Abstract
Granular cell tumors (GCT) are rare soft tissue tumors that involve the head and neck in 50% of patients. Two distinct variants of GCT, one benign (bGCT) and the other malignant (mGCT), involving the hypopharynx, a subsite of the larynx, are presented here. The clinical presentations, radiographic features, pathologic diagnosis in these two variants of GCT are discussed. The mGCT was diagnosed only after complete tumor excision. This report highlights the importance of complete excision of the tumor mass, as diagnosis of mGCT can be exceedingly difficult to make on a small biopsy specimen. Therefore, complete excision is recommended for definitive diagnosis and treatment of GCTs.
Keywords: Granular cell tumor, Hypopharynx, Surgery, Malignant, Benign, Atypia, Necrosis, Metastasis
Introduction
In 1926, Russian pathologist Dr. Alexei Abrikossoff first described the myoblastenmyome tumor today termed granular cell tumor (GCT) [1]. These tumors were originally thought to be myogenic in origin based on microscopic appearance, but now are recognized as neural or Schwann cell etiology based on electron microscope studies [2, 3]. GCTs are rare, representing 0.5% of all soft tissue tumors; they are more common in men in general with a predilection for women in oral subsites; and typically occur between the fourth and sixth decades of life [3]. Approximately 50% of these tumors occur in the head and neck with 75% of oral cavity tumors occurring in the anterior two-thirds of the oral tongue [1]. Oral cavity tumors tend to be asymptomatic; however, when the larynx or hypopharynx are involved, globus sensation, hoarseness and dysphagia are not uncommon at presentation [4]. The majority of these tumors are solitary, but in 5–16% of patients there can be multiple tumors present [1, 4]. Less than 2% of tumors demonstrate malignant characteristics and tumors arising in the head and neck have less malignant potential than other sites such as the lower extremities [3]. In the review of 110 patients with GCT by Lack et al., there was an 8% recurrence rate following surgical resection, the recommended treatment modality [1].
GCT is a non-encapsulated, locally infiltrative tumor that is positive for S100, which helps to differentiate it from other tumors such as rhabdomyoma, leiomyoma, and oncocytomas [3, 5, 6]. Pathologic determination of malignancy is important and the Fanburg-Smith criteria are used to help define this. Fanburg-Smith criteria include the following: necrosis, tumor cell spindling, vesicular nuclei with large nucleoli, increased rate of mitosis with more than 2 mitosis per 10 hpf, a high nuclear to cystoplasmic ratio, and pleomorphism [7]. Tumors with none of the criteria above or focal pleomorphism are considered benign. Two criteria indicate an atypical GCT (aGCT) and three or more indicate a mGCT [7]. The majority (98%) of GCTs are benign in nature, however, it is important to identify malignant cases as 50% of the cases metastasize to regional lymph nodes, lung or bone [7–9]. Computed Tomography (CT) and Magnetic Resonance Imaging (MRI) of the head and neck with contrast may be used to evaluate these patients. GCT is characterized as a solid mass with homogenous enhancement on contrast enhanced CT imaging and is characterized by a hypointense homogenous signal on contrast enhanced T1 sequence MRI [4]. There is not a unified treatment guideline dictating the interval for follow-up surveillance visits. Therefore, the timing of follow-up visits is largely left to the discretion of the treating physician. In general, it is our practice to see patients with bGCT two weeks post-operatively for a wound check, then again at 3 months to evaluate for any signs of recurrent disease. In patients who are diagnosed with mGCT it is our practice to see these patients at a two-week post-operative interval and then every 6–8 weeks for the first year following resection to closely monitor for signs of recurrent disease.
In the following case reports, we describe two distinct variants of GCT of the head and neck with unusual presentation in the hypopharyngeal/paralaryngeal location. The first patient presented with multifocal benign pathology. The second patient’s diagnosis was determined to be malignant only on final pathology after complete surgical resection.
Case Reports
Case 1: Benign Granular Cell Tumor (bGCT)
A 35 year old female, with hypertension presented with a 2-year history of an asymptomatic slowly growing oral tongue mass. On physical exam, she was found to have a 1 cm × 1.5 cm submucosal mobile dorsal oral tongue mass. An MRI of the neck with and without intravenous contrast revealed an incidental left 6.5 cm neck mass extending in a submucosal plane along the left lateral laryngeal surface to the left pyriform sinus. CT of neck with intravenous contrast (Figs. 1 and 2) additionally demonstrated possible prevertebral involvement and a Positron Emission Tomography (PET) CT was recommended. PET CT demonstrated hypermetabolic activity in the mass with SUV of 5.3. She was taken to the operating room for direct laryngoscopy with biopsy confirming bGCT on final pathology. Surgical resection was recommended by multidisciplinary tumor board for both the oral tongue lesion as well as the hypopharyngeal mass.
Fig. 1.
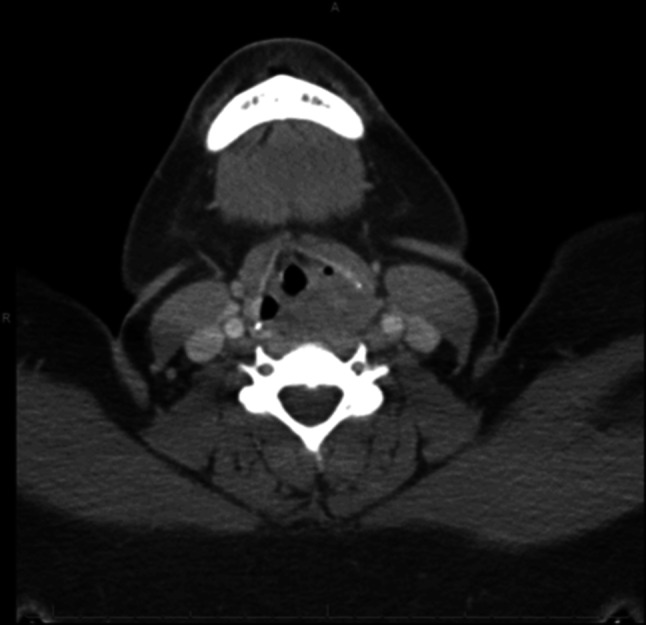
Case 1,- Benign granular cell tumor, Axial section CT with contrast demonstrates paralaryngeal involvement
Fig. 2.
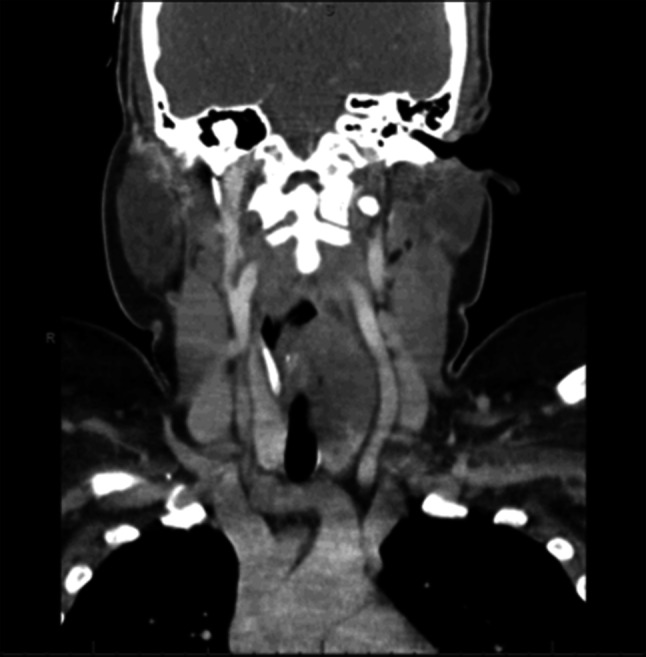
Case 1- Benign granular cell tumor, Coronal section CT with contrast demonstrates lateral displacement of common carotid artery
A right partial glossectomy was performed to remove the oral tongue mass with bGCT measuring 0.8 cm in largest diameter and negative margins on final pathology. In addition, the hypopharyngeal mass was excised with a partial pharyngectomy using a transcervical approach. This was closed primarily. On final pathology, bGCT was confirmed measuring 4.5 cm in greatest diameter with negative margins and no morphologic features to suggest malignancy using Fanburg-Smith criteria (Figs. 3, 4). The two masses were distinct. A limited neck dissection was performed for surgical access purposes. Eight lymph nodes were reviewed without signs of metastatic disease.
Fig. 3.
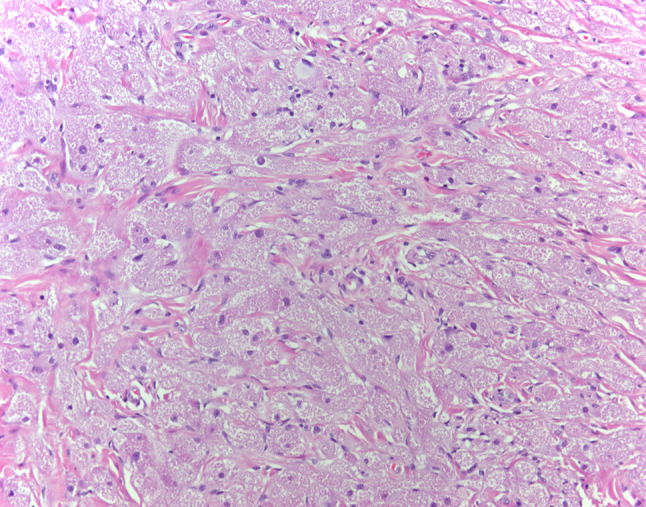
Case 1- Benign granular cell tumor, Sheets of polygonal cells with abundant granular eosinophilic cytoplasm. Nuclei are small with dense chromatin (HES × 20)
Fig. 4.
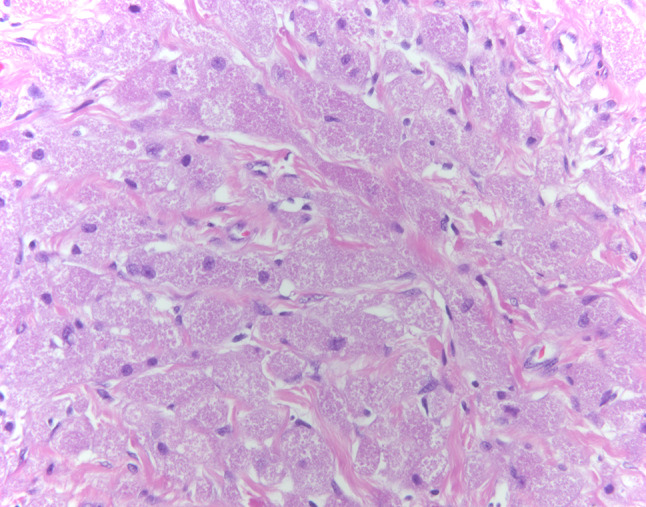
Case 1-Benign granular cell tumor, Polygonal cells with abundant granular cytoplasm and inconspicuous nucleoli. No atypical features are present (HES × 40)
The patient is currently 6 months out from surgery without signs of recurrence and has normal speech and swallowing function.
Case 2: Malignant Granular Cell Tumor (mGCT)
An otherwise healthy 39 year-old female, with a remote tobacco use history, was evaluated in our clinic for a 3-month history of symptomatic left neck mass. She reported dysphagia to solids, odynophagia, left sided otalgia and weight loss. Physical exam revealed a large left neck mass that was palpable in the upper and mid neck regions. Intraoral exam revealed fullness at the inferior pole of the left palatine tonsil with tongue mobility intact. Flexible laryngoscopy was performed and showed submucosal fullness involving the left lateral pharynx, pyriform sinus and left aryepiglottic fold. There was mass effect on the larynx and the left true vocal fold could not be visualized. CT neck with contrast identified a 5.2 cm left parapharyngeal space mass extending in a submucosal plane to the left pyriform sinus and left aryepiglottic fold. There was lateral displacement of the hyoid and carotid sheath (Figs. 5, 6). No enlarged lymph nodes were identified. A MRI confirmed the findings on CT imaging, but could not confirm direct true vocal fold involvement. A core biopsy was obtained from her referring center and was read as aGCT, suspicious for malignancy. Given her presenting symptom of progressive airway obstruction, tracheostomy for airway protection was performed. At the same time, a biopsy of the mass in the left pharynx was obtained with final pathology reporting aGCT without overt signs of malignancy.
Fig. 5.
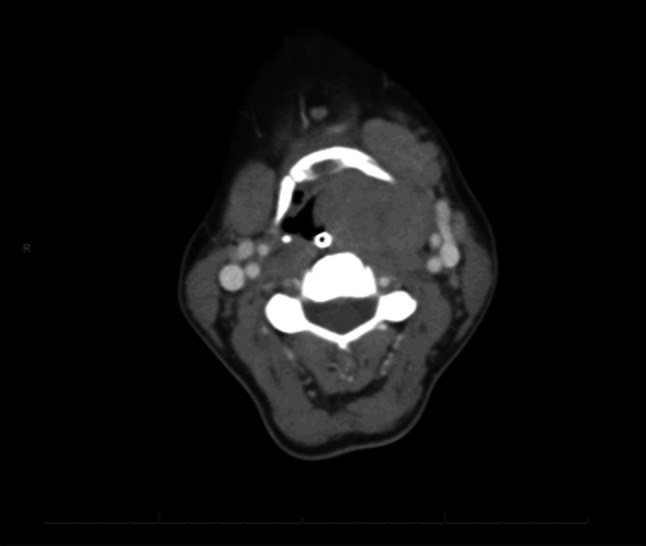
Case 2- Malignant granular cell tumor, Axial section CT with contrast demonstrates rotation of the hyoid, nasogastric tube visualized
Fig. 6.
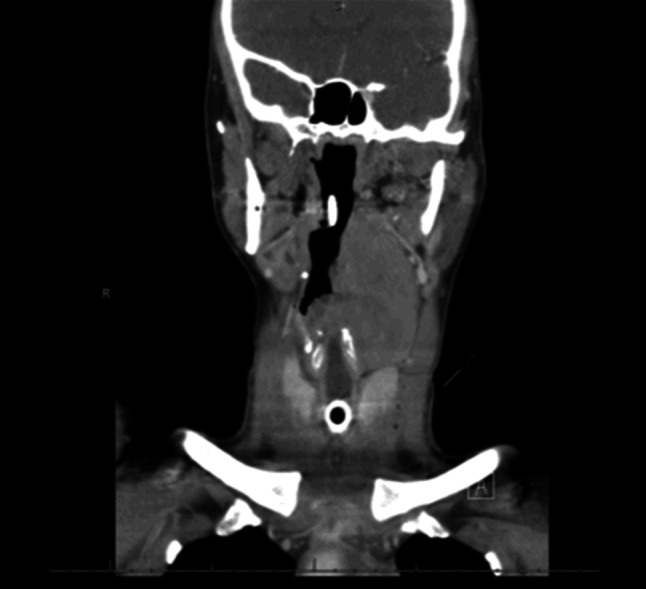
Case 2- Malignant granular cell tumor, Coronal section CT with contrast demonstrates paralaryngeal involvement with tracheostomy tube in place
She underwent laryngeal-sparing surgery with left partial pharyngectomy, partial vertical laryngectomy and left neck exposure. She underwent pharyngeal reconstruction with an ulnar free flap. On final pathology, the tumor was diagnosed as mGCT measuring 6.8 × 4.5 × 3.2 cm in dimension (Fig. 7). The mass was graded as 2/3 and staged pT2N0M0 based on AJCC 8th edition sarcoma staging of the head and neck. Two lymph nodes were reviewed without metastatic deposits. Pathological examination of the tumor exhibited cellular pleomorphism, increased nuclear:cytoplasmic ratio, necrosis and spindling; consistent with mGCT (Figs. 8, 9).
Fig. 7.
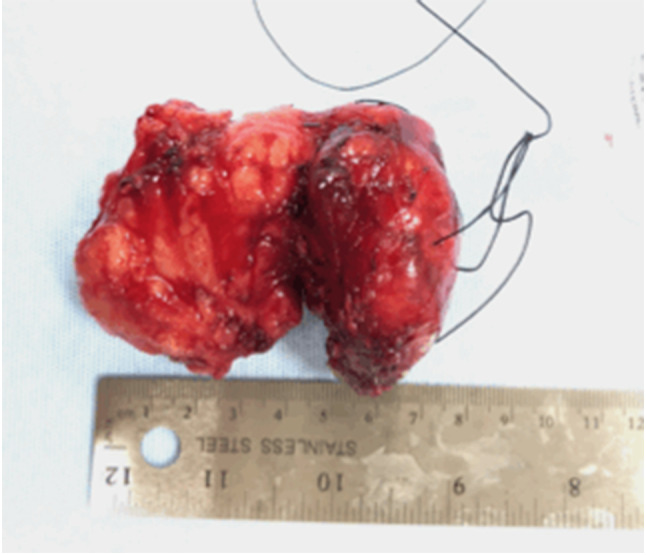
Case 2- Malignant granular cell tumor, Gross Specimen removed from neck, pharynx and hypopharynx
Fig. 8.
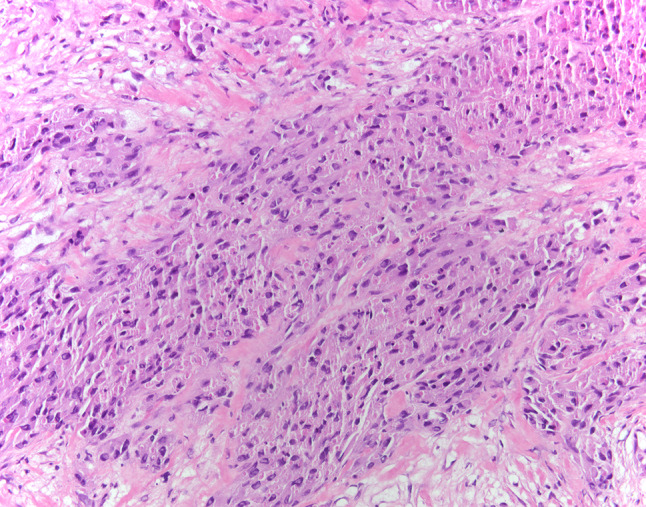
Case 2- Malignant granular cell tumor, sheets of spindle and polygonal cells with granular cytoplasm. (HES × 20)
Fig. 9.
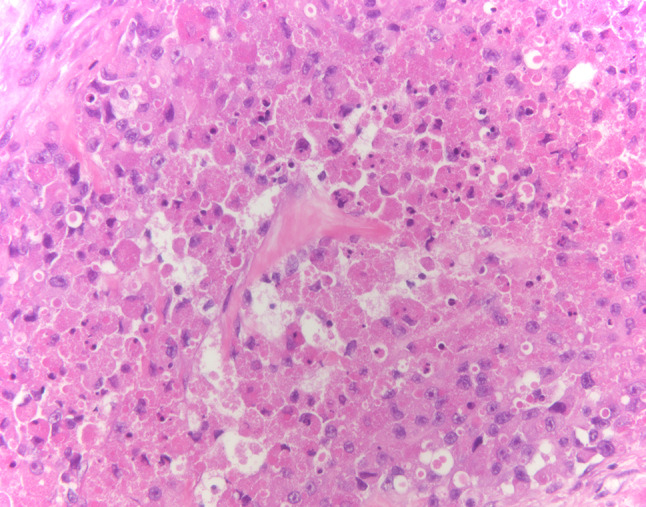
Case 2- Malignant granular cell tumor with areas of spindling and nuclear atypia (HES × 40)
Post-operative radiation therapy was recommended and, on four-week post-operative simulation planning CT, a newly enlarged contralateral lymph node was identified. This was biopsied and confirmed as metastatic mGCT. Approximately six weeks after her initial surgical resection, she underwent contralateral right neck dissection with one of 12 lymph nodes positive for disease with extranodal extension identified; therefore, she was upstaged to pT2bN1M0. Following her neck dissection, she completed a course of adjuvant radiation therapy of 66 Gy and has started surveillance.
Discussion
We present two distinct cases of GCT that share similar demographics, but differ in clinical presentation and tumor histopathology. The first case was bGCT with multifocal presentation involving the anterior 2/3 of the oral tongue and contralateral hypopharynx. The patient was asymptomatic despite the large size of the hypopharyngeal mass. The second case with mGCT presented with more acute symptoms including dysphagia, odynophagia and ipsilateral otalgia. The tumor in the patient with mGCT also had epicenter in the hypopharynx, and exhibited compression on the larynx requiring airway management with placement of a tracheostomy. The first important point to make in this discussion is that, in head and neck, GCT can present with variable symptomatology and presentation. Both patients underwent surgical resection of their tumors and fully regained swallowing function post-operatively, as evaluated by both subjective and objective means.
The second important point to make in this discussion is that in our second case, mGCT was firmly diagnosed by histopathology only after complete tumor resection. This highlights the fact that, even in the hands of very experienced head and neck pathologists, the diagnosis of mGCT can be challenging because large tumors can be histologically heterogeneous contributing to sampling error. Therefore, complete resection is recommended for definitive diagnosis.
Surgical resection is the standard treatment for GCT regardless of histopathology finding. GCT grows submucosally, unlike squamous cell carcinoma, which grows along the mucosa. Large mucosal resections, as done for squamous cell cancers may not be necessary and importantly, laryngeal preservation should be considered when possible. The risk of recurrence reported for both benign and malignant GCT is 2–3%, but can rise to 21% if there is a positive margin after excision [10]. The third important point to make in this discussion is that complete tumor excision with negative margins is recommended and the potential use of free tissue microvascular reconstruction should be considered to optimize functional outcome in the patients with head and neck GCT. In the case described by Krishnamurthy et al., the patient was diagnosed with mGCT, requiring a total glossectomy for tumor extirpation followed by free tissue reconstruction [9]. Similarly, in our second case, the large resection of pharyngeal mucosa resected required free tissue microvascular reconstruction to optimize swallowing outcome. This required careful surgical planning by experienced surgical and reconstructive teams.
A number of institutional as well as SEER based retrospective studies have been published, evaluating overall survival, risk of lymph node metastasis, and rate of distal metastasis in patients with mGCT of all sites. These studies and their pertinent findings are summarized below in Table 1. Head and neck mGCT are rare and comprise a very small percentage of patients represented in these studies. However, there are no population-based studies to determine head and neck specific recurrence and/or metastasis rates of mGCT. With that said, mGCT involving all sites have a reported mortality rate of up to 40% with 5 and 10 year overall survival of 52% and 26% respectively [10, 11]. In the series published by Imanishi et al., all patients diagnosed with aGCT or mGCT who had recurrence or distant metastatic disease died of their disease. Moreover, the authors found that necrosis was statistically associated with local recurrence [11].
Table 1.
Literature review of Studies on Granular Cell Tumor between 1980 and 2018
| Author | Title | Journal | Year | Findings |
|---|---|---|---|---|
| Mirza FN, Tuggle CT, Zogg CK, Mirza HN, Narayan D | Epidemiology of Malignant Cutaneous Granular Cell Tumors: A United States population-based cohort analysis using the Surveillance, Epidemiology, and End Results (SEER) database | J Am Acad Dermatol | 2018 | DSS for local, regional and distant mGTC: 20yrs, < 5 yrs, < 0.5yrs respectively |
| Moten AS, Movva S, von Mehren M, Wu H, Esnaola NF, Reddy SS, Farma JM | Granular cell tumor experience at a comprehensive cancer center | J Surg Res | 2018 | Institutional review of 50 GTC patients: 4% malignancy rate, 6% multifocal on presentation |
| Moten AS, Zhao H, Wu H, Farma JM | Malignant granular cell tumor: Clinical features and long-term survival | J Surg Oncol | 2018 | 113 mGCT patients: 4.0 cm average T size, 13% lymph node metastasis, 11% distal metastasis, poorer survival in patients with T size > 5 cm |
| Imanishi J, Yazawa Y, Saito T, Shimizu M, Kawashima H, Ae K, Matsumine A, Torigoe T, Sugiura H, Joyama S | Atypical and malignant granular cell tumors in Japan: a Japanese musculoskeletal oncology group (JMOG) study | Int J Clin Oncol | 2016 | 18 atypical & mGCTs reviewed: lymph node metastasis in 4/18 cases; 5 & 10 yr OS 52%, 26% respectively. 5 yr LRFS 69% with wide resection and 43% with marginal or intralesional resection |
| Fanburg-Smith JC, Meis-Kindblom JM, Fante R, Kindblom LG | Malignant granular cell tumor of soft tissue: diagnostic criteria and clinicopathologic correlation | Am J Surg Pathol | 1998 | Reviewed 46 mGCT, 21 atypical GCT, 6 GCT: median T size in mGCT 4 cm, 1.5 cm in atypical and 2 cm in benign. 39% of mGCT died of disease at 3 yr median, local recurrence in 32%, metastasis in 50% (including lymph nodes) |
| Lack EE, Worsham GF, Callihan MD, Crawford BE, Klappenbach S, Rowden G, Chun B | Granular cell tumor: A clinicopathologic study of 110 patients | J Surg Onc | 1980 | Review of 110 GTC patients: 10% “clinically simulated a malignant neoplasm”-non pathologic diagnosis of mGTC, 5% multifocal on presentation |
Local nodal dissection has been advocated in cases of malignant tumors for local regional control. Our patient with mGCT developed contralateral cervical lymph node metastasis while under surveillance and prior to start of post-operative radiation therapy. Although, this is an unusual presentation, patients with hypopharyngeal cancers from squamous cell carcinoma can develop bilateral nodal disease due to abundant bilateral cervical lymphatic drainage in the hypopharynx [12]. The fourth important point to make in this discussion is that hypopharyngeal primary mGCT may have a significant risk of regional metastatic nodal disease, which can be bilateral. Therefore, management of the neck should be incorporated in the overall management of mGCT of the hypopharynx whether by surveillance or neck dissections for clinically evident disease. The literature is not clear, however, on whether elective nodal dissections for occult disease should be uniformly performed. In the SEER database review of 113 patients with mGCT of all sites, Moten et al. concluded that patients with no clinically evident lymph node metastasis do not warrant lymph node dissection, as only 12% had positive disease identified in pathology specimens [13]. Moten’s group also identified tumor size greater than 5 cm as a risk factor for death from disease as compared to smaller tumors [13]. Tumor size has been identified as an important risk factor for metastatic potential, specifically tumors greater than 5.5 cm have been shown to have greater risk of metastasis, as seen in our patient with mGCT with a tumor size of 6.8 cm [14]. The patient with mGCT also underwent adjuvant radiation therapy. This has been described in the literature in cases of mGCT with positive margins or with metastasis [15, 16].
The fifth most important point to make in this discussion is that hypopharyngeal GCT is rare and diagnosis of mGCT is even more challenging. However, it is important to keep this diagnosis in the working differential for submucosal hypopharyngeal masses. The GCT case reports illustrated here highlight the spectrum of clinical presentation, multifocality, and potential for metastatic disease in the cervical lymph nodes. Complete surgical resection is the standard of care, and appropriate surgical pre-planning by an experienced head and neck surgical and reconstructive team cannot be over-emphasized. Management by an experienced multidisciplinary head and neck team comprising of a pathologist, radiologist, radiation and medical oncologist, and speech and swallowing therapist is paramount for maximizing functional and oncologic outcomes of patients with GCT.
Author contributions
EBB and GRT contributed to the study conception and design. Material preparation, data collection and analysis were performed by EBB. EBB and GRT wrote the first draft of the manuscript. JMVT provided micrographs. All authors read and approved the final manuscript.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Elizabeth Jo Bradford Bell, Email: e.bradfordbell@unmc.edu.
Giovana R. Thomas, Email: gthomas@med.miami.edu
Jason Leibowitz, Email: jleibowitz@med.miami.edu.
Jaylou M. Velez Torres, Email: jveleztorres@med.miami.edu
References
- 1.Lack EE, Worsham GF, Callihan MD, Crawford BE, Klappenbach S, Rowden G, Chun B. Granular cell tumor: a clinicopathologic study of 110 patients. J Surg Onc. 1980;13:301–316. doi: 10.1002/jso.2930130405. [DOI] [PubMed] [Google Scholar]
- 2.Sobel H, Marquet E, Avrin E, Schwarz R. Granular cell myoblastoma an electron microscopic and cytochemical study illustrating the genesis of granules and aging of myoblastoma cells. Am J Pathol. 1971;65(1):59–78. [PMC free article] [PubMed] [Google Scholar]
- 3.Mirza FN, Tuggle CT, Zogg CK, Mirza HN, Narayan D. Epidemiology of malignant cutaneous granular cell tumors: a United States population-based cohort analysis using the Surveillance, Epidemiology, and End Results (SEER) database. J Am Acad Dermatol. 2018;78(3):490–497. doi: 10.1016/j.jaad.2017.09.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Koltsidopoulos P, Chaidas K, Chlopsidis P, Skoulakis C. Granular cell (Abrikossof) tumor in the head and neck: a series of 5 cases. Ear Nose Throat J. 2016;95(1):36–39. doi: 10.1177/014556131609500108. [DOI] [PubMed] [Google Scholar]
- 5.Tobouti PL, Pigatti FM, Martins-Mussi MC, Sedassari BT, de Sousa SC. Extra-tongue oral granular cell tumor: histological and immunohistochemical aspect. Med Oral Patol Oral Cir Bucal. 2017;22(1):31–35. doi: 10.4317/medoral.21401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cui Y, Tong SS, Zhang YH, Li HT. Granular cell tumor: a report of three cases and review of literature. Cancer Biomark. 2018;23:173–178. doi: 10.3233/CBM-170556. [DOI] [PubMed] [Google Scholar]
- 7.Fanburg-Smith JC, Meis-Kindblom JM, Fante R, Kindblom LG. Malignant granular cell tumor of soft tissue: diagnostic criteria and clinicopathologic correlation. Am J Surg Pathol. 1998;22(7):779–794. doi: 10.1097/00000478-199807000-00001. [DOI] [PubMed] [Google Scholar]
- 8.Moten AS, Movva S, von Mehren M, Wu H, Esnaola NF, Reddy SS, Farma JM. Granular cell tumor experience at a comprehensive cancer center. J Surg Res. 2018;226:1–7. doi: 10.1016/j.jss.2018.01.027. [DOI] [PubMed] [Google Scholar]
- 9.Krishnamurthy A, George R, Majhi U. Malignant granular cell tumor of the tongue: a clinico-pathological challenge. Indian J Surg Oncol. 2014;5(1):71–74. doi: 10.1007/s13193-013-0283-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jobrack AD, Goel S, Cotlar AM. Granular cell tumor: report of 13 cases in a Veterans Administration hospital. Mil Med. 2018;183(9):e589–e593. doi: 10.1093/milmed/usx237. [DOI] [PubMed] [Google Scholar]
- 11.Singh VA, Gunasagaran J, Pailoor J. Granular cell tumor: malignant or benign? Singapore Med J. 2015;56(9):513–517. doi: 10.11622/smedj.2015136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Imanishi J, Yazawa Y, Saito T, Shimizu M, Kawashima H, Ae K, Matsumine A, Torigoe T, Sugiura H, Joyama S. Atypical and malignant granular cell tumors in Japan: a Japanese musculoskeletal oncology group (JMOG) study. Int J Clin Oncol. 2016;21:808–816. doi: 10.1007/s10147-016-0949-1. [DOI] [PubMed] [Google Scholar]
- 13.Olzowy B, Hillebrand M, Harreus U. Frequency of bilateral cervical metastases in hypopharyngeal squamous cell carcinoma: a retrospective analysis of 203 cases after bilateral neck dissection. Eur Arch Otorhinolaryngol. 2017;274(11):3965–3970. doi: 10.1007/s00405-017-4724-3. [DOI] [PubMed] [Google Scholar]
- 14.Moten AS, Zhao H, Wu H, Farma JM. Malignant granular cell tumor: clinical features and long-term survival. J Surg Oncol. 2018;118(6):891–897. doi: 10.1002/jso.25227. [DOI] [PubMed] [Google Scholar]
- 15.Rosenthal SA, Livolsi VA, Turrisi AT., III Adjuvant radiotherapy for recurrent granular cell tumor. Cancer. 1990;65:897–900. doi: 10.1002/1097-0142(19900215)65:4<897::AID-CNCR2820650413>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 16.Crety CM, Garbar C, Madelis G, Guillemin F, Oudot PS, Eymard JC, Vernat SS. Adjuvant radiation therapy for malignant Abrikossoff’s tumor: a case report about a femoral triangle localisation. Radiat Oncol. 2018;13:115–119. doi: 10.1186/s13014-018-1064-4. [DOI] [PMC free article] [PubMed] [Google Scholar]


