Abstract
Carcinomas of the thyroid with Ewing family tumor elements (CEFTEs) are small cell thyroid tumors characterized by epithelial differentiation and EWSR1-FLI1 rearrangements. In contrast to primary thyroid Ewing sarcomas, these rare tumors have a favorable prognosis. CEFTEs may co-exist with papillary thyroid carcinoma (PTC) foci and are thought to arise from either PTCs or main cells of solid cell nests (SCN). Due to their rare occurrence, characteristic clinical presentations, preoperatory sonographic (US) findings, and fine-needle aspiration (FNA) cytologic features were ill-defined until now. We report a case of a 40-year-old male who was referred to the thyroid clinic for a progressively enlarging, hard, painless, cervical mass. US examination revealed a hypoechoic nodule with lobulated margins and scant intranodular vascular signals of the right thyroid lobe. Evidence of extracapsular spread was not identified. FNA provided a Bethesda V cytology classification on conventional smears. Repeat FNA sampling with the use of a CytoFoam Core allowed a preoperative diagnosis consistent with undifferentiated thyroid carcinoma. Total thyroidectomy without lymph node dissection was performed. Histologic examination with subsequent molecular studies provided the diagnosis of papillary carcinoma of the thyroid with Ewing family tumour elements (CEFTEs). No additional treatment was rendered and the patient showed no evidence of local or distant disease by clinical examination, US, and 18FDG-TAC/PET after 6 months of follow-up. This is the first reported case of CEFTE with complete clinical, US, cytologic, and immunohistochemical preoperatory assessment.
Keywords: Papillary thyroid carcinoma, CEFTE, Small cell carcinoma, EWSR1 rearrangement, Ewing sarcoma, CytoFoam Core
Introduction
Small cell tumors represent a heterogeneous group of thyroid malignancies that are nearly indistinguishable from each other on cytology samples and lack a uniformly accepted classification [1–4]. The most frequent small cell tumors of the thyroid gland are poorly differentiated carcinoma and thyroid lymphoma. The distinction of these from other primary or metastatic small cell tumors requires confirmation with immunohistochemical and molecular studies [4, 5].
Primary carcinomas of the thyroid with Ewing family tumor elements (CEFTEs) are exceptionally rare small cell tumors of the thyroid with epithelial differentiation and, unlike primary thyroid Ewing sarcoma, a favorable prognosis [4, 5]. Due to their rare occurrence, the characteristic clinical presentation, sonographic (US) findings, and cytologic features were previously ill-defined. We report a case of a patient with a progressively enlarging, non-painful neck mass. Fine needle aspiration biopsy (FNA) demonstrated a cellular smear of densely dispersed, small, monomorphic, round cells with fine nuclear chromatin, round nuclei, and scant cytoplasm. No features of papillary carcinoma were identified, and the cytologic diagnosis was suspicious for malignancy (Bethesda V) [6]. The histopathologic sections performed on a CytoFoam Core demonstrated small blue cells with a monomorphic appearance that were consistent with an undifferentiated thyroid carcinoma (UTC). Notably, immunohistochemistry performed on the same foam support ruled out thyroid lymphoma and a small cell metastatic lesion. The patient was treated with total thyroidectomy and the histologic and molecular assessment confirmed a diagnosis of papillary thyroid carcinoma (PTC) with CEFTE. No evidence of persistent disease was present at follow-up.
Case Report
Clinical Presentation
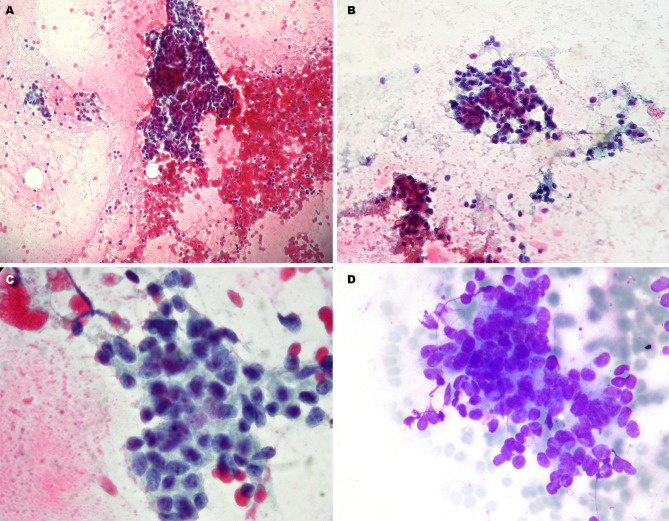
A 40-year-old man was referred to our thyroid outpatient clinic for the progressive growth of an anterior neck mass. US examination revealed a solid, hypoechoic lesion with heterogenous structure and lobulated margins in the right thyroid lobe. It measured 65 × 44 × 40 mm without sonographic evidence of infiltration of the thyroid capsule and surrounding tissues (Fig. 1a). No lymph node pathology was identified by neck US examination, and color-Doppler evaluation showed only scant intranodular vascular signals (Fig. 1b). Due to the worrisome clinical and sonographic findings, the patient underwent US-guided FNA according to previously described techniques [7]. Cytologic smears showed tissue fragments with clusters of small cells demonstrating a high nuclear-to-cytoplasmic ratio, round nuclei, coarse chromatin, inconspicuous nucleoli, and scant cytoplasm (Fig. 2). Cytologic features consistent with PTC were not identified. The cytologic sample was classified as Bethesda V: suspicious for malignancy [8].
Fig. 1.
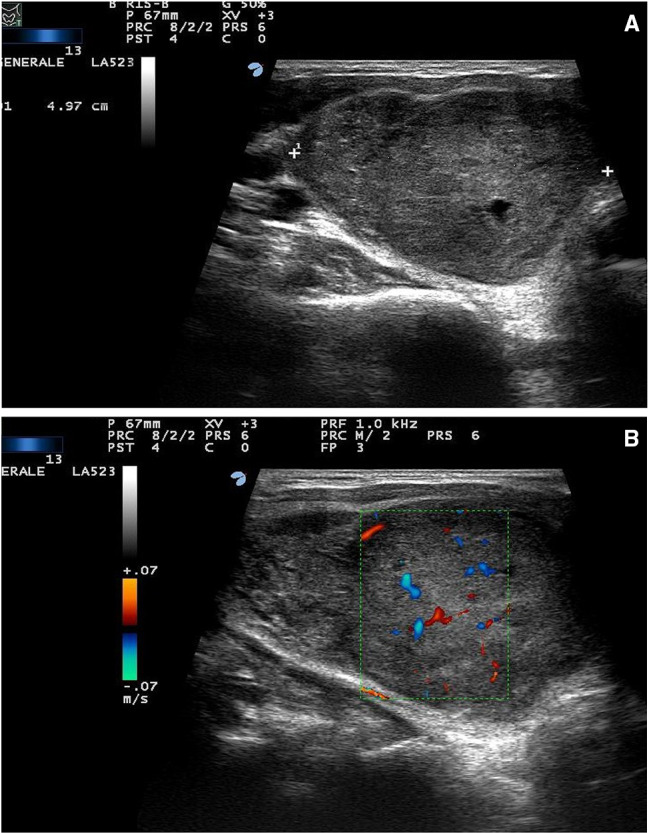
Ultrasound scan of the right thyroid lobe. a Deeply hypoechoic nodule, 50 × 60 × 35 mm, with heterogeneous structure and slightly lobulated margins; b Color-doppler evaluation demonstrates scant intranodular vascular images. No sonographic evidence of thyroid capsule infiltration
Fig. 2.
Cellular smear from fine needle aspiration (FNA) sampling (23 gauge needle) (Papanicolaou staining): a the cellular specimen is hypercellular with presence of a tissue fragment (× 100); b clustered cell groups with poorly defined cytoplasm (× 200); c groups of small cells with high nuclear-to-cytoplasmic ratio (× 400); d clear evidence of nuclear overlapping and crush artifacts (May Grunwald Giemsa staining × 400)
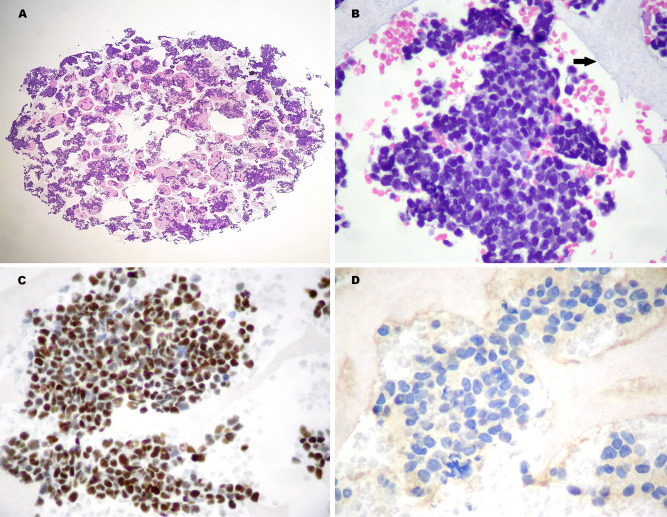
In effort to better define the preoperative diagnosis, a CytoFoam Core sample (Exmoor Innovations Ltd, Bio-Optica, Milan) was obtained with a second US-guided FNA of the thyroid lesion [9]. The CytoFoam material, collected on a synthetic foam support, was formalin-fixed and processed as a micro-histologic sample. The tissue fragments showed densely packed small, blue, round cells characterized by syncytial architecture and nuclear overlapping (Fig. 3a, b). The immunophenotypic profile was as follows: synaptophysin (−), CD56 (−), CK19 (+), p63 (+), TTF1 (−), calcitonin (−), Bcl2 (+), LCA (−), thyroglobulin (−), and Pax8 (−) (Fig. 3c, d). Due to a strong suspicion of UTC without US evidence of extracapsular growth or cervical lymph node involvement, a total thyroidectomy without neck lymph node dissection was performed.
Fig. 3.
CytoFoam Core from FNA sampling (21 gauge needle). Hematoxilin & Eosin staining (H&E): a high-power view of the specimen (× 40); b the neoplastic aggregates that are entrapped in the foam structure (arrow) demonstrate a crowded cell population with nuclear overlapping (× 200). Immunohistochemistry: c the cells express strong nuclear p63 positivity (× 200); d complete absence of TTF1 staining (× 400)
Macroscopic Examination
The surgical specimen showed a large tumor measuring 65 × 44 × 40 mm that nearly replaced the right lobe of the gland entirely. The surface was a yellowish colour without grossly apparent cystic or necrotic areas (Fig. 4).
Fig. 4.

Gross examination of the surgical specimen. The tumour nearly replaces the entire right lobe of thyroid gland. The cut surface shows a yellowish colour without relevant cystic or necrotic areas. No evidence of gross extracapsular growth
Histology and Immunohistochemistry
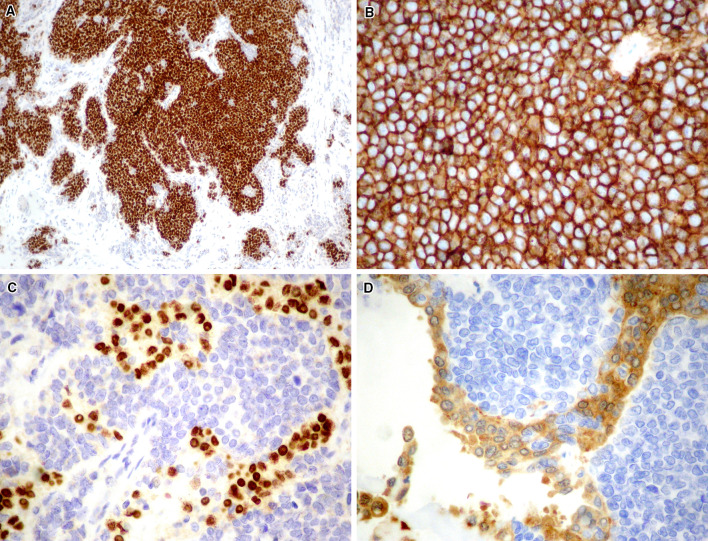
The tumor was composed of small, blue, round cells growing in sheets, nests, cords, and trabeculae that were divided into irregular lobules by fibrous strands (Fig. 5a). Cells were uniform in shape, small to medium in size, and had round nuclei with evenly distributed chromatin and inconspicuous nucleoli. The cytoplasm was scant and eosinophilic. The mitotic rate was brisk at 10–20 mitoses/10 HPF, and abundant apoptotic debris and multifocal necrosis were present (Fig. 5b). The small cells were closely associated to papillary (Fig. 5c) and follicular (Fig. 5d) structures that showed PTC-type nuclei (Fig. 5e, f). Immunohistochemical stains revealed epithelial differentiation, as demonstrated by diffuse positivity for pankeratin and p63 (Fig. 6a), as well as diffuse, strong, and membranous positivity for CD99 (Fig. 6b). The following stains were negative: thyroglobulin, TTF1, PAX8, calcitonin, CK19, synaptophysin, and CD56. The follicular and papillary structures expressed TTF-1 (Fig. 6c), thyroglobulin, cytokeratin 19, and galectin3 (Fig. 6d), confirming the coexistence of a PTC component. Immunohistochemistry was negative for VE1 (BRAFV600E) in both the papillary and small cell carcinoma areas [10].
Fig. 5.
Histopathology (H&E staining). a The small cell component is characterized by tumor cell nests separated from the surrounding fibrous stroma (× 200). b The cells show scant cytoplasm, monotonous, roundish nuclei and presence of foci of necrosis (× 400). c The small cell component closely coexists with papillary thyroid carcinoma (PTC) areas (× 400), disclosing a follicular (d, × 400) and a papillary (e, × 200) growth characterized by typical PTC-like nuclei (f, × 400)
Fig. 6.
Immunohistochemical features. The small cell component expresses a p63 (× 200), and b CD99 (× 400). The PTC cells express TTF1 (c, × 200) and galectin 3 (d, × 400)
Molecular Assessment
RNA was extracted from five to eight unstained 5 μm-thick tissue sections of formalin-fixed paraffin embedded (FFPE) specimens using the QIAamp DNA/RNA FFPE Tissue Kit (Qiagen, Hilden, Germany), according to the manufacturer’s instructions. Libraries were created using the FusionPlex Sarcoma assay in conjunction with the Archer MBC Adapters (ArcherDX, Boulder, CO). The FusionPlex Sarcoma Panel is a targeted sequencing assay that simultaneously detects and identifies fusions of 26 genes (ALK, FUS, NTRK3, TCF12, CAMTA1, GLI1, PDGFB, TFE3, CCNB3, HMGA2, PLAG1, TFG, CIC, JAZF1, ROS1, USP6, EPC1, MEAF6, SS18, YWHAE, EWSR1, MKL2, STAT6, FOXO1, NCOA2, TAF15) associated with soft tissue cancers using Archer’s proprietary Anchored Multiplex PCR™ based enrichment. With the Archer analysis software version 5.0 (ArcherDX), the produced libraries were analyzed for presence of relevant fusions. The results were confirmed by real time PCR and by fluorescent in situ hybridation (FISH).
Given the morphologic, immunohistochemical, and molecular findings, a diagnosis of CEFTE was rendered. Substitution therapy with levothyroxine 125 mcg/day resulted in a satisfactory general condition. Neck US examination and 18FDG-PET/CT imaging, along with biochemical assessment, did not reveal any regional or distant persistence of disease at the 3- and 9-months follow-up visits (Fig. 7).
Fig. 7.
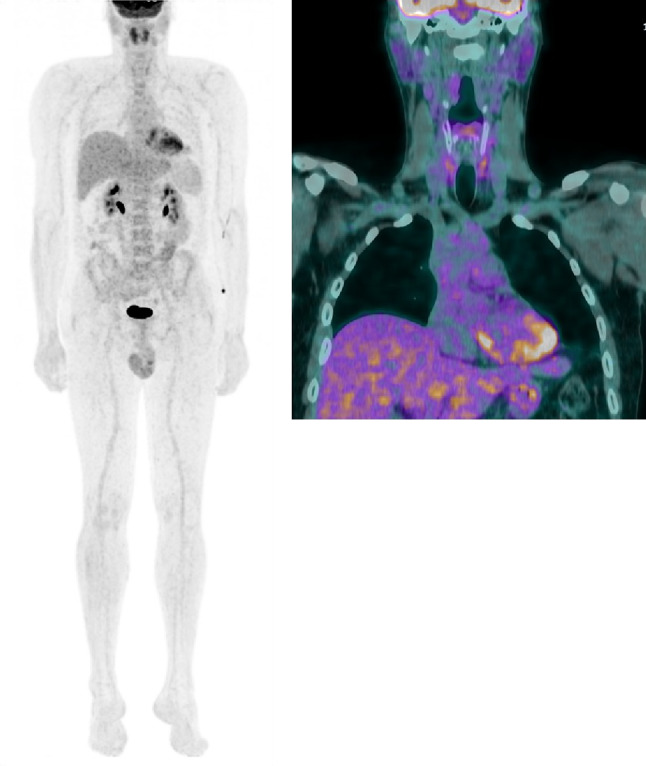
18F-FDG-PET/CT scan performed for post-surgical staging. Left panel: maximum intensity projection (MIP) showing no evidence of disease localisation in all body segments. Right panel: coronal view showing a faint, diffuse non-specific FDG uptake in the thyroid bed
Discussion
CEFTE is an exceptionally rare small cell tumour with uncertain histogenesis [3, 11]. One theory suggests a dedifferentiation of PTC cells whereby they acquire the EWSR1-FLI1 rearrangement and lose thyroid differentiation, explaining the negativity for TTF-1 and thyroglobulin [5]. Alternatively, as evidenced by the diffuse expression of p63, this tumor could originate from the main cells of solid cell nests (SCN) [12, 13]. As the EWSR1 rearrangement is quite common in PTCs and never detected in SCN, CEFTEs most likely originate from PTC foci. Although this hypothesis is not proven, the close structural relationship between CEFTE and PTC identified in our case suggests dedifferentiation of a pre-existing PTC [5, 14].
The most frequent thyroid tumor histotypes to display a small cell phenotype are poorly differentiated thyroid carcinoma (PDTC), small cell variant of medullary thyroid carcinoma (MTC), primary extraskeletal Ewing family of tumors (PEEFTs), lymphomas, and a few other rare tumors [14–16]. These include squamous cell carcinoma, carcinoma showing thymus-like elements (CASTLE), primary small cell neuroendocrine carcinoma, small cell secondary neoplasms, and rare primary tumours such as neuroblastoma and basaloid neoplasm with SCN features [12, 17]. Finally, “primary carcinomas of the thyroid with Ewing family tumor elements” (CEFTE) were recently described [3–5]. CEFTEs are exceptionally rare and difficult to distinguish from aggressive PEEFTs as they share the same morphology, immunophenotype, and EWSR1-FLI1 rearrangement [5, 17]. In contrast with PEEFTs, CEFTEs generally have a minor PTC component concurrent with the small cell population [3, 4]. Interestingly, in the present case, US-guided FNA did not provide a correct preoperative diagnosis due to a lack of PTC component in the sample. The second FNA obtained with the CytoFoam Core revealed a small, blue, round cell component with an immunophenotype showing diffuse expression of p63, pankeratin, and CD99 [15].
In the histologic differential diagnosis, MTC was excluded due to the absence of amyloid and lack of calcitonin and TTF1 expression [3, 15, 16]. Although spindle epithelial tumor with thymus-like element (SETTLE), a rare entity of presumed branchial origin, shows diffuse keratin expression, this entity was ruled out based on the absence of its characteristic spindled and glandular histologic features. Furthermore, these tumors are negative for the EWSR1-FLI1 rearrangement. Intrathyroid thymic carcinoma, like CEFTE, shows the expression of epithelial markers but is more squamous in appearance with variable lymphoid infiltrates. It may also express CD5 and CD117 [3]. Finally, CEFTE was distinguished from PEEFT by the positivity of p63 and cytokeratins [4].
As the PEEFT of the thyroid follows an aggressive clinical course, similar to that of the PEEFTs in other sites of the body, an accurate preoperative differential diagnosis is of crucial importance for defining the prognosis and guiding the therapeutic approach [16, 17]. The present case, in accordance with others previously reported, emphasizes that the presence of epithelial differentiation in a thyroid tumor does not conclusively rule out the possibility of an Ewing family tumor [3, 4, 16]. Poorly differentiated head and neck tumors that show nuclear monotony, fine chromatin, epithelial differentiation, and positivity for CD99 should prompt further immunohistochemical and, possibly, molecular workup to exclude the presence of a CEFTE [2, 14, 18]. For cases with inconclusive cytologic features and an ambiguous immunophenotype on the FNA sample, both a core needle biopsy, performed with a 20G cutting needle, and a CytoFoam Core, obtained with a less traumatic 21G FNA, may provide relevant information [8]. Molecular confirmation remains necessary for achieving a definitive diagnosis of CEFTE [14, 18].
While too few cases of CEFTE of the thyroid are reported to elucidate the true prognosis, this case showed complete remission of disease at 9 months, suggesting a possible improved prognosis as compared to primary Ewing sarcoma of the thyroid.
A few potential pitfalls pertaining to the clinical approach to a rapidly enlarging thyroid mass with cytologic findings of solely small cell clusters were illustrated. Specifically, the possibility of a thyroid metastasis was not preoperatively ruled out through an extensive search for a primary tumor, no studies for a EWSR1-FLI1 rearrangement were performed on the initial cytology specimen, and, finally, multiple samplings in different areas of the large thyroid lesion, to potentially demonstrate the coexistence of both PTC and small cell features, were not carried out.
Conclusions
CEFTE is a peculiar type of small cell thyroid carcinoma characterized by PEEFT features interspersed with foci of PTC. Although the clinical presentation suggested an aggressive cancer, the absence of extrathyroid spread and the lack of disease relapse after surgery demonstrated favourable behavior of the tumor. Notably, the correct diagnosis prevented cervical lymph node dissection and the use of adjuvant chemotherapy and/or external beam radiation therapy. This is the first reported case of CEFTE with a complete clinical, US, cytologic, and immunohistochemical preoperatory assessment and post-surgical follow-up.
Acknowledgements
The authors are grateful to the laboratory staff for excellent technical assistance: M. D’Angelo, P. Carone and G. Gaglianò. The authors would also like to thank A. Cerrito for his assistance with the preparation of the images.
Compliance with Ethical Standards
Conflict of interest
The authors have no conflicts of interest or financial relationships to disclose.
Ethical Approval
This article does not contain any studies with animals performed by any of the authors.
Informed Consent
Informed consent was waived for this study as part of the corresponding author’s TS.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Kabata P, Kaniuka-Jakubowska S, Kabata W, Lakomy J, Biernat W, Krzysztof S, Kiewicz JJ, Swierblewski M. Primary Ewing sarcoma of the thyroid—eight cases in a decade: a case report and literature review front. Endocrinol. 2017 doi: 10.3389/fendo.2017.00257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sanati S, Lu DW, Schmid E, Perry A, Dehner LP, Pfeifer JD. Cytologic diagnosis of Ewing sarcoma/peripheral neuroectodermal tumor with paired prospectivemolecular genetic analysis. Cancer Cytopathol. 2007;111:192–199. doi: 10.1002/cncr.22692. [DOI] [PubMed] [Google Scholar]
- 3.Eloy C, Cameselle-Teijeiro JM, AmendoeiraI, Soares P, Caneiro-Gómez J, Melo M, Sobrinho-Simões M. In: Cameselle-Teijeiro JM, Eloy C, Sobrinho-Simões M, editors. Rare tumors of the thyroid gland. Diagnosis and WHO Classification. Berlin: Springer; 2018. p. 53–56.
- 4.Eloy C, Cameselle-Teijeiro JM, Rousseau E, Sobrinho-Simões M. Small cell tumors of the thyroid gland: a review. Int J SurgPathol. 2014;22(3):197–201. doi: 10.1177/1066896913510029. [DOI] [PubMed] [Google Scholar]
- 5.De Oliveira CGS. ‘Study of EWSR1 rearrangement in thyroid carcinoma’ Guilherme Santos De Oliveira 2015 Dissertação De Mestrado em Oncologia. https://repositorio-aberto.up.pt/bitstream/10216/86650/2/36991.
- 6.Cibas ES, Ali SZ. The 2017 bethesda system for reporting thyroid cytopathology. Thyroid. 2017;27(11):1341–1346. doi: 10.1089/thy.2017.0500. [DOI] [PubMed] [Google Scholar]
- 7.Kim MJ, Kim EK, Park SI, Kim BM, Kwak JY, Kim SJ, Youk JH, Park SH. US-guided fine-needle aspiration of thyroid nodules: indications, techniques, results. RadioGraphics. 2008;28:1869–1889. doi: 10.1148/rg.287085033. [DOI] [PubMed] [Google Scholar]
- 8.Pusztaszeri M, Rossi ED, Auger M, Baloch Z, et al. The Bethesda system for reporting thyroid cytopathology: proposed modifications and updates for the second edition from an international panel. ActaCytologica. 2016;60:399–405. doi: 10.1159/000451020. [DOI] [PubMed] [Google Scholar]
- 9.Erra E. A novel approach towards fine needle aspiration cytology with ultrasound-guide and cyto-assistance: utility of cytofoam-core in differential diagnosis of nodular mass in parenchymatous superficial organs. MOJ Anat Physiol. 2017;4(1):208–209. [Google Scholar]
- 10.Singarayer R, Mete M, Perrier L, Thabane L, Asa SL, Van Uum S, Ezzat S, Goldstein DP, Sawka AM. A systematic review and meta-analysis of the diagnostic performance of BRAF V600E immunohistochemistry in thyroid histopathology. Endocr Pathol. 2019 doi: 10.1007/s12022-019-09585-2. [DOI] [PubMed] [Google Scholar]
- 11.Eloy C, Cameselle-Teijeiro J, Vieira J, Teixeira MR, Cruz J, Sobrinho-Simões M. Carcinoma of the thyroid with Ewing/PNET family tumor elements: a tumor of unknown histogenesis. Int J SurgPathol. 2014;22(6):579–581. doi: 10.1177/1066896913486697. [DOI] [PubMed] [Google Scholar]
- 12.Cruz J, Eloy C, Aragüés JM, Vinagre J, Sobrinho-Simões M. Small-cell (basaloid) thyroid carcinoma: a neoplasm with a solid cell nest histogenesis? Int J SurgPathol. 2011;19(5):620–626. doi: 10.1177/1066896911405320. [DOI] [PubMed] [Google Scholar]
- 13.Eloy C, Vinagre J, Cameselle-Teijeiro J, Paiva ME, Soares P, Sobrinho-Simões M. Tumor-in-tumor of the thyroid with basaloid differentiation: a lesion with a solid cell nest neoplastic component? Int J SurgPathol. 2011;19:276–280. doi: 10.1177/1066896910393506. [DOI] [PubMed] [Google Scholar]
- 14.Oliveira G, Polónia A, Cameselle-Teijeiro JM, Leitão D, Sapia S, Sobrinho-Simões M, Eloy C. EWSR1 rearrangement is a frequent event in papillary thyroid carcinoma and in carcinoma of the thyroid with Ewing family tumor elements (CEFTE) Virchows Arch. 2017;470(5):517–552. doi: 10.1007/s00428-017-2095-1. [DOI] [PubMed] [Google Scholar]
- 15.Morlote D, Harada S, Lindeman B, Stevens TM. Adamantinoma-like Ewing sarcoma of the thyroid: a case report and review of the literature. Head Neck Pathol. 2019 doi: 10.1007/s12105-019-01021-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chan JM, Bilodeau E, Celin S, Nikiforov Y, Johnson JT. Ewing sarcoma of the thyroid: report of 2 cases and review of the literature. Head Neck. 2013;35(11):E346–E350. doi: 10.1002/hed.23240. [DOI] [PubMed] [Google Scholar]
- 17.Bishop JA, Alaggio R, Zhang L, Seethala RR, Antonescu CR. Adamantinoma-like Ewing family tumors of the head and neck: a pitfall in the differential diagnosis of basaloid and myoepithelial carcinomas. Am J Surg Pathol. 2015;39:1267. doi: 10.1097/PAS.0000000000000460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Owosho AA, Estilo C, Huryn JM, et al. Head and neck. Round cell sarcomas: a comparative clinicopathologic analysis of 2 molecular subsets: Ewing and CIC-rearranged sarcomas. Head Neck Pathol. 2017;11:450–459. doi: 10.1007/s12105-017-0808-z. [DOI] [PMC free article] [PubMed] [Google Scholar]