Abstract
The first detailed description of calcifying epithelial odontogenic tumor (CEOT) are ascribed to Jens Pindborg, but this tumor was described some years previously. Subsequently, CEOT was included in the 1971 WHO classification of odontogenic tumors and a since then number of variants have been described, which have added confusion to the diagnostic criteria. We aimed to survey the literature on the variants of CEOT, in parallel with a review of our single institution experience of CEOTs. Cases identified were collated, including available clinical, radiological and histological information and then reviewed, taking into account changes in the understanding and classifications of odontogenic tumors since initial diagnosis. We identified 26 cases from 1975 to 2017 for which histological material was available. Of these, only 13 (50%) showed the “classic” histological appearance, whilst two cases were identified as recognized variants. In 11 cases, other diagnoses or a differential diagnosis were preferred, with no agreed diagnosis in four of these. The proliferation fraction (Ki67) in the 10 cases tested was 2.1% ± 0.18. These findings illustrate the diagnostic challenges in this group of tumors and highlight the gaps in knowledge. Techniques, such as EWSR1 gene cytogenetic analysis, may be helpful in cases with clear cells. However, in other areas of controversy, including the non-calcifying and Langerhans cell rich variants, further investigation, perhaps utilizing sequencing technologies may be needed to refine the classification. Owing to the relative rarity of these lesions it would be beneficial if future work could be pursued as an international collaboration.
Keywords: Odontogenic, Tumor, CEOT, Calcifying epithelial odontogenic tumor, Clear cell, Amyloid, EWSR1
Introduction and Review of the Literature
Jens Pindborg described the calcifying epithelial odontogenic tumor (CEOT), a rare epithelial odontogenic tumor, in detail in 1958 [1]. Many authorities suggest, however, that the first description was by Thoma and Goldman ten years previously, who termed it adenoid-type adamantoblastoma [2], although earlier descriptions do exist [3]. Various synonyms have been used to describe this lesion, such as adamantoblastoma [4], ameloblastoma of unusual type with calcification [5], malignant odontoma [6], and cystic complex odontoma [7]. In 1963, the term ‘Pindborg tumor’ was first used by Shafer and this is a well-recognized eponym for this neoplasm [8]. Twenty years after the original CEOT description, Pindborg and Franklin reviewed 113 cases reported in the literature [9].
Since the original descriptions, the number of cases has continued to increase and, to date, more than 362 cases have been reported [10]. According to this recent review of published cases, there was an almost equal distribution among males and females and the peak age of occurrence of central lesions was in the 3rd and 4th decades, similar to that presented in our recent series of odontogenic tumors [11]. The majority occurred in the body of the mandible, but some were large lesions, extending widely antero-posteriorly and involving the ramus [10, 11]. Most presentations are intraosseous but in 1966, Pindborg described an extra-osseous/peripheral CEOT [12].
Radiologically, CEOTs vary from small, unilocular radiolucent lesions to extensive multilocular, mixed radio-dense lesions often associated with an impacted tooth (in 61% of central cases [10]). Some authors have considered the presence of radio-opaque flecks in the pericoronal tissues of an impacted tooth (as originally described by Pindborg) as characteristic for CEOT [13]. Half of the central lesions show evidence of cortical bone perforation whilst 40% of peripheral CEOTs have subjacent bone erosion [10]. On Computed tomography (CT) scans, there is diffuse high attenuation, suggesting calcification and/or ossification. On magnetic resonance imaging (MRI), CEOT is a hypointense tumor on T1-weighted images and a mixed hyper intense tumor on T2-weighted images [14]. CT scans and 3D reconstructions may be useful in delineating the extent of the lesion, which is essential for surgical treatment planning [15]. Whilst CEOT is considered a benign epithelial neoplasm, evidence of clinically aggressive behavior, malignant transformation with multiple recurrences and cases with metastasis have been reported [10, 16].
The histological hallmarks of the “classic” CEOT are sheets of polyhedral epithelial cells with distinct cell borders, prominent intercellular bridges, nuclear pleomorphism, and few mitoses (Fig. 1) [1, 9, 12]. Also common are concentric calcifications (Liesegang rings) and the presence of deposits of amorphous ‘amyloid-like’ eosinophilic material which stains with Congo Red (Fig. 2) and demonstrates apple-green birefringence on polarization. This material is largely PAS negative prior to calcification [9].
Fig. 1.
Photomicrograph illustrating the histological features described the original publication by Pindborg [1]
Fig. 2.
Photomicrograph of the characteristic appearance of CEOT amyloid, as stained by Congo Red (a)
It has been suggested that CEOTs originate from remnants of the dental lamina [17] or stratum intermedium [18]. Two cell types have been demonstrated by electron microscopy: polyhedral epithelial cells and myoepithelial-like cells containing electron-dense tonofilament bundles, electron-dense bodies, and fine lamina dense filaments [19]. Immunohistochemically, the polyhedral cells of CEOT express laminins 1 and 5, cytokeratins, fibronectin and vimentin [20]. High levels of alkaline phosphatase and ATPase localization to the cell membrane are significant findings [21]. The amyloid material has been shown to contain a number of ameloblast associated proteins, most consistently Odontogenic Ameloblast-Associated Protein (ODAM) [22].
Apart from the classic features, a number of CEOT variants have been reported, with various proportions of clear cells, Langerhans cells and some cases without calcification. Furthermore, hybrid tumors with adenomatoid odontogenic tumor or ameloblastoma [10, 23, 24], and cystic/microcystic variants have been reported [25, 26]. Ai-Ru et al. proposed a sub-classification comprising four histological patterns, indicating that some tumors might show a cribriform appearance without clear cell borders; others may contain multinucleated giant cells or cells with abundant eosinophilic cytoplasm or clear/vacuolated cells with centrally placed nuclei [27]. However, this sub-classification was based on only nine cases and has not been widely adopted or otherwise assessed in a larger study population.
In this case series, we aimed to review all of our diagnoses of CEOT in the diagnostic archive (either definitive or in differential diagnosis) and review them in light of the three WHO classifications published during this time (1991, 2005 and 2017) and the current literature on this entity.
Materials and Methods
The diagnostic database of the department of Oral and Maxillofacial Pathology, Charles Clifford Dental Hospital/School of Clinical Dentistry, University of Sheffield, was searched for cases either with the diagnostic code of CEOT (as a definitive diagnosis) or by keyword search where CEOT was raised as a differential diagnosis in more challenging cases from 1975 to 2017. Clinical information including age, gender and location of the tumor were recorded, and plain film radiology was reviewed where available. Very limited clinical follow-up data was available, and none of the cases for which this was available recurred.
Given the passage of time since the original diagnoses in the series (a span of 42 years: and three intervening WHO classifications), the original slides were re-evaluated using contemporary diagnostic criteria, with attention to the 2017 WHO classification of odontogenic lesions [28]. Hematoxylin and Eosin and Congo Red stained sections of the selected cases from the database were re-evaluated by 3 experienced OMF Pathologists (PMS, KDH and SAK), and consensus diagnoses recorded. Cases with multiple biopsies (incisional and resection) were considered as single cases.
Immunohistochemical analysis of the expression of Ki67 (Rabbit polyclonal Abcam ab16667 at 1:50; to assess the proliferation fraction) and Amelogenin/AMELX (Rabbit monoclonal, Abcam ab129418 at 1:150; to assess ameloblastic differentiation) was conducted on 10 and 8 cases respectively, where sufficient formalin fixed paraffin embedded (FFPE) material remained. Slides were dewaxed and rehydrated before quenching of endogenous peroxidase using H2O2. Heat-induced epitope retrieval in 0.01 M sodium citrate was undertaken before blocking with normal serum. After primary antibody incubation, biotinylated secondary antibodies were used and specific staining demonstrated using the Vector Nova Red kit (Vector Laboratories Inc, Burlingame, CA, USA). Ki67 was assessed as % of cells positive and AMELX expression was assessed using a modified quickscore method [29], with a maximum possible score of 24.
Results
Thirty two cases had been coded as CEOT in the diagnostic database from 1975 to 2017. Histological slides (H&E and Congo Red) were available for 26 cases (Table 1). In one additional case, whilst a differential diagnosis of CEOT was suggested in the incisional biopsy, the resection showed an unequivocally malignant odontogenic tumor. This case was excluded. A variety of other histochemical (largely PAS) and immunohistochemical stains were available in some cases, conducted as part of the original diagnostic work-up. Of the 26 cases, 18 were referral/consult cases, so the FFPE blocks were not available for further analysis. In 15 cases, a definitive diagnosis of CEOT had been made, whilst in the remaining 11, it was part of a differential diagnosis.
Table 1.
Demographic and histological data of the cohort of 26 CEOTs
| Case no | Year of Dx | Age | Sex | Site | Central/peripheral |
|---|---|---|---|---|---|
| 1 | 1975 | 32 | Male | Not known | Central |
| 2 | 1978 | 38 | Female | Not known | Central |
| 3 | 1980 | 50 | Female | Not known | Peripheral |
| 4 | 1982 | 38 | Male | Mid Mandible | Central |
| 5 | 1988 | 25 | Male | Mid to post mandible | Peripheral |
| 6 | 1992 | 23 | Male | Ant to mid maxilla | Peripheral |
| 7 | 1993 | 39 | Female | Mid to post mandible | Central |
| 8 | 1993 | 31 | Female | Ant to mid mandible | Central |
| 9 | 1997 | 44 | Male | Mid to post mandible | Central |
| 10 | 1998 | 52 | Male | Mid mandible | Central |
| 11 | 1999 | 49 | Female | Mid maxilla | Central |
| 12 | 2003 | 32 | Female | Ant mandible | Peripheral |
| 13 | 2004 | 69 | Female | Mid maxilla | Central |
| 14 | 2004 | 25 | Male | Maxillary antrum | Central |
| 15 | 2007 | 48 | Female | Post mandible | Central |
| 16 | 2008 | 53 | Male | Maxillary antrum | Central |
| 17 | 2009 | 30 | Male | Post maxilla | Central |
| 18 | 2010 | 47 | Male | Mid to post mandible | Peripheral |
| 19 | 2010 | 27 | Female | Ant to mid mandible | Peripheral |
| 20 | 2011 | 46 | Male | Mid to post maxilla | Central |
| 21 | 2011 | 49 | Male | Mid to post mandible | Central |
| 22 | 2012 | 74 | Female | Ramus of mandible | Central |
| 23 | 2013 | 52 | Male | Mid Mandible | Central |
| 24 | 2015 | 32 | Female | Ant maxilla | Peripheral |
| 25 | 2015 | 55 | Female | Maxillary antrum | Central |
| 26 | 2016 | 34 | Female | Maxillary antrum | Peripheral |
Dx diagnosis
The age range was 23–74 years with a mean age of 42 ± 2.6 (Table 1). There was an equal gender distribution. 62% occurred in the mandible and, of the mandibular tumors, the majority were in the posterior mandible (54%). Of those in the maxilla, 3/10 (30%) involved the maxillary sinus. The majority of CEOTs were intraosseous (18/26; 69%), whilst 8 were peripheral lesions (31%). Association with unerupted teeth was not consistently recorded.
Histologically, a variety of appearances were seen and many cases met the criteria for diagnosis originally described by Pindborg (13/26; 50%), but a number of other histological appearances were also observed. Clear cell clusters (of varying extent) were observed in 46% (12/26), more commonly in peripheral tumors (6/8; 75%). Out of the total sample, 10 cases had no identifiable calcifications (Table 2). Three of the cases (7, 24 and 26) contained dentin-like material (dentinoid).
Table 2.
Histological features of the cohort of 26 CEOTs
| Case no | Epithelium description | Distinct cellular outline | Prominent intercellular bridges | Eosinophilic cytoplasm | Nuclear/cellular pleomorphism | Mitotic figures | Calcifications/ Liesegang rings | Amyloid | Clear cells | Original diagnosis | Review consensus diagnosis | IHC |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Nests | Y | Y | Y | Y | N | Y | Y | N | Typical CEOT | CEOT | |
| 2 | Nests | Y | N | Y | Y | N | N | Y | N | Typical CEOT | CEOT | ki67 6% |
| 3 | Small nests and thin strands | Y | N | Y | Y | N | N | Y | Y (focal) | Unusual, maybe CEOT or OD hamartoma | CEOT vs OdF | |
| 4 | Nests | Y | Y | Y | Y | N | Y | Y | N | Typical CEOT | CEOT | |
| 5 | Small nests and thin strands | N | N | Y | Y | N | Y (focal) | N | Y (focal) | Unusual, CEOT (preferred) vs OdF | CEOT vs CCOC | ki67 5% |
| 6 | Small nests and thin strands | Y | N | Y | Y | N | N | Y | Y (focal) | CEOT | CEOT vs OdF | |
| 7 | Sheets and thin strands | Y | N | Y | Y | N | Y* | N | Y | CEOT, clear cell variant | No consensus | ki67 < 1% |
| 8 | Sheets and small nests | Y | Y | Y | Y | N | Y | Y | N | Typical CEOT | CEOT | ki67 2% |
| 9 | Sheets and thin strands | N | N | Y | Y | N | N | Y | N | Unusual OT, CEOT vs OF | No consensus | |
| 10 | Sheets and thin strands | Y | N | Y | Y | N | Y | Y | Y (focal) | CEOT | CEOT, clear cell variant | ki67 < 1% |
| 11 | Small nests and thin strands | Y | N | Y | Y | N | N | Y | N | CEOT | CEOT vs OdF/SOC | ki67 2% |
| 12 | Small nests and thin strands | Y | Y | N | Y | N | Y | Y | Y | CEOT | CEOT | |
| 13 | Small nests | Y | N | Y | Y | N |
Y (min) |
Y | Y | CEOT | CEOT | ki67 < 1% |
| 14 | Small nests | Y | Y | Y | Y | N | Y | Y | Y (focal) | CEOT, maybe arising from dentigerous cyst | CEOT | ki67 1% |
| 15 | Small nests | Y | Y | Y | Y | N | Y | Y | N | Typical CEOT | CEOT | |
| 16 | Sheets and thin strands | Y | N | Y | Y | N | N | N | Y | Unusual, maybe CEOT variant | Ameloblastoma with clear cells | |
| 17 | Small nests and thin strands | Y | Few | ? | Y | N | N | N | N | Unusual, perhaps non-calcifying CEOT | No consensus | |
| 18 | Small nests and thin strands | Y | In some areas | Y | Y | N | Y | Equiv | Y (most) | Clear cell CEOT | CEOT, clear cell variant | |
| 19 | Small nests and thin strands | Y | Y | Y | Y | N | N | Y | N | CEOT | CEOT | |
| 20 | Small nests |
Y (few) |
N | Y | Y | N |
Y (few) |
Y | Y (focal) | CEOT | CEOT vs OdF | |
| 21 | Sheets | Y | N (few) | Y | Y | N | Y | Y | Y | Unusual, perhaps CEOT | No consensus | ki67 < 1% |
| 22 | Sheets | Y | N (few) | Y | Y | N | Y | Y | N | CEOT | CEOT | |
| 23 | Small nests and thin strands | Y | N | Y | Y | N | N | Y | N | CEOT | CEOT | |
| 24 | Sheets and thin strands | N | N | Y | Y | N |
Y* (few) |
Equiv | n | CEOT | CEOT vs OC with dentinoid | |
| 25 | Small nests and thin strands | Y | N | Y | Y | N | Y | Y | N | CEOT | CEOT | |
| 26 | Small nests and thin strands | Y | N | Y | Y | N | Y* | Y | Y (most) | OT, perhaps CEOT | CEOT, Clear Cell variant vs OC with dentinoid | Ki67 < 1% |
OT odontogenic tumor, OdF odontogenic fibroma, OC odontogenic carcinoma, CCOC clear cell odontogenic carcinoma, SOC sclerosing odontogenic carcinoma, equiv equivocal
*Calcifications assessed as “dentinoid”
The relationship of the review diagnoses to the original diagnoses is presented in Table 2. Of the 26 cases, 14 were confirmed as CEOT (12 “classic” CEOT, and 2 of the clear cell variant of CEOT). In 6 cases, CEOT was part of a differential diagnosis, which variably included central odontogenic fibroma, clear cell odontogenic carcinoma (CCOC), sclerosing odontogenic carcinoma and odontogenic carcinoma with dentinoid. In two cases, other diagnoses were favored (one clear cell odontogenic carcinoma, and one ameloblastoma with clear cells), and four were odontogenic tumors which were difficult to classify with no consensus achieved.
Immunohistochemistry for Ki67 expression was available for 10 of the cases with a mean of 2.1% of positive cells (SEM = 0.18; range 1–6%; Fig. 3a). This reinforces the concept that despite frequent nuclear and pleomorphism, the proliferation rate is low. There was no discernible pattern of ki67 expression with regard to histological subtype, nor in those cases where a malignant diagnosis was considered. The lowest (1%) and highest (6%) Ki67 expression were both found in “classic” subtypes. AMELX (amelogenin) was expressed in the epithelium in all 8 cases tested, with the histoscore varying between 5 and 18 (Fig. 3b), indicating that this may be of use, similar to ODAM, in demonstrating ameloblastic differentiation in the epithelial cells.
Fig. 3.
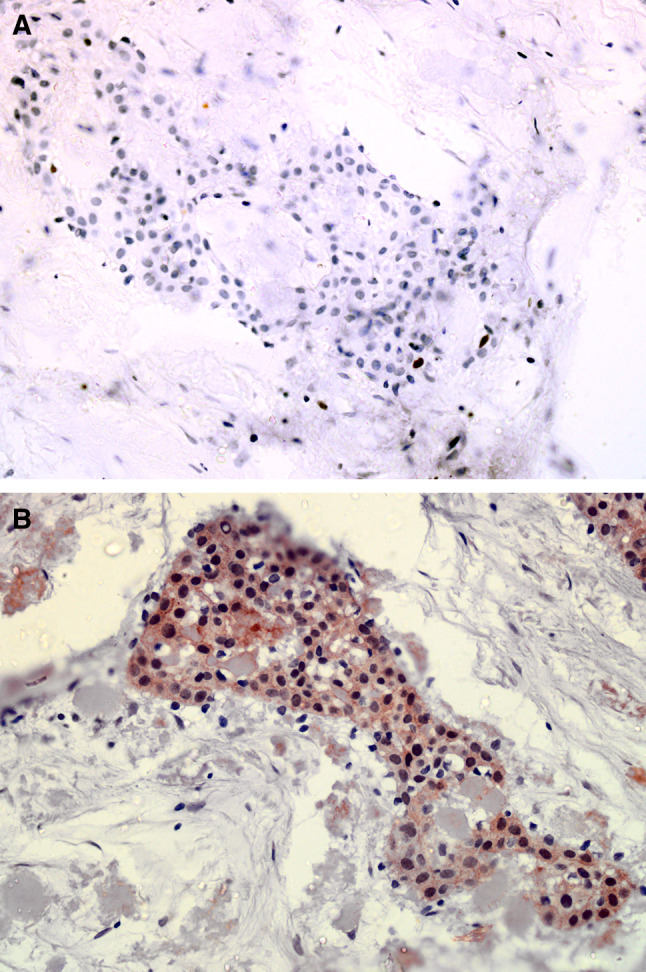
Photomicrograph of Ki67 (a) and AMELX expression (b) in a selected CEOT case from the cohort
Discussion
A summary of the main histological variants of CEOT, which have been described in the literature, is presented in Table 3 and a summary of the histochemical and immunohistochemical staining characteristics of these different cell types is presented in Table 4. In addition to these main variants, others, such as melanin-containing lesions have also been described [24, 30]. The reported variation in clinical outcomes may represent a spectrum of biological behavior in CEOT, but conversely may merely represent a group of heterogeneous entities which have, for various reasons discussed below, been classified together as “variants” of CEOT, which are briefly reviewed below.
Table 3.
A summary of reported cases and case series of variants of CEOT
| Authors | Age/sex | Location | Radiographic features | Histopathological findings | C/P |
|---|---|---|---|---|---|
| CEOT cystic variant | |||||
| Gopalakrishnan et al. [26] | 15M | Left posterior maxilla | Unilocular radiolucency with radiopacities | Cyst lining varying from NKSSE to thickened epithelium with characteristics of CEOT | C |
| Channappa et al. [48] | 30M | Left posterior maxilla | Unilocular radiolucency with calcifications in association with impacted tooth #13 | Cyst lined by odontogenic epithelium, majority with uniform thickness, with classic features of CEOT | C |
| Urias Barreras et al. [49] | 31M | Left Posterior mandible | Unilocular radiopaque/lucent area | Lining of odontogenic epithelium with necrosis, featuring clear cells (PASD positive and osteodentin | C |
| Dantas et al. [50] | 22M | Right posterior mandible | Unilocular, mixed radiodensity lesion, root resorption | Microcystic lined by typical CEOT with abundant clear cells | C |
| Sánchez-Romero et al. [25] | 42F | Right posterior mandible | Well-defined mixed radiodense lesion in relation to an un-erupted third molar | Microcystic compartments of varying size and occasional clear cells with classic features of CEOT | C |
| CEOT clear cell variant | |||||
| Abrams and Howell [31] | 50M | Posterior mandible | Unilocular mixed radiodense/radiolucent | Prominent clear cells with classic features of CEOT | C |
| Anderson et al. [37] | 68F | Left mandible molar area | Unilocular radiolucent/radiopacity | Prominent clear cells with classic features of CEOT | C |
| Oikarinen et al. [59] | 36F | Mandible Left molar to right premolar | Multiloculated radiolucent with radiopaque central region | Prominent clear cells with classic features of CEOT. Amyloid diagnosed under electron microscopy | C |
| Yamaguchi et al. [60] | 36M | Right mandible from anterior to premolar region | Unilocular radiolucency | Prominent clear cells with classic features of CEOT. PAS positive granules in clear cells | C |
| Ai-Ru et al. [27] | 64F | Anterior mandible | Not recorded | Prominent clear cells with classic features of CEOT | C |
| Asano et al. [43] | 44F | Right maxilla | Unilocular, radiolucent area with root resorption | Islands that frequently contained clear cells with typical features of CEOT | C |
| Schmidt-Westhausen et al. [36] | 38M | Right premolar to left incisor region in mandible | Radiolucency with diffuse radiopacities in part of the lesion | Central necrosis of large epithelial islands and clusters of clear cells | C |
| Hicks et al. [61] | 59F | Right posterior mandible | Unilocular mixed radiolucency and radiopacity | Prominent clear cells with classic features of CEOT | C |
| Kumamoto et al. [62] | 14F | Right maxillary 3rd molar region | Unilocular radiolucency impacted upper right 3rd molar | Prominent clear cells, few mitotic figures and typical features of CEOT | C |
| Anavi et al. [33] | 27M | Left mandibular canine and first premolar | Unilocular well-circumscribed radiolucency | Sheets of clear cells, amyloid and few small oval calcifications | C |
| Germanier et al. [63] | 44F | Right angle of the mandible enclosing the 3rd molar | Multiloculated radiolucency with calcifications | Clear cells in some places and with typical CEOT | C |
| Mohtasham et al. [64] | 18M | Right anterior maxilla | Radiolucency with calcification | Scattered clear cells with typical features of CEOT | C |
| Rangel et al. [32] | 65M | Right mandible between lateral incisor and canine | Unilocular radiolucency with radio-opacities | Significant portion of cells are clear and other areas with typical features of CEOT | C |
| Sahni et al. [65] | 52M | Right maxilla | Mixed radiodensity lesion | Areas of clear cells within epithelial islands and with typical features of CEOT | C |
| Chen et al. [66] | 59F | Posterior mandible/ ramus | Unilocular radiolucency | Nests of clear cells in a pseudoglandular pattern. Other areas with typical features of CEOT | C |
| Turatti et al [67] | 25F | Left mandible | Unilocular radiolucency with root displacement | Sheets and nests of clear cells with areas of calcifications and amyloid | C |
| Rydin et al. [68] | 40F | Left mandible | Unilocular radiolucency with scattered calcifications | Central portion of the tumor composed of clear cells and periphery with typical CEOT | C |
| Chatterjee et al. [69] | 73F | Left maxillary molar region | A large mixed radiodense/RL area spearing maxillary antrum | Typical CEOT with Clear cells. PAS positive | C |
| Sabir et al. [70] | 63F | Angle of the mandible | Radiolucent lesion in ramus distal to 3rd molar | Almost all islands are clear cells amyloid in between | C |
| Júnior et al. [71] | 42M | Mandibular symphysial region | Unilocular radiolucency with patchy radio density | Most clusters with clear cells and abundant small calcifications and amyloid | C |
| Wertheimer et al. [35] | 20M | Right maxillary gingiva | Premolar region cup-shaped area | Typical areas of CEOT with some areas with clear cells | P |
| Ai-Ru et al. [27] |
32F 47F |
mandibular gingiva | No signs of bone involvement | Typical areas of CEOT with some areas with clusters of clear cells | P |
| Houston and Fowler [72] | 27M | Gingiva of right posterior mandible | Underlying bone was normal | Prominent clear cells with classic features of CEOT | P |
| Orsini et al. [73] | 32M | Maxillary gingiva | NA | Typical areas of CEOT with some areas with clusters of clear cells | P |
| Mesquita et al. [74] | 48F | Right maxilla, canine region | NA | Polyhedral and clear epithelial cells associated with amyloid-like deposition | P |
| Anavi et al. [33] | 27M | Left mandible | Alveolar crest resorption | Sheets of clear cells, focal mild atypia with amyloid in between cells and clusters | P |
| de Oliveira et al. [75] | 43F |
Lesion 1: Left mandible Lesion 2: Left maxilla |
Superficial cupping in canine area | some clusters are composed with clear cells with typical features of CEOT | P |
| Habibi et al. [76] | 70F | Left maxilla | Normal underlying alveolar bone | Typical areas of CEOT with some areas with clusters of clear cells | P |
| Gadodia et al. [77] | 18M | Left mandible | Alveolar crest resorption | Scattered clear cells with classic features of CEOT | P |
| CEOT Non-calcified with Langerhans cells | |||||
| Asano et al. [43] | 44F | Right maxilla | Unilocular radiolucency | Less cellular, clear cells within polyhedral cell clusters. Birbeck granules seen. No calcification | C |
| Takata et al. [44] | 58M | Left maxillary canine premolar region | Unilocular radiolucency | Scattered small islands of epithelial cells. Within islands many spherical bodies seen. Amyloid present. S-100 positive. Birbeck granules identified | C |
| Wang et al. [78] | 52F | Right maxilla, central incisor canine region | Unilocular radiolucency | Small nests of polyhedral cells and amyloid deposition. Clear cells present. CD1a positive cells are frequent. No calcification | C |
| Wang et al. [79] |
38M 39F |
Right mandible Left maxilla |
Unilocular radiolucency with patchy radiopacities | Small nests and cords of epithelial cells. Few clear cells. Amyloid present. CD1a + , Birbeck granules identified. No calcification |
C P |
| Afroz et al. [40] | 20F | Right maxilla, lateral incisor area | Normal underlying alveolar bone | Scattered small islands of polygonal cells and occasional clear cells. Amyloid present. No calcifications. Clear cells confirmed as Langerhans cells (S100) | P |
| Chen et al. [45] |
40F 58M |
Maxilla |
Unilocular radiolucency with root resorption Multilocular radiolucency with root resorption |
Small nests and cords of epithelial islands with some clear cells. Amyloid present, CD1a + , langerin + , No calcification | Both C |
| Tseng et al. [80] | 24M | Left maxilla, canine premolar area | Unilocular radiolucency with root resorption in canine and premolar | Strands and island of epithelial cells and some clear cells. Scant amyloid, CD1a + , No calcification | C |
| Santosh et al. [81] | 44M | Left anterior maxilla | Large unilocular radiolucency | Bland epithelial islands with admixed amyloid. CD1a + cells. No calcification was present | C |
| Combined epithelial odontogenic tumor. CEOT /AOT | |||||
| Damm et al. [18] |
18M 15F |
Mandible | Unilocular predominantly radiolucent, one case with radiopacities | A cystic tumor lined with areas of typical AOT. And some CEOT-like areas | C |
| Bingham et al. [82] | 14F | Right mandible | Unilocular radiolucent lesion related to impacted first premolar tooth | Cystic tumor with multiple intraluminal nodules. Some typical AOT and others are CEOT. Amyloid positive. Calcifications noted | C |
| Takeda and Kudo [83] | 17F | Right maxilla between incisors | Unilocular radiolucent lesion with flakes of radio densities | Encapsulated solid tumor with areas of typical AOT and CEOT. Amyloid positive | C |
| Siar and Ng [51] |
13–28 2M, 3F |
3 in maxilla, 2 in mandible | Radiolucent lesion | Thick walled cystic tumor lined with areas of typical AOT and variable amounts of CEOT-like areas | All C |
| Ledesma et al. [84] |
10–21 10F, 2M |
9 in maxilla (most canine region), 2 mandible | Radiolucent lesion most related to impacted canine tooth. Some have radiopacities | Typical AOT areas with CEOT-like areas of variable sizes |
11 C 1 P |
| Miyake et al. [85] | 16F | Left maxilla, canine region | Radiolucent lesion related to impacted canine tooth | Encapsulated solid tumor composed with areas of typical AOT and CEOT. Amyloid positive | C |
| Rosa et al. [86] | 17 | Anterior mandible | Unilocular radiolucent lesion with radio-opacity centrally | A cystic tumor with solid mural nodules with typical AOT and CEOT areas. Amyloid positive | C |
C Central, P Peripheral
Table 4.
Histochemical and immunohistochemical stains in CEOT
| Epithelial cells | Amyloid- like material | Calcification | Clear cells | Langerhans cell | Stromal cells | |
|---|---|---|---|---|---|---|
| Histochemical stains | ||||||
| Congo red | ✔ | |||||
| Thioflavin T | ✔ | |||||
| PAS | ✔a | |||||
| Tryptophan | ✔ | |||||
| IHC stains | ||||||
| Pan-cytokeratin | ✔ | ✔ | ||||
| Cytokeratin cocktail | ✔ | |||||
| EGFR | ✔ | |||||
| p63 | ✔ | ✔ | ||||
| CK7 | ✔ | ✔ | ||||
| CK14 | ✔ | ✔ | ||||
| CK8 | ✔ | ✔ | ||||
| CK13 | ✔ | ✔ | ||||
| CK19 | ✔ | ✔ | ||||
| Vimentin | ✔ | |||||
| Ameloblast-associated protein | ✔ | ✔ | ✔ | |||
| Amelotin | ✔ | |||||
| Ameloblastin | ✔ | |||||
| Amelogenin | ✔ | ✔ | ||||
| S100 protein | ✔ | |||||
| CD1a | ✔ | |||||
| Langerin | ✔ | |||||
| Enamelin | ✔ | |||||
|
Syndecan-1 (CD138) |
✔ | ✔ | ✔ | |||
| E-Cadherin | ✔ | |||||
| Amyloid A | ✔ | ✔ | ✔ | |||
Clear Cell Variant
In 1967, Abrams and Howell described the first case of a CEOT with a clear cell component [31]. Many case reports and series have followed, some of which are summarized in Table 3. Most of the clear cell CEOTs are intraosseous lesions and are most commonly found in the mandible [10]. The mean age is 44 years, which is 8 years older than for conventional CEOT. Unlike conventional CEOT, there is a female predilection and an association with unerupted teeth was found in only six out of the 24 patients, compared with nearly 50% of the conventional CEOTs. It has been suggested that clear cell CEOTs are clinically more aggressive as they tend to perforate the cortex and recur more frequently than other CEOT variants [32–34].
In almost all the reported cases, there were areas with histological features of conventional CEOT including polyhedral sheets of epithelial cells with prominent intercellular bridges, amyloid-like material and calcifications. The clear cells contain PAS positive material which is diastase labile, consistent with glycogen, and does not stain with Alcian Blue [35]. This finding is consistent with suggestions that the clear cells form by epithelial cell degradation [36, 37]. Although the presence of typical areas of conventional CEOT, with minor cellular atypia and absence of mitoses helps in diagnosis, special stains and cytogenetics may be helpful in arriving at a final diagnosis. CEOTs with prominent clear cells must be diagnosed with caution, as many clear cell neoplasms are malignant and further investigations are needed to exclude clear cell malignancies such as CCOC and other carcinomas with a clear cell component (for example, of renal or salivary origin) [38]. It is unclear to what extent difficulties in distinguishing clear cell CEOTs from CCOC has contributed to the reported apparent increased aggressiveness of clear cell CEOT.
Non-Calcified and Langerhans Cell-Rich Variants of CEOT
The non-calcified variant of CEOT is the least reported variant (Table 3). To date, eight intraosseous cases and two extraosseous cases of non-calcified CEOT have been reported [39, 40]. The absence of calcification in CEOT may be due to the relative immaturity of the lesion, as long-standing tumors tend to have more calcifications than young, underdeveloped ones [41]. In a study of 19 patients with CEOT by Azevedo et al., the age of patients at the time of diagnosis was linked to the amount of calcification; older patients showing more calcifications [42]. This variant of CEOT usually appears as a radiolucent area on radiographs that may be misdiagnosed as an odontogenic cyst.
Many of these cases contain Langerhans cells (LC), which are antigen-presenting immune cells that are normally found in oral epithelium but have also been described in conventional CEOT in small numbers. If abundant, LC-rich lesions are considered a variant of CEOT [43, 44]. They appear histologically as clear cells, which contain Birbeck granules, within the tumor’s conventional pattern of polyhedral sheets of epithelial cells and amyloid-like material. Five of the cases reported so far were without associated calcification, all of whom presented in patients of Asian origin [45]. However, a Langerhans cell–rich case with calcification has been reported in one black individual [46], challenging the concept that ‘all CEOTs with a Langerhans cell component are non-calcified variants’. Diagnosis of this variant is based on either electron microscopic examination of the LC structure or positive staining of LCs for S100 and CD1a [46]. The natural history of this variant is not well described.
Histological examination was important in all of the reported cases of non-calcified CEOT, in order to evaluate the presence of the classic features of epithelial sheets and amyloid-like material. In one reported case there was a “poorly differentiated non-calcified CEOT” [41]. Others contained Langerhans cells. Takata et al. reported a case with a histologic appearance consistent with “pattern four” in the Ai-Ru subtypes of conventional CEOT [44]. It was suggested by Kaushal et al. that the non-calcified variant of CEOT behaves more aggressively than calcified CEOTs [39]. However, this contrasted with suggestions made in previous studies that most non-calcified CEOTs contain Langerhans cells, which may indicate a less aggressive lesion. More research in non-calcified CEOT cases with and without LCs is required to address this issue. There has been recent discussion regarding the nature of these non-calcifying, Langerhans cell-rich lesions [47]. This issue will be explored further later.
Cystic/Microcystic Variant
Recently, a number of reports of cystic and microcystic variants of CEOT have been published. The initial report was of a large cystic lesion in a 15 year-old male, in which the lining demonstrated CEOT features [26]. The lesion was enucleated. A number of similar cases have been reported [48–50], and subsequently, a microcystic variant has also been described [25]. In this lesion, a pseudo-glandular appearance was reported in association with otherwise rather conventional CEOT histology. The natural history of these lesions is not known, but there have been no reports of recurrences so far.
Combined CEOT-Adenomatoid Odontogenic Tumor
Although it is not a variant of CEOT, Adenomatoid odontogenic tumor (AOT) is worth mentioning in this context, as some contain CEOT-like areas. AOT is a separate odontogenic tumor with its own distinctive histological features. In 1983 Damm et al. reported an AOT that contained CEOT-like features and named it ‘combined epithelial odontogenic tumor’ [18]. Philipsen and Reichart reported 24 AOTs with some areas of CEOT-like components [23]. None of these combined AOTs /CEOT were dominated by CEOT-like areas. According to Ng and Siar, the behavior of these forms of AOT was no different from that of the conventional AOT and suggested they were benign hamartomas without any evidence of CEOT-like aggressive behavior, and none recurred [51]. Thus, combined CEOT-AOTs should be managed as conventional AOTs.
The designation of these cases as variants of CEOT has resulted in a dramatic widening of the histological spectrum of appearances that fall under the diagnostic umbrella of CEOT, far beyond the original histological description [1]. Furthermore, there are some odontogenic tumors that do not fit very well into the diagnostic criteria of the existing classification. This includes a number of lesions containing dentinoid and dispersed nests of tumor cells within a hyalinized stroma, which can share some histological features of CEOT. This raises an important issue as to the usefulness of tumor sub-classifications that develop incrementally, without periodic review of the variations in histological appearances in other tumors and integration of new insights from other molecular features including genomic analyses. It also raises questions regarding the usefulness of historical surveys of variants of this tumor, as, given progress in knowledge of the biology of odontogenic tumors, some variants which have been labelled as part of the CEOT family, may not be so.
In the present report, 26 sequentially accessioned cases from a single Oral and Maxillofacial Pathology Diagnostic Service from 1975 to 2017 have been analyzed. In these cases, diverse histomorphology was seen, but the index diagnosis was of a CEOT, or CEOT was included in the differential diagnosis. The whole cohort has been reviewed taking into account a number of other entities which have been described since the original diagnoses were made, particularly those in the early years of the cohort. In one case the resection specimen showed an odontogenic malignancy, with necrosis, a high mitotic rate and areas of de-differentiation. We excluded this as there was limited evidence of CEOT in the biopsy or resection. However, this does raise the issue of malignant CEOT, which we did not identify in the review of our diagnostic archive. A small number of individual case reports have been published, most of which show areas of conventional CEOT with associated malignant transformation [16, 52]. A detailed discussion of diagnostic features is beyond the scope of this review, however, as with ameloblastic carcinoma, this is fraught with difficulty. A combination of the use of a proliferation marker, such as Ki67, with histological features of malignancy may be useful, but this has not been assessed in a cohort of these lesions.
In our cohort, the “classic” appearance, as described in the initial Pindborg paper [1], was found in only 13/26 cases (50%). In our series, we defined this as a tumor demonstrating the described epithelial features (polyhedral cells with clear boundaries), and containing amyloid, in keeping with the WHO 2017 classification [28]. Other features, such as calcification and nuclear pleomorphism were variably present. Tumors with these histological features present little difficulty in diagnosis. Two other tumors were diagnosed as clear cell CEOT as, although they were dominated by a clear cell population, they also contained areas of “classic” CEOT, with amyloid.
The main differential diagnosis to be considered in the tumors with a significant clear cell component is Clear Cell Odontogenic Carcinoma (CCOC). CCOC is an intraosseous malignant neoplasm consisting of sheets, nests and cords of polygonal to round clear cells, usually separated by fibrous septa and often showing peripheral palisading [53]. The lesional clear cells are usually PAS positive, diastase sensitive and negative for mucicarmine (mucin). Congo Red (amyloid) is also negative. Histologically, CC-CEOTs that contain few epithelial islands with clear cells in an eosinophilic homogenous stroma need careful investigations in order to confirm them as CEOT. It is mandatory to identify the presence of amyloid for confirmation. Metastatic tumors that contain clear cells are most likely renal cell carcinoma, clear cell breast carcinoma or thyroid carcinoma and, therefore, immunomarkers such as RCC, CD10, PAX8, ER/PR, TTF-1 are useful [54].
In difficult cases or small biopsies, fluorescence in situ hybridization (FISH) for EWSR1 gene rearrangement can be used to resolve this dilemma. EWSR1 gene rearrangement is absent in CEOT, clearly separating CC-CEOT from CCOC. Bilodeau et al. analyzed 12 CCCa and 8 CCOCs for EWSR-ATF1 FISH with 92% and 63% positive respectively. Subsequent Congo Red staining revealed that two of the CCOC that were negative for EWSR1 rearrangement contained amyloid; therefore these were more likely to be hypocellular CEOTs rather than CCOC with hyalinized stroma [55]. A key element in this analysis is the availability of tissue which has not been decalcified. Unfortunately, a combination of unavailability of FFPE blocks, very old tissue and a high frequency of decalcification in our cohort meant that EWRSR1 rearrangement studies were either not possible, or failed, in our cohort.
In cases where a differential diagnosis was agreed after review, four included odontogenic fibroma (OdF) and sclerosing odontogenic carcinoma as differential diagnoses. On H&E, these cases resemble “pattern 4” in the subtypes described by Ai-Ru et al. [27], with dominance of a fibrous stroma component. The difficulties in distinguishing these entities have been recently discussed in the literature and are very relevant to addressing the issues of the uncertain nature of the non-calcifying CEOT variants. As highlighted recently by Ide et al. [47], differential diagnosis of odontogenic fibroma (OdF) has been raised in these lesions and, indeed, there is much to suggest (including a lack of recurrence) that they may represent odontogenic fibromas, rather than non-calcifying CEOTs. This is reinforced in the case series reported by Eversole [56], where a small number of the 65 OdFs described contained both ODAM positive amyloid and Langerhans cells. It is worth noting that this issue was raised in the 1971 WHO classification, in relation to the differential diagnosis of non-calcifying CEOT and cellular OdF [57].
We considered sclerosing odontogenic carcinoma as a differential diagnosis in some cases (Table 1). This tumor has now been added to the WHO classification [28], but is somewhat controversial, and clear diagnostic criteria have not been established. Perineural invasion was not seen in any of these cases where this was considered as a diagnosis.
Three of these cases contained dentinoid. The significance of this is unclear, but in two cases, we included odontogenic carcinoma with dentinoid in the differential diagnosis, as these tumors presented some features similar to the case reports of this entity [58]. In particular, this was considered in cases where the original diagnosis was rather uncertain, where CEOT was a suggested diagnosis whilst acknowledging the tumor was difficult to classify. This indicates that the classification, and what may be considered to fall within the diagnostic remit of CEOT, may further evolve as other odontogenic entities are described and their diagnostic criteria established.
Conclusion
The development of diagnostic criteria for a tumor is an iterative process and the description and acceptance of tumor variants is limited to some degree by the lack of appropriate molecular tools to confirm or refute the placing of a particular tumor into its place on the classification. The description of a number of the variants of CEOT very much falls into this trap. Whilst some of the variants are most likely true variants of CEOT, it is becoming increasingly apparent that others are most likely a part of the spectrum of other odontogenic entities. This includes CCOC (now with EWSR1 cytogenetics to aid diagnosis) and odontogenic fibroma. Further refinement will most likely require a collaborative international approach to collect sufficiently large cohorts of these cases allow a more comprehensive molecular characterization of this group of lesions. In this way, more variants may be defined as other entities, whilst the true spectrum of CEOT is established. Such analysis may also aid in defining the histogenesis of these lesions.
This will not be without its challenges: many of the cases of CEOT are decalcified, which may significantly compromise the quality of genomic information which can be obtained from these specimens. To this end, careful consideration will have to be given to a concerted international effort to collect samples which have been optimally collected, stored and processed. The development of an international prospective database, with associated availability of both fixed and fresh material, which has not undergone harsh decalcification will be needed, and this could be coordinated via various international specialist societies. This will then allow for a program of translational research, which can include multi-omics analyses of these tumors.
Author Contributions
KH, PS and CF contributed to the study conception and design. The literature review was undertaken by BSMSS and RA. Material preparation, data collection and analysis were performed by BSMSS, PS, RA, SAH and KH. The first draft of the manuscript was written by BSMSS and KH and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Funding
No external funding was received for this project.
Compliance with Ethical Standards
Conflict of interest
None of the authors have any conflict of interest to declare.
Ethics Approval
Approval for the project was granted by West Glasgow Research Ethics Committee (Reference: 08/S0709/70). All cases were pseudoanonymised before analysis.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Pindborg JJ. A calcifying epithelial odontogenic tumor. Cancer. 1958;11:838–843. doi: 10.1002/1097-0142(195807/08)11:4<838::aid-cncr2820110423>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 2.Thoma KH, Goldman HM. Odontogenic tumors: classification based on observations of the epithelial, mesenchymal, and mixed varieties. Am J Pathol. 1946;22:433–471. [PubMed] [Google Scholar]
- 3.Ide F, Matsumoto N, Kikuchi K, Kusama K. Who originally described pindborg tumor? Head Neck Pathol. 2019;13:485–486. doi: 10.1007/s12105-018-0950-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Smith RA, Roman RS, Hansen LS, Lundell WJ, Riley RW. Oral surgery program university of california, san francisco. J Oral Surg (Chic) 1977;35:160–166. [PubMed] [Google Scholar]
- 5.Ivy RH. Unusual case of ameloblastoma of mandible; resection followed by restoration of continuity by iliac bone graft. Oral Surg Oral Med Oral Pathol. 1948;1:1074–1082. doi: 10.1016/0030-4220(48)90225-4. [DOI] [PubMed] [Google Scholar]
- 6.Wunderer S. The problem of malignant, odontomas. Osterr Z Stomatol. 1953;50:567–571. [PubMed] [Google Scholar]
- 7.Stoopack JC. Cystic odontoma of the mandible. Oral Surg Oral Med Oral Pathol. 1957;10:807–812. doi: 10.1016/0030-4220(57)90108-1. [DOI] [PubMed] [Google Scholar]
- 8.Shafer W, Hine M, Levy B. A textbook of oral pathology. 2. Philadelphia: Saunders; 1963. [Google Scholar]
- 9.Franklin CD, Pindborg JJ. The calcifying epithelial odontogenic tumor. Oral Surg Oral Med Oral Pathol. 1976;42:753–765. doi: 10.1016/0030-4220(76)90098-0. [DOI] [PubMed] [Google Scholar]
- 10.Chrcanovic BR, Gomez RS. Calcifying epithelial odontogenic tumor: an updated analysis of 339 cases reported in the literature. J Cranio-Maxillofac Surg. 2017;45:1117–1123. doi: 10.1016/j.jcms.2017.05.007. [DOI] [PubMed] [Google Scholar]
- 11.Siriwardena BSMS, Crane H, O’Neill N, Abdelkarim R, Brierley DJ, Franklin CD, et al. Odontogenic tumors and lesions treated in a single specialist oral and maxillofacial pathology unit in the United Kingdom in 1992–2016. Oral Surg Oral Med Oral Pathol Oral Radiol. 2019;127:151–166. doi: 10.1016/j.oooo.2018.09.011. [DOI] [PubMed] [Google Scholar]
- 12.Pindborg JJ. The calcifying epithelial odontogenic tumor: review of literature and report of an extraosseous case. Acta Odontol Scand. 1966;24:419–430. [Google Scholar]
- 13.Kaplan I, Buchner A, Calderon S, Kaffe I. Radiological and clinical features of calcifying epithelial odontogenic tumour. Dentomaxillofac Radiol. 2001;30:22–28. doi: 10.1038/sj/dmfr/4600566. [DOI] [PubMed] [Google Scholar]
- 14.Cross JJ, Pilkington RJ, Antoun NM, Adlam DM. Value of computed tomography and magnetic resonance imaging in the treatment of a calcifying epithelial odontogenic (Pindborg) tumour. Br J Oral Maxillofac Surg. 2000;38:154–157. doi: 10.1054/bjom.1999.0148. [DOI] [PubMed] [Google Scholar]
- 15.Uchiyama Y, Murakami S, Kishino M, Furukawa S. CT and MR imaging features of a case of calcifying epithelial odontogenic tumor. J Belgian Soc Radiol 2012;95:315. [DOI] [PubMed]
- 16.Kawano K, Ono K, Yada N, Takahashi Y, Kashima K, Yokoyama S, et al. Malignant calcifying epithelial odontogenic tumor of the mandible: report of a case with pulmonary metastasis showing remarkable response to platinum derivatives. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2007;104:76–81. doi: 10.1016/j.tripleo.2006.04.014. [DOI] [PubMed] [Google Scholar]
- 17.Philipsen H, Reichart P. Calcifying epithelial odontogenic tumour: biological profile based on 181 cases from the literature. Oral Oncol. 2000;36:17–26. doi: 10.1016/s1368-8375(99)00061-5. [DOI] [PubMed] [Google Scholar]
- 18.Damm DD, White DK, Drummond JF, Poindexter JB, Henry BB. Combined epithelial odontogenic tumor: adenomatoid odontogenic tumor and calcifying epithelial odontogenic tumor. Oral Surg Oral Med Oral Pathol. 1983;55:487–496. doi: 10.1016/0030-4220(83)90235-9. [DOI] [PubMed] [Google Scholar]
- 19.El-Labban NG, Lee KW, Kramer IR. The duality of the cell population in a calcifying epithelial odontogenic tumour (CEOT) Histopathology. 1984;8:679–691. doi: 10.1111/j.1365-2559.1984.tb02380.x. [DOI] [PubMed] [Google Scholar]
- 20.Sauk JJ, Cocking-Johnson D, Warings M. Identification of basement membrane components and intermediate filaments in calcifying epithelial odontogenic tumors. J Oral Pathol. 1985;14:133–140. doi: 10.1111/j.1600-0714.1985.tb00476.x. [DOI] [PubMed] [Google Scholar]
- 21.Morimoto C, Tsujimoto M, Shimaoka S, Shirasu R, Takasu J. Ultrastructural localization of alkaline phosphatase in the calcifying epithelial odontogenic tumor. Oral Surg Oral Med Oral Pathol. 1983;56:409–414. doi: 10.1016/0030-4220(83)90352-3. [DOI] [PubMed] [Google Scholar]
- 22.Crivelini MM, Felipini RC, Miyahara GI, de Sousa SCOM. Expression of odontogenic ameloblast-associated protein, amelotin, ameloblastin, and amelogenin in odontogenic tumors: immunohistochemical analysis and pathogenetic considerations. J Oral Pathol Med. 2012;41:272–280. doi: 10.1111/j.1600-0714.2011.01079.x. [DOI] [PubMed] [Google Scholar]
- 23.Philipsen HP, Reichart PA, Siar CH, Ng KH, Lau SH, Zhang X, et al. An updated clinical and epidemiological profile of the adenomatoid odontogenic tumour: a collaborative retrospective study. J Oral Pathol Med. 2007;36:383–393. doi: 10.1111/j.1600-0714.2007.00536.x. [DOI] [PubMed] [Google Scholar]
- 24.Priya S, Madanagopaal LR, Sarada V. Pigmented pindborg tumor of the maxilla: a case report. J Oral Maxillofac Pathol. 2016;20:548. doi: 10.4103/0973-029X.190970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sánchez-Romero C, Carlos R, de Almeida OP, Romañach MJ. Microcystic calcifying epithelial odontogenic tumor. Head Neck Pathol. 2018;12:598–603. doi: 10.1007/s12105-017-0868-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gopalakrishnan R, Simonton S, Rohrer MD, Koutlas IG. Cystic variant of calcifying epithelial odontogenic tumor. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;102:773–777. doi: 10.1016/j.tripleo.2005.09.029. [DOI] [PubMed] [Google Scholar]
- 27.Ai-Ru L, Zhen L, Jian S. Calcifying epithelial odontogenic tumors: a clinicopathologic study of nine cases. J Oral Pathol. 1982;11:399–406. doi: 10.1111/j.1600-0714.1982.tb00181.x. [DOI] [PubMed] [Google Scholar]
- 28.El-Naggar AK, Chan JKC, Rubin Grandis J, Takata T, Slootweg PJ, International Agency for Research on Cancer, et al. WHO classification of head and neck tumours. 4th Edition. El-Naggar A, Chan J, Grandis J, Takata T, Slootweg P, editors. Lyon: International Agency for Research on Cancer (IARC); 2017
- 29.Detre S, Saclani Jotti G, Dowsett M. A “quickscore” method for immunohistochemical semiquantitation: validation for oestrogen receptor in breast carcinomas. J Clin Pathol 1995;48:876–8. [DOI] [PMC free article] [PubMed]
- 30.Richardson JF, Balogh K, Merk F, Booth D. Pigmented odontogenic tumor of jawbone: a previously undescribed expression of neoplastic potential. Cancer. 1974;34:1244–1251. doi: 10.1002/1097-0142(197410)34:4<1244::aid-cncr2820340434>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 31.Abrams AM, Howell FV. Calcifying epithelial odontogenic tumors: report of four cases. J Am Dent Assoc. 1967;74:1231–1240. doi: 10.14219/jada.archive.1967.0426. [DOI] [PubMed] [Google Scholar]
- 32.Rangel ALCA, da Silva AA, Ito FA, Lopes MA, de Almeida OP, Vargas PA. Clear cell variant of calcifying epithelial odontogenic tumor: is it locally aggressive? J Oral Maxillofac Surg. 2009;67:207–211. doi: 10.1016/j.joms.2007.11.029. [DOI] [PubMed] [Google Scholar]
- 33.Anavi Y, Kaplan I, Citir M, Calderon S. Clear-cell variant of calcifying epithelial odontogenic tumor: clinical and radiographic characteristics. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2003;95:332–339. doi: 10.1067/moe.2003.8. [DOI] [PubMed] [Google Scholar]
- 34.Bouckaert MMR, Raubenheimer EJ, Jacobs FJ. Calcifying epithelial odontogenic tumor with intracranial extension: report of a case and review of the literature. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2000;90:656–662. doi: 10.1067/moe.2000.106577. [DOI] [PubMed] [Google Scholar]
- 35.Wertheimer FW, Zielinski RJ, Wesley RK. Extraosseous calcifying epithelial odontogenic tumor (Pindborg tumor) Int J Oral Surg. 1977;6:266–269. doi: 10.1016/s0300-9785(77)80037-9. [DOI] [PubMed] [Google Scholar]
- 36.Schmidt-Westhausen A, Philipsen HP, Reichart PA. Clear cell calcifying epithelial odontogenic tumor: A case report. Int J Oral Maxillofac Surg. 1992;21:47–49. doi: 10.1016/s0901-5027(05)80453-9. [DOI] [PubMed] [Google Scholar]
- 37.Anderson HC, Kim B, Minkowitz S. Calcifying epithelial odontogenic tumor of Pindborg: an electron microscopic study. Cancer. 1969;24:585–596. doi: 10.1002/1097-0142(196909)24:3<585::aid-cncr2820240325>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 38.Datar U, Kamat M, Kanitkar S, Byakodi S. Clear cell odontogenic carcinoma: A rare case report with emphasis on differential diagnosis. J Cancer Res Ther. 2017;13:374. doi: 10.4103/0973-1482.187381. [DOI] [PubMed] [Google Scholar]
- 39.Kaushal S, Mathur SR, Vijay M, Rustagi A. Calcifying epithelial odontogenic tumor (Pindborg tumor) without calcification: a rare entity. J Oral Maxillofac Pathol. 2012;16:110–2. [DOI] [PMC free article] [PubMed]
- 40.Afroz N, Jain A, Maheshwari V, Ahmad SS. Non-calcifying variant of calcifying epithelial odontogenic tumor with clear cells-first case report of an extraosseous (Peripheral) presentation. Eur J Gen Dent. 2013;2:80–82. [Google Scholar]
- 41.Hafian H, Mauprivez C, Furon V, Pluot M, Lefevre B. Pindborg tumor: a poorly differentiated form without calcification. Rev Stomatol Chir Maxillofac. 2004;105:227–230. doi: 10.1016/s0035-1768(04)72313-x. [DOI] [PubMed] [Google Scholar]
- 42.Azevedo RS, Mosqueda-Taylor A, Carlos RR, Cabral MGMG, Romanach MJ, de Almeida OP, et al. Calcifying epithelial odontogenic tumor (CEOT): a clinicopathologic and immunohistochemical study and comparison with dental follicles containing CEOT-like areas. Oral Surg Oral Med Oral Pathol Oral Radiol. 2013;116:759–768. doi: 10.1016/j.oooo.2013.08.023. [DOI] [PubMed] [Google Scholar]
- 43.Asano M, Takahashi T, Kusama K, Iwase T, Hori M, Yamanoi H, et al. A variant of calcifying epithelial odontogenic tumor with Langerhans cells. J Oral Pathol Med. 1990;19:430–434. doi: 10.1111/j.1600-0714.1990.tb00874.x. [DOI] [PubMed] [Google Scholar]
- 44.Takata T, Ogawa I, Miyauchi M, Ijuhin N, Nikai H, Fujita M, et al. Non-calcifying Pindborg tumor with Langerhans cells. J Oral Pathol Med. 1993;22:378–383. doi: 10.1111/j.1600-0714.1993.tb01093.x. [DOI] [PubMed] [Google Scholar]
- 45.Chen Y, Wang T-TT, Gao Y, Li T-JJ. A clinicopathologic study on calcifying epithelial odontogenic tumor: with special reference to Langerhans cell variant. Diagn Pathol. 2014;9:1–8. doi: 10.1186/1746-1596-9-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Afrogheh A, Schneider J, Mohamed N, Hille J. Calcifying Epithelial odontogenic tumour with clear langerhans cells: a novel variant, report of a case and review of the literature. Head Neck Pathol. 2014;8:214–219. doi: 10.1007/s12105-013-0490-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ide F, Matsumoto N, Miyazaki Y, Kikuchi K, Kusama K. What is the non-calcifying langerhans cell-rich variant of calcifying epithelial odontogenic tumor? Head Neck Pathol. 2019;13:489–491. doi: 10.1007/s12105-018-0968-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Channappa NK, Krishnapillai R, Rao JB. Cystic variant of calcifying epitelial odontogenic tumor. Clin Dent. 2012;3:152–156. doi: 10.1111/j.2041-1626.2011.00092.x. [DOI] [PubMed] [Google Scholar]
- 49.Urias Barreras CM, Quezada Rivera D, Koutlas IG, Gaitan Cepeda LA, Gaitán Cepeda LA. Clear cell cystic variant of calcifying epithelial odontogenic tumor. Head Neck Pathol. 2014;8:229–233. doi: 10.1007/s12105-013-0494-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dantas RCM, Ramos-Perez FM de MFM de M, Perez DE da C, Durighetto AFJ, Vargas PA. Cystic variant of calcifying epithelial odontogenic tumor. J. Craniofac. Surg. 2015, 1722–3. [DOI] [PubMed]
- 51.Siar CH, Ng KH. The combined epithelial odontogenic tumour in Malaysians. Br J Oral Maxillofac Surg. 1991;29:106–109. doi: 10.1016/0266-4356(91)90093-k. [DOI] [PubMed] [Google Scholar]
- 52.Demian N, Harris RJ, Abramovitch K, Wilson JW, Vigneswaran N. Malignant transformation of calcifying epithelial odontogenic tumor is associated with the loss of p53 transcriptional activity: a case report with review of the literature. J Oral Maxillofac Surg. 2010;68:1964–1973. doi: 10.1016/j.joms.2010.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Avninder S, Rakheja D, Bhatnagar A. Clear cell odontogenic carcinoma: a diagnostic and therapeutic dilemma. World J Surg Oncol. 2006;4:91. doi: 10.1186/1477-7819-4-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Eversole LR. On the differential diagnosis of clear cell tumours of the head and neck. Eur J Cancer B Oral Oncol. 1993;29B:173–179. doi: 10.1016/0964-1955(93)90019-b. [DOI] [PubMed] [Google Scholar]
- 55.Bilodeau EA, Weinreb I, Antonescu CR, Zhang L, Dacic S, Muller S, et al. Clear cell odontogenic carcinomas show EWSR1 rearrangements. Am J Surg Pathol. 2013;37:1001–5. [DOI] [PubMed]
- 56.Eversole LR. Odontogenic Fibroma, including amyloid and ossifying variants. Head Neck Pathol. 2011;5:335–343. doi: 10.1007/s12105-011-0279-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pindborg JJ, Kramer IR. Histological typing of odontogenic tumours, jaw cysts, and allied lesions. 1. London: World Health Organisation; 1971. [Google Scholar]
- 58.Mosqueda-Taylor A, Neville BW, Tatemoto Y, Ogawa I, Takata T. Odontogenic carcinoma with dentinoid: a new odontogenic carcinoma. Head Neck Pathol. 2014;8:421–431. doi: 10.1007/s12105-014-0586-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Oikarinen VJ, Calonius PE, Meretoja J. Calcifying epithelial odontogenic tumor (Pindborg tumor) case report. Int J Oral Surg. 1976;5:187–191. doi: 10.1016/s0300-9785(76)80043-9. [DOI] [PubMed] [Google Scholar]
- 60.Yamaguchi A, Kokubu JM, Takagi M. Calcifying odontogenic tumor: histochemical and electron microscopic observation of a case. Bull Tokyo Med Dent Univ. 1980;27:129–135. [PubMed] [Google Scholar]
- 61.Hicks MJ, Flaitz CM, Wong ME. Clear cell variant of calcifying epithelial odontogenic tumor: case report and review of the literature. Head Neck. 1994;16:272–277. doi: 10.1002/hed.2880160311. [DOI] [PubMed] [Google Scholar]
- 62.Kumamoto H, Sato I, Tateno H, Yokoyama J, Takahashi T, Ooya K. Clear cell variant of calcifying epithelial odontogenic tumor (CEOT) in the maxilla: report of a case with immunohistochemical and ultrastructural investigations. J Oral Pathol Med. 1999;28:187–191. doi: 10.1111/j.1600-0714.1999.tb02022.x. [DOI] [PubMed] [Google Scholar]
- 63.Germanier Y, Bornstein MM, Stauffer E. Calcifying epithelial odontogenic (Pindborg) tumor of the mandible with clear cell component treated by conservative surgery: report of a case. J Oral Maxillofac Surg. 2005;63:1377–1382. doi: 10.1016/j.joms.2005.05.298. [DOI] [PubMed] [Google Scholar]
- 64.Mohtasham N, Habibi A, Jafarzadeh H, Amirchaghmaghi M. Extension of Pindborg tumor to the maxillary sinus: a case report. J Oral Pathol Med. 2007;37:59–61. doi: 10.1111/j.1600-0714.2007.00567.x. [DOI] [PubMed] [Google Scholar]
- 65.Sahni P, Nayak MT, Singhvi A, Sharma J. Clear cell calcifying epithelial odontogenic (Pindborg) tumor involving the maxillary sinus: a case report and review of literature. J Oral Maxillofac Pathol. 2012;16:454–459. doi: 10.4103/0973-029X.102520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chen CY, Wu CW, Wang WC, Lin LM, Chen YK. Clear-cell variant of calcifying epithelial odontogenic tumor (Pindborg tumor) in the mandible. Int J Oral Sci. 2013;5:115–119. doi: 10.1038/ijos.2013.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Turatti E, Brasil J, de Andrade BB-A-B, Romanach M-JM, de Almeida O-PO. Clear cell variant of calcifying epithelial odontogenic tumor: case report with immunohistochemical findings. J Clin Exp Dent 2015;7:e163–6. [DOI] [PMC free article] [PubMed]
- 68.Rydin K, Sjöström M, Warfvinge G, Sjostrom M, Warfvinge G. Clear cell variant of intraosseous calcifying epithelial odontogenic tumor: a case report and review of the literature. Oral Surg Oral Med Oral Pathol Oral Radiol. 2016;122:e125–e130. doi: 10.1016/j.oooo.2016.01.001. [DOI] [PubMed] [Google Scholar]
- 69.Chatterjee RP, Gayen S, Kundu S, Chattaraj M, Pal M, Das S. A unique case of clear cell variant of calcifying epithelial odontogenic tumor involving the maxilla. Dent Res J (Isfahan) 2017;14:293–296. doi: 10.4103/1735-3327.211623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sabir H, Kumbhare S, Redij S, Gajbhiye N. Clear cell variant of calcifying epithelial odontogenic tumor: a rare clinical entity. Gulf J Oncolog. 2017;1:55–60. [PubMed] [Google Scholar]
- 71.Júnior BC, Muniz VRVM, Vidal MTA, Gurgel CA, Leon JE, De Azevedo RA, et al. Clear cell variant of calcifying epithelial odontogenic tumor. Appl Immunohistochem Mol Morphol 2017;25:e95–9. [DOI] [PubMed]
- 72.Houston GD, Fowler CB. Extraosseous calcifying epithelial odontogenic tumor: report of two cases and review of the literature. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1997;83:577–83. [DOI] [PubMed]
- 73.Orsini G, Favia G, Piattelli A. Peripheral clear cell calcifying epithelial odontogenic tumor. Report of a case. Periodontol. 2000;71:1177–1180. doi: 10.1902/jop.2000.71.7.1177. [DOI] [PubMed] [Google Scholar]
- 74.Mesquita RA, Lotufo MA, Sugaya NN, De Araújo NS, De Araújo VC. Peripheral clear cell variant of calcifying epithelial odontogenic tumor: report of a case and immunohistochemical investigation. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2003;95:198–204. doi: 10.1067/moe.2003.63. [DOI] [PubMed] [Google Scholar]
- 75.Gaiger de Oliveira M, Chaves ACM, Visioli F, Rojas EU, Moure SP, Romanini J, et al. Peripheral clear cell variant of calcifying epithelial odontogenic tumor affecting 2 sites: report of a case. Oral Surg, Oral Med Oral Pathol Oral Radiol Endod. 2009;107:407–411. doi: 10.1016/j.tripleo.2008.11.004. [DOI] [PubMed] [Google Scholar]
- 76.Habibi A, Saghravanian N, Zare R, Jafarzadeh H. Clear cell variant of extraosseous calcifying epithelial odontogenic tumor: a case report. J Oral Sci. 2009;51:485–488. doi: 10.2334/josnusd.51.485. [DOI] [PubMed] [Google Scholar]
- 77.Gadodia P, Wadhwani R, Murgod V, Vinodkumar MP, Panda A, Sabnis R, et al. Clear cell variant of extraosseous calcifying epithelial odontogenic tumor: report of a case and review of literature. J Int Oral Res. 2016;8:973–977. [Google Scholar]
- 78.Wang L, Wang S, Chen X. Langerhans cells containing calcifying epithelial odontogenic tumour: report of two cases and review of the literature. Oral Oncol Extra. 2006;42:144–146. [Google Scholar]
- 79.Wang Y-P, Lee J-J, Wang J-T, Liu B-Y, Yu C-H, Kuo R-C, et al. (2007) Non-calcifying variant of calcifying epithelial odontogenic tumor with Langerhans cells. J Oral Pathol Med, 36:436–9. [DOI] [PubMed]
- 80.Tseng C-H, Wang Y-P, Lee J-J, Chang JYF, Tseng YP, Wang JJ, et al. Noncalcifying variant of calcifying epithelial odontogenic tumor with Langerhans cells. J Formos Med Assoc. 2015;114:781–782. doi: 10.1016/j.jfma.2015.04.004. [DOI] [PubMed] [Google Scholar]
- 81.Santosh N, McNamara KK, Kalmar JR, Iwenofu OH. Non-calcifying langerhans cell-rich variant of calcifying epithelial odontogenic tumor: a distinct entity with predilection for anterior maxilla. Head Neck Pathol. 2019;13:718–721. doi: 10.1007/s12105-018-0958-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Bingham RA, Adrian JC. Combined epithelial odontogenic tumor-adenomatoid odontogenic tumor and calcifying epithelial odontogenic tumor: report of a case. J oral Maxillofac Surg 1986;44:574–7. [DOI] [PubMed]
- 83.Takeda Y, Kudo K. Adenomatoid odontogenic tumor associated with calcifying epithelial odontogenic tumor. Int J Oral Maxillofac Surg. 1986;15:469–73. [DOI] [PubMed]
- 84.Ledesma CM, Taylor AM, de Leon ER, J. Adenomatoid odontogenic tumour with features of calcifying epithelial odontogenic tumour. (The so-called combined epithelial odontogenic tumour.) Clinico-pathological report of 12 cases. Eur J Cancer Part B Oral Oncol. 1993;29:221–4. [DOI] [PubMed]
- 85.Miyake M, Nagahata S, Nishihara J, Ohbayashi Y. Combined adenomatoid odontogenic tumor and calcifying epithelial odontogenic tumor: report of case and ultrastructural study. J Oral Maxillofac Surg. 1996;54:788–793. doi: 10.1016/s0278-2391(96)90706-5. [DOI] [PubMed] [Google Scholar]
- 86.Rosa ACG, Soares AB, Furuse C, Lima SRR, de Araújo VC, Passador-Santos F. A combined epithelial odontogenic tumor? A 7-year follow-up case. Head Neck Pathol. 2017;11:519–524. doi: 10.1007/s12105-016-0767-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Poomsawat S, Punyasingh J. Calcifying epithelial odontogenic tumor: an immunohistochemical case study. J Mol Histol. 2007;38:103–109. doi: 10.1007/s10735-007-9082-9. [DOI] [PubMed] [Google Scholar]




