Abstract
Ectomesenchymal chondromyxoid tumor is a rare neoplasm of uncertain histogenesis that typically occurs in the anterior dorsal tongue. Recent reports in the literature have described rare examples of gingival, palatal and tonsillar lesions. Histologically, ectomesenchymal chondromyxoid tumors are typically well-circumscribed, lacking overtly aggressive features. Herein we report a tumor arising in the right mandible that is morphologically and molecularly consistent with ectomesenchymal chondromyxoid tumor. This case furthers awareness of the extra-glossal distribution of this neoplasm; moreover, it suggests that a subset of these tumors have the potential for locally aggressive behaviour.
Electronic supplementary material
The online version of this article (10.1007/s12105-020-01169-5) contains supplementary material, which is available to authorized users.
Keywords: Ectomesenchymal chondromyxoid tumor, Case report, RREB1, MRTFB, MKL2, Gene rearrangement
Introduction
Ectomesenchymal chondromyxoid tumor, which was first described in a case series by Smith et al., is considered a rare benign neoplasm that has a striking predilection for the anterior dorsal tongue [1]. The histogenesis of this neoplasm remains uncertain, although it has been postulated to arise from an undifferentiated ectomesenchymal cell [1]. Recently, it has been demonstrated that virtually all cases are characterized by a RREB1-MKL2 fusion product [2]. To date, all of the cases reported in the literature have occurred in soft tissue, with the vast majority arising in the tongue [1, 2]. Rare examples of lesions involving the gingiva [3], hard palate [4, 5] and tonsillar bed [6] have also been reported. Herein, we describe the first case of a molecularly confirmed primary intraosseous ectomesenchymal chondromyxoid tumor arising in the mandible.
Case Report
A previously healthy 37-year-old woman presented with a tumor in the right body of the mandible. Computed tomographic (CT) imaging of the area revealed an expansile radiolucency in the right body of the mandible with a sharp zone of transition (Fig. 1). The lesion measured 2.4 cm × 1.4 cm and extended from the root of the right mandibular first premolar to the root of the right mandibular second permanent molar. Thinning of the buccal and lingual cortices of the right mandible was noted, particularly involving the buccal cortex in which soft tissue extension was identified. No significant lymphadenopathy was observed on imaging.
Fig. 1.

Computed tomographic (CT) imaging of the right mandibular lesion demonstrates thinning of the cortical bone with soft tissue extension
The incisional biopsy, representing only soft tissue extension of the lesion, showed a bland spindle-stellate cell neoplasm with a vague storiform pattern within a myxo-hyaline stroma (Fig. 2). No ductal or glandular formation is identified. The tumor cells have pale and eosinophilic cytoplasm with occasional vacuolation. The nuclei were ovoid and monomorphic with occasional binucleation. The overlying squamous epithelium was unremarkable. Differential diagnoses were considered including an ECM, chondromyxoid fibroma, myoepithelioma, pleomorphic adenoma, myxoid neurofibroma and extraskeletal myxoid chondrosarcoma. Based on the characteristic morphology of the oral submucosal neoplasm, the findings were favoured to represent ectomesenchymal chondromyxoid tumour, and the case was submitted for confirmatory molecular testing. RNA-sequencing (RNA-Seq) confirmed the presence of an RREB1-MRTFB (formerly MKL2) fusion product; this involved RREB1 (exon 8 of 13; NCBI Reference Sequence: NM_001003699.3), and MRTFB (exon 11 of 17; NM_001308142.1). Based on the morphology and molecular features, a diagnosis of ectomesenchymal chondromyxoid tumor was rendered. The tumor was subsequently resected with curative intent.
Fig. 2.

Ectomesenchymal chondromyxoid tumor, incisional biopsy. a A spindle cell neoplasm with myxo-hyaline stroma in the submucosa. The surface squamous epithelium is unremarkable. b The tumor cells have pale, vacuolated cytoplasm and small nuclei with occasional binucleation
Examination of the resection specimen revealed a 3.0 cm lobulated neoplasm replacing the marrow cavity and associated with soft tissue extension (Fig. 3). The morphology was similar to that of the initial biopsy, and within the continuum of findings seen in ectomesenchymal chondromyxoid tumor. A broad immunohistochemical panel (Supplementary Table 1) demonstrated patchy immunoreactivity for S100, smooth muscle actin (Fig. 4), CD56, with very rare immunoreactivity for desmin and glial fibrillary acid protein. The tumor was negative for myogenin, SOX10, keratin (AE1/AE3), chromogranin and calponin. Mitotic activity and necrosis were not identified.
Fig. 3.
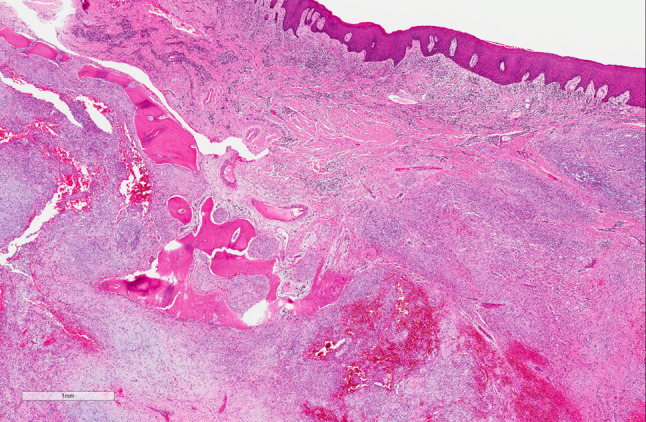
Ectomesenchymal chondromyxoid tumor, resection specimen. The tumor is replacing the marrow cavity and associated with soft tissue extension
Fig. 4.
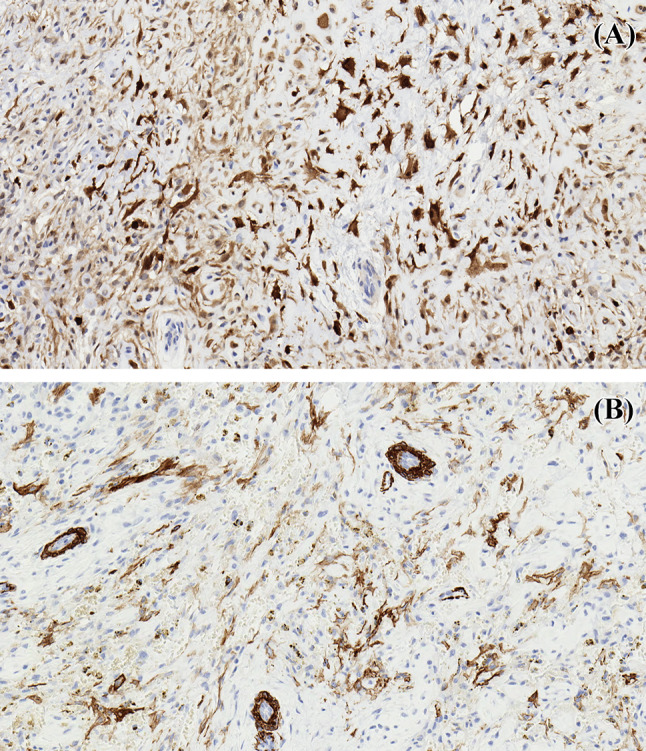
Immunohistochemistry demonstrates patchy immunoreactivity of the tumor for a S100 and b smooth muscle actin
Discussion
Since its initial description by Smith et al., the reported cases of ectomesenchymal chondromyxoid tumor have largely been confined to the tongue [1, 2]. Rare cases demonstrating a gingival, palatal or tonsillar location have also been described in the literature [3–6]; however, the diagnosis of one case has been previously questioned due to a lack of immunohistochemical data [3, 7]. Two of the previously reported ectomesenchymal chondromyxoid tumors involving the palatal gingiva demonstrated no radiographic evidence of bone involvement [3, 4].
To date, ectomesenchymal chondromyxoid tumor have been reported to follow a benign course with no metastases reported. In most instances, conservative excision is curative; however, local recurrence has been reported in a minority of cases, even with incomplete excision [1, 2, 8].
Histologically, ectomesenchymal chondromyxoid tumors are typically well-circumscribed and unencapsulated with tumor lobules separated by thin fibrous septa [1]. Although frank invasion is not a feature of this tumor, several authors have reported muscle fiber entrapment or “pseudo-infiltration” of muscle at the periphery of some lesions [1, 2, 8–12]. The tumor cells are variably described as spindled, stellate, fusiform or polygonal with small nuclei and moderate amounts of pale cytoplasm [1, 2]. The cells are typically arranged in cords, sheets, globoid or reticular patterns in a chondromyxoid stromal background with occasional hyalinized areas [1, 2]. There may be some atypical features, such as pleomorphism and nuclear atypia and might be confused with other malignant processes. However, the absence of mitotic figures, tumor necrosis, perineural and/or angiolymphatic invasion hitherto supports a benign tumor. Immunohistochemistry frequently demonstrates immunoreactivity for S100, glial fibrillary acid protein and smooth muscle actin, while keratin, and desmin are less commonly expressed [1, 2].
The molecular pathogenesis of ectomesenchymal chondromyxoid tumor has only recently been characterized. Argyris et al. previously demonstrated EWSR1 rearrangement by fluorescence in-situ hybridization (FISH) in 3/11 (27%) cases of ectomesenchymal chondromyxoid tumor [13]. More recently, Dickson et al. detected a RREB1-MRTFB (formerly MKL2) fusion gene in 19/21 (90%) tumors by RNA-Seq and/or FISH as well as an EWSR1-CREM fusion product in a single tumor [2]. In addition to the ectomesenchymal chondromyxoid tumor, the RREB1-MRTFB fusion product has also been reported in a “biphenotypic oropharyngeal sarcoma” [14] as well as two mediastinal masses described as “extra-glossal ectomesenchymal tumors” [15]. The biphenotypic oropharyngeal sarcoma appears to be morphologically dissimilar to the current case; thus, the relationship of this report to ectomesenchymal chondromyxoid tumor remains uncertain. The mediastinal tumors shared morphologic and immunophenotypic features with ectomesenchymal chondromyxoid tumor, thereby expanding the clinical distribution of this entity.
In summary, to our knowledge this is the first example of an intraosseous tumor exhibiting the RREB1-MRTFB fusion. In conjunction with morphologic and immunohistochemical features consistent with an ectomesenchymal chondromyxoid tumor, this case expands our understanding of the anatomic distribution of this tumor as well as its biologic potential as an intermediate, locally aggressive neoplasm. Further studies are required in order to better characterize the anatomic distribution and behaviour of this rare and enigmatic neoplasm.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Compliance with Ethical Standards
Conflict of interest
The authors declare that there is no conflict of interest to disclose
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Smith BC, Ellis GL, Meis-Kindblom JM, Williams SB. Ectomesenchymal chondromyxoid tumor of the anterior tongue. Nineteen cases of a new clinicopathologic entity. Am J Surg Pathol. 1995;19(5):519–30. doi: 10.1097/00000478-199505000-00003. [DOI] [PubMed] [Google Scholar]
- 2.Dickson BC, Antonescu CR, Argyris PP, et al. Ectomesenchymal chondromyxoid tumor: a neoplasm characterized by recurrent RREB1-MKL2 fusions. Am J Surg Pathol. 2018;42(10):1297–305. doi: 10.1097/PAS.0000000000001096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Truschnegg A, Acham S, Kqiku L, Jakse N, Beham A. Ectomesenchymal chondromyxoid tumor: a comprehensive updated review of the literature and case report. Int J Oral Sci. 2018;10(1):4. doi: 10.1038/s41368-017-0003-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gouvêa AF, Díaz KP, Léon JE, Vargas PA, de Almeida OP, Lopes MA. Nodular lesion in the anterior hard palate. Oral Surg Oral Med Oral Pathol Oral Radiol. 2012;114(2):154–9. doi: 10.1016/j.oooo.2011.11.005. [DOI] [PubMed] [Google Scholar]
- 5.Nigam S, Dhingra KK, Gulati A. Ectomesenchymal chondromyxoid tumor of the hard palate—a case report. J Oral Pathol Med. 2006;35(2):126–8. doi: 10.1111/j.1600-0714.2006.00368.x. [DOI] [PubMed] [Google Scholar]
- 6.Stecco A, Quagliozzi M, Pino M, et al. An unusual case of ectomesenchymal chondromyxoid tumour of the left tonsillar bed: imaging and histopathologic features. BJR Case Rep. 2016;2(3):20150183. doi: 10.1259/bjrcr.20150183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ide F. Chondromyxoid tumor of palate. J Oral Pathol Med. 2006;35(8):523–4. doi: 10.1111/j.1600-0714.2006.00452_1.x. [DOI] [PubMed] [Google Scholar]
- 8.Portnof JE, Friedman JM, Reich R, Freedman PD, Behrman DA. Oral ectomesenchymal chondromyxoid tumor: case report and literature review. Oral Surg Oral Med Oral Pathol Oral Radiol Endocrinol. 2009;108(4):e20–4. doi: 10.1016/j.tripleo.2009.05.040. [DOI] [PubMed] [Google Scholar]
- 9.Aldojain A, Jaradat J, Summersgill K, Bilodeau EA. Ectomesenchymal chondromyxoid tumor: a series of seven cases and review of the literature. Head Neck Pathol. 2015;9(3):315–22. doi: 10.1007/s12105-014-0578-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sengul D, Sengul I, Ozdol MU, Astarci MH, Ustun H. Ectomesenchymal chondromyxoid tumor of the anterior tongue: a rare case. Kaohsiung J Med Sci. 2011;27(5):203–5. doi: 10.1016/j.kjms.2010.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Seo SH, Shin DH, Kang HJ, et al. Reticulated myxoid tumor of the tongue: 2 cases supporting an expanded clinical and immunophenotypic spectrum of ectomesenchymal chondromyxoid tumor of the tongue. Am J Dermatopathol. 2010;32(7):660–4. doi: 10.1097/DAD.0b013e3181d7d3bf. [DOI] [PubMed] [Google Scholar]
- 12.de Visscher JG, Kibbelaar RE, van der Waal I. Ectomesenchymal chondromyxoid tumor of the anterior tongue. Report of two cases. Oral Oncol. 2003;39(1):83–6. doi: 10.1016/S1368-8375(01)00117-8. [DOI] [PubMed] [Google Scholar]
- 13.Argyris PP, Bilodeau EA, Yancoskie AE, et al. A subset of ectomesenchymal chondromyxoid tumours of the tongue show EWSR1 rearrangements and are genetically linked to soft tissue myoepithelial neoplasms: a study of 11 cases. Histopathology. 2016;69(4):607–13. doi: 10.1111/his.12973. [DOI] [PubMed] [Google Scholar]
- 14.Siegfried A, Romary C, Escudié F, et al. RREB1-MKL2 fusion in biphenotypic “oropharyngeal” sarcoma: new entity or part of the spectrum of biphenotypic sinonasal sarcomas? Genes Chromosomes Cancer. 2018;57(4):203–10. doi: 10.1002/gcc.22521. [DOI] [PubMed] [Google Scholar]
- 15.Makise N, Mori T, Kobayashi H, et al. Mesenchymal tumors with RREB1-MRTFB fusion involving the mediastinum: extra-glossal ectomesenchymal chondromyxoid tumors? Histopathology. 2020 doi: 10.1111/his.14080. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


