGL2low cells had the lowest self-renewal ability, with FGL2hi and FGL2mid cells in between (Fig. 1C). Cell proliferation assays showed that FGL2 did not promote glioma cell proliferation in vitro (Fig. 1D). Collectively, these data demonstrated that FGL2 has no direct impact on classical stem-like properties such as expression of transcription factors, sphere formation, or proliferation in vitro.
Fig. 1. FGL2 promotes stem-like properties of glioma cells in vivo, but not in vitro.
FGL2KO, FGL2low, FGL2mid, and FGL2hi GL261 cells were generated for following assays. (A), Expression of FGL2 and stemness-associated transcription factors were detected by Western blotting. Top: Representative blots. Bottom: Quantitative analysis of expression. (B), Expression levels of GSC markers on GL261 cells detected by FACS. (C), In vitro limiting dilution sphere-forming assay. (D), In vitro cell proliferation assay. Data are presented as the mean ± SD (n = 3). (E), Tumor incidence of GL261 cells in WT mice. Cells were orthotopically implanted into the brains of C57BL/6 mice at the indicated numbers. (F), Limiting dilution tumorigenesis assay of GL261 cells in C57BL/6 mice. **P < 0.01, ***P < 0.001, likelihood ratio test. (G-J), Survival curves of C57BL/6 mice (n = 6) implanted with FGL2KO (G), FGL2low (H), FGL2mid (I), or FGL2hi (J) cells at indicated numbers. (K and L), Survival curves of NSG mice (K) and Scid mice (L) implanted with 2000 FGL2KO or FGL2Hi cells (n = 6). All data are representative of at least 2 independent experiments. The survival curves were generated by Kaplan–Meier analysis, and the log-rank test was used to compare overall survival between groups.
3.2. FGL2 enhances the tumorigenesis of glioma cells
The tumorigenicity of glioma cells at low numbers serves as the key defining function of GSCs. To determine the impact of FGL2 on the stem-like functionality of glioma cells in vivo, we examined glioma incidence and progression by inoculating a range of low numbers of glioma cells with variable expression levels of FGL2 into immune-competent mice. This in vivo limiting dilution assay showed that as few as 100 FGL2hi glioma cells, 500 FGL2mid glioma cells, or 2000 FGL2low glioma cells were sufficient to initiate intracranial glioma growth. In contrast, as many as 5 × 104 FGL2KO glioma cells were incapable of forming tumors in immune-competent mice (Fig. 1E). Statistical analyses of the glioma formation ability showed a positive correlation between glioma incidence and the expression level of FGL2 in tumors (Fig. 1F). Moreover, mice bearing FGL2hi cell-derived gliomas exhibited markedly shortened survival compared to those bearing FGL2mid or FGL2low cell-derived gliomas (Fig. 1G–J), demonstrating that FGL2 promotes tumor growth and progression in vivo. Our published data showed that FGL2 increased stem marker CD44 expression in glioma in immune-competent mice [7]. These results indicate that FGL2hi glioma cells may be more effective in inducing stem-like populations or increasing stem-like functionality to augment tumorigenic capacity than the FGL2KO or FGL2low/mid cells when transplanted into immune-competent mice.
3.3. Myeloid cells are required for FGL2-regulated tumorigenesis
The data above pose a paradox: FGL2 promotes the stem-like populations or stem-like functionality of glioma cells in vivo but not in vitro. One logical explanation is that the FGL2 secreted by glioma cells may regulate the immune system, which then upregulates the stem-like function of the glioma cells [4, 13]. To clarify whether immune cells contribute to the regulation of stem-like transition potential and functionality, we implanted FGL2hi and FGL2KO glioma cells into both NOD scid gamma (NSG) and Scid mice. Implantation of FGL2hi or FGL2KO glioma cells yielded the same survival in NSG mice, which are deficient in T, B, and natural killer (NK) cells and have defective myeloid cells (macrophages and DCs), indicating that the immune system plays a pivotal role in FGL2-mediated glioma stem-like transition (Fig. 1K). In contrast, in Scid mice, which have a functional innate immune system including NK cells, macrophages, and granulocytes, mice implanted with FGL2KO glioma cells showed increased survival and decreased tumor incidence relative to mice implanted with FGL2hi glioma cells (Fig. 1L). These results indicate that the stem-like functionality of glioma cells is dependent on innate immunity. Our previous study showed that NK depletion had no significant effect on survival of C57 mice bearing GL261-FGL2KO gliomas [8]. Thus, FGL2 likely regulates the stem-like functionality of glioma cells in vivo via the presence of functional myeloid cells.
3.4. FGL2 affects the composition of specific subtypes of GAMs
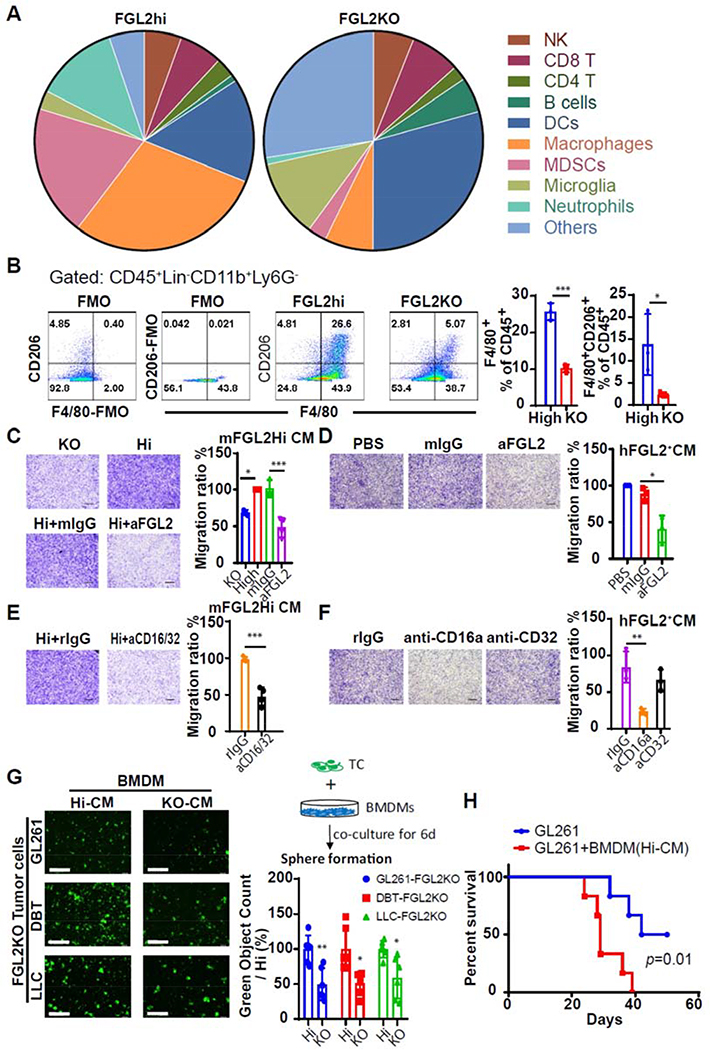
In clinical samples, GAMs are the most abundant cells, accounting for up to 30% of the tumor mass of gliomas. GAMs facilitate tumor-cell proliferation, survival, and migration [14]. To identify the specific types of immune cells that are regulated by FGL2 and affect stem-like function, we designed an antibody panel to identify different populations of macrophages, microglia, MDSCs, DCs, NK cells, B cells, and T cells [15, 16] and performed mass cytometry analysis of infiltrated leukocytes in FGL2high and FGL2KO glioma-bearing brains in wild-type mice. This analysis enabled classification of brain-infiltrating leukocytes into clusters representing 17 populations and identified macrophages (CD11b+F4/80+CD49d+ P2RY12-), microglias (CD45lowCD11b+F4/80lowCD49d-P2RY12+), MDSCs (CD11b+CD49d+P2RY12-Ly6ChiLy6Glow), neutrophils (CD11b+CD49dlowP2RY12lowLy6ClowLy6Ghi), and DCs (CD11b+CD11c+) (Fig. 2A,Supplemental Fig. 1A). The macrophages constituted the largest proportion (29.26%) of brain-infiltrating leukocytes induced by FGL2hi tumor cells. This proportion was dramatically lower, 7.16%, in FGL2KO tumor-bearing brains. MDSC and neutrophil populations were also significantly higher in FGL2hi than in FGL2KO glioma-bearing brains (Fig. 2A, Supplemental Fig. 1B). Meanwhile, the DC and microglia populations were dramatically lower in the FGL2hi glioma-bearing brains than in the FGL2KO glioma-bearing brains (Fig. 2A, Supplemental Fig. 1B). Additional flow cytometry analysis verified the difference in macrophage infiltration between FGL2hi and FGL2KO gliomas (Fig. 2B, Supplemental Fig. 1C). Furthermore, more than half of GAMs in tumors derived from FGL2hi cells expressed CD206, a marker that is used to denote an association with the M2 phenotype. These CD206+ GAMs were markedly less abundant in FGL2KO tumors (Fig. 2B). In summary, there were dramatic differences in the composition of the infiltrating immune cell populations between FGL2hi and FGL2KO gliomas, and the presence of FGL2 increased the populations of myeloid cells and especially macrophages.
Fig. 2. Macrophages infiltrate into glioblastoma via the FGL2-CD16a axis and increase the ability of sphere formation of glioma cells.
(A), Pie charts showing percentages of a variety of immune cells in GL261-FGL2hi or GL261-FGL2KO-tumor bearing brains at 7 days after tumor implantation. (B), F4/80+CD206+ populations in BIL (n = 3).(C and E), Representative photos and quantification of relative migration of RAW264.7 macrophages following stimulation with conditioned media (CM) pretreated with/without anti-FGL2, anti-CD16/32 blocking antibody, or IgG (10 μg/mL) (n = 4). Scale bar, 500 μm. (D and F), Representative photos and quantification of relative migration of THP-1 macrophages following stimulation with CM pretreated with/without anti-FGL2, anti-CD16a, anti-CD32 blocking antibody, or IgG (10 μg/mL) (n = 3). Scale bar, 500 μm. (G), Sphere-formation capacity of tumor cells cocultured with CM-rewired BMDMs in cancer stem cell culture media. Left: representative photos of sphere formation in the indicated system. Right Top: schematic diagram of BMDM-driven sphere formation. Right Bottom: quantification of relative sphere formation. Scale bar, 400 μm. Data are presented as the mean ± SD and were analyzed by 1-way ANOVA (n = 6). (H), Survival curves of C57BL/6 mice (n = 6) implanted with GL261 or GL261+CM-rewired BMDMs. The survival curves were generated by Kaplan–Meier analysis, and the log-rank test was used to compare overall survival between groups. Representative data from 2–3 independent experiments are shown. *P < 0.05, **P < 0.01, ***P < 0.001.
3.5. FGL2 derived from tumor cells directly facilitates recruitment of macrophages
To show the direct impact of FGL2 on macrophage recruitment, conditioned medium (CM) from FGL2hi and FGL2KO glioma cells was used in macrophage migration assay. As expected, almost no FGL2 was detected in CM from murine (m) FGL2KO glioma cells, but approximately 20 ng/mL of FGL2 was produced by the FGL2hi cells (Supplemental Fig. 2). CM from FGL2hi glioma cells attracted significantly more RAW264.7 macrophage cells than did mFGL2KO CM. Neutralizing FGL2 in mFGL2hi CM with an mFGL2-blocking antibody significantly reduced this chemoattractant effect (Fig.2C). Moreover, the capacity of FGL2 to attract macrophages was further demonstrated with PMA-primed human (h) macrophage-like THP-1 cells. Migration was significantly suppressed when hFGL2 in the CM was neutralized with an hFGL2 antibody (compared with IgG-treated CM from U-87 human glioma cells) (Fig. 2D). Collectively, these data showed that FGL2 preferentially secreted by glioma cells exhibits a potent capacity to attract macrophages.
To verify this direct effect on FGL2 CM-mediated macrophage migration, we also tested the effect of blocking FcγRIIb (CD32) and FcγRIII (CD16), 2 known receptors for FGL2 binding on the surface of macrophages [17]. After blocking CD16/32 on RAW264.7 cells with the cognate antibodies (clone 2.4G2 antibody), the migration of macrophages induced by mFGL2hi CM was significantly suppressed (Fig. 2E). Furthermore, blocking the CD16a receptor, but not the CD32 receptor, abolished the hFGL2+ CM-enhanced migration of human THP-1 macrophages, showing that CD16a is the receptor involved in FGL2-enhanced human macrophage migration (Fig. 2F). Together with in vivo data, these findings demonstrate that FGL2 functions as a potent chemokine to recruit macrophages to the tumor microenvironment (TME) of glioma, and CD16a is the receptor mediating this chemoattractant effect.
3.6. FGL2-wired GAMs increased tumorigenesis of glioma cells
To evaluate the effect of FGL2 derived from glioma cells on the GAM-supported the stem-like transition of glioma cells, we compared the impact of mFGL2hi vs mFGL2KO CM-treated macrophages on glioma sphere formation. Sphere-formation ability of FGL2KO tumor cells was enhanced when cocultured with mFGL2hi CM-exposed macrophages than with mFGL2KO CM -exposed macrophages (Fig.2G), indicating increased self-renewal capacity of tumor cells. Moreover, co-implanted mFGL2hi CM-exposed macrophages with tumor cells increased the tumor incidence from 3 of 6 mice to 6 of 6 and significantly shorten the survival time of GL261-tumor bearing mice (Fig. 2H). Together, FGL2-wired macrophages are important for glioma tumorigenicity.
3.7. CXCL7 is preferentially expressed by FGL2-wired GAMs
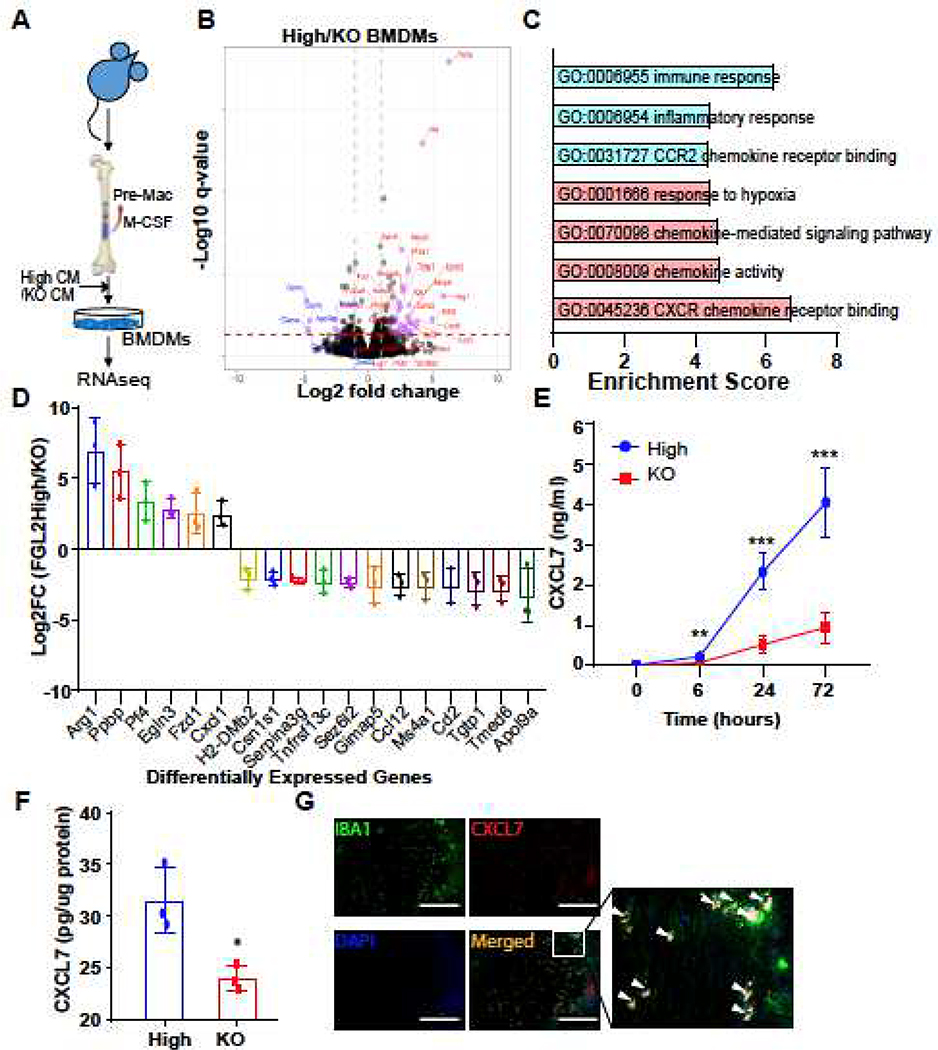
To gain insight into the molecular programs by which FGL2 treatment facilitates GAM-mediated stem-like transition of tumor cells, we performed bulk RNA-seq on total macrophages derived from mFGL2hi and mFGL2KO CM-exposed BMDMs (Fig. 3A). Comparison of the gene expression profiles of BMDMs treated with either mFGL2hi CM or mFGL2KO CM revealed that 136 protein-coding genes were differentially expressed in the BMDMs (fold change > 2 and q < 0.05) (Fig. 3B). Gene ontology analysis reported enrichment in the domains of immune response, chemokine activity, chemokine signaling, and response to hypoxia, indicating that these domains are regulated by FGL2 in BMDMs (Fig. 3C). Waterfall plots showed that ARG1 and PPBP (CXCL7) were the 2 primary candidate genes whose abundance was increased the most in BMDMs by mFGL2hi CM treatment (Fig. 3D). Arg1 is recognized as an M2 macrophage marker [18]. The expression of Arg1 was dramatically higher in BMDMs exposed to mFGL2hi CM than in those exposed to mFGL2KO CM (Supplemental Fig. 3A, 3B), but the induced Arg1 expression could not be reversed by blocking FGL2 in vitro (Supplemental Fig. 3C, D). ELISA of CXCL7 in medium derived from mFGL2hi CM- and mFGL2KO CM-exposed BMDMs confirmed that CXCL7 expression was induced in a time-dependent manner in both groups of BMDMs, but the levels of CXCL7 was dramatically lower in the mFGL2KO CM-exposed than mFGL2hi CM-exposed BMDMs (Fig. 3E). In agreement with this in vitro observation, our in vivo study revealed that CXCL7 expression in mFGL2hi glioma-bearing brain tissues was significantly higher than that in mFGL2KO glioma-bearing brain tissues (Fig. 3F); this difference was tightly associated with the increased GAMs in mFGL2hi gliomas but not in mFGL2KO ones. The relationship between CXCL7 and GAMs was further validated by the colocalization of CXCL7 and IBA1 in human GBM tumors (Fig. 3G). Together, these results demonstrate that CXCL7 is induced in GAMs by FGL2+ tumor cells.
Fig. 3. FGL2 induces CXCL7 expression in macrophages.
(A), Experimental scheme for RNA-seq analysis of BMDMs treated with FGL2hi conditioned media (CM) vs FGL2KO CM. (B), Volcano plots showing DEGs in BMDMs exposed to FGL2hi CM as compared with FGL2KO CM. (C), KEGG pathway enrichment analysis of DEGs selected in the heatmap in (C). (D), Waterfall plot of DEGs. The criteria were: (1) genes in the heatmap and (2) paired FC > 2 or < -2. (E), Protein levels of CXCL7 in medium of cultured BMDMs. Data were analyzed by 1-way ANOVA (n = 3). (F), Protein levels of CXCL7 in brain lysates at day 7 after tumor implantation. Data are presented as the mean ± SD and were analyzed by t test (n = 3). (G), Representative immunofluorescent staining of CXCL7 (in red) and IBA1 (in green) in human glioblastoma tissues. Area indicated with rectangle is enlarged and shown on the right side. Scale bar, 200 μm. White arrows: co-immunofluorescent staining. Representative data from 2–3 independent experiments. *P < 0.05, **P < 0.01, ***P < 0.001.
3.8. CXCL7 regulates the self-renewal and tumorigenicity of glioma cells
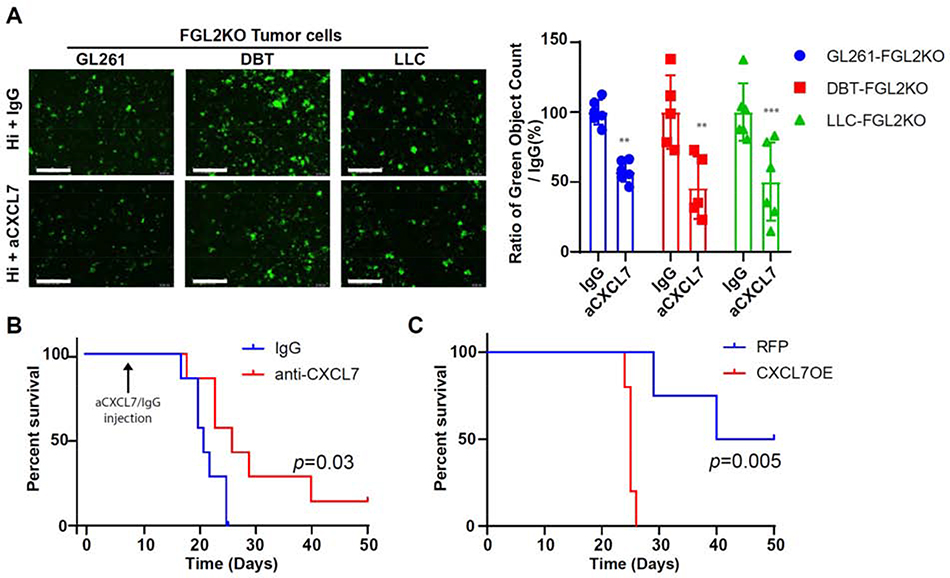
Chemokines in the TME can directly target tumor cells and regulate tumor-cell proliferation, cancer-cell stem-like properties, cancer invasiveness, and metastasis [19]. To clarify the effects of CXCL7 produced by GAMs on glioma cells, we tested whether neutralizing the CXCL7 produced by BMDMs could suppress the sphere formation and migration of tumor cells in vitro. The results showed that neutralizing CXCL7 produced by mFGL2hi CM-exposed BMDMs suppressed sphere formation more effectively than did IgG in macrophage-tumor cell coculture assays across 3 different tumor cell lines (GL261-FGL2KO, DBT-FGL2KO, and LLC-FGL2KO) (Fig. 4A). mFGL2hi CM-exposed BMDMs with higher levels of CXCL7 attracted more tumor-cell migration than did mFGL2KO CM-exposed BMDMs, and neutralizing CXCL7 reversed the migration of tumor cells (Supplemental Fig. 4). To further address the effect of CXCL7 on the tumorigenesis of glioma cells, we investigated whether blocking CXCL7 expression in the TME by co-implanting an anti-CXCL7 neutralizing antibody with FGL2hi glioma cells could attenuate tumor incidence and suppress tumor progression. The results showed that blocking CXCL7 expression in the TME of FGL2hi tumors reduced tumor incidence from 7 of 7 mice to 6 of 7 and prolonged the survival time of FGL2hi tumor-bearing mice compared with IgG control (Fig. 4B). These findings indicated that CXCL7 is a critical factor promoting the stem-cell function of FGL2hi tumor cells in immune-competent mice. Moreover, overexpression of CXCL7 in FGL2low tumor cells increased the tumor incidence from 2 of 4 mice to 5 of 5 and dramatically shortened the survival time of FGL2low tumor-bearing mice (Fig. 4C). Taken together, these results indicate that CXCL7 secreted by BMDMs exposed to FGL2+ tumor cells can induce stem-like transition in vitro and promote the stem-like functionality and migration of glioma cells in vivo.
Fig. 4. CXCL7 promotes sphere formation and gliomagenesis of glioma cells.
(A), Sphere formation capacity of GL261-FGL2KO, DBT-FGL2KO, and LLC-FGL2KO tumor cells in cancer stem cell culture media when cocultured with mFGL2hi conditioned media (CM)-wired BMDMs pretreated with/without IgGoranti-CXCL7. Left: representative photos of sphere formation in the indicated system. Right: quantification of relative sphere formation. Scale bar, 400 μm. Data are presented as the mean ± SD and were analyzed by 1-way ANOVA (n = 6 per group). Representative data from 2–3 independent experiments are shown. **P < 0.01, ***P < 0.001. (B), Survival curves of C57BL/6 mice (N = 7) implanted with FGL2hi tumor cells treated with anti-CXCL7 or IgG antibody. Mice were implanted with GL261-FGL2hi tumor cells (2000 cells). At day 7 post implantation, 50 μg anti-CXCL7 or 50 μg IgG antibody were injected into the brain and survival time of mice was monitored. The survival curves were generated by Kaplan-Meier analysis, and the log-rank test was used to compare overall survival between groups. (C), Survival curves of C57BL/6 mice (N = 4) implanted with 5 × 104 GL261-FGL2low-RFP or GL261-FGL2low-CXCL7OE cells. The survival curves were generated by Kaplan–Meier analysis, and the log-rank test was used to compare overall survival between groups.
3.9. CXCL7 is included by the FGL2-CD16/Syk/HIF1α signaling pathway in macrophages
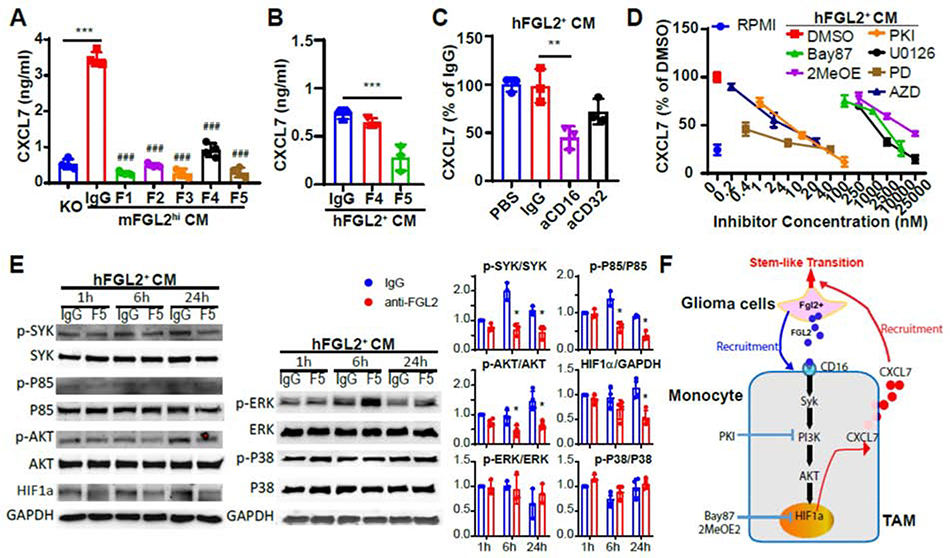
To explore the molecular mechanisms underlying induced CXCL7 expression in GAMs by FGL2hi tumors, we first detected CXCL7 levels in BMDMs using different clones of anti-FGL2 antibodies to neutralize mFGL2 in CM. The results showed that 5 clones of antibodies targeting mFGL2 blocked CXCL7 production induced by mFGL2hi CM in BMDMs (Fig. 5A). Likewise, 1 clone of antibodies targeting human FGL2 significantly reduced the CXCL7 production induced by hFGL2+ CM in human THP-1 macrophages (Fig. 5B), indicating that CXCL7 induced by CM is exclusively mediated by FGL2 in macrophages. Blocking the 2 receptors of FGL2, CD16a and CD32, showed that CD16a is the receptor on human macrophages that mediates FGL2-induced CXCL7 production in THP-1 cells (Fig. 5C). The downstream signaling events following activation of Fcγ receptors are Syc activation and, subsequently, activation of the PI3K pathway; late MEK and MAP family kinases; and the Ras pathway [20]. We used inhibitors that target the PI3K-AKT, MAPK, HIF, JAK2-STAT3, and NF-κB signaling pathways to test the effect of these pathways on FGL2-induced CXCL7 production in macrophages. We found that CXCL7 production was suppressed in a dose-dependent manner when the PI3K-AKT/MAPK/HIF1 signaling pathways were inhibited in hFGL2+ CM-exposed THP-1 macrophages (Fig. 5D, Supplemental Fig. 5). Western blotting data showed that phosphorylated Syk, PI3Kp85, and AKT were suppressed by neutralizing FGL2 in hFGL2+ CM-exposed THP-1 macrophages at 1, 6, and 24 h after culture, while phosphorylated P38 and ERK showed no difference after FGL2 was neutralized at these time points (Fig. 5E). Expression of the transcription factor HIF1α was decreased after FGL2 was neutralized in hFGL2+ CM-exposed THP-1 macrophages. Together, these findings demonstrated a critical role of the CD16a/Syk/PI3K/AKT/HIF1 signaling pathway in regulating FGL2-induced CXCL7 expression in macrophages (Fig. 5F).
Fig. 5. FGL2-induced CXCL7 production is regulated by the CD16/Syk/PI3K/HIF1α pathway.
(A), Blocking FGL2 in mFGL2hi-conditioned media (CM) leads to a decrease in CXCL7 production in BMDMs. F1, F2, F3, F4, and F5 are different hybridoma clones of anti-mFGL2 antibodies. (B), Blocking FGL2 by F5 in hFGL2+-CM leads to a decrease in CXCL7 production in THP-1 macrophages. F4 and F5 are different hybridoma clones of anti-hFGL2 antibodies. ### P < 0.001 vs IgG. (C), Blocking CD16a receptor decreases FGL2-induced CXCL7 production in THP-1 macrophages. (D), Dose-response curve of CXCL7 production. CXCL7 produced by THP-1 macrophages treated with hFGL2+ CM in the presence or absence of different dosages of the HIF1 inhibitor BAY87–2243 (Bay87), pan-HIF inhibitor 2-MeOE2, PI3K/mTOR inhibitor PKI-587 (PKI), MEK1/ERK inhibitor U0126, MEK1/2 inhibitor PD0325901 (PD), MEK1/ERK inhibitor AZD6244 (AZD), or p38 inhibitor PH797804 (PH). (E), Immunoblots for SYK, PI3Kp85, AKT, HIF1α, ERK, and P38 in THP-1 macrophages treated with hFGL2+ CM pretreated with IgG and an anti-FGL2 antibody at the indicated time points. Representative blots are shown in the left. Quantitative analysis of expression is shown in the right. (F), Schematic representation of the crosstalk between glioma cells and glioma-associated macrophages (GAMs). Representative data from 3 independent experiments are shown. **P < 0.01, ***P < 0.001.
3.10. CXCL7+ GAMs are associated with poor clinical outcomes and FGL2 expression
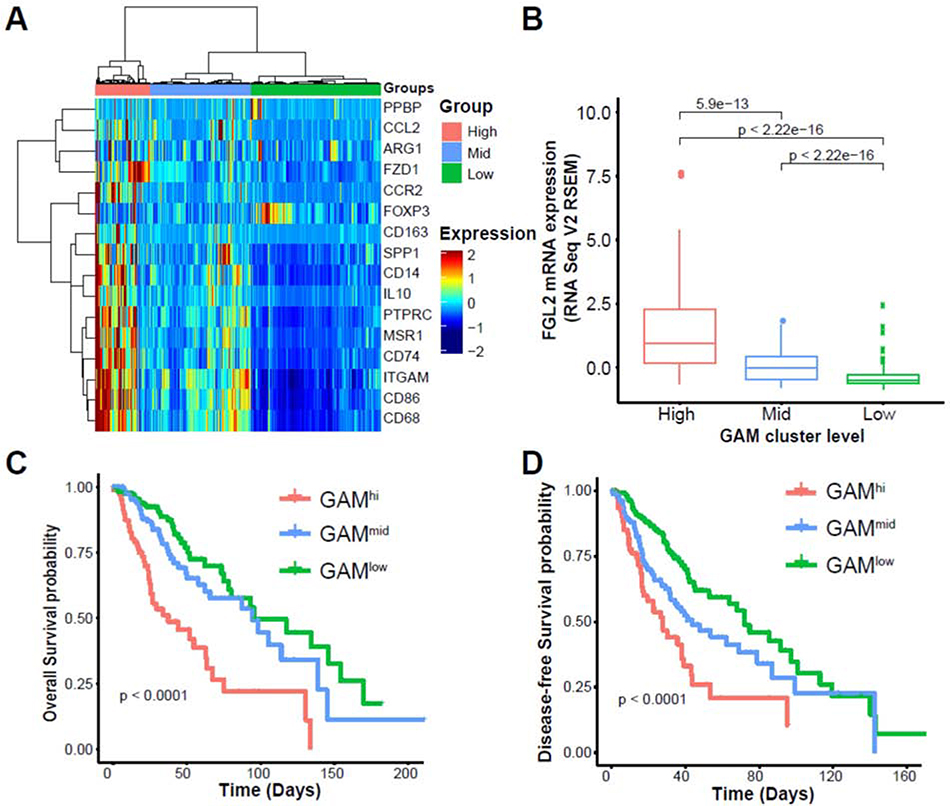
Our previous studies showed that FGL2 expression negatively correlates with the overall survival of patients with low-grade glioma (LGG) and glioblastoma [6, 7]. To interrogate whether this correlation was due to CXCL7+ macrophage infiltration in glioma patients, we investigated the association between FGL2 and GAMs by examining the LGG and glioblastoma datasets of The Cancer Genome Atlas (TCGA) for a panel of GAM-associated genes including CXCL7 [11, 15]. On the basis of the expression levels of these genes, samples were clustered to GAMhi, GAMmid, and GAMlow groups (Fig. 6A, Supplemental Fig. 6B). In line with our findings in mouse model systems and invitro experiments, the expression level of FGL2 was positively correlated with the GAM signature in both LGG and glioblastoma patients (Fig.6B, Supplemental Fig. 6C). Immunofluorescence staining confirmed that IBA+FGL2+ cells were preferentially distributed in the area around FGL2+ tumor cells in human glioblastoma surgical specimens (Supplemental Fig. 6A). Furthermore, LGG patients with the GAMhi signature exhibited worse disease-free and overall survival than did patients with the GAMmid or GAMlow signatures (Fig. 6C, 6D), indicating that GAM levels in tumors are positively correlated with disease progression and recurrence. Glioblastoma patients with the GAMhi signature exhibited the same trend of poorer disease-free and overall survival as LGG patients did, but the differences were not statistically significant (Supplemental Fig. 6D, 6E). Taken together, our findings from TCGA datasets and human surgical specimens highlighted the relevance of FGL2 expression to CXCL7+ GAM infiltration and poor clinical outcomes.
Fig. 6. CXCL7+ glioma-associated macrophages track with FGL2 expression and poor outcomes in human low-grade glioma.
(A), Heatmap of TCGA provisional low-grade glioma dataset clustered into GAMhi (n = 100), GAMmid (n = 181), and GAMlow (n = 235) groups on the basis of a gene signature for monocytic MDSCs/M2 macrophages. (B), Expression levels of FGL2 in 3 clustered groups of samples. FGL2 gene expression levels were compared among the TAM gene signature groups using t-tests, and P values were adjusted using the Bonferroni multiple testing correction. (C), Overall survival in the 3 clustered groups of patients. (D), Disease-free survival in the 3 clustered groups of patients. Kaplan-Meier survival analysis and the log-rank test were used to compare survival among the different TAM gene signature groups.
4. Discussion
GAMs and GSCs are crucial components promoting tumor growth in glioma, and their reciprocal interactions are pivotal for glioma propagation, therapeutic resistance, and tumor recurrence [3]. Our study mechanistically showed that glioma cell-secreted FGL2 plays a key role in this interaction by inducing CXCL7 production by GAMs, which then orchestrates stem-like transition of glioma cells in vivo. FGL2 expressed by glioma cells functions as a chemoattractant and a phenotype regulator of GAMs by interacting with the CD16a receptor in the TME of gliomas. This triggers the Syk/PI3K/AKT/HIF1α signaling pathway in the GAMs, thereby inducing release of CXCL7 (Fig. 5F). CXCL7 then induces the stem-like functionality of glioma cells to promote gliomagenesis and progression. As such, the glioma cell-secreted FGL2 and the GAM-secreted CXCL7 constitute a critical paracrine signaling loop in induced stem-like functionality. Disrupting FGL2-CXCL7 signaling by knock out of FGL2 or by CXCL7-neutralizing antibodies largely abrogates the tumor-supportive effects of FGL2 and suppresses glioma development.
GSC markers are useful for enriching stem cells, but by themselves are not sufficient to determine stem-cell function. Others have shown that GSCs isolated from various patients displayed equivalent stem-cell properties and functions in vitro but marked differences in tumor development in vivo owing to differences in microenvironmental cues [21]. Similarly, our data showed that glioma cells with variable levels of FGL2 expression, but with equivalent GSC markers and expression of transcription factors, had similar stem-cell phenotypic properties in vitro. The expression level of GSC markers was not associated with the ability to initiate tumors. However, the FGL2 levels directly correlated with gliomagenesis in vivo, indicating that environmental cues are an essential component of this process. In addition, sphere formation assays showed that the self-renewal capacity of FGL2KO glioma cells was promoted by FGL2hi CM-wired macrophages, and reversed by neutralizing CXCL7 in the system, indicating that stem-like functionality is a dynamic process of reversible transition regulated by GAMs. Thus, our work further reinforces the importance of the immune compartment in studies of the functional differences of GSCs and reveals that FGL2 is required for supporting the stem-like functionality of glioma cells in the presence of an immune response.
CXCL7 is a platelet-derived growth factor that belongs to the CXC chemokine family produced by platelets, monocytes, and macrophages. CXCL7 expression by macrophages and monocytes has been shown to be increased in response to bacterial or microbial components and to activation of protease-activated receptors by thrombin [22], and significantly suppressed by glucocorticoids, IL-4, andIL-10[23]. Others have shown that treatment of glioblastoma with a CSF1R inhibitor significantly decreased CXCL7 expression in myeloid cells [24]. CD16 and CD32 have both been reported to play a role in the regulation of chemokine production in human monocytes [17, 25], and the ERK-MAPK, PI3K-AKT, and NF-κB pathways have been previously shown to be involved in regulating CXCL7 expression [23, 26]. Through both inhibitor experiments and Western blotting, we uncovered that the Syk/PI3K/AKT/HIF1α signaling pathway, but not NF-κB, specifically regulates FGL2-induced CXCL7 production in macrophages. As such, we are the first group to show that the CD16/Syk/PI3K/AKT/HIF1α signaling pathway mediates FGL2-induced CXCL7 production in GAMs.
Dysregulation of CXCL7 has been observed in some common human cancers, including lung [27], renal [28], colon [29], and breast cancer [30], and is associated with faster tumor development and shorter disease-free and overall survival. The importance of CXCL7 in stemness regulation is supported by recent studies demonstrating that CXCL7 has the capacity to recruit stem-cell migration [31] and suppress mesenchymal stem-cell differentiation [32]. We found that CXCL7 promotes the migration and sphere formation of glioma cells in vitro. CXCL7 expression is positively associated with higher tumor incidence and faster tumor progression in vivo. Moreover, blocking CXCL7 decreased glioma incidence, whereas overexpression of CXCL7 promoted tumorigenesis in immune-competent mice. These findings support that consideration of strategies targeting the crosstalk between GAM and glioma cells to reverse the stem-like functionality of tumor cells and further consideration of GAM-targeting strategies as therapeutic modalities that may particularly applicable to glioma.
Supplementary Material
Highlights.
FGL2 promotes tumorigenesis of GL261 cells in an expression level-dependent manner in immunocompetent mice.
FGL2 induces CXCL7 expression via CD16/SyK/PI3K/HIF1α pathways in glioma -associated macrophages.
CXCL7 promotes sphere formation and tumorigenesis of glioma cells.
5. Acknowledgments
We appreciate Amy L Ninetto in Scientific Publications, Research Medical Library at The University of Texas MD Anderson Cancer Center for helping us edit our manuscript. This research used the Flow Cytometry and Cellular Imaging Facility and Monoclonal Antibody Core Facility at MD Anderson, whichare supported in part by the National Institutes of Health through MD Anderson's Cancer Center Support Grant P30 CA016672. This work was also supported by National Institutes of Health Grants R01 CA120895, R01 CA203493, and R01 NS094615.
Footnotes
Conflict of Interest:
The authors declare no potential conflicts of interest.
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests:
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
6. References
- [1].Bao S, Wu Q, McLendon RE, Hao Y, Shi Q, Hjelmeland AB, Dewhirst MW, Bigner DD, Rich JN, Glioma stem cells promote radioresistance by preferential activation of the DNA damage response, Nature, 444 (2006) 756–760. [DOI] [PubMed] [Google Scholar]
- [2].Lan X, Jorg DJ, Cavalli FMG, Richards LM, Nguyen LV, Vanner RJ, Guilhamon P, Lee L, Kushida MM, Pellacani D, Park NI, Coutinho FJ, Whetstone H, Selvadurai HJ, Che C, Luu B, Carles A, Moksa M, Rastegar N, Head R, Dolma S, Prinos P, Cusimano MD, Das S, Bernstein M, Arrowsmith CH, Mungall AJ, Moore RA, Ma Y, Gallo M, Lupien M, Pugh TJ, Taylor MD, Hirst M, Eaves CJ, D. B ns Simo, Dirks PB, Fate mapping of human glioblastoma reveals an invariant stem cell hierarchy, Nature, 549 (2017) 227–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Lathia JD, Mack SC, Mulkearns-Hubert EE, Valentim CL, Rich JN, Cancer stem cells in glioblastoma, Genes Dev, 29 (2015) 1203–1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Lenos KJ, Miedema DM, Lodestijn SC, Nijman LE, van den Bosch T, Romero Ros X, Lourenco FC, Lecca MC, van der Heijden M, van Neerven SM, van Oort A, Leveille N, Adam RS, de Sousa EMF, Otten J, Veerman P, Hypolite G, Koens L, Lyons SK, Stassi G, Winton DJ, Medema JP, Morrissey E, Bijlsma MF, Vermeulen L, Stem cell functionality is microenvironmentally defined during tumour expansion and therapy response in colon cancer, Nat Cell Biol, 20 (2018) 1193–1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Plaks V, Kong N, Werb Z, The cancer stem cell niche: how essential is the niche in regulating stemness of tumor cells?, Cell Stem Cell, 16 (2015) 225–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Yan J, Kong LY, Hu J, Gabrusiewicz K, Dibra D, Xia X, Heimberger AB, Li S, FGL2 as a Multimodality Regulator of Tumor-Mediated Immune Suppression and Therapeutic Target in Gliomas, J Natl Cancer Inst, 107 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Latha K, Yan J, Yang Y, Gressot LV, Kong LY, Ezhilarasan GR,Wang Q,Sulman EP, Eric Davis R, Huang S, Fuller GN, Rao A, Heimberger AB, Li S, Rao G, The Role of Fibrinogen-Like Protein 2 on Immunosuppression and Malignant Progression in Glioma, J Natl Cancer Inst, (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Yan J, Zhao Q, Gabrusiewicz K, Kong LY, Xia X, Wang J, Ott M, Xu J, Davis RE, Huo L, Rao G, Sun SC, Watowich SS, Heimberger AB, Li S, FGL2 promotes tumor progression in the CNS by suppressing CD103(+) dendritic cell differentiation, Nat Commun, 10 (2019) 448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Zhou W, Ke SQ, Huang Z, Flavahan W, Fang X, Paul J, Wu L, Sloan AE, McLendon RE, Li X, Rich JN, Bao S, Periostin secreted by glioblastoma stem cells recruits M2 tumour-associated macrophages and promotes malignant growth, Nat Cell Biol, 17 (2015) M170–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Kim D, Pertea G, Trapnell C, Pimentel H, Kelley R, Salzberg SL, TopHat2: accurate alignment of transcriptomes in the presence of insertions, deletions and gene fusions, Genome Biol, 14 (2013) R36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Bezzi M, Seitzer N, Ishikawa T, Reschke M, Chen M, Wang G, Mitchell C, Ng C, Katon J, Lunardi A, Signoretti S, Clohessy JG, Zhang J, Pandolfi PP, Diverse genetic-driven immune landscapes dictate tumor progression through distinct mechanisms, Nat Med, 24 (2018) 165–175. [DOI] [PubMed] [Google Scholar]
- [12].Gu Z, Eils R, Schlesner M, Complex heatmaps reveal patterns and correlations in multidimensional genomic data, Bioinformatics, 32 (2016) 2847–2849. [DOI] [PubMed] [Google Scholar]
- [13].Capp JP, Cancer Stem Cells: From Historical Roots to a New Perspective, J Oncol, 2019 (2019) 5189232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Quail DF, Joyce JA, The Microenvironmental Landscape of Brain Tumors, Cancer Cell, 31 (2017) 326–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Korin B, Dubovik T, Rolls A, Mass cytometry analysis of immune cells in the brain, Nat Protoc, 13 (2018) 377–391. [DOI] [PubMed] [Google Scholar]
- [16].Gubin MM, Esaulova E, Ward JP, Malkova ON, Runci D, Wong P, Noguchi T, Arthur CD, Meng W, Alspach E, Medrano RFV, Fronick C, Fehlings M, Newell EW, Fulton RS, Sheehan KCF, Oh ST, Schreiber RD, Artyomov MN, High-Dimensional Analysis Delineates Myeloid and Lymphoid Compartment Remodeling during Successful Immune-Checkpoint Cancer Therapy, Cell, 175 (2018) 1014–1030 e1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Bournazos S, Wang TT, Ravetch JV, The Role and Function of Fcgamma Receptors on Myeloid Cells, Microbiol Spectr, 4 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Geiger R, Rieckmann JC, Wolf T, Basso C, Feng Y, Fuhrer T, Kogadeeva M, Picotti P, Meissner F, Mann M, Zamboni N, Sallusto F, Lanzavecchia A, L-Arginine Modulates T Cell Metabolism and Enhances Survival and Anti-tumor Activity, Cell, 167 (2016) 829–842 e813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Nagarsheth N, Wicha MS, Zou W, Chemokines in the cancer microenvironment and their relevance in cancer immunotherapy, Nat Rev Immunol, 17 (2017) 559–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Bournazos S, Ravetch JV, Fcgamma receptor pathways during active and passive immunization, Immunol Rev, 268 (2015) 88–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Dirkse A, Golebiewska A, Buder T, Nazarov PV, Muller A, Poovathingal S, Brons NHC, Leite S, Sauvageot N, Sarkisjan D, Seyfrid M, Fritah S, Stieber D, Michelucci A, Hertel F, Herold-Mende C, Azuaje F, Skupin A, Bjerkvig R, Deutsch A, Voss-Bohme A, Niclou SP, Stem cell-associated heterogeneity in Glioblastoma results from intrinsic tumor plasticity shaped by the microenvironment, Nat Commun, 10 (2019) 1787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Schaffner A, King CC, Schaer D, Guiney DG, Induction and antimicrobial activity of platelet basic protein derivatives in human monocytes, J Leukoc Biol, 76 (2004) 1010–1018. [DOI] [PubMed] [Google Scholar]
- [23].El-Gedaily A, Schoedon G, Schneemann M, Schaffner A, Constitutive and regulated expression of platelet basic protein in human monocytes, J Leukoc Biol, 75 (2004) 495–503. [DOI] [PubMed] [Google Scholar]
- [24].Achyut BR, Shankar A, Iskander AS, Ara R, Angara K, Zeng P, Knight RA, Scicli AG, Arbab AS, Bone marrow derived myeloid cells orchestrate antiangiogenic resistance in glioblastoma through coordinated molecular networks, Cancer Lett, 369 (2015) [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Sironi M, Martinez FO, D'Ambrosio D, Gattorno M, Polentarutti N, Locati M, Gregorio A, Iellem A, Cassatella MA, Van Damme J, Sozzani S, Martini A, Sinigaglia F, Vecchi A, Mantovani A, Differential regulation of chemokine production by Fcgamma receptor engagement in human monocytes: association of CCL1 with a distinct form of M2 monocyte activation (M2b, Type 2), J Leukoc Biol, 80 (2006) 342–349. [DOI] [PubMed] [Google Scholar]
- [26].Taniguchi K, Karin M, NF-kappaB, inflammation, immunity and cancer: coming of age, Nat Rev Immunol, 18 (2018) 309–324. [DOI] [PubMed] [Google Scholar]
- [27].Du Q, Li E, Liu Y, Xie W, Huang C, Song J, Zhang W, Zheng Y, Wang H, Wang Q, CTAPIII/CXCL7: a novel biomarker for early diagnosis of lung cancer, Cancer Med, 7 (2018) 325–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Kinouchi T, Uemura M, Wang C, Ishizuya Y, Yamamoto Y, Hayashi T, Matsuzaki K, Nakata W, Yoshida T, Jingushi K, Kawashima A, Ujike T, Nagahara A, Fujita K, Imamura R, Ueda Y, Kitae K, Tsujikawa K, Nonomura N, Exlevel of CXCL7 in peripheral blood cells is a potential biomarker for the diagnosis of renal cell carcinoma, Cancer Sci, 108 (2017) 2495–2502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Li L, Zhang L, Tian Y, Zhang T, Duan G, Liu Y, Yin Y, Hua D, Qi X, Mao Y, Serum Chemokine CXCL7 as a Diagnostic Biomarker for Colorectal Cancer, Front Oncol, 9 (2019) 921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Yu M, Berk R, Kosir MA, CXCL7-Mediated Stimulation of Lymphangiogenic Factors VEGF-C, VEGF-D in Human Breast Cancer Cells, J Oncol, 2010 (2010) 939407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Almeida CR, Caires HR, Vasconcelos DP, Barbosa MA, NAP-2 Secreted by Human NK Cells Can Stimulate Mesenchymal Stem/Stromal Cell Recruitment, Stem Cell Reports, 6 (2016) 466–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Kalwitz G, Neumann K, Ringe J, Sezer O, Sittinger M, Endres M, Kaps C, Chondrogenic differentiation of human mesenchymal stem cells in micro-masses is impaired by high doses of the chemokine CXCL7, J Tissue Eng Regen Med, 5 (2011) 50–59. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.