Abstract
Oligometastatic disease is a hypothesized intermediate stage of disease between localized and widespread metastatic cancer. Localized treatment of oligometastatic lesions may offer survival advantages in addition to systemic treatment. In this case report, we describe a patient who presented with small cell neuroendocrine carcinoma “Merkel type” (SNECM) of the parotid gland which had metastasized to the brain and adrenal gland. He was treated with chemotherapy followed by stereotactic radiotherapy and volumetric modulated arc therapy for oligometastasis. He maintains good functional status with low burden of disease at 20-months after diagnosis. SNECM is a rare and aggressive parotid cancer with immunohistochemical and morphologic similarities to Merkel cell carcinoma (MCC). There are only 44 cases of parotid SNECM in the English literature. This is the first case to describe management of oligometastatic SNECM and we review literature on management of both SNECM and metastatic MCC.
Keywords: Merkel cell type, Merkel cell carcinoma, Small cell neuroendocrine carcinoma, Parotid gland, Oligometastasis, Brain metastasis, Stereotactic radiotherapy, Case report
Introduction
Oligometastasis refers to a hypothetical intermediate stage of disease between localized and widespread metastatic cancer. The number of metastases that define “oligo” is controversial and differs between studies [1–4]. There is accumulating evidence that local ablative treatments for oligometastatic patients may provide survival benefits. However, most of the evidence is based on phase II studies, and the sites of the studies are mainly limited to lung and prostate cancers.
Primary small cell neuroendocrine carcinoma “Merkel type” (SNECM) of the parotid gland is a very rare and aggressive cancer. There have been only 44 cases recorded in the English literature based on case studies and series (Table 1). As such, there is little data to guide management. Parotid SNECM stains positively for cytokeratin 20 (CK20) in a perinuclear pattern and is indistinguishable from Merkel cell carcinoma (MCC) from a morphologic and immunohistochemical perspective [5]. Since the parotid gland is also a frequent site of metastasis for MCC, differentiating SNECM from MCC can present a diagnostic challenge. It has been proposed that some parotid SNECM may in fact be MCCs for which the primary tumor went unrecognized or regressed.
Table 1.
Clinical data of 44 cases parotid small cell neuroendocrine carcinoma “Merkel type” published from 1983 to 2019, coming from 14 sources
| Source | n | Age | Sex | Size (cm) | Treatment | Local recurrence | LN metastasis | Distant metastasis | Status at last follow-up |
|---|---|---|---|---|---|---|---|---|---|
| Kraemer [6] | 2 | 54 | M | Chemo + RT (50 Gy) | No | Brain + bone | 3 year, died | ||
| 78 | M | 4.0 | SP + rND + RT (50 Gy) | No | Subdigastric + midjugular | 3 year, died | |||
| Gnepp [7] | 7 | 5 | F | 6 year, alive | |||||
| 72 | M | Unspecified | 20 months, died | ||||||
| 60 | M | Surgery (unspecified) | 53 months, died | ||||||
| 80 | F | 13 months, died | |||||||
| 42 | F | 75 months, alive | |||||||
| 82 | F | 12 months, died | |||||||
| 69 | M | Unknown | |||||||
| Fornelli [8] | 2 | 65 | M | 4.0 | SP + TP + RND | Yes | Widespread | 28 months, died | |
| 70 | M | 2.5 | TP + chemo + RT | Yes | None | Possible | 24 months, alive | ||
| Nagao [9] | 11 | 77 | F | 1.8 | TP + rND + RT | No | 28 months, alive | ||
| 78 | M | 1.5 | TP + rND + RT | No | Axillary, supraclavicular | Liver, lung | 45 months, died | ||
| 81 | F | 3 | TP + rND | No | Cervical | Liver, lung | 17 months, died | ||
| 50 | M | 0.7 | TP + rND | No | Cervical | 155 months, alive | |||
| 66 | M | 5.0 | SP + rND + chemo | No | Brain, abdomen | 20 months, died | |||
| 76 | M | 11.0 | TP + rND | No | Cervical, mediastinal | 2 months, died | |||
| 72 | M | 2.9 | TP | No | 4 months, alive | ||||
| 72 | M | 2.0 | TP + rND + RT + chemo | No | Axillary | Pleura, pericardium | 34 months, died | ||
| 67 | M | 8.0 | TP + rND + RT + chemo | No | Systemic | Brain, chest wall | 18 months, alive | ||
| 52 | F | 1.5 | TP + rND + RT | No | 4 months, alive | ||||
| Jorcano [10] | 1 | 91 | M | 4.0 | RT | No | Cervical | 3 years, alive | |
| Ghaderi [11] | 1 | 35 | F | 2.0 | SP + RT | ||||
| Mulder [12] | 1 | 78 | M | SP + mND + RT (30 Gy) | Cervical | 3 year, died | |||
| Chernock [13] | 4 | 66 | M | Surgery + chemo | Unspecified | 9 year, alive | |||
| 74 | M | 2 years, alive | |||||||
| 22 | M | 6 months, died | |||||||
| 60 | M | 13 years, alive | |||||||
| de Biase [14] | 1 | 64 | M | Cervical | 12 months, died | ||||
| Kanazawa [15] | 1 | 87 | F | 5.5 | TP + mND | No | 9 year, alive | ||
| Fisher [16] | 3 | 64 | F | 1.4 | SP + rND | No | 41 months, alive | ||
| Knopf [17] | 8 | Mean age 75 |
3/8 M 5/8F |
8/8 TP + RND 3/8 RT 1/8 chemo |
5-year recurrence-free interval = 73% 5-year OS = 54% |
||||
| Bizzaro [18] | 1 | 65 | M | 2 years, alive | |||||
| Giroulet [19] | 1 | 87 | M | Chemo (carboplatin-etoposide) | Unspecified LN | Bone |
TP total paritodectomy, SP superficial parotidectomy, rND radical neck dissection, mND modified neck dissection, RT radiotherapy, LN lymph node, chemo chemotherapy
In this case study, we describe a HIV-positive patient who presented with oligometastatic parotid SNECM to the adrenal gland and the brain. Physical exam and PET/CT scans did not demonstrate any cutaneous primary tumor. He was subsequently treated with a combination of chemotherapy and salvage radiotherapy and is still alive and with excellent performance status, 20 months from initial diagnosis. This is the first case study to describe volumetric-modulated arc therapy (VMAT) and brain stereotactic radiotherapy (SRT) for oligometastatic parotid SNECM.
Case Report
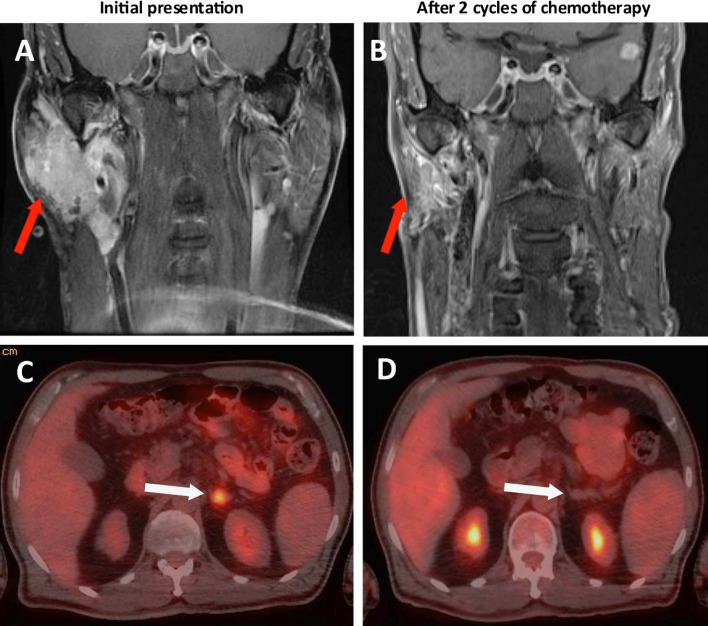
A 69-year-old man presented in April 2018 to the emergency department with a painless mass below his right ear that had been enlarging for a month. The patient’s past medical history was significant for well-controlled HIV with an undetectable viral load, dyslipidemia, and twice resected basal cell carcinoma. Examination revealed a small, immobile, firm, and non-tender lesion under the right ear. Pan-endoscopy was unremarkable. Computed tomography (CT) and magnetic resonance imaging (MRI) revealed a 4.9 × 4.5 × 2.8 cm mass centered within the right superficial parotid gland, with extension into the deep lobe medial to the vascular bundle (Fig. 1a).
Fig. 1.
Initial response to chemotherapy. a Coronal neck MRI revealing a 4.9 × 4.5 × 2.8 cm mass centered within the right superficial parotid gland, with extension into the deep lobe medial to the vascular bundle. b Coronal neck MRI after 2 cycles of chemotherapy, showing partial response. Right parotid mass now measures 3.2 × 1.2 × 1.8 cm. c PET/CT axial slice reveals a discrete 1.5 cm, FDG-avid (SUV 6.7) lesion in the left adrenal gland, suspicious for metastasis. d PET/CT axial slice shows complete response to 2 cycles of chemotherapy. The previous left adrenal FDG-avid focus has resolved
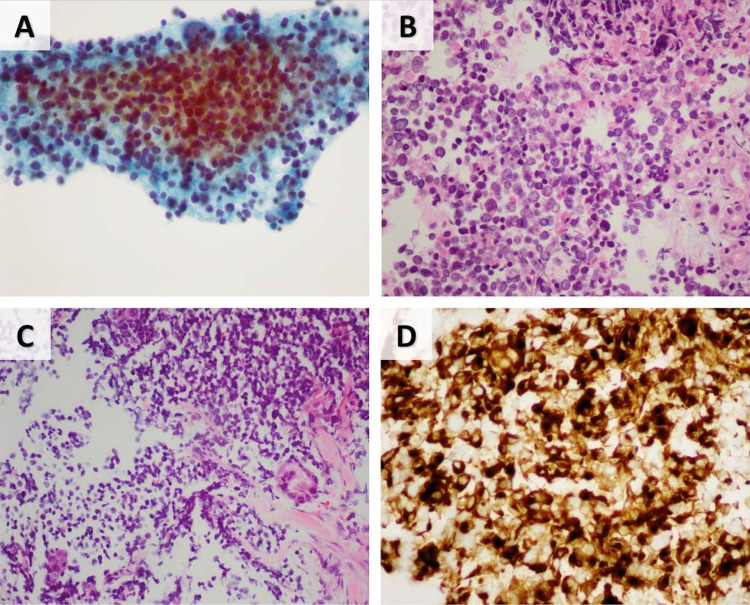
Core needle biopsy of the mass revealed a lesion composed of sheets of round to ovoid cells with scant cytoplasm. The nuclei were hyperchromatic showing indistinct nucleoli, fine chromatin and focal molding, typical of neuroendocrine differentiation. Immunohistochemically, the tumor cells were positive for synaptophysin and CK20 in a typical perinuclear dot-like pattern, which is characteristic of both Merkel cell carcinoma and SNECM. The cells were negative for CK7 and p63. Additionally, TTF-1was immunonegative, arguing against the possibility of a metastatic deposit from small cell lung carcinoma. The cells lacked expression of Merkel Cell Polyomavirus (MCPyV). (Fig. 2) These findings were consistent with a primary parotid gland “Merkel cell type” small cell neuroendocrine carcinoma. 18F-fluorodeoxyglucose (FDG) positron emission tomogram (PET) showed an FDG-avid lesion in the left adrenal gland, suspicious for metastasis (Fig. 1c). There was no lung or lymph node involvement.
Fig. 2.
Fine needle aspiration and core need biopsy of mass lesion. a FNA specimen showing a moderately cellular specimen with atypical small cells with occasional large bizarre cells. b Cell block showing atypical small monomorphic cells with neuroendocrine features. Adjacent unremarkable salivary glands are seen on the right. c Core needle biopsy showing atypical small and monomorphic cell proliferation. d Immunohistochemistry positive for CK20 in a perinuclear dot-like pattern
The patient received four cycles of cisplatin and etoposide chemotherapy from June to August 2018, with favorable response of the parotid mass (Fig. 1b) and complete resolution of the left adrenal lesion demonstrated on PET/CT and MRI (Fig. 1d). However, 9 weeks after completion of chemotherapy, the adrenal lesion was found to recur based on PET.
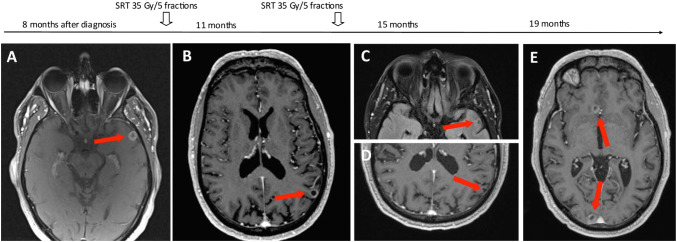
On re-staging neck CT, two small rim-enhancing brain metastases were found incidentally. The largest measured 1.1 × 1.1 cm in the left temporal lobe and a smaller focus in the right paramedic occipital lobe on MRI (Fig. 4a). The patient remained well and asymptomatic. His case was discussed at the brain multidisciplinary tumor conference and palliative brain radiotherapy was recommended to take place before further systemic treatment. Ultimately, stereotactic radiotherapy (SRT) was chosen over whole brain radiotherapy (WBRT) due to the patient’s excellent performance status and preferences. The patient received 35 Gy in 5 fractions to both lesions in December 2018.
Fig. 4.
Metastatic brain lesions and treatments with VMAT/SRT. Two metastatic brain lesions initially diagnosed on neck MRI incidentally, 8 months after diagnosis of MCC. This timeline shows the resolution of brain metastatic lesions with brain-directed radiotherapy treatments as well as the development of new lesions over time. a Metastatic 1.1 × 1.1 cm rim-enhancing lesion in the left temporal lobe (shown) and focus in the right paramedic occipital lobe (not shown). b New 1.0 × 1.0 cm metastatic lesion appears in the left parieto-occipital lobe (shown). Another 0.3 cm right occipital lobe focus (not shown). Previously treated lesions have resolved. c, d Resolution of previously irradiated metastatic lesions. No new metastases. e Development of 3 new metastases. A rim-enhancing lesion in the right occipital lobe (1.2 × 1.6 cm) and right frontal lobe (1.0 × 0.8 cm) (shown). A 0.4 cm focus in the vermis (not shown). Previously treated lesions have sustained response
Follow-up MRI 3-months post-SRT showed complete resolution of the treated lesions, though a new 1.0 × 1.0 cm metastasis in the left parieto-occipital lobe and 0.3 cm focus in the right occipital lobe had now appeared (Fig. 4b). Each new lesion was treated with targeted SRT (35 Gy in 5 fractions) in April 2019. Complete resolution was seen on brain MRI in 3-month follow-up, with no evidence of recurrence or new disease (Fig. 4c).
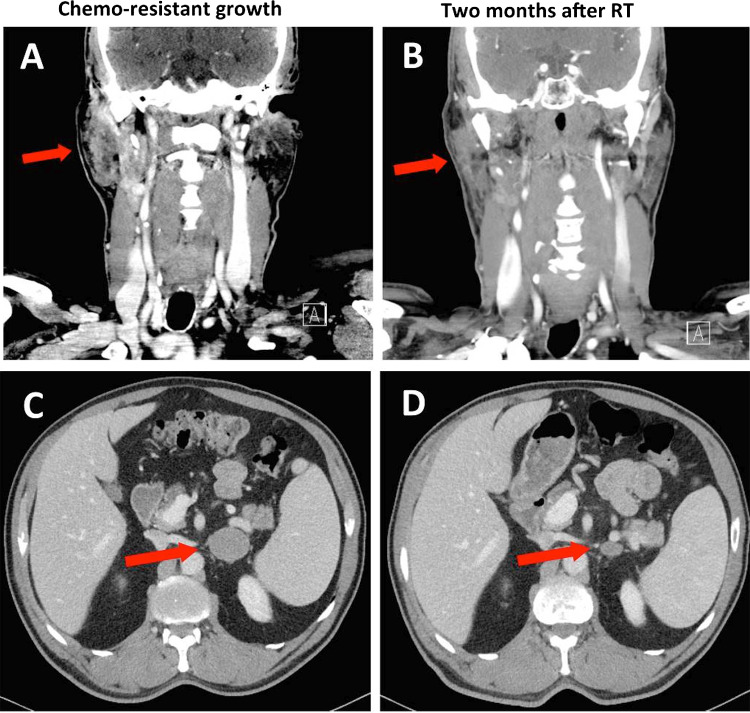
CT scan in May 2019 showed continued progression of the right parotid and left adrenal masses, as well as new ipsilateral lymphadenopathy. He was treated with another 2 cycles of cisplatin and etoposide with limited effect. CT scan in July 2019, post-chemotherapy, showed the parotid mass continued to increase in size to 3.7 × 2.9 × 5.4 cm (Fig. 3a), the left adrenal mass to 3.7 × 4.5 cm (Fig. 3c), and the right 1B and 2A cervical lymph nodes to 1.4 × 1.9 cm and 0.9 × 1.0 cm respectively.
Fig. 3.
Computed tomography showing chemo-resistant growth of the parotid and left adrenal masses and the response to VMAT. a Right parotid mass measuring 3.7 × 2.9 × 5.4 cm and right cervical lymph nodes 1B and 2A measuring 1.4 × 1.9 cm and 0.9 × 1.0 cm respectively. b Two months after receiving VMAT to a total of 40 Gy in 15 fractions, CT shows partial response with the mass decreasing to 2.3 × 1.5 cm. c Left adrenal metastatic lesion measuring 3.7 × 4.5 cm. d Two months after receiving VMAT to a total of 20 Gy in 4 fractions, CT shows partial response with the mass decreasing to 1.9 × 1.5 cm
Second-line systemic therapy with avelumab was considered, yet given the limited burden of metastasis, it was felt that a trial of targeted radiotherapy would be a reasonable initial approach. From July to August 2019, he received conformal VMAT to the left adrenal mass (20 Gy in 5 fractions), right parotid (40 Gy in 15 fractions) and involved lymph nodes. Partial response to each site was demonstrated on a CT, 2-months following radiotherapy (Oct 2019) (Fig. 3b, d). However, follow-up brain MRI in October 2019 showed 3 new asymptomatic intracranial lesions (Fig. 4d).
The patient is now 20 months from initial presentation, with excellent performance status and function. He is now being considered again for avelumab.
Methods
Literature review was conducted using a search of PubMed and OVID Medline from inception to March 25, 2020, using the terms “primary parotid” AND “Merkel cell carcinoma” OR “small cell neuroendocrine carcinoma”. Reference lists from sources were hand-searched for additional references. Included references were limited to the English language. Articles were excluded if the disease had metastasized from another site and if there were no details of CK20 testing as an indication of SNECM.
Results
In our literature review, we found 44 cases of parotid SNECM from 14 sources between 1983 and 2019. Aggregating data from these sources, patients presented at a mean age of 66.4 years (range 5–91) with a male-to-female ratio of 3.4 to 1. The majority of patients presented with a rapidly enlarging mass in the parotid region with an average size of 3.7 cm at presentation (range 0.7–8.0 cm). Although immunosuppression such as HIV/AIDs is a reported risk factor and associated with more aggressive disease in Merkel cell carcinoma [20], there is no existing literature regarding immunosuppression in SNECM.
Combining data from existing case reports and case series (Table 1) yields a 1-year survival rate of 78.1% from 32 patients, 2-year survival rate of 66.7% from 27 patients, and a 5-year survival rate of 23.8% from 21 patients. The 5-year survival of parotid gland SNECM appears to be better than the < 10% survival of small cell carcinoma of the lung, but worse than the 40–60% 5-year survival rate of Merkel cell carcinoma [21–23] .
Discussion
Parotid SNEC “Merkel type” is a very rare cancer, with significant morphologic and immunohistochemical overlap with MCC. Although there are now 44 recorded cases of parotid SNECM (Table 1), emphasis in literature has been on the pathology of the disease and prognostic factors. The literature on management is quite limited, especially in the metastatic setting. Nagao et al. described 4 patients with metastasis to distant lymph nodes and 5 patients with distant solid organ metastasis, including the brain, liver and lung [9]. Median overall survival was 21.6 months, although metastatic disease was not discovered upon initial presentation in any of the patients [9].
A chemotherapy regimen of carboplatin-etoposide is typically used for head and neck cancer. Giroulet et al. describes complete response of bone and lymph node metastasis to this regimen in an 87-year old male, though duration of response is unknown [19]. Data from MCC has shown that though some patients respond initially to carboplatin-etoposide (53–61% response rate), responses are short-lived and there is no evidence it provides survival advantage [23]. This is similar to our experience, where our patient had initial partial response that lasted 9 weeks before chemo-resistance developed.
Though there is no literature on the use of biologics in parotid SNECM, we may be able to apply findings from MCC clinical trials, due to their similar morphologic and immunohistochemical characteristics. There are now 3 MCC phase II clinical trials showing high and durable response rates for programmed death 1/programmed death ligand 1 (PD1-PDL1) checkpoint pathway inhibitors (CPI) [1]. In 2016, Nghiem et al. first showed pembrolizumab to have an impressive response rate of 56% involving 26 patients with previously untreated metastatic MCC [24]. Shortly after, Kaufman et al. demonstrated a 33% response rate to avelumab as salvage therapy for 88 patients, with the majority achieving sustained remission at 12 months following treatment.[25, 26] Our patient is now being considered for avelumab for salvage systemic treatment. In oligometastatic disease, it has been suggested that in addition to systemic therapy, local ablative therapy may provide further survival benefit [27].
This is the first paper to describe local treatment of oligometastic parotid SNECM. The oligometastasic state was hypothesized in the 1990s, and since then there has been a growing number of studies showing benefits of local treatment to both intracranial and extracranial oligometastasis. Brain metastasis of any cancer is typically treated with neurosurgery, whole-brain radiotherapy (WBRT) or stereotactic radiosurgery/radiotherapy (SRS/SRT) depending on the size, location and number of lesions. SRS has been especially validated in the setting of limited brain metastasis (1–4 lesions) as an equal and often preferred alternative to WBRT with less neurotoxic effects [28–30]. SRT was chosen in our patient who maintained high function, and we were able to achieve excellent local control with minimal toxicity. Metastasis that occurs outside the irradiated field can be treated as they appear with salvage SRT. The ideal dose and fractionation schemes for SRT are unknown and differ amongst institutions, although we prefer the dose of 35 Gy in 5 fractions. Though uncommon, neurometastatic MCC has been studied in a case series of 40 patients, where all three modalities (WBRT, SRS and neurosurgery) were associated with improved overall survival [31].
Evidence for local treatment of extracranial oligometastasis has been limited to non-randomized observational studies until recent years. There are now several randomized phase II trials showing improved survival in extracranial oligometastasis in cancers such as prostate, colorectal, and non-small cell lung cancer (NSCLC). Two randomized trials involving patients with oligometastatic NSCLC have demonstrated tripling of progression-free survival with locally ablative therapy [2, 32]. In 2018, Ost et al. showed that patients with oligometastatic prostate cancer had an 8-month improvement in androgen deprivation therapy-free survival, when treated with metastatectomy and/or stereotactic body radiotherapy (SBRT) [4]. In 2019, the randomized, phase II, multi-centre SABR-COMET trial involving 99 patients demonstrated a 13-month improvement in median overall survival and doubling of median progression-free survival in patients treated [3]. At this point, phase III trials are yet to be completed, but the evidence is quite promising in support of local therapies for oligometastasis of various cancers, including MCC [26].
One of the limitations of our case is that our patient had a total of 7 metastases at the time of VMAT to the adrenal and parotid gland, whereas the above trials have stricter definitions of oligometastasis, such as under 3–5 total metastases [20–23]. It is currently unknown what the maximum number of metastases is where local therapy provides a benefit, though secondary analysis of clinical trial data from colorectal cancer has suggested that there may be survival benefit with radiofrequency ablation of up to 9 hepatic metastases [27]. The ongoing SABR-COMET-10 trial is currently investigating oligometastasis with 4–10 tumors [33]. Another limitation is that our radiation doses were not ablative, though hypofractionated conformal VMAT also provides local control and may delay disease progression. Finally, due to diagnostic challenges of separating parotid SNECM and MCC, there is likely a cross-contamination of the two entities in the literature and possibly cases that we reported. Many of the parotid SNECM cases in our literature review were named “primary parotid Merkel cell” due to their similarities. A significant percentage (50%) of unknown primary Merkel cell carcinomas occur in the cervical lymph nodes, and it is possible that a subset of these cases actually represents parotid SNECM. On the other hand, a subset of the cases reported as parotid SNECM may be metastatic MCC where the primary skin tumor had regressed [34].
This is the first report of SRT and VMAT treatment in oligometastatic parotid MCC and it adds to the mounting evidence that local conformal RT may help in controlling both intracranial and extracranial oligometastatic cancer.
Conclusion
This study describes a rare case of a patient with a parotid SNECM with oligometastasis to the brain, lymph nodes and adrenal gland. The patient was treated with chemotherapy, SRT and conformal VMAT. At the 20-month follow-up, our patient continues to maintain good functional status with a low burden of disease. There is increasing evidence for improved oncologic outcomes and side effect profiles with conformal and locally ablative therapies for both intracranial and extracranial oligometastases. Further research into the benefits of SRT, SBRT and conformal VMAT for metastatic parotid SNECM should be considered.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Otake S, Goto T. Stereotactic radiotherapy for oligometastasis. Cancers (Basel) 2019;11:133. doi: 10.3390/cancers11020133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gomez DR, Blumenschein GR, Lee JJ, Hernandez M, Ye R, Camidge DR, et al. Local consolidative therapy versus maintenance therapy or observation for patients with oligometastatic non-small-cell lung cancer without progression after first-line systemic therapy: a multicentre, randomised, controlled, phase 2 study. Lancet Oncol. 2016;17:1672–1682. doi: 10.1016/S1470-2045(16)30532-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Palma DA, Olson R, Harrow S, Gaede S, Louie AV, Haasbeek C, et al. Stereotactic ablative radiotherapy versus standard of care palliative treatment in patients with oligometastatic cancers (SABR-COMET): a randomised, phase 2, open-label trial. Lancet. 2019;393:2051–2058. doi: 10.1016/S0140-6736(18)32487-5. [DOI] [PubMed] [Google Scholar]
- 4.Ost P, Reynders D, Decaestecker K, Fonteyne V, Lumen N, De Bruycker A, et al. Surveillance or metastasis-directed therapy for oligometastatic prostate cancer recurrence: a prospective, randomized, multicenter phase II trial. J Clin Oncol. 2018;36:446–453. doi: 10.1200/JCO.2017.75.4853. [DOI] [PubMed] [Google Scholar]
- 5.Chernock RD, Duncavage EJ. Proceedings of the NASHNP Companion Meeting, March 18th, 2018, Vancouver, BC, Canada: salivary neuroendocrine carcinoma-an overview of a rare disease with an emphasis on determining tumor origin. Head Neck Pathol. 2018;12:13–21. doi: 10.1007/s12105-018-0896-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kraemer BB, Mackay B, Batsakis JG. Small cell carcinomas of the parotid gland. A clinicopathologic study of three cases. Cancer. 1983;52:2115–21. doi: 10.1002/1097-0142(19831201)52:11<2115::AID-CNCR2820521124>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 7.Gnepp DR, Wick MR. Small cell carcinoma of the major salivary glands. An immunohistochemical study. Cancer. 1990;66:185–92. doi: 10.1002/1097-0142(19900701)66:1<185::AID-CNCR2820660133>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 8.Fornelli A, Eusebi V, Pasquinelli G, Quattrone P, Rosai J. Merkel cell carcinoma of the parotid gland associated with Warthin tumour: Report of two cases. Histopathology. 2001;39:342–6. doi: 10.1046/j.1365-2559.2001.01240.x. [DOI] [PubMed] [Google Scholar]
- 9.Nagao T, Gaffey TA, Olsen KD, Serizawa H, Lewis JE. Small cell carcinoma of the major salivary glands: clinicopathologic study with emphasis on cytokeratin 20 immunoreactivity and clinical outcome. Am J Surg Pathol. 2004;28:762–770. doi: 10.1097/00000478-200403000-00004. [DOI] [PubMed] [Google Scholar]
- 10.Jorcano S, Casado A, Berenguer J, Arenas M, Rovirosa A, Colomo L. Primary neuroendocrine small cell undifferentiated carcinoma of the parotid gland. Clin Transl Oncol. 2008;10:303–6. doi: 10.1007/s12094-008-0203-z. [DOI] [PubMed] [Google Scholar]
- 11.Ghaderi M, Coury J, Oxenberg J, Spector H. Primary Merkel cell carcinoma of the parotid gland. Ear Nose Throat J. 2010;89:E24–27. doi: 10.1177/014556131008900705. [DOI] [PubMed] [Google Scholar]
- 12.Mulder DC, Rosenberg AJWP, Storm-Bogaard PW, Koole R. Spontaneous regression of advanced Merkel-cell-like small cell carcinoma of the parotid gland. British Journal of Oral and Maxillofacial Surgery. 2010;48:199–200. doi: 10.1016/j.bjoms.2009.06.008. [DOI] [PubMed] [Google Scholar]
- 13.Chernock RD, Duncavage EJ, Gnepp DR, El-Mofty SK, Lewis JS. Absence of Merkel cell polyomavirus in primary parotid high-grade neuroendocrine carcinomas regardless of cytokeratin 20 immunophenotype. Am J Surg Pathol. 2011;35:1806–11. doi: 10.1097/PAS.0b013e318236a9b0. [DOI] [PubMed] [Google Scholar]
- 14.de Biase D, Ragazzi M, Van Asioli S, Eusebi V. Extracutaneous Merkel cell carcinomas harbor polyomavirus DNA. Hum Pathol. 2012;43:980–5. doi: 10.1016/j.humpath.2011.08.014. [DOI] [PubMed] [Google Scholar]
- 15.Kanazawa T, Fukushima N, Tanaka H, Shiba J, Nishino H, Mineta H, et al. Parotid small cell carcinoma presenting with long-term survival after surgery alone: A case report. J Med Case Rep. 2012;6:431. doi: 10.1186/1752-1947-6-431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fisher CA, Harms PW, McHugh JB, Edwards PC, Siddiqui J, Palanisamy N, et al. Small cell carcinoma in the parotid harboring Merkel cell polyomavirus. Oral Surg Oral Med Oral Pathol Oral Radiol. 2014;118:703–12. doi: 10.1016/j.oooo.2014.09.012. [DOI] [PubMed] [Google Scholar]
- 17.Knopf A, Bas M, Hofauer B, Mansour N, Stark T. Clinicopathological characteristics of head and neck Merkel cell carcinomas. Head & Neck. 2017;39:92–7. doi: 10.1002/hed.24540. [DOI] [PubMed] [Google Scholar]
- 18.Bizzarro T, Buda R, Ricci M, Bernardi L. Cytological diagnosis of a rare case of primary Merkel cell carcinoma of the parotid gland. Cytopathology. 2017;28:552–4. doi: 10.1111/cyt.12485. [DOI] [PubMed] [Google Scholar]
- 19.Giroulet F, Tabotta F, Pomoni A, Prior J. Primary parotid Merkel cell carcinoma: a first imagery and treatment response assessment by 18F-FDG PET. BMJ Case Reports. 2019;12. [DOI] [PMC free article] [PubMed]
- 20.Engels EA, Frisch M, Goedert JJ, Biggar RJ, Miller RW. Merkel cell carcinoma and HIV infection. Lancet. 2002;359:497–8. doi: 10.1016/S0140-6736(02)07668-7. [DOI] [PubMed] [Google Scholar]
- 21.Behera M, Ragin C, Kim S, Pillai RN, Chen Z, Steuer CE, et al. Trends, predictors, and impact of systemic chemotherapy in small cell lung cancer patients between 1985 and 2005. Cancer. 2016;122:50–60. doi: 10.1002/cncr.29674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liang E, Brower JV, Rice SR, Buehler DG, Saha S, Kimple RJ. Merkel Cell Carcinoma Analysis of Outcomes: A 30-Year Experience. PLoS One. 2015;10. [DOI] [PMC free article] [PubMed]
- 23.Becker JC, Stang A, DeCaprio JA, Cerroni L, Lebbé C, Veness M, et al. Merkel cell carcinoma. Nat Rev Dis Primers. 2017;1:17077. doi: 10.1038/nrdp.2017.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nghiem PT, Bhatia S, Lipson EJ, Kudchadkar RR, Miller NJ, Kudchadkar RR, et al. PD-1 Blockade with Pembrolizumab in Advanced Merkel-Cell Carcinoma. N Engl J Med. 2016;374:2542–52. doi: 10.1056/NEJMoa1603702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kaufman HL, Russell J, Hamid O, Bhatia S, Terheyden P, D’Angelo SP, et al. Avelumab in patients with chemotherapy-refractory metastatic Merkel cell carcinoma: a multicentre, single-group, open-label, phase 2 trial. Lancet Oncol. 2016;17:1374–85. doi: 10.1016/S1470-2045(16)30364-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kaufman HL, Russell JS, Hamid O, Bhatia S, Terheyden P, D’Angelo SP, et al. Updated efficacy of avelumab in patients with previously treated metastatic Merkel cell carcinoma after ≥1 year of follow-up: JAVELIN Merkel 200, a phase 2 clinical trial. J Immunother Cancer. 2018;6:7. doi: 10.1186/s40425-017-0310-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ruers T, Punt C, Van Coevorden F, Pierie JPEN, Borel-Rinkes I, Ledermann JA, et al. Radiofrequency ablation combined with systemic treatment versus systemic treatment alone in patients with non-resectable colorectal liver metastases: a randomized EORTC Intergroup phase II study (EORTC 40004) Ann Oncol. 2012;23:2619–26. doi: 10.1093/annonc/mds053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Andrews DW, Scott CB, Sperduto PW, Flanders AE, Gaspar LE, Schell MC, et al. Whole brain radiation therapy with or without stereotactic radiosurgery boost for patients with one to three brain metastases: phase III results of the RTOG 9508 randomised trial. Lancet. 2004;363:1665–72. doi: 10.1016/S0140-6736(04)16250-8. [DOI] [PubMed] [Google Scholar]
- 29.Aoyama H, Shirato H, Tago M, Nakagawa K, Toyoda T, Toyoda K, et al. Stereotactic radiosurgery plus whole-brain radiation therapy vs stereotactic radiosurgery alone for treatment of brain metastases: A randomized controlled trial. JAMA. 2006;295:2483–91. doi: 10.1001/jama.295.21.2483. [DOI] [PubMed] [Google Scholar]
- 30.Mazzola R, Corradini S, Gregucci F, Figlia V, Fiorentino A, Alongi F. Role of Radiosurgery/Stereotactic Radiotherapy in Oligometastatic Disease: Brain Oligometastases. Front Oncol. 2019;9. [DOI] [PMC free article] [PubMed]
- 31.Harary M, Kavouridis VK, Thakuria M, Smith TR. Predictors of survival in neurometastatic Merkel cell carcinoma. Eur J Cancer. 2018;101:152–9. doi: 10.1016/j.ejca.2018.07.002. [DOI] [PubMed] [Google Scholar]
- 32.Iyengar P, Wardak Z, Gerber DE, Tumati V, Ahn C, Hughes RS, et al. Consolidative Radiotherapy for Limited Metastatic Non-Small-Cell Lung Cancer: A Phase 2 Randomized Clinical Trial. JAMA Oncol. 2018;4:e173501. doi: 10.1001/jamaoncol.2017.3501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Palma DA, Olson R, Harrow S, Correa RJM, Schneiders F, Haasbeek CJA, et al. Stereotactic ablative radiotherapy for the comprehensive treatment of 4–10 oligometastatic tumors (SABR-COMET-10): Study protocol for a randomized phase III trial. BMC Cancer. 2019;19:816. doi: 10.1186/s12885-019-5977-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ahmadi Moghaddam P, Cornejo KM, Hutchinson L, Tomaszewicz K, Dresser K, Deng A, et al. Complete Spontaneous Regression of Merkel Cell Carcinoma After Biopsy: A Case Report and Review of the Literature. Am J Dermatopathol. 2016;38:e154–8. doi: 10.1097/DAD.0000000000000614. [DOI] [PubMed] [Google Scholar]