Abstract
Vitamin D has an immunomodulating property that regulates the inflammatory response. In this study, the aim was to evaluate the relationship between vitamin D levels and clinical severity and inflammation markers in children and adolescents with COVID-19. The clinical and laboratory records of 103 pediatric cases with COVID-19, whose vitamin D levels had been measured, were retrospectively reviewed. The cases were divided into groups according to their clinical severity (asymptomatic, mild, and moderate-to-severe) and vitamin D levels. The moderate-to-severe clinical group had significantly higher inflammation markers (CRP, procalcitonin, fibrinogen, d-dimer) and a lower lymphocyte count compared to both the mild and asymptomatic groups. The 25 OH vitamin D levels were also significantly lower (p < 0.001), and the ratio of vitamin D deficiency was 70.6% in the moderate-to-severe group. The vitamin D–deficient group had a significantly higher age and fibrinogen levels while also having a lower lymphocyte count compared to the insufficient and normal groups. The 25 OH vitamin D level was correlated positively with the lymphocyte count (r = 0.375, p = <0.001), and negatively with age (r = −0.496, p = <0.001), CRP (r = −0.309, p = 0.002) and fibrinogen levels (r = −0.381, p = <0.001). In a logistic regression analysis, vitamin D deficiency, d-dimer, and fibrinogen levels on admission were independent predictors of severe clinical course.
Conclusion: This study revealed an association between vitamin D deficiency and clinical severity, in addition to inflammation markers in pediatric COVID-19 cases. Prophylactic vitamin D supplementation may be considered, especially in the adolescent age group.
|
What is Known: • • The pathology of COVID-19 involves a complex interaction between the SARS-CoV-2 and the immune system. Hyperinflammation/cytokine storm is held responsible for the severity of the disease. • Vitamin D has multiple roles in the immune system that can modulate the body reaction to an infection. | |
|
What is New: • • Clinically more severe group had significantly lower vit D levels and significantly higher inflammation markers. • Lower 25 OH vit D levels were associated with higher inflammation markers, suggesting an important role of vitamin D in the clinical course of COVID-19 in children and adolescents probably by regulating the systemic inflammatory response. |
Supplementary Information
The online version contains supplementary material available at 10.1007/s00431-021-04030-1.
Keywords: COVID-19, Vitamin D, Inflammation, Children, Pediatric
Introduction
Coronavirus disease 2019 (COVID-19), which is caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) first appeared in Wuhan, China, and was announced as a pandemic in March 2020 [1, 2]. The clinical severity of the infection varies from a simple cold to severe acute respiratory syndrome (ARDS) or even death [3]. The infection is reported to be rarely seen in childhood and commonly affects ≥15 years old [4]; however, the number of pediatric COVID-19 cases has increased rapidly with the global spread of the infection.
Although the clinical course has been observed to be milder in children than in adults, the prevalence of serious disease has been reported as 3–10% and is especially high in children under 1 year old [5].
Although basic guidelines on infection control have been published and studies on vaccination are ongoing, time is needed for an effective vaccine to become available for most people. This has led researchers to focus on a variety of drugs currently available to find an effective treatment. Vitamin D has been proven to increase the expression of genes associated with antioxidation, strengthen cellular immunity, and balance adaptive immunity [6–8]. Based on the data, it has been suggested that vitamin D supplements can alleviate the clinical course of COVID-19 and be used as an effective treatment method [9, 10]. A few adult studies have demonstrated an association between vitamin D deficiency and COVID-19 severity and mortality [11, 12]; however, pediatric data are scarce.
In this study, the aim was to determine the potential association between the level of vitamin D (25 OH vitamin D), clinical course, and inflammatory markers (C-reactive protein, fibrinogen, d-dimer, and procalcitonin) in children and adolescents with COVID-19.
Material and method
Patients
In this retrospective cohort study, pediatric cases aged between 1 and 18 years, attending to the department of pediatrics of Haseki Training and Research Hospital between March and May 2020 and whose SARS-CoV-2 infection was confirmed by a polymerase chain reaction (PCR) analysis using a nasopharyngeal swab were consecutively evaluated (n = 356). Patients with a measured 25 OH vitamin D (25 OH vit D) level were included (n = 175) while patients with comorbidities (diabetes, asthma, tuberculosis, chronic renal failure, etc.), which may affect the clinical course of COVID-19, and those under 1 year of age (n = 72) were excluded from the study. Ultimately, a total of 103 patients were enrolled in the study.
Data collection
Clinical data, laboratory, and imaging (chest X-ray and computed tomography) findings on admission were recorded retrospectively. According to the clinical findings, patients were divided into three groups: (1) asymptomatic group: patients who underwent a PCR test due to only contact history without any complaints; (2) mild group: patients with nonspecific symptoms such as cough, fever, malaise, and myalgia; and (3) moderate-to-severe group: patients whose pneumonia was confirmed by physical examination and imaging (chest X-ray and/or computed tomography) with or without oxygen need. The moderate-to-severe group consisted of hospitalized patients. Asymptomatic and mild cases were followed by telephone or outpatient clinic visits, and none of them had a clinical deterioration.
Laboratory data were recorded retrospectively from the patients’ files. At the beginning of the pandemic, all possible COVID-19 cases were routinely tested for a complete blood count: C-reactive protein (CRP), procalcitonin, fibrinogen, d-dimer, ferritin, calcium, phosphorus, and alkaline phosphatase (ALP). At the beginning of the pandemic, all patients with a positive PCR test were invited to the outpatient clinic for a second evaluation to assess the disease’s progress, which was within 1 week after the first visit. Due to its possible effect on the course of COVID-19, vitamin D (25 OH vit D) and parathyroid hormone (PTH) levels were measured in patients who accepted the invitation in addition to hospitalized patients in this second visit.
SARS-CoV-2 was investigated using reverse-transcriptase quantitative PCR by detection kit on nasopharyngeal swabs (Bioksen ArGe Teknik Co., Ltd., Turkey; Biospeedy®). Levels of serum 25 OH vit D were detected with an DXI800 instrument (Beckman Coulter, Brea, CA, USA) via an immunoinhibition assay. Patients were also grouped according to 25 OH vit D serum levels as sufficient (> 20 ng/mL), deficient (12–20 ng/mL), and insufficient (<12 ng/mL) [13]. This study was approved by the local ethical committee (Haseki Training and Research Hospital, 2020-56, 14.05.2020).
Statistical analysis
The SPSS software version 20.0 (SPSS, Inc., Chicago, IL, USA) was used in the analysis. Continuous data were given as the mean ± SD or median (25th; 75th percentile) and categorical variables were given as numbers (percentages). Patients were divided into groups according to clinical severity and vitamin D levels. The Kruskal-Wallis and chi-square tests were used for intragroup comparisons. Bonferroni correction was performed for post hoc analysis. The Spearman’s rank correlation test was used to analyze the association between inflammatory markers and vitamin D levels. To identify predictors of clinical severity, all parameters that showed a p value of ≤ 0.1 in the univariate analysis were tested using the multivariable logistic regression analysis. A two-tailed p value of ≤ 0.05 was defined as statistically significant.
Results
A total of 103 children whose PCR test revealed SARS-CoV-2 and had a measured 25 OH vit D level were included in the study. The mean age was 12.2 ± 4.92 (range 1–17) years and 52.4% (n = 54) were male. The frequency of 25 OH vit D deficiency was 41.7% (n = 43), insufficiency was 38.4% (n = 41), and sufficiency was only 18.4% (n= 19). Of all cases, 69.9% (n = 72) consisted of adolescents (10–19 years old). Among the adolescents, the frequency of vitamin D deficiency, insufficiency, and sufficiency were 55.6%, 37.5%, and 6.9%, respectively. Vitamin D deficiency was found in only three (9.7%) of the patients under 10 years of age, while vitamin D was sufficient in 45.2% (n = 14) of these children.
Comparison of vitamin D levels and inflammatory markers between patient groups defined by clinical course as asymptomatic, mild, and moderate-to-severe groups
Patient characteristics and laboratory findings of the three clinical groups are demonstrated in Table 1. The prevalence of vitamin D deficiency in the asymptomatic, mild, and moderate-to-severe groups were 17.2%, 35.4%, and 70.6%, respectively. Consistent with this, the moderate-to-severe group had by far the lowest median 25 OH vit D levels and highest inflammation markers compared to both the asymptomatic and mild groups. The mild group had similar laboratory findings to the asymptomatic group in terms of both inflammation and calcium metabolism markers.
Table 1.
Comparison of patients according to clinical groups as asymptomatic, mild, and moderate-to-severe groups
| Variables | Asymptomatic (n = 29) | Mild (n = 40) | Moderate-to-severe (n = 34) | p* | p** | p*** |
|---|---|---|---|---|---|---|
| Age (year) | 11.3 ± 5.05 | 11.9 ± 5.01 | 13.2 ± 4.68 | NS | NS | NS |
| Male gender, n (%) | 19 (65.5) | 22 (55) | 13 (38.2) | NS | NS | NS |
| Hemoglobin (%) | 13.5 (12.8–15.2) | 13.4 (12.6–14.4) | 12.9 (11.8–11.4) | NS | NS | NS |
| Leukocyte (103 μL) | 6.52 (5.3–8.74) | 5.46 (4.37–6.46) | 5.56 (4.0–7.1) | NS | NS | NS |
| Neutrophil (103μL) | 3.02 (2.21–4.33) | 2.56 (1.75–3.31) | 2.71 (1.84–3.68) | NS | NS | NS |
| Lymphocyte (103 μL) | 2.3 (2.00–3.38) | 2.2 (1.8–2.6) | 1.64 (1.12–2.38) | NS | 0.001 | 0.04 |
| Platelet (103 μL) | 226 (209–287) | 244 (207–285) | 218 (187–279) | NS | NS | NS |
| C-reactive protein (mg/L) | 0.8 (0.5–2.7) | 1.5 (0.7–2.5) | 8.45 (1.45–33.4) | NS | 0.001 | 0.001 |
| Procalcitonin (ng/mL) | 0.02 (0.02–0.035) | 0.03 (0.02–0.03) | 0.04 (0.2–0.13) | NS | 0.03 | 0.01 |
| d-dimer (mg/L) | 0.31 (0.25–0.63) | 0.35 (0.26–0.46) | 0.74 (0.35–1.28) | NS | 0.009 | 0.02 |
| Fibrinogen (mg/dL) | 242 (227–300) | 266 (236–332) | 372 (314–459) | NS | <0.001 | <0.001 |
| Calcium (mg/dL) | 10 (9.6–10.25) | 9.8 (9.6–10.1) | 9.4 (9.1–9.82) | NS | 0.001 | 0.003 |
| Phosphorus (mg/dL) | 4.8 (4–5.35) | 4.5 (3.7–5) | 3.85 (2.5–4.37) | NS | 0.004 | NS |
| Alkaline phosphatase (U/L) | 186 (103–240) | 171 (91–248) | 110 (86–182) | NS | NS | NS |
| Parathormone (pg/mL) | 32.5 (25–44.2) | 33 (24–47.5) | 43 (29–46.2) | NS | NS | NS |
| 25-OH vitamin D (ng/mL) | 16.3 (12.6–19.1) | 13.95 (10.0–17.2) | 9.95 (7.9–12.9) | NS | <0.001 | 0.001 |
| 25-OH vitamin D levels, n (%) | 0.001 | |||||
| Normal (>20 ng/ mL) | 7 (24.1)a | 9 (22.5)a | 3 (8.6)a | |||
| Insufficiency (12–20 ng/mL) | 17 (58.6)a | 17 (42.5)a,b | 7 (20.6)b | |||
| Deficiency (<12 ng/mL) | 5 (17.2)a | 14 (35.2)a | 24 (70.6)b | |||
Data was presented as mean ± standard deviation, median (25th–75th percentiles) or n (%). Kruskal-Wallis test and chi-square test with a Bonferroni correction were performed to compare continuous and categorical data, respectively
NS not significant
p*, asymptomatic vs mild group
p**, asymptomatic vs moderate-to-severe group
p***, mild vs moderate-to-severe group
a,bWithin each row, percentages that do not share a subscript are significantly different
The comparison of patients according to vitamin D levels (normal, insufficient, and deficient groups) revealed that 55.8% of the deficient group, 17.1% of the insufficient group, and 15.8% of the sufficient group applied with a moderate-to-severe clinical course (p < 0.001). The deficient group had the highest mean age and had by far the highest fibrinogen and lowest lymphocyte levels compared to both the insufficient and normal groups (Supplementary Table 1).
Correlation analysis
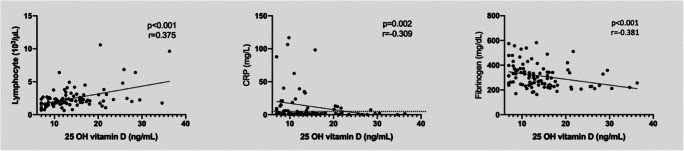
25 OH Vit D was positively correlated with a lymphocyte count (r = 0.375, p = <0.001) and negatively correlated with age ((r = −0.496, p = <0.001), CRP (r = −0.309, p = 0.002), and fibrinogen levels (r = −0.381, p = <0.001), (Fig. 1). The lymphocyte count was negatively correlated with CRP (p = <0.001, r = −0.428) and fibrinogen (p = 0.001, r = −0.330).
Fig. 1.
Correlations of 25 OH vitamin D with inflammation markers. Vitamin D was correlated positively with lymphocyte count and negatively with C-reactive protein and fibrinogen levels
In the multivariate logistic regression analysis, vitamin D deficiency (OR 6.16; 95% CI 1.66–22.81; p = 0.006), d-dimer (OR 2.03; 95% CI 1.11–3.70; p = 0.02), and fibrinogen levels (OR 1.01; 95% CI 1.0–1.02; p = 0.004) on admission were found as the independent predictors of moderate-to-severe clinical course in a model including age, gender, lymphocyte count, CRP, procalcitonin, d-dimer, fibrinogen, and vitamin D deficiency.
Discussion
In the study, in which the relationship between the serum level of vitamin D and COVID-19 clinical severity and markers of inflammation in children and adolescents was evaluated, it is shown that lower vitamin D levels were associated with clinical severity in addition to higher inflammation markers (i.e., CRP and fibrinogen) and a lower lymphocyte count. Interestingly, these associations were observed especially when there was a deficiency (i.e., 25 OH vit D <12 ng/mL) rather than insufficiency.
Vitamin D has multiple roles in the immune system that can modulate the body’s reaction to an infection. Activation of the vitamin D receptor, which is expressed in many cells of the immune system, regulates the expression of many genes involved in congenital and adaptive immunity [14]. The immunomodulatory effect of vitamin D has been demonstrated by reducing plasma cells, proinflammatory cytokine production, and immunoglobulin release and increasing anti-inflammatory cytokine production, activating Tall-like receptors (TLR), and increasing the release of anti-microbial peptides such as cathelicidin and β-defensin [15–18]. In addition, it has been reported that vitamin D balances inflammatory response by increasing the expression of angiotensin converting enzyme 2 (ACE2), which is an anti-inflammatory component “ACE2/angiotensin (1-7)/Mas receptor (MaSR) axis” [19].
Studies conducted to date have shown that vitamin D plays an important immunomodulatory role in reducing both the incidence and severity of bacterial and viral infections. Vitamin D deficiency has been found to increase the risk of respiratory infections by respiratory syncytial virus, tuberculosis, and influenza [20]. Furthermore, vitamin D supplementation, especially if there is a deficiency, has been shown to reduce the risk of acute respiratory infections [21]. In terms of severity, vitamin D deficiency has been shown to be a risk factor for exaggerated and persistent inflammation and play a role in the development of acute respiratory distress syndrome [22–24].
The pathology of COVID-19 involves a complex interaction between SARS-CoV-2 and the immune system. The renin-angiotensin system (RAS) is thought to play a central role in the pathophysiology of COVID-19, which balances the inflammation response with two opposing regulatory routes: the classical inflammation pathway formed by the ACE/angiotensin 2 receptor and the alternative anti-inflammation pathway formed by the ACE2/angiotensin (1-7)/MaSR. The ACE2 is the host receptor for the entry of SARS-CoV-2 into the cell [25]. It has been suggested that the introduction of SARS-CoV-2 into the cell decreases angiotensin (1-7)/MaSR levels, causing downregulation of ACE2. Thereby, suppression of the anti-inflammatory response and excessive activation of the classical pathway may result in hyperinflammation/cytokine storm, which is held responsible for the severity of the disease [26]. Children experience COVID-19 more mildly compared to adults. Older age and chronic illnesses are also risk factors for severe disease in the pediatric age group. In our cohort, age and gender were not significantly different between patient groups according to clinical severity.
There are a few, mostly observational, studies evaluating the relationship between vitamin D and COVID-19 severity or mortality in adults. The cause of a higher hospitalization and mortality rate of COVID-19 cases in northern latitudes was attributed to a common vitamin D deficiency in people living in northern countries [27]. A negative correlation has also been found between mean vitamin D levels and the number of COVID-19 cases in different countries [10]. Vitamin D deficiency has been reported to be associated with worse clinical outcomes and mortality in adult COVID-19 cases [11, 12]. Pediatric research evaluating vitamin D levels and COVID-19 in terms of clinical severity and inflammation markers is scarce. In this study, vitamin D deficiency (<12 ng/mL) was significantly higher in patients with a moderate-to-severe clinical course (24/34, 70.6%) compared to both mild (14/40, 35.2%) and asymptomatic (5/29, 17.2%) cases. In line with this, 55.8% of the vitamin D–deficient patient group had a moderate-to-severe clinical course. The multivariate logistic regression analysis demonstrated that vitamin D deficiency was associated with six times an increase in having a moderate-to-severe clinical course. These findings demonstrate the association between vitamin D deficiency and clinical severity in pediatric COVID-19 cases.
The cutoff values to define vitamin D deficiency and insufficiency have been established according to vitamin D (25 Oh vit D) levels associated with abnormal laboratory and imaging findings of bone metabolism (i.e., risk for rickets) (13). However, there are controversial reports regarding the serum level of vitamin D at which the immune system functions optimally (4). In the current study, interestingly, vitamin D insufficiency (12–20 ng/mL) was found to be similar to the normal group in terms of both clinical severity and inflammation markers, whereas vitamin D deficiency was associated with both clinical severity and markers of inflammation.
In COVID-19 cases, a decrease in the number of peripheral lymphocytes has been widely observed and associated with a severe clinical course [28]. The proposed mechanism is functional depletion of ACE2 expressing and SARS-CoV-2-infected lymphocytes in the early stages of the disease, which in turn causes impairment of viral immunity. It has also been shown that the lymphocyte count reaches the lowest values when the inflammatory cytokine levels are the highest on days 4 to 6 [29]. Moreover, the association of vitamin D deficiency with hyperinflammation has been reported in animal studies [22, 24]. A decrease in hyperinflammation with vitamin D supplementation has been also reported [23]. Increased CRP levels in response to inflammatory cytokines have been associated with disease severity and mortality in COVID-19 cases [30]. In the present study, the clinically more severe group, which also had significantly lower vitamin D levels and lymphocyte count, had significantly higher inflammation markers. Furthermore, the lower 25 OH vit D level was associated with higher inflammation markers (i.e., CRP and fibrinogen) and a lower lymphocyte count. Therefore, vitamin D deficiency may have a role in hyperinflammation and an associated low lymphocyte count in COVID-19.
Vitamin D deficiency is still a common public health problem worldwide. In some countries, prophylactic vitamin D supplementation is given in both infancy and adolescents [31]. In our country, prophylactic vitamin D supplementation is given only in infancy. In the present cohort, sufficient 25 OH vit D levels were observed only in 18.4% of all cases. Vitamin D deficiency was more evident in the adolescent age group. The high ratio of vitamin D insufficiency (38.4%) and deficiency (41.7%) may be explained by decreased synthesis of natural vitamin D due to the low exposure to the sun because of the quarantine and the beginning of the pandemic, which started just at the end of winter in our country. In addition, children under 1 year old were excluded as routine vitamin D supplementation is applied. Considering the high prevalence of vitamin D insufficiency and deficiency in the adolescent age group and the given relationship between vitamin D levels and the clinical severity of the COVID-19 in this study, prophylactic vitamin D supplementation to adolescents should be considered during the COVID-19 pandemic as a health policy.
This is a single-center study, and the findings may not be attributable to other populations as COVID-19 is a pandemic affecting all nations. Genetic variability of ACE2 or the vitamin D receptor may also affect the disease’s course. As the anthropometric measures of all patients were unavailable, the effect of obesity on the clinical course and inflammation markers could not be evaluated. This is the first study to evaluate the relationship between vitamin D and clinical severity and inflammatory markers in pediatric COVID-19 cases. Although the relationship between COVID-19 and vitamin D deficiency has been demonstrated, the cause and effect relationship has not been fully explained due to the retrospective nature of the study. Thus, randomized-controlled prospective studies are needed.
Conclusion
Although the effects of vitamin D on the immune system are quite complex, the available data support that adequate 25 OH vit D levels facilitate the defense process against bacterial and viral infections and prevent hyperinflammation. In this study, it has been shown that the serum vitamin D level was associated with clinical severity and markers of inflammation in children and adolescents with COVID-19. Interestingly, these associations were observed especially when there was a deficiency (i.e., 25 OH vit D <12 ng/mL). Although it has been demonstrated that vitamin D insufficiency has a role in bone metabolism, in the present study, vitamin D insufficiency was not found to be associated with disease severity or inflammation markers in COVID-19. Lastly, we suggest that considering the ongoing lockdown measures, prophylactic vitamin D supplementation may be considered especially for the adolescent age group during the COVID-19 pandemic as a health policy.
Supplementary information
(PDF 139 kb)
Abbreviations
- ACE2
Angiotensin converting enzyme 2
- ALP
Alkaline phosphatase
- ARDS
Severe acute respiratory syndrome
- CRP
C-reactive protein
- COVID-19
Coronavirus disease 2019
- PCR
Polymerase chain reaction
- PTH
Parathyroid hormone
- RAS
Renin-angiotensin system
- TLR
Tall-like receptors
- SARS-CoV-2
Severe acute respiratory syndrome coronavirus 2
- 25 OH vit D
25 Hydroxy vitamin D
Authors’ contributions
EB, GA, and AA design the study. EB, GA, KD, and OA data acquisition. EB and AA performed the statistical analysis. EB, AA, and GA wrote the manuscript. EB, GA, AA, HNSD, and ME reviewed the manuscript.
Availability of data and material
The authors declare that (the/all other) data supporting the findings of this study are available within the article (and its Supplementary information files).
Code availability
Not applicable
Declarations
Conflict of interest
The authors declare no competing interests.
Ethics approval
This study was approved by the local ethical committee (Haseki Training and Research Hospital, 2020-56, 14.05.2020).
Consent to participate
None (retrospective study)
Consent for publication
All authors consent to the publication of the manuscript in European Journal of Pediatrics, should the article be accepted by the Editor-in-chief upon completion of the refereeing process.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Elvan Bayramoğlu, Email: elvanbayramoglu@gmail.com.
Gülşen Akkoç, Email: agulsenakkoc@gmail.com.
Ayşe Ağbaş, Email: yurtayse@hotmail.com.
Özlem Akgün, Email: drozlemakgun@hotmail.com.
Kamer Yurdakul, Email: kamer_dogan@yahoo.com.
Hatice Nilgün Selçuk Duru, Email: nilgunduru@yahoo.com.
Murat Elevli, Email: muratelevli@gmail.com.
References
- 1.Lu H, Stratton CW, Tang YW. Outbreak of pneumonia of unknown etiology in Wuhan, China: the mystery and the miracle. J Med Virol. 2020;92(4):401–402. doi: 10.1002/jmv.25678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cucinotta D, Vanelli M. WHO declares COVID-19 a pandemic. Acta Biomed. 2020;91(1):157–160. doi: 10.23750/abm.v91i1.9397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, Wang B, Xiang H, Cheng Z, Xiong Y, Zhao Y, Li Y, Wang X, Peng Z. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. Jama. 2020;323(11):1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.She J, Liu L, Liu W. COVID-19 epidemic: disease characteristics in children. J Med Virol. 2020;92(7):747–754. doi: 10.1002/jmv.25807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dong Y, Mo X, Hu Y, Qi X, Jiang F, Jiang Z, Tong S. Epidemiology of COVID-19 among children in China. Pediatrics. 2020;145(6):e20200702. doi: 10.1542/peds.2020-0702. [DOI] [PubMed] [Google Scholar]
- 6.Cantorna MT. Mechanisms underlying the effect of vitamin D on the immune system. Proc Nutr Soc. 2010;69(3):286–289. doi: 10.1017/s0029665110001722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lei GS, Zhang C, Cheng BH, Lee CH. Mechanisms of action of vitamin D as supplemental therapy for pneumocystis pneumonia. Antimicrob Agents Chemother. 2017;61(10):e01226–e01217. doi: 10.1128/aac.01226-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sharifi A, Vahedi H, Nedjat S, Rafiei H, Hosseinzadeh-Attar MJ. Effect of single-dose injection of vitamin D on immune cytokines in ulcerative colitis patients: a randomized placebo-controlled trial. Apmis. 2019;127(10):681–687. doi: 10.1111/apm.12982. [DOI] [PubMed] [Google Scholar]
- 9.Grant WB, Lahore H, McDonnell SL, Baggerly CA, French CB, Aliano JL, Bhattoa HP. Evidence that vitamin D supplementation could reduce risk of influenza and COVID-19 infections and deaths. Nutrients. 2020;12(4):988. doi: 10.3390/nu12040988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ilie PC, Stefanescu S, Smith L. The role of vitamin D in the prevention of coronavirus disease 2019 infection and mortality. Aging Clin Exp Res. 2020;32(7):1195–1198. doi: 10.1007/s40520-020-01570-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alipio M (2020) Vitamin D supplementation could possibly improve clinical outcomes of patients infected with coronavirus-2019 (COVID-19). Available at SSRN 3571484
- 12.Raharusun P (2020) Patterns of COVID-19 mortality and vitamin D: an Indonesian study. Available at SSRN 3585561
- 13.Munns CF, Shaw N, Kiely M, Specker BL, Thacher TD, Ozono K, Michigami T, Tiosano D, Mughal MZ, Mäkitie O, Ramos-Abad L, Ward L, DiMeglio LA, Atapattu N, Cassinelli H, Braegger C, Pettifor JM, Seth A, Idris HW, Bhatia V, Fu J, Goldberg G, Sävendahl L, Khadgawat R, Pludowski P, Maddock J, Hyppönen E, Oduwole A, Frew E, Aguiar M, Tulchinsky T, Butler G, Högler W. Global consensus recommendations on prevention and management of nutritional rickets. J Clin Endocrinol Metab. 2016;101(2):394–415. doi: 10.1210/jc.2015-2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Panfili FM, Roversi M, D’Argenio P, Rossi P, Cappa M, Fintini D. Possible role of vitamin D in Covid-19 infection in pediatric population. J Endocrinol Investig. 2020;44(1):27–35. doi: 10.1007/s40618-020-01327-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen S, Sims GP, Chen XX, Gu YY, Chen S, Lipsky PE. Modulatory effects of 1, 25-dihydroxyvitamin D3 on human B cell differentiation. J Immunol. 2007;179(3):1634–1647. doi: 10.4049/jimmunol.179.3.1634. [DOI] [PubMed] [Google Scholar]
- 16.Lemire JM. Immunomodulatory role of 1, 25-dihydroxyvitamin D3. J Cell Biochem. 1992;49(1):26–31. doi: 10.1002/jcb.240490106. [DOI] [PubMed] [Google Scholar]
- 17.Zhang Y, Leung DY, Richers BN, Liu Y, Remigio LK, Riches DW, Goleva E. Vitamin D inhibits monocyte/macrophage proinflammatory cytokine production by targeting MAPK phosphatase-1. J Immunol. 2012;188(5):2127–2135. doi: 10.4049/jimmunol.1102412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu PT, Stenger S, Li H, Wenzel L, Tan BH, Krutzik SR, Ochoa MT, Schauber J, Wu K, Meinken C. Toll-like receptor triggering of a vitamin D-mediated human antimicrobial response. Science. 2006;311(5768):1770–1773. doi: 10.1126/science.1123933. [DOI] [PubMed] [Google Scholar]
- 19.Cui C, Xu P, Li G, Qiao Y, Han W, Geng C, Liao D, Yang M, Chen D, Jiang P. Vitamin D receptor activation regulates microglia polarization and oxidative stress in spontaneously hypertensive rats and angiotensin II-exposed microglial cells: role of renin-angiotensin system. Redox Biol. 2019;26:101295. doi: 10.1016/j.redox.2019.101295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zisi D, Challa A, Makis A. The association between vitamin D status and infectious diseases of the respiratory system in infancy and childhood. Hormones (Athens) 2019;18(4):353–363. doi: 10.1007/s42000-019-00155-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Martineau AR, Jolliffe DA, Hooper RL, Greenberg L, Aloia JF, Bergman P, Dubnov-Raz G, Esposito S, Ganmaa D, Ginde AA, Goodall EC, Grant CC, Griffiths CJ, Janssens W, Laaksi I, Manaseki-Holland S, Mauger D, Murdoch DR, Neale R, Rees JR, Simpson S, Jr, Stelmach I, Kumar GT, Urashima M, Camargo CA., Jr Vitamin D supplementation to prevent acute respiratory tract infections: systematic review and meta-analysis of individual participant data. Bmj. 2017;356:i6583. doi: 10.1136/bmj.i6583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dancer RC, Parekh D, Lax S, D’Souza V, Zheng S, Bassford CR, Park D, Bartis DG, Mahida R, Turner AM, Sapey E, Wei W, Naidu B, Stewart PM, Fraser WD, Christopher KB, Cooper MS, Gao F, Sansom DM, Martineau AR, Perkins GD, Thickett DR. Vitamin D deficiency contributes directly to the acute respiratory distress syndrome (ARDS) Thorax. 2015;70(7):617–624. doi: 10.1136/thoraxjnl-2014-206680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Khare D, Godbole NM, Pawar SD, Mohan V, Pandey G, Gupta S, Kumar D, Dhole TN, Godbole MM. Calcitriol [1, 25[OH]2 D3] pre- and post-treatment suppresses inflammatory response to influenza A (H1N1) infection in human lung A549 epithelial cells. Eur J Nutr. 2013;52(4):1405–1415. doi: 10.1007/s00394-012-0449-7. [DOI] [PubMed] [Google Scholar]
- 24.Kong J, Zhu X, Shi Y, Liu T, Chen Y, Bhan I, Zhao Q, Thadhani R, Li YC. VDR attenuates acute lung injury by blocking Ang-2-Tie-2 pathway and renin-angiotensin system. Mol Endocrinol. 2013;27(12):2116–2125. doi: 10.1210/me.2013-1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wan Y, Shang J, Graham R, Baric RS, Li F. Receptor recognition by the novel coronavirus from Wuhan: an analysis based on decade-long structural studies of SARS coronavirus. J Virol. 2020;94(7):e00127–e00120. doi: 10.1128/jvi.00127-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lanza K, Perez LG, Costa LB, Cordeiro TM, Palmeira VA, Ribeiro VT, Simões ESAC. Covid-19: the renin-angiotensin system imbalance hypothesis. Clin Sci (Lond) 2020;134(11):1259–1264. doi: 10.1042/cs20200492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Panarese A, Shahini E (2020) Letter: Covid-19, and vitamin D. Alimentary Pharmacology & Therapeutics 51(10):993–995 [DOI] [PMC free article] [PubMed]
- 28.Zheng M, Gao Y, Wang G, Song G, Liu S, Sun D, Xu Y, Tian Z. Functional exhaustion of antiviral lymphocytes in COVID-19 patients. Cell Mol Immunol. 2020;17(5):533–535. doi: 10.1038/s41423-020-0402-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu J, Li S, Liu J, Liang B, Wang X, Wang H, Li W, Tong Q, Yi J, Zhao L, Xiong L, Guo C, Tian J, Luo J, Yao J, Pang R, Shen H, Peng C, Liu T, Zhang Q, Wu J, Xu L, Lu S, Wang B, Weng Z, Han C, Zhu H, Zhou R, Zhou H, Chen X, Ye P, Zhu B, Wang L, Zhou W, He S, He Y, Jie S, Wei P, Zhang J, Lu Y, Wang W, Zhang L, Li L, Zhou F, Wang J, Dittmer U, Lu M, Hu Y, Yang D, Zheng X. Longitudinal characteristics of lymphocyte responses and cytokine profiles in the peripheral blood of SARS-CoV-2 infected patients. EBioMedicine. 2020;55:102763. doi: 10.1016/j.ebiom.2020.102763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Luo X, Zhou W, Yan X, Guo T, Wang B, Xia H, Ye L, Xiong J, Jiang Z, Liu Y, Zhang B, Yang W. Prognostic value of C-reactive protein in patients with COVID-19. Clin Infect Dis. 2020;71(16):2174–2179. doi: 10.1093/cid/ciaa641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lips P, Cashman KD, Lamberg-Allardt C, Bischoff-Ferrari HA, Obermayer-Pietsch B, Bianchi ML, Stepan J, El-Hajj Fuleihan G, Bouillon R. Current vitamin D status in European and Middle East countries and strategies to prevent vitamin D deficiency: a position statement of the European Calcified Tissue Society. Eur J Endocrinol. 2019;180(4):23–54. doi: 10.1530/EJE-18-0736. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF 139 kb)
Data Availability Statement
The authors declare that (the/all other) data supporting the findings of this study are available within the article (and its Supplementary information files).
Not applicable