Abstract
Despite higher stages at presentation, patients with high-risk (HR) HPV-related (HPV+) head and neck squamous cell carcinomas (HNSCCs) have better survival rates compared to those with non-HPV-related (HPV−) disease. However, significant comorbidity and the number of patients who suffer failed treatment, recurrent disease, late metastasis, and death are increasing along with the incidence of HPV+ HNSCC. A cytotoxic T-cell-dependent immune response is required to clear these antigenic cancers. This provides a unique opportunity to employ immune modulators in therapy. Galectin-3 (Gal-3) is a lectin and glycoprotein involved in numerous immunosuppressive functions. Inhibitors are currently under clinical investigation for various diseases. Gal-3 expression was evaluated in HR-HPV+ and HPV− HNSCCs and regional lymph node metastases by tissue microarray. HR-HPV+ cases were more likely to be Gal-3-positive (Gal+) [50% (14/28)] than HPV− cases [18% (9/50), p = 0.004]. No difference in the number of Gal+ cases was identified between primary [30% (16/53)] and metastatic [28% (7/25)] cancers (p = 1); 53% (9/17) of primary HPV+ cancers were Gal+ and 45% (5/11) of metastatic HPV+ cancers were Gal+ (p = 1). Nineteen percent (7/36) of primary HPV− cancers were Gal+ and 14% (2/14) of metastatic HPV− cancers were Gal+ (p = 1). Gal-3 positivity was observed in a subset of HNSCC, suggesting a potential role for therapeutic inhibition in this tumor type. The significantly higher rates of expression seen in HR-HPV+ versus HPV− HNSCC suggest particular promise in the setting of HPV infection. The relatively consistent Gal-3 expression rates observed between metastatic and primary tumors argues against progressive Gal-3 expression in metastasis.
Keywords: Galectin 3, Head and neck cancer, Oropharyngeal cancer, Squamous cell carcinoma, Human papilloma virus, Metastasis
Introduction
As the sixth most common cancer in the world, head and neck squamous cell carcinoma (HNSCC) annually accounts for nearly 600,000 new cancer cases worldwide and 10,000 deaths in the United States alone [1–3]. High-risk (HR) human papillomavirus-related (HPV+) HNSCC accounts for up to 25% of all HNSCCs, and HPV type-16 (HPV-16) is recognized as the driving etiologic factor for the majority of oropharyngeal HNSCCs in North America and Europe [4, 5]. In the oropharynx, at least 80% of cancers are HPV+ [6]. Despite their more advanced clinical stage at presentation and often poorly differentiated morphology, patients with HPV+ HNSCCs have a clear survival advantage over those with non-HPV-related (HPV−) cancers [7, 8]. Treatment is successful in approximately 80% of patients but often imparts significant comorbidities associated with eating and speaking [9]. Furthermore, approximately 10% of HPV+ HNSCC patients develop metastases that often culminate in incurable disease [10]. Considering the continuous increase in total numbers of HPV+ HNSCC, improved therapies and novel targets are desperately needed [3, 6].
HPV+ oropharyngeal squamous cell carcinomas (HPV+ OPSCC) are more curable following standard cisplatin/radiation therapy (CRT) than their HPV− counterparts, and an immune response is responsible and required for long-term cure [11]. HPV+ HNSCCs are not more curable due to increased sensitivity to cisplatin or radiation, rather CRT induces an immune response that leads to immune-mediated clearance. This process is CD8+ cytotoxic T-lymphocyte (CTL) dependent, and CTLs predominate the intratumoral immune cell infiltrate in HPV+ HNSCC [12–14]. It has been postulated that immune evasion plays a significant role in the recurrence, progression, and metastasis of these cancers, leading to a piqued interest in immunotherapies in their treatment.
Galectin-3 (Gal-3) is a mammalian lectin and glycoprotein with numerous, dynamic intra- and extracellular functions. It is influenced by cleavage status by matrix metalloproteases and demonstrated to have a regulatory role in cell cycle progression and apoptosis. Gal-3 also contributes to angiogenesis and tumor growth in various cancer types, including head and neck carcinomas [15, 16]. In oral tongue squamous cell carcinomas, Gal-3′s contribution to tumor progression is via a Wnt/β-catenin-dependent mechanism [17]. Specific to the tumor microenvironment, Gal-3 contributes to M2 macrophage-mediated local immune tolerance, induces apoptosis of lymphocytes, and decreases anti-tumor natural killer cell action. It also suppresses T-cell function and expansion, including that of CD8-positive T-cells, a particularly intriguing function in the context of HPV+ HNSCC [15, 16, 18]. Its expression has also been reported to increase with cancer progression including metastasis [15, 19]. Gal-3 inhibitors are currently available and under clinical investigation for treatment of various cancers and diseases.
To better understand the potential role of Gal-3 in tumor progression and as an immunotherapy target in HNSCC, we evaluate the expression of Gal-3 in primary and metastatic (regional lymph node) HNSCCs relative to HR-HPV status using a tissue microarray (TMA).
Materials and Methods
Tissue Microarray
This work was approved by the Institutional Review Board of the University of Virginia (IRB #13310). Seventy-eight HNSCC cases were evaluated using a tissue microarray constructed from the pathology archives of the University of Virginia Department of Pathology with material from the years of 2008–2017. Cases were selected based on the availability of sufficient non-frozen, formalin-fixed, paraffin-embedded tumor material for research purposes. Both HPV-positive and negative oral cavity and oropharyngeal cases were selected based on prior p16 expression status, with all metastatic cases involving regional neck lymph nodes. All cases were reviewed and those with sufficient amounts of tumor were used in construction of the TMA. A study pathologist (J.D.C.) was responsible for array design including the selection of tumor area for core punching. The arrays were then constructed by the University of Virginia Biorepository and Tissue Research Facility. The TMA consisted of four replicate 0.6 mm punches of each tumor with sampling of different areas within the original tumor section. This included the tumor periphery and center, as well as ten 0.6 mm punches each of normal spleen, liver, kidney, and placenta as controls. Upon confirmation of HPV status as below, HPV− cases consisted of 17 oral tongue, 3 floor of mouth, 1 buccal, 1 pharyngeal, 6 laryngeal, 2 anterior tonsillar pillar, 1 tonsillar, 3 tongue base, 2 soft palate cancers, and 14 regional neck lymph nodes harboring metastatic cancer. HPV+ cases consisted of 14 tonsillar and 3 tongue base cancers and 11 regional neck lymph nodes harboring metastatic cancer. Patients ranged in age from 17–94 years, with a median age of 61 years, and 81% were male, 92.3% Caucasian, 5.1% African American, and 2.6% Middle Eastern.
Immunohistochemistry & RNA In Situ Hybridization
HPV status at the time of diagnosis and from which initial case selection was made was established using p16 immunohistochemistry (IHC) at a dilution of 1:2 (CINtec Plus Histology kit, Roche/Ventana, Oro Valley, AZ). Per College of American Pathologists (CAP) guidelines, cases were considered p16 positive, and thus HPV+, if ≥ 70% nuclear and cytoplasmic tumor cell staining was observed. Those with ≤ 70% nuclear and cytoplasmic staining were considered negative.
HR-HPV status was confirmed by RNA in situ hybridization (RNA ISH) (cocktail probe, RNAscope, Advanced Cell Diagnostics, Newark, CA) which detects E6/E7 specific to the HR-HPV types 16, 18, 26, 31, 33, 35, 39, 45, 51, 52, 53, 56, 58, 59, 66, 68, 73, and 82. RNA ISH was performed on unbaked slides using an automated staining system (Leica Bond III). All slides were cut within 24 h of staining to avoid RNA degradation, and an ubiquitin control slide was performed to control for RNA degradation. The chromogenic stain was brown with a pale blue counterstain. Positive signal was recorded in the presence of dark brown, dot-like cytoplasmic positivity with or without nuclear positivity. All cases showed intact ubiquitin control staining.
Gal-3 expression was determined manually by evaluating percentage of positive tumor cells by IHC (Cell Marque, 255-M15, Rocklin, CA) at a 1:50 dilution. No consensus cutoff for Gal-3 positivity exists at this time. While prior studies have considered as little as any tumor cell staining or tumor cell staining higher than that of background cells positive, an inclusive cutoff that mitigates the difficult distinction between only background or tumor-infiltrating immune cell staining was utilized in this study [18, 20]. Tumor staining was classified as positive when definitive cytoplasmic with or without nuclear staining was present in ≥ 5% of tumor cells.
Statistical Analysis
Comparisons were performed using the two-tailed Fisher exact probability test.
Results
Seventy-eight primary and metastatic (regional neck lymph node) squamous cell carcinomas were evaluated via TMA: 17 primary HPV+, 36 primary HPV−, 11 metastatic HPV+, and 14 metastatic HPV−. HPV status was confirmed via HR-HPV RNA ISH (see Materials and Methods) (Fig. 1).
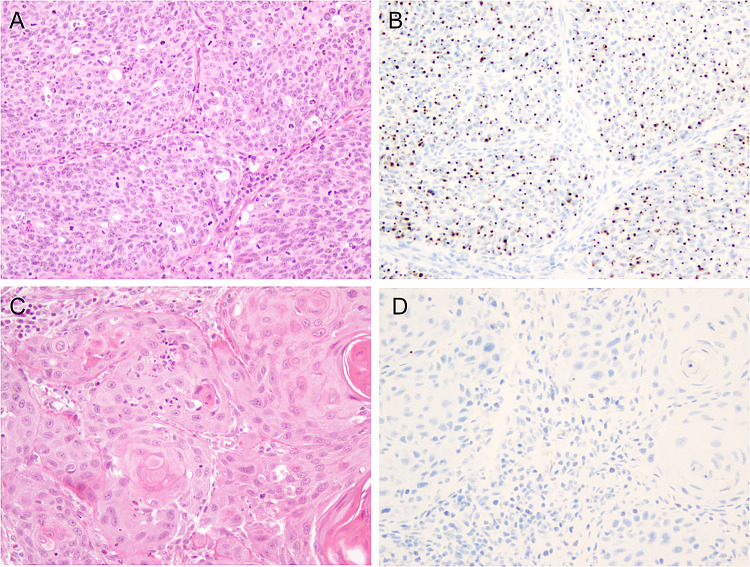
Fig. 1.
High-risk HPV status by RNA in situ hybridization. a HPV-positive squamous cell carcinoma (H&E, × 200 total magnification). b HR-HPV RNA ISH with nuclear and cytoplasmic dot-like positivity (× 200 total magnification). c HPV-negative squamous cell carcinoma (H&E, × 200 total magnification). d Negative HR-HPV RNA ISH (× 200 total magnification)
Gal-3 IHC demonstrated ≥ 5% cytoplasmic tumor cell staining in 29% (23/78) of all HNSCC cases evaluated (Table 1). No significant difference in the overall number of Gal+ cases was identified between primary [30% (16/53)] (Fig. 2) and metastatic [28% (7/25)] (Fig. 3) cancers (p = 1). Fifty-three percent (9/17) of primary HPV+ cancers were Gal+ and 45% (5/11) of metastatic HPV+ cancers were Gal+ (p = 1). Nineteen percent (7/36) of primary HPV− cancers were Gal+ and 14% (2/14) of metastatic HPV− cancers were Gal+ (p = 1). However, 50% (14/28) of total HPV+ cases were Gal-3-positive (Gal+) while only 18% (9/50) of total HPV− cases were Gal+ (p = 0.004). Overall, HR-HPV+ cases were significantly more likely to be Gal+ than HPV− cases.
Table 1.
Gal-3 expression in primary and metastatic, HPV+ and HPV− HNSCCs
| HPV RNA ISH+ (28) | HPV RNA ISH− (50) | |
|---|---|---|
| Primary (53) | ||
| GAL+ | 9 | 7 |
| GAL− | 8 | 29 |
| Metastasis (25) | ||
| GAL+ | 5 | 2 |
| GAL− | 6 | 12 |
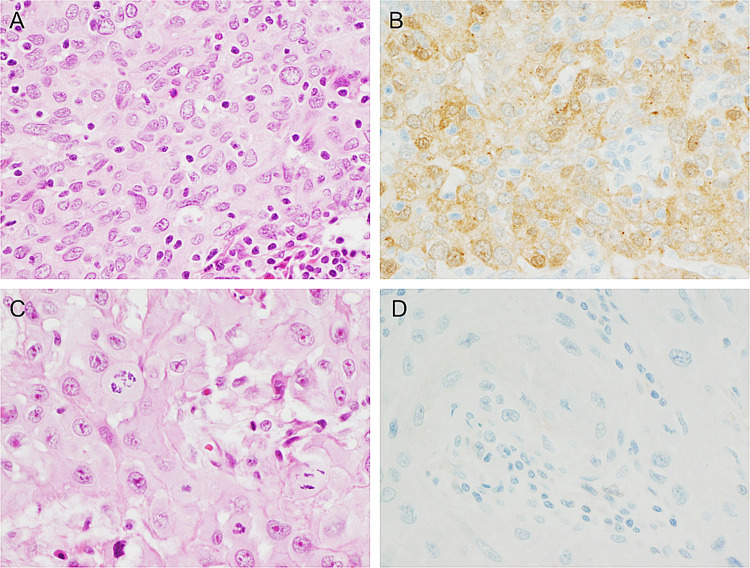
Fig. 2.
Gal-3 expression by immunohistochemistry in primary HNSCC. a An example of a Gal-3 positive primary oral SCC (H&E, × 400 total magnification) and b Gal-3 IHC demonstrating cytoplasmic and some nuclear staining in a majority of tumor cells (× 400 total magnification). c A contrasting example of a Gal-3 negative primary oral SCC (H&E, × 400 total magnification) and D) Gal-3 IHC demonstrating a complete lack of staining (× 400 total magnification)
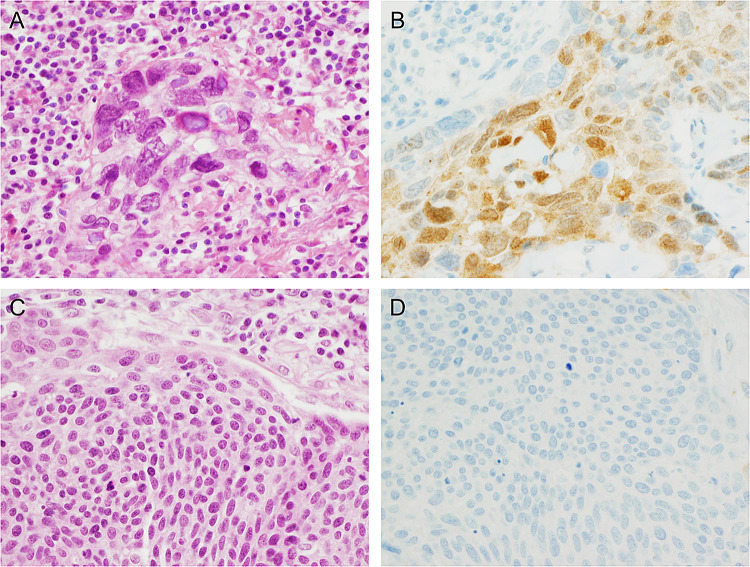
Fig. 3.
Gal-3 expression by immunohistochemistry in metastatic HNSCC. a An example of a Gal-3 positive HNSCC lymph node metastasis (H&E, × 400 total magnification) and b Gal-3 IHC demonstrating staining in a majority of tumor cells (× 400 total magnification). c In contrast, a Gal-3 negative HNSCC lymph node metastasis (H&E, × 400 total magnification) and d Gal-3 IHC demonstrating a complete lack of staining (× 400 total magnification)
Discussion
Despite the essential role of the antitumor immune response in effective treatment of HPV-related HNSCC and numerous mechanistic descriptions of Gal-3′s suppression of such an immune response, Gal-3 expression in HNSCC has been only minimally investigated [15, 16, 18, 19]. HPV status is the strongest prognostic indicator in HNSCC regardless of histomorphology, and the data presented here demonstrate higher Gal-3 expression in HPV-related disease compared to HPV-negative disease. Interestingly, a high percentage of cervical squamous cell carcinomas (84%) have also been demonstrated to express Gal-3 [21]. This suggests Gal-3 may contribute to immune escape. This is particularly critical in HPV-related disease and may provide a potential therapeutic target, though it could be more widely applicable to the entire subset of all HNSCC found to express Gal-3. This may be approximately a third of cases based on the data presented here. Future work evaluating this possibility is warranted, especially considering that Gal-3 inhibitors are currently available and under clinical investigation for other cancer types and diseases.
The Gal-3 inhibitor, GM-CT-01, is currently being evaluated for its ability to restore CTLs from anergy in melanoma (NCT01723813). Another Gal-3 inhibitor, GR-MD-02, is currently being studied in patients with liver fibrosis (NCT02462967) and also in combination with the immune modulator and CTLA-4 inhibitor, ipilumumab, in metastatic melanoma (NCT02117362). In a syngeneic mouse model of non-small cell lung cancer (NSCLC), the orally active and well-tolerated Gal-3 inhibitor, GB1107, reduced human and mouse lung adenocarcinoma growth and metastasis, increased CTL tumor infiltration, and potentiated the effects of PD-L1 inhibition [22]. While data on such combination therapies, including Gal-3 antagonists, are currently limited, identifying a treatment augmenting target for HNSCC with well-tolerated inhibition may allow for reduction of the more toxic components of an otherwise effective treatment regimen in CRT [23].
While Gal-3 expression in regional lymph node metastases was suggested to potentially be associated with disease progression, the data presented here do not support this notion [19]. No significant difference in Gal-3 expression was identified in primary tumors versus regional lymph node metastases. While the contributions of Gal-3 to immune escape could contribute to tumor progression, progressive Gal-3 expression was not present in the metastases. Furthermore, while clinical staging data is not available for the cohort of patients in this study, a few prior studies provided no compelling evidence for the stratification of Gal-3 expression in HNSCC by stage [18–20].
In conclusion, Gal-3 positivity is observed in a subset of HNSCC, suggesting a potential role for therapeutic Gal-3 inhibition. The significantly higher rates of expression seen in HR-HPV+ versus HPV− HNSCC suggest particular promise in the setting of HPV infection. The relatively consistent Gal-3 expression rates observed between metastases and primaries argue against progressive Gal-3 expression in metastasis.
Acknowledgments
The authors would like to acknowledge and thank the University of Virginia Biorepository & Tissue Research Facility for their skill and expertise in the construction of the tissue microarray and performance of the in situ hybridization and immunohistochemical assays used in this study.
Funding
No funding obtained.
Compliance with Ethical Standards
Conflict of interest
No conflict of interest to disclose.
Ethical Approval
This work was approved by the Institutional Review Board of the University of Virginia (IRB #13310).
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Leemans CR, Braakhuis BJM, Brakenhoff RH. The molecular biology of head and neck cancer. Nat Rev Cancer. 2011;11:9–22. doi: 10.1038/nrc2982. [DOI] [PubMed] [Google Scholar]
- 2.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2015;66:7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- 3.Marur S, Forastiere AA. Head and neck squamous cell carcinoma: update on epidemiology, diagnosis, and treatment. Mayo Clin Proc. 2016;91:386–396. doi: 10.1016/j.mayocp.2015.12.017. [DOI] [PubMed] [Google Scholar]
- 4.Lee DW, Anderson ME, Wu S, Lee JH. Development of an adenoviral vaccine against E6 and E7 oncoproteins to prevent growth of human papillomavirus-positive cancer. Arch Otolaryngol Head Neck Surg. 2008;134:1316–1323. doi: 10.1001/archoto.2008.507. [DOI] [PubMed] [Google Scholar]
- 5.Gooi Z, Chan JYK, Fakhry C. The epidemiology of the human papillomavirus related to oropharyngeal head and neck cancer. Laryngoscope. 2016;126:894–900. doi: 10.1002/lary.25767. [DOI] [PubMed] [Google Scholar]
- 6.Marur S, D’Souza G, Westra WH, Forastiere A. HPV-associated head and neck cancer: a virus-related cancer epidemic. Lancet Oncol. 2010;11:781–789. doi: 10.1016/S1470-2045(10)70017-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Koch WM. Clinical features of HPV-related head and neck squamous cell carcinoma: presentation and work-up. Otolaryngol Clin North Am. 2012;45:779–793. doi: 10.1016/j.otc.2012.04.004. [DOI] [PubMed] [Google Scholar]
- 8.Marklund L, Hammarstedt L. Impact of HPV in oropharyngeal cancer. J Oncol. 2011;2011:509036. doi: 10.1155/2011/509036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.O’Rorke MA, Ellison MV, Murray LJ, Moran M, James J, Anderson LA. Human papillomavirus related head and neck cancer survival: a systematic review and meta-analysis. Oral Oncol. 2012;48:1191–1201. doi: 10.1016/j.oraloncology.2012.06.019. [DOI] [PubMed] [Google Scholar]
- 10.Ruzevick J, Olivi A, Westra WH. Metastatic squamous cell carcinoma to the brain: an unrecognized pattern of distant spread in patients with HPV-related head and neck cancer. J Neurooncol. 2013;112:449–454. doi: 10.1007/s11060-013-1075-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Spanos WC, Nowicki P, Lee DW, Hoover A, Hostager B, Gupta A, et al. Immune response during therapy with cisplatin or radiation for human papillomavirus-related head and neck cancer. Arch Otolaryngol Head Neck Surg. 2009;135:1137–1146. doi: 10.1001/archoto.2009.159. [DOI] [PubMed] [Google Scholar]
- 12.Williams R, Lee DW, Elzey BD, Anderson ME, Hostager BS, Lee JH. Preclinical models of HPV+ and HPV− HNSCC in mice: an immune clearance of HPV+ HNSCC. Head Neck. 2009;31(7):911–918. doi: 10.1002/hed.21040. [DOI] [PubMed] [Google Scholar]
- 13.Krupar R, Robold K, Gaag D, Spanier G, Kreutz M, Renner K, et al. Immunologic and metabolic characteristics of HPV-negative and HPV-positive head and neck squamous cell carcinomas are strikingly different. Virchows Arch. 2014;465:299–312. doi: 10.1007/s00428-014-1630-6. [DOI] [PubMed] [Google Scholar]
- 14.Allen CT, Clavijo PE, Van Waes C, Chen Z. Anti-tumor immunity in head and neck cancer: understanding the evidence, how tumors escape and immunotherapeutic approaches. Cancers (Basel) 2015;7:2397–2414. doi: 10.3390/cancers7040900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Farhad M, Rolig AS, Redmond WL. The role of Galectin-3 in modulating tumor growth and immunosuppression within the tumor microenvironment. Oncoimmunology. 2018;7:e1434467. doi: 10.1080/2162402X.2018.1434467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kindt N, Journe F, Ghanem GE, Saussez S. Galectins and carcinogenesis: their role in head and neck carcinomas and thyroid carcinomas. Int J Mol Sci. 2017;18:2745. doi: 10.3390/ijms18122745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang LP, Chen SW, Zhuang SM, Li H, Song M. Galectin-3 accelerates the progression of oral tongue squamous cell carcinoma via a Wnt/β-catenin-dependent pathway. Pathol Oncol Res. 2013;19(3):461–474. doi: 10.1007/s12253-013-9603-7. [DOI] [PubMed] [Google Scholar]
- 18.Weber M, Büttner-Herold M, Distel L, Ries J, Moebius P, Preidl R, et al. Galectin 3 expression in primary oral squamous cell carcinomas. BMC Cancer. 2017;17:906. doi: 10.1186/s12885-017-3920-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wehrhan F, Büttner-Herold M, Distel L, Ries J, Moebius P, Preidl R, et al. Galectin 3 expression in regional lymph nodes and lymph node metastases of oral squamous cell carcinomas. BMC Cancer. 2018;18:823. doi: 10.1186/s12885-018-4726-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mesquita JA, Queiroz LMG, Silveira ÉJD, Gordon-Nunez MA, Godoy GP, Nonaka CFW, et al. Association of immunoexpression of the galectins-3 and -7 with histopathological and clinical parameters in oral squamous cell carcinoma in young patients. Eur Arch Oto-Rhino-Laryngol. 2016;273:237–243. doi: 10.1007/s00405-014-3439-y. [DOI] [PubMed] [Google Scholar]
- 21.Punt S, Thijssen VL, Vrolijk J, De Kroon CD, Gorter A, Jordanova ES. Galectin-1, -3 and -9 expression and clinical significance in squamous cervical cancer. PLoS ONE. 2015;10:e0128119. doi: 10.1371/journal.pone.0129119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vuong L, Kouverianou E, Rooney CM, McHugh BJ, Howie SEM, Gregory CD, et al. An orally active galectin-3 antagonist inhibits lung adenocarcinoma growth and augments response to PD-L1 blockade. Cancer Res Cancer Res. 2019;79:1480–1492. doi: 10.1158/0008-5472.CAN-18-2244. [DOI] [PubMed] [Google Scholar]
- 23.Coppock JD, Lee JH. mTOR, metabolism, and the immune response in HPV-positive head and neck squamous cell cancer. World J Otorhinolaryngol Neck Surg. 2016;2:76–83. doi: 10.1016/j.wjorl.2016.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]