Abstract
Diffusion MRI paired with tractography has facilitated a non-invasive exploration of many association, projection, and commissural fiber tracts. However, there is still a scarcity of research studies related to intralobar association fibers. The Dejerines’ (two of the most notable neurologists of 19th century France) gave an in-depth description of the intralobar fibers of the occipital lobe. Unfortunately, their exquisite work has since been sparsely cited in the modern literature. This work gives a modern description of many of the occipital intralobar lobe fibers described by the Dejerines. We perform a virtual dissection and reconstruct the tracts using diffusion MRI tractography. The dissection is guided by the Dejerines’ treatise, Anatomie des Centres Nerveux. As an accompaniment to this article, we provided a French-to-English translation of the treatise portion concerning five intra-occipital tracts, namely: the stratum calcarinum, the stratum proprium cunei, the vertical occipital fasciculus of Wernicke, the transverse fasciculus of the cuneus and the transverse fasciculus of the lingual lobule of Vialet. It was possible to reconstruct all but one of these tracts. For completeness, the recently described sledge runner fasciculus, although not one of the Dejerines’ tracts, was identified and successfully reconstructed.
Subject terms: Neuroscience, Visual system
Bugain et al perform a virtual dissection and reconstruct the occipital intralobar tracts using diffusion MRI tractography. They provide a modern anatomical depiction of what had originally been described by the Dejerines in the 19th century, as well as a complete translation of the original text.
Introduction
The advent of in vivo magnetic resonance imaging (MRI) techniques has heightened the desire to construct a comprehensive atlas of the human brain connective network—the so-called human connectome1–3. In humans, diffusion MRI paired with tractography permits a noninvasive three-dimensional reconstruction of large-scale white matter system organisation, which can then be compiled into an atlas4 (see reviews: refs. 5–7). Diffusion MRI has contributed to the verification of many association fiber pathways8,9, as well as to the redefinition of their boundaries and relations10–17. Recent success in discovering novel or forgotten association fibers has stoked interest in the less conspicuous short-range fiber connections, which can be more challenging to delineate18–21.
Despite the rapid advancement in the technology and the connectome mapping techniques available, there is a paucity in research related to short-range association fibers in the occipital lobe. The initial focus of this new field was geared towards the more prominent, and seemingly more physiologically crucial, long-range association fibers. A more generalised issue with MRI-based tractography studies is that the findings tend to remain speculative, devoid of tangible experimental evidence and ground truth, unlike those of axonal tracer studies22.
To partially overcome these inherent limitations, contemporary researchers can guide their findings through postmortem dissection studies, by consulting the tract tracing literature or historical neuroanatomy texts. The 19th-century neurologists were masterfully skilled in gross dissection and fastidious in the documentation of their composite observations. Numerous diffusion tractography studies have successfully used these classical dissections to interpret imaging results and identify false positives or false negatives18,23–28. Pioneering texts have also often been an initial source of inspiration for explorative neuroanatomical studies18,23. Indeed, we had previously studied dissections by 19th-century neurologists Joseph and Augusta Dejerine with a view to enriching the field’s knowledge and perspectives on long-range association fiber anatomy29. It showcased the pertinence of these historical studies and prompted further examination into the Dejerines’ other work on association short-range occipital fibers.
One of the biggest divisions across the neuroanatomical field towards the end of the 19th century was the definition and categorisation of association fibers. Prominent authorities at the time, such as Meynert, postulated that true association fibres only projected in an anterior-posterior dierection18,30, while Wernicke defended the existence of dorsal-ventral projecting association fibres, particularly in the occipital region18,31,32. Other neurologists, like the Dejerines and Sachs, supported the school of thought of Wernicke by acknowledging the existence of the vertical occipital fasciculus of Wernicke (VOF)33,34 and several other intralobar tracts with a dorsal-ventral trajectory. Whereas the work of Heinrich Sachs on the anatomy of intralobar pathways has previously been explored and translated25,31, the equally insightful contributions and perspectives of the Dejerines have yet to be explored and made more accessible.
Joseph Jules Dejerine (1849–1917) and his wife, Augusta Dejerine-Klumpke (1859–1927) frequently collaborated and are both individually celebrated as two of France’s most renowned 19th-century neurologists29,35,36. Arguably, the Dejerines’ seminal work is their two-volume neuroanatomical treatise, Anatomie des Centres Nerveux, originally published in 1895 and 1902 and then reprinted in 1980 by Mason-Elsevier33,37. The Dejerines’ impressive histological sections, accompanied by beautifully intricate illustrations by H. Gillet, are relevant even in this technological era, and are reference points for anatomical findings26,38. Of particular interest to us, the Dejerines dedicated a portion of the first volume to occipital intralobar association fibers. The Dejerines observed five fiber bundles specific to the occipital lobe: the stratum calcarinum, the VOF, the transverse bundle of the lingual lobule of Vialet, the transverse fasciculus of the cuneus and the stratum proprium cunei. Over 100 years later and these short, intralobar, association tracts remain poorly understood and surrounded by controversies due to inconsistent classification and nomenclature18,39.
Of late, the VOF, the largest of the occipital intralobar fibers dissected by the Dejerines, has been the focus of an increasing number of papers10,16,18,40–42. As of December 2019, a PubMed search generated thirty hits relating to the “vertical occipital fasciculus” when used as a key term. In comparison, the other intraoccipital tracts generate no results and remain overlooked, mostly being listed in passing (Table 1). An article by our group revisited the Dejerines’ work and provided access to an English translation of the portion of the Dejerines’ book related to the long-range association fibers of the human brain29. Here, we provide the complete translation of the description by the Dejerines of the occipital originating short-range fibers trajectories, from the original French taken from Anatomie des Centres Nerveux (1895 p. 780–784) (Supplementary note). The aim of this study is to use the observations made by the Dejerines for guidance through a virtual dissection of the intralobar tracts of the occipital lobe, based on in vivo diffusion imaging data. The virtual dissections enable us, to better discern the three-dimensional relations of these once-forgotten tracts.
Table 1.
Summary of studies10,12,16–18,24,25,31,32,34,41,42,44–46,51–53,56,59,62,63,91–99 commenting on the intralobar occipital fibers in human or Old-World monkeys.
| Intralobar occipital fibers | Studies identifying the fiber pathways | Studies mentioning the fiber pathways |
|---|---|---|
| Stratum calcarinum | Déjerine & Déjerine (1892), Koutsarnakis et al. (2019), Sachs & Wernicke (1892), Schmahmann & Pandya (2006), Takemura et al. (2020), Vergani et al. (2014), Vialet (1893), Von Boin et al. (1942) | Barker (1899), Campbell et al. (1905), Forkel et al. (2015), Maunsell & Van Essen (1983), Vergani et al. (2014) |
| Stratum proprium cunei | Déjerine & Déjerine (1892), Sachs & Wernicke (1892), Schmahmann & Pandya (2006), Vergani et al. (2014), Vialet (1893) | Barker (1899), Campbell et al. (1905), Forkel et al. (2015), Greenblatt (1973), Vergani et al. (2014) |
| Sledge runner fasciculus | Güngör et al. (2017), Koutsarnakis et al. (2019), Muftah Lahirish et al. (2020), Vergani et al. (2014) | |
| Transverse fasciculus of the cuneus | Dejerine & Dejerine (1892), Sachs & Wernicke (1892), Schmahmann & Pandya (2006), Vergani et al. (2014), Vialet (1893), Von Boin et al. (1942) | Barker (1899), Campbell et al. (1905) Forkel et al. (2015), Greenblatt (1973), Takemura et al. (2019), Vergani et al. (2014), Yeatman et al. (2014) |
| Transverse fasciculus of the lingual lobule of vialet | Dejerine & Dejerine (1892), Schmahmann & Pandya (2006), Vialet (1893), Von Boin et al. (1942) | Barker (1899), Campbell et al. (1905), Greenblatt (1973), Takemura et al. (2019), Yeatman et al. (2014) |
| Vertical occipital fasciculus | Bartsch et al. (2013), Briggs et al. (2018), Dejerine & Dejerine (1892), Duan et al. (2015), Güngör et al. (2017), Keser et al. (2016), Martino & García-Porrero (2013), Muftah Lahirish et al. (2020), Oishi et al. (2018), Palejwala et al. (2019), Panesar et al. (2019), Rokem et al. (2017), Sachs & Wernicke (1892), Schmahmann & Pandya (2006), Schurr & Mezer (2019), Takemura et al. (2016), Takemura et al. (2017), Takemura et al. (2020), Vergani et al. (2014), Vialet (1893), Von Boin et al. (1942), Weiner et al. (2017), Wernicke (1876), Wu et al. (2016), Yeatman et al. (2013), Yeatman et al. (2014) | Barker (1899), Campbell et al. (1905), Forkel et al. (2015), Greenblatt (1973), Vergani et al. (2014) |
Results
Using constrained spherical deconvolution (CSD) tractography, this study successfully identified and mapped four of the five occipital intralobar white matter tracts observed by the Dejerines (Fig. 1). The translation of the Dejerines’ work on these fibers, from 19th-century French to accessible English (Supplementary Note), helped guide the tractography findings. Tractography reconstruction of the occipital intralobar tracts have been overlaid on a 3D T1-weighted MNI template image and presented in Figs. 2–5. The relation of each tract to each other are provided in Fig. 6, while Fig. 7 shows individual tracts in a representative sample of the subjects.
Fig. 1. The Dejerines’ intraoccipital fibres.
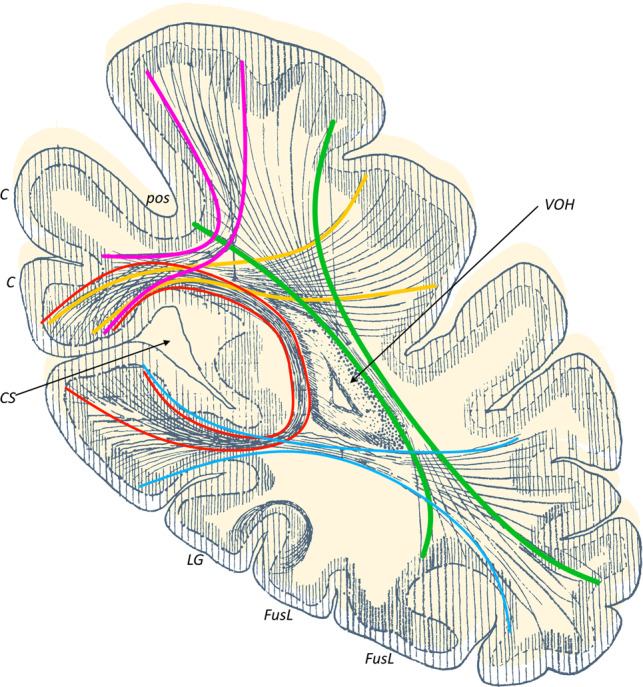
A coronal slice of the occipital lobe depicting the five occipital intralobar fibres identified by Dejerine. Green, vertical occipital fasciculus; blue, transverse fasciculus of the lingual lobule of Vialet; red, stratum calcarinum; magenta, stratum proprium cunei; yellow, transverse fasciculus of the cunei; C cuneus, CS calcarine sulcus, LG lingual gyrus, FusL fusiform lobule, pos parieto-occipital sulcus, VOH occipital horn of the lateral ventricle. Image adapted and reproduced from the illustration by H. Gillet in Dejerine & Dejerine (1895, p. 781).
Fig. 2. The stratum calcarinum (SC).
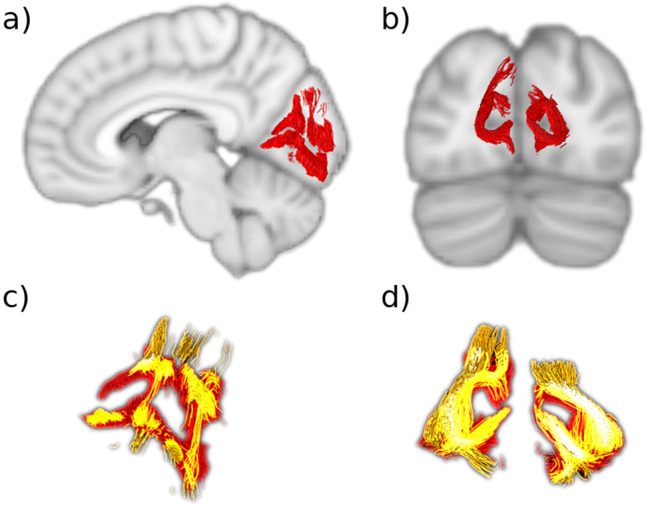
a Sagittal slice showing the right SC (red) curved around the calcarine fissure. b Coronal slice showing the left and right SC. c, d The two layers of the SC. The deeper and longer layer (orange) lies under the more superficial and u-shaped layer (red). Where they intersect is shown in bright yellow.
Fig. 5. Dorsal to ventral intralobar fibers.
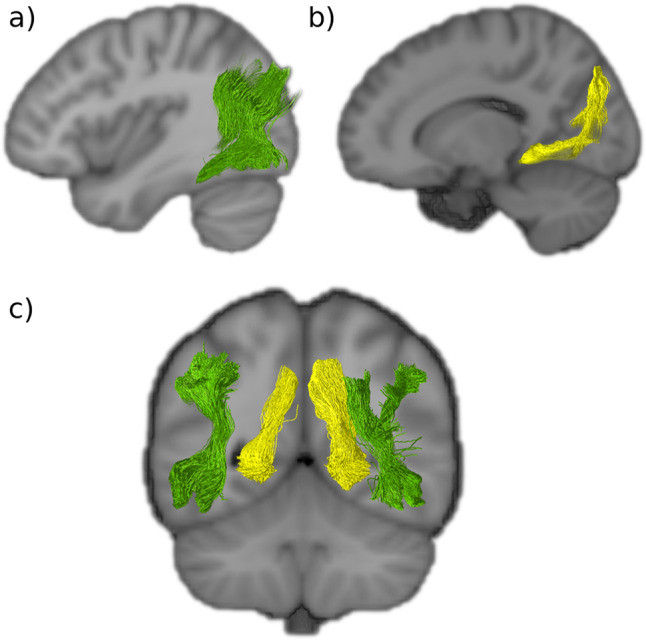
a Sagittal view of the right vertical occipital fasciculus (VOF in green). b Sagittal view of the right sledge runner fasciculus (SRF in yellow). c Coronal slice of the VOF and SRF showing how they lie lateral and medial to the lateral ventricles, respectively. Both connect dorsal aspects of the occipital lobe to ventral aspects. VOF vertical occipital fasciculus, SRF sledge runner fasciculus.
Fig. 6. The five intralobar fibers identified in the occipital lobe.
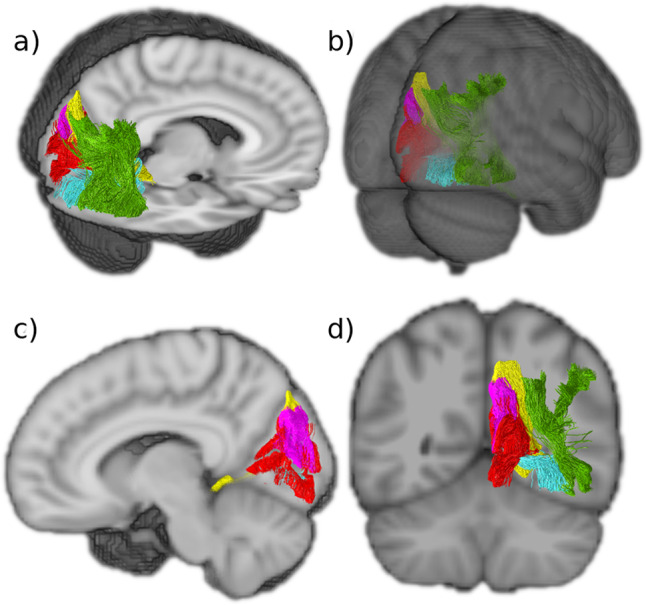
Tracts generated from group averaged data of 24 subjects. a Sagittal and axial view. b Posterior view. c Sagittal view showing that the SPC is more medial than the SRF and that the SC extends more widely than the other medial intralobar tracts. d Coronal view showing that the intralobar fibers connect all aspects of the occipital lobe. VOF vertical occipital fasciculus (green), SRF sledge runner fasciculus (yellow), SPC stratum proprium cunei (magenta), SC stratum calcarinum (red), TFV putative transverse fasciculus of the lingual lobule of Vialet (blue).
Fig. 7. Intralobar fibers in the occipital lobe presented in individual subjects.
Representation of the identified occipital intralobar tracts in five of the 24 subjects used in this study. The same methodology and regions of interest (ROIs) used to isolate the tracts in the group averaged tractography data. The ROIs were wrapped to native subject spaces. This generates tractography findings that support the presence of the tracts in distinct individuals and depict the subject specific trajectory of the intralobar tracts. VOF vertical occipital fasciculus (green), SRF sledge runner fasciculus (yellow), SPC stratum proprium cunei (magenta), SC stratum calcarinum (red), TFV putative transverse fasciculus of the lingual lobule of Vialet (blue).
The following descriptions of the tracts are based on a virtual dissection derived from averaged orientation distribution functions (CSD ODFs) from 24 healthy subjects (Figs. 2–6). To probe the individual level results, the same dissections were also performed on the individual level data. The individualized dissections were found to be generally consistent with the average dissection (see Fig. 7 for a representative sample or the online material for the full dataset).
Occipital diffusion tractography
With regard to short-range association fibers arising from the occipital lobe, the Dejerines described the vertical occipital fasciculus (VOF), which spans the entirety of the occipital lobe lateral to the posterior ventricle, and four shorter association fiber tracts projecting superiorly and inferiorly from the banks of the calcarine fissure: the stratum calcarinum (SC), stratum proprium cunei (SPC), transverse fasciculus of the cuneus (TFC), and the transverse fasciculus of the lingual lobule of Vialet (TFV) (Fig. 1).
Stratum calcarinum (SC)
The stratum calcarinum (SC) consists of several U-shaped fibers that connect the two banks of the calcarine fissure, a major landmark that medially divides each occipital lobe into the wedge-shaped cuneus superiorly, and the lingual lobule inferiorly, and represents the boundaries of the primary visual cortex. This group of U-shaped fibers extends all along the calcarine fissure from the occipital pole to its junction with the parieto-occipital fissure at anterior border of the occipital lobe (Fig. 2). The SC is an unusually large U-fiber system because it directly connects adjacent gyri across a long and deep fissure—perhaps the reason the Dejerines devoted considerable attention to it, describing it alongside other intralobar fibers.
The SC fibers are composed of two superimposed layers (Fig. 2c). The first, most superficial layer has a vertical and horizontal orientation that embraces the superior and inferior edges of the fissure. The second has longer and more deeply penetrating fibers that join the medial-central aspects of the cuneus to the medial-central aspects of the lingual gyrus. SC layers lie directly on top of the longitudinal fibers of the inferior longitudinal fasciculus that joins them to carpet the floor of the calcarine fissure. These fibers run perpendicular to the vertical SC fibers and interlink the different U-fiber units along the fissure like rails on a track. This relation between the inferior longitudinal fasciculus and the U-fibers of the occipital lobe was depicted by Ffytch and Catani43, however, they did not explicitly mention the SC system or distinguish it from other U-fibers.
The horizontal aspect of the SC projects away from the midline into the bank of the fissure and becomes intertwined with the other intralobar tracts that originate from the lips of the calcarine fissure: the stratum proprium cunei in the superior lip and the putative transverse fasciculus of the lingual lobule of Vialet in the inferior lip. In the anterior part of the fissure, the vertical portion of the SC is on the same plane, and slightly overlaps the sledge runner fibers (not described by the Dejerines) that travel vertically downwards, beneath the calcarine fissure, prior to continuing transversely to the anterior lingual gyrus (Fig. 6).
Stratum proprium cunei (SPC)
The stratum proprium cunei (SPC) arises from the superior bank of the anterior calcarine fissure, just posterior to the junction with the parieto-occipital sulcus (Fig. 3). The tract connects the inferior aspect of the cuneus with the most anteromedial convexity of the occipital lobe, just posterior to the precuneus. The SPC runs deep to the parieto-occipital sulcus, at the level of the calcar avis, initially coursing laterally away from the calcarine fissure for a short distance, before ascending vertically to terminate in what is classically referred to as the first occipital convolution. Unlike the vertical occipital fasciculus that fans out across the entirety of the first occipital convolution, the SPC projections remain on a plane medial to the posterior horn of the ventricle. Although the SPC connects distal regions of the same lobe, it is a relatively short and narrow association fiber tract. Our findings align with the Dejerines’ and show that the SPC runs neatly along the anteromedial margins but is constrained to the occipital lobe.
Fig. 3. The stratum proprium cunei (SPC).

a Coronal view of the SPC (magenta) that lines the midline of the cuneus. b Sagittal view of the SPC showing how it arises from the superior bank of the calcarine fissure. cf: calcarine fissure.
Transverse fasciculus of the cuneus of Sachs (TFC)
The transverse fasciculus of the cuneus of Sachs (TFC) is described as a tight, slender bundle, consisting of relatively few fibers that initially move away from the upper fold of the calcarine fissure with a strong oblique trajectory. They course laterally and superiorly to the roof of the posterior lateral ventricle and cross the cuneus to terminate in the lateral convexities of the occipital lobe24,25,34,44–46. Unfortunately, in the present study, it was not possible to identify a bundle resembling the TFC.
Transverse fasciculus of the lingual lobule of Vialet (TFV)
A tract resembling the many aspects of the Dejerines’ description of the transverse fasciculus of the lingual lobule of Vialet (TFV) was successfully isolated (Fig. 4). The Dejerines depicted the TFV as a mirror counterpart to the transverse fasciculus of the cuneus, as it connects the inferior gyrus of the calcarine fissure to the infero-lateral aspects of the occipital lobe, akin to the way in which the transverse fasciculus of the cuneus connects the fissure to the external surface of the cuneus. In contrast, the stratum proprium cunei connects the fissure to the medial surface of the hemisphere and does not appear to have an inferior lingual gyrus equivalent. Interestingly, the putative TFV origin can be found all along the calcarine fissure with a span similar to the stratum proprium cunei.
Fig. 4. Putative transverse fasciculus of the lingual lobule of Vialet (TFV).

a Axial view of the TFV (blue) showing it projects anteriorly before it reflects in the anterior aspect of the lingual gyrus. b Coronal view of TFV showing it arises from the inferior gyri of the calcarine fissure and terminates infero-laterally. c Sagittal and axial view of the TFV showing how it does a hairpin loop at the anterior edge of the lingual gyrus.
The putative TFV has a morphology that resembles an extended U-fiber; the tract travels laterally away from the fissure prior to adopting an intense anterior-oblique trajectory that sharply curves back on itself and continues in a posterior-oblique course toward the lateral convexity of the occipital lobe. Due to the squatness of the lingual lobule, the tract appears quite flat and two ends of the fiber tract are nearly on the same coronal plane, with the lateral terminations slightly inferior to its calcarine fissure origin. The putative TFV starts superiorly and medially to the inferior longitudinal fasciculus and occipital projections of the inferior fronto-occipital fasciculus, and crosses both laterally and superior to these two major association fiber bundles, before descending just laterally to them.
Vertical occipital fasciculus (VOF)
Using the Dejerines’ classical description, we identified the vertical occipital fasciculus (VOF) (Fig. 5a), and it aligns well with modern tractography findings. Of the five occipital tracts the Dejerines observed, the VOF is the longest and most prominent, and consequently has been the focus of many more studies. Often described as a short, vertical, and ‘sheet-like’ bundle of fibers, the VOF “connects the superior aspect of occipital lobe to its inferior surface” (Dejerine & Dejerine-Klumpke33, p. 779) maintaining a dorsal-ventral course lateral to the posterior ventricle. More specifically, the inferior terminations of the VOF extend from the ventral temporo-occipital cortex region, encompassing the inferior temporal gyrus and its intersection with the anterior aspects of the middle and inferior occipital gyri. The superior projections of the VOF reach the lateral gyri of the occipital lobe, including the transverse occipital sulcus, with several fibers reaching the posterior end of the intraparietal sulcus and the angular gyrus.
The ‘sheet-like’ stem of the VOF tract, that courses through the central part of the occipital lobe, is a wide bundle that has a predominantly vertical trajectory, apart from the ventral and dorsal terminations that flick out laterally and transversely to reach the convexity of the lateral occipital lobe across a wide area. Whereas the stratum calcarinum is covered laterally by the inferior longitudinal fasciculus, the VOF passes laterally to the inferior longitudinal fasciculus along with the inferior fronto-occipital fasciculus, continuing superiorly to both and coursing vertically, posterior to the arcuate fasciculus.
Sledge runner fasciculus (SRF)
Tracts resembling the recently discovered sledge runner fasciculus (SRF)25 were identified in both hemispheres and reconstructed successfully (Fig. 5b, c). The SRF connects the posterior precuneus to the anterior lingual gyrus of the lingual lobule, taking a strongly inferior anterior-oblique trajectory with a couple of convexities—giving it a sledge-like morphology. It is a long occipital tract that traverses from the dorsal to ventral aspects of the cerebrum like the vertical occipital fasciculus. However, unlike the vertical occipital fasciculus, it is medial to the ventricles. With a compact central stalk that widens as the tract descends anteriorly and narrows superiorly, it is a prominent bundle of similar length to the vertical occipital fasciculus. As in the first observations of the SRF obtained through fiber microdissections, the results showed that the dorsal SRF terminations were located at the anterior border of the cuneus, adjacent to the midline of the cerebrum. The dorsal SRF terminations are entangled with the anterior fibers of the stratum proprium cunei bundle (Fig. 6). The ventral terminations are more anteriorly located and project deep into the lingual gyrus, posterior to the cingulate isthmus and the posterior part of the parahippocampal gyrus.
Discussion
The anatomical findings described in the present study suggests that the occipital lobe has at least five intrinsic white matter structures that fall between the long association fibers and the very short U-shaped fibers. These are: the stratum calcarinum, the transverse fasciculus of the lingual lobule of Vialet, the stratum proprium cunei, the vertical occipital fasciculus of Wernicke (VOF)—which were described by the Dejerines, and the recently discovered sledge runner fasciculus (SRF). Apart from the vertical occipital fasciculus of Wernicke and the sledge runner fasciculus, none have been the focus of dedicated studies since the turn of the 20th century. Certain, expansive, U-fibers studies have previously presented findings that are suggestive of some of the shorter occipital intralobar fibres identified in this current study47–50. Yet, the scope of these U-fiber studies is vast and they do not provide precise descriptions of the trajectory of the occipital U-fiber tracts. This makes it difficult to gauge to what extent pathways depicted in their figures are equivalent to our findings. As such, dedicated and focused anatomical studies are still required.
This current study also highlights the persistent utility of historical studies for guidance of neuroimaging findings.
Vertical occipital fasciculus (VOF)
The VOF’s existence as an association fiber bundle has been extensively verified, through diffusion MRI (dMRI) studies12,16,18,40,51, postmortem dissections25,41,52, and it has even been identified and compared in macaques53. Yet, the boundaries of the pathway remain unclear and disputed54. Its vertical trajectory, like that of the stratum proprium cunei, distinguishes it from the other long association fibers that project into the occipital lobe, and its long dorso-ventral trajectory, located lateral to the ventricle, sets it apart from the intraoccipital tracts. The Dejerines, like other early neuroanatomists, admitted to poorly defining the anterior limits of the VOF. Their descriptions suggest that it extended anteriorly beyond the boundaries of the occipital lobe, descriptions borne out by most recent tractography studies, including this present one. Hence, it is arguable that the VOF is not strictly an autochthonous tract restricted to the occipital lobe10. Depending on how anterior the ventral terminations of the VOF were, it assumed either an oblique dorsal and posterior trajectory, or in the case of more posterior ventral terminations, a vertical trajectory to the occipital cortex. We did not find strong ventral VOF termination within the ventromedial-fusiform gyrus or the dorsomedial portion of the occipital lobe. Our findings show that the VOF extends from the dorsal to ventral aspects of the lateral parietal and occipital lobe as a thin sheet and terminates at the lateral-posterior end of the temporo-occipital region (Fig. 5). As described by the Dejerines, the VOF courses laterally to the occipital horn and the inferior longitudinal fasciculus.
Studies now aim to better differentiate the VOF fibers from the neighbouring larger bundles. The VOF projects superior to the inferior longitudinal fasciculus. Their proximity once made the VOF difficult to distinguish from the terminal portions of the inferior longitudinal fasciculus, leading to its misidentification in one of the early studies on the macaque24. Recent findings by Takemura et al.53 demonstrate a clear separation between the VOF and the inferior longitudinal fasciculus and optic radiations streamlines in humans through dMRI. This supports what had first been indicated in classical studies of the human and macaque by the Dejerines and Wernicke, respectively33,55.
Similarly, the anterior dorsal terminations of the VOF closely borders the posterior and the ventral aspect of the arcuate fasciculus10,12,16,54. This has generated debate about the anatomical boundaries and distinctive functions17,56 of VOF and arcuate fasciculus. The proximity of the VOF to the posterior arcuate fasciculus has been seen to be dependent on species, subject variability, local model, and tractography algorithms10,57.
Borders for the VOF, or any other occipital intralobar tract, can manifest small discrepancies across studies partly due to brain borders being a human construct, high subject variability, the limits of imaging resolution, and the larger number of more prominent tracts that cross the intralobar tracts58. The occipital intralobar tracts, notably the VOF, have a strong dorsal-ventral trajectory, whereas many of the major long association fiber pathways that enter the occipital lobe have an anterior-posterior trajectory. Hence, the intralobar fibers cross paths with more prominent bundles that can cancel out their signal. The Dejerines themselves remarked on the limits of even masterful brain dissection skills for identification of terminations in pathway-dense regions, limiting the extent of guidance that postmortem dissections can provide. The continuous improvement in MRI tractography angular resolution, and the development of alternative models, have made some headway53,54,59.
It is important to make these distinctions, for all association fibers, in order to generate a truly representative map—that is, one that does not include pathways that appear longer or wider than they truly are because they artificially contain subsections of adjacent tracts. Knowing the extent of a distinct white matter system is useful for future work concerning functional specialization and pathology. It has been suggested that certain U-fibers, situated between the VOF and other long association pathways, form artificial connections, or U-fiber ‘bridges’, between the pathways that can make the two tracts look like one larger tract16. The intermingling of U-fibers is known to obstruct the visualisation and interpretation of the deeper underlying subcortical tracts in both fiber tractography and dissections60.
The difficulty that arises when studying the work of the Dejerines, like other 19th-century anatomists, relates to translating their definition of structures into the current school of thought. Inconsistent landmarks, nomenclatures, and methods were used to define and visualise structures. This discordance makes it difficult to reference a classic text and persists to the present day. Nevertheless, the congruency between the Dejerines’ dissection and modern imaging suggests that the other tracts they described should also be successfully visualised in vivo.
Stratum calcarinum (SC)
In vivo and in vitro dissections of the calcarine fissure reveal a large continuous bundle of U-fibers, named the stratum calcarinum (SC), connecting the upper and lower edges of calcarine cortex (Fig. 2). U-fibers typically do not form a clear pathway or large fiber system. The Dejerines chose to omit the SC from the portion of their book that concerns U-fibers, in favor of discussing it alongside the association fibers of the occipital lobe to highlight the prominence and size of the SC. The Dejerines emphasised that it is not a true association fiber or a single entity. Instead, it represents a series of tracts forming an oversized U-fiber system that curves around the whole calcarine fissure and is specific to the primary visual cortex. It extends from the posterior end of the occipital horn to the intersection with parieto-occipital fissure and circumnavigates the calcar avis in its full extension. The SC intermingles with the other association fibers like the transverse fasciculus of the lingual lobule of Vialet and stratum proprium cunei, which link the primary visual cortex with the convexity of the occipital lobe.
The SC system forms an atypical bundle as it consists of a long and deep layer underlying a short and more superficial layer. The superficial SC layer, as can be seen from the results (Fig. 2c), has short projections that tightly carpet the floor of the calcarine fissure and join the superior and inferior gyri that directly border the fissure. This gives the layer the characteristic ‘U’ appearance. The second underlying layer has wider projections and a weaker U shape. Its longer fibers connect the medial aspect of the central cuneus to the inferior-medial aspect of the lingual lobule.
The calcarine fissure receives several white matter bundle pathways that share similar orientations at the level of the fissure, making it particularly difficult to differentiate them. Campbell46, postulated that the majority of the fibers labelled by the Dejerines, such as the SC, were actually terminal fibers of the optic radiation. Indeed, the Dejerines33 acknowledged that the histological staining method employed was insufficient to confidently evaluate the degree to which association fibers, like the SC, contribute to the projection fiber pathways that are situated along the ventricles. However, more recent studies, conducted with the improved Klingler’s technique, are congruent with the Dejerines’ description of the SC20,25. Unfortunately, to date, most mentions of the human SC, transverse fasciculus of the lingual lobule of Vialet, transverse fasciculus of the cuneus, and stratum proprium cunei in the literature have been in reference to historical dissections (Table 1).
A human postmortem dissection study had shown the three-dimensional relationship between the sledge runner fasciculus and SC, with the sledge runner fasciculus running obliquely, almost perpendicularly, superior to the SC fibers that lie in the anterior portion of the calcarine fissure just posterior to the junction it shares with the parieto-occipital sulcus20. There is a similar overlap that occurs between the SC and the stratum proprium cunei (Fig. 6). The posterior inferior longitudinal fasciculus projects obliquely toward the calcarine fissure and passes superior to the SC, before descending and coating the internal wall of the occipital horn, separating the ventricle from the SC24,33.
The Dejerines considered the SC as one of the most important short-range connections in the occipital lobe. The proposed importance of the SC arises from its role in connecting the superior and inferior vision field regions all along the fissure, likely implicating it in the visual pathways of the macula and the retina. It has been postulated that the SC, stratum proprium cunei and transverse fasciculus of the cuneus contribute to coordination and propagation of the visual stimuli of color, shape, and motion from the primary cortex to higher order association areas, permitting both dorsal and ventral outflow to the rest of the parietal and temporal cortex45.
Stratum proprium cunei (SPC)
The SPC is a short and seemingly inconsequential tract that rises in the sagittal plane from the calcarine fissure at the medial border of the hemisphere (Fig. 3). Since the first reports by Sachs34, it has only been vaguely mentioned in passing24,25,45,46,61. Our current findings align strongly with the Dejerines’ observation that the SPC “originates from the superior lip of the calcarine fissure” and projects dorsally to the “cortical region of the superior border of the hemisphere”33, (p. 782). The results show a bundle with a clear vertical projection that links the upper border of the anterior calcarine fissure to the medial aspect of the cuneus convexity. Like the stratum calcarinum, the SPC extends along the entire length of the calcarine fissure. It differs, however, by being limited to the cuneus. Schmahmann & Pandya24, speculated that the SPC bundle corresponds to fibers they identified in rhesus macaque, located in the superior parietal lobule and the dorsal occipital white matter, caudal to the dorsal component of the superior longitudinal fasciculus. The fibers connected the medial pre-occipital gyrus of the occipital lobe with the medial part of the superior parietal lobule. However, the SPC we observed does not extend past the parieto-occipital fissure and does not correspond with the observations of Schmahmann & Pandya24 who describe it as more anteriorly projecting compared to the system we have identified. The SPC shares a similar trajectory to the dorsal half of the stratum calcarinum and sledge runner fasciculus. This study specifically followed the Dejerines’ descriptions, which limits the SPC to the medial surface of the cuneus to differentiate the SPC from the stratum calcarinum and sledge runner fasciculus. In a postmortem dissection, Vergani et al.25 depict both the SPC and their discovery of the sledge runner fasciculus distinctively. Interestingly, another study examining the sledge runner fasciculus discusses its relation to the stratum calcarinum, but not the SPC. It is unclear as to whether the authors have interpreted the SPC as forming part of the sledge runner fasciculus62. Due to the limited literature on the SPC, it is not clear to what extent diffusion tractography’s limitations have affected the reconstruction.
Transverse fasciculus of the lingual lobule of Vialet (TFV)
The Dejerines named the TFV after Nehemie Vialet who described it in detail and published a paper arguing for its existence as a distinct fascicle44. The Dejerines were able to successfully reproduce the observations made by Vialet in their own dissection studies. Our study succeeded in reconstructing in several individual subjects a putative TFV bundle (Fig. 7). This was unexpected, as like the transverse fasciculus of the cuneus, the TFV tract has not been mentioned in recent occipital fiber dissection or tractography studies. The TFV tract can be seen as a counterpart to the transverse fasciculus of the cuneus, as it connects the inferior gyri of the calcarine fissure to the convexity of the lingual lobule. The TFV can be differentiated from the inferior longitudinal fasciculus that courses alongside it at the level of the lingual gyrus and posterior temporal lobe, by its slightly thinner bulk and paler Pal-haematoxylin staining33,44. Vialet considered this bundle to be functionally important as it seems to represent the inferior portion of the occipital lobe association fiber system.
The existence of the TFV, like the transverse fasciculus of the cuneus and vertical occipital fasciculus, as a distinct entity was heavily contested shortly after its discovery. This resulted in their omission from much of the literature, such as the neuroatlases of notable and influential neurologists like that of Constantin Von Monakow. Von Monakow described various subcortical structures with the locations and gross trajectories that match the TFV, transverse fasciculus of the cuneus and vertical occipital fasciculus, but was unable to differentiate them from neighbouring bundles and so negated their existence as distinct tracts18. Indeed, Schmahman & Pandya24, professed uncertainty as to the existence of the TFV as a distinct tract and considered the TFV as described by the Dejerines to be equivalent to the “transverse inferior longitudinal fasciculus fibers that lie within the ventral part of the occipital and temporal lobe and that link the lateral cortices with the medial cortices” (p. 450). However, Schmahmann & Pandya24 had made a similar comment in relation to the vertical occipital fasciculus and had interpreted it as part of the vertical component of the inferior longitudinal fasciculus23, which was later disputed by more focused tractography studies. Vergani et al.25, conducted a thorough postmortem exploration of the intralobar fibers of the occipital lobe, in an effort to replicate the work of Sachs34. Interestingly, like Sachs, the authors did not mention a tract that matches the morphology of the TFV as described later by Vialet, the Dejerines, and as identified in this study. A recently resurfaced photograph of a Weigert-stained histological slide of the macaque occipital lobe by von Bonin et al.61,63 clearly depicts the vertical occipital fasciculus and stratum calcarinum, and somewhat less clearly, the TFV and transverse fasciculus of the cuneus named the fasciculus transversus lingualis and fasciculus transversus cunei, respectively63. The brevity of the description makes it hard to ascertain in greater detail the course of the TFV in the macaque.
Although identified in multiple subjects of this present study, limitations inherent to this study and past ones, puts into question the existence of the TFV and whether it is a misconstrued artifact formed in part by the optic radiations, or other such neighbouring pathways. Validation of the TFV will depend on future studies with higher resolution and varied methodology, and it remains putative until such a time.
The transverse fasciculus of the cuneus (TFC)
Our study did not identify a consistent tract in either hemisphere that matches any previous descriptions. Yet, the Dejerines remarked that the calcarine cortex is attached to the cuneus and first occipital convolution through both the stratum proprium cunei and TFC and this observation of the TFC was also made by previous works25,34,44–46,61. The Dejerines, with reference to Sachs’ work, described the TFC as having a trajectory that “runs slightly obliquely, anteriorly and laterally, and radiate[s] in the superior parietal lobule and in the angular gyrus” (Dejerine & Dejerine-Klumpke33, pg. 781). It was specified by the Dejerines, Sachs and Vialet33,34,44 that the origin of the TFC is at the level of the calcarine fissure among the stratum calcarinum and stratum proprium cunei. This was more recently supported by Vergani et al., 2014,25 who conducted an in vitro dissection and identified segments of the TFC, which they labelled stratum transverse cunei. The cuneus is very compact with a high density of white matter fibers crossing it. Therefore, there are a large amount of fibers with an antero-posterior trajectory, crossing paths with the thinner TFC, either perpendicular or parallel to it, disrupting the fiber orientation. Indeed, a recent effort to follow the full trajectory of this bundle was unsuccessful25.
The transverse fasciculus of the lingual lobule of Vialet and TFC are described by the Dejerines as having opposing origins along the calcarine fissures and mirroring trajectories. This implies the possibility of complementary functions related to the upper and lower quadrants of the visual field, and integration of visual stimuli to the accessory visual integration area and the rest of the cerebrum45,46. It follows that if the transverse fasciculus of Vialet exists to connect the areas of the cortex involved in the superior visual fields with distal cortical regions, a similar subcortical system would need to be in place for the inferior visual fields.
The sledge runner fasciculus (SRF)
The SRF had not been described by the Dejerines or other neuroanatomists until Vergani and colleagues25. The tract was initially named the ‘sledge-runner fasciculus’ to highlight its undulating shape due to its anteriorly projecting curve at the level of the calcarine fissure25. Later, in a focused tractography study, the SRF was alternatively named as the Medial Occipital Longitudinal Tract (MOLT) to better reflect its anatomy64. We continue to use the term SRF as it is the more widely known term. The SRF is a thin tract that lies within the medial aspect of the anterior calcarine fissure relaying the anterior border of the dorsal occipital lobe to the antero-superior aspect of the lingual gyrus (Fig. 5). Previous tractography studies align with our observation that the SRF lies deep to the anterior third of the stratum calcarinum (Fig. 6). The SRF connects the anterior border of the medial cuneus to the lingula, isthmus of the cingulum and posterior parahippocampal gyrus20,25,41. Vergani et al.25, supposed that previous anatomists failed to identify the SRF because they classically examined the brain using coronal sections. This allowed U-fibers, running in the coronal plane along the medial and inferior aspects of the hemisphere, to mask the tract. Subsequently, studies combining both postmortem dissections and tractography confirmed the dorsomedial-ventrolateral trajectory of the SRF, specifying that it connects the posterior part of the precuneus to the lingual gyrus and that it lies predominantly medial to the occipital horn and the posterior two-thirds of the atrium20,41. The SRF abuts the posterior cingulum and passes medial to the forceps major with a convex anterior projection.
Furthermore, based on current functional understanding of the regions near the SRF tract dorsal origins, specifically the posterior precuneus, it is postulated that the SRF has a role in processing visual stimuli for visuospatial attention and the recognition of places20,41. The present study is in concordance with past findings and shows the SRF’s relations to other intraoccipital tracts (Fig. 6).
Limitations
As we have shown throughout this study, dMRI has permitted the explorative and comparative studies of subcortical architecture and morphology in large pools of healthy patients, producing both qualitative and quantifiable in vivo data. Regardless, it is necessary to acknowledge the inherent limitations of dMRI based tractography and the specific shortcomings of the technique used in our study. First, it is well documented that the foundation of all successful white matter studies is a strong anatomical background to limit tract misinterpretation and erroneous definitions58,65. Interpretation of the results depends on the expertise of the operator in both physical and virtual dissections. Second, literature has extensively noted that tractography struggles to discern crossing and kissing fibers58. Third, the inaccurate localisation of tract endpoints is a persistent issue despite the improvement in image resolution66,67. Identification of endpoints requires heuristic selection techniques and uses varied termination criteria, introducing a considerable operator bias. Fourth, selecting a method that prevents spurious inferences, by limiting the trade-off between sensitivity and specificity, when reconstructing tracts is an unsolved problem65,68. Furthermore, the anatomical accuracy of tractography relies on the selected algorithm parameters65,69, with their optimisation varying from bundle to bundle. The choice of these parameters can lead to biased characterisation of connectome topography and therefore remains a source of controversy.
One of the standard approaches to try to reduce the spurious results of tractography, particularly false positives, is the implementation of anatomical knowledge into the interpretation of the results. A form of anatomical confirmation is invasive postmortem human brain dissection. This study consulted the work of classical neuroanatomists, such as the Dejerines, to guide in vivo dissections.
With the growing sparsity of appropriate brain cadavers, the development of tractography has used the historic work of past neuroanatomists to supplement their imaging findings26,54,70–72. The 19th-century neuroanatomists were masterful in their blunt postmortem dissections and histological staining studies. Nevertheless, the Dejerines, and the other neuroanatomists they reviewed in their work, used brains that were not necessarily healthy, did not use the Klingler’s technique, nor was there a clear consensus on the definition of association fibers. The first two likely led to artefacts and unclear structures, and the latter incorporated a bias into the interpretation of their findings. There are therefore limits to using historical dissection work to guide new findings. This is especially the case since different neuroanatomists omitted certain tracts from their atlases that they judged, rightly or wrongly, to be inaccurate. Historical work has proven to be a useful starting point for reviving unexploited concepts. It also serves as a strong guideline in helping to determine the potential value of a study. Regardless, they do not circumvent the need for subsequent validation from the modern postmortem dissection literature.
Tractography has generated findings shrouded in controversies, many dating from the 19th century that are still unresolved. Yet, great strides have been made in acknowledging and reducing biases. In this study we have shown that there is a concordance between the original anatomical work of the Dejerines and the recently well-defined VOF, giving us confidence to extrapolate trust in our findings of the other lesser-known tracts. The results indicate gaps in current knowledge and encourage future studies based on this perspective of what the association fibers of the occipital lobe entail.
Methods
The study consisted of two principal methods: the first being the translation from French into English of the Dejerines’ classical study of occipital intralobar fibers. This was followed by a diffusion MRI (dMRI) based virtual reconstruction of the Dejerines’ dissection in healthy subjects.
Translation
The masterful description of intraoccipital fiber tracts in Chapter 5: pp. 780–784 by J.J. Dejerine and A. Dejerine-Klumpke in Anatomie Centre Nerveux; Volume I (1895) was fully translated (MB) from its original 19th-century French to more universally accessible English and reviewed for its accuracy by the experts in this field (CB, MTS). The complete translation is available as a Supplementary note.
Subject selection
A template-based, probabilistic, fiber tractography study was conducted in 24 healthy and unrelated consenting volunteers (range 22–35 years, 12 males). The analysed diffusion MRI dataset was derived from the S1200 release of the publicly available WU-Minn Human Connectome Project (HCP) database (https://db.humanconnectome.org/). To ensure data homogeneity, the subjects selected had none of the following features/criteria: endocrine abnormalities including hyper and hypothyroidism, a handedness score below zero (people with a left-handed tendency), color vision abnormalities, illegal drug use, history of psychiatric problems, hypertensive individuals, alcohol detected by a breathalyser, data with quality control (QC) issues A or B. This study uses data collected and processed by the HCP and approved by the Washington University IRB. The additional data analysis conducted in this present study was approved by the local ethics committee of the University of Malta.
Image acquisition
The full details of image acquisition can be found in the following articles73–77. In short, the HCP group generated the in vivo T1-weighted and diffusion weighted magnetic resonance imaging (MRI) of the human brains through a customised high-resolution 3-Tesla Siemens Skyra scanner equipped with a 32-channel head coil. Structural imaging utilised an axial 3D MPRAGE pulse sequence, while the dMRI used a monopolar Sejskal-Tanner sequence78,79. Following the algorithm in ref. 80 DICOM image files were defaced and deidentified. For further data analysis, dcm2nii MRIcron was used to convert DICOM files to nifti81 (http://www.nitrc.org/projects/mricron).
Diffusion data were rectified for head motion and geometrical distortion, and preprocessed through the HCP minimally processed pipeline15,79,82–86.
MRI data processing
All additional processing was accomplished with the fiber tractography MRtrix3 (RC_3) software toolkit and iFOD2 algorithm87,88. The fiber orientation distribution (FOD) functions from the diffusion signal in each voxel were computed using the multi-shell-multi-tissue CSD.
The individual FODs across the 24 subjects were used to create a population template within MRtrix3. A manually defined occipital lobe mask on the template was then used to produce occipital lobe specific connectomes. The safety margins between the occipital lobe and the exclusion border were large and included posterior aspects of the temporal and parietal lobe. The mask excluded the brainstem, the cerebellum. The anterior border of the mask was defined by a coronal line at the anterior aspect of the splenium of the corpus callosum that extended straight to the lateral convexity of the cerebrum. Figures of the mask are provided in https://osf.io/5v92x/. The connectome derived from 10 million randomly seeded fibers from within it (iFOD2, Lmax = 8, Length: 10–150 mm, Max angle = 45 degrees, output step size = 0.625 mm, FOD cutoff = 0.05, FOD power = 3)89,90. Fibers traversing beyond the occipital lobe were manually assessed. Inclusion and exclusion masks are available through the osf site. Each ROI is titled in a way that reflects the order in which it was applied with a number, the name of tract it is identifying, and in which hemisphere. The first ROI selected was always an inclusion ROI to reduce the bulk of the tract and narrow it around an anatomical landmark as described in the work of the Dejerines. Within the convention of the MRtrix3, inclusion ROIs retain any tracts passing through the MRI mask regardless of their origin and termination. Inclusion ROIs were generally followed by exclusions to remove spurious fibers.
Following template space dissections, the process was repeated in all 24 individual subjects where all masks and ROIs defined in template space were warped into native space (with linear interpolation followed by a binarization with no thresholding to overestimate the native regions).
Reporting summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Supplementary information
Acknowledgements
This article is based upon work from COST Action CA18106, supported by COST (European Cooperation in Science and Technology). S.C. received funding via the Helmholtz Initiative and Networking Fund as well as from the European Union’s Horizon 2020 Research and Innovation Program under Grant Agreement 785907 (Human Brain Project SGA2) and 945539 (Human Brain Project SGA3). M.T.S. is funded by the European Research Council (ERC) under the European Union’s Horizon 2020 research and innovation programme (Grant agreement No. 818521). We would like to thank Krista Bonello Rutter Giappone for her meticulous critique on language and style of this manuscript. Data were provided by the Human Connectome Project, WU-Minn Consortium (Principal Investigators: David Van Essen and Kamil Ugurbil; 1U54MH091657) funded by the 16 NIH Institutes and Centers that support the NIH Blueprint for Neuroscience Research; and by the McDonnell Center for Systems Neuroscience at Washington University.
Author contributions
M.B.: data analysis, translation, visualisation, writing, and editing first draft, and revisions. Y.D.: data analysis, writing and editing first draft, and revisions, N.T.: writing and editing first draft. M.T.S.: translation revision, writing and editing first draft, and revisions. S.C.: writing and editing first draft and revisions. R.M.: resources, writing and editing first draft, and revisions. C.J.B.: conceptualisation, resources, data analysis, data curation, supervision, translation revision, writing and editing first draft, and revisions. All authors reviewed and approved of the final version of this manuscript.
Data availability
The datasets used and analysed in this manuscript, including results and scripts, are available in the OSF repository, https://osf.io/5v92x/. Further unprocessed, and minimally preprocessed, data can also be found through the Human Connectome Project Database, http://db.humanconnectome.org/.
Competing interests
M.T.S. is an Editorial Board Member for Communications Biology. The remaining authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version contains supplementary material available at 10.1038/s42003-021-01935-3.
References
- 1.Sotiropoulos SN, Zalesky A. Building connectomes using diffusion MRI: why, how and but. NMR Biomed. 2019;32:e3752. doi: 10.1002/nbm.3752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shibata S, et al. Connectomics: comprehensive approaches for whole-brain mapping. Reprod. Syst. Sex. Disord. 2015;64:57–67. doi: 10.1093/jmicro/dfu103. [DOI] [PubMed] [Google Scholar]
- 3.Van Essen DC, Ugurbil K. The future of the human connectome. Neuroimage. 2012;62:1299–1310. doi: 10.1016/j.neuroimage.2012.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wakana S, Jiang H, Nagae-Poetscher LM, van Zijl PCM, Mori S. Fiber tract-based atlas of human white matter anatomy. Radiology. 2004;230:77–87. doi: 10.1148/radiol.2301021640. [DOI] [PubMed] [Google Scholar]
- 5.Jbabdi S, Behrens TE. Long-range connectomics. Ann. N. Y. Acad. Sci. 2013;1305:83–93. doi: 10.1111/nyas.12271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jbabdi S, Sotiropoulos SN, Haber SN, Van Essen DC, Behrens TE. Measuring macroscopic brain connections in vivo. Nat. Neurosci. 2015;18:1546–1555. doi: 10.1038/nn.4134. [DOI] [PubMed] [Google Scholar]
- 7.Jeurissen B, Descoteaux M, Mori S, Leemans A. Diffusion MRI fiber tractography of the brain. NMR Biomed. 2019;32:e3785. doi: 10.1002/nbm.3785. [DOI] [PubMed] [Google Scholar]
- 8.Meola A, Comert A, Yeh F-C, Stefaneanu L, Fernandez-Miranda JC. The controversial existence of the human superior fronto-occipital fasciculus: Connectome-based tractographic study with microdissection validation. Hum. Brain Mapp. 2015;36:4964–4971. doi: 10.1002/hbm.22990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bao Y, Wang Y, Wang W, Wang Y. The superior fronto-occipital fasciculus in the human brain revealed by diffusion spectrum imaging tractography: an anatomical reality or a methodological artifact? Front. Neuroanat. 2017;11:119. doi: 10.3389/fnana.2017.00119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Weiner KS, Yeatman JD, Wandell BA. The posterior arcuate fasciculus and the vertical occipital fasciculus. Cortex. 2017;97:274–276. doi: 10.1016/j.cortex.2016.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kamali A, Flanders AE, Brody J, Hunter JV, Hasan KM. Tracing superior longitudinal fasciculus connectivity in the human brain using high resolution diffusion tensor tractography. Brain Struct. Funct. 2014;219:269–281. doi: 10.1007/s00429-012-0498-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schurr R, Filo S, Mezer AA. Tractography delineation of the vertical occipital fasciculus using quantitative T1 mapping. Neuroimage. 2019;202:116121. doi: 10.1016/j.neuroimage.2019.116121. [DOI] [PubMed] [Google Scholar]
- 13.Wu Y, Sun D, Wang Y, Wang Y, Ou S. Segmentation of the cingulum bundle in the human brain: a new perspective based on DSI tractography and fiber dissection study. Front. Neuroanat. 2016;10:84. doi: 10.3389/fnana.2016.00084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hau J, et al. Cortical terminations of the inferior fronto-occipital and uncinate fasciculi: anatomical stem-based virtual dissection. Front. Neuroanat. 2016;10:58. doi: 10.3389/fnana.2016.00058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sampson JN, et al. Analysis of heritability and shared heritability based on genome-wide association studies for thirteen cancer types. J. Natl Cancer Inst. 2015;107:djv279. doi: 10.1093/jnci/djv279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Panesar SS, Belo JTA, Yeh F-C, Fernandez-Miranda JC. Structure, asymmetry, and connectivity of the human temporo-parietal aslant and vertical occipital fasciculi. Brain Struct. Funct. 2019;224:907–923. doi: 10.1007/s00429-018-1812-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bartsch AJ, Geletneky K, Jbabdi S. The temporoparietal fiber intersection area and wernicke perpendicular fasciculus. Neurosurgery. 2013;73:E381–E382. doi: 10.1227/01.neu.0000430298.25585.1d. [DOI] [PubMed] [Google Scholar]
- 18.Yeatman JD, et al. The vertical occipital fasciculus: a century of controversy resolved by in vivo measurements. Proc. Natl Acad. Sci. USA. 2014;111:E5214–E5223. doi: 10.1073/pnas.1418503111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.David S, et al. The superoanterior fasciculus (SAF): a novel white matter pathway in the human brain? Front. Neuroanat. 2019;13:24. doi: 10.3389/fnana.2019.00024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Koutsarnakis C, et al. Sledge runner fasciculus: anatomic architecture and tractographic morphology. Brain Struct. Funct. 2019;224:1–16. doi: 10.1007/s00429-018-01822-4. [DOI] [PubMed] [Google Scholar]
- 21.Uesaki M, Takemura H, Ashida H. Computational neuroanatomy of human stratum proprium of interparietal sulcus. Brain Struct. Funct. 2018;223:489–507. doi: 10.1007/s00429-017-1492-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Donahue CJ, et al. Using diffusion tractography to predict cortical connection strength and distance: a quantitative comparison with tracers in the monkey. J. Neurosci. 2016;36:6758–6770. doi: 10.1523/JNEUROSCI.0493-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Forkel SJ, et al. The anatomy of fronto-occipital connections from early blunt dissections to contemporary tractography. Cortex. 2014;56:73–84. doi: 10.1016/j.cortex.2012.09.005. [DOI] [PubMed] [Google Scholar]
- 24.Schmahmann, J. D. & Pandya, D. N. Fiber Pathways of The Brain. (Oxford University Press, 2006).
- 25.Vergani F, Mahmood S, Morris CM, Mitchell P, Forkel SJ. Intralobar fibres of the occipital lobe: a post mortem dissection study. Cortex. 2014;56:145–156. doi: 10.1016/j.cortex.2014.03.002. [DOI] [PubMed] [Google Scholar]
- 26.Catani M, et al. Short frontal lobe connections of the human brain. Cortex. 2012;48:273–291. doi: 10.1016/j.cortex.2011.12.001. [DOI] [PubMed] [Google Scholar]
- 27.Catani M, Jones DK, Donato R, Ffytche DH. Occipito-temporal connections in the human brain. Brain. 2003;126:2093–2107. doi: 10.1093/brain/awg203. [DOI] [PubMed] [Google Scholar]
- 28.Catani, M. in Diffusion MRI: Theory, Methods, and Applications (ed. Jones, D. K. PhD) 5–18 (Oxford University Press, 2010).
- 29.Bajada CJ, Banks B, Lambon Ralph MA, Cloutman LL. Reconnecting with Joseph and Augusta Dejerine: 100 years on. Brain. 2017;140:2752–2759. doi: 10.1093/brain/awx225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Meynert T. Neue Studien über die Associations-Bündel des Hirnmantels. Math. Nat. Sci. Cl. 1892;101:361–380. [Google Scholar]
- 31.Forkel SJ, Mahmood S, Vergani F, Catani M. The white matter of the human cerebrum: part I The occipital lobe by Heinrich Sachs. Cortex. 2015;62:182–202. doi: 10.1016/j.cortex.2014.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wernicke C. Das Urwindungssystem des menschlichen Gehirns. Arch. f. Psychiatr. 1876;6:298–326. doi: 10.1007/BF02230815. [DOI] [Google Scholar]
- 33.Déjerine, J. J. & Déjerine-Klumpke, A. Anatomie des centres nerveux: Tome 1, vol. 1, (Rueff et Cie, 1895).
- 34.Sachs, H. & Wernicke, C. Das Hemisphärenmark des menschlichen Grosshirns: 1. Der Hinterhauptlappen https://archive.org/details/dashemisphrenm00sach/page/n7/mode/2up (G. Thieme, Leipzig, Germany, 1892).
- 35.Bogousslavsky J. The Klumpke family–memories by Doctor Déjerine, born Augusta Klumpke. Eur. Neurol. 2005;53:113–120. doi: 10.1159/000085554. [DOI] [PubMed] [Google Scholar]
- 36.Shoja MM, Tubbs RS. Augusta Déjerine-Klumpke: the first female neuroanatomist. Clin. Anat. 2007;20:585–587. doi: 10.1002/ca.20474. [DOI] [PubMed] [Google Scholar]
- 37.Dejerine, J. J. & Dejerine-Klumpke, A. Anatomie des Centres Nerveux. Tome 2, vol. 2, (Rueff et Cie, 1902).
- 38.Chou W, Salamon G, Orr NB, Salamon N. Neuroanatomic analysis of diffusion tensor imaging of white matter tracts with dejerine sections and neuroimaging. Neuroradiol. J. 2009;22:499–517. doi: 10.1177/197140090902200501. [DOI] [PubMed] [Google Scholar]
- 39.Mandonnet E, Sarubbo S, Petit L. The nomenclature of human white matter association pathways: proposal for a systematic taxonomic anatomical classification. Front. Neuroanat. 2018;12:94. doi: 10.3389/fnana.2018.00094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Briggs RG, et al. A connectomic atlas of the human cerebrum-Chapter 16: Tractographic description of the vertical occipital fasciculus. Oper. Neurosurg. 2018;15:S456–S461. doi: 10.1093/ons/opy270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Güngör A, et al. The white matter tracts of the cerebrum in ventricular surgery and hydrocephalus. J. Neurosurg. 2017;126:945–971. doi: 10.3171/2016.1.JNS152082. [DOI] [PubMed] [Google Scholar]
- 42.Oishi H, Takemura H, Aoki SC, Fujita I, Amano K. Microstructural properties of the vertical occipital fasciculus explain the variability in human stereoacuity. Proc. Natl Acad. Sci. USA. 2018;115:12289–12294. doi: 10.1073/pnas.1804741115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.ffytche DH, Catani M. Beyond localization: from hodology to function. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2005;360:767–779. doi: 10.1098/rstb.2005.1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vialet, N. Les centres cérébraux de la vision et l’appareil nerveux visuel intra-cérébral. (F. Alcan, 1893).
- 45.Greenblatt SH. Alexia without agraphia or hemianopsia. Anatomical analysis of an autopsied case. Brain. 1973;96:307–316. doi: 10.1093/brain/96.2.307. [DOI] [PubMed] [Google Scholar]
- 46.Campbell, A. Histological Studies on The Localisation of Cerebral Function. (Cambridge University Press, 1905).
- 47.Oishi K, et al. Human brain white matter atlas: identification and assignment of common anatomical structures in superficial white matter. Neuroimage. 2008;43:447–457. doi: 10.1016/j.neuroimage.2008.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Oishi K, et al. Superficially located white matter structures commonly seen in the human and the macaque brain with diffusion tensor imaging. Brain Connect. 2011;1:37–47. doi: 10.1089/brain.2011.0005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhang Y, et al. Atlas-guided tract reconstruction for automated and comprehensive examination of the white matter anatomy. Neuroimage. 2010;52:1289–1301. doi: 10.1016/j.neuroimage.2010.05.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Román C, et al. Clustering of whole-brain white matter short association bundles using HARDI data. Front Neuroinformatics. 2017;11:73. doi: 10.3389/fninf.2017.00073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Keser Z, Ucisik-Keser FE, Hasan KM. Quantitative mapping of human brain vertical-occipital fasciculus. J. Neuroimaging. 2016;26:188–193. doi: 10.1111/jon.12268. [DOI] [PubMed] [Google Scholar]
- 52.Palejwala AH, et al. Anatomy and white matter connections of the lateral occipital cortex. Surg. Radiol. Anat. 2020;42:315–328. doi: 10.1007/s00276-019-02371-z. [DOI] [PubMed] [Google Scholar]
- 53.Takemura H, et al. Occipital white matter tracts in human and macaque. Cereb. Cortex. 2017;27:3346–3359. doi: 10.1093/cercor/bhx070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bullock D, et al. Associative white matter connecting the dorsal and ventral posterior human cortex. Brain Struct. Funct. 2019;224:2631–2660. doi: 10.1007/s00429-019-01907-8. [DOI] [PubMed] [Google Scholar]
- 55.Wernicke, C. Lehrbuch der Gehirnkrankheiten für Aerzte und Studirende. (Kassel Theodor Fischer, 1881).
- 56.Martino J, García-Porrero JA. Wernicke perpendicular fasciculus and vertical portion of the superior longitudinal fasciculus. Neurosurgery. 2013;73:E382–E383. doi: 10.1227/01.neu.0000430303.56079.0e. [DOI] [PubMed] [Google Scholar]
- 57.Kaneko T, et al. Spatial organization of occipital white matter tracts in the common marmoset. Brain Struct. Funct. 2020;225:1313–1326. doi: 10.1007/s00429-020-02060-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Maier-Hein KH, et al. The challenge of mapping the human connectome based on diffusion tractography. Nat. Commun. 2017;8:1349. doi: 10.1038/s41467-017-01285-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Takemura H, et al. Anatomy of nerve fiber bundles at micrometer-resolution in the vervet monkey visual system. elife. 2020;9:e55444. doi: 10.7554/eLife.55444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Reveley C, et al. Superficial white matter fiber systems impede detection of long-range cortical connections in diffusion MR tractography. Proc. Natl Acad. Sci. USA. 2015;112:E2820–E2828. doi: 10.1073/pnas.1418198112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Von Bonin GE, Garol HW, McCulloch WS. The functional organization of the occipital lobe. Biol. Symposia. 1942;7:165. [Google Scholar]
- 62.Koutsarnakis, C. et al. Mapping the superficial morphology of the occipital lobe: proposal of a universal nomenclature for clinical and anatomical use. Neurosurg. Rev.10.1007/s10143-019-01212-2 (2019). [DOI] [PubMed]
- 63.Takemura H, Pestilli F, Weiner KS. Comparative neuroanatomy: integrating classic and modern methods to understand association fibers connecting dorsal and ventral visual cortex. Neurosci. Res. 2019;146:1–12. doi: 10.1016/j.neures.2018.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Beyh, A., Dell’Aqua, F., Ffytche, D. & Catani, M. The Medial Occipital Longitudinal Tract (MOLT): A Novel Pathway for Visual Spatial Learning. (OHBM, 2019).
- 65.Thomas C, et al. Anatomical accuracy of brain connections derived from diffusion MRI tractography is inherently limited. Proc. Natl Acad. Sci. USA. 2014;111:16574–16579. doi: 10.1073/pnas.1405672111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Girard G, Whittingstall K, Deriche R, Descoteaux M. Towards quantitative connectivity analysis: reducing tractography biases. Neuroimage. 2014;98:266–278. doi: 10.1016/j.neuroimage.2014.04.074. [DOI] [PubMed] [Google Scholar]
- 67.Smith RE, Tournier J-D, Calamante F, Connelly A. Anatomically-constrained tractography: improved diffusion MRI streamlines tractography through effective use of anatomical information. Neuroimage. 2012;62:1924–1938. doi: 10.1016/j.neuroimage.2012.06.005. [DOI] [PubMed] [Google Scholar]
- 68.Zalesky A, et al. Connectome sensitivity or specificity: which is more important? Neuroimage. 2016;142:407–420. doi: 10.1016/j.neuroimage.2016.06.035. [DOI] [PubMed] [Google Scholar]
- 69.Bastiani M, Shah NJ, Goebel R, Roebroeck A. Human cortical connectome reconstruction from diffusion weighted MRI: the effect of tractography algorithm. Neuroimage. 2012;62:1732–1749. doi: 10.1016/j.neuroimage.2012.06.002. [DOI] [PubMed] [Google Scholar]
- 70.Schmahmann JD, Smith EE, Eichler FS, Filley CM. Cerebral white matter: neuroanatomy, clinical neurology, and neurobehavioral correlates. Ann. N. Y. Acad. Sci. 2008;1142:266–309. doi: 10.1196/annals.1444.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Schmahmann JD, Pandya DN. The complex history of the fronto-occipital fasciculus. J. Hist. Neurosci. 2007;16:362–377. doi: 10.1080/09647040600620468. [DOI] [PubMed] [Google Scholar]
- 72.Dick AS, Tremblay P. Beyond the arcuate fasciculus: consensus and controversy in the connectional anatomy of language. Brain. 2012;135:3529–3550. doi: 10.1093/brain/aws222. [DOI] [PubMed] [Google Scholar]
- 73.Moeller S, et al. Multiband multislice GE-EPI at 7 tesla, with 16-fold acceleration using partial parallel imaging with application to high spatial and temporal whole-brain fMRI. Magn. Reson. Med. 2010;63:1144–1153. doi: 10.1002/mrm.22361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Feinberg DA, et al. Multiplexed echo planar imaging for sub-second whole brain FMRI and fast diffusion imaging. PLoS ONE. 2010;5:e15710. doi: 10.1371/journal.pone.0015710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Setsompop K, et al. Blipped-controlled aliasing in parallel imaging for simultaneous multislice echo planar imaging with reduced g-factor penalty. Magn. Reson. Med. 2012;67:1210–1224. doi: 10.1002/mrm.23097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sotiropoulos SN, et al. Effects of image reconstruction on fiber orientation mapping from multichannel diffusion MRI: reducing the noise floor using SENSE. Magn. Reson. Med. 2013;70:1682–1689. doi: 10.1002/mrm.24623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Xu J, et al. Highly accelerated whole brain imaging using aligned-blipped-controlled-aliasing multiband EPI. Proc. Intl. Soc. Mag. Reson. Med. 2012;20:2306. [Google Scholar]
- 78.Van Essen DC, et al. The Human Connectome Project: a data acquisition perspective. Neuroimage. 2012;62:2222–2231. doi: 10.1016/j.neuroimage.2012.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Glasser MF, et al. The minimal preprocessing pipelines for the Human Connectome Project. Neuroimage. 2013;80:105–124. doi: 10.1016/j.neuroimage.2013.04.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Milchenko M, Marcus D. Obscuring surface anatomy in volumetric imaging data. Neuroinformatics. 2013;11:65–75. doi: 10.1007/s12021-012-9160-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Rorden C, Karnath HO, Bonilha L. Improving lesion-symptom mapping. J. Cogn. Neurosci. 2007;19:1081–8. doi: 10.1162/jocn.2007.19.7.1081. [DOI] [PubMed] [Google Scholar]
- 82.Jenkinson M, Bannister P, Brady M, Smith S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage. 2002;17:825–841. doi: 10.1006/nimg.2002.1132. [DOI] [PubMed] [Google Scholar]
- 83.Jenkinson M, Beckmann CF, Behrens TE, Woolrich MW, Smith SM. FSL. Neuroimage. 2012;62:782–790. doi: 10.1016/j.neuroimage.2011.09.015. [DOI] [PubMed] [Google Scholar]
- 84.Fischl B. FreeSurfer. Neuroimage. 2012;62:774–781. doi: 10.1016/j.neuroimage.2012.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Andersson JLR, Skare S, Ashburner J. How to correct susceptibility distortions in spin-echo echo-planar images: application to diffusion tensor imaging. Neuroimage. 2003;20:870–888. doi: 10.1016/S1053-8119(03)00336-7. [DOI] [PubMed] [Google Scholar]
- 86.Andersson JLR, Sotiropoulos SN. Non-parametric representation and prediction of single- and multi-shell diffusion-weighted MRI data using Gaussian processes. Neuroimage. 2015;122:166–176. doi: 10.1016/j.neuroimage.2015.07.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Tournier J-D, Calamante F, Connelly A. MRtrix: Diffusion tractography in crossing fiber regions. Int. J. Imaging Syst. Technol. 2012;22:53–66. doi: 10.1002/ima.22005. [DOI] [Google Scholar]
- 88.Tournier J-D, et al. MRtrix3: A fast, flexible and open software framework for medical image processing and visualisation. Neuroimage. 2019;202:116137. doi: 10.1016/j.neuroimage.2019.116137. [DOI] [PubMed] [Google Scholar]
- 89.Jeurissen B, Tournier J-D, Dhollander T, Connelly A, Sijbers J. Multi-tissue constrained spherical deconvolution for improved analysis of multi-shell diffusion MRI data. Neuroimage. 2014;103:411–426. doi: 10.1016/j.neuroimage.2014.07.061. [DOI] [PubMed] [Google Scholar]
- 90.Tournier J-D, Calamante F, Connelly A. Robust determination of the fibre orientation distribution in diffusion MRI: non-negativity constrained super-resolved spherical deconvolution. Neuroimage. 2007;35:1459–1472. doi: 10.1016/j.neuroimage.2007.02.016. [DOI] [PubMed] [Google Scholar]
- 91.Duan Y, Norcia AM, Yeatman JD, Mezer A. The structural properties of major white matter tracts in strabismic amblyopia. Invest. Ophthalmol. Vis. Sci. 2015;56:5152–5160. doi: 10.1167/iovs.15-17097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Muftah Lahirish IA, et al. Comparison between transcortical and interhemispheric approaches to the atrium of lateral ventricle using combined white matter fiber dissections and magnetic resonance tractography. World Neurosurg. 2020;138:e478–e485. doi: 10.1016/j.wneu.2020.02.161. [DOI] [PubMed] [Google Scholar]
- 93.Rokem A, et al. The visual white matter: the application of diffusion MRI and fiber tractography to vision science. J. Vis. 2017;17:4. doi: 10.1167/17.2.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Takemura H, et al. A major human white matter pathway between dorsal and ventral visual cortex. Cereb. Cortex. 2016;26:2205–2214. doi: 10.1093/cercor/bhv064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Wu Y, Sun D, Wang Y, Wang Y, Wang Y. Tracing short connections of the temporo-parieto-occipital region in the human brain using diffusion spectrum imaging and fiber dissection. Brain Res. 2016;1646:152–159. doi: 10.1016/j.brainres.2016.05.046. [DOI] [PubMed] [Google Scholar]
- 96.Yeatman JD, Rauschecker AM, Wandell BA. Anatomy of the visual word form area: adjacent cortical circuits and long-range white matter connections. Brain Lang. 2013;125:146–155. doi: 10.1016/j.bandl.2012.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Barker, L. in The Nervous system and its Constituent Neurones 1059–1061 (Appleton and Company, 1899).
- 98.Dejerine, J. J. In Comptes rendus des séances de la Société de Biologie et de ses filiales (ed. Masson, G.) (la Société de Biologie, 1892).
- 99.Maunsell JH, van Essen DC. The connections of the middle temporal visual area (MT) and their relationship to a cortical hierarchy in the macaque monkey. J. Neurosci. 1983;3:2563–2586. doi: 10.1523/JNEUROSCI.03-12-02563.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and analysed in this manuscript, including results and scripts, are available in the OSF repository, https://osf.io/5v92x/. Further unprocessed, and minimally preprocessed, data can also be found through the Human Connectome Project Database, http://db.humanconnectome.org/.



