Abstract
Ascaridoid nematodes comprise a wide range of heteroxenous parasites infecting top fish predators and marine mammals as definitive hosts, with crustaceans, squids, and fishes acting as intermediate/paratenic hosts. Limited data exist on the species and role of several intermediate and paratenic hosts in the life cycle of these parasites. In the aim of adding knowledge on the role of squid species in their life cycle, we have here investigated the larval ascaridoid nematodes collected from the deep-sea umbrella squid Histioteuthis bonnelli and the reverse jewel squid Histioteuthis reversa captured in the Central Mediterranean Sea (Tyrrhenian Sea). Morphological study and sequence analysis of the internal transcribed spacer (ITS) regions of the ribosomal DNA (rDNA) and the mitochondrial cytochrome c oxidase subunit 2 (mtDNA cox2) gene locus revealed the occurrence of Anisakis physeteris and of an unidentified species of the genus Lappetascaris. Sequence analysis revealed that specimens of Lappetascaris from both squid species matched at 100% sequences previously deposited in GenBank from larval ascaridoids collected in octopuses of the genus Eledone of the Mediterranean Sea. The Bayesian inference tree topology obtained from the analysis of the fragments amplified showed that Lappetascaris specimens were included in a major clade comprising Hysterothylacium species collected in fishes of the families Xiphiidae and Istiophoridae. As regards the site of infection in the squid host species, A. physeteris larvae predominated (60.7%) in the gonads, while those of Lappetascaris (76.3%) were found infecting the mantle musculature. The overall high values of parasitic load suggest both squid species as transmitting hosts of third stage larvae of Lappetascaris to top predator fishes, as well as the umbrella squid as an intermediate/paratenic host in the life cycle of A. physeteris in the Mediterranean Sea.
Subject terms: Ecology, Molecular biology
Introduction
Histioteuthidae Verrill, 1881 is a family of pelagic cephalopods distributed circumglobally in the midwaters of the oceans, from the subarctic to the subantarctic regions1. The umbrella squid Histioteuthis bonnellii (Férussac, 1835) and the reverse jewel squid Histioteuthis reversa (Verrill, 1880) are the only species inhabiting the Mediterranean Sea2, where they are usually found between 500 and 1500 m depth1,3–6. Both species are important prey-resources for higher trophic levels, such as those constituted by marine mammals and top fish predators; they are also voracious consumers of crustaceans, other cephalopods, and fishes1,6–12.
Host-species interaction through food webs allows transmission and maintenance of biological cycles of most of the parasites in marine ecosystems13,14. Parasites constitute an important component of every marine community showing a high diversity in their life-cycles15–17. Cephalopods, as intermediate or paratenic hosts in the life cycle of heteroxenous parasites, can accumulate them throughout their lifespan, thus increasing the chance of predation by the next host and, consequently, the probability of parasite transmission. This is especially relevant for ascaridoid nematodes, which use squids as intermediate and/or paratenic hosts14,18,19 and marine mammals or teleostean fishes as definitive ones20.
Likely due to their elusiveness, most of the records so far available in the literature about the umbrella and reverse jewel squids from the Mediterranean Sea have been limited to occasional captures with the description of morphometric features or data derived from the gastric contents of teuthophagous predators21–23, or because they were included within studies on the cephalopod faunas of certain geographic areas6,24,25. Moreover, the only published data regarding ascaridoid nematodes in the Mediterranean histioteuthids is a morphological study by Culurgioni et al.26, reporting low prevalence and abundance levels of larval forms of Lappetascaris sp. Type A in the umbrella squid and the reverse jewel squid, as well as the occurrence of third stage larvae of Anisakis sp. morphotype II (sensu Berland, 1961) in the umbrella squid from the Sardinian Channel (Western Mediterranean).
In the present paper, a genetic/molecular approach was applied to identify, at the lower possible taxonomic level, larval ascaridoid nematodes collected from poorly known squids species (i.e., the umbrella squid and reverse jewel squid) from the Tyrrhenian Sea in order to: i) add knowledge on the role of these squids species in nematode parasites having in top predators of a marine food webs their definitive hosts; ii) provide data on their infection level and site of infection in the hosts. Identification of the examined squid hosts was also included by means of genetic/molecular analysis.
Materials and methods
Sampling
A total of 10 specimens of the genus Histioteuthis d'Orbigny [in Férussac & d'Orbigny], 1841 were collected from off Campania coast (Tyrrhenian Sea, Mediterranean), during July and August 2020. Nine specimens were obtained from off Ischia Island (Gulf of Naples) (~ 40°35′30″ N, 14°00′37″ E) and a single individual from the Gulf of Salerno (~ 40°34′30″ N, 14°40′00″ E). In particular, they constituted the by-catch of commercial and scientific trawling operations (red shrimp’s fishery) held with commercial fishing vessels equipped with bottom trawl nets (mouth of 3 × 4 m in height and width, respectively; 40 mm mesh size), towed at ~ 2–2.5 kn on muddy bottoms at ~ 450–600 m depth see27. Procedures for this study were performed in accordance with the permit n. 0008453 (issued May 15, 2020) by the Italian Ministry of Agricultural, Food and Forestry Policies, guide for the care and use of animals by the Italian Ministry of Health and the ARRIVE guidelines.
Morphological and molecular identification of the squids
After the sampling, squids were transferred, in iceboxes, to the laboratory, where the specimens were identified to the species level according to their morphological characters3,5,28. Subsequently, they were weighed (Wt) to the nearest 0.1 g and measured (dorsal mantle length, DML) to the nearest 0.1 cm. Sex was determined before the parasitological inspection by gonadal examination. The identification to the species level was then supported by direct sequencing of PCR products for the barcode gene locus.
Total genomic DNA was extracted from squid muscle samples using the DNeasy® Blood & Tissue kit (QIAGEN), following the manufacturer’s protocol. A partial sequence of the mitochondrial cytochrome c oxidase subunit 1 gene locus (mtDNA cox1) was amplified from each specimen using both the primers developed by Folmer et al.29 [LCO-1490 (forward) 5′-GGTCAACAAATCATAAAGATATTGG-3′; HCO-2198 (reverse) 5′-TAAACTTCAGGGTGACCAAAAATCA-3′] and their degenerated version by Meyer30 [dgLCO-1490 (forward) 5′-GGTCAACAAATCATAAAGAYATYGG-3′; dgHCO-2198 (reverse) 5′-TAAACTTCAGGGTGACCAAARAAYCA-3′]. The polymerase chain reactions (PCRs) were conducted in 25 μL volume reaction, containing 2.5 μL of Roche buffer (10 ×), 2.5 μL (2 mM) of dNTPack Mixture (Roche), 1 μL of each forward and reverse primers (10 µM), 0.25 μL (5 U/μL) of Roche Taq DNA polymerase, 1 μL of DNA (15 ng/μL) and sterilized distilled water up to 25 μL. Amplifications were performed with the following conditions: initial denaturation at 95 °C (5 min), followed by 39 cycles of denaturation at 95 °C (1 min), annealing at 45 °C (1 min), extension at 72 °C (1 min), with a final extension at 72 °C (5 min). The successful PCR products were purified, and Sanger sequenced through an Automated Capillary Electrophoresis Sequencer 3730 DNA Analyzer (Applied Biosystems), using the BigDye® Terminator v3.1 Cycle Sequencing Kit (Life Technologies). Forward and reverse sequences obtained were assembled using Sequencher v. 5.0.1 (GeneCodes Co.) and compared with reference sequences using BLASTn31.
Parasitological analysis of the squids
For the parasitological examination, each squid specimen was cut along the ventral mid-line of the mantle, the organs were removed, placed individually in plastic Petri dishes (200 mm in diameter), opened, and studied for metazoan parasites under a dissecting microscope. The mantle of each specimen was dissected in small pieces (1 cm × 1 cm) and examined under the dissecting microscope. Parasites found embedded in the mantle tissue were extracted using scissors and tweezers. All the removed nematodes were subsequently counted, washed in physiological saline solution, and preserved in 70% ethanol or frozen at − 20 °C for morphological and molecular identification, respectively. Larval nematodes were studied and photographed using a dissecting microscope and a compound microscope both equipped with ZEN 3.1 imaging system (Zeiss). They were morphologically assigned to the genus level according to the morphological features32–34. Descriptors of the parasite distribution used in the present study follow Bush et al.35.
Molecular identification of ascaridoid parasites
Total genomic DNA from ∼2 mg of each parasite was extracted using Quick-gDNA Miniprep Kit (ZYMO RESEARCH) following the standard manufacturer-recommended protocol. The ITS region of rDNA including the first internal transcribed spacer (ITS-1), the 5.8S gene, the second transcribed spacer (ITS-2), and ∼70 nucleotides of the 28S gene, was amplified using the primers NC5 (forward; 5′-GTAGGTGAACCTGCGGAAGGATCATT-3′) and NC2 (reverse; 5′-TTAGTTTCTTTTCCTCCGCT-3′)36. PCRs were carried out in a 15 µL volume containing 0.3 µL of each primer 10 mM, 2.5 µL of MgCl2 25 mM (Promega), 15 µL of 5 × buffer (Promega), 0.3 µL of DMSO, 0.3 µL of dNTPs 10 mM (Promega), 0.3 µL (5 U/μL) of Go-Taq Polymerase (Promega) and 2 µL of total DNA. PCR temperature conditions were the following: 94 °C for 5 min (initial denaturation), followed by 30 cycles at 94 °C for 30 s (denaturation), 55 °C for 30 s (annealing), 72 °C for 30 s (extension) and followed by post-amplification at 72 °C for 5 min. Additionally, the mtDNA cox2 locus was sequenced in a subsample of 10 larvae of both the genera Anisakis Dujardin, 1845 and Lappetascaris Rasheed, 1965, randomly selected among those sequenced at the ITS region of rDNA, using the primers 211F (5′-TTTTCTAGTTATATAGATTGRTTYAT-3′) and 210R (5′-CAC CAACTCTTAAAATTATC-3′)37,38. PCRs were carried out in a 25 µL volume containing 2 µL of each primer 10 mM, 4 µL of MgCl2 25 mM (Promega), 5 µL of 5 × buffer (Promega), 2 µL of dNTPs 10 mM (Promega), 0.25 µL (5 U/μL) of Go-Taq Polymerase (Promega) and 3 µL of total DNA. The amplification protocol was performed using the following conditions: 94 °C for 3 min (initial denaturation), followed by 35 cycles at 94 °C for 30 s (denaturation), at 46 °C for 1 min (annealing), at 72 °C for 90 s (extension), and a final extension at 72 °C for 10 min.
The successful PCR products were purified, and Sanger sequenced through an Automated Capillary Electrophoresis Sequencer 3730 DNA Analyzer (Applied Biosystems), using the BigDye® Terminator v3.1 Cycle Sequencing Kit (Life Technologies).
The obtained sequences were analysed, edited, and assembled by Sequence Matrix v. 1.7.839 and compared with those available in GenBank using BLASTn31 (see Tables 1, 2). JModelTest v. 2.1.1040,41 was used to select the best fit model using the Akaike Information Criterion (AIC)42–44. Phylogenetic trees of the ITS-1 and ITS-2 region and cox2 gene locus were constructed using Bayesian inference (BI) with MrBayes, v. 3.2.745. The Bayesian posterior probability analysis was performed using the MCMC algorithm, with four chains, 0.2 as the temperature of heated chains, 1,000,000 generations, with a subsampling frequency of 100 and a burn-in fraction of 0.25. Posterior probabilities were estimated and used to assess support for each branch. Values with a 0.90 posterior probability were considered well-supported. Genetic distances were computed using the Kimura 2-Parameters (K2P) model46 with 1000 bootstrap re-samplings, using MEGA Software, version 7.047.
Table 1.
Species, stage (A: adult, L4: fourth larval stage, L3: third larval stage), host, geographical location, and accession number of sequences of ITS rDNA of Hysterothylacium species included in the Bayesian inference shown in Fig. 2.
| Species | Stage | Host | Geographical location | Accession number | References |
|---|---|---|---|---|---|
| H. aduncum (Rudolphi, 1802) | A | Melanogrammus aeglefinus | Northeast Atlantic Ocean | MW131976 | 76 |
| H. amoyense (Hsü, 1933) Deardorff & Overstreet, 1980 | L3 | Lophius litulon | Chinese waters | MH211527 | 77 |
| H. auctum (Rudolphi, 1802) Deardorff & Overstreet, 1981 | L3 | Zoarces viviparus | Baltic Sea | AF115571 | 78 |
| H. australe Shamsi, 2016 | A | Seriola lalandi | Australian waters | HE862216-HE862225 | 63 |
| H. bidentatum (Linstow, 1899) Deardorff & Overstreet, 1981 | – | – | – | AY603539 | GenBank unpublished |
| H. brucei Shamsi, 2016 | A | Kajikia audax | Australian waters | HE862222-HE862230 | 63 |
| H. deardoffoverstreetorum Knoff, Felizardo, Iñiguez, Maldonado, Torres, Magalhães Pinto & Gomes, 2012 | – | Cynoscion nebulosu | South Carolina coast | MF668866 | GenBank unpublished |
| H. fabri (Rudolphi, 1819) Deardorff & Overstreet, 1980 | L4 | Lophius litulon | Chinese waters | MH211492 | 77 |
| H. fortalezae (Klein, 1973) Deardorff & Overstreet, 1981 | L3 | Maurolicus weitzmani | Gulf of Mexico | KX098563 | 79 |
| H. liparis Li, Xu & Zhang, 2007 | L4 | Lophius litulon | Chinese waters | MH211547 | 77 |
| H. longilabrum Li, Liu & Zhang, 2012 | A | Siganus sp. | Chinese waters | JQ520159 | 80 |
| H. persicum Shamsi, Ghadam, Suthar, Mousavi, Soltani & Mirzargar, 2016 | A | Scomberomorus commerson | Persian Gulf | LT576367-LT576370 | 81 |
| H. reliquiens (Norris & Overstreet, 1975) Deardorff & Overstreet, 1981 | A | Brachirus orientalis | Persian Gulf | MF061682 | 82 |
| H. rigidum (Rudolphi, 1809) Deardorff & Overstreet, 1980 | – | Lophius piscatorius | Ireland, Porcupine Bank | HF680323 | GenBank unpublished |
| H. sinense Li, An & Zhang, 2007 | L3 | Conger myriaster | Chinese waters | MF539804 | 82 |
| H. tetrapteri (Bruce & Cannon, 1989) Moravec & Justine, 2005 | – | – | Chinese waters | KF601901 | GenBank unpublished |
| H. thalassini Bruce, 1990 | A | Priacanthus macracanthus | Chinese waters | JX982129 | 83 |
| H. kajikiae Shamsi, 2016 | A | Kajikia audax | New Caledonia | HE862220-HE862229 | 63 |
| H. zhoushanense Li, Liu & Zhang, 2012 | L3 | Lophius litulon | Chinese waters | MH211555 | 77 |
| H. larval type III | L3 | Lutjanus sp. | Queensland waters (Australia) | FN811721-FN811678 | 84 |
| H. larval type IV | L3 | Halieutaea stellata | Chinese waters | KP203840 | 85 |
| H. larval type IV-A | L3 | Apogonichthyoides taeniatus | Chinese waters | KP326500 | 86 |
| H. larval type IV-B | L3 | Sardionops sagax | Australian waters | MK161418-MK161443 | 87 |
| H. larval type IV-C | L3 | Sillago flindersi | Australian waters | JN631798-JN631805 | 88 |
| H. larval type IV-D | L3 | Sillago flindersi | Australian waters | JN631799-JN631806 | 88 |
| H. larval type V | L3 | Lutjanus carponotatus | Queensland waters | FN811738-FN811699 | 84 |
| H. larval type VI | L3 | Chaetodon lineolatu | Queensland waters | FN811740-FN811701 | 84 |
| H. larval type VII | L3 | Caesio cunning | Queensland waters | FN811749-FN811709 | 84 |
| H. larval type VIII | L3 | Engraulis australis | Australian waters | MK161423-MK161448 | GenBank unpublished |
| H. larval type X | L3 | Upeneichthys lineatus | Australian waters | KC437340-KC437350 | 89 |
| H. larval type XI | L4 | Seriola lalandi | Australian waters | FN811763-FN811717 | 84 |
| H. larval type XII | L4 | Lutjanus carponotatus | Queensland waters | FN811767-FN811720 | 84 |
| H. larval type XIV | L3 | Engraulis australis | Australian waters | MK161424-MK161449 | 87 |
| H. larval type XV | L3 | Otolithes ruber | Persian Gulf, Iran | LT576354-LT576363 | 81 |
| H. larval type XVII | L3 | – | Queensland waters | MG594313-MG594336 | 90 |
| H. larval type XVIII | L3 | Engraulis australis | Australian waters | MK161426-MK161451 | 87 |
| Ascaris lumbricoides Linnaeus, 1758 | A | Homo sapiens | Japan | AB571298 | 91 |
(–: data not stated).
Table 2.
Species, stage (A: adult, L4: fourth larval stage, L3: third larval stage), host, geographical location, and accession number of sequences of mtDNA cox2 of Hysterothylacium species included in the Bayesian inference shown in Fig. 3.
| Species | Stage | Host | Geographical location | Accession number | References |
|---|---|---|---|---|---|
| H. aduncum (Rudolphi, 1802) | – | Theragra chalcogramma | South Korea | KY270874 | GenBank unpublished |
| H. amoyense (Hsü, 1933) Deardorff & Overstreet, 1980 | A | Muraenesox cinereus | Chinese waters | MF120253 | 64 |
| H. corrugatum Deardorff & Overstreet, 1981 | A | Xiphias gladius | Mediterranean Sea | MW456072 | 92 |
| H. deardoffoverstreetorum Knoff, Felizardo, Iñiguez, Maldonado, Torres, Magalhães Pinto & Gomes, 2012 | L3 | Mullus argentinae | Southeast coast of Brazil | MF189875 | 93 |
| H. fabri (Rudolphi, 1819) Deardorff & Overstreet, 1980 | L4 | Zeus faber | Turkey Mediterranean coast | KC862609 | 94 |
| H. fortalezae (Klein, 1973) Deardorff & Overstreet, 1981 | – | – | – | AF179914 | 95 |
| H. liparis Li, Xu & Zhang, 2007 | A | Liparis tanakae | Chinese waters | MF120251 | 64 |
| H. longilabrum Li, Liu & Zhang, 2012 | A | Siganus fuscescens | Chinese waters | MF120247 | 64 |
| H. reliquiens (Norris & Overstreet, 1975) Deardorff & Overstreet, 1981 | A | Brachirus orientalis | Arabian Gulf | KX825845 | 95 |
| H. sinense Li, An & Zhang, 2007 | A | Conger myriaster | Chinese waters | MF120254 | 64 |
| H. tetrapteri (Bruce & Cannon, 1989) Moravec & Justine, 2005 | A | Kajikia audax | Chinese waters | MF120256 | 64 |
| H. thalassini Bruce, 1990 | Priacanthus macracanthus | Chinese waters | MF120250 | 64 | |
| H. zhoushanense Li, Liu & Zhang, 2012 | A | Pseudorhombus oligodon | Chinese waters | MF120248 | 64 |
| Ascaris lumbricoides Linnaeus, 1758 | A | Homo sapiens | Denmark, Odense | KY368760 | GenBank unpublished |
| Toxocara canis Werner, 1782 | – | – | – | AF179923 | 95 |
(–: data not stated).
Results
Identification of squid species
Based on their external morphology (mantle length, development of the inner web, and buccal membrane), the Histioteuthis specimens were identified as belonging to the umbrella squid (8 specimens, of which 7 from the Gulf of Naples and 1 from the Gulf of Salerno) and the reverse jewel squid (2 specimens from the Gulf of Naples). All squids were males showing Wt and DML ranges as it follows 930–2450 g and 111–177 mm for the umbrella squids, and 140–206 g and 86–95 mm for the reverse jewel squids, respectively.
Partial sequences of the mtDNA cox1 were obtained from all the specimens analysed here [8 umbrella squids (691 bp) and 2 reverse jewel squids (693 bp)]. The sequences of umbrella squids here obtained from the Mediterranean Sea showed > 99.5% of identity with sequences already deposited for the same species from the Atlantic Ocean48,49, whereas those from the reverse jewel squid showed 98.45–100% of identity with those of the same species from the Atlantic Ocean48,49, thus confirming their identity as achieved also by morphological analysis.
Parasitological general data
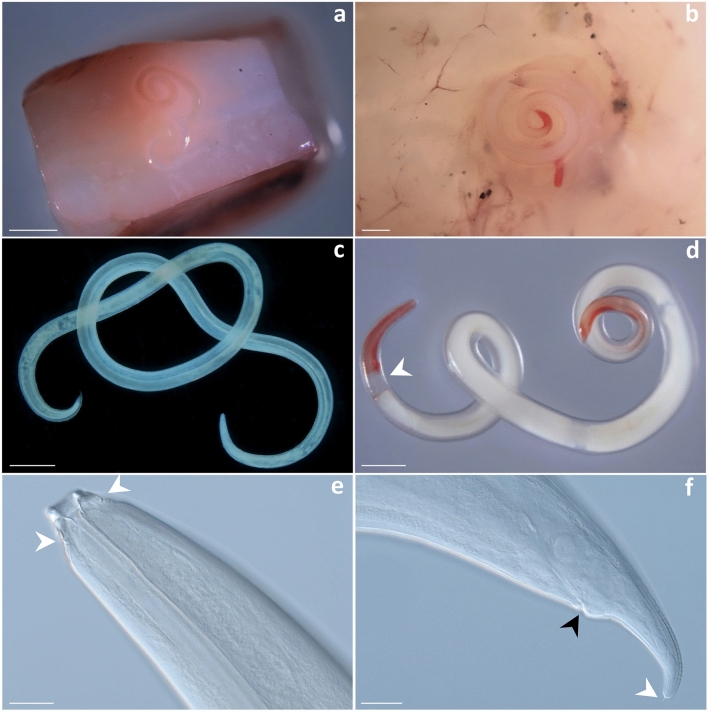
An overall of 161 ascaridoid nematode larvae was collected from the two squid species. Out of them, 133 (82.6%) were morphologically assigned to the genus Lappetascaris showing morphological features of the third stage larvae (L3) Type A (Fig. 1a,c,e,f). The following measurements were achieved on 10 larvae. They were: 26.05 ± 3.16 mm in body length (range: 20.60–29.27 mm) and 0.45 ± 0.10 mm in body width (range: 0.32–0.63 mm), and whitish in colour (Fig. 1a,c). In addition, a total of 28 (17.4%) nematode larvae were morphologically assigned to the genus Anisakis showing features of the L3 Type II larvae (sensu Berland, 1961) (Fig. 1b,d). The measurements obtained on 10 larvae were 26.24 ± 1.94 mm in body length (range: 21.40–28.35 mm) and 0.63 ± 0.01 mm in body width (range: 0.62–0.75 mm), with both extremities reddish in colour (Fig. 1b,d).
Figure 1.
Lappetascaris sp. and Anisakis physeteris in Histioteuthis squids collected from the Tyrrhenian Sea. Lappetascaris larva in the mantle musculature of an umbrella squid (a). Anisakis physeteris in the testis of an umbrella squid. Note the worm extremities reddish in colour (b). Microscopic view of Lappetascaris sp. larva (c) and A. physeteris showing the extremities reddish in colour and ventriculus (arrow) (d). Cephalic extremity of Lappetascaris sp. larva showing sclerotized formations (arrows) (e). Caudal extremity of Lappetascaris sp. larva showing anus (black arrow) and cuticular spike (white arrow) (f). Scale bar: 2000 µm (a); 1000 µm (b,c,d); 50 µm (e,f).
Molecular/genetic analysis of the ascaridoid nematodes
According to the obtained sequences (850 bp) at the ITS region of the rDNA, 28 Anisakis sp. Type II larvae showed 100% identity with the sequences of A. physeteris (Baylis, 1923) previously deposited in GenBank (accession numbers MF668924–MF668926). The mtDNA cox2 gene locus (580 bp), sequenced in a subsample of 10 larvae, also identified the larvae as A. physeteris. Those sequences matched at 99–100% with the mtDNA cox2 sequences of A. physeteris obtained in previous works and deposited in GenBank (accession number KY595212). Sequences of the species A. physeteris here obtained were deposited in GenBank with the accession numbers MW697752-53 (ITS region of the rDNA) and MW691145-46 (mtDNA cox2).
The sequences (850 bp) of the ITS region of rDNA obtained from the 133 Lappetascaris Type A larvae showed 100% identity with the sequences of larvae morphologically indicated as Lappetascaris sp. from octopuses of the genus Eledone Leach, 1817 sequenced by Guardone et al.50 and deposited by the same authors in GenBank as Hysterothylacium sp. (accession numbers MT365530–37). The BLAST analysis of the sequences at mtDNA cox2 gene locus (580 bp) obtained from 10 Lappetascaris sp. larvae showed 88–89% similarity with H. corrugatum Deardorff & Overstreet, 1981 (accession number MW456072).
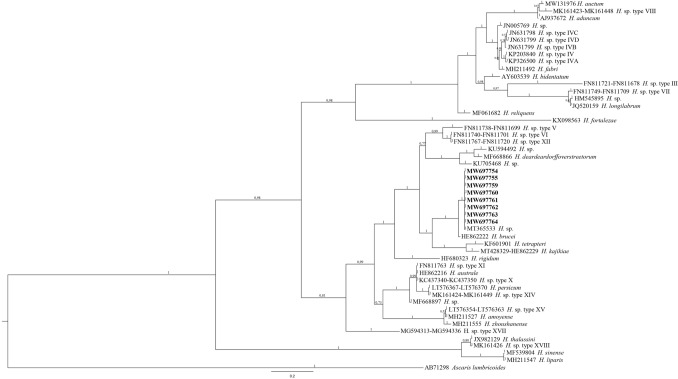
The BI tree topology as inferred from the phylogenetic analysis of the sequences obtained at the ITS region of rDNA of Lappetascaris larvae showed that they are all clustering in the same clade, supported with high probability value, which also includes the sequences MT365530–37 deposited by Guardone et al.50 (Fig. 2). The sequences included in this clade showed a close relationship with the sequence of H. brucei Shamsi, 2016 retrievable in GenBank (Fig. 2). Indeed, the distance values resulted to be K2P = 0.007 and K2P = 0.11, respectively at the ITS-1 and ITS-2 loci, between Lappetascaris sp. and H. brucei. The sequences of Lappetascaris sp. showed a higher K2P value with respect to the species H. deardoffoverstreetorum Knoff, Felizardo, Iniguez, Maldonado, Torres, Magalhaes Pinto & Gomes, 2012 (K2P = 0.06 at ITS-1, K2P = 0.18 at the ITS-2). On the other hand, the BI tree also showed that the clade comprising Lappetascaris sp. and some species of Hysterothylacium here considered and available in GenBank [i.e., H. brucei, H. tetrapteri (Bruce & Cannon, 1989) Moravec & Justine, 2005, H. deardoffoverstreetorum], is well-supported with high value of probability, well distinct from the other clades (Fig. 2) including other species of the genus Hysterothylacium. Indeed, the highest values of K2P between Lappetascaris sp. and other Hysterothylacium species were found with respect to H. fortalezae (Klein, 1973) Deardorff & Overstreet, 1981 (K2P = 0.21 at the ITS-1, KP2 = 0.59 at the ITS-2) (Fig. 2).
Figure 2.
Phylogenetic concatenated tree from Bayesian inference based on ITS-1 and ITS-2 sequences of Lappetascaris sp. obtained in the present study, with respect to the sequences of raphidascaridid species at the same gene loci available in GenBank. The analysis was performed by MrBayes, v. 3.2.7, using the GTR + G substitution model, as implemented in jModeltest 2.1.10. Ascaris lumbricoides was used as outgroup. Sequences obtained in the present study are in bold. Tree was drawn using FigTree v. 1.3.1 (http://tree.bio.ed.ac.uk/software/figtree/).
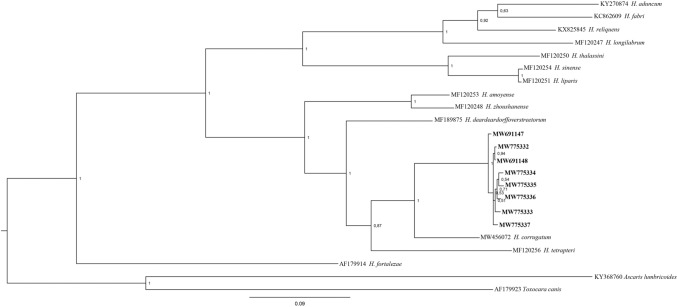
Similar tree topology was shown by the BI inference using the sequence’s analysis of the mtDNA cox2 gene locus. In particular, the sequences of Lappetascaris sp. clustered in a well-supported distinct phylogenetic lineage (Fig. 3) with other species of Hysterothylacium, such as H. deardoffoverstreetorum, H. amoyense (Hsu, 1933) Deardorff & Overstreet, 1980, H. zhoushanense Li, Liu & Zhang, 2012, H. tetrapteri, and H. corrugatum. The closer sequence similarity (K2P = 0.12 ± 0.016) was found between Lappetascaris and H. corrugatum. Higher level of K2P distance was found between the sequences of Lappetascaris and H. fortalezae (K2P = 0.26 ± 0.004) and with respect to H. thalassini Bruce, 1990 (K2P = 0.26 ± 0.006), which are, instead, included in the other well supported clade, with a high probability value (Fig. 3).
Figure 3.
Phylogenetic tree from Bayesian inference on cox2 sequences of Lappetascaris sp. obtained in the present study, with respect to the sequences of raphidascaridid species at the same gene loci available in GenBank. The analysis was performed by MrBayes, v. 3.2.7, using the TrN + I + G substitution model, as implemented in jModeltest 2.1.10. Ascaris lumbricoides and Toxocara canis were used as outgroup. Sequences obtained in the present study are in bold. Tree was drawn using FigTree v. 1.3.1 (http://tree.bio.ed.ac.uk/software/figtree/).
Sequences of Lappetascaris sp. obtained were deposited in GenBank with the accession numbers MW697754-55, MW750359-64 (ITS region) and MW691147-48, MW775332-37 (mtDNA cox2).
Parasitic infection and site preferences
Squids were all found infected by at least 1 nematode larva. The maximum number of larvae was 32 and 11 in the umbrella squid and the reverse jewel squid, respectively. The umbrella squids were found to be infected by both nematode larval forms with prevalence of 87% and abundance (± standard deviation) of infection of 3.5 ± 3.2 for A. physeteris, and prevalence of 100% and abundance of 13.25 ± 9.2 for larvae of Lappetascaris respectively. The reverse jewel squids were infected only with Lappetascaris larvae with prevalence and abundance of 100% and 11 ± 0.0, respectively.
Regarding tissue distribution in the umbrella squid, out of the 28 larvae of A. physeteris, 17 (60.7%) were recorded in the testis (Fig. 1b), 6 (21.4%) were free in the body cavity, 2 (7.1%) were in the gills; the remaining larvae were respectively collected in the nidamental gland, the wall of the stomach, and the connective tissue surrounding the mantle muscle. Out of the 133 Lappetascaris larvae collected from both the umbrella and the reverse jewel squids, 100 (76.3%) were found in the mantle (Fig. 1a), 22 (16.5%) in the body cavity, 4 (3%) in the gills, 2 (1.5%) in the nidamental gland, and 1 (0.7%) was in the wall of the stomach.
Discussion
Squids are considered the trophic bridge for many marine heteroxenous parasites, including the ascaridoid nematodes14,51. The heteroxenous biological cycle of marine ascaridoids is entirely embedded within the food web of marine ecosystems as it follows the trophic relationships among their hosts, as based on a prey-predatory system52,53. Adults of the family Raphidascarididae Hartwich, 1954 are common parasites of predatory teleosts and squids, while crustaceans and various species of fish act, respectively, as intermediate and paratenic hosts33,54. Definitive hosts of Anisakis nematodes of the family Anisakidae Railliet & Henry, 1912 are marine mammals (mainly cetaceans), while the intermediate and/or paratenic hosts are crustaceans, fishes, and squids52,53. Several parasitological available data on squids are based strictly on morphological studies. This does not allow the identification of most of the parasite larvae to the species level, which is of pivotal importance for understanding the host-parasite relationships. This is the case of larvae of anisakid nematodes previously found in the Mediterranean histioteuthids and morphologically assigned to Anisakis sp. Type II (sensu Berland, 1961).
In the present study, Anisakis Type II larvae detected in the umbrella squid were identified, by genetic/molecular markers, as A. physeteris, whose main definitive hosts are cetaceans of the family Physeteridae Gray, 182153,55. This finding suggests the umbrella squid as a transport host in transmitting A. physeteris to Physeteridae in the Mediterranean Sea. Histioteuthid squids are numerically among the prey items most important for the sperm whale from different geographical areas56,57. This is also supported by the large amounts of beaks of umbrella squid and adult specimens of A. physeteris detected as co-occurring in the stomach of the sperm whales Physeter macrocephalus Linnaeus, 1758 and the pygmy sperm whale Kogia breviceps (de Blainville, 1838), definitive hosts of the parasite species in the Mediterranean basin, recently stranded along the Mediterranean coasts53,55,58,59.
Moreover, the occurrence of an unidentified species of the genus Lappetascaris, both in the umbrella and reverse jewel squids, was studied by morphological and genetic analysis. To date the genus Lappetascaris comprises three species: L. lutjani Rasheed, 1965, L. suraiyae Kalyankar, 1975, and L. chandipurensis Gupta & Masoodi, 1990, reported in a wide range of freshwater and brackish fishes from Pakistan, India, and Brazil33,60,61. Unfortunately, no sequences of L. lutjani (type species of the genus) are so far available in GenBank for comparison with the Lappetascaris larvae here sequenced. The morphology of the larvae of the present material was identical with that of Lappetascaris described by Nagasawa & Moravec33. These authors supposed that those larvae found in the mantle of the Japanese flying squid Todarodes pacificus (Steenstrup, 1880) from the western North Pacific Ocean would represent an undescribed species of Lappetascaris whose definitive host, according to the same authors, would be a yet unknown predatory marine fish; unfortunately, the same authors did not perform genetic/molecular analysis of those specimens. However, according to the tree topologies inferred from the BI analyses at both the nuclear and mitochondrial regions, it appears that species of genus Lappetascaris are phylogenetically closely related to other ascaridoid nematodes having in teleost fish of the family Xiphiidae Rafinesque, 1815 and Istiophoridae Rafinesque, 1815 their definitive hosts. Among them, there are the species H. corrugatum and H. tetrapteri; interestingly, these two species appear to be closely related in the BI tree, inferred from the mtDNA cox2, to the Lappetascaris larvae here studied (Fig. 3), and are parasites found at the adult stage in the swordfish Xiphias gladius Linnaeus, 1758 (i.e., H. corrugatum) and in the striped marlin Kajikia audax (Philippi, 1887) (i.e., H. tetrapteri)62. In addition, the BI tree inferred from the ITS region of rDNA sequences analysis showed that Lappetascaris larvae here sequenced are phylogenetically related to H. brucei, which also matures into fish species of the family Istiophoriidae, i.e., in the striped marlin63. Unfortunately, sequences at the mtDNA cox2 gene locus are not available for the species H. brucei; therefore, it was not possible to include them in the BI here obtained at that gene locus. Interestingly, the finding of a close phylogenetic relationship between Lappetascaris sp., H. corrugatum, H. brucei, and H. tetrapteri seems to suggest that the definitive host of the Lappetascaris specimens sequenced here would be a top predator teleost fish belonging to the family Xiphiidae and/or Istiophoriidae, whose species members are known to commonly prey on histioteutiid squids9,10,21.
Moreover, both the BI tree topologies (Figs. 2, 3) also show that all those raphidascaridid species here considered and Lappetascaris species are clustering in two major clades; they include raphidascaridid ascaridoid species maturing in teleost fishes, as also recently shown by a multilocus phylogenetic analysis of ascaridoid nematodes64. However, in the last study, species of genus Lappetascaris were not included. The phylogenetic and morphological analyses performed on the species so far included in the genus Lappetascaris, in comparison with as many as possible raphidascaridid species, as well as by using a multilocus genetic approach, will help to clarify the taxonomy of this group of marine nematodes.
Taking into account that a high parasitic load with A. physeteris larvae has been previously identified in the swordfish65, the finding of several A. physeteris larvae in the examined squid species seems to support that hypothesis. In addition, in our previous parasitological analysis, the swordfish was found to harbour, in its stomach lumen, several squid beaks of the species of genus Histiotheuthis as a residual part of their prey items65.
The supposed life-history strategy of this parasite might explain the finding of A. physeteris in the umbrella squid alone. It has been suggested that different species of Anisakis have evolved different life-history strategies occupying different ecological niches, also in terms of vertical distribution53,64–67. Indeed, each parasite species has its depth preferences, following the most common feeding ecology and depth range of its definitive host. In turn, the depth preferences determine the spectrum of paratenic and intermediate hosts52,53,66,67. For example, Mattiucci & Nascetti52, Klimpel et al.70 and Mattiucci et al.53 suggested a deeper water life cycle for the species A. paggiae, A. physeteris, A. ziphidarum, and A. nascettii, in contrast to an epipelagic life cycle for A. pegreffii and A. simplex (s. s.). The finding of third stage larvae of A. simplex (s. s.) in pelagic squid species corroborates that hypothesis68,69. While, only L3 larvae of A. nascettii were genetically identified in the deep greater hooked squid species Moroteuthopsis ingens (Smith, 1881)71. Both the umbrella and the reverse jewel squids here analyzed are opportunistic deep-sea predators; however, the umbrella squid usually reaches higher depths1. Thus, the present findings could be correlated to a different ecology, in terms of feeding behaviour, and/or to a different spatial and bathymetric distribution of the two histioteuthid species. However, the parasitological analysis carried out on a higher number of specimens of these squid species, as well as other deep squid species, would in future support this hypothesis.
In the Mediterranean Sea, the co-occurrence of A. physeteris and Hysterothylacium larvae was recently recorded in the southern shortfin squid Illex coindetii (Vérany, 1839), with prevalence ranging from 1 to 17%72,73. Unfortunately, the sequences derived from these studies are not available in GenBank for comparison. A total of 9 Lappetascaris larvae were also reported in 5 (6.7%) individuals of the curled octopus Eledone cirrhosa (Lamarck, 1798) and of the musky octopus Eledone moschata (Lamarck, 1798)50. In the present study, the overall prevalence of ascaridoid larvae found in the Histioteuthis squids was higher (100%) than that reported by Culurgioni et al.26 (from 1.83 to 4.5%). This difference could be addressed to some ecological drivers, such as geographical sampling area, prey availability, season or year of sampling, and size of the host as well as to the method of squid inspection. Likewise, the distribution and abundance of the definitive hosts have been suggested as pivotal factors capable to influence the prevalence of infection and parasite abundance74,75. For instance, definitive hosts of A. physeteris (i.e., mysticetes of the family Physeteridae and Kogiidae) release a large amount of parasite eggs into the seawater with their faeces, so host distribution largely determines where infection with this nematode occurs74,75. However, the reasons for the higher prevalence and abundance in the present study are impaired by the lack of data on the biological cycle of these nematodes and in general by missing data on the ecology and biology of Histhioteuthis squids in the Mediterranean basin.
In the present study, different preference for the site of infection were recorded for the two ascaridoid taxa. Larvae of A. physeteris were mainly found in the gonads (testes) (60.7%) of squids; in contrast, the Lappetascaris larvae were mainly found in the mantle musculature (76.3%). Different site preferences for larval forms of Anisakis spp. and Lappetascaris spp. are in accordance with previous studies. Localization of Anisakis larvae in gonads of squids with parasitic castration was the most important pathological change observed by Abollo et al.14, where nematodes caused the partial destruction and alteration of gonad tissue and partial inhibition of gamete formation in hosts. In contrast, the localization of Lappetascaris larvae in the mantle of both Histioteuthis squids agrees with Nagasawa & Moravec33, that found this site preference as the most common for the genus Lappetascaris.
The main limitation of this study can be considered the low number of squids examined which makes our results not definitive. However, due to the difficulty to obtain specimens of these species, and the scarce published data on both Histioteuthis squids and their ascaridoid nematodes from the Mediterranean Sea we believe this study provides ecological, molecular and phylogenetic data that allow for a better characterisation of these poorly known hosts and their parasites.
In conclusion, although further studies are still necessary to understand which is the source of infection of both parasite taxa that infected the present Histioteuthis squids, this study provided for the first time the molecular identification of ascaridoid nematodes found in the umbrella and reverse jewel squids, and highlight the importance of both squids as transmitting hosts of Lappetascaris larvae to still unknown top predator fishes, and of the umbrella squid as vector host of A. physeteris to Physeteridae cetaceans. Studies are currently under way to identify the definitive host for the present larval forms of Lappetascaris, according to the present data and known fishes which commonly feed on Histioteuthis squids in the Mediterranean basin.
Acknowledgements
Sampling in the Gulf of Naples was supported by the project ADViSE (PG/2018/0494374). The Maglione family (Fabrizio, Francesco, Giuseppe, Salvatore, and Vincenzo) (Giovanni Padre fishing vessel, Naples, Italy) offered the highest possible support during trawling activities.
Author contributions
M.P. performed host dissection, genetic/molecular analysis of nematodes and wrote the main manuscript text. S.M. supervised genetic/molecular data of nematodes and wrote the main manuscript text. F.C. and D.O. performed genetic identification of the hosts. M.S. conceived and financed the study, performed host dissection, parasitological analysis, supervised genetic/molecular analysis of nematodes, and wrote the main manuscript text. All authors reviewed and approved the final manuscript.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Voss, N. A., Nesis, K. N. & Rodhouse, P. G. The cephalopod family Histioteuthidae (Oegopsida): systematics, biology, and biogeography in Systematics and Biogeography of Cephalopods (eds. Voss, N. A., Vecchione, M., Toll, R. B. & Sweeney M. J.) 277–291 (Smithsonian Contributions to Zoology, 1998).
- 2.Crocetta F, et al. Biogeographical homogeneity in the eastern Mediterranean Sea - III: new records and a state of the art of Polyplacophora, Scaphopoda and Cephalopoda (Mollusca) from Lebanon. Spixiana. 2014;37(2):183–206. [Google Scholar]
- 3.Guerra, A. Mollusca, cephalopoda in Fauna Iberica (eds. Ramos, M. A. et al.) 327 (Museo Nacional de Ciencias Naturales, 1992).
- 4.Cuccu D, Mereu M, Loi B, Sanna I, Cau A. The squid family Histioteuthidae in the Sardinian waters. Biol. Mar. Mediterr. 2007;13:262–263. [Google Scholar]
- 5.Jereb, P. & Roper, C. F. E. Cephalopods of the world. An annotated and illustrated catalogue of cephalopod species known to date. Myopsid and Oegopsid Squids in FAO species catalogue for fishery purposes (ed. FAO) 649 (FAO, 2010).
- 6.Quetglas A, de Mesa A, Ordines F, Grau A. Life history of the deep-sea cephalopod family Histioteuthidae in the western Mediterranean. Deep Sea Res. Part I. 2010;57:999–1008. doi: 10.1016/j.dsr.2010.04.008. [DOI] [Google Scholar]
- 7.Oshima T, Shimazu T, Koyama H, Akahane HJJ. On the larvae of the genus Anisakis (Nematoda: Anisakidaae) from euphausiids. Jpn. J. Parasitol. 1969;18:241–248. [Google Scholar]
- 8.Hochberg FG. The parasites of cephalopods: a review. Mem. Nat. Mus. Vict. 1983;44:109–145. doi: 10.24199/j.mmv.1983.44.10. [DOI] [Google Scholar]
- 9.Bello G. Role of cephalopods in the diet of the swordfish, Xiphias gladius, from the eastern Mediterranean Sea. Bull. Mar. Sci. 1991;49:312–324. [Google Scholar]
- 10.Bello G. Teuthophagous predators as collectors of oceanic cephalopods: the case of the Adriatic Sea. Boll. Malacol. 1996;32:71–78. [Google Scholar]
- 11.Santos M, et al. Stomach contents of sperm whales Physeter macrocephalus stranded in the North Sea 1990–1996. Mar. Ecol. Prog. Ser. 1999;183:281–294. doi: 10.3354/meps183281. [DOI] [Google Scholar]
- 12.Xavier J, et al. Current status of using beaks to identify cephalopods: III International Workshop and training course on Cephalopod beaks, Faial island, Azores, April 2007. Arquipélago-Life Mar. Sci. 2007;24:41–48. [Google Scholar]
- 13.Marcogliese DJ, Cone DK. Food webs: a plea for parasites. Trends Ecol. Evol. 1997;12:320–325. doi: 10.1016/S0169-5347(97)01080-X. [DOI] [PubMed] [Google Scholar]
- 14.Abollo E, et al. Squid as trophic bridges for parasite flow within marine ecosystems: the case of Anisakis simplex (Nematoda: Anisakidae), or when the wrong way can be right. Afr. J. Mar. Sci. 1998;20:223–232. doi: 10.2989/025776198784126575. [DOI] [Google Scholar]
- 15.Klimpel, S., Seehagen, A., Palm, H. W. & Rosenthal, H. Deep-water metazoan fish parasites of the world. (eds. Klimpel, S., Seehagen, A., Palm, H. W. & Rosenthal, H.) (Logos Verlag, 2001).
- 16.Parker GA, Chubb JC, Ball MA, Roberts GN. Evolution of complex life cycles in helminth parasites. Nature. 2003;425:480–484. doi: 10.1038/nature02012. [DOI] [PubMed] [Google Scholar]
- 17.Santoro M, Iaccarino D, Bellisario B. Host biological factors and geographic locality influence predictors of parasite communities in sympatric sparid fishes off the southern Italian coast. Sci. Rep. 2020;10(1):13283. doi: 10.1038/s41598-020-69628-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pascual S, González A, Arias C, Guerra A. Helminth infection in the short-finned squid Illex coindetii (Cephalopoda, Ommastrephidae) off NW Spain. Dis. Aquat. Org. 1995;23:71–75. doi: 10.3354/dao023071. [DOI] [Google Scholar]
- 19.Petrić M, Mladineo I, Šifner S. Insight into the short-finned squid Illex coindetii (Cephalopoda: Ommastrephidae) feeding ecology: is there a link between helminth parasites and food composition? J. Parasitol. 2011;97:55–62. doi: 10.1645/GE-2562.1. [DOI] [PubMed] [Google Scholar]
- 20.Klimpel S, Rückert S. Life cycle strategy of Hysterothylacium aduncum to become the most abundant anisakid fish nematode in the North Sea. Parasitol. Res. 2005;97:141–149. doi: 10.1007/s00436-005-1407-6. [DOI] [PubMed] [Google Scholar]
- 21.Tursi A, D'Onghia A, Matarrese A, Panetta P, Maiorano P. Finding of uncommon cephalopods (Ancistroteuthis lichtensteinii, Histioteuthis bonnellii, Histioteuthis reversa) and first record of Chiroteuthis veranyi in the Ionian Sea. Cah. Biol. Mar. 1994;35:339–346. [Google Scholar]
- 22.Koutsoubas D, Boyle P. Histioteuthis bonnelli (Férussac, 1835) (Cephalopoda) in the Eastern Mediterranean: new record and biological considerations. J. Mollus. Stud. 1999;65:380–383. doi: 10.1093/mollus/65.3.380. [DOI] [Google Scholar]
- 23.Bello G. How rare is Histioeuthis bonnellii (Cephalopoda: Histioteuthidae) in the eastern Mediterranean Sea? J. Mollus. Stud. 2000;66:575–576. doi: 10.1093/mollus/66.4.575. [DOI] [Google Scholar]
- 24.Belcari P, Sartor P. Bottom trawling teuthofauna of the northern Tyrrhenian Sea. Sci. Mar. 1993;57:145–152. [Google Scholar]
- 25.Quetglas A, Carbonell A, Sánchez P. Demersal continental shelf and upper slope cephalopod assemblages from the Balearic Sea (North-Western Mediterranean). Biological aspects of some deep-sea species. Estuar. Coast. Shelf Sci. 2000;50:739–749. doi: 10.1006/ecss.1999.0603. [DOI] [Google Scholar]
- 26.Culurgioni J, Cuccu D, Mereu M, Figus V. Larval anisakid nematodes of Histioteuthis reversa (Verril, 1880) and H. bonnellii (Férussac, 1835) (Cephalopoda: Teuthoidea) from Sardinian Channel (western Mediterranean) Bull. Eur. Ass. Fish Pathol. 2010;30:217. [Google Scholar]
- 27.Capua, D. I cefalopodi delle coste e dell'Arcipelago Toscano: sistematica, anatomia, fisiologia e sfruttamento delle specie presenti nel Mediterraneo. 446 (Evolver, 2004).
- 28.Crocetta F, et al. Bottom-trawl catch composition in a highly polluted coastal area reveals multifaceted native biodiversity and complex communities of fouling organisms on litter discharge. Mar. Environ. Res. 2020;155:104875. doi: 10.1016/j.marenvres.2020.104875. [DOI] [PubMed] [Google Scholar]
- 29.Folmer O, Black M, Hoeh W, Lutz R, Vrijenhoek R. DNA primers for amplification of mitochondrial cytochrome c oxidase subunit I from diverse metazoan invertebrates. Mol. Mar. Biol. Biotechnol. 1994;3:294–299. [PubMed] [Google Scholar]
- 30.Meyer CP. Molecular systematics of cowries (Gastropoda: Cypraeidae) and diversification patterns in the tropics. Biol. J. Linn. Soc. 2003;79:401–459. doi: 10.1046/j.1095-8312.2003.00197.x. [DOI] [Google Scholar]
- 31.Morgulis A, et al. Database indexing for production MegaBLAST searches. Bioinformatics. 2008;24:1757–1764. doi: 10.1093/bioinformatics/btn322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Berland B. Nematodes from some Norwegian marine fishes. Sarsia. 1961;2:1–50. doi: 10.1080/00364827.1961.10410245. [DOI] [Google Scholar]
- 33.Nagasawa K, Moravec F. Larval anisakid nematodes of Japanese common squid (Todarodes pacificus) from the Sea of Japan. J. Parasitol. 1995;81:69–75. doi: 10.2307/3284008. [DOI] [PubMed] [Google Scholar]
- 34.Nagasawa K, Moravec F. Larval anisakid nematodes from four species of squid (Cephalopoda: Teuthoidea) from the central and western North Pacific Ocean. J. Nat. Hist. 2002;36:8. doi: 10.1080/00222930110051752. [DOI] [Google Scholar]
- 35.Bush AO, Lafferty KD, Lotz JM, Shostak AW. Parasitology meets ecology on its own terms: Margolis et al. revisited. J. Parasitol. 1997;83(4):575–583. doi: 10.2307/3284227. [DOI] [PubMed] [Google Scholar]
- 36.Zhu X, Gasser RB, Podolska M, Chilton N. Characterisation of anisakid nematodes with zoonotic potential by nuclear ribosomal DNA sequences. Int. J. Parasitol. 1998;28:1911–1921. doi: 10.1016/S0020-7519(98)00150-7. [DOI] [PubMed] [Google Scholar]
- 37.Nadler SA, Hudspeth DS. Phylogeny of the Ascaridoidea (Nematoda: Ascaridida) based on three genes and morphology: hypotheses of structural and sequence evolution. J. Parasitol. 2000;86:380–393. doi: 10.1645/0022-3395(2000)086[0380:POTANA]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 38.Valentini A, et al. Genetic relationships among Anisakis species (Nematoda: Anisakidae) inferred from mitochondrial cox2 sequences, and comparison with allozyme data. J. Parasitol. 2006;92:156–166. doi: 10.1645/GE-3504.1. [DOI] [PubMed] [Google Scholar]
- 39.Vaidya G, Lohman DJ, Meier R. SequenceMatrix: concatenation software for the fast assembly of multi-gene datasets with character set and codon information. Cladistics. 2011;27:171–180. doi: 10.1111/j.1096-0031.2010.00329.x. [DOI] [PubMed] [Google Scholar]
- 40.Darriba D, Taboada GL, Doallo R, Posada D. jModelTest 2: more models, new heuristics and parallel computing. Nat. Methods. 2012;9(8):772. doi: 10.1038/nmeth.2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Guindon S, Gascuel O. A simple, fast and accurate method to estimate large phylogenies by maximum-likelihood. Syst. Biol. 2003;52:696–704. doi: 10.1080/10635150390235520. [DOI] [PubMed] [Google Scholar]
- 42.Akaike, H. Information theory and an extension of the maximum likelihood principle in Proceeding of the second international symposium on information theory (eds. Petrov, T. & Caski, F.) 267–281 (Akademiai Kiado, 1973).
- 43.Posada D. jModelTest: phylogenetic model averaging. Mol. Biol. Evol. 2008;25:1253–1256. doi: 10.1093/molbev/msn083. [DOI] [PubMed] [Google Scholar]
- 44.Posada D, Buckley TR. Model selection and model averaging in phylogenetics: advantages of Akaike Information Criterion and Bayesian approaches over likelihood ratio tests. Syst. Biol. 2004;53:793–808. doi: 10.1080/10635150490522304. [DOI] [PubMed] [Google Scholar]
- 45.Ronquist F, Huelsenbeck J. MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics. 2003;19:1572–1574. doi: 10.1093/bioinformatics/btg180. [DOI] [PubMed] [Google Scholar]
- 46.Kimura M. A simple method for estimating evolutionary rates of base substitutions through comparative studies of nucleotide sequences. J. Mol. Evol. 1980;16:111–120. doi: 10.1007/BF01731581. [DOI] [PubMed] [Google Scholar]
- 47.Kumar S, et al. MEGA X: molecular evolutionary genetics analysis across computing platforms. Mol. Biol. Evol. 2018;35:1547–1549. doi: 10.1093/molbev/msy096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lindgren AR. Molecular inference of phylogenetic relationships among Decapodiformes (Mollusca: Cephalopoda) with special focus on the squid Order Oegopsida. Mol. Phylogenet. 2010;56(1):77–90. doi: 10.1016/j.ympev.2010.03.025. [DOI] [PubMed] [Google Scholar]
- 49.Taite M, Vecchione M, Fennell S, Allcock LA. Paralarval and juvenile cephalopods within warm-core eddies in the North Atlantic. Bul. Mar. Sci. 2020;96(2):235–262. doi: 10.5343/bms.2019.0042. [DOI] [Google Scholar]
- 50.Guardone L, et al. Larval ascaridoid nematodes in horned and musky octopus (Eledone cirrhosa and E. moschata) and longfin inshore squid (Doryteuthis pealeii): safety and quality implications for cephalopod products sold as fresh on the Italian market. Int. J. Food Microbiol. 2020;333:108812. doi: 10.1016/j.ijfoodmicro.2020.108812. [DOI] [PubMed] [Google Scholar]
- 51.Pascual, S., Abollo, E., Mladineo, I. & Gestal, C. Metazoa and Related Diseases in Handbook of Pathogens and Diseases in Cephalopods (eds. Gestal, C., Pascual S., Guerra A., Fiorito G. & Vieites, J. M.) 169–179 (2019).
- 52.Mattiucci S, Nascetti G. Advances and trends in the molecular systematics of anisakid nematodes, with implications for their evolutionary ecology and host—parasite co-evolutionary processes. Adv. Parasitol. 2008;66:47–148. doi: 10.1016/S0065-308X(08)00202-9. [DOI] [PubMed] [Google Scholar]
- 53.Mattiucci S, Cipriani P, Levsen A, Paoletti M, Nascetti G. Molecular epidemiology of Anisakis and Anisakiasis: an ecological and evolutionary road map. Adv. Parasitol. 2018;99:93–263. doi: 10.1016/bs.apar.2017.12.001. [DOI] [PubMed] [Google Scholar]
- 54.Kie M. Aspects of the life cycle and morphology of Hysterothylacium aduncum (Rudolphi, 1802) (Nematoda, Ascaridoidea, Anisakidae) Can. J. Zool. 1993;71:1289–1296. doi: 10.1139/z93-178. [DOI] [Google Scholar]
- 55.Santoro M, et al. Helminth parasites of the dwarf sperm whale Kogia sima (Cetacea: Kogiidae) from the Mediterranean Sea, with implications on host ecology. Dis. Aquat. Organ. 2018;14:175–182. doi: 10.3354/dao03251. [DOI] [PubMed] [Google Scholar]
- 56.Kawakami T. A review of sperm whale food. Sci. Rep. Whales Res. Inst. 1980;32:199–218. [Google Scholar]
- 57.Garibaldi F, Podestà M. Stomach contents of a sperm whale (Physeter macrocephalus) stranded in Italy (Ligurian Sea, northwestern Mediterranean) JMBA. 2014;94(6):1087–1091. doi: 10.1017/S0025315413000428. [DOI] [Google Scholar]
- 58.Mattiucci S, Nascetti G, Bullini L, Orecchia P, Paggi L. Genetic structure of Anisakis physeteris and its differentiation from the Anisakis simplex complex (Ascaridida: Anisakidae) Parasitology. 1986;93:383–387. doi: 10.1017/S0031182000051544. [DOI] [PubMed] [Google Scholar]
- 59.Mattiucci S, et al. Genetic divergence and reproductive isolation between Anisakis brevispiculata and Anisakis physeteris (Nematoda: Anisakidae) Int. J. Parasitol. 2001;31:9–14. doi: 10.1016/S0020-7519(00)00125-9. [DOI] [PubMed] [Google Scholar]
- 60.Gupta PC, Masoodi BA. Three new and one known nematode (Family: Anisakidae) from marine fishes of India. Indian J. Parasitol. 1990;14(2):157–164. [Google Scholar]
- 61.Vicente JJ, Mincarone MM, Pint RM. First report of Lappetascaris lutjani Rasheed, 1965 (Nematoda, Ascaridoidea, Anisakidae) parasitizing Trachipterus arawatae (Pisces, Lampridiformes) on the Atlantic coast of Brazil. Mem. Inst. Oswaldo Cruz. 2002;97:93–94. doi: 10.1590/s0074-02762002000100015(2002). [DOI] [PubMed] [Google Scholar]
- 62.Bruce NL, Cannon LRG. Hysterothylacium, Iheringascaris and Maricostula new genus, nematodes (Ascaridoidea) from Australian pelagic marine fishes. J. Nat. Hist. 1989;23(6):1397–1441. doi: 10.1080/00222938900770771. [DOI] [Google Scholar]
- 63.Shamsi S. Morphometric and molecular descriptions of three new species of Hysterothylacium (Nematoda: Raphidascarididae) from Australian marine fish. J. Helminthol. 2016;91:1–12. doi: 10.1017/S0022149X16000596. [DOI] [PubMed] [Google Scholar]
- 64.Li L, et al. Molecular phylogeny and dating reveal a terrestrial origin in the early carboniferous for ascaridoid nematodes. Syst. Biol. 2018;67(5):888–900. doi: 10.1093/sysbio/syy018. [DOI] [PubMed] [Google Scholar]
- 65.Garcia A, Mattiucci S, Santos MN, Damiano S, Nascetti G. Metazoan parasites of Xiphias gladius (L. 1758) (Pisces: Xiphiidae) from the Atlantic Ocean: implications for host stock identification. ICES J. Mar. Sci. 2010;68:175–182. doi: 10.1093/icesjms/fsq147. [DOI] [Google Scholar]
- 66.Klimpel, S. & Palm, H. W. Anisakid Nematode (Ascaridoidea) Life Cycles and Distribution: Increasing Zoonotic Potential in the Time of Climate Change? in Progress in Parasitology. Parasitology Research Monographs (ed. Mehlhorn, H.) 10.1007/978-3-642-21396-0_11 (Springer, 2011).
- 67.Kuhn T, Cunze S, Kochmann J, Klimpel S. Environmental variables and definitive host distribution: a habitat suitability modelling for endohelminth parasites in the marine realm. Sci. Rep. 2016;6:30246. doi: 10.1038/srep30246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Cipriani P, et al. Occurrence of larval ascaridoid nematodes in the Argentinean short-finned squid Illex argentinus from the Southwest Atlantic Ocean (off Falkland Islands) Int. J. Food. Microbiol. 2019;297:27–31. doi: 10.1016/j.ijfoodmicro.2019.02.019. [DOI] [PubMed] [Google Scholar]
- 69.Cipriani P, et al. Anisakis simplex (s.s.) larvae (Nematoda: Anisakidae) hidden in the mantle of European flying squid Todarodes sagittatus (Cephalopoda: Ommastrephidae) in NE Atlantic Ocean: food safety implications. Int. J. Food Microbiol. 2021;339:109021. doi: 10.1016/j.ijfoodmicro.2020.109021. [DOI] [PubMed] [Google Scholar]
- 70.Klimpel S, Kellermanns E, Palm HW. The role of pelagic swarm fish (Myctophidae: Teleostei) in the oceanic life cycle of Anisakis sibling species at the Mid-Atlantic Ridge, Central Atlantic. Parasitol. Res. 2008;104:43–53. doi: 10.1007/s00436-008-1157-3. [DOI] [PubMed] [Google Scholar]
- 71.Mattiucci S, Paoletti M, Webb SC. Anisakis nascettii n. sp. (Nematoda: Anisakidae) from beaked whales of the southern hemisphere: morphological description, genetic relationships between congeners and ecological data. Syst. Parasitol. 2009;74:199–217. doi: 10.1007/s11230-009-9212-8. [DOI] [PubMed] [Google Scholar]
- 72.Pico-Duran G, Pulleiro-Potel L, Abollo E, Pascual S, Munoz P. Molecular identification of Anisakis and Hysterothylacium larvae in commercial cephalopods from the Spanish Mediterranean coast. Vet. Parasitol. 2016;220:47–53. doi: 10.1016/j.vetpar.2016.02.020. [DOI] [PubMed] [Google Scholar]
- 73.Menconi V, et al. Occurrence of ascaridoid nematodes in Illex coindetii, a commercially relevant cephalopod species from the Ligurian Sea (Northwest Mediterranean Sea) Food Control. 2020 doi: 10.1016/j.foodcont.2020.107311. [DOI] [Google Scholar]
- 74.Blazekovic K, et al. Three Anisakis spp. isolated from toothed whales stranded along the eastern Adriatic Sea coast. Int. J. Parasitol. 2015;45(1):17–31. doi: 10.1016/j.ijpara.2014.07.012. [DOI] [PubMed] [Google Scholar]
- 75.Santoro M, et al. Epidemiology of Sulcascaris sulcata (Nematoda: Anisakidae) ulcerous gastritis in the Mediterranean loggerhead sea turtle (Caretta caretta) Parasitol. Res. 2019;118:1457–1463. doi: 10.1007/s00436-019-06283-0. [DOI] [PubMed] [Google Scholar]
- 76.Bao M, Cipriani P, Giulietti L, Drivenes N, Levsen A. Quality issues related to the presence of the fish parasitic nematode Hysterothylacium aduncum in export shipments of fresh Northeast Arctic cod (Gadus morhua) Food Control. 2020;121:107724. doi: 10.1016/j.foodcont.2020.107724. [DOI] [Google Scholar]
- 77.Zhang K, Xu Z, Chen HX, Guo N, Li L. Anisakid and raphidascaridid nematodes (Ascaridoidea) infection in the important marine food-fish Lophius litulon (Jordan) (Lophiiformes: Lophiidae) Int. J. Food Microbiol. 2018;284:105–111. doi: 10.1016/j.ijfoodmicro.2018.08.002. [DOI] [PubMed] [Google Scholar]
- 78.Szostakowska B, Myjak P, Kur J, Sywula T. Molecular evaluation of Hysterothylacium auctum (Nematoda, Ascaridida, Raphidascarididae) taxonomy from fish of the southern Baltic. Acta Parasitol. 2001;46(3):194–201. [Google Scholar]
- 79.Andres MJ, Peterson MS, Overstreet RM. Endohelminth parasites of some midwater and benthopelagic stomiiform fishes from the northern Gulf of Mexico. Gulf Caribb. Res. 2016;27:11–19. doi: 10.18785/gcr.2701.02. [DOI] [Google Scholar]
- 80.Li L, Liu YY, Zhang LP. Morphological and molecular identification of Hysterothylacium longilabrum sp. Nov. (Nematoda: Anisakidae) and larvae of different stages from marine fishes in the South China Sea. Parasitol. Res. 2012;111(2):767–777. doi: 10.1007/s00436-012-2897-7. [DOI] [PubMed] [Google Scholar]
- 81.Shamsi S, et al. Occurrence of ascaridoid nematodes in selected edible fish from the Persian Gulf and description of Hysterothylacium larval type XV and Hysterothylacium persicum n. sp. (Nematoda: Raphidascarididae) Int. J. Food Microbiol. 2016;236:65–67. doi: 10.1016/j.ijfoodmicro.2016.07.006. [DOI] [PubMed] [Google Scholar]
- 82.Chen HX, et al. Detection of ascaridoid nematode parasites in the important marine food-fish Conger myriaster (Brevoort) (Anguilliformes: Congridae) from the Zhoushan fishery, China. Parasites Vectors. 2018;11:274. doi: 10.1186/s13071-018-2850-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Liu YY, Xu Z, Zhang LP, Li L. Redescription and genetic characterization of Hysterothylacium thalassini Bruce, 1990 (Nematoda: Anisakidae) from marine fishes in the South China Sea. J. Parasitol. 2013;99:655–661. doi: 10.1645/12-136.1. [DOI] [PubMed] [Google Scholar]
- 84.Shamsi S, Gasser R, Beveridge I. Description and genetic characterisation of Hysterothylacium (Nematoda: Raphidascarididae) larvae parasitic in Australian marine fishes. Parasitol. Int. 2013;62:320–328. doi: 10.1016/j.parint.2012.10.001. [DOI] [PubMed] [Google Scholar]
- 85.Li L, Zhao WT, Guo YN, Zhang LP. Nematode parasites infecting the starry batfish Halieutaea stellata (Vahl) (Lophiiformes: Ogcocephalidae) from the East and South China Sea. J. Fish. Dis. 2016;39(5):515–529. doi: 10.1111/jfd.12374. [DOI] [PubMed] [Google Scholar]
- 86.Zhao WT, et al. Ascaridoid parasites infecting in the frequently consumed marine fishes in the coastal area of China: a preliminary investigation. Parasitol. Int. 2016;65(2):87–98. doi: 10.1016/j.parint.2015.11.002. [DOI] [PubMed] [Google Scholar]
- 87.Hossen MS, Shamsi S. Zoonotic nematode parasites infecting selected edible fish in New South Wales, Australia. Int. J. Food Microbiol. 2019;308:108306. doi: 10.1016/j.ijfoodmicro.2019.108306. [DOI] [PubMed] [Google Scholar]
- 88.Jabbar A, et al. Mutation scanning-based analysis of anisakid larvae from Sillago flindersi from Bass Strait, Australia. Electrophoresis. 2012;33:499–505. doi: 10.1002/elps.201100438. [DOI] [PubMed] [Google Scholar]
- 89.Jabbar A, et al. Molecular characterization of anisakid nematode larvae from 13 species of fish from Western Australia. Int. J. Food Microbiol. 2013;161(3):247–253. doi: 10.1016/j.ijfoodmicro.2012.12.012. [DOI] [PubMed] [Google Scholar]
- 90.Shamsi S, Stellar E, Chen Y. New and known zoonotic nematode larvae within selected fish species from Queensland waters in Australia. Int. J. Food Microbiol. 2018;272:73–82. doi: 10.1016/j.ijfoodmicro.2018.03.007. [DOI] [PubMed] [Google Scholar]
- 91.Arizono N, et al. Ascariasis in Japan: is pig-derived ascaris infecting humans? Jpn. J. Infect. Dis. 2010;63(6):447–448. [PubMed] [Google Scholar]
- 92.Mattiucci S, et al. Metazoan parasitic infections of swordfish (Xiphias gladius) from the Mediterranean Sea and Atlantic Gibraltar waters: implications for stock assessment. Col. Vol. Sci. Pap. ICCAT. 2005;58(4):1470–1482. [Google Scholar]
- 93.Di Azevedo MIN, Iñiguez AM. Nematode parasites of commercially important fish from the southeast coast of Brazil: morphological and genetic insight. Int. J. Food Microbiol. 2018;267:29–41. doi: 10.1016/j.ijfoodmicro.2017.12.014. [DOI] [PubMed] [Google Scholar]
- 94.Pekmezci GZ, Yardimci B, Onuk EE, Umur S. Molecular characterization of Hysterothylacium fabri (Nematoda: Anisakidae) from Zeus faber (Pisces: Zeidae) caught off the Mediterranean coasts of Turkey based on nuclear ribosomal and mitochondrial DNA sequences. Parasitol. Int. 2014;63(1):127–131. doi: 10.1016/j.parint.2013.10.006. [DOI] [PubMed] [Google Scholar]
- 95.Zhao JY, Zhao WT, Ali AH, Chen HX, Li L. Morphological variability, ultrastructure and molecular characterisation of Hysterothylacium reliquens (Norris & Overstreet, 1975) (Nematoda: Raphidascarididae) from the oriental sole Brachirus orientalis (Bloch & Schneider) (Pleuronectiformes: Soleidae) Parasitol. Int. 2016;66(1):831–838. doi: 10.1016/j.parint.2016.09.012. [DOI] [PubMed] [Google Scholar]