Abstract
Purpose
Extracorporeal membrane oxygen (ECMO) is increasingly used as an advanced form of life support for cardiac and respiratory failure. Unfortunately, in infrequent instances, circulatory and/or respiratory recovery is overshadowed by neurologic injury that can occur in patients who require ECMO. As such, knowledge of ECMO and its implications on diagnosis and treatment of neurologic injuries is indispensable for intensivists and neurospecialists.
Recent findings
The most common neurologic injuries include intracerebral hemorrhage, ischemic stroke, seizure, cerebral edema, intracranial hypertension, global cerebral hypoxia/anoxia, and brain death. These result from events prior to initiation of ECMO, failure of ECMO to provide adequate oxygen delivery, and/or complications that occur during ECMO. ECMO survivors also experience neurological and psychological sequelae similar to other survivors of critical illness.
Summary
Since many of the risk factors for neurologic injury cannot be easily mitigated, early diagnosis and intervention are crucial to limit morbidity and mortality from neurologic injury during ECMO.
Keywords: ECMO, Neurological disorder, Acute brain injury, Complications, Hemorrhage, Extracorporeal membrane oxygenation
Introduction to extracorporeal membrane oxygenation
Extracorporeal membrane oxygenation (ECMO) is the most advanced level of life support used in the setting of cardiac and/or respiratory failure refractory to conventional medical therapies [1, 2]. By temporarily performing the work of the heart and/or lungs, ECMO preserves and promotes recovery of vital organ function and allows time for possible recovery of the cardiovascular and/or respiratory systems. Although definitive mortality data are lacking for many conditions in which ECMO is employed, there is growing interest in its use, and the clinical indications for which ECMO is initiated have expanded in recent years.
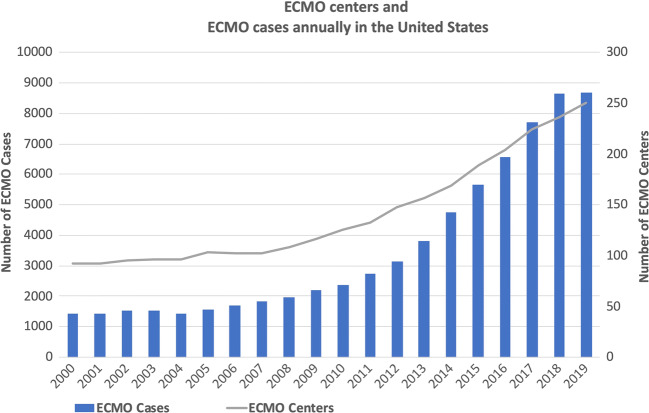
The utilization of ECMO in adult patients in the USA increased substantially in the last decade from 1830 cases in 2007 to 8673 in 2019 [3, 4] (see Fig. 1). Its use has further increased in 2020 due to severe acute respiratory distress syndrome (ARDS) associated with the SARS-CoV-2 pandemic [5, 6]. Many patients who might otherwise have died are able to return to a meaningful quality of life as a result of this revolutionary technology. However, neurologic injuries can occur while on ECMO and can be devastating. The reported incidence of such injuries ranges widely from 1 to 78% with a median of 13% [7–10]. As utilization of ECMO becomes more mainstream, the overall prevalence of associated neurologic injuries is also expected to increase.
Fig. 1.
Annual numbers of ECMO centers and ECMO cases in the USA reported to Extracorporeal Life Support Organization. The number of ECMO cases in 2019 is under-reported at the time of this review.
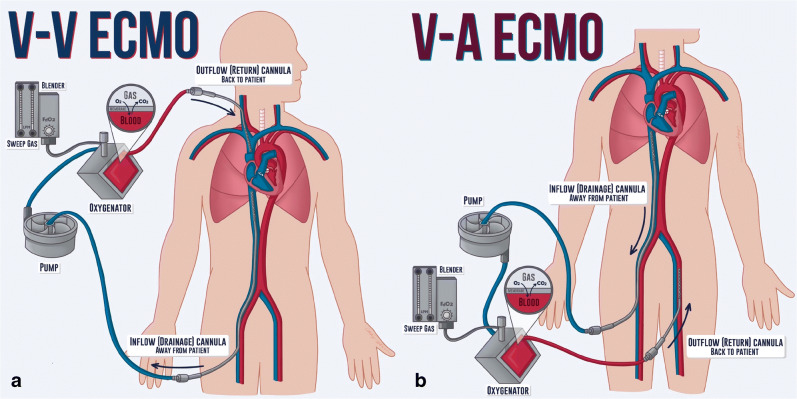
There are two types of ECMO therapy: veno-venous ECMO (VV ECMO) and veno-arterial (VA ECMO). VV ECMO removes blood from the venous system and returns it to the venous system. It is used in severe respiratory failure and provides only respiratory support (adding oxygen to and removing carbon dioxide from the blood). Therefore, the use of VV ECMO requires sufficient intrinsic cardiac output to perfuse vital organs [4]. In contrast, VA ECMO removes blood from the venous system and returns it to the arterial system [5]. VA ECMO is used in patients with impaired cardiac function. In addition to providing organ perfusion when the heart is unable to do so, it also provides respiratory support.
The ECMO circuit consists of a drainage (inflow or venous) cannula, a mechanical pump, a membrane oxygenator, and a return (outflow or arterial) cannula. Venous blood enters the circuit via the inflow cannula and is driven forward by the mechanical pump. Oxygen (O2) and sweep gas (air) flow into the membrane oxygenator where oxygen is added and carbon dioxide (CO2) is removed from the blood. Gas bubbles, thrombi, and other particulate matter are also filtered out in the membrane oxygenator. Blood is then reinfused into a large vein (e.g., superior vena cava) or large artery (e.g., abdominal aorta) via the outflow cannula (see Fig. 2). Blood O2 and CO2 levels are modulated by adjusting the fraction of delivered O2 and the sweep gas flow rate (typically 1–15 l/min), respectively. CO2 removal is proportional to the sweep gas flow rate.
Fig. 2.
(a) Veno-venous (V-V) ECMO with a duel site cannulation. Drainage cannula inserted into the right femoral vein and return cannula in the right internal jugular vein. (b) Veno-arterial (V-A) ECMO, with a duel site cannulation, with the drainage cannula inserted into the right femoral vein, and the return cannula to the left femoral artery. Credit: Catherine Cichon, MD, MPH.
Initiation and maintenance of ECMO alter normal blood flow, O2 delivery, CO2 clearance, and hemostasis. These changes affect cardiovascular and cerebral physiology, as well as coagulation in ways that increase risk for neurologic injury. Additionally, there are intrinsic aspects of ECMO that make it more difficult to diagnose and treat these injuries if they do arise. Therefore, an understanding of ECMO and its implications on diagnosis and management of associated neurologic conditions is essential for intensivists and neurological specialists.
Implications of ECMO on diagnosis and treatment of neurologic injuries
As mentioned above, there are aspects of ECMO that make it more difficult to diagnose and treat acute neurologic injury.
Sedation
Frequent neurologic examination of an awake, interactive patient is the optimal monitor for acute neurologic injury. Unfortunately, the ability to perform and accurately interpret the neurologic examination is limited in many ECMO patients due to deep levels of sedation and, in some cases, by the administration of neuromuscular blockade (NMB). Deep sedation and NMB may be required in some patients prior to consideration for ECMO. However, it is often possible to discontinue NMB and dramatically reduce the depth of sedation once ECMO is initiated. The goal of sedation is to keep the patient calm and comfortable while preserving the neurologic exam. In this context, most of the cranial nerve reflexes and limited motor and sensory function can be tested even while on low-to-moderate levels of sedation. These examinations should be performed at regular intervals to verify the integrity of the central nervous system. Therefore, in addition to the benefits of minimizing sedation in all ICU patients [11], maintaining the neurologic exam allows for early diagnosis of neurologic injuries in ECMO patients.
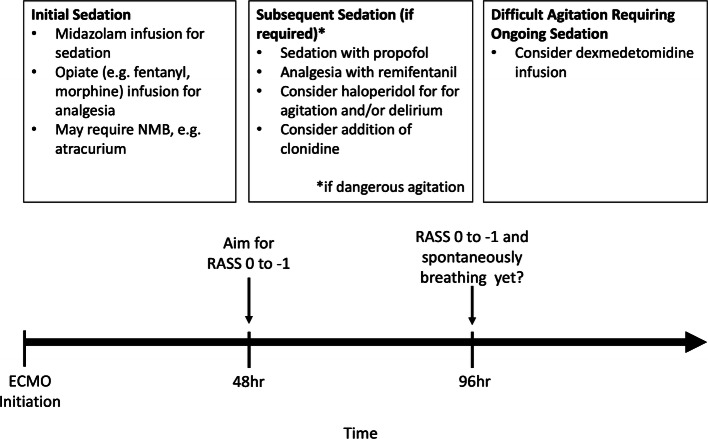
There is now momentum among many ECMO centers toward discontinuing NMB, reducing sedation, and even keeping patients completely awake when feasible. However, there is a paucity of data to guide sedation and NMB in ECMO patients, and the limited guidelines that currently exist are based largely on expert opinion (see Fig. 3). Sedation strategies must take into account changes in plasma concentrations of opioid, anxiolytic, and sedative-hypnotic agents caused by hemodilution due to addition of priming solution, sequestration due to nonspecific binding to circuit components, and impaired metabolism and clearance due to hepatic and renal dysfunction. The volume of distribution of commonly used sedation agents such as fentanyl, propofol, and midazolam is increased, and much higher than normal doses are often required to achieve sedation goals [12]. Furthermore, after continuous infusions of such agents are discontinued, sequestered drug is released back into the plasma, which prolongs sedation in an unpredictable manner [13]. Importantly, changes to the circuit, such as replacing a clogged filter or oxygenator, can reset this process in unpredictable ways. Given these complexities, a more practical approach is to preserve the neurologic exam and use it as a guide to depth of sedation when feasible.
Fig. 3.
Sedation guidelines, adapted from Extracorporeal Life Support Organization recommendations with modifications. NMB, neuromuscular blockade.
Challenges to obtaining neuroimaging
Neuroimaging modalities such as magnetic resonance imaging (MRI), computed tomography (CT), CT angiography, and CT perfusion scans are standard diagnostic tools used to assess acute neurologic injuries. However, obtaining these studies in ECMO patients must take into account a number of considerations, including incompatibility of ECMO circuit components with MRI, logistics of transport, timing of contrast administration, and interpretation of contrast-enhanced CT imaging in the setting of altered blood flow patterns.
The ECMO circuit has necessary components that are not compatible with MRI. Therefore, this valuable imaging modality is unobtainable until after decannulation and removal of the ECMO pump. Confirmation of a clinical diagnosis of cerebral ischemia, hypoxic/ischemic encephalopathy, or subtle intracranial hemorrhage is limited, and clinicians must rely on non-MRI modalities such as CT head to evaluate acute brain injury.
While portable CT scanners exist, they are not widely available. As such, patients must be transported to a stationary CT scanner in many cases. Similar to other critically ill patients, this requires substantial staffing resources and time to achieve [14]. The skillsets of multiple providers are required including bedside nurses, respiratory therapists, perfusionists/ECMO specialists, and sometimes physicians. Additional personnel are usually needed to assist in the movement of the bed, ventilator, ECMO circuit, and pumps for intravenous infusions. Care must be taken to not dislodge vital life support devices during movement through the halls, elevators, and onto and off of the imaging table. Our center performs annual ECMO transfer training and simulation with our nursing ECMO specialists.
Contrast-enhanced head CT is useful for evaluation of acute ischemic stroke and other enhancing abnormalities such as abscess. Under normal physiological conditions, contrast is injected into a peripheral vein, passes through the right heart, pulmonary vascular system, and left heart before reaching the cerebral vasculature; the timing of contrast enhancement of cerebral arteries and veins depends on intrinsic cardiac output, and a bolus tracking system is used to identify the arterial and venous phases of contrast enhancement.
In ECMO patients, intravenously injected contrast is directed into the inflow cannula, transits the ECMO circuit, and returns to patient circulation via the outflow cannula. In VV ECMO, the contrast returns to the central veins. Since cardiac output is relatively preserved, bolus tracking is performed in the usual manner [15]. However, imaging technicians who protocol these studies should be made aware that there is a delay of contrast enhancement due to ECMO circuit transit time [16]. Reducing ECMO flows briefly, if tolerated, during the contrast injection mitigates this effect.
In VA ECMO, intravenous contrast diverted to the ECMO circuit is returned to the aorta, and contrast enhancement of cerebral vessels depends on retrograde flow into the aortic arch. Retrograde filling is proportional to ECMO pump flow rate and is opposed by residual left ventricular (LV) function [16]. If there is little-to-no intrinsic LV function, enhancement of cerebral vessels is more rapid and symmetric. Conversely, if LV function is relatively preserved, un-opacified cardiac output will preferentially fill the right-sided cerebral circulation, while the opacified ECMO flow preferentially fills the left-sided cerebral circulation. This results in asymmetric enhancement of the left and right head and neck vessels [15]. As with VV-ECMO, this can be partially mitigated by briefly decreasing the ECMO flow rate if hemodynamically tolerated [15].
Lastly, air bubbles generated during contrast administration pose a unique challenge in VA ECMO. An air bubble detector senses the presence of air in the circuit and turns off the pump to avoid introducing arterial air emboli. Patients that are highly reliant on ECMO flow for mechanical circulatory support will not tolerate this transient loss of flow. However, if this function is turned off during contrast injection, air bubbles might enter the arterial circulation and embolize to the brain.
Anticoagulation strategies on ECMO
Despite advances in biocompatibility and reduced size of ECMO circuits, interaction between the blood and circuit still results in a prothrombotic state. As a result, anticoagulation is needed to maintain the circuit’s integrity and minimize clot formation in the membrane oxygenator. There are multiple anticoagulation strategies employed when initiating ECMO therapy, and these vary by center. In general, therapeutic anticoagulation is necessary in VA ECMO to prevent arterial thromboembolism. Anticoagulation is also used for VV ECMO; however, it can be held for prolonged periods of time or sometimes not started at all. The Extracorporeal Life Support Organization (ELSO) publishes general guidelines on anticoagulation during the initiation and maintenance of ECMO [17–20].
Neurologic injuries in ECMO patients
The same major neurological injuries occur with both VV and VA ECMO, but at different rates. According to a retrospective review of patient data collected in the ELSO registry for VV ECMO (includes 4988 adults treated from 1992 to 2015), the most commonly reported neurologic injuries were intracranial hemorrhage (3.6%), brain death (2%), ischemic stroke (1.7%), and seizure (1.2%) [21••]. Contrast this with ELSO registry data for VA ECMO (includes 4522 adults treated between 1992 and 2013), where the most commonly reported neurologic injuries were brain death (7.9%), ischemic stroke (3.6%), seizure (1.8%), and intracerebral hemorrhage (1.8%) [22]. Despite significant improvements in ECMO technology and understanding of its application, more recent observational studies in adult patients treated at an advanced ECMO center indicate that both intracranial hemorrhage and ischemic injury are still common: the reported incidences of intracranial hemorrhage and ischemic stroke in VV ECMO (135 patients) are 7.5% and 2%, respectively, and 2.8% and 5.3% in VA ECMO (878 patients), respectively [23, 24••].
Intracranial hemorrhage
Intracranial hemorrhage (ICH) includes non-traumatic, spontaneous intraparenchymal hemorrhage, subarachnoid hemorrhage, and subdural hemorrhage. Interestingly, it is reported that 85% of ICH are present on the head CT performed shortly after cannulation [25]. However, the percentage of ICH that is present prior to cannulation and anticoagulation is unknown. For this reason, some institutions now include a head CT in their screening protocol prior to cannulation (as safety permits). If a patient is too unstable or was placed on mobile ECMO at an outside institution, then many centers will obtain a head CT after ECMO cannulation.
The exact mechanisms by which ICH occurs are unknown. Cavayas et al. propose a two-step process wherein there is first some injury to the blood-brain barrier followed by propagation of hemorrhage due to impaired hemostasis [26]. This is certainly true in hemorrhagic conversion of ischemic strokes which occur on ECMO and is also consistent with many—but not all—of the risk factors which have been identified in retrospective analyses. Some risk factors are unfortunately intrinsic to ECMO itself, such as the use of systemic anticoagulation, duration of ECMO, hematologic derangements due to hemolysis and platelet consumption, and rapid correction of arterial CO2 (PaCO2) levels after initiation of ECMO (which may result in an ischemic area that is more susceptible to hemorrhagic conversion). Other identified risk factors are intrinsic to the patient or related to the nature and severity of the underlying disease process. These include younger age, female gender, use of anticoagulation or antiplatelet medications prior to ECMO, pre-ECMO cardiac arrest, sepsis, influenza, renal failure, and renal replacement therapy [21••, 22, 23, 24••, 26–31].
Treatment for ICH should generally follow standard guidelines, including stopping anticoagulation and strict blood pressure control. There is little data regarding reversal of anticoagulation on ECMO as part of the treatment of ICH. Generally, our institutional practice is to avoid reversal of anticoagulation; however, this is a decision that must be made with caution, weighing the relative risks of hematoma expansion against the risk of thrombosing the ECMO circuit which may cause an abrupt and likely life-threatening pause in both VV and VA ECMO. Conversely, cessation of anticoagulation with gradual correction of coagulopathy is generally tolerated for periods of time. However, there is increased risk of arterial thromboembolic events with prolonged cessation of anticoagulation on VA ECMO [32–35]. The optimal time period for reinstitution of anticoagulation has not been well-studied. Visual inspection of the ECMO circuit for clot formation and monitoring circuit pressure to identify impending oxygenator failure may provide guidance for ongoing discussions regarding the risk/benefit ratio of resuming anticoagulation.
Ischemic stroke
Ischemic stroke occurs more commonly in VA ECMO [21••, 22, 23, 24••] and often results from either disruption of arterial atheromas during cannulation of large arteries or from embolic thrombi that originate within the circuit or at the tip of the outflow cannula. In patients with atrial fibrillation or VA ECMO patients with poor cardiac function, thrombi may be cardioembolic in origin. Ischemia can also result from microthrombi or more rarely from arterial air emboli.
There are no formal guidelines on the best practice for treatment of ischemic stroke in ECMO patients. In many cases, it is impossible to determine a last known well time due to sedation, NMB, or lack of detailed neurologic examinations. Furthermore, thrombolytic therapy is usually contraindicated in patients with active anticoagulation. While experience is limited, there are case reports of improved outcomes with mechanical thrombectomy in VA ECMO patients [36]. Therefore, in cases where CT angiography reveals a large vessel occlusion, mechanical thrombectomy should be considered.
Seizure
Abnormal electroencephalography (EEG) is noted in 50–80% of ECMO patients with electrographic seizures reported in 8–20% of patients [37]. While it may be necessary to monitor continuous EEG for 24–48 h to capture electrographic seizures, many centers do not have the resources to do so. A common practice is to perform intermittent EEG monitoring for 30 min to several hours. While it may be difficult to obtain, continuous EEG should be considered in patients with focal and global neurologic deficits which are not completely explained by brain imaging.
Seizure activity can be secondary to ICH, embolic infarcts, or global cerebral hypoxia/anoxia. When detected it should be aggressively treated with antiepileptic medications (AEDs) and by increasing sedative hypnotic agents (e.g., propofol). Severe cases of status epilepticus may require prolonged burst suppression and multiple AEDs to adequately treat. Other epileptiform activity such as triphasic waves, polyspikes, and generalized and lateralized epileptiform discharges suggests focal or global cerebral injury and may warrant treatment depending on the clinical scenario. As with sedative medications, AEDs have altered pharmacokinetics in ECMO patients [12], and plasma drug levels should be monitored if clinically available.
Cerebral edema and intracranial hypertension
Large hematomas and ischemic strokes may result in cerebral edema which puts the patient at risk for cerebral herniation syndromes. Additionally, cerebral artery dysautoregulation is associated with non-pulsatile arterial flow during short runs of cardiopulmonary bypass [38, 39]. This phenomenon may be even more pronounced during long periods of VA ECMO and could predispose to abrupt changes in cerebral blood flow, thereby contributing to cerebral edema and intracranial hypertension. Intracranial pressure monitoring may be indicated, and elevated pressures should be aggressively managed when necessary. Protocols exist for the treatment of malignant edema and intracranial hypertension [40, 41]. In severe, refractory cases, craniectomy may be helpful to decompress the affected intracranial compartments [36]. While systemic anticoagulation does increase the risk of severe bleeding complications associated with neurosurgical intervention, it is not an absolute contraindication. Furthermore, in most cases, anticoagulation can be stopped temporarily to reduce this risk.
Hypoxic ischemic encephalopathy
Inadequate oxygen delivery to the brain results in hypoxic ischemic encephalopathy (HIE). HIE may be due to conditions that occur before ECMO is initiated (e.g., severe respiratory failure) or to failure of ECMO to provide adequate oxygen delivery to the brain [42]. In normal circumstances, the primary determinants of cerebral oxygen delivery are arterial oxygenation and cerebral blood flow (CBF).
Arterial oxygen content can be reduced in patients on VV ECMO by high cardiac output states (which will overwhelm the ability of ECMO to provide oxygenated blood) and by severe lung disease (in which there is increased intrapulmonary shunting). Reduced cerebral arterial oxygen content can also be seen in VA ECMO, especially when there is preserved or improving intrinsic LV function and persistent respiratory failure. This results in a condition known as Harlequin syndrome, wherein the native LV output perfuses the brain with poorly oxygenated blood (returning to the heart from the diseased lungs), while ECMO-driven flow of oxygenated blood is directed to the abdominal organs and lower extremities. For this reason, arterial blood is usually sampled away from the return cannula (e.g., right radial artery for a femoral return cannula) to determine gas exchange abnormalities experienced by the brain. Cerebral hypoxia may be a factor in developing cerebral injury, and patients with cerebral desaturations have worse outcomes [43].
Cerebral blood flow results from a dynamic balance of mean arterial pressure (MAP), intracranial pressure, cerebral venous outflow, PaCO2, and vasoreactivity. Insufficient CBF results in cerebral ischemia, while excess CBF causes cerebral hyperemia. It is generally accepted that cerebral autoregulation maintains steady CBF over a wide range of MAPs through cerebral blood vessel vasoreactivity. Insufficient CBF occurs when the MAP falls below the lower limit of autoregulation (unique to each person). Importantly, the cerebral autoregulation curve may be right shifted in patients with chronic hypertension, such that a higher MAP is required to maintain CBF and avoid ischemia. Impaired autoregulation presents an additional challenge. Up to 24% of patients on cardiac bypass have signs of impaired autoregulation [39], and this may be applicable in ECMO as well because of non-pulsatile blood flow (VA ECMO), increased cerebral vascular resistance from rapid correction of arterial CO2 (VV ECMO), or other unknown causes [7, 18]. In these patients with impaired autoregulation, CBF is reliant on systemic blood pressure, and the range of safe pressures (resulting in sufficient perfusion without hyperemia) is much narrower.
Brain death
Despite the best therapeutic interventions, patients with acute brain injury may progress to irreversible coma and brainstem areflexia consistent with brain death. However, the diagnosis of brain death while on ECMO poses some challenges. First, VA ECMO patients with severely reduced intrinsic cardiac function do not have a measurable systolic blood pressure (SBP); MAP is used in these patients to monitor perfusion. However, current AAN guidelines for brain death stipulate a minimum SBP measurement with no mention of MAP criteria [44, 45]. Secondly, the apnea test may not be technically feasible in patients on ECMO because it is either impossible to achieve a sufficient baseline PaO2 in patients with severe respiratory failure or it is challenging to achieve the required rise in PaCO2 without turning the sweep gas flow rate significantly down or off. However, traditional oxygenators require a minimal sweep flow, and oxygenation will not occur when sweep flow rate equals 0 L/min. Although some authors have suggested methods for successful apnea testing on ECMO [46], these strategies are not often employed. In accordance with AAN guidelines, if an apnea test is aborted, inconclusive, or cannot be completed, ancillary testing is required. However, the routinely used ancillary tests are also complicated by ECMO and have not been specifically validated in this population [46]. Current brain death guidelines do not account for use of supportive therapies such as ECMO, and additional research is required to devise a national standardized protocol.
Neuromonitoring adjuncts in the setting of ECMO
In addition to the neurologic exam, technologies that provide continuous or intermittent neurologic monitoring include serial computed tomography (CT) scans, transcranial Doppler (TCD), near-infrared spectroscopy (NIRS), and EEG (quantitative or otherwise). Alone or in combination, these provide useful information regarding neurologic function. Given the increased risk posed by undetected brain injury in patients on ECMO, the use of such neuromonitoring adjuncts should be considered. Although a full review of neuromonitoring techniques is outside the scope of this manuscript, it has been explored in detail in other literature [47•]. Table 1 notes some modalities that may serve as surrogates for the neurologic exam. However, none of these has yet to be widely adopted nor has any shown a significant impact on outcomes measures.
Table 1.
Monitoring strategies for patients undergoing ECMO therapy, including advantages and limitations
| Utility and regions monitored | Advantages | Limitations | |
|---|---|---|---|
| Neurologic exam | Cranial nerves, language comprehension, motor, sensory, rarely gait | Clinically accurate reflection of neurologic function, can trend over time to detect neurologic deterioration | Often limited by sedation or other pharmacological therapy |
| Computed tomography (CT) and CT angiography | Brain parenchyma, ventricular system, and brain vasculature | Portable CT if available, rapid acquisition | Transport, IV contrast timing must be coordinated based on VA or VV cannulation, does not rule out acute ischemia, no information on dynamic cerebral hemodynamics |
| Magnetic resonance (MR) imaging and magnetic resonance angiography | Brain parenchyma, ventricular system, and brain vasculature | Characterizes edema, masses, and early ischemia with higher sensitivity than CT and may be useful for evaluation of neurologic injury post-ECMO | MR modalities incompatible with ECMO circuit. Long duration, no information on dynamic cerebral hemodynamics |
| Transcranial Doppler ultrasound | Blood flow in cerebral vasculature: internal, middle cerebral, posterior cerebral, and basilar arteries | Provides blood flow velocity. Real-time microemboli detection, non-invasive, portable | Only rarely offers continuous monitoring. Can be operator dependent |
| NIRS | Frontal lobe, measures regional cerebral oxygenation | Continuous, non-invasive scalp electrode | Uses regional oxygenation to estimate global function |
| EEG | Superficial cortical areas | Seizure detection, minimally invasive | Limited resource, artifact from ECMO circuitry |
IV intravenous, VA veno-arterial, VV veno-venous, NIRS near-infrared spectroscopy, EEG electroencephalogram
CT and MR imaging—as well as the limitations of these modalities—have been discussed elsewhere is this review. TCD and NIRS monitor surrogate measures of cerebral flow and autoregulation [48–50]. While TCD measures flow velocity, NIRS measures regional oxygen saturation by determining the relative concentrations of oxygenated and deoxygenated hemoglobin in the cerebral circulation [51–53]. While definitive data is lacking, TCDs and NIRS might be utilized in ECMO patients to guide optimization of cerebral arterial oxygenation and CBF. TCD can also detect microemboli arising from the ECMO circuit in real time.
EEG monitoring can be used intermittently or continuously to evaluate for changes in either generalized or focal background. Modules that quantitatively process EEG waveforms may reduce the time needed to review hours of recordings and can alert providers to early problems such as seizures and cerebral dysfunction (due to ischemia and other causes). For example, a reduction of the ratio between alpha (8–13 Hz) and delta (<4 Hz) frequencies along with an increase in slower frequencies can identify cerebral ischemia before neurologic deficits or changes in head CT become evident [54, 55]. However, any findings identified by a non-epileptologist will need to be confirmed by those credentialed to read EEG.
Neurocognitive outcomes following ECMO
Depending on the underlying reason for initiation of ECMO, published survival rates are variable and dependent on multiple factors [56–61]; however, recovery after ECMO means more to many patients than just surviving their critical illness. Adverse events such as the ones described within this review impact outcomes greatly, and any of these events may individually or synergistically jeopardize brain integrity [47•]. For the purposes of this section, we will focus on long-term neuropsychological outcomes in ECMO survivors with or without cerebral injury.
Long-term neurological outcomes that are associated with critical illness are also seen in ECMO patients. These include impaired memory, psychiatric disturbance, chronic pain, motor disability, neuropathy and sensory deficits, hearing loss, and visual deficits [61–63]. All of these can potentially impact health-related quality of life and social recovery. That said, in the CESAR trial [64], no difference was demonstrated in health-related quality of life measures between surviving patients with severe respiratory failure randomized to ECMO versus the control group. However, only 50% of survivors in both groups had follow-up information available for analysis. In another study evaluating multi-modality outcomes in 28 adult patients at 5-year (on average) follow-up, 43% of patients had impaired neuropsychological performance (especially in the domains of attention and verbal memory), 52% had abnormal neuroimaging (seen more frequently following VA ECMO than VV ECMO), and 43% had pathological electrophysiological studies, even though EEG findings did not correlate with neuropsychological performance or neuroradiographic abnormalities [62]. Neuroradiographic complications were associated with poorer cognitive performance, though radiographic abnormalities are not always associated with overt clinical syndromes.
Post-intensive care syndrome is a described phenomenon in critically ill patients [65], and the neuropsychological impact of critical illness is also seen in ECMO patients with or without overt neurologic injury. Although their 36-Item Short Form Survey psychological domain is comparable to the general population [66], studies have identified that patients surviving to 6-month follow-up or longer have persistent emotional and mental health difficulties [61, 66–68, 69•, 70]: depression (20–42%), anxiety (20–55%), and post-traumatic stress (PTS) symptoms (5–47%). The rates of psychological sequelae are unrelated to the duration of ECMO or length of follow-up [61, 67]. Return to work is only seen in 50–65% of patients receiving ECMO for ARDS [61–63]. Moreover, informal caregivers (often family members) suffer from increased rates of depression, anxiety, and PTS disorders as well [65], with significant correlation between mental health sequelae in patients and their informal caregivers following VV ECMO for ARDS [69•]. These rates of neuropsychological sequelae may not differ substantially when compared with survivors of severe ARDS without ECMO [70], but they do highlight the need for intense follow-up that includes physiological and psychological evaluation and support for patients and their loved ones [71]. Such follow-up can be performed in an ICU recovery clinic [72, 73], which is the standard of practice at our institution.
Conclusions
ECMO is a lifesaving technology that is increasingly utilized for mechanical circulatory and respiratory support. While it has many benefits, uncommon devastating neurologic injuries contribute to significant morbidity and mortality. Overall, the incidence and severity of these injuries are likely underestimated due to lack of continuous monitoring of neurologic function, inability to obtain frequent, reliable neurologic examinations, and limitations of diagnostic imaging in ECMO patients. Moreover, some neurologic injuries may never be identified due to withdrawal of ECMO without any attempt at neurologic evaluation in patients who fail to recover cardiac or pulmonary function.
Clinicians should be vigilant in minimizing sedation, performing frequent neurologic assessments, and utilizing available monitoring technologies. Suspected and confirmed neurologic injuries should be proactively monitored and managed to minimize further injury. The role of highly trained neurologists—and, where available, neurointensivists—in evaluating and guiding management of neurologic injuries associated with ECMO cannot be overemphasized. Further research is necessary to determine optimal sedation and anticoagulation strategies, validate neuromonitoring adjuncts, identify interventions to improve long-term outcomes in survivors, and develop a standardized protocol for diagnosis and declaration of brain death.
Funding
Dr. Odish is supported by an institutional training grant through the Department of Anesthesiology (T32 - T32GM121318).
Compliance with Ethical Standards
Conflict of interest
Dr. Illum reports no disclosures. Dr. Odish reports no disclosures. Dr. Minokadeh reports no disclosures. Dr. Owens reports funding from the NIH. Cassia Yi reports no disclosures. Dr. Pollema reports no disclosures. Dr. LaBuzetta reports no disclosures.
Footnotes
This article is part of the Topical Collection on Critical Care Neurology
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References and Recommended Reading
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
- 1.Rao P, Khalpey Z, Smith R, Burkhoff D, Kociol RD. Venoarterial extracorporeal membrane oxygenation for cardiogenic shock and cardiac arrest. Circ Heart Fail. 2018;11(9):e004905. doi: 10.1161/CIRCHEARTFAILURE.118.004905. [DOI] [PubMed] [Google Scholar]
- 2.Brodie D, Slutsky AS, Combes A. Extracorporeal life support for adults with respiratory failure and related indications: a review. JAMA. 2019;322(6):557–568. doi: 10.1001/jama.2019.9302. [DOI] [PubMed] [Google Scholar]
- 3.Kulat B. The American Board of Cardiovascular Perfusion, Annual Report 20192019.
- 4.Registry [database on the Internet]. Available from: https://www.elso.org/Registry.aspx. Accessed: 2020 Jun 22.
- 5.MacLaren G, Fisher D, Brodie D. Preparing for the most critically ill patients with COVID-19: the potential role of extracorporeal membrane oxygenation. JAMA. 2020;323:1245–1246. doi: 10.1001/jama.2020.2342. [DOI] [PubMed] [Google Scholar]
- 6.Organization ELS. ECMO in COVID-19. https://www.elso.org/COVID19.aspx. Accessed August 17 2020.
- 7.Cavayas YA, Munshi L, Del Sorbo L, Fan E. The early change in PaCO2 after extracorporeal membrane oxygenation initiation is associated with neurological complications. Am J Respir Crit Care Med. 2020;201(12):1525–1535. doi: 10.1164/rccm.202001-0023OC. [DOI] [PubMed] [Google Scholar]
- 8.Cho S, Canner J, Chiarini G, Calligy K, Caturegli G, Rycus P, et al. Modifiable risk factors and mortality from ischemic and hemorrhagic strokes in patients receiving venoarterial extracorporeal membrane oxygenation: results from the Extracorporeal Life Support Organization Registry. Crit Care Med. 2020;48:e897–e905. doi: 10.1097/CCM.0000000000004498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lynn PV. Neurologic complications and neuromonitoring on ECMO. Advances in Extracorporeal Membrane Oxygenation: IntechOpen; 2019.
- 10.Sutter R, Tisljar K, Marsch S. Acute neurologic complications during extracorporeal membrane oxygenation: a systematic review. Crit Care Med. 2018;46(9):1506–1513. doi: 10.1097/CCM.0000000000003223. [DOI] [PubMed] [Google Scholar]
- 11.Devlin JW, Skrobik Y, Gelinas C, Needham DM, Slooter AJC, Pandharipande PP, et al. Clinical practice guidelines for the prevention and management of pain, agitation/sedation, delirium, immobility, and sleep disruption in adult patients in the ICU. Crit Care Med. 2018;46(9):e825–ee73. doi: 10.1097/CCM.0000000000003299. [DOI] [PubMed] [Google Scholar]
- 12.Cheng V, Abdul-Aziz MH, Roberts JA, Shekar K. Optimising drug dosing in patients receiving extracorporeal membrane oxygenation. J Thorac Dis. 2018;10(Suppl 5):S629–SS41. doi: 10.21037/jtd.2017.09.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hahn J, Choi JH, Chang MJ. Pharmacokinetic changes of antibiotic, antiviral, antituberculosis and antifungal agents during extracorporeal membrane oxygenation in critically ill adult patients. J Clin Pharm Ther. 2017;42(6):661–671. doi: 10.1111/jcpt.12636. [DOI] [PubMed] [Google Scholar]
- 14.Knight PH, Maheshwari N, Hussain J, Scholl M, Hughes M, Papadimos TJ, Guo WA, Cipolla J, Stawicki SP, Latchana N. Complications during intrahospital transport of critically ill patients: focus on risk identification and prevention. Int J Crit Illn Inj Sci. 2015;5(4):256–264. doi: 10.4103/2229-5151.170840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Acharya J, Rajamohan AG, Skalski MR, Law M, Kim P, Gibbs W. CT angiography of the head in extracorporeal membrane oxygenation. AJNR Am J Neuroradiol. 2017;38(4):773–776. doi: 10.3174/ajnr.A5060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lambert L, Grus T, Balik M, Fichtl J, Kavan J, Belohlavek J. Hemodynamic changes in patients with extracorporeal membrane oxygenation (ECMO) demonstrated by contrast-enhanced CT examinations - implications for image acquisition technique. Perfusion. 2017;32(3):220–225. doi: 10.1177/0267659116677308. [DOI] [PubMed] [Google Scholar]
- 17.Chlebowski MM, Baltagi S, Carlson M, Levy JH, Spinella PC. Clinical controversies in anticoagulation monitoring and antithrombin supplementation for ECMO. Crit Care. 2020;24(1):19. doi: 10.1186/s13054-020-2726-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kazmi SO, Sivakumar S, Karakitsos D, Alharthy A, Lazaridis C. Cerebral pathophysiology in extracorporeal membrane oxygenation: pitfalls in daily clinical management. Crit Care Res Pract. 2018;2018:3237810–3237811. doi: 10.1155/2018/3237810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Organization ELS. ELSO Anticoagulation Guideline. 2014.
- 20.Sklar MC, Sy E, Lequier L, Fan E, Kanji HD. Anticoagulation practices during venovenous extracorporeal membrane oxygenation for respiratory failure. A systematic review. Ann Am Thorac Soc. 2016;13(12):2242–2250. doi: 10.1513/AnnalsATS.201605-364SR. [DOI] [PubMed] [Google Scholar]
- 21.Lorusso R, Gelsomino S, Parise O, Di Mauro M, Barili F, Geskes G, et al. Neurologic injury in adults supported with veno-venous extracorporeal membrane oxygenation for respiratory failure: findings from the Extracorporeal Life Support Organization Database. Crit Care Med. 2017;45(8):1389–1397. doi: 10.1097/CCM.0000000000002502. [DOI] [PubMed] [Google Scholar]
- 22.Lorusso R, Barili F, Mauro MD, Gelsomino S, Parise O, Rycus PT, Maessen J, Mueller T, Muellenbach R, Belohlavek J, Peek G, Combes A, Frenckner B, Pesenti A, Thiagarajan RR. In-hospital neurologic complications in adult patients undergoing venoarterial extracorporeal membrane oxygenation: results from the Extracorporeal Life Support Organization Registry. Crit Care Med. 2016;44(10):e964–e972. doi: 10.1097/CCM.0000000000001865. [DOI] [PubMed] [Google Scholar]
- 23.Luyt CE, Brechot N, Demondion P, Jovanovic T, Hekimian G, Lebreton G, et al. Brain injury during venovenous extracorporeal membrane oxygenation. Intensive Care Med. 2016;42(5):897–907. doi: 10.1007/s00134-016-4318-3. [DOI] [PubMed] [Google Scholar]
- 24.Le Guennec L, Cholet C, Huang F, Schmidt M, Brechot N, Hekimian G, et al. Ischemic and hemorrhagic brain injury during venoarterial-extracorporeal membrane oxygenation. Ann Intensive Care. 2018;8(1):129. doi: 10.1186/s13613-018-0475-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lockie CJA, Gillon SA, Barrett NA, Taylor D, Mazumder A, Paramesh K, Rowland K, Daly K, Camporota L, Meadows CIS, Glover GW, Ioannou N, Langrish CJ, Tricklebank S, Retter A, Wyncoll DLA. Severe respiratory failure, extracorporeal membrane oxygenation, and intracranial hemorrhage. Crit Care Med. 2017;45(10):1642–1649. doi: 10.1097/CCM.0000000000002579. [DOI] [PubMed] [Google Scholar]
- 26.Cavayas YA, Del Sorbo L, Fan E. Intracranial hemorrhage in adults on ECMO. Perfusion. 2018;33(1_suppl):42–50. doi: 10.1177/0267659118766435. [DOI] [PubMed] [Google Scholar]
- 27.Fletcher-Sandersjoo A, Bartek J, Jr, Thelin EP, Eriksson A, Elmi-Terander A, Broman M, et al. Predictors of intracranial hemorrhage in adult patients on extracorporeal membrane oxygenation: an observational cohort study. J Intensive Care. 2017;5:27. doi: 10.1186/s40560-017-0223-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fletcher-Sandersjoo A, Bartek J, Jr, Thelin EP, Eriksson A, Elmi-Terander A, Broman M, et al. Correction to: predictors of intracranial hemorrhage in adult patients on extracorporeal membrane oxygenation: an observational cohort study. J Intensive Care. 2020;8:2. doi: 10.1186/s40560-019-0423-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fletcher-Sandersjoo A, Thelin EP, Bartek J, Jr, Broman M, Sallisalmi M, Elmi-Terander A, et al. Incidence, outcome, and predictors of intracranial hemorrhage in adult patients on extracorporeal membrane oxygenation: a systematic and narrative review. Front Neurol. 2018;9:548. doi: 10.3389/fneur.2018.00548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kasirajan V, Smedira NG, McCarthy JF, Casselman F, Boparai N, McCarthy PM. Risk factors for intracranial hemorrhage in adults on extracorporeal membrane oxygenation. Eur J Cardiothorac Surg. 1999;15(4):508–514. doi: 10.1016/s1010-7940(99)00061-5. [DOI] [PubMed] [Google Scholar]
- 31.Omar HR, Mirsaeidi M, Mangar D, Camporesi EM. Duration of ECMO is an independent predictor of intracranial hemorrhage occurring during ECMO support. ASAIO J. 2016;62(5):634–636. doi: 10.1097/MAT.0000000000000368. [DOI] [PubMed] [Google Scholar]
- 32.Arlt M, Philipp A, Voelkel S, Rupprecht L, Mueller T, Hilker M, Graf BM, Schmid C. Extracorporeal membrane oxygenation in severe trauma patients with bleeding shock. Resuscitation. 2010;81(7):804–809. doi: 10.1016/j.resuscitation.2010.02.020. [DOI] [PubMed] [Google Scholar]
- 33.Ryu KM, Chang SW. Heparin-free extracorporeal membrane oxygenation in a patient with severe pulmonary contusions and bronchial disruption. Clin Exp Emerg Med. 2018;5(3):204–207. doi: 10.15441/ceem.17.252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lee YY, Baik HJ, Lee H, Kim CH, Chung RK, Han JI, Joo H, Woo JH. Heparin-free veno-venous extracorporeal membrane oxygenation in a multiple trauma patient: a case report. Medicine (Baltimore) 2020;99(5):e19070. doi: 10.1097/MD.0000000000019070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Muellenbach RM, Kredel M, Kunze E, Kranke P, Kuestermann J, Brack A, Gorski A, Wunder C, Roewer N, Wurmb T. Prolonged heparin-free extracorporeal membrane oxygenation in multiple injured acute respiratory distress syndrome patients with traumatic brain injury. J Trauma Acute Care Surg. 2012;72(5):1444–1447. doi: 10.1097/TA.0b013e31824d68e3. [DOI] [PubMed] [Google Scholar]
- 36.Le Guennec L, Schmidt M, Clarencon F, Elhfnawy AM, Baronnet F, Kalamarides M, et al. Mechanical thrombectomy in acute ischemic stroke patients under venoarterial extracorporeal membrane oxygenation. J Neurointerv Surg. 2020;12(5):486–488. doi: 10.1136/neurintsurg-2019-015407. [DOI] [PubMed] [Google Scholar]
- 37.Gannon CM, Kornhauser MS, Gross GW, Wiswell TE, Baumgart S, Streletz LJ, Graziani LJ, Spitzer AR. When combined, early bedside head ultrasound and electroencephalography predict abnormal computerized tomography or magnetic resonance brain images obtained after extracorporeal membrane oxygenation treatment. J Perinatol. 2001;21(7):451–455. doi: 10.1038/sj.jp.7210593. [DOI] [PubMed] [Google Scholar]
- 38.Veraar CM, Rinosl H, Kuhn K, Skhirtladze-Dworschak K, Felli A, Mouhieddine M, et al. Non-pulsatile blood flow is associated with enhanced cerebrovascular carbon dioxide reactivity and an attenuated relationship between cerebral blood flow and regional brain oxygenation. Crit Care. 2019;23(1):426. doi: 10.1186/s13054-019-2671-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Joshi B, Brady K, Lee J, Easley B, Panigrahi R, Smielewski P, Czosnyka M, Hogue CW., Jr Impaired autoregulation of cerebral blood flow during rewarming from hypothermic cardiopulmonary bypass and its potential association with stroke. Anesth Analg. 2010;110(2):321–328. doi: 10.1213/ANE.0b013e3181c6fd12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cadena R, Shoykhet M, Ratcliff JJ. Emergency neurological life support: intracranial hypertension and herniation. Neurocrit Care. 2017;27(Suppl 1):82–88. doi: 10.1007/s12028-017-0454-z. [DOI] [PubMed] [Google Scholar]
- 41.Koenig MA, Bryan M, Lewin JL, 3rd, Mirski MA, Geocadin RG, Stevens RD. Reversal of transtentorial herniation with hypertonic saline. Neurology. 2008;70(13):1023–1029. doi: 10.1212/01.wnl.0000304042.05557.60. [DOI] [PubMed] [Google Scholar]
- 42.Mateen FJ, Muralidharan R, Shinohara RT, Parisi JE, Schears GJ, Wijdicks EF. Neurological injury in adults treated with extracorporeal membrane oxygenation. Arch Neurol. 2011;68(12):1543–1549. doi: 10.1001/archneurol.2011.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pozzebon S, Blandino Ortiz A, Franchi F, Cristallini S, Belliato M, Lheureux O, Brasseur A, Vincent JL, Scolletta S, Creteur J, Taccone FS. Cerebral near-infrared spectroscopy in adult patients undergoing veno-arterial extracorporeal membrane oxygenation. Neurocrit Care. 2018;29(1):94–104. doi: 10.1007/s12028-018-0512-1. [DOI] [PubMed] [Google Scholar]
- 44.Russell JA, Epstein LG, Greer DM, Kirschen M, Rubin MA, Lewis A, et al. Brain death, the determination of brain death, and member guidance for brain death accommodation requests: AAN position statement. Neurology. 2019;92:228–232. doi: 10.1212/WNL.0000000000006750. [DOI] [PubMed] [Google Scholar]
- 45.Wijdicks EF, Varelas PN, Gronseth GS, Greer DM, American Academy of N Evidence-based guideline update: determining brain death in adults: report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2010;74(23):1911–1918. doi: 10.1212/WNL.0b013e3181e242a8. [DOI] [PubMed] [Google Scholar]
- 46.Beam WB, Scott PD, Wijdicks EFM. The physiology of the apnea test for brain death determination in ECMO: arguments for blending carbon dioxide. Neurocrit Care. 2019;31(3):567–572. doi: 10.1007/s12028-019-00784-7. [DOI] [PubMed] [Google Scholar]
- 47.Lorusso R, Taccone FS, Belliato M, Delnoij T, Zanatta P, Cvetkovic M, et al. Brain monitoring in adult and pediatric ECMO patients: the importance of early and late assessments. Minerva Anestesiol. 2017;83(10):1061–1074. doi: 10.23736/S0375-9393.17.11911-5. [DOI] [PubMed] [Google Scholar]
- 48.Brady K, Joshi B, Zweifel C, Smielewski P, Czosnyka M, Easley RB, Hogue CW., Jr Real-time continuous monitoring of cerebral blood flow autoregulation using near-infrared spectroscopy in patients undergoing cardiopulmonary bypass. Stroke. 2010;41(9):1951–1956. doi: 10.1161/STROKEAHA.109.575159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ono M, Joshi B, Brady K, Easley RB, Zheng Y, Brown C, Baumgartner W, Hogue CW. Risks for impaired cerebral autoregulation during cardiopulmonary bypass and postoperative stroke. Br J Anaesth. 2012;109(3):391–398. doi: 10.1093/bja/aes148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Steiner LA, Pfister D, Strebel SP, Radolovich D, Smielewski P, Czosnyka M. Near-infrared spectroscopy can monitor dynamic cerebral autoregulation in adults. Neurocrit Care. 2009;10(1):122–128. doi: 10.1007/s12028-008-9140-5. [DOI] [PubMed] [Google Scholar]
- 51.Cui X, Bray S, Reiss AL. Functional near infrared spectroscopy (NIRS) signal improvement based on negative correlation between oxygenated and deoxygenated hemoglobin dynamics. Neuroimage. 2010;49(4):3039–3046. doi: 10.1016/j.neuroimage.2009.11.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dehghani H, White BR, Zeff BW, Tizzard A, Culver JP. Depth sensitivity and image reconstruction analysis of dense imaging arrays for mapping brain function with diffuse optical tomography. Appl Opt. 2009;48(10):D137–D143. doi: 10.1364/ao.48.00d137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ferrari M, Quaresima V. A brief review on the history of human functional near-infrared spectroscopy (fNIRS) development and fields of application. Neuroimage. 2012;63(2):921–935. doi: 10.1016/j.neuroimage.2012.03.049. [DOI] [PubMed] [Google Scholar]
- 54.Claassen J, Hirsch LJ, Kreiter KT, Du EY, Connolly ES, Emerson RG, et al. Quantitative continuous EEG for detecting delayed cerebral ischemia in patients with poor-grade subarachnoid hemorrhage. Clin Neurophysiol. 2004;115(12):2699–2710. doi: 10.1016/j.clinph.2004.06.017. [DOI] [PubMed] [Google Scholar]
- 55.Rots ML, van Putten MJ, Hoedemaekers CW, Horn J. Continuous EEG monitoring for early detection of delayed cerebral ischemia in subarachnoid hemorrhage: a pilot study. Neurocrit Care. 2016;24(2):207–216. doi: 10.1007/s12028-015-0205-y. [DOI] [PubMed] [Google Scholar]
- 56.Bermudez CA, Adusumilli PS, McCurry KR, Zaldonis D, Crespo MM, Pilewski JM, et al. Extracorporeal membrane oxygenation for primary graft dysfunction after lung transplantation: long-term survival. Ann Thorac Surg. 2009;87(3):854–860. doi: 10.1016/j.athoracsur.2008.11.036. [DOI] [PubMed] [Google Scholar]
- 57.Falk L, Hultman J, Broman LM. Extracorporeal membrane oxygenation for septic shock. Crit Care Med. 2019;47(8):1097–1105. doi: 10.1097/CCM.0000000000003819. [DOI] [PubMed] [Google Scholar]
- 58.Khorsandi M, Dougherty S, Bouamra O, Pai V, Curry P, Tsui S, Clark S, Westaby S, al-Attar N, Zamvar V. Extra-corporeal membrane oxygenation for refractory cardiogenic shock after adult cardiac surgery: a systematic review and meta-analysis. J Cardiothorac Surg. 2017;12(1):55. doi: 10.1186/s13019-017-0618-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mehta A, Ibsen LM. Neurologic complications and neurodevelopmental outcome with extracorporeal life support. World J Crit Care Med. 2013;2(4):40–47. doi: 10.5492/wjccm.v2.i4.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Migdady I, Rice C, Deshpande A, Hernandez AV, Price C, Whitman GJ, Geocadin RG, Cho SM. Brain injury and neurologic outcome in patients undergoing extracorporeal cardiopulmonary resuscitation: a systematic review and meta-analysis. Crit Care Med. 2020;48(7):e611–e6e9. doi: 10.1097/CCM.0000000000004377. [DOI] [PubMed] [Google Scholar]
- 61.Schmidt M, Zogheib E, Roze H, Repesse X, Lebreton G, Luyt CE, et al. The PRESERVE mortality risk score and analysis of long-term outcomes after extracorporeal membrane oxygenation for severe acute respiratory distress syndrome. Intensive Care Med. 2013;39(10):1704–1713. doi: 10.1007/s00134-013-3037-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Risnes I, Wagner K, Nome T, Sundet K, Jensen J, Hynas IA, et al. Cerebral outcome in adult patients treated with extracorporeal membrane oxygenation. Ann Thorac Surg. 2006;81(4):1401–1406. doi: 10.1016/j.athoracsur.2005.10.008. [DOI] [PubMed] [Google Scholar]
- 63.Stoll C, Haller M, Briegel J, Meier M, Manert W, Hummel T, Heyduck M, Lenhart A, Polasek J, Bullinger M, Schelling G. Health-related quality of life. Long-term survival in patients with ARDS following extracorporeal membrane oxygenation (ECMO) Anaesthesist. 1998;47(1):24–29. doi: 10.1007/s001010050518. [DOI] [PubMed] [Google Scholar]
- 64.Peek GJ, Mugford M, Tiruvoipati R, Wilson A, Allen E, Thalanany MM, Hibbert CL, Truesdale A, Clemens F, Cooper N, Firmin RK, Elbourne D. Efficacy and economic assessment of conventional ventilatory support versus extracorporeal membrane oxygenation for severe adult respiratory failure (CESAR): a multicentre randomised controlled trial. Lancet. 2009;374(9698):1351–1363. doi: 10.1016/S0140-6736(09)61069-2. [DOI] [PubMed] [Google Scholar]
- 65.LaBuzetta JN, Rosand J, Vranceanu AM. Review: post-intensive care syndrome: unique challenges in the neurointensive care unit. Neurocrit Care. 2019;31(3):534–545. doi: 10.1007/s12028-019-00826-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wang ZY, Li T, Wang CT, Xu L, Gao XJ. Assessment of 1-year outcomes in survivors of severe acute respiratory distress syndrome receiving extracorporeal membrane oxygenation or mechanical ventilation: a prospective observational study. Chin Med J. 2017;130(10):1161–1168. doi: 10.4103/0366-6999.205847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Muller G, Flecher E, Lebreton G, Luyt CE, Trouillet JL, Brechot N, et al. The ENCOURAGE mortality risk score and analysis of long-term outcomes after VA-ECMO for acute myocardial infarction with cardiogenic shock. Intensive Care Med. 2016;42(3):370–378. doi: 10.1007/s00134-016-4223-9. [DOI] [PubMed] [Google Scholar]
- 68.Risnes I, Heldal A, Wagner K, Boye B, Haraldsen I, Leganger S, Møkleby K, Svennevig JL, Malt UF. Psychiatric outcome after severe cardio-respiratory failure treated with extracorporeal membrane oxygenation: a case-series. Psychosomatics. 2013;54(5):418–427. doi: 10.1016/j.psym.2013.02.008. [DOI] [PubMed] [Google Scholar]
- 69.Sanfilippo F, Ippolito M, Santonocito C, Martucci G, Carollo T, Bertani A, et al. Long-term functional and psychological recovery in a population of acute respiratory distress syndrome patients treated with VV-ECMO and in their caregivers. Minerva Anestesiol. 2019;85(9):971–980. doi: 10.23736/S0375-9393.19.13095-7. [DOI] [PubMed] [Google Scholar]
- 70.Sylvestre A, Adda M, Maltese F, Lannelongue A, Daviet F, Parzy G, Coiffard B, Roch A, Loundou A, Baumstarck K, Papazian L. Long-term neurocognitive outcome is not worsened by of the use of venovenous ECMO in severe ARDS patients. Ann Intensive Care. 2019;9(1):82. doi: 10.1186/s13613-019-0556-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Auzinger G. Long-term outcome after VV ECMO: like the iceberg beneath the Titanic. Minerva Anestesiol. 2019;85(9):934–936. doi: 10.23736/S0375-9393.19.13688-7. [DOI] [PubMed] [Google Scholar]
- 72.McPeake J, Boehm LM, Hibbert E, Bakhru RN, Bastin AJ, Butcher BW, Eaton TL, Harris W, Hope AA, Jackson J, Johnson A, Kloos JA, Korzick KA, MacTavish P, Meyer J, Montgomery-Yates A, Quasim T, Slack A, Wade D, Still M, Netzer G, Hopkins RO, Mikkelsen ME, Iwashyna TJ, Haines KJ, Sevin CM. Key components of ICU recovery programs: what did patients report provided benefit? Crit Care Explor. 2020;2(4):e0088. doi: 10.1097/CCE.0000000000000088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Meyer J, Brett SJ, Waldmann C. Should ICU clinicians follow patients after ICU discharge? Yes. Intensive Care Med. 2018;44(9):1539–1541. doi: 10.1007/s00134-018-5260-3. [DOI] [PubMed] [Google Scholar]