Abstract
Intraductal carcinoma (IDC) is a rare salivary gland tumor that is considered analogous to ductal carcinoma in-situ of the breast, demonstrating a complex neoplastic epithelial proliferation surrounded by a continuous layer of presumed non-neoplastic myoepithelial cells. It is subcategorized into intercalated duct, apocrine, and hybrid subtypes based on morphologic and immunohistochemical features, with frequent NCOA4-RET and TRIM27-RET fusions, respectively, seen in intercalated duct and hybrid tumors. However, as an expanding clinicopathologic spectrum of IDC has been documented, controversy has emerged as to whether this tumor type is best defined by its intraductal growth pattern or distinctive molecular and immunophenotypic differentiation. Here, we further explore the nature of IDC by evaluating four cases that arose within intraparotid lymph nodes. These intercalated-duct phenotype tumors with diffuse S100 protein expression demonstrated a crowded and complex epithelial proliferation arranged in cystic, cribriform, and micropapillary architecture, surrounded by an intact myoepithelial cell layer, and were completely intranodal. Of two tumors with tissue available for molecular analysis, one demonstrated a NCOA4-RET fusion and one harbored a STRN-ALK fusion that is novel to IDC. Not only does the intranodal presence of IDC present a challenging differential diagnosis, but the complex nature of this proliferation within lymph node tissue raises questions as to whether the myoepithelial component of IDC is actually non-neoplastic in nature. Furthermore, identification of a STRN-ALK fusion expands the genetic spectrum of IDC and adds to evidence of an emerging role for ALK in salivary gland tumors. Further attention to the nature of the myoepithelial cells and documentation of alternate fusion events in IDC may inform continued discussion about its appropriate classification.
Keywords: Salivary gland neoplasms, Intraductal carcinoma, Intranodal tumors, NCOA4-RET, STRN-ALK
Background
Intraductal carcinoma (IDC) is a rare salivary gland neoplasm that had previously been termed low-grade salivary duct carcinoma and low-grade cribriform cystadenocarcinoma [1–5]. Conceptually regarded as similar to ductal carcinoma in-situ or atypical ductal hyperplasia of the breast, IDC is defined by an expansile neoplastic epithelial proliferation surrounded by a continuous layer of myoepithelial cells presumed to be non-neoplastic. It usually demonstrates low-grade cytology and indolent behavior, although high-grade examples and overtly invasive growth can rarely be seen [6–9]. Recent studies have placed IDC into at least three variants based on morphology and immunohistochemistry: the intercalated duct-like type, which is positive for S100 protein and SOX10; the apocrine type, which expresses AR and GCDFP; and a hybrid or mixed type which demonstrates both intercalated duct-like and apocrine features [7–10]. Emerging molecular data suggests that these variants also have distinct molecular profiles, with recurrent NCOA4-RET rearrangements in the majority of cases with intercalated duct differentiation, TRIM27-RET fusions in cases with hybrid features, and a complex genetic profile including HRAS and PIK3CA hotspot mutations in the apocrine type [7–9, 11–14].
These divergent molecular findings, as well as increasing understanding of the clinicopathologic spectrum of IDC, have engendered controversy regarding its classification. While most cases that show intercalated duct differentiation and NCOA4-RET mutations do demonstrate an intact surrounding myoepithelial layer diagnostic of IDC, several cases with similar morphologic and immunohistochemical findings have been documented with widely invasive growth including lymph node metastasis [7–9]. Skalova, et al. proposed that this subtype of IDC should be renamed “intercalated duct carcinoma” to account for this variability, with further designation of non-invasive or invasive types, and mixed apocrine features [7]. Furthermore, although the purely apocrine subtype of IDC has an excellent prognosis in the absence of high-grade cytology or invasive growth, its molecular profile demonstrates more similarities to salivary duct carcinoma (SDC) than other IDC variants [15–17]. Both Bishop, et al. and Hsieh, et al. recently argued that these findings make low-grade apocrine IDC distinct from tumors with intercalated duct-like features, although its relationship to high-grade apocrine IDC and SDC remains clouded [11, 13].
Additional evaluation of morphologic and molecular variability in the IDC spectrum has the potential to further refine our evolving understanding of this tumor type and to inform ongoing discussion about its optimal taxonomy. One unique finding briefly noted in an early series of IDC was a tumor centered in an intraparotid lymph node with focal extension into adjacent salivary tissue [4]. Two similar case reports of intranodal IDC have been published [18, 19]. Given that several other benign and malignant salivary tumor types are known to rarely arise within intraparotid lymph nodes [20–24], it is tempting to regard this growth pattern as little more than a histologic curiosity and potential diagnostic pitfall. However, extensive involvement of lymph node parenchyma by IDC raises fundamental questions about the nature of a tumor that is currently thought to arise entirely within existing salivary ducts. Here, we present a series of four cases of IDC involving intraparotid lymph nodes, including one that harbored a STRN-ALK fusion novel to this tumor type. We discuss the implications of these findings for diagnosis and classification.
Methods
After institutional review board approval, we identified 4 cases of IDC involving lymph nodes from the authors' consultation files, one of which was published previously [19]. We reviewed all available histologic sections for each case and identified the morphologic features. We documented clinical and demographic information, including any available follow-up data, for each patient.
We performed immunohistochemistry for S100 protein (clone 4C4.9; prediluted; Ventana Medical Systems, Tucson, AZ), smooth muscle actin (clone 1A4; prediluted; Ventana), and either p40 (clone BC28; 1:100 dilution, BioCare Medical, Concord, CA) or p63 (clone 4A4; prediluted; BioCare) on all cases. We also performed Cam 5.2 (clone Cam 5.2; prediluted; Ventana) on 2 cases that had tissue available. Staining was performed on 4 μm whole-slide sections using standardized automated protocols on Ventana BenchMark Ultra autostainers (Ventana) in the presence of appropriate controls.
On 2 cases that had sufficient tissue available, we also performed fluorescence in-situ hybridization (FISH) for RET rearrangement utilizing a dual color DNA probe set (Agilent Technologies, Inc., Santa Clara, CA) that hybridizes to each end of the RET gene in chromosome band 10q11.21, according to manufacturer's instructions. A total of 200 interphase nuclei within an area of tumor were manually enumerated, with separation of the 5′ and 3′ signals in greater than 10% of cells considered positive for RET rearrangement.
We also performed targeted RNA sequencing (RNA-seq) on these 2 cases as described previously [25]. Briefly, whole-slide tissue sections were cut at 10 μm, and Qiagen AllPrep kits (Qiagen, Germantown, MD) were used for RNA isolation. A sequencing library was generated using a modified TruSight RNA Pan-Cancer kit (Illumina, San Diego, CA) with 1425 genes. Sequencing was performed on the NextSeq 550 (Illumina, San Diego, CA) with a minimum of 6 million mapped reads. Fusions are called using the Star-Fusion algorithm [26]. All fusions were manually reviewed via the Integrated Genomics Viewer (Broad Institute, Cambridge,MA).
Results
Clinical and demographic information is summarized in Table 1. The four cases of IDC were from three females and one male with a median age of 73 years (range 59–84 years). All patients initially presented with a mass or slow-growing swelling in the parotid region and underwent surgical resection. The tumors had an average size of 3.2 cm (range 1.2–5.3 cm). While two tumors were prospectively diagnosed as IDC involving intraparotid lymph nodes, one was initially called atypical papillary cystadenoma and one was initially diagnosed as carcinoma ex-lymphadenoma. Neither of the two patients with follow-up information available developed recurrence or metastasis, with one patient having no evidence of disease at 20 months and one patient dead of unrelated causes at 44 months.
Table 1.
Clinical and demographic information of intranodal salivary gland IDC
| Case | Age | Sex | Presentation | Size (cm) | Original diagnosis | Follow-up |
|---|---|---|---|---|---|---|
| 1 | 59 | F | Left parotid mass | 3.5 | Intraductal carcinoma arising in an intraparotid LN | NA |
| 2 | 68 | M | Right parotid mass | 2.8 | Carcinoma ex-lymphadenoma | DUC at 44 months, NED |
| 3 | 80 | F | Left parotid mass | 1.2 | Atypical papillary cystadenoma | NA |
| 4 | 84 | F | Slow-growing parotid swelling | 5.3 | Intraductal carcinoma arising in an intraparotid LN | NED at 20 months |
F female; M male; DUC dead of unrelated causes; LN lymph node; NA not available; NED No evidence of disease
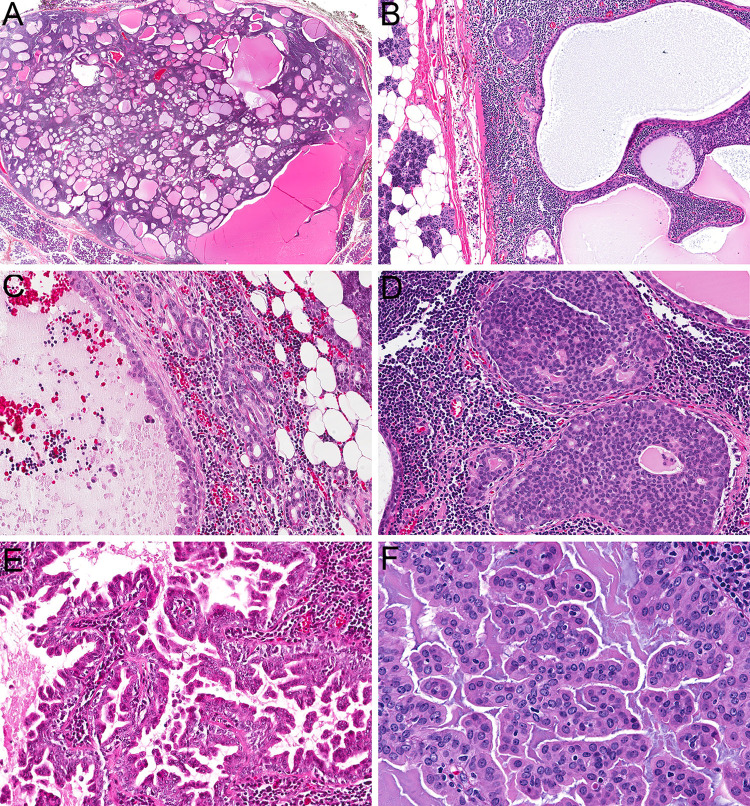
Histologically, all four lesions were well-circumscribed (Fig. 1a) and demonstrated dense lymphoid stroma with recognizable underlying architecture diagnostic for a true lymph node, namely an intact capsule, subcapsular and medullary sinuses, and sinus histiocytes (Fig. 1b). Benign salivary epithelial inclusions were identifiable within the lymph nodes adjacent to the tumor in three cases, in a predominantly paracapsular distribution (Fig. 1c). All four lymph nodes contained a complex epithelial proliferation composed of tubules, nests, cribriform sheets (Fig. 1d), and micropapillary structures with extensive interanastomosing cyst formation (Fig. 1e). While no stromal desmoplasia or hyalinization was noted, the tumors had a crowded and irregular configuration without a clear lobulated growth pattern. In one case, this proliferation extended beyond the lymph node parenchyma to focally involve surrounding tissue. The tumor cells had abundant eosinophilic to amphophilic cytoplasm with bland oval nuclei that demonstrated delicate chromatin and indistinct nucleoli (Fig. 1f).
Fig. 1.
The tumors were well-circumscribed and consisted of a complex epithelial proliferation embedded in dense lymphoid stroma (a, 2x). A well-developed lymph node capsule with sinus histiocytes was recognizable at the periphery of all lesions (b, 10x). Benign salivary inclusions were also present within the lymph node parenchyma adjacent to the tumor cells in three cases (c, 10x). Tumors showed a variety of architectural patterns, including cribriform (d, 20x), cystic, and micropapillary growth (e, 20x). Tumor cells were bland with a moderate amount of eosinophilic cytoplasm and oval nuclei with delicate chromatin and inconspicuous nucleoli (f, 40x)
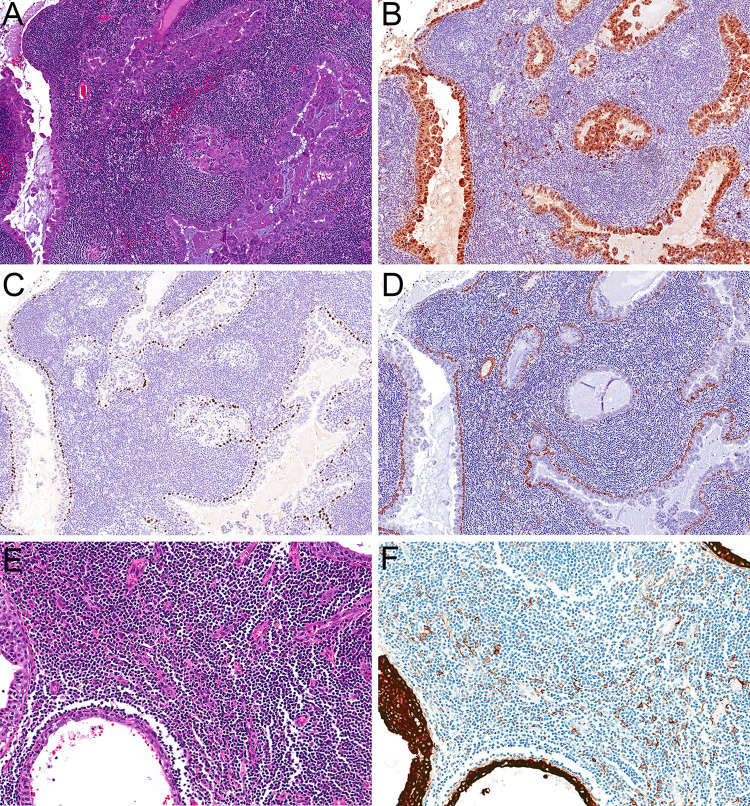
The results of ancillary studies are tabulated in Table 2. Immunohistochemical stains confirmed the diagnosis of salivary gland IDC in all cases (Fig. 2a). The luminal cells in all four tumors were diffusely positive for S100 protein (Fig. 2b), consistent with an intercalated duct-type phenotype. They also showed a continuous peripheral myoepithelial layer throughout, as highlighted by abluminal positivity for p63 or p40 (Fig. 2c) and SMA (Fig. 2d). In two cases that had tissue available for staining (Fig. 2e), low-molecular weight cytokeratin Cam 5.2 highlighted extrafollicular reticulum cells within the background lymphoid stroma (Fig. 2f), confirming the presence of true lymph node architecture [27].
Table 2.
Ancillary testing of intranodal salivary gland IDC
| Case | S100 protein | p63/p40 | SMA | Cam 5.2 | RET FISH | RNA sequencing |
|---|---|---|---|---|---|---|
| 1 | Diffuse | Peripheral | Peripheral | NA | NA | NA |
| 2 | Diffuse | Peripheral | Peripheral | Extrafollicular reticulum cells | Positive | NCOA4-RET |
| 3 | Diffuse | Peripheral | NA | NA | NA | NA |
| 4 | Diffuse | Peripheral | Peripheral | Extrafollicular reticulum cells | Negative | STRN-ALK |
FISH Fluorescence in-situ hybridization; NA not available
Fig. 2.
Immunohistochemistry confirmed classification as the intercalated duct subtype of IDC (a, 20x), with tumor cells diffusely positive for S100 protein (b, 20x) and an intact myoepithelial layer, positive with p40 (c, 20x) and SMA (d, 20x). Immunohistochemistry demonstrated that the lymphoid stroma represented lymph node parenchyma rather than tumor-associated lymphoid proliferation (e, 20x) with positivity for low molecular-weight cytokeratin Cam 5.2 in extrafollicular reticulum cells (f, 20x)
Tissue was also available for molecular testing in two cases. FISH highlighted RET rearrangement in 23.5% of cells in one case, which was subsequently shown to harbor an NCOA4-RET fusion by RNA-Seq. The canonical fusion breakpoints included exons 1–8 of NCOA4 and exons 12–20 of RET. The other case was negative for RET rearrangement by FISH, and demonstrated an alternate STRN-ALK fusion by RNA-Seq. The fusion breakpoints included exon 3 of STRN and exon 20 of ALK. Neither case demonstrated any other fusions.
Discussion
IDC is a rare salivary gland neoplasm that is defined by expansile epithelial growth within an intact myoepithelial layer. It is currently subcategorized into at least three variants—intercalated duct-like, apocrine, and hybrid—on the basis of morphologic and immunohistochemical features. However, as recent publications have better characterized this unique tumor type at a clinicopathological and molecular level, controversy has ensued as to whether IDC is best classified as a single entity based on its intraductal growth pattern or whether its divergent intercalated duct and apocrine differentiation should result in two separate diagnostic categories. Here, we further explore the characteristics and classification of this tumor type by evaluating four cases of IDC that arose within intraparotid gland lymph nodes, including one case with a STRN-ALK fusion novel to this entity.
First, the findings in this series confirm previous case reports that a subset of IDC truly do arise within the parenchyma of intraparotid lymph nodes [4, 18, 19]. While the presence of dense lymphoid tissue intermixed with the IDC cells raised consideration of tumor-associated lymphoid proliferation (TALP), which has previously been described adjacent to IDC [28], all four tumors described here demonstrated well-developed lymph node architecture, including an intact capsule and sinuses with sinus histiocytes. Moreover, immunohistochemistry for low-molecular weight cytokeratin performed on two cases highlighted extrafollicular reticulum cells, confirming that the lymphoid tissue represented true lymph nodes rather than TALP [27]. With this unique intranodal growth pattern, IDC joins a wide range of benign and malignant salivary tumors, including pleomorphic adenoma, Warthin tumor, mucoepidermoid carcinoma, and acinic cell carcinoma, that have all been reported to occasionally arise within lymph node parenchyma [20–24]. In these other tumor types, the ectopic salivary tissue that is frequently seen within intraparotid lymph nodes is speculated to give rise to the intranodal location [29–31]. The presence of benign salivary inclusions adjacent to the tumor in three of our IDC cases further supports this interpretation.
Of course, the presence of IDC within lymph node parenchyma raises a broad differential diagnosis including entities that do not usually overlap with this tumor type. In general, the main diagnostic considerations for IDC include secretory carcinoma and SDC, which, respectively, show morphologic and immunohistochemical overlap with the intercalated duct-like and apocrine variants. Not only must these entities still be distinguished from intranodal IDC via recognition of its myoepithelial component, but involvement of lymph node parenchyma must not be misinterpreted as metastatic disease. However, intranodal IDC also needs to be differentiated from benign neoplasms that are defined by their dense lymphoid stroma. The presence of prominent cystic and papillary architecture raises consideration of Warthin tumor. However, IDC lacks the perfectly paired, well-organized oncocytic bilayer seen in Warthin tumor and shows well-developed myoepithelial (rather than simply basal) differentiation in its abluminal cells. Additionally, areas of nested growth within lymphoid stroma raise consideration of a non-sebaceous lymphadenoma. IDC generally shows more complex microcystic and micropapillary architecture than is acceptable in lymphadenoma and lacks areas of overt squamous differentiation. Moreover, a well-defined layer of myoepithelial cells is not present in lymphadenoma.
More importantly, the complex architecture of the proliferation within these intraparotid lymph nodes also raises fundamental questions about the nature of the myoepithelial cells in IDC. Since its initial description, the characteristic expansile epithelial proliferation in IDC has been compared to ductal carcinoma in-situ or atypical ductal hyperplasia of the breast, with the surrounding myoepithelial layer assumed to be non-neoplastic [1–5]. While the morphologic parallels between these entities are undeniable, it must be pointed out that several other salivary tumor types, including adenoid cystic carcinoma, epithelial-myoepithelial carcinoma, and pleomorphic adenoma are composed of biphasic tumor cell populations that include neoplastic myoepithelial cells. Despite their probable origin from salivary inclusions in the lymph nodes, intranodal IDC show greater architectural confluence and crowding than can easily be accounted for by simple colonization of these scant remnant ducts and even acini. Intuitively, it seems virtually inconceivable that the sheer number of myoepithelial cells demonstrated in these tumors (which ranged up to 5.3 cm) could ever be present within benign lymph node epithelial inclusions, which are almost always less than a few millimeters. This apparent disconnect raises the possibility that the myoepithelial component of the intercalated duct variant of IDC could be neoplastic as well. Further molecular investigation of the origin of these myoepithelial cells may be informative when assessing whether the IDC terminology, or proposed variants thereof, is truly appropriate for this tumor type.
Identification of a novel STRN-ALK fusion in one of the cases in this series also expands the genetic spectrum of salivary gland IDC. As noted above, the molecular profile of IDC appears closely linked to its histologic subtype. The majority of IDC with intercalated duct-like differentiation have recently been shown to harbor recurrent NCOA4-RET fusions, which can produce false-negative FISH results because they involve a subtle intrachromosomal inversion that is easily missed [7–9, 12, 13]. One of the intranodal IDC in this series did demonstrate this characteristic fusion. TRIM27-RET fusions have also been reported as a recurrent genetic event in a few cases of IDC, occurring in those tumors with hybrid intercalated duct and apocrine features [7, 8, 14]. Meanwhile, IDC with pure apocrine differentiation often show more complex genetic profiles with multiple mutations including PIK3CA and HRAS hotspot mutations that overlap with those seen in SDC [9, 11, 13]. Only rare cases with alternate fusions have been reported to date, including a TUT1-ETV5 fusion in a tumor with intercalated duct-like differentiation and a KIAA1217-RET fusion in a tumor with hybrid intercalated duct and apocrine features [7]. The STRN-ALK fusion identified in this series represents a third alternate fusion event in the intercalated duct-like variant of IDC. Identification of ALK rearrangement in a tumor that shows well-developed morphologic and immunohistochemical features of the intercalated duct-type variant of IDC adds to increasing evidence that this tumor cannot be entirely defined by one or two fusions.
This finding also expands the small but emerging role for ALK fusions in salivary gland carcinomas. Given that RET and ALK rearrangements play an interchangeable oncogenic role in several tumor types, including papillary thyroid carcinoma and lung adenocarcinoma, it is not entirely surprising that an ALK fusion can drive IDC as well. Indeed, a STRN-ALK fusion was initially reported as a recurrent genetic event in papillary thyroid carcinoma, where it is associated with aggressive behavior [32–35]. The same fusion has since been documented to occur in lung adenocarcinomas, colon adenocarcinomas, renal cell carcinomas and pancreatic intratubular papillary neoplasms [36–40]. Although ALK involvement has never previously been reported in IDC, ALK rearrangements have recently been identified in a few other salivary carcinomas, including one case of secretory carcinoma with a CTNNA1-ALK fusion and two cases of SDC with HNRNPH3-ALK and EML4-ALK fusions [16, 41]. Examples of SDC reported with NCOA4-RET fusions have previously been speculated to represent the high grade end of the spectrum of tumors with intercalated duct-like differentiation [7]; it is possible that these SDC with ALK fusions may be part of a similar phenomenon. Regardless, evaluation for ALK as well as RET fusions in rare cases of IDC with aggressive disease may allow for additional targeted therapy options.
In summary, this study highlights a series of IDC that demonstrate intercalated duct-type differentiation and arise within the parenchyma of intraparotid gland lymph nodes. The presence of a complex epithelial and myoepithelial proliferation involving lymph node tissue in these cases not only presents a challenging differential diagnosis that includes benign and malignant entities, but it also raises questions as to whether the myoepithelial component of IDC is actually non-neoplastic in nature. This study also reports the first case of IDC with a STRN-ALK fusion. The presence of this alternate rearrangement confirms that IDC cannot be entirely defined by specific molecular events and expands an emerging role for ALK in salivary gland tumors. Further attention to molecular findings within the myoepithelial cells and documentation of alternate fusion patterns may inform continued discussion about the appropriate classification of IDC.
Funding
This study was funded in part by the Jane B. and Edwin P. Jenevein, MD Endowment for Pathology at UT Southwestern Medical Center.
Compliance with Ethical Standards
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Brandwein-Gensler M, Gnepp DR. Low-grade cribriform cystadenocarcinoma. In: Barnes L, Eveson JW, Reichart P, Sidransky D, editors. Pathology and genetics of head and neck tumours. World Health Organization classification of tumours. Lyon: IARC; 2005. p. 430. [Google Scholar]
- 2.Brandwein-Gensler M, Hille J, Wang BY, Urken M, Gordon R, Wang LJ, et al. Low-grade salivary duct carcinoma: description of 16 cases. Am J Surg Pathol. 2004;28(8):1040–1044. doi: 10.1097/01.pas.0000128662.66321.be. [DOI] [PubMed] [Google Scholar]
- 3.Chen KT. Intraductal carcinoma of the minor salivary gland. J Laryngol Otol. 1983;97(2):189–191. doi: 10.1017/S002221510009397X. [DOI] [PubMed] [Google Scholar]
- 4.Delgado R, Klimstra D, Albores-Saavedra J. Low grade salivary duct carcinoma. A distinctive variant with a low grade histology and a predominant intraductal growth pattern. Cancer. 1996;78(5):958–967. doi: 10.1002/(SICI)1097-0142(19960901)78:5<958::AID-CNCR4>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 5.Loening T, Leivo I, Simpson RHW, Weinreb I. Intraductal carcinoma. In: El-Naggar A, Chan JK, Grandis JR, Takata T, Slootweg PJ, editors. WHO classification of head and neck tumours. Lyon: International Agency for Research on Cancer; 2017. pp. 170–171. [Google Scholar]
- 6.Simpson RH, Desai S, Di Palma S. Salivary duct carcinoma in situ of the parotid gland. Histopathology. 2008;53(4):416–425. doi: 10.1111/j.1365-2559.2008.03135.x. [DOI] [PubMed] [Google Scholar]
- 7.Skalova A, Ptakova N, Santana T, Agaimy A, Ihrler S, Uro-Coste E, et al. NCOA4-RET and TRIM27-RET are characteristic gene fusions in salivary intraductal carcinoma, including invasive and metastatic tumors: is "intraductal" correct? Am J Surg Pathol. 2019;43(10):1303–1313. doi: 10.1097/PAS.0000000000001301. [DOI] [PubMed] [Google Scholar]
- 8.Skalova A, Vanecek T, Uro-Coste E, Bishop JA, Weinreb I, Thompson LDR, et al. Molecular profiling of salivary gland intraductal carcinoma revealed a subset of tumors harboring NCOA4-RET and novel TRIM27-RET fusions: a report of 17 cases. Am J Surg Pathol. 2018;42(11):1445–1455. doi: 10.1097/PAS.0000000000001133. [DOI] [PubMed] [Google Scholar]
- 9.Weinreb I, Bishop JA, Chiosea SI, Seethala RR, Perez-Ordonez B, Zhang L, et al. Recurrent RET Gene Rearrangements in Intraductal Carcinomas of Salivary Gland. Am J Surg Pathol. 2018;42(4):442–452. doi: 10.1097/PAS.0000000000000952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Weinreb I, Tabanda-Lichauco R, Van der Kwast T, Perez-Ordonez B. Low-grade intraductal carcinoma of salivary gland: report of 3 cases with marked apocrine differentiation. Am J Surg Pathol. 2006;30(8):1014–1021. doi: 10.1097/00000478-200608000-00013. [DOI] [PubMed] [Google Scholar]
- 11.Bishop JA, Gagan J, Krane JF, Jo VY. Low-grade apocrine intraductal carcinoma: expanding the morphologic and molecular spectrum of an enigmatic salivary gland tumor. Head Neck Pathol. 2020 doi: 10.1007/s12105-020-01128-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guilmette J, Dias-Santagata D, Nose V, Lennerz JK, Sadow PM. Novel gene fusions in secretory carcinoma of the salivary glands: enlarging the ETV6 family. Hum Pathol. 2019;83:50–58. doi: 10.1016/j.humpath.2018.08.011. [DOI] [PubMed] [Google Scholar]
- 13.Hsieh MS, Lee YH, Jin YT, Kuo YJ. Clinicopathological study of intraductal carcinoma of the salivary gland, with emphasis on the apocrine type. Virchows Arch. 2020 doi: 10.1007/s00428-020-02823-7. [DOI] [PubMed] [Google Scholar]
- 14.Lu H, Graham RP, Seethala R, Chute D. Intraductal carcinoma of salivary glands harboring TRIM27-RET fusion with mixed low grade and apocrine types. Head Neck Pathol. 2020;14(1):239–245. doi: 10.1007/s12105-018-0996-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dalin MG, Desrichard A, Katabi N, Makarov V, Walsh LA, Lee KW, et al. Comprehensive molecular characterization of salivary duct carcinoma reveals actionable targets and similarity to apocrine breast cancer. Clin Cancer Res. 2016;22(18):4623–4633. doi: 10.1158/1078-0432.CCR-16-0637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dogan S, Ng CKY, Xu B, Kumar R, Wang L, Edelweiss M, et al. The repertoire of genetic alterations in salivary duct carcinoma including a novel HNRNPH3-ALK rearrangement. Hum Pathol. 2019;88:66–77. doi: 10.1016/j.humpath.2019.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Luk PP, Weston JD, Yu B, Selinger CI, Ekmejian R, Eviston TJ, et al. Salivary duct carcinoma: Clinicopathologic features, morphologic spectrum, and somatic mutations. Head Neck. 2016;38(Suppl 1):E1838–E1847. doi: 10.1002/hed.24332. [DOI] [PubMed] [Google Scholar]
- 18.Lin SC, Ko RT, Kang BH, Wang JS. Intraductal carcinoma of salivary gland originating from an intraparotid lymph node: a case report. Malays J Pathol. 2019;41(2):207–211. [PubMed] [Google Scholar]
- 19.Weinreb I. Intraductal carcinoma of salivary gland (so-called low-grade cribriform cystadenocarcinoma) arising in an intraparotid lymph node. Head Neck Pathol. 2011;5(3):321–325. doi: 10.1007/s12105-011-0256-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Barakat N, Salman S, Nassar VH. Mucoepidermoid carcinoma in a lymph node of the parotid sheath simulating condylar tumor. Int J Oral Surg. 1973;2(1):26–30. doi: 10.1016/S0300-9785(73)80014-6. [DOI] [PubMed] [Google Scholar]
- 21.Minic AJ. Acinic cell carcinoma arising in a parotid lymph node. Int J Oral Maxillofac Surg. 1993;22(5):289–291. doi: 10.1016/S0901-5027(05)80518-1. [DOI] [PubMed] [Google Scholar]
- 22.Perzin KH, Livolsi VA. Acinic cell carcinoma arising in ectopic salivary gland tissue. Cancer. 1980;45(5):967–972. doi: 10.1002/1097-0142(19800301)45:5<967::AID-CNCR2820450522>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 23.Smith A, Winkler B, Perzin KH, Wazen J, Blitzer A. Mucoepidermoid carcinoma arising in an intraparotid lymph node. Cancer. 1985;55(2):400–403. doi: 10.1002/1097-0142(19850115)55:2<432::AID-CNCR2820550223>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 24.Wedell B, Burian P, Dahlenfors R, Stenman G, Mark J. Cytogenetical observations in a mucoepidermoid carcinoma arising from heterotopic intranodal salivary gland tissue. Oncol Rep. 1997;4(3):515–516. doi: 10.3892/or.4.3.515. [DOI] [PubMed] [Google Scholar]
- 25.Bishop JA, Gagan J, Baumhoer D, McLean-Holden AL, Oliai BR, Couce M, et al. Sclerosing polycystic "adenosis" of salivary glands: a neoplasm characterized by PI3K pathway alterations more correctly named sclerosing polycystic adenoma. Head Neck Pathol. 2019 doi: 10.1007/s12105-019-01088-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Haas BJ, Dobin A, Li B, Stransky N, Pochet N, Regev A. Accuracy assessment of fusion transcript detection via read-mapping and de novo fusion transcript assembly-based methods. Genome Biol. 2019;20(1):213. doi: 10.1186/s13059-019-1842-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kurian EM, Miller R, McLean-Holden AL, Oliai BR, Bishop JA. Low Molecular weight cytokeratin immunostaining for extrafollicular reticulum cells is an effective means of separating salivary gland tumor-associated lymphoid proliferation from true lymph node involvement. Head Neck Pathol. 2019 doi: 10.1007/s12105-019-01080-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nishijima T, Yamamoto H, Nakano T, Hatanaka Y, Taguchi KI, Masuda M, et al. Low-grade intraductal carcinoma (low-grade cribriform cystadenocarcinoma) with tumor-associated lymphoid proliferation of parotid gland. Pathol Res Pract. 2017;213(6):706–709. doi: 10.1016/j.prp.2017.02.019. [DOI] [PubMed] [Google Scholar]
- 29.Shinohara M, Harada T, Nakamura S, Oka M, Tashiro H. Heterotopic salivary gland tissue in lymph nodes of the cervical region. Int J Oral Maxillofac Surg. 1992;21(3):166–171. doi: 10.1016/S0901-5027(05)80787-8. [DOI] [PubMed] [Google Scholar]
- 30.Teymoortash A. Back to the roots of Warthin's tumor of the parotid gland. Eur Arch Otorhinolaryngol. 2013;270(9):2397–2402. doi: 10.1007/s00405-012-2309-8. [DOI] [PubMed] [Google Scholar]
- 31.Weiler C, Agaimy A, Zengel P, Zenk J, Kirchner T, Ihrler S. Nonsebaceous lymphadenoma of salivary glands: proposed development from intraparotid lymph nodes and risk of misdiagnosis. Virchows Arch. 2012;460(5):467–472. doi: 10.1007/s00428-012-1225-z. [DOI] [PubMed] [Google Scholar]
- 32.Bastos AU, de Jesus AC, Cerutti JM. ETV6-NTRK3 and STRN-ALK kinase fusions are recurrent events in papillary thyroid cancer of adult population. Eur J Endocrinol. 2018;178(1):83–91. doi: 10.1530/EJE-17-0499. [DOI] [PubMed] [Google Scholar]
- 33.Kelly LM, Barila G, Liu P, Evdokimova VN, Trivedi S, Panebianco F, et al. Identification of the transforming STRN-ALK fusion as a potential therapeutic target in the aggressive forms of thyroid cancer. Proc Natl Acad Sci USA. 2014;111(11):4233–4238. doi: 10.1073/pnas.1321937111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Panebianco F, Nikitski AV, Nikiforova MN, Kaya C, Yip L, Condello V, et al. Characterization of thyroid cancer driven by known and novel ALK fusions. Endocr Relat Cancer. 2019;26(11):803–814. doi: 10.1530/ERC-19-0325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Perot G, Soubeyran I, Ribeiro A, Bonhomme B, Savagner F, Boutet-Bouzamondo N, et al. Identification of a recurrent STRN/ALK fusion in thyroid carcinomas. PLoS ONE. 2014;9(1):e87170. doi: 10.1371/journal.pone.0087170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Basturk O, Berger MF, Yamaguchi H, Adsay V, Askan G, Bhanot UK, et al. Pancreatic intraductal tubulopapillary neoplasm is genetically distinct from intraductal papillary mucinous neoplasm and ductal adenocarcinoma. Mod Pathol. 2017;30(12):1760–1772. doi: 10.1038/modpathol.2017.60. [DOI] [PubMed] [Google Scholar]
- 37.Kusano H, Togashi Y, Akiba J, Moriya F, Baba K, Matsuzaki N, et al. Two cases of renal cell carcinoma harboring a novel STRN-ALK fusion gene. Am J Surg Pathol. 2016;40(6):761–769. doi: 10.1097/PAS.0000000000000610. [DOI] [PubMed] [Google Scholar]
- 38.Yakirevich E, Resnick MB, Mangray S, Wheeler M, Jackson CL, Lombardo KA, et al. Oncogenic ALK fusion in rare and aggressive subtype of colorectal adenocarcinoma as a potential therapeutic target. Clin Cancer Res. 2016;22(15):3831–3840. doi: 10.1158/1078-0432.CCR-15-3000. [DOI] [PubMed] [Google Scholar]
- 39.Yang Y, Qin SK, Zhu J, Wang R, Li YM, Xie ZY, et al. A rare STRN-ALK fusion in lung adenocarcinoma identified using next-generation sequencing-based circulating tumor DNA profiling exhibits excellent response to crizotinib. Mayo Clin Proc Innov Qual Outcomes. 2017;1(1):111–116. doi: 10.1016/j.mayocpiqo.2017.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nakanishi Y, Masuda S, Iida Y, Takahashi N, Hashimoto S. Case report of non-small cell lung cancer with STRN-ALK translocation: a nonresponder to alectinib. J Thorac Oncol. 2017;12(12):e202–e204. doi: 10.1016/j.jtho.2017.08.009. [DOI] [PubMed] [Google Scholar]
- 41.Sasaki E, Masago K, Fujita S, Suzuki H, Hanai N, Hosoda W. Salivary secretory carcinoma harboring a novel ALK fusion: expanding the molecular characterization of carcinomas beyond the ETV6 gene. Am J Surg Pathol. 2020;44:962–969. doi: 10.1097/PAS.0000000000001471. [DOI] [PubMed] [Google Scholar]