Abstract
Segmental odontomaxillary dysplasia (SOD) is a developmental condition of the middle and posterior maxilla featuring dysplastic bone overgrowth, dental abnormalities and, occasionally, various homolateral cutaneous manifestations. Herein, we describe an individual with maxillary abnormality akin to SOD and associated ipsilateral segmental odontomandibular dysplasia. Also, the result of the evaluation of lesional mandibular gingival tissue for overgrowth-related gene variants is reported. An 8-year-old girl presented clinically with congenital maxillary and mandibular alveolar soft tissue enlargement in the area of the premolars. A panoramic radiograph revealed abnormal trabeculation essentially similar to SOD in the maxilla and mandible with congenitally missing maxillary and mandibular first and second premolars and mandibular canines. Diagnostic mandibular bone biopsy was performed and lesional mandibular gingival hyperplastic tissue was obtained for variant analysis of somatic overgrowth genes PIK3CA, AKT1, AKT3, GNAQ, GNA11, MTOR, PIK3R2. Cone beam computerized tomography (CBCT) disclosed osseous abnormalities on the left side of the maxilla and mandible and very mild osseous expansion in the mandible. Histologically, abnormal bone exhibiting prominent reversal lines was present and associated with fibrocollagenous tissue. Genomic DNA analysis disclosed PIK3CAc.1571G>A; pArg524Lys which was seen at a low mosaic level in the blood, indicating a post-zygotic change. Although this case may be a unique disorder, by sharing features with SOD, one can suggest the possibility of mandibular involvement in SOD. The presence of a PIK3CA variant may support the hypothesis that these segmental disorders could be part of the PIK3CA-related overgrowth spectrum.
Keywords: Segmental odontomaxillary dysplasia, Mandible, PIK3CA, Somatic mutation, Segmental odontognathic dysplasia
Segmental odontomaxillary dysplasia (SOD) was first described by Miles et al. [1] as “hemimaxillofacial dysplasia” and its clinicopathologic characteristics further refined and detailed by Danforth et al. [2] who coined the term SOD. Briefly, observed are unilateral maxillary bone enlargement with associated hyperplasia of the gingival/alveolar soft tissue, missing teeth, usually premolars, dental hard tissue defects, and, radiographically, thickened trabeculation with vertical orientation. Radiographically, the lesions may be misinterpreted as fibrous dysplasia, however, histopathologic features exclude this possibility. Homolateral cutaneous abnormalities, frequently subtly, can be encountered in less than 50% of the patients evaluated for skin involvement, and include variations in pigmentation, e.g. facial erythema, melanin hyperpigmentation and hypertrichosis, features of Becker nevus and, rarely, hypopigmentation. A comprehensive review by Smith et al. [3] on SOD summarizes all legitimate reports in the literature on the subject to this date. The term hemimaxillary enlargement, asymmetry of the face, tooth abnormalities and skin findings (HATS) has been also proposed to describe the condition but it is not widely accepted [4, 5]. Furthermore, extension of bone involvement to affect the zygomatic and base of the orbit bones [6] has been reported justifying the term segmental hemimaxillofacial dysplasia used occasionally.
In the present report, an individual is described with maxillary abnormality akin to SOD and ipsilateral segmental odontomandibular dysplasia and soft tissue enlargement of the gingiva and alveolar mucosal of the affected sites. Being a disorder of asymmetry and apparent overgrowth, genomic evaluation of mandibular lesional gingival/alveolar soft tissue for genes related to overgrowth was performed to possibly elucidate the pathogenesis of the condition.
Case Report
An 8-year-old Caucasian girl presented clinically with left maxillary and mandibular gingival enlargement in the area of premolars in (Fig. 1a and b). The facial enlargement was apparently congenital and accompanied by homolateral hypopigmentation and palsy which have persisted to this date. It was initially thought that the overgrowth was the result of traumatic delivery due to shoulder dystocia that resulted in left sided facial bruising and bilateral subconjunctival hemorrhages.
Fig. 1.
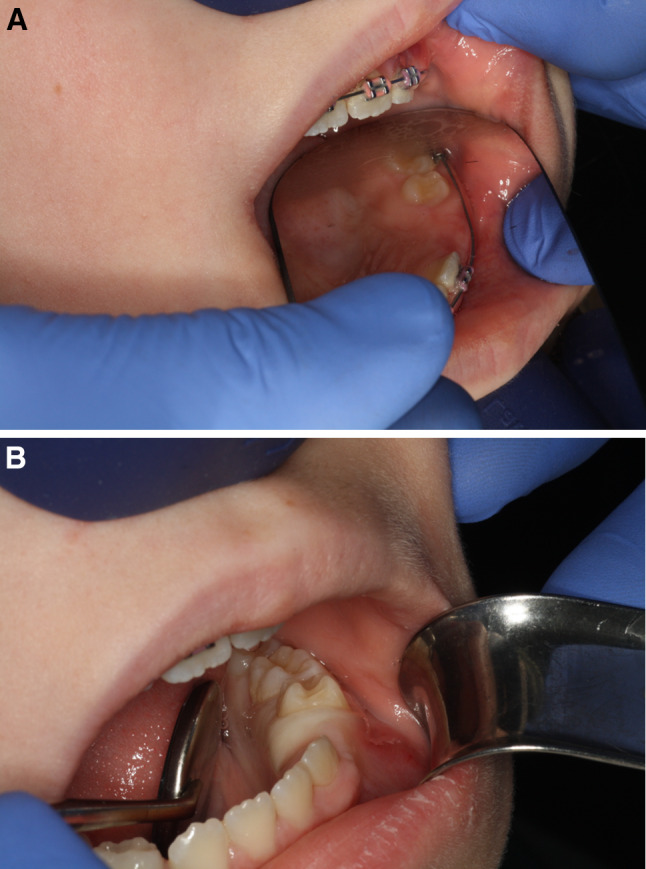
a Maxillary gingivoalveolar enlargement. b Mandibular gingivoalveolar enlargement
A panoramic radiograph (Fig. 2) at age 9 revealed abnormal trabeculation with characteristics similar to SOD, and congenitally missing left maxillary and mandibular first and second premolars and both mandibular canines. Cone beam computerized tomography disclosed mild enlargement of the left side of the mandible in the area of the premolars with abnormal trabeculation (Fig. 3a) and abnormal maxillary bone in the area of the premolars exhibiting loss of lingual plate definition and lingual concavity when compared to the contralateral side (Fig. 3b). Bone biopsy revealed dysplastic woven bone with prominent reversal lines, features that were interpreted similar to SOD (Fig. 4a and b).
Fig. 2.
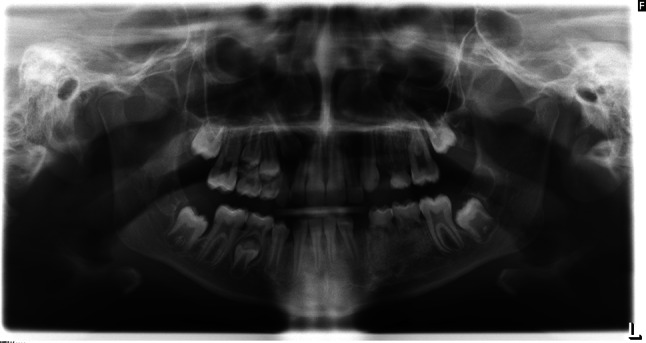
Panoramic radiograph at age 9 reveals abnormal trabeculation in the middle aspect of left side of the maxilla in association with a retained primary molar and congenitally missing first and second premolars. In the mandible there is abnormal trabeculation of the left middle segment in the area of retained primary molars. Both mandibular left premolars and both canines are also missing. There is also evidence of developing third molars
Fig. 3.
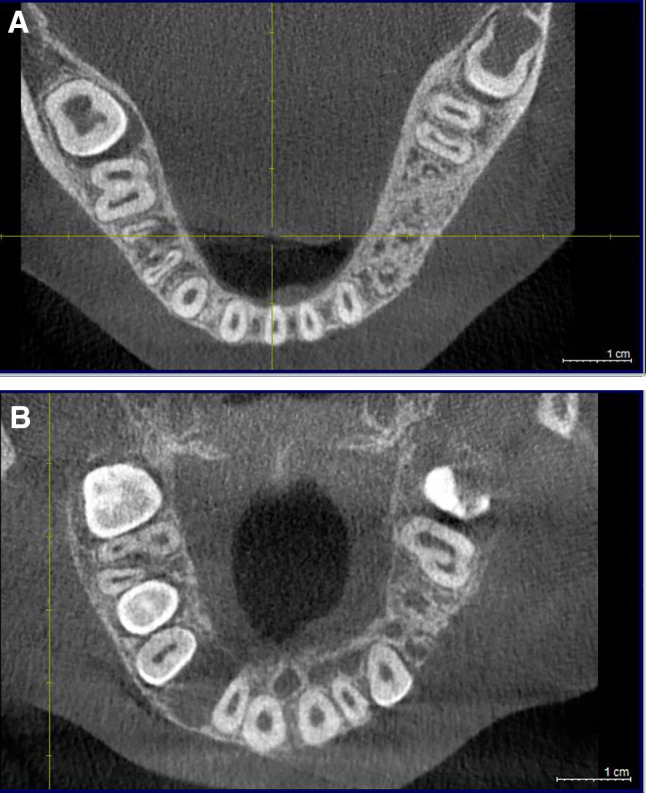
a Very slight enlargement of the left side of the mandible and abnormal trabeculation present. b Abnormal trabeculation of the left side of the maxillary bone in the area of the premolars exhibiting loss of lingual plate definition and lingual concavity when compared to the contralateral side
Fig. 4.
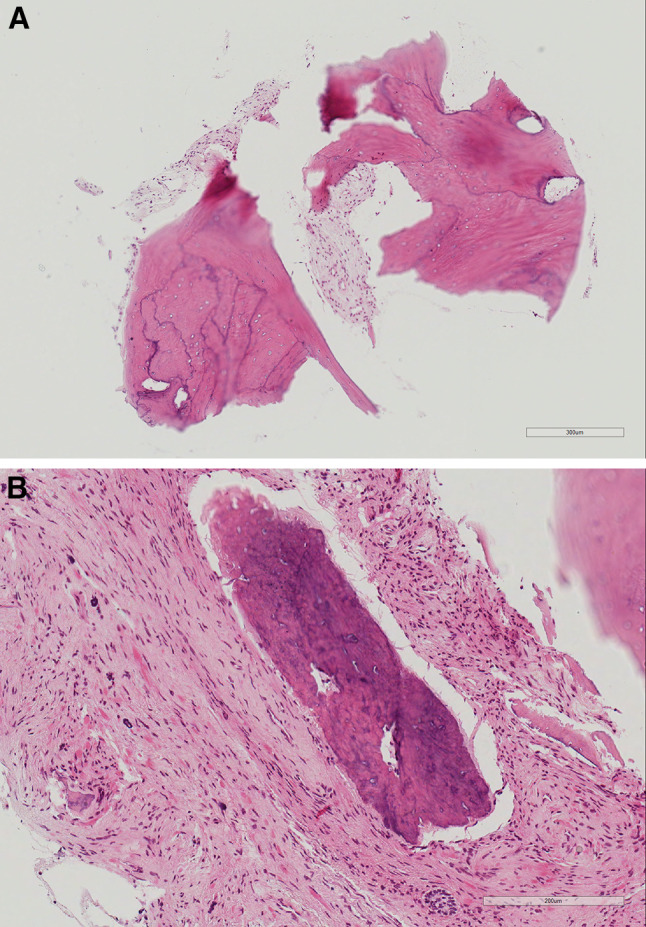
a Bone biopsy revealed dysplastic woven bone with prominent reversal lines in association with fibrocollagenous connective tissue. b Woven bone spicules in a loose and dense fibrocollagenous stroma
For purposes of genomic DNA evaluation, soft tissue from mandibular gingival/alveolar hyperplastic mucosa was surgically obtained. Mutation analysis of somatic overgrowth related genes PIK3CA, AKT1, AKT2, AKT3, GNAQ, GNA11, MTOR, and PIK3R2 was undertaken using next generation sequencing (NGS) on the IonTorrent PGM platform. The test assesses all coding regions of PIK3CA and flanking intron sequences (± 20 bp) and specific regions of the AKT1, AKT2, AKT3, GNAQ, GNA11, MTOR, and PIK3R2. The minimum threshold for variant allele detection is set at 2% at 2000 × coverage and ≥ 10 reads without strand bias. Repeat sequencing of an independent replicate sample was also performed on the same platform to confirm mutations. For appropriate variant filtering the Ion Reporter Software suite was used. The confidence score was calculated using the Phred quality score [7, 8]. Known variants with minor allele frequencies greater than 5% in the Exome Aggregation Consortium (https://exac.broadinstitute.org) and variants with a Phred quality score < 20 were filtered out of analysis.
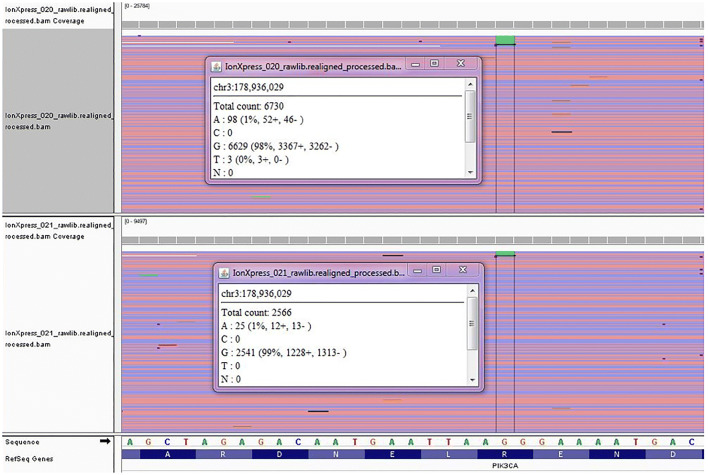
DNA analysis of two gingival tissue portions resulted in identification of heterozygosity at a mosaic level (0.5–1.8%) for a single base substitution, c.1571G>A, in exon 10 of the coding sequence of PIK3CA (Phosphatidylinositol-4,5-Bisphosphate 3-Kinase Catalytic Subunit Alpha) which translates to a missense mutation, p.Arg524Lys, in the translated PIK3CA protein (Fig. 5). The variant was seen at a low mosaic level of 2.0–3.7% in the blood. The frequency indicates that this variant was a post-zygotic change and not germline.
Fig. 5.
Below is a summary of sequencing reads (visualized via Integrative Genomics Viewer) at the c.1571 position in PIK3CA for two gingival hyperplastic tissue biopsies. The c.1571G>A variant was detected an at allele frequency of 1.0–1.5% in the results shown here. The presence of the variant in these biopsies was further confirmed by additional replicates on the same sequencing platform. No significant strand bias was observed among the mutant alleles
The girl has completed phase one of orthodontic treatment which consisted of alignment of the anterior teeth. The retained primary teeth will be maintained for as long as possible in order to preserve space in the posterior jaw. After puberty, the quality of bone will be assessed and the possibility of dental implants with or without bone grafting will be considered. If the patient is not a good candidate for dental implants then a fixed partial prosthesis will be fabricated. Surgical contouring of the jaws will be performed only if cosmetically necessary. Lastly, the palsy affecting the left cheek has been improving over time.
Discussion
Herein, we presented the clinicopathologic findings of an individual with clinical lesions exhibiting similarities with SOD who, in addition, revealed mandibular involvement. This may be a unique disorder. However, one can suggest the possibility of the mandible being also affected, however rarely, in SOD. SOD is an uncommon, apparently underrecognized, developmental disorder characterized by posterior maxillary enlargement, dental and osseous abnormalities and occasional homolateral cutaneous findings [3].
Because the condition is non-hereditary, segmental, and partially characterized by enlargement, we considered the possibility of somatic mutation in one of the genes related to overgrowth as a causative mechanism. We were able to identify somatic PIK3CAc.1571G>A; pArg524Lys albeit in a small percentage in the lesional mandibular gingival tissue. Interestingly, the variant existed in a low mosaic level in the blood.
Phosphatidylinositide 3 kinases (PI3K), members of intracellular lipid kinases, and their downstream mediators, AMP/GMP/protein kinase C (AKT) kinases and mammalian target of rapamycin (mTOR), comprise the PI3K/AKT/mTOR signaling pathway which is essential in the regulation of cell proliferation, growth, migration, cell survival and angiogenesis [9]. PIK3CA encodes the p110α catalytic subunit of class IA PI3Ks which, when activated, phosphorylates AKT to initiate downstream cellular effects [9].
Somatic PIK3CA mutations commonly occur in many human cancers including breast and colon, as well as, overgrowth disorders, a group of conditions characterized by combinations of skeletal and soft tissue overgrowth, vascular malformations, epidermal nevi, megalencephaly and skin lesions collectively referred to as PIK3CA-related overgrowth spectrum (PROS) [10, 11]. Most activating PIK3CA mutations are clustered at three hot-spots, two glutamates (E) at 542 and 545 in the helical domain, and histidine (H) 1047 near the C-terminus of the kinase domain. Surprisingly, the same hot spots are observed in cancers with participating PIK3CA mutations, as well as, epidermal nevi and seborrheic keratoses, two benign conditions (for a comprehensive review see [12]).
The presence of somatic mutations in PROS supports Rudolph Happle’s principle that non-hereditary syndromic conditions affecting a segment or segments of the body are caused by somatic mosaic mutations while complete heterozygosity of the same mutations should be considered detrimental for development resulting in lethal phenotype [13]. For example, mice with germline PIK3CAH1047R (p110α) mutation, a hot-spot mutation occurring in the kinase region of the gene, which is frequently encountered in patients with PROS, die prior to E10 due to disruption of the vascular endothelial growth factor A (VEGF-A) signaling [14].
Although rarely observed, presence of low level PIK3CA somatic variants in peripheral blood of individuals with PROS, has been described [15, 16].
Apparently, mutations in blood are less infrequently encountered in non-hotspot variants, than in hotspot [15, 16]. Also, higher levels of PIK3CA variants in blood compared to lesional tissue occur in individuals with megalencephaly or hemimegalencephaly [16]. Although we do not have information on the individual presented herein regarding the brain size or brain abnormality, she presented with homolateral palsy.
The missense PIK3CAc.1571G>A found herein has not yet been encountered in reported patients with PROS and should be considered of unknown significance according to Standards and Guidelines by the American College of Medical Genetics. As a variant, PIK3CAc.1571G>A is absent from both the Genome Aggregation Database (https://gnomad.broadinstiture.org) and the 1000 Genome Project (https://browser.1000genomes.org). It has a single literature-only entry in ClinVar (https://ncbi.nlm.nih.gov/clinvar) [17]. Bioinformatic software predicts this change to be tolerated or benign. However, PIK3CAc.1571G>A has been observed in neoplasms including HPV+oropharyngeal carcinoma [18], esophageal squamous cell carcinoma [19], breast cancer [20] and ovarian cancer [21]. It is unknown if PIK3CAc.1571G>A is a driver mutation in these neoplasms and therefore its significance cannot be determined.
One of the cardinal characteristics of SOD is the occasional absence of permanent teeth, retention of primary, and morphologic variations observed in dental hard tissues [3]. In the experience of the senior author, an individual with proven PIK3CA-related segmental orofacial overgrowth (in-frame PIK3CA c.1353_1364del, p.Glu453_Leu456del) and oral pseudo-perineuriomas has presented also with SOD/SOD-like osseous dysplasia and dental abnormalities [22]. Individuals with infiltrating lipomatosis, a type of PROS, are characterized by macrodontia [23]. A PIK3CA variant has been detected in DNA extracted from the pulp of a tooth of a patient with megalencephaly-capillary malformation syndrome also a PROS condition [24]. One can suggest that the dental changes observed in SOD as it may be the case in the individual presented here, could be the result of PIK3CA variants.
It is known that SOD can occasionally present homolateral cutaneous manifestations such as hypertrichosis and hyperpigmentation including Becker nevus and rarely hypopigmentation [3]. Becker nevus is defined as a cutaneous hamartomatous lesion appearing in childhood. It affects approximately 1 in 200 individuals. It is characterized by unilateral hyperpigmented patch which increases in thickness, pigmentation and growth during adolescence [25]. Also, it may be that the homolateral hyperpigmented and hypertrichotic cutaneous lesions, at least in some patients with SOD, represent clinical variations of Becker nevus [5].
Becker nevus can be associated with hypoplasia of the breast in both males and females, absence of the pectoralis major muscle (pectoral), underdevelopment of the shoulder girdle, scoliosis, vertebral defects, fused ribs, pectus excavatum or carinatum, supernumerary nipples, abnormally sparse hair under the armpit, ipsilateral shortness of a limb and underdeveloped teeth and jaws, defining what is known as Becker syndrome [26, 27]. Recently, it was confirmed that Becker nevus and Becker nevus syndrome have been associated with somatic mutation of ACTB, a gene coding for beta-actin which may interfere with the Hedgehog signaling pathway [28]. This confirms the postulate by Happle and Koopman that both Becker nevus and Becker syndrome are mosaic disorders representing a paradominant trait which implies that loss of heterozygosity occurs at an early developmental stage leading to mosaic population of heterozygous or hemizygous cells [28]. Based on the above, one can theorize that SOD and Becker nevus may share a common gene etiology and it should be of interest to evaluate patients with SOD for ACTB mutations in addition to PIK3CA.
An interesting report of an Asian patient with facial Becker nevus and homolateral involvement of the maxilla and mandible by “polyostotic fibrous dysplasia” has been reported in a dermatologic journal [29]. The patient’s facial asymmetry was accompanied by absence of the maxillary permanent lateral incisor and the right canine. The authors provided only a clinical picture of the affected part of the face and a representative CT scan image of the maxilla and mandible depicting homolateral enlargement and radiopaque lesions. There were no clinical intraoral pictures available, no dental x-rays and no reference on histopathology. Given that lesions of SOD may be misdiagnosed as fibrous dysplasia and the fact that gnathic fibrous dysplasia is not expected to be associated with hypodontia, it is possible that the patient reported may have SOD or an SOD-like process with mandibular involvement similar to our patient.
Lastly, we do not argue against the possibility of trauma being the cause of the clinical variant observed in the individual presented herein, as well as in patients with SOD. Although trauma has been included as a potential etiologic factor, confirmation in reported cases is lacking [3].
In summary, we presented a female pediatric individual with features akin to SOD and mandibular involvement. We also identified low somatic level PIK3CA variant in lesional hyperplastic gingival tissue and in peripheral blood. Given that the etiology of SOD remains to be elucidated, we are of the opinion that future research should concentrate on (a) verifying the presence of PIK3CA mutations in conventional (classic) patients with the disorder, and (b) evaluate such patients for ACTB mutations, especially if they present with cutaneous lesions suggestive or consistent with Becker nevus.
Funding
This article was not supported by any external funding.
Compliance with Ethical Standards
Conflict of interest
The authors do not have any conflict of interest.
Research Involving Human and Animal Rights
This article does not contain any studies with human participants or animals performed by any of the authors. Mutation analysis was performed for diagnostic purposes.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Miles DA, Lovas JL, Cohen MM. Hemimaxillofacial dysplasia: a newly recognized disorder of facial asymmetry, hypertrichosis of the facial skin, unilateral enlargement of the maxilla, and hypoplastic teeth in two patients. Oral Surg Oral Med Oral Pathol. 1987;64:445–448. doi: 10.1016/0030-4220(87)90150-2. [DOI] [PubMed] [Google Scholar]
- 2.Danforth RA, Melrose RJ, Abrams AM, Handlers JP. Segmental odontomaxillary dysplasia. Report of eight cases and comparison with hemimaxillofacial dysplasia. Oral Surg Oral Med Oral Pathol. 1990;70:81–85. doi: 10.1016/0030-4220(90)90183-S. [DOI] [PubMed] [Google Scholar]
- 3.Smith MH, Cohen DM, Katz J, Bhattacharyya I, Islam NM. Segmental odontomaxillary dysplasia: An underrecognized entity. J Am Dent Assoc. 2018;149:153–162. doi: 10.1016/j.adaj.2017.08.007. [DOI] [PubMed] [Google Scholar]
- 4.Welsch MJ, Stein SL. A syndrome of hemimaxillary enlargement, asymmetry of the face, tooth abnormalities, and skin findings (HATS) Pediatr Dermatol. 2004;21:448–451. doi: 10.1111/j.0736-8046.2004.21405.x. [DOI] [PubMed] [Google Scholar]
- 5.Alshaiji JM, Handler MZ, Huo R, Freedman A, Schachner LA. HATS syndrome: hemimaxillary enlargement, asymmetry of the face, tooth abnormalities, and skin findings. Cutis. 2014;94:E18–21. [PubMed] [Google Scholar]
- 6.Prusack N, Pringle G, Scotti V, Chen SY. Segmental odontomaxillary dysplasia: a case report and review of the literature. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2000;90:483–488. doi: 10.1067/moe.2000.108807. [DOI] [PubMed] [Google Scholar]
- 7.Ewing B, Hillier L, Wendl MC, Green P. Base-calling of automated sequencer traces using phred. I. Accuracy assessment. Genome Res. 1998;8:175–185. doi: 10.1101/gr.8.3.175. [DOI] [PubMed] [Google Scholar]
- 8.Ewing B, Green P. Base-calling of automated sequencer traces using phred. II. Error probabilities. Genome Res. 1998;8:186–194. doi: 10.1101/gr.8.3.186. [DOI] [PubMed] [Google Scholar]
- 9.Engelman JA, Luo J, Cantley LC. The evolution of phosphatidylinositol 3-kinases as regulators of growth and metabolism. Nat Rev Genet. 2006;7:606–619. doi: 10.1038/nrg1879. [DOI] [PubMed] [Google Scholar]
- 10.Keppler-Noreuil KM, Sapp JC, Lindhurst MJ, Parker VE, Blumhorst C, Darling T, et al. Clinical delineation and natural history of the PIK3CA-related overgrowth spectrum. Am J Med Genet A. 2014;164A:1713–1733. doi: 10.1002/ajmg.a.36552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Keppler-Noreuil KM, Parker VE, Darling TN, Martinez-Agosto JA. Somatic overgrowth disorders of the PI3K/AKT/mTOR pathway & therapeutic strategies. Am J Med Genet C. 2016;172:402–421. doi: 10.1002/ajmg.c.31531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Madsen RR, Vanhaesebroeck B, Semple RK. Cancer-associated PIK3CA mutations in overgrowth disorders. Trends Mol Med. 2018;24:856–870. doi: 10.1016/j.molmed.2018.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Happle R. Lethal genes surviving by mosaicism: a possible explanation for sporadic birth defects involving the skin. J Am Acad Dermatol. 1987;16:899–906. doi: 10.1016/S0190-9622(87)80249-9. [DOI] [PubMed] [Google Scholar]
- 14.Hare LM, Schwarz Q, Wiszniak S, Gurung R, Montgomery KG, Mitchell CA, et al. Heterozygous expression of the oncogenic Pik3ca(H1047R) mutation during murine development results in fatal embryonic and extraembryonic defects. Dev Biol. 2015;404:14–26. doi: 10.1016/j.ydbio.2015.04.022. [DOI] [PubMed] [Google Scholar]
- 15.Mirzaa G, Timms AE, Conti V, et al. PIK3CA-associated developmental disorders exhibit distinct classes of mutations with variable expression and tissue distribution. JCI Insight. 2016;1(9):e87623. doi: 10.1172/jci.insight.87623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kuentz P, St-Onge J, Duffourd Y, et al. Molecular diagnosis of PIK3CA-related overgrowth spectrum (PROS) in 162 patients and recommendations for genetic testing. Genet Med. 2017;19:989–997. doi: 10.1038/gim.2016.220. [DOI] [PubMed] [Google Scholar]
- 17.https://preview.ncbi.nlm.nih.gov/clinvar/variation/132954.
- 18.Chiosea SI, Grandis JR, Lui VW, Diergaarde B, Maxwell JH, Ferris RL, et al. PIK3CA, HRAS and PTEN in human papillomavirus positive oropharyngeal squamous cell carcinoma. BMC Cancer. 2013;13:602. doi: 10.1186/1471-2407-13-602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Munari FF, Cruvinel-Carloni A, Lacerda CF, de Oliveira ATT, Scapulatempo-Neto C, da Silva SRM, et al. PIK3CA mutations are frequent in esophageal squamous cell carcinoma associated with chagasic megaesophagus and are associated with a worse patient outcome. Infect Agent Cancer. 2018;13:43. doi: 10.1186/s13027-018-0216-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Elwy F, Helwa R, El Leithy AA, Shehab El din Z, Assem MM, Hassan NH. PIK3CA mutations in HER2-positive breast cancer patients; frequency and clinicopathological perspective in Egyptian Patients. Asian Pac J Cancer Prev. 2017;18:57–64. doi: 10.22034/APJCP.2017.18.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen S, Cavazza E, Barlier C, Salleron J, Filhine-Tresarrieu P, Gavoilles C, et al. Beside P53 and PTEN: Identification of molecular alterations of the RAS/MAPK and PI3K/AKT signaling pathways in high-grade serous ovarian carcinomas to determine potential novel therapeutic targets. Oncol Lett. 2016;12:3264–3272. doi: 10.3892/ol.2016.5083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Koutlas I, Anbinder A, Alshagroud R. Hemiorofacial asymmetry (hyperplasia/hypoplasia) with associated perineurial hyperplasia and perineuriomatous pseudo-onion bulb proliferations is a segmental orofacial variant of the PIK3CA-related overgrowth spectrum (PROS). Abstract #62; American Academy of Oral and Maxillofacial Pathology Annual Meeting, 2019, Miami FL.
- 23.Couto JA, Konczyk DJ, Vivero MP, Kozakewich HPW, Upton J, Fu X, et al. Somatic PIK3CA mutations are present in multiple tissues of facial infiltrating lipomatosis. Pediatr Res. 2017;82:850–854. doi: 10.1038/pr.2017.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McDermott JH, Byers H, Clayton-Smith J. Detection of a mosaic PIK3CA mutation in dental DNA from a child with megalencephaly capillary malformation syndrome. Clin Dysmorphol. 2016;25:16–18. doi: 10.1097/MCD.0000000000000099. [DOI] [PubMed] [Google Scholar]
- 25.Tymen R, Forestier JF, Boutet B. Colomb D [Late Becker's nevus. One hundred cases (author's transl)] Ann Dermatol Venereol. 1981;108:41–46. [PubMed] [Google Scholar]
- 26.Danarti R, König A, Salhi A, Bittar M, Happle R. Becker's nevus syndrome revisited. J Am Acad Dermatol. 2004;51:965–969. doi: 10.1016/j.jaad.2004.06.036. [DOI] [PubMed] [Google Scholar]
- 27.Happle R, Koopman RJ. Becker nevus syndrome. Am J Med Genet. 1997;68:357–361. doi: 10.1002/(SICI)1096-8628(19970131)68:3<357::AID-AJMG20>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 28.Cai ED, Sun BK, Chiang A, Rogers A, Bernet L, Cheng B, et al. Postzygotic mutations in beta-actin are associated with Becker's nevus and Becker's nevus syndrome. J Invest Dermatol. 2017;137:1795–1798. doi: 10.1016/j.jid.2017.03.017. [DOI] [PubMed] [Google Scholar]
- 29.Kim HJ, Kim KD, Lee MH. Becker's melanosis associated with fibrous dysplasia. Int J Dermatol. 2002;41:384–386. doi: 10.1046/j.1365-4362.2002.01439_1.x. [DOI] [PubMed] [Google Scholar]