Abstract
The goals of this chapter in keeping with the overall general themes of this special edition will be (1) to highlight aspects of development of the thyroid and parathyroid glands with particular focus on the role and contribution of the neural crest (or not) and how this may impact on the pathology that is seen, (2) to emphasize those lesions particularly more commonly arising in the pediatric population that actually generate specimens that the surgical pathologist would encounter, and (3) highlight more in depth specific lesions associated with heritable syndromes or specific gene mutations since the heritable syndromes tends to manifest in the pediatric age group. In this light, the other interesting areas of pediatric thyroid disease including medical thyroid diseases, congenital hypothyroidism, anatomic variants and aberrations of development that lead to structural anomalies will not be emphasized here.
Keywords: Pediatric, Thyroid, Parathyroid, Development, C-cells, Ultimobranchial bodies, Heritable syndromes
Thyroid Gland: A Few Epidemiological Considerations
It is interesting to note that in the arena of cancer alone, based on United States Cancer Statistics [National Program of Cancer Registries (NPCR) and Surveillance, Epidemiology & End Results (SEER)] from 2001 to 2016, the frequency of thyroid cancer in the 19 years old and under population was 4.1% which was on par with the overall frequency of malignant bone tumors (4.5%) and neuroblastoma (4.3%) and more frequent than retinoblastoma, hepatic, or renal tumors [1]. Contrasting this, the frequency of thyroid cancer in adults was 2.3%. The actual number of cases, however, is greatly skewed towards adults with approximately 600,000 cases constituting the 2.3% compared to 10,973 cases in the pediatric population. Another interesting note along these lines is that the rate of pediatric thyroid cancer has increased substantially in the last 15 years. From 1973 to 2006, the incidence rates of pediatric thyroid cancer increased 1.1% per year while from 2006 to 2013 the incidence rate jumped to 9.6% per year [2]. When scrutinized further, the increased incidence rates were reflected in increased incidence in females, in the adolescent age range, and in papillary thyroid carcinoma while rates for medullary carcinoma remained the same. In some studies, this trend was not limited by gender or ethnic designation [3]. This sharp increase in incidence has been attributed to an increase in detection and diagnosis and environmental exposures particularly medical radiation [4]. Differentiated thyroid cancer (DTC, papillary thyroid and follicular cancers and their variants) has an incidence of 0.2–1 case per million children. The incidence increases sharply at adolescence for both sexes; however the female to male ratio in adolescence is approximately 2.5–6:1 making thyroid cancer the second most common malignancy in adolescent females [5]. The other epidemiological note worth mentioning here and one that is probably well known is that while thyroid nodules are actually rare in the pediatric population (1–2%), the incidence of a nodule harboring a malignancy is much higher; approximately 26% compared to 5–15% for adults [6]. So, in the very practical sense, for the surgical pathologist, the pathology that comes across the microscope from the pediatric population (through 21 years of age or so) seems somewhat restricted and not tremendously distinguishable from that of adults; although will tend towards being malignant. In general, a higher proportion of cancer, particularly differentiated epithelial tumors typically papillary thyroid carcinomas and medullary carcinomas as part of multiple endocrine neoplasia are seen in the pediatric population compared to adults. Some follicular lesions, usually adenomas, and multinodular hyperplasia are also seen but in lesser numbers, but along with epithelial cancers such as papillary thyroid carcinoma and follicular carcinomas are more common in adults [5]. The spectrum of parathyroid gland pathology that crosses the surgical pathology bench in any setting whether a general adult-pediatric hospital or tertiary children’s referral hospital is extremely limited in my experience. The goals of this chapter in keeping with the overall general themes of this special edition will be (1) to highlight aspects of development of the thyroid and parathyroid glands with particular focus on the role and contribution of the neural crest (or not) and how this may impact on the pathology that is seen, (2) to emphasize those lesions particularly more commonly arising in the pediatric population that actually generate specimens that the surgical pathologist would encounter, and (3) highlight more in depth specific lesions associated with heritable syndromes or specific gene mutations since the heritable syndromes tends to manifest in the pediatric age group. In this light, the other interesting areas of pediatric thyroid disease including medical thyroid diseases, congenital hypothyroidism, anatomic variants and aberrations of development that lead to structural anomalies will not be emphasized here and multiple other sources and excellent reviews are available for these topics [7–11].
Thyroid Development: The Neural Crest is Important But Not Ontogenic
In this section, the goal will not be to present an exhaustive summary of the thyroid development. That would be far from the scope of this chapter and overall mission of this special edition. We will briefly touch on the highlights, hopefully, as they pertain to pathologies that arise in the thyroid with a particular emphasis on recent developments regarding the ontology of C-cells in the thyroid and its implications for medullary carcinoma. An excellent review of thyroid development can be found in the publication by Nilsson and Fagman [12].
The thyroid gland is the only source of thyroid hormone in our physical bodies. Suffice to say that thyroid hormone has multiple roles in organ development and critical roles in physiological homeostasis of body growth and metabolism. The thyroid is composed of thyroid follicles lined by epithelial cells. These cells are responsible for the production of hormones, T3 and T4 (triiodothyronine and thryroxine, respectively) under the feedback control of pituitary thyroid stimulating hormone, TSH [12]. However, thyroid organogenesis and follicle formation occur independent of TSH. The other main cellular component is the C-cells or somewhat misnamed parafollicular cells, since these cells can not only be parafollicular in location, but intrafollicular and form interfollicular nests [13]. These cells produce calcitonin, a natural antagonist to parathyroid hormone, that interestingly is not particularly “missed” by the body after total thyroidectomy and calcitonin supplements or other means of compensating for its loss are not necessary in post-total thyroidectomy therapeutic regimens or in children with congenital agenesis of the thyroid [13]. The thyroid has an intricate network of capillaries and stroma that surround and septate the thyroid that is composed of ectomesenchymal fibroblasts clearly derived from neural crest [14].
The thyroid is the earliest endocrine organ to develop and anterior most pharyngeal organ (the other anterior pharyngeal organs being the ultimobranchial bodies (UBB), thymus, and parathyroids) and is derived from the median thyroid anlage arising from the foregut endoderm at the level between the first and second pharyngeal arch and does not require retinoic acid for early specification and development compared to other foregut derivatives [15]. The thyroid gland is a fusion at the bilobation stage of the thyroid proper with the UBBs derived from the lateral anlages of the caudal pharyngeal pouches (fourth pharyngeal pouch) and thus the central medial concentration of the UBBs in the thyroid gland proper. Hoxa3 expression is paramount for specifying cell identity in the third pharyngeal pouch and null mutants can lack thymus and parathyroids as well as failure of the UBB to fuse with the thyroid often resulting in cystic UBB remnants [16]. Fibroblast growth factors and bone morphogenetic proteins are crucial for induction of thyroid fate. Athyreosis (lack of a thyroid gland) occurs in Fgfr2b and Fgf10 deficient mice and cardiac mesoderm may also have inductive roles in thyroid development. This condition may be specified by lack of primordial precursors or regression of said precursors. The lingual thyroid sometimes encountered on the surgical pathology bench is a failure of migration of the primordium from the pharyngeal endoderm. Hemiagenesis (usually left-sided) is a later defect and often accompanied by lack of ipsilateral thyroid arteries and right-side arch abnormalities strongly suggesting a link between thyroid and cardiovacular development. Differentiated thyroid cells can be produced from embryoid bodies derived from mouse embryonic stem cells (ESCs) also indicating that thyroid development is independent of TSH signaling [17]. Four transcription factors are key and specific to thyroid development acting both independently and in concert: Hhex, Nkx2-1, Pax8, and Foxe1. These are all expressed elsewhere but the combination and interplay of these four in specific ways confer thyroid development such that deletion of one of these genes produces athyreosis or severe hypoplasia. Hhex is ubiquitously expressed in foregut endoderm but unlike elsewhere, Hhex is not necessary for positioning of precursors in the thyroid compared to the liver or pancreas but Hhex-/- mice have decreased progenitor numbers. Nkx2-1 (thyroid transcription factor, TTF-1) appears to have key roles in epithelial cell phenotype and folliculogenesis. Foxe1 is important in migration of precoursors and Pax8, known as the master gene of thyroid differentiation, is key in precursor survival and folliculogenesis. Practically speaking in humans, mutations in NKX2-1 give rise to brain-thyroid-lung syndrome (chorea, respiratory distress, congenital hypothyroidism). FOXE1 mutations are the cause of Bamforth-Lazarus syndrome (thyroid dysgenesis, cleft palate, bifid epiglottis, choanal atresia, spiky hair) while gain of function mutations cause athyreosis and craniofacial malformations suggesting that gene dosage is critical for proper development. PAX8 mutations can cause thyroid hypoplasia and urinary tract abnormalities [12]. A pictorial summary of thyroid development is given in Fig. 1.
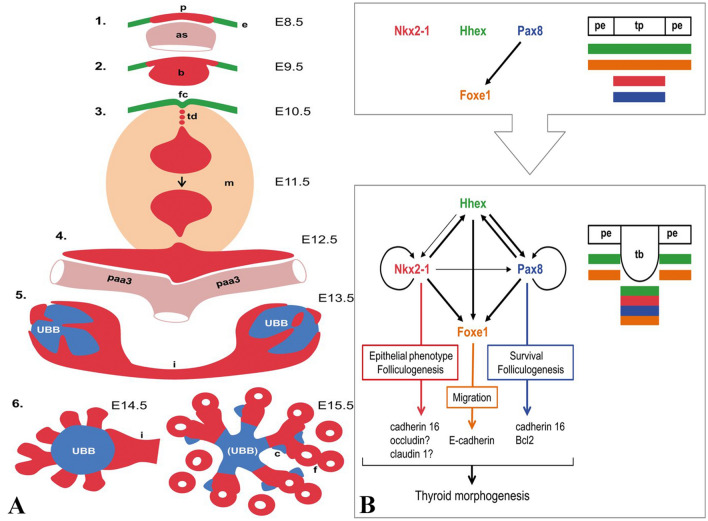
Fig. 1.
THYROID DEVELOPMENT: a Mouse thyroid development by day and stage beginning with the pharyngeal endoderm (e) coalescing into the placode (p) on the cranial aspect of the aortic sac (as) then to the bud (b). The bud detaches and leaves the foramen caecum (fc) with a thyroglossal duct attached (td) and surrounded by mesenchyme (m) followed by bifurcation along the third pharyngeal arteries (paa3). The endodermally derived UBBs from the lateral thyroid anlagen fuse with the thyroid or more appropriately are surrounded by the thyroid and the isthmus forms (i). Lobe growth occurs by the projection of parenchymal cords emanating from the UBB and the conversion of these solid cords into microfollicles (f). C-cells (c) then migrate along the cords at E15.5. b The major transcription factors involved in all aspects of thyroid development include Nkx2-1, Hhex, Pax8, and Foxe1. At the placode (p) stage surrounded by the pharyngeal endoderm (pe), these factors are co-expressed independently with the exception of Foxe1 that requires Pax8. As the bud forms (tb), all factors except for Foxe1 co-regulate the others and Nkx2-1 and Pax8 having autoregulation. Nkx2-1, Pax8, and Foxe1 control the expression of adhesion and junctional proteins that may mediate the functions of these three transcription factors. Figures used with permission from the author and publisher: Mikael Nilsson, Henrik Fagman. Development of the thyroid gland. Development 2017 Jun 15;144(12):2123–2140. https://doi.org/10.1242/dev.145615
As mentioned above, recent developments have turned “upside down” the previously held notions of the origin of C-cells, the other major epithelial cell constituent within the thyroid (although comprising less than 1% of the epithelial mass of the thyroid). C-cells are of great interest since they are the cell of origin of medullary thyroid carcinoma (MTC), a key player in pediatric thyroid pathology (MEN2A (mainly), MEN2B, and familial medullary thyroid cancer [FMTC]). C-cells were given their name in 1966 by Dr. Pearse who discovered they produced the hormone calcitonin and who also proposed the notion that C-cells were of the APUD (amine precursor uptake and decarboxylation) cell concept; a now defunct notion. The concept that C-cells were derived from neural crest comes from the fact that neural crest migrating from the dorsal neural tube populates the pharyngeal apparatus leading to segmentation and endodermal pouch formation. C-cells are more numerous in the medial central portions since now it is known that they hail from the ultimobranchial bodies (UBB) that carry them and can be found in solid cell nests associated with remnants of the UBB. C-cells populate the thyroid lobes during bilobation by migrating from the UBBs. Nkx2-1 is necessary for calcitonin expression whereas Nkx2-1 and Pax8 drive thyroid hormone synthesis. These two transcription factors are functional very early as thyroid precursors are committed from the pharyngeal endoderm [13, 18].
An important backdrop note, as an umbrella to this developmental story, but perhaps a fact that has clouded this issue for many years, is phylogenetically UBBs and thyroid homologues are physically separate structures in non-mammalian vertebrates and only in mammals do these structures “fuse” together in what we call the “thyroid gland”. Back to the neural crest; UBB and neural crest cells are known to share biomarkers and fusion of the thyroid with the UBB (purportedly carrying the neural crest) is required to transport C-cell precursors that ultimately have neuroendocrine and neuronal features. Le Douarin showed that transplanted quail crest cells ended up in the UBB and that some of these cells were immunoreactive for calcitonin neglecting the fact that in all vertebrates but mammals, the UBB is a separate endocrine organ with no connection to the thyroid [18, 19]. This same finding was also confirmed in fishes and birds. Calcitonin-producing cells have been found in invertebrates before a true neural crest existed. Lower chordates have C-cells that are immediately adjacent to a zone of iodine-binding cells that comprise a thyroid homologue [18, 20]. In the gut, through lineage tracing, the notion that APUD cells were of neural crest origin was debunked demonstrating that the entire enteroendocrine cell compartments were derived from gut endoderm. As this pertains to the C-cells and thyroid in mammals, Wnt1 and connexin 43 (Cx43), a gap junction protein, are highly expressed in neural crest [21]. Transgenic mice with Cx43-lacZ show that cells identified by anti-B-galactosidase immunohistochemistry are localized to the ectomesenchymal neural crest of the pharyngeal arches [20, 22]. Wnt1 is solely restricted to the dorsal neural tube and required for expansion of the neural crest. Wnt1 + cre mice crossed with a double fluorescent cre reporter (mT/mG) allows tracking of cells derived from the neural crest by activation of the cre reporter labeling cells fluorescent green. These experiments clearly show that mG positive cells (neural crest) were confined to the pharyngeal ectomesenchyme that surrounded the UBB. No mG positive cells are seen in the UBB at this early stage or later when UBBs fuse with the thyroid. Differentiated C-cells remained in the mT compartment (Wnt 1-). The confirmation that C-cells are endodermal derivatives came from experiments using Sox17 cre reporter mice. SRY (sex-determining region Y)-box 17 (Sox17) is expressed in early endoderm only and these experiments definitively demonstrate that Sox17 is expressed in the entire pharyngeal endoderm including the thyroid bud as expected and the UBBs while the mesenchyme was negative. Calcitonin expressing cells at E15 were confined to the B-gal expressing thyroid parenchyma [18, 22–24]. These recent findings appear to negate the longstanding notion that C-cells are derived from the neural crest. At the time of delamination from the pouch endoderm and merging with the developing thyroid, all cells in UBBs express Nkx2-1 and Foxa1 (proximally expressed near the pouch, + Ki67) and Foxa2 (distally expressed, -Ki67), factors known to be critical to the determination of definitive endoderm. When UBBs merge with the thyroid they are Foxa1 + Foxa2 + and Ki67- and C-cells expressing calcitonin are Foxa1 + Foxa2 + [22]. Mash1 is also critical to C-cell neuroendocrine development as Mash1−/− null mutants show normal UBB development from the pharyngeal endoderm but the merged thyroid (thyroid anlage and UBB) lacks cells with C-cell markers (calcitonin, PGP9.5, TuJ1, and NeuroD) and lack secretory granules on ultrastructural examination. It is postulated that in these null mutants, the C-cell progenitors degenerated before final differentiation into C-cells. Mash1 is also critical for differentiation of neuronal and neuroendocrine lineages (sympathetic ganglia, olfactory epithelium [neural crest derived] and pulmonary neuroendocrine cells, glomus cells [endodermal derived]) [25]. The implications of this as it relates to MTC will be dealt with later. To date then, the neural crest appears responsible for the ectomesenchymal connective tissue within the thyroid and likely has a role in the bilobation of the thyroid gland, perhaps moreso than the third pharyngeal arch arteries that the thyroid is intimately associated with on its descent [22]. C-cell development can be summarized as progressing through a patterning of the fourth pharyngeal pouch endoderm, budding of the UBB anlage, migration of the UBB, fusion of the UBB with thyroid anlage, C-cell differentiation and proliferation within the fused thyroid. The excellent and comprehensive review by Kameda is recommended for those wishing to further delve into this very interesting developmental story [20]. The role of RET in this C-cell story will be addressed a bit later. Collectively, these data demonstrate that tumors derived from C-cells (i.e. medullary thyroid carcinoma) should not be considered as “neural-crest” derived or as “neurocristopathies” as has previously been stated.
As a transition, I would be amiss here if I did not include a bit of perinatology here given my previous career as a newborn intensive care physician. It should be noted here that recent evidence suggests that maternal factors such as smoking and obesity during pregnancy affect thyroid development. Maternal high body mass index was associated with an immature histological phenotype, and decreased expression of FOXA2 and FOXE1 predominantly in second trimester female fetuses and decreased PAX8 expression overall. These three transcription factors as you recall are critical to thyroid cell development, survival, and folliculogenesis. Maternal smoking was associated with decreased expression of GATA6 and NKX2-1 which may contribute to lower levels of thyroid stimulating hormone receptor [26].
Thyroid Neoplasia
In this section we will address some neoplasias that arise in the thyroid with focused attention on cancer and lesions with syndromic associations. In this light, we will address the role of RET in the pathogenesis of thyroid cancer, specific subtypes of papillary thyroid carcinoma, medullary thyroid carcinoma, and briefly touch on some other lesions. In general, most thyroid cancers in children are PTC (80–90%) with follicular thyroid carcinoma (FTC, 10%), MTC (3–5%) and rarely anaplastic or poorly differentiated cancers. Risk factors for epithelial derived cancers in children include autoimmune thyopathies, particularly Hashimoto’s, iodine deficiency, and radiation exposure. Despite at times having more aggressive features (ETE, nodal disease, higher recurrence rates, aggressive histological subtypes), the outcome, in general, for pediatric DTC is better than adults. FTC in children tends to be minimally invasive (90%) compared to adults [27–29]. The American Thyroid Association guidelines published in 2015 stress these important differences in clinical, pathological, and molecular differences between DTC in children and adults [30].
RET is a receptor tyrosine kinase that has important missions in the development of neural crest lineage derived tissues, the kidney, and male germ cells. The RET gene on 10q11.2 encodes a transmembrane protein whose intracellular domain is the tyrosine kinase portion and whose glycosylated receptor is home for several neurotrophic growth factors of the glial cell line-derived neurotrophic factors (GDNF family). Importantly, GDNFs are presented to RET by glycosylphosphatidylinositol (GPI) anchored co-receptors, GDNF family receptor alpha 1–4 GFR-alpha). Differential tissue expression will then determine the specificity of action by alternative GDNF-GFR-alpha pairs. RET is a dependence receptor and in absence of ligand RET exerts proapoptotic activity. More on the intricacies of the RET receptor can be found elsewhere [31]. RET is expressed in enteric ganglia, adrenal medulla chromaffin cells, thyroid C-cells, sensory and autonomic ganglia of the peripheral nervous system, some central nervous system nuclei, kidney, and testis germ cells. In mice more specifically (Pachnis et al.), RET is expressed differentially at specific locations and times of development and is seen in a population of neural crest cells from rhombomere 4 that will form the facioacoustic ganglion, all cranial ganglia, autonomic ganglia and dorsal root ganglia, enteric neuroblasts of the vagal crest, myenteric ganglia, undifferentiated neuroepithelial cells of the ventral neural tube, motor neurons of the spinal cord and hindbrain, embryonic neuroretina, nephric duct, ureteric bud, tips of the renal collecting duct. Interestingly, RET was also expressed in a closely associated domain of surface ectoderm near rhombomere 4 and pharyngeal endoderm. This is one of the few, if not the only study, to show that RET is expressed in pharyngeal endoderm and this result is mentioned in passing only in this paper [32]. The latter has implications for MEN2A where parathyroid hyperplasia is seen (parathyroid gland clearly being of endodermal origin) and in conjunction with C-cells (now demonstrated to be of endodermal origin), the pathogenesis of MTC. Germline loss of function mutations of RET can result in intestinal aganglionosis (Hirschsprung disease; usually quite severe up to and including total intestinal aganglionosis) and congenital anomalies of the kidney and urinary tract, alone or in combination. Select mutations can convert RET into an oncogene that can cause different tumors in the thyroid and parathyroid [33–36].
Multiple risk factors have been identified with subsequent development of thyroid nodules in children. These are iodine deficiency (increased risk of autoimmune thyroiditis particularly Hashimoto’s with an increased risk of cancer), radiation exposure (environmental and iatrogenic related to treatment for other primary malignancies (Hodgkin lymphoma, leukemia, CNS tumors), history of thyroid diseases, and genetic syndromes [30, 37]. We will discuss in detail more fully those tumors associated with genetic syndromes as the thyroid tumors associated with them arise in the pediatric age range and/or the other manifestations of the syndrome manifest during childhood.
Papillary thyroid carcinoma and variants are by far the most common cancers seen in the pediatric population accounting for 90% of thyroid cancers as noted. In approximately, 5–10% of cases there is a family history of PTC and PTC is seen in association with familial adenomatous polyposis syndrome (cribriform-morular variant), DICER1, PTEN hamartoma syndrome, Carney complex, and Cowden Disease. The main risk for PTC in young children is radiation exposure as evidenced from the Chernobyl Nuclear Power Plant accident. Main differences between DTC in the pediatric population and adults are that thyroid nodules in pediatrics have a fourfold higher risk of being malignant, higher risk of lymph node and pulmonary metastases (80% vs 20–50% for LN and 9–30% vs 2–9% for pulmonary mets). In young children (< 10 years), tumors tend to be larger, multifocal, bilateral and with extracapsular extension. Recurrence rates are higher in children (> 25% vs 2–25%) and yet mortality is lower (< 5% vs 2–40%) and with a higher response to radioactive iodide therapy. This in part may be related to less genomic instability with RET/PTC fusions compared to BRAF mutations [27–30, 38].
A brief comment regarding cytological evaluation of pediatric thyroid nodules will be made here. Fine needle aspiration of thyroid nodules is more often the first procedure leading to pathological diagnoses and has a sensitivity and specificity of 94% and 100% respectively [5]. The risk of malignancy is 28% for AUS/FLUS (aytpia of undetermined significance/follicular lesion of undetermined significance) and 58% for FN/SFN (follicular neoplasm/suspicious for follicular neoplasm) with these categories constituting about 35% of pediatric thyroid FNA. These figures are higher than reported in adults (6–18% for AUS/FLUS and 10–40% in the FN/SFN category (excluding non-invasive follicular neoplasm with papillary-like nuclear features (NIFTP) [39]. Current ATA guidelines for children recommend definitive surgery (lobectomy and isthmusectomy) for indeterminate cytology (AUS/FLUS and FN/SFN) and SM categories given the overall higher rates of malignancy in pediatric thyroid nodules [30]. However, more recent studies reviewing institutional data on thyroid FNAs in children seem to indicate a much lower incidence of indeterminate diagnoses (13–18%) and lower malignancy rates for nodules with these cytological diagnoses. Data has also emerged showing that the malignancy rates vary considerably between the indeterminate categories, with the highest rates of malignancy in the SM category (80%) leading some to consider segregating this category from the other indeterminate categories for definitive surgery [39]. These recommendations will certainly change as these data and molecular data are correlated in the pediatric population.
From the molecular genetic perspective, overall, PTCs have genetic alterations in the RTK-RAS-MAPK pathway with half having BRAF mutations (usually V600E), RAS mutations (usually follicular variant PTC), and NTRK1. PAX8 and PPARG mutations would also be included in those seen more frequently in adults. However, in children, chromosomal rearrangements including RET/PTC, AKAP9-BRAF, and NTRK1 are found more commonly. In comparison children show a higher incidence of RET/PTC rearrangements (30–70% vs 10–20%) and lower incidence of BRAF mutations (10% vs 40–50%), although the overall prevalence of molecular alterations reported in some pediatric cohorts with DTC is considerably lower (26%) compared to adults (70%) [27–29, 40]. In young children, however, at least in one study from China, BRAF V600E mutations were found in 35% of pediatric PTCs and associated with age less than 10 years and aggressive features [41]. Approximately twenty different RET/PTC rearrangements have been noted. The RET/PTC rearrangements are more frequently seen in young patients and those with a history of ionizing radiation exposure (41% for sporadic PTC vs 58% for radiation-exposed PTC) with RET/PTC3 (RET/NCOA4) having an association with more aggressive tumors than the classic form of PTC with RET/PTC1 (RET/CCDC6) [28, 31]. The advent of more sophisticated molecular techniques, particularly next-gen sequencing (NGS) has allowed for greater detection of molecular alterations in sporadic pediatric DTC. In our study, we observed three cases of sporadic DTC with ETV6/NTRK3 fusion and one case with PAX8/PPARγ fusion all with somewhat aggressive histological features (unencapsulated FVPTC with solid and/or insular growth and fibrosis) similar to those lesions seen in radiation-induced PTC [40].
While it will not be considered extensively here due to space limitations, autoimmune thyroid disease (autoimmune thyropathies) are the most common manifestations of autoimmune disease in children, typically in the forms of Hashimoto’s thyroiditis and Graves disease responsible for 95% of pediatric hyperthyroidism. There may be an association between the more aggressive presentation of DTC in children and adolescents and the concurrence of Hashimoto’s thyroiditis, perhaps through persistently elevated TSH levels [30, 37].
It is not the goal of this chapter to thoroughly cover every form of thyroid neoplasia that can occur in children as practically speaking the diagnosis of multinodular hyperplasia, dominant hyperplastic nodules, follicular adenomas, and the differentiated thyroid carcinomas (mostly classical PTC) in their most common presentations have been exhaustively covered in the literature. In fact, speaking frankly, it is difficult to come up with fresh angles for some of these pathologies. However, in reference to this and really to the heart of this special edition, this author will alert the pathologist that a diagnosis of any of these entities in the pediatric age population, that is 18 years or younger, and particularly in children less than 10 years old, should at least give a pause to search the medical record for association with other elements of a syndrome or to at least look for a defined cause. So, the goal here will be to be reasonably comprehensive in review of those lesions associated with specific genetic syndromes and even in that we will not cover every heritable syndrome associated with thyroid neoplasia. Rather, this review, although somewhat unorthodox, will highlight cases that have crossed this author’s desk in practice with those thyroid neoplasias that are part of genetic syndromes and/or that present in childhood/adolescence. Other cancers including poorly differentiated, anaplastic carcinoma, sarcomas, hematolymphoid tumors, and others will not be discussed here. Heritable genetic syndromes associated with thyroid neoplasia are shown in Table 1 [42–47].
Table 1.
Genetic syndromes associated with thyroid neoplasia
| Genetic syndrome | Genetic defect/inheritance | Clinical manifestations | Associated thyroid neoplasia |
|---|---|---|---|
| APC-associated polyposis (familial adenomatous polyposis, FAP, attenuated FAP, Gardner syndrome, Turcot Syndrome | Mutations of APC (5q21-q22) [particularly germline exon 15 mutations for thyroid cancer]/AD | Gastrointestinal polyposis (colon, duodenum, stomach), osteomas, nasopharyngeal angiofibroma, odontomas, congenital hypertrophy of retinal epithelium, epidermal cysts, fibromas, medulloblastoma, hepatoblastoma | PTC (cribriform-morular variant in 70–90% of patients with APC gene mutation)* |
| Carney complex, LAMB (lentigines, atrial myxoma mucocutaneous myxoma, blue nevi), NAME (nevi, atrial myxoma, myxoid neurofibroma, ephelide) |
Mutations of PRKAR1A [CNC1] (17q22-24) CNC2 (2p16)/AD |
Primary pigmented nodular adrenocortical disease), cardiac myxoma, skin myxoma, lentiginosis, blue nevi, breast ductal adenoma, Large-Cell calcifying Sertoli cell tumor, melanotic schwannoma, osteochondromyxoma |
Benign, non-toxic adenomas Multinodular goiter PTC (multiple, aggressive) FTC |
| DICER1 | Pathogenic germline variants of DICER1 (14q32.13, c.5441C > T) Rnase III endonuclease/AD | Pleuropulmonary blastoma, cystic nephroma, Sertoli-Leydig cell tumor of ovary, cervix embryonal rhabdomyosarcoma, Wilms tumor, ciliary body medulloepithelioma, nasal chondromesenchymal hamartoma |
Multinodular goiter (frequent) FTC (macrofollicular variant) Papillary adenoma Malignant teratomas Poorly differentiated carcinoma PTC, Carcinosarcoma |
| PTEN hamartoma tumor syndrome (Cowden syndrome, Bannayan-Riley-Ruvalcaba syndrome, PTEN-related Proteus syndrome, adult Lhermitte-Duclos, autism spectrum with macrocephaly) | Heterozygous germline pathogenic variants of 10q23.31 (phosphatase and tensin homolog)/AD | Macrocephaly, gastrointestinal hamartomas, macular pigmentation of the glans penis, multiple trichilemmomas, mucocutaneous neuromas, endometrial cancer, acral keratoses |
Multinodular goiter Adenomas Papillary thyroid carcinoma (follicular variant), 60% Follicular carcinoma (oncocytic [Hurthle cell]) 25–40% |
| Werner syndrome | Mutations in WRN [RECQL DNA helicase] (8p11.1–21.1)/AR | Premature aging syndrome, progeria-like, short stature, meningiomas, osteosarcomas |
PTC, FTC Anaplastic carcinoma (18% of Japanese patients develop thyroid cancer) |
| Multiple endocrine neoplasia (MEN) | MEN1 [Wermer] (11q13, menin)/AD | Hyperparathyroidism, pancreatic islet tumors, anterior pituitary tumors | Multinodular goiter, adenomas, carcinoma |
| MEN2A [Sipple] (RET codon 634)/AD | Pheochromocytoma, hyperparathyroidism, variants with cutaneous lichen amyloidosis or Hirschsprung disease, familial MTC | Medullary thyroid carcinoma | |
| MEN2B(3) [multiple mucosal neuroma syndrome] (RET codon 918)/AD | Multiple ganglioneuromas, marfanoid habitus, mucosal neuroma, pheochromocytoma | Medullary thyroid carcinoma | |
| MEN4 (CDNK1B) (very rare)/AD | Parathyroid and anterior pituitary, adrenal, gonadal tumors |
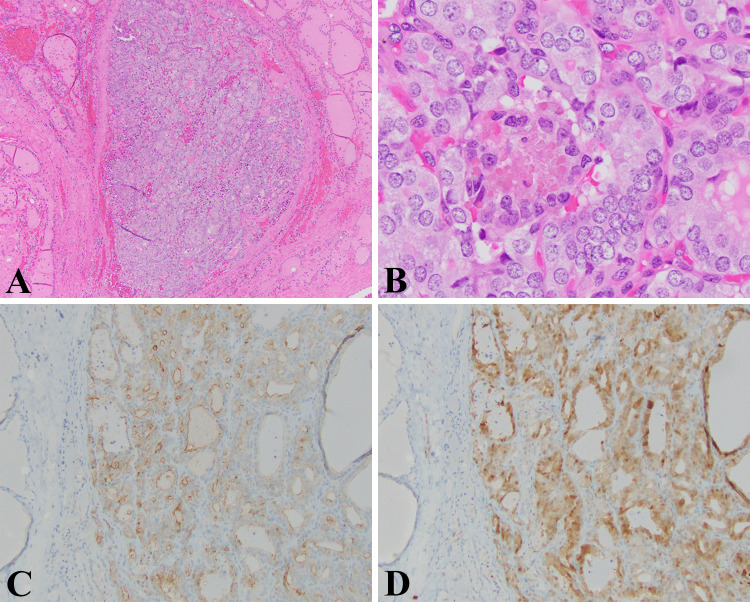
Case 1: Papillary thyroid carcinoma, diffuse sclerosing variant (DSV-PTC) (WHO Classification of Tumours of Endocrine Organs, 4th Edition, 2017; ICD-O 8260/3) (Fig. 2).
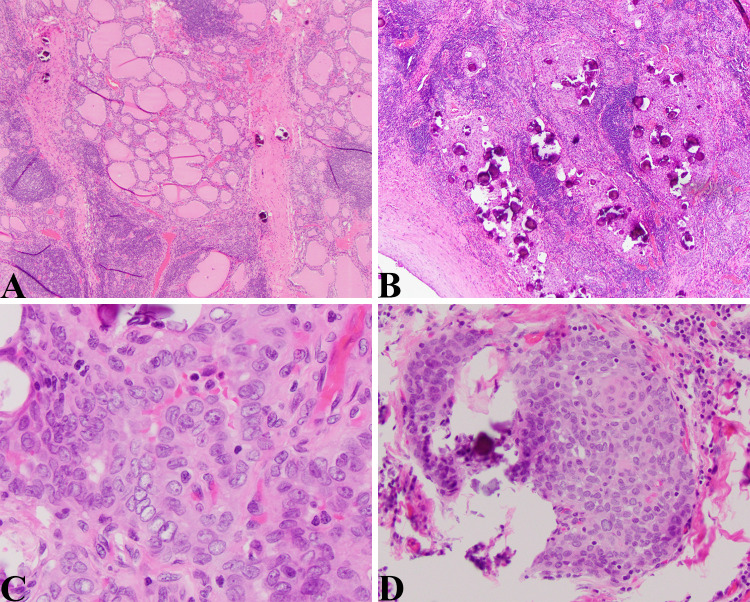
Fig. 2.
DSV-PTC: a Low magnification view of this tumor showing an area with a heavy lymphocytic infiltrate, thick bands of fibrous tissue that divide the thyroid into lobules, and numerous psammoma bodies. This is a deceptive field of view given that the thyroid tissue that is visible is non-neoplastic thyroid (HE 40X). b Numerous psammoma bodies characteristic of this aggressive variant of PTC. Here in this field the psammoma bodies are concentrated in the more solid appearing and neoplastic foci (HE 40X). c High magnification view showing the characteristic nuclear morphology of PTC; nuclear crowding, pleomorphism, central clearing, and nuclear grooves (HE, 400X). d Solid squamous area with psammoma bodies (HE, 200X)
The patient was a 15-year-old boy who was found to have a “thyroid nodule” on a routine sports physical. The ultrasound examination demonstrated a heterogeneous mass (4 × 2.4 × 3.6 cm) involving entire right lobe, extending across isthmus with involvement of the inferior portion of the left thyroid lobe (3 × 1.1 × 1.8 cm) with increased vascularity and fine areas of increased echogenicity likely representing calcifications. Fine needle aspiration of the right lobe confirmed a papillary thyroid carcinoma with extensive psammoma bodies and chronic lymphocytic thyroiditis that was cytokeratin 19, HBME, thyroglobulin and galectin-3 positive. Molecular testing (ThyroSeq v2) demonstrated a RET/PTC1 fusion. The pathological examination showed a papillary thyroid carcinoma diffusely involving the right lobe and with multifocal involvement of the left lobe with broad areas of fibrosis, a brisk lymphocytic infiltrate, squamous metaplasia, and numerous psammoma bodies. Extrathyroidal extension and lymph node metastases were observed, and the pathological stage was pT3, N1b.
Discussion: (DSV-PTC)
This tumor demonstrated nearly all the classic features for DSV-PTC. This variant constitutes 0.3–1.8% (WHO) of PTCs with other investigators reporting an incidence of 0.7–6.6%. DSV-PTC occurs mainly in children particularly late adolescent and young adult females (2–4:1 F:M, 18–29 years old, mean age 23 years) and is the most common variant of PTC in patients under 20 years old [48]. This tumor is also associated with the presence of serum anti-thyroidal and anti-microsomal autoantibodies [49]. The presentation, as in our case, was that of a diffusely enlarged gland without a discrete mass. Under the microscope, the tumor shows a mixed solid/papillary architecture with the characteristic nuclear morphology of PTC, fibrosis/sclerosis, extensive lymphocytic infiltration, psammoma bodies, and focal squamous metaplasia. The fibrosis is typically in bands with nodular areas and areas with a more hyalinized appearance. This variant could be confused or diagnosed as chronic lymphocytic thyroiditis because of the extensive lymphocytic infiltrate, diffuse nature of the tumor, and serological evidence of autoimmune thyropathy [48, 49]. The lymphocytes within DSV are primarily CD8 + and attached to venules similar to high-endothelial venules that express sLeX glycans and ICAM-1 in lymph nodes and may contribute to the metastatic potential of this variant [50]. Genetic alterations in DSV-PTC include RET/PTC1 or RET/PTC3 rearrangements (62%) and BRAF V600E mutations (24%) [51]. Tumors with RET/PTC3 rearrangements are associated with more aggressive (high stage) and persistent disease. This variant does not seem to be associated with a genetic syndrome. A thyroid ultrasound showing a diffusely enlarged gland with diffusely scattered microcalcifications (“snowstorm”, “punctate”) should raise a high degree of suspicion for DSV-PTC [52]. Thyroiditis and diffuse microcalcifications are seen in the majority (approximately two-thirds) and diffuse microcalcifications are more often associated with this variant [52]. In contrast to Hashimoto’s thyroiditis or other thyroid malignancies, DSV-PTC may show markedly elevated “stiffness” by shear wave elastography and may be a helpful adjunct in addition to the pattern of microcalcifications in ultrasonic diagnosis of DSV-PTC [53]. In comparison to classical PTC in a large series, DSV-PTCs were larger (4.2 cm vs 2.4 cm), multifocal (72% vs 39%), had capsular invasion (92% vs 54%), central nodal disease (92% vs 34%), pT3 (96% vs 39%), and a much higher recurrence rate (62% vs 7%) [49]. Decreased membranous E-cadherin expression is also seen perhaps correlating with more aggressive behaviors [54]. Thus, along with hobnail, tall cell, columnar, and solid variants, DSV is categorized as a variant form of PTC with clearly aggressive features (ETE, nodal involvement, lymphovascular invasion, distant metastases) and decreased disease-free survival compared to classical PTC. However, somewhat controversially, while many studies conclude that overall survival is the same for DSV, some studies show worse overall survival in conjunction with other invasive features [49, 55–59].
Case 2: Papillary thyroid carcinoma, cribriform-morular variant (CMV-PTC) (WHO Classification of Tumours of Endocrine Organs, 4th Edition, 2017; ICD-O 8260/3) (Fig. 3).
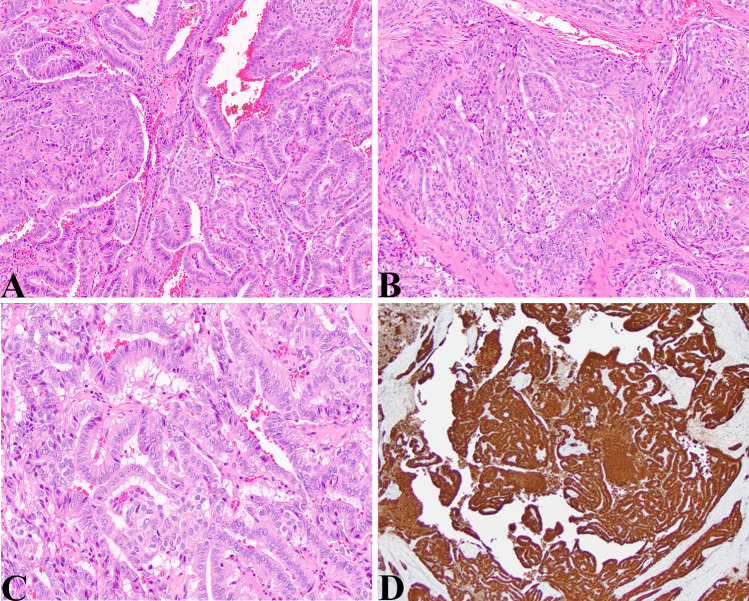
Fig. 3.
CMV-DTC: a Medium magnification view of CMV-PTC showing a cribriform pattern in the left half of the image, a more papillary pattern in the right half and a small central morule (HE, 100X). b Medium magnification view showing a solid squamous morula embedded in a cribriform pattern of PTC (HE, 100X). c Higher magnification showing an area with typical nuclear features of PTC (crowding, elongated and enlarged nuclei, grooved nuclei, nuclear clearing) (HE, 200X). d Beta-catenin stain shows diffuse nuclear and cytoplasmic staining (Beta-catenin, 40X)
The patient was a 13-year-old girl with a solid thyroid nodule of the right upper pole, 2.7 cm. A fine needle aspiration was positive for malignant cells. A thyroidectomy was performed and showed a somewhat circumscribed lesion with papillary, solid, and cribriform architectural pattern. Solid areas demonstrated oncocytic cytology and papillary areas did reveal nuclear morphology coinciding with diagnostic criteria for papillary thyroid carcinoma. A beta-catenin stain showed diffuse cytoplasmic and nuclear staining within the tumor compared to the surrounding normal thyroid showing diffuse membranous staining. This girl had a history of Gardner syndrome and was post colectomy for adenomatous polyposis.
Discussion: CMV-PTC
CMV-PTC represents approximately 0.2% of PTC and may be sporadic or associated with FAP. Roughly a quarter to one-half of all CMV-PTCs occur in patients with FAP [48, 60]. This variant almost invariably occurs in females with an approximate age of presentation between 24 and 28 years of age (median 24 years, range 15–61 years). No differences in age of presentation or tumor size are seen between FAP-associated tumors and sporadic [61]. In general about 80% of CMV-PTC present as solitary nodules but some indications show that in patients with FAP associated CMV-PTC, the tumors tend to be multifocal and bilateral [61, 62]. PTCs in patients with FAP appear to have unique genetic signatures compared to sporadic PTCs. In addition to the germline mutations in the APC gene, PTCs in patients with FAP harbor second hit mutations in APC, BRAF V600E mutations and mutations in methyltransferase genes, KMTD2 and KMT2C [60]. The finding of BRAF mutations as a second hit in FAP is unique and probably signifies that PTC arising in the setting of germline APC mutations can have the same second hit driver mutation as sporadic PTC. Patients with non-silent somatic mutations in APC were female and their PTC had CMV-PTC histology [60]. A rare case of two somatic inactivating mutations of APC producing a CMV-PTC phenotype in the absence of a germline APC mutation has been reported [63].
The tumors are generally 2–3 cm on presentation and can demonstrate several microarchitectural patterns that include cribriform, trabecular, solid, papillary, and follicular growth with morules composed of squamoid/spindle cells typically. The epithelial component may have oncocytic features with tall cuboidal cells and nuclear features of PTC may be focal. Psammoma bodies are not a feature. This variant can be difficult to recognize as the noted architectural patterns (particularly cribriform pattern), presence of squamous morules, and characteristic nuclear features of PTC can be quite variable. The lack of colloid generally seen in this variant is helpful. As noted, beta-catenin by IHC is positive in both the nucleus and cytoplasm of both FAP-associated and sporadic (compared to membranous in cPTC) and tumors are positive for galectin-3, estrogen and progesterone receptors, and LEF-1 [64, 65]. The squamous morula (not squamous metaplasia) may show expression of p53, CDX2, CD10, E-cadherin, and bcl-2 in addition to beta-catenin and galectin-3 [61, 64]. Ki-67 labeling is higher in CMV-PTC compared to classical PTC [66]. Lymph node metastases occur in 10–20% and ETE is rare. In general this variant has an excellent prognosis similar to that of classical PTC [43, 61, 64]. In the study by Perrier et al., thyroid carcinoma, the majority of which is PTC (with more than half of these showing histology with cribriform features), in the setting of FAP has a 90% and 77%, 5 and 20 year survival rates respectively [67]. Rare reports of a poorly differentiated component with the CMV-PTC (age 45 years, [68]) and lung metastases in patients with CMV-PTC have been reported (ages 24 and 28 years, [69, 70].
Case 3: Follicular carcinoma and multiple follicular microadenomas in the setting of PTEN Hamartoma syndrome (WHO Classification of Tumours of Endocrine Organs, 4th Edition, 2017; ICD-O 8330/0) (Fig. 4).
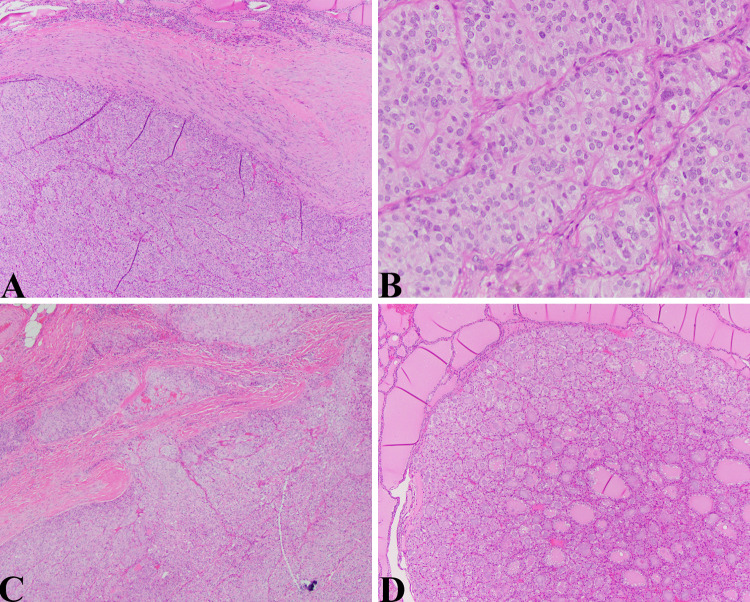
Fig. 4.
Follicular carcinoma/adenomas in PTEN Hamartoma syndrome: a Low magnification view of cellular follicular neoplasm with a thick fibrous capsule (HE, 40X). b The lesional cells demonstrated a “tight” microfollicular pattern bordering on solid/trabecular microarchitecture. The nuclei show mild pleomorphism and abundant eosinophilic to clear cytoplasm (HE, 200X). c Distinctive areas of capsular and vascular invasion were appreciated (HE, 40X). d Multiple small but distinctive and mostly unencapsulated hyperplastic nodules/microadenomas were scattered throughout both lobes (HE, 40X)
The patient was an 18-year-old-female with a complex medical history that included colonic ganglioneuromas. Thyroid ultrasound demonstrated multiple nodules (0.5–1.7 cm mostly solid nodules). Fine needle aspiration of two left thyroid nodules were diagnosed as suspicious for oncocytic neoplasm. Pathological examination revealed a 1 cm follicular carcinoma with capsular and angiolymphatic invasion, a 1.3 cm partially calcified adenomatous nodule, and numerous adenomatous nodules (microadenomas). The patient had a known PTEN mutation (253 + 1G > A mutation PTEN gene, intron 4).
Discussion: PTEN Hamartoma Syndrome Associated Follicular Neoplasms
Germline heterozygous PTEN mutations (Table 1) cause PTEN Hamartoma Tumor Syndrome (PTHS) which includes Cowden syndrome (CS), Bannayan-Riley-Ruvalcaba syndrome (BRRS) Proteus (PS) and Proteus-like syndrome. CS is characterized by multiple hamartomas, macrocephaly, trichilemmomas, papillomatous papules and high risk for breast, endometrial and thyroid cancers. The lifetime risk of thyroid cancer is 35% and usually presents before 20 years of age. BRRS consists of macrocephaly, intestinal hamartomatous polyposis, lipomas, and pigmented macules of the glans penis. PS can have hamartomas, tissue overgrowth, epidermal and connective tissue nevi and hyperostoses. Consensus criteria have been established by the National Comprehensive Cancer Network for the diagnosis of CS and include pathognomonic, major (of which thyroid cancer is included), and minor criteria [44, 45, 47, 71, 72]. Up to 10% of CS patients do not have an identifiable PTEN sequence variant in the coding/flanking regions of the PTEN gene (30% may have a germline KLLN methylation epimutation). Also, up to 10% of PTEN-CS, CS-like syndrome or BRRS also have germline mutations in the succinate dehydrogenase (SDHx), mitochondrial complex II gene that then modifies cancer risk [44, 72, 73]. Patients with germline variants of SDHx and PTEN have perhaps a twofold increased risk of thyroid cancer compared to PTEN-CS and a 50% decreased prevalence of thyroid cancer compared to SDHx-CS. Certain SDHx variants can cause phosphorylation of FOXO3a via ubiquitination of PTEN and acetylation of FOXO3a that results in nuclear export and down regulation of FOXO3a target autophagy gene regulation perhaps promoting thyroid carcinogenesis [73]. PHTS patients also can exhibit immune dysregulation with hypogammaglobulinemia and insufficient priming of CD8 + cells by dendritic cells contributing to carcinogenesis by decreasing tumor eradication capabilities [74].
Thyroid disease in CS encompasses multinodular goiter and follicular adenomas (75% of CS) as well as a lifetime risk of non-medullary thyroid cancer of 35% occurring at a median age of 37 years but also presenting in childhood with follicular histology being the dominant form. The histology of these lesions is not different from that described in general for these lesions [9]. Staining for PTEN can be used as an adjunct. However, the history and pattern of lesions, in particular, multiple follicular lesions in a child/adolescent should certainly raise suspicion if no other information is available. For those children with an identified pathogenic variant of PTEN, yearly thyroid ultrasound is recommended [30, 46, 75].
Case 4: Non-invasive follicular thyroid neoplasm with papillary-like nuclear features (NIFTP) (WHO Classification of Tumours of Endocrine Organs, 4th Edition, 2017; ICD-O 8349/1) (Fig. 5).
Fig. 5.
NIFTP: a Low magnification view of a minimally encapsulated but well-circumscribed follicular lesion surrounded by normal thyroid parenchyma (HE, 40X). b The nuclei clearly demonstrated morphological features of papillary thyroid carcinoma. In this field, we see mild nuclear anisocytosis, focal chromatin clearing, crowding with overlapping, nuclear grooves (HE, 400X). c The tumor cells mostly express HBME-1 in the characteristic membranous pattern (HBME-1, 100X). d Similarly, the tumor shows nearly diffuse expression of Galectin-3 with appropriate cytoplasmic and nuclear staining (Galectin-3, 100X)
The patient is a 15-year-old-girl with thryomegaly and normal thyroid function tests. Ultrasound demonstrated a left lower pole complex cystic and solid mass, 1.3 × 0.9 × 1.0 cm and an upper pole hypoechoic mass, 0.6 × 0.3 × 0.5 cm. FNA of the left superior pole was diagnosed as suspicious for follicular neoplasm. Molecular testing was positive for NRAS mutation.
The pathology revealed an encapsulated 0.5 cm follicular lesion with no papillary features and demonstrated nuclear features characteristic for PTC. The tumor diffusely expressed HBME-1 and Galectin-3 by IHC and CK19 was variably positive.
Discussion: NIFTP
I might suppose that we would be somewhat amiss if our review of thyroid pathology in children did not include NIFTP as this has been one of the substantial changes in the designation of thyroid tumors in the 4th edition of the WHO Classification of Tumours of Endocrine Organs; this tumor having been previously termed “non-invasive encapsulated follicular variant of papillary thyroid carcinoma”. However, in the line of thyroid tumors and cancer in children, the incidence of NIFTP is quite uncommon in children. In three of the largest series publications by Nikiforov et al. (n = 210), Thompson et al. (n = 94), and Cho et al. (n = 152), no cases of pediatric NIFTP were included [76–78]. One cytology study reported an incidence of 1.9% for all thyroid resected nodules and 4.5% within their PTC cohort [79]. In that study, the 2 NIFTP were diagnosed on FNA as follicular neoplasm whereas the invasive FVPTC were either suspicious or positive for malignancy. Cytological features were not specific but a microfollicular pattern and nuclear size intermediate between follicular adenoma and PTC were consistently seen [79]. To date, there is even less information regarding any meaningful differences in clinical behavior or pathological characteristics between NIFTP in the pediatric population compared to the adult. A few studies have shown that NIFTP in children behave indolently similar to the experience in adults [80, 81].
The separation of this category from invasive encapsulated follicular variant of papillary carcinoma and its description with more “indolent” terminology (not carcinoma) is the realization that its clinicopathological behavior (indolent with clinical behavior like an adenoma and without capsular/vascular invasion) and molecular characteristics differ from its invasive counterpart (invasive encapsulated FVPTC). The criteria for the diagnosis of NIFTP have been established [77, 82] and only very key elements will be highlighted here. They must be well-demarcated, have nuclear features of PTC, be without capsular/vascular/lymphatic invasion, predominantly follicular architecture (can have arbitrarily up to 30% of solid/trabecular/insular), no aggressive histological features (tall cell, columnar cell, cribriform architecture), no well-formed papillae (none at all), no psammoma bodies, no necrosis, no increased mitotic count (somewhat arbitrary) [77, 82]. Of note, the criterion of no papillae is a change from previous criterion due to some lesions diagnosed as “NIFTP” progressing to have behavior more in line with an invasive tumor. Currently, there is no defined size criteria and even tumors 1 cm or less might be included although authors on this topic note that size less than 1 cm, multiple tumors, and oncocytic follicular patterns will require more investigation as to whether they should be included under the rubric of NIFTP [82]. Practically and anecdotally speaking, I have seen pathologists (including myself as the case illustrates) designate lesions less than 1 cm as NIFTP on a somewhat regular basis. HBME-1 and Galectin-3 can be useful adjuncts, particularly if the nuclear features are not robustly manifest, but in my experience, may show more variable staining such as that seen in FVPTCs. About 80% of NIFTP show clonal alterations including RAS mutations most frequently and others (PAX8/PPARG, THADA fusions and BRAF K601E, EIF1AX mutations). RET/PTC and BRAF V600E are not seen in NIFTP and these and other mutations (TERT, ALK, others) if confirmed should provide reason for serious reconsideration of the diagnosis [77, 82]. As this entity is further reported and its features refined, the clinicopathological landscape of pediatric tumors with this designation will become clearer.
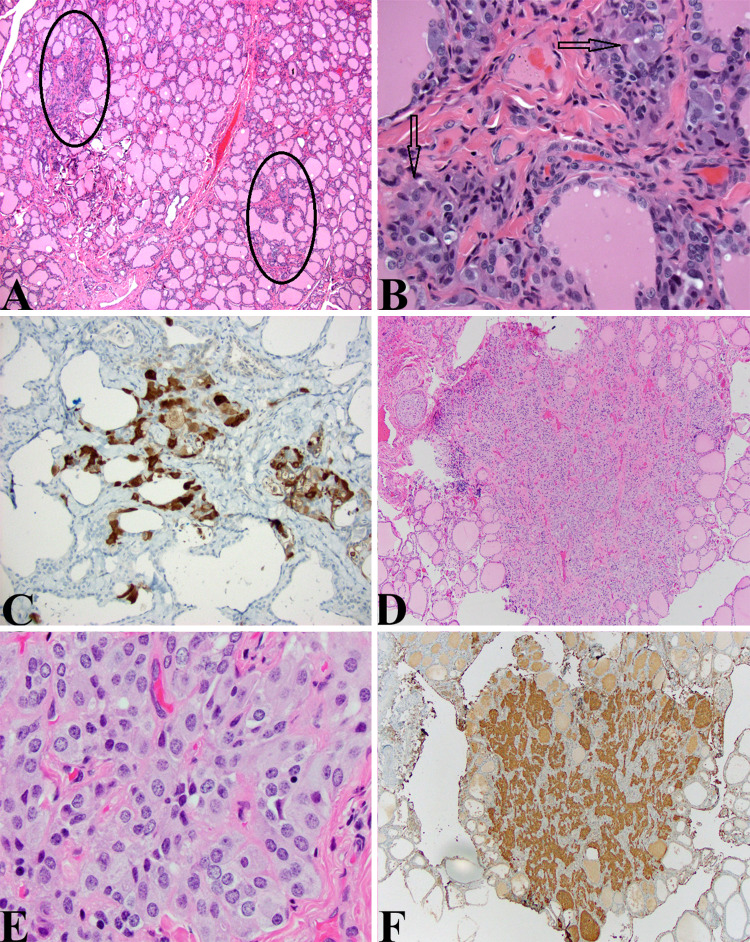
Case 5: Total thyroidectomy in a patient with MEN2A with C-Cell hyperplasia (WHO Classification of Tumours of Endocrine Organs, 4th Edition, 2017; ICD-O E07.0) (Fig. 6).
Fig. 6.
CCH and MTC: a Thyroidectomy specimen from the 3-year-old boy presented above showing vague hypercellular areas having a grayish tinge intercalated within the thyroid parenchyma (ovals) (HE, 40X). b High magnification view of one of the highlighted areas in (A) showing large cells with relatively indistinct nuclei and abundant amphophilic cytoplasm in clusters and around follicles. While the nuclei are relatively indistinct even compared to normal thyroid follicular epithelial cells, they are quite noticeable given that under normal circumstances, C-cells are difficult to ascertain on routine HE sections. These features qualify for cytological atypia and should be considered as such (HE, 400X). c These cells noted in (b) are easily highlighted by a calcitonin stain (Calcitonin, 200X). d Small relatively uncircumscribed lesion in an adolescent with MEN2A. This cellular lesion could be mistaken for an intrathyroidal parathyroid gland or small hyperplastic thyroid epithelial cell-derived nodule (HE, 40X). e The cells have a “neuroendocrine” appearance and form small organoid nests. The cytological features include small uniform nuclei, bland chromatin, and amphophilic cytoplasm (HE, 400X). f A calcitonin stain again confirms the ontogeny from C-cells and this lesion as a medullary thyroid microcarcinoma in a patient undergoing prophylactic thyroidectomy for MEN2A (Calcitonin, 40X)
The patient is a 3-year-old boy who presented for total thyroidectomy. His father has MEN2A and the patient demonstrated a 634 (A > C) mutation in exon 11 of the RET gene. Serum calcitonin levels have slowly but steadily risen to 13.6 at the time of surgery.
The thyroidectomy specimen demonstrated focal areas of C-cell hyperplasia (CCH) with increased numbers of atypical C-cells demonstrated on the HE and calcitonin stained sections (Fig. 6a–c).
An adolescent girl with MEN2A was referred for prophylactic thyroidectomy due to rising calcitonin levels (Fig. 6d–f).
Discussion: MTC and CCH
Approximately 25% of MTC cases are associated with hereditary syndromes as noted (MEN2A, MEN2B, and familial medullary thyroid cancer) and due to germline activating point mutations of the RET receptor tyrosine kinase gene [83]. RET is also mutated in approximately 50% of sporadic MTC. Sporadic MTC can also have other mutations including KRAS, HRAS, and rarely NRAS and rarely rearrangements in ALK and RET genes [84]. Over 95% of MTC in children is hereditary with germline activating mutations in RET unlike FTC and MTC in adults where 65–75% of cases are sporadic with somatic RET mutations. Up to 10% of sporadic cases of MTC in children are due to de novo germline activation mutations in RET. Most mutations in MEN2A are in the extracellular cysteine-rich domains and in cysteine codons in exon 10 (609, 611, 618, 620) or 11 (634). Other rare mutations in codons 11, 13, and 15 have been reported. FMTC patients have mutations in 618, 620, and 634 and rarely in non-cysteine parts of the intracellular domain in exon 13, 14, and 15. More than 95% of MEN2B patients have the M918T mutation in exon 16 [33]. Compared to adults, pediatric patients present with less advanced disease and better cancer-free survival and overall survival compared to adults [83, 85]. American Thyroid Association guidelines for management of hereditary MTC (RET germline mutation) are prophylactic thyroidectomy (if possible depending on ability to preserve parathyroid glands) for children with MEN2B in the first months of life (ATA-highest risk), thyroidectomy in children less than 5 years of age with MEN2A based on serum calcitonin levels (ATA-high risk) or when serum calcitonin levels become elevated or in childhood if parents do not wish to proceed with a prolonged clinical follow-up protocol (ATA-moderate risk) [86]. A variety of treatment options exist or are under investigation including treatment with tyrosine kinase inhibitor small molecules such as cabozantinib, vandetanib, and apatinib. These molecules target key elements of proliferation, growth, and angiogenesis [84, 87].
The differential pattern of Foxa1 (high proliferation zone) and Foxa2 seen in embryonic C-cells seems to show a recrudescence in MTC suggesting that oncogenic transformation by Ret may switch on embryonic expression patterns seen in developing C-cells from the UBB (see discussion of endodermal origin of C-cell from UBB). The ontogenesis of rare mixed tumors consisting of MTC and follicular epithelial elements; mixed medullary-follicular thyroid carcinoma (MMFTC) has always been a bit puzzling if C-cells were of neural crest derivation. These tumors could be the result of two different ontogenic and clonal populations that came together. Since C-cells are of endodermal origin, it may be more plausible that such tumors may arise from the UBB alone. A similar line of reasoning might be invoked for polyclonal mixed tumors where tumors of C-cells and follicular epithelial cells co-exist with varying degrees of fusion between the two neoplastic tissues. In a minority of MMFTC (MTC with PTC), bilateral tumors occur with a single RET mutation and show tumor cells that express both calcitonin and thyroglobulin [13]. Some reports show that PTC co-occurring with MTC is more common and in most of these cases they are not clonally related but can have mutations in RET (MTC, germline and PTC, rearrangement or perhaps low level RET mutation). It should not be surprisingly plausible that tumors with neuroendocrine and follicular epithelial phenotypes might occur (endocrine and exocrine) since these have been well-described elsewhere, for instance, in the gastrointestinal tract where both elements are endodermally derived (e.g. mixed adenoneuroendocrine carcinoma (MANEC)).
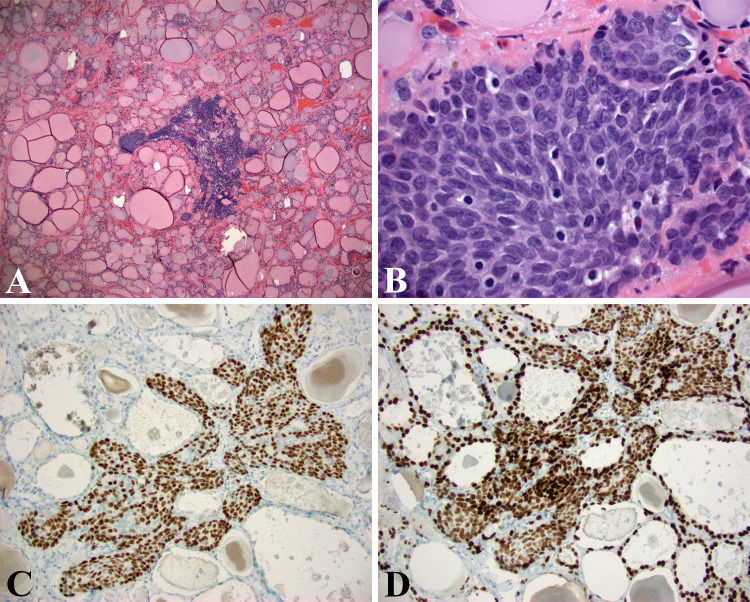
While putative cancer stem cells have been isolated from thyroid cancer cell lines, it is unclear if there are cells in the thyroid capable of differentiating into C-cells and follicular epithelial cells [17]. Evidence does exist that cells from UBBs have pluripotent capabilities and in animals where the fusion of UBB and thyroid is incomplete, tumors with a mixture of C-cells and follicular cells have been described [13]. UBBs in thyroid consistently and uniformly express cytokeratin 19, galectin-3, p63, bcl-2, and telomerase; the latter three markers considered hallmarks of putative basal/stem cells [88–90]. P63 appears to not be essential for thyroid or UBB development [88]. It is interesting how the cytomorphology of UBBs (Fig. 7) resembles that of Walthard cell nests (WCN) and Brenner tumors (thought to derive from WCN). WCNs are also show expression of p63 and GATA3 by immunohistochemistry. These are endodermally derived remnants from the developing urogenital sinus from which urothelium is derived. Urothelial development, in fact, is under the control of many of the same transcription factors noted in thyroid development [91].
Fig. 7.
UBB: a Densely cellular and somewhat ill-defined focus within the thyroid of an elderly gentleman with multiple follicular thyroid parenchymal lesions (HE, 40X). b High magnification view shows tightly packed oval cells with some nuclear overlapping, smooth chromatin, and nuclear grooves characteristic of the UBB epithelium. These cells demonstrate cytomorphology reminiscent of Walthard cell nests (see text) (HE, 600X). c UBBs showing diffuse expression of p63 and TTF-1 d (p63, 200X, TTF-1, 200X)
The histopathology of MTC is highly variable with classical solid and nesting patterns seen in neuroendocrine tumors. However, other patterns can be seen including pseudopapillary (confused with PTC) and follicular. Deposition of amyloid or proteinaceous material can mimic colloid in follicles. Tumor cells have ample amphophilic granular cytoplasm with generally eccentrically place nuclei with relative anisocytosis and “neuroendocrine” salt and pepper chromatin patterns. Pseudoinclusions and multinucleated cells can be seen. Oncocytic, plasmacytoid, spindle-cell, giant-cell, amphicrine (mucin/vacuolated cells), melanotic, clear cell, and small cell variants/types have all been described and can cause diagnostic confusion with follicular carcinomas, plasmacytomas, sarcomas, and lymphomas. The tumor cells express chromogranin, synaptophysin calcitonin, and carcinoembryonic antigen by immunohistochemistry. Some tumors can be calcitonin negative and TTF-1 and Pax8 usually show weaker staining [48, 83, 92].
A bit more discussion regarding precursor lesions of MTC, specifically C-cell hyperplasia (CCH), is warranted. CCH has been divided into three morphological forms: focal (partial follicle involvement), diffuse (surrounding follicle), and nodular (obliteration of follicle). Furthermore, CCH has been designated as physiologic or neoplastic. Defining CCH, however, is a bit of a moving target due to baselines that vary by age, sex, and other concomitant thyroid diseases. Published criteria have included 50 cells per 100 × field of view, 50 cells in 50 100 × fields of view, 40 cells/cm2 [93]. Neoplastic CCH (medullary carcinoma in situ or C-cell dysplasia) is considered a precursor lesion for lesion for MTC and could be defined as intrafollicular proliferation of cytologically atypical C-cells recognized on hematoxylin and eosin stained sections. Their recognition on HE sections is due to their cytological atypia with cells becoming larger in size and more prominent nuclei [18]. In this author’s experience, they are observed as a grayish tinge that disrupts and intercalates between the eosinophilic continuity of the thyroid at low magnification (40x). Staining with specific antibodies to neural cell adhesion molecule may reliably distinguish between neoplastic and reactive CCH [94]. Reactive CCH has been associated with aging, thyroiditis, nodular hyperplasia, adenomas, and hyperparathyroidism. The distinction between CCH and medullary microcarcinoma can be problematic with microinvasion signified by focal loss of basement membrane staining with collagen IV and local stromal fibrosis perhaps helpful but sometimes equivocal [48]. In the context of known MEN2A, any “significant” CCH should be considered as “neoplastic” particularly in a younger child where C-cells should not be prominently visible in the thyroid under most circumstances.
Parathyroid Gland: Embryology:
In humans, two bilateral pairs of parathyroid glands develop from the third and fourth pharyngeal pouch endoderm. In mice, the anterior-dorsal region of each third pouch forms a parathyroid gland and the posterior-ventral region develops into a lobe of the thymus. GCM2 expression under the influence of Sonic Hedgehog and GATA3 portends parathyroid differentiation and survival while FOXN1 and likely BMP4 expression determines thymic fate. As usual, the story is more complex and an array of other transcription factors also play roles in parathyroid differentiation and cell survival including Hoxa3, Pax1, Eya1, Pbx1, and Six1. Separation from the pharyngeal endoderm occurs via FGF and BMP4 signaling and this parathyroid-thymic primordium migrates ventrally and posteriorly mediated by Ephrin from the neural crest and thymic epithelium. Separation of the two occurs via differential expression of E-cadherin (thymic cells > parathyroid cells) and the thymus continues to migrate caudally while the parathyroid stops. CaSR and CCL21 genes are important for parathyroid differentiation and MABF1 gene is important for parathyroid hormone (PTH) production. Casr and Ccl21 expression is Gcm-2 independent but maintenance of their expression is Gcm-2 dependent [95, 96]. Genetic causes for primary hypoparathyroidism are listed in Table 2 [97]. These conditions most often will present during childhood, perhaps not with symptoms or signs of hypoparathyroidism but with other manifestations, particularly when hypoparathyroidism is associated with a syndrome. In these cases, the clinician should be alert to the possibility of hypoparathyroidism as an association and evaluate accordingly. For the practicing pathologist, these conditions rarely translate to specimens that will traverse his/her microscope, except perhaps at postmortem examination and then careful investigation for the parathyroid glands would be important. They are presented here to complete the genetic spectrum of parathyroid disease in children.
Table 2.
Genetic syndromes associated with primary hypoparathyroidism
| Genetic defect | Syndrome | Clinical manifestations | Parathyroid pathology |
|---|---|---|---|
|
GCM2 (6p23-24) SOX3 (insertion-deletion 2p25.3 and Xq26-27) |
Isolated parathyroid aplasia |
Primary hypoparathyroidism Infantile hypocalcemic seizures |
Agenesis of parathyroid glands |
|
TBX1(22q11.2) NEBL (10p13) |
DiGeorge Syndrome (DGS, type 1) DGS, type 2 |
Thymic aplasia, T-cell defects, conotruncal anomalies, cleft palate, dysmorphic facies | Hypoalcemia due to parathyroid hypoplasia |
|
CHD7 (8q12.2) SEMA3E (7q21.11) |
CHARGE syndrome | Coloboma, heart defects, choanal atresia, retarded growth, genital hypoplasia, ear anomalies | Hypocalcemia due to parathyroid hypoplasia |
| GATA3 haploinsufficiency | HDR/Barakat syndrome | Transient to severe neonatal hypocalcemia, deafness, renal dysplasia | Hypocalcemia due to parathyroid hypoplasia |
| TBCE (1q42-43) |
HRD/Sanjad-Sakati Syndrome (Arabic descent mostly) KCS/Kenny-Caffey syndrome |
Hypoparathyroidism, retardation, dysmorphism | Hypocalcemia due to parathyroid hypoplasia |
| Mitochondrial disease (Kearns-Sayre, Pearson Marrow-pancreas syndromes, MELAS, LCHAD, MCADD) | Various manifestations based on the specific disease | Hypoparathyroidism | |
| PTH (11p15.3-p15.1) | Isolated familial hypoparathyroidism | Hypocalcemia | Hypocalcemia due to defective PTH |
|
CASR (ADH1) (3q13.3-q21.1) GNA11 (ADH2) (19p13) |
Autosomal dominant hypocalcemia, types 1 and 2 | Neonatal to adult presentation of hypocalcemia (increased urinary calcium excretion, decreased PTH synthesis) | Increased sensitivity of the calcium sensing receptor to extracellular ionized calcium |
| AIRE (21q22.3) | Autoimmune polyendrocrinopathy candidiasis ectodermal dysplasia (APECED) | Mucocutaneous candidiasis, hypoparathyroidism, adrenal insufficiency, endocrinopathies, hepatitis, vitiligo, alopecia, nail/dental dystrophy | Hypocalcemia due to autoimmune destruction of parathyroid glands |
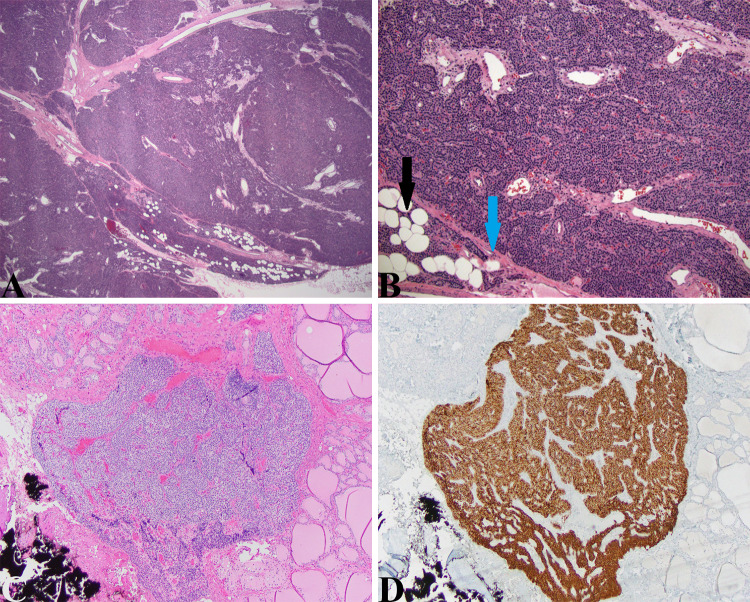
Case 6: Parathyroid adenoma (WHO Classification of Tumours of Endocrine Organs, 4th Edition, 2017; ICD-O 8140/0) (Fig. 8).
Fig. 8.
Parathyroid adenoma and parathyroid gland: a Large parathyroid adenoma
taken from the 11-year-old boy presented above. This uniformly hypercellular gland is finely encapsulated (blue arrow in (b)) and abuts normal parathyroid tissue characteristic of adenomas (black arrow in (b)). Some thicker fibrous bands are seen traversing the adenoma proper but other features to suggest malignancy (capsular/vascular invasion, trabecular growth, atypia, atypical mitoses) were not seen (HE, 20X, 40X). c Intrathyroidal lesion seen in the thyroidectomy specimen from the young girl with MEN2A presented above that has similar cytological features to that of the medullary thyroid microcarcinoma. This lesion is clearly parathyroid gland confirmed by the diffuse staining with anti-PTH antibody in (d) (HE, 40X and PTH, 40X)
This patient is an 11-year-old male who presented with right flank pain and vomiting. Laboratory evaluation showed a serum calcium of 13.4, phosphate 3.0, vitamin D level 16, and parathyroid hormone level of 408 pg/ml. A CT scan of the abdomen showed a 3 mm nephrolith obstructing the right ureter and multiple non-obstructing renal calculi. Thyroid ultrasound revealed a 1 × 2 cm hypoechoic nodule posterior to the right upper lobe of the thyroid confirmed by technetium-99 m sestamibi parathyroid scan (Tc-99 m MIBI). Interestingly, the father had a history of kidney “stones”. At the time of surgery, a single large parathyroid gland was removed with appropriate reduction of intraoperative PTH values. Pathological examination was consistent with a parathyroid adenoma.
Discussion: Parathyroid Tumors
Genetic causes of primary hyperparathyroidism presenting with signs and symptoms of hypercalcemia primarily or in association with other syndromic manifestations are listed in Table 3 [98]. Once again, for the practicing clinician who encounters a patient, particularly a young patient, with a constellation of findings or perhaps one suspicious syndromic finding should prompt referral for genetic testing and be alerted for the possibility of parathyroid associated disease. Practically speaking, for the surgical pathologist, the pathology will consist of benign tumors mostly adenomas/microadenomas and very rarely carcinoma. For the most part, with these genetic syndromes, while manifestations of primary hyperparathyroidism can present earlier, MEN1 is one syndrome where parathyroid pathology (adenoma) may be seen in the pediatric age range. Also, patients with the severe form of neonatal PHPT, requiring urgent parathyroidectomy will typically have multigland hyperplasia. An additional note, patients with HPT-JT are particularly predisposed to parathyroid carcinoma and about 10% will have jaw tumors with histology of ossifying fibromas as opposed to osteitis cystica fibrosa seen in some patients with PHPT [48, 97, 98].
Table 3.
Genetic syndromes associated with primary hyperparathyroidism
| Genetic defect/Inheritance | Chromosomal location | Clinical Manifestations | Parathyroid pathology |
|---|---|---|---|
| MEN1/AD | See above | Can present very young with parathyroid tumors; 17% less than 21 years old have symptomatic hypercalcemia | Hypercalcemia first manifestation in 90%, multigland disease (microadenomatosis) |
| MEN2A/AD | See above | See above | Parathyroid tumors in 20% (hyperplasia/adenoma) |
| MEN2B (3)/AD | See above | See above | Rare |
| MEN4 | See above | See above | Parathyroid tumors (adenomas) |
| FHH1/FHH2/FHH3/AD |
3q21.1/19p13/19q13.2-q13.3 CASR/GNA11/AP2S1 |
Hypercalcemia | PTH-independent decreased calcium excretion |
| NSHPT/AR | 3q21.1 (homozygous or compound heterozygous mutations of CASR gene) | Severe neonatal hypercalcemia, hypotonia, fatal by 3 months untreated | Urgent parathyroidectomy; multigland hyperplasia |
| FIHP/AD | 11q13/1q31.2/3q21.1/6p24.2 | Hereditary primary hyperparathyroidism without other tumors, hypercalcemia after first decade; diagnosis of exclusion | Parathyroid tumors |
| HPT-JT/AD | 1q31.2 (CDC73; cell division cycle 73, parafibromin) | Multiple parathyroid tumors, ossifying fibromas of jaw, renal cysts perhaps other renal tumors | Adenomas, synchronous/asynchronous parathyroid tumors, lifetime risk of parathyroid carcinoma 20%, PC cause of hypercalcemia in 15–37.5%), PC reported in adolescents |
At the time of this writing, this author conjured up approximately 19 cases of parathyroid carcinoma in children reported separately or embedded within larger series (certainly underestimated) with 8 years of age the earliest presentation [99–116]. Patients from two more recent cases demonstrated mutations in the CDC73 gene (one germline, one somatic) and one patient had an intragenic deletion in the CDC73 gene in a familial cohort [106, 109, 113]. An atypical parathyroid adenoma in a 13-year-ol boy with FIHP with CDC73 mutation has been reported [117]. The CDC73 gene (formerly HRPT2; chromosome 1q25) encodes parafibromin, a tumor suppressor gene, down regulates the expression of cyclin D1. Mutations in CDC73 have been found in cases of sporadic parathyroid carcinoma (70% of cases), HPT-JT, and FIHP. Parathyroid adenomas rarely demonstrate CDC73 mutations [113].
This review will not serve as an exhaustive review of the histopathology of parathyroid glands as this subject is treated comprehensively in many other sources. No specific histopathological features will distinguish parathyroid pathology in the pediatric population compared to the adult population. The diagnosis of parathyroid carcinoma is made by observing a tumor of parathyroid origin with invasive growth (adjacent structures, capsule, blood vessels, perineurial) or metastases [48]. Other features; fibrous bands, trabecular growth pattern, cytological atypia, mitoses (unless atypical), and Ki-67 indices are not reliable enough discriminating features from adenomas [48, 112]. The tumors designated as “atypical adenomas” may have some of the other ancillary features listed but lack true invasive growth and thus not qualifying diagnostically as carcinomas. Microadenomas are 0.6 cm or less in diameter and less than 100 mg but may be difficult to distinguish from hyperplasias or may share some features of adenomas (thinly encapsulated with adjacent normal parathyroid). Parathyroid hyperplasia is multigland diffuse increase in parenchyma without a known cause or as noted may be part of a heritable condition or syndrome. The pathological distinction between hyperplasia and adenoma can be very challenging and the intraoperative PTH values may be helpful as well as use of Oil red O stains on two glands intraoperatively may reliably distinguish these entities [118]. Multiple hyperplastic parathyroid rests in the neck soft tissues or mediastinum (parathyromatosis) may be the cause of persistent hypercalcemia in patients having undergone subtotal parathyroidectomy for hyperparathyroidism. Since most young patients (< 18 years) with primary HPT without a family history of parathyroid disease have adenomas, most experienced surgeons will opt for a minimally invasive approach to removing the single enlarged gland since the complication rates for parathyroidectomy are higher in children than adults [95].
Compliance with Ethical Standards
Conflict of interest
The author has no disclosures or conflicts of interest.
Ethical Approval
This study does not use human or animal subjects.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Benard V (2018) NPCR and SEER Incidence—U.S. Cancer Statistics 2001–2016 Public Use Database Data Standards and Data Dictionary. Centers for Disease Control (pp. 33–48). https://www.cdc.gov/cancer/uscs/public-use/pdf/npcr-seer-public-use-database-data-dictionary-2001-2016-508.pdf
- 2.Qian ZJ, Jin MC, Meister KD, Megwalu UC. Pediatric Thyroid Cancer Incidence and Mortality Trends in the United States, 1973–2013. JAMA Otolaryngol Head Neck Surg. 2019;145(7):617–623. doi: 10.1001/jamaoto.2019.0898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bernier MO, Withrow DR, de Gonzalez AB, Lam CJK, Linet MS, Kitahara CM, et al. Trends in pediatric thyroid cancer incidence in the United States, 1998–2013. Cancer. 2019;125(14):2497–2505. doi: 10.1002/cncr.32125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vanderpump MP. The epidemiology of thyroid disease. Br Med Bull. 2011;99:39–51. doi: 10.1093/bmb/ldr030. [DOI] [PubMed] [Google Scholar]
- 5.Paulson VA, Rudzinski ER, Hawkins DS. Thyroid cancer in the pediatric population. Genes (Basel). 2019 doi: 10.3390/genes10090723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guille JT, Opoku-Boateng A, Thibeault SL, Chen H. Evaluation and management of the pediatric thyroid nodule. Oncologist. 2015;20(1):19–27. doi: 10.1634/theoncologist.2014-0115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hong HS, Lee EH, Jeong SH, Park J, Lee H. Ultrasonography of various thyroid diseases in children and adolescents: a pictorial essay. Korean J Radiol. 2015;16(2):419–429. doi: 10.3348/kjr.2015.16.2.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Koch CA, Sarlis NJ. The spectrum of thyroid diseases in childhood and its evolution during transition to adulthood: natural history, diagnosis, differential diagnosis and management. J Endocrinol Invest. 2001;24(9):659–675. doi: 10.1007/BF03343911. [DOI] [PubMed] [Google Scholar]
- 9.Nikiforov Y, Biddinger PW, Thompson LDR. Diagnostic pathology and molecular genetics of the thyroid. 2. Philadelphia: Wolters Kluwer Health/Lippincott Williams & Wilkins; 2012. [Google Scholar]
- 10.Ozolek JA. Selective pathologies of the head and neck in children: a developmental perspective. Adv Anat Pathol. 2009;16(5):332–358. doi: 10.1097/PAP.0b013e3181b50571. [DOI] [PubMed] [Google Scholar]
- 11.Subramanyam P, Palaniswamy SS. Pictorial essay of developmental thyroid anomalies identified by Technetium thyroid scintigraphy. Indian J Nucl Med. 2015;30(4):323–327. doi: 10.4103/0972-3919.159694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nilsson M, Fagman H. Development of the thyroid gland. Development. 2017;144(12):2123–2140. doi: 10.1242/dev.145615. [DOI] [PubMed] [Google Scholar]
- 13.Nilsson M, Williams D. On the origin of cells and derivation of thyroid cancer: C cell story revisited. Eur Thyroid J. 2016;5(2):79–93. doi: 10.1159/000447333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kameda Y, Nishimaki T, Chisaka O, Iseki S, Sucov HM. Expression of the epithelial marker E-cadherin by thyroid C cells and their precursors during murine development. J Histochem Cytochem. 2007;55(10):1075–1088. doi: 10.1369/jhc.7A7179.2007. [DOI] [PubMed] [Google Scholar]
- 15.Bohnsack BL, Kahana A. Thyroid hormone and retinoic acid interact to regulate zebrafish craniofacial neural crest development. Dev Biol. 2013;373(2):300–309. doi: 10.1016/j.ydbio.2012.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gordon J. Hox genes in the pharyngeal region: how Hoxa3 controls early embryonic development of the pharyngeal organs. Int J Dev Biol. 2018;62(11–12):775–783. doi: 10.1387/ijdb.180284jg. [DOI] [PubMed] [Google Scholar]
- 17.Nagayama Y, Shimamura M, Mitsutake N. Cancer stem cells in the thyroid. Front Endocrinol (Lausanne). 2016;7:20. doi: 10.3389/fendo.2016.00020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Scognamiglio T. C cell and follicular epithelial cell precursor lesions of the thyroid. Arch Pathol Lab Med. 2017;141(12):1646–1652. doi: 10.5858/arpa.2016-0399-RA. [DOI] [PubMed] [Google Scholar]
- 19.Dupin E, Calloni G, Real C, Goncalves-Trentin A, Le Douarin NM. Neural crest progenitors and stem cells. C R Biol. 2007;330(6–7):521–529. doi: 10.1016/j.crvi.2007.04.004. [DOI] [PubMed] [Google Scholar]
- 20.Kameda Y. Cellular and molecular events on the development of mammalian thyroid C cells. Dev Dyn. 2016;245(3):323–341. doi: 10.1002/dvdy.24377. [DOI] [PubMed] [Google Scholar]
- 21.Lo CW, Cohen MF, Huang GY, Lazatin BO, Patel N, Sullivan R, et al. Cx43 gap junction gene expression and gap junctional communication in mouse neural crest cells. Dev Genet. 1997;20(2):119–132. doi: 10.1002/(SICI)1520-6408(1997)20:2<119::AID-DVG5>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 22.Johansson E, Andersson L, Ornros J, Carlsson T, Ingeson-Carlsson C, Liang S, et al. Revising the embryonic origin of thyroid C cells in mice and humans. Development. 2015;142(20):3519–3528. doi: 10.1242/dev.126581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chisaka O, Kameda Y. Hoxa3 regulates the proliferation and differentiation of the third pharyngeal arch mesenchyme in mice. Cell Tissue Res. 2005;320(1):77–89. doi: 10.1007/s00441-004-1042-z. [DOI] [PubMed] [Google Scholar]
- 24.Kameda Y, Arai Y, Nishimaki T, Chisaka O. The role of Hoxa3 gene in parathyroid gland organogenesis of the mouse. J Histochem Cytochem. 2004;52(5):641–651. doi: 10.1177/002215540405200508. [DOI] [PubMed] [Google Scholar]
- 25.Kameda Y, Nishimaki T, Miura M, Jiang SX, Guillemot F. Mash1 regulates the development of C cells in mouse thyroid glands. Dev Dyn. 2007;236(1):262–270. doi: 10.1002/dvdy.21018. [DOI] [PubMed] [Google Scholar]
- 26.Filis P, Hombach-Klonisch S, Ayotte P, Nagrath N, Soffientini U, Klonisch T, et al. Maternal smoking and high BMI disrupt thyroid gland development. BMC Med. 2018;16(1):194. doi: 10.1186/s12916-018-1183-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jarzab B, Handkiewicz-Junak D. Differentiated thyroid cancer in children and adults: same or distinct disease? Hormones (Athens). 2007;6(3):200–209. [PubMed] [Google Scholar]
- 28.Karapanou O, Tzanela M, Vlassopoulou B, Kanaka-Gantenbein C. Differentiated thyroid cancer in childhood: a literature update. Hormones (Athens). 2017;16(4):381–387. doi: 10.14310/horm.2002.1758. [DOI] [PubMed] [Google Scholar]
- 29.Leboulleux S, Baudin E, Hartl DW, Travagli JP, Schlumberger M. Follicular cell-derived thyroid cancer in children. Horm Res. 2005;63(3):145–151. doi: 10.1159/000084717. [DOI] [PubMed] [Google Scholar]
- 30.Francis GL, Waguespack SG, Bauer AJ, Angelos P, Benvenga S, Cerutti JM, et al. Management guidelines for children with thyroid nodules and differentiated thyroid cancer. Thyroid. 2015;25(7):716–759. doi: 10.1089/thy.2014.0460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Santoro M, Carlomagno F. Central role of RET in thyroid cancer. Cold Spring Harb Perspect Biol. 2013;5(12):a009233. doi: 10.1101/cshperspect.a009233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pachnis V, Mankoo B, Costantini F. Expression of the c-ret proto-oncogene during mouse embryogenesis. Development. 1993;119(4):1005–1017. doi: 10.1242/dev.119.4.1005. [DOI] [PubMed] [Google Scholar]
- 33.Khatami F, Tavangar SM. Multiple endocrine neoplasia syndromes from genetic and epigenetic perspectives. Biomark Insights. 2018;13:1177271918785129. doi: 10.1177/1177271918785129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Marini F, Falchetti A, Del Monte F, Carbonell Sala S, Gozzini A, Luzi E, et al. Multiple endocrine neoplasia type 1. Orphanet J Rare Dis. 2006;1:38. doi: 10.1186/1750-1172-1-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Martucciello G, Lerone M, Bricco L, Tonini GP, Lombardi L, Del Rossi CG, et al. Multiple endocrine neoplasias type 2B and RET proto-oncogene. Ital J Pediatr. 2012;38:9. doi: 10.1186/1824-7288-38-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Millar S, Bradley L, Donnelly DE, Carson D, Morrison PJ. Familial pediatric endocrine tumors. Oncologist. 2011;16(10):1388–1396. doi: 10.1634/theoncologist.2011-0120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zirilli G, Salzano G, Corica D, Pajno GB, Mignosa C, Pepe G, et al. Thyrotropin serum levels and coexistence with Hashimoto's thyroiditis as predictors of malignancy in children with thyroid nodules. Ital J Pediatr. 2019;45(1):96. doi: 10.1186/s13052-019-0693-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hincza K, Kowalik A, Kowalska A. Current knowledge of germline genetic risk factors for the development of non-medullary thyroid cancer. Genes (Basel). 2019 doi: 10.3390/genes10070482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang H, Mehrad M, Ely KA, Liang J, Solorzano CC, Neblett WW, 3rd, et al. Incidence and malignancy rates of indeterminate pediatric thyroid nodules. Cancer Cytopathol. 2019;127(4):231–239. doi: 10.1002/cncy.22104. [DOI] [PubMed] [Google Scholar]
- 40.Picarsic JL, Buryk MA, Ozolek J, Ranganathan S, Monaco SE, Simons JP, et al. Molecular characterization of sporadic pediatric thyroid carcinoma with the DNA/RNA ThyroSeq v2 next-generation sequencing assay. Pediatr Dev Pathol. 2016;19(2):115–122. doi: 10.2350/15-07-1667-OA.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Geng J, Wang H, Liu Y, Tai J, Jin Y, Zhang J, et al. Correlation between BRAF (V600E) mutation and clinicopathological features in pediatric papillary thyroid carcinoma. Sci China Life Sci. 2017;60(7):729–738. doi: 10.1007/s11427-017-9083-8. [DOI] [PubMed] [Google Scholar]
- 42.Bertherat J. Carney complex (CNC) Orphanet J Rare Dis. 2006;1:21. doi: 10.1186/1750-1172-1-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dinarvand P, Davaro EP, Doan JV, Ising ME, Evans NR, Phillips NJ, et al. Familial adenomatous polyposis syndrome: an update and review of extraintestinal manifestations. Arch Pathol Lab Med. 2019;143(11):1382–1398. doi: 10.5858/arpa.2018-0570-RA. [DOI] [PubMed] [Google Scholar]
- 44.Eng C, Peacocke M. PTEN and inherited hamartoma-cancer syndromes. Nat Genet. 1998;19(3):223. doi: 10.1038/897. [DOI] [PubMed] [Google Scholar]
- 45.Garofola C, Jamal Z, Gross GP. Cowden Disease (Multiple Hamartoma Syndrome). StatPearls. Treasure Island (FL)2020.
- 46.Pilarski R. PTEN hamartoma tumor syndrome: a clinical overview. Cancers (Basel). 2019 doi: 10.3390/cancers11060844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schultz KAP, Rednam SP, Kamihara J, Doros L, Achatz MI, Wasserman JD, et al. PTEN, DICER1, FH, and their associated tumor susceptibility syndromes: clinical features, genetics, and surveillance recommendations in childhood. Clin Cancer Res. 2017;23(12):e76–e82. doi: 10.1158/1078-0432.CCR-17-0629. [DOI] [PubMed] [Google Scholar]
- 48.Lloyd RV, Osamura RY, Klöppel Gn, Rosai J, World Health O, International Agency for Research on C. WHO classification of tumours of endocrine organs. 2017
- 49.Spinelli C, Strambi S, Bakkar S, Nosiglia A, Elia G, Bertocchini A, et al. Surgical management of diffuse sclerosing variant of papillary thyroid carcinoma: experience in 25 patients. World J Surg. 2020;44(1):155–162. doi: 10.1007/s00268-019-05230-5. [DOI] [PubMed] [Google Scholar]
- 50.Low S, Sakai Y, Hoshino H, Hirokawa M, Kawashima H, Higuchi K, et al. High endothelial venule-like vessels and lymphocyte recruitment in diffuse sclerosing variant of papillary thyroid carcinoma. Pathology. 2016;48(7):666–674. doi: 10.1016/j.pathol.2016.08.002. [DOI] [PubMed] [Google Scholar]
- 51.Joung JY, Kim TH, Jeong DJ, Park SM, Cho YY, Jang HW, et al. Diffuse sclerosing variant of papillary thyroid carcinoma: major genetic alterations and prognostic implications. Histopathology. 2016;69(1):45–53. doi: 10.1111/his.12902. [DOI] [PubMed] [Google Scholar]
- 52.Kobayashi K, Fujimoto T, Ota H, Hirokawa M, Yabuta T, Masuoka H, et al. Calcifications in thyroid tumors on ultrasonography: calcification types and relationship with histopathological type. Ultrasound Int Open. 2018;4(2):E45–E51. doi: 10.1055/a-0591-6070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Xue N, Xu Y, Huang P, Zhang S, Wang H, Yu F. Shear wave elastography diagnosis of the diffuse sclerosing variant of papillary thyroid carcinoma: a case report. Mol Clin Oncol. 2016;5(2):333–336. doi: 10.3892/mco.2016.932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Andriescu EC, Caruntu ID, Giusca SE, Lozneanu L, Ciobanu Apostol DG. Prognostic significance of cell-adhesion molecules in histological variants of papillary thyroid carcinoma. Rom J Morphol Embryol. 2018;59(3):721–727. [PubMed] [Google Scholar]
- 55.Feng J, Shen F, Cai W, Gan X, Deng X, Xu B. Survival of aggressive variants of papillary thyroid carcinoma in patients under 55 years old: a SEER population-based retrospective analysis. Endocrine. 2018;61(3):499–505. doi: 10.1007/s12020-018-1644-y. [DOI] [PubMed] [Google Scholar]
- 56.Holoubek SA, Yan H, Khokar AH, Kuchta KM, Winchester DJ, Prinz RA, et al. Aggressive variants of papillary thyroid microcarcinoma are associated with high-risk features, but not decreased survival. Surgery. 2020;167(1):19–27. doi: 10.1016/j.surg.2019.03.030. [DOI] [PubMed] [Google Scholar]
- 57.Limberg J, Ullmann TM, Stefanova D, Buicko JL, Finnerty BM, Zarnegar R, et al. Does aggressive variant histology without invasive features predict overall survival in papillary thyroid cancer?: A national cancer database analysis. Ann Surg. 2019 doi: 10.1097/SLA.0000000000003632. [DOI] [PubMed] [Google Scholar]
- 58.Malandrino P, Russo M, Regalbuto C, Pellegriti G, Moleti M, Caff A, et al. Outcome of the diffuse sclerosing variant of papillary thyroid cancer: a meta-analysis. Thyroid. 2016;26(9):1285–1292. doi: 10.1089/thy.2016.0168. [DOI] [PubMed] [Google Scholar]
- 59.Russo M, Malandrino P, Moleti M, Vermiglio F, Violi MA, Marturano I, et al. Tall cell and diffuse sclerosing variants of papillary thyroid cancer: outcome and predicting value of risk stratification methods. J Endocrinol Invest. 2017;40(11):1235–1241. doi: 10.1007/s40618-017-0688-9. [DOI] [PubMed] [Google Scholar]
- 60.Nieminen TT, Walker CJ, Olkinuora A, Genutis LK, O'Malley M, Wakely PE, et al. Thyroid carcinomas that occur in familial adenomatous polyposis patients recurrently harbor somatic variants in APC, BRAF, and KTM2D. Thyroid. 2020;30(3):380–388. doi: 10.1089/thy.2019.0561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Akaishi J, Kondo T, Sugino K, Ogimi Y, Masaki C, Hames KY, et al. Cribriform-morular variant of papillary thyroid carcinoma: clinical and pathological features of 30 cases. World J Surg. 2018;42(11):3616–3623. doi: 10.1007/s00268-018-4644-4. [DOI] [PubMed] [Google Scholar]
- 62.Park J, Kim JW, Park H, Park SY, Kim TH, Kim SW, et al. Multifocality in a patient with cribriform-morular variant of papillary thyroid carcinoma is an important clue for the diagnosis of familial adenomatous polyposis. Thyroid. 2019;29(11):1606–1614. doi: 10.1089/thy.2019.0261. [DOI] [PubMed] [Google Scholar]
- 63.Aydemirli MD, van der Tuin K, Hes FJ, van den Ouweland AMW, van Wezel T, Kapiteijn E, et al. A unique case of two somatic APC mutations in an early onset cribriform-morular variant of papillary thyroid carcinoma and overview of the literature. Fam Cancer. 2020;19(1):15–21. doi: 10.1007/s10689-019-00146-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Brehar AC, Terzea DC, Ioachim DL, Procopiuc C, Brehar FM, Bulgar AC, et al. Cribriform-morular variant of papillary thyroid carcinoma at pediatric age - case report and review of the literature. Rom J Morphol Embryol. 2016;57(2):531–537. [PubMed] [Google Scholar]
- 65.Mohindra S, Sakr H, Sturgis C, Chute DJ. LEF-1 is a sensitive marker of cribriform morular variant of papillary thyroid carcinoma. Head Neck Pathol. 2018;12(4):455–462. doi: 10.1007/s12105-017-0873-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hirokawa M, Matsuda K, Kudo T, Higuchi M, Suzuki A, Takada N, et al. Cribriform-morular variant of papillary thyroid carcinoma shows high Ki-67 labeling indices, despite its excellent prognosis. Pathobiology. 2019;86(5–6):248–253. doi: 10.1159/000501097. [DOI] [PubMed] [Google Scholar]
- 67.Perrier ND, van Heerden JA, Goellner JR, Williams ED, Gharib H, Marchesa P et al. Thyroid cancer in patients with familial adenomatous polyposis. World J Surg. 1998;22(7):738–42; discussion 43. doi:10.1007/s002689900462. [DOI] [PubMed]
- 68.Tsuji H, Yasuoka H, Nakamura Y, Hirokawa M, Hiroshima T, Sakamaki Y, et al. Aggressive cribriform-morular variant of papillary thyroid carcinoma: report of an unusual case with pulmonary metastasis displaying poorly differentiated features. Pathol Int. 2018;68(12):700–705. doi: 10.1111/pin.12728. [DOI] [PubMed] [Google Scholar]
- 69.Ito Y, Ishikawa H, Kihara M, Hirokawa M, Kiyota N, Kasahara T, et al. Control of lung metastases and colon polyposis with lenvatinib therapy in a patient with Cribriform-Morular variant of papillary thyroid carcinoma and an APC gene mutation: a case study. Thyroid. 2019;29(10):1511–1517. doi: 10.1089/thy.2019.0121. [DOI] [PubMed] [Google Scholar]
- 70.Laforga JB, Pedro T, Gasent JM. Pulmonary metastasis of cribriform-morular variant of thyroid carcinoma mimicking primary adenocarcinoma of the lung: a potential pitfall. Diagn Cytopathol. 2020;48(1):78–81. doi: 10.1002/dc.24312. [DOI] [PubMed] [Google Scholar]
- 71.Eng C. PTEN Hamartoma Tumor Syndrome. In: Adam MP, Ardinger HH, Pagon RA, Wallace SE, Bean LJH, Stephens K et al., editors. GeneReviews((R)). Seattle (WA)1993.
- 72.Tan MH, Eng C. RE: Cowden syndrome and PTEN hamartoma tumor syndrome: systematic review and revised diagnostic criteria. J Natl Cancer Inst. 2014;106(6):130. doi: 10.1093/jnci/dju130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Yu W, Ni Y, Saji M, Ringel MD, Jaini R, Eng C. Cowden syndrome-associated germline succinate dehydrogenase complex subunit D (SDHD) variants cause PTEN-mediated down-regulation of autophagy in thyroid cancer cells. Hum Mol Genet. 2017;26(7):1365–1375. doi: 10.1093/hmg/ddx037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Eissing M, Ripken L, Schreibelt G, Westdorp H, Ligtenberg M, Netea-Maier R, et al. PTEN hamartoma tumor syndrome and immune dysregulation. Transl Oncol. 2019;12(2):361–367. doi: 10.1016/j.tranon.2018.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Tosur M, Brandt ML, Athanassaki ID, Rednam SP. Considerations for total thyroidectomy in an adolescent with PTEN mutation. Ther Adv Endocrinol Metab. 2018;9(9):299–301. doi: 10.1177/2042018818784517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Cho U, Mete O, Kim MH, Bae JS, Jung CK. Molecular correlates and rate of lymph node metastasis of non-invasive follicular thyroid neoplasm with papillary-like nuclear features and invasive follicular variant papillary thyroid carcinoma: the impact of rigid criteria to distinguish non-invasive follicular thyroid neoplasm with papillary-like nuclear features. Mod Pathol. 2017;30(6):810–825. doi: 10.1038/modpathol.2017.9. [DOI] [PubMed] [Google Scholar]
- 77.Nikiforov YE, Seethala RR, Tallini G, Baloch ZW, Basolo F, Thompson LD, et al. Nomenclature revision for encapsulated follicular variant of papillary thyroid carcinoma: a paradigm shift to reduce overtreatment of indolent tumors. JAMA Oncol. 2016;2(8):1023–1029. doi: 10.1001/jamaoncol.2016.0386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Thompson LD. Ninety-four cases of encapsulated follicular variant of papillary thyroid carcinoma: a name change to Noninvasive Follicular Thyroid Neoplasm with Papillary-like Nuclear Features would help prevent overtreatment. Mod Pathol. 2016;29(7):698–707. doi: 10.1038/modpathol.2016.65. [DOI] [PubMed] [Google Scholar]
- 79.Rossi ED, Mehrotra S, Kilic AI, Toslak IE, Lim-Dunham J, Martini M, et al. Noninvasive follicular thyroid neoplasm with papillary-like nuclear features in the pediatric age group. Cancer Cytopathol. 2018;126(1):27–35. doi: 10.1002/cncy.21933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Mariani RA, Kadakia R, Arva NC. Noninvasive encapsulated follicular variant of papillary thyroid carcinoma: Should it also be reclassified in children? Pediatr Blood Cancer. 2018;65(6):e26966. doi: 10.1002/pbc.26966. [DOI] [PubMed] [Google Scholar]
- 81.Wang H, Correa H, Sanders M, Neblett WW, Liang J. Noninvasive follicular thyroid neoplasm with papillary-like nuclear features in children: an institutional experience and literature review. Pediatr Dev Pathol. 2020;23(2):121–126. doi: 10.1177/1093526619866584. [DOI] [PubMed] [Google Scholar]
- 82.Seethala RR, Baloch ZW, Barletta JA, Khanafshar E, Mete O, Sadow PM, et al. Noninvasive follicular thyroid neoplasm with papillary-like nuclear features: a review for pathologists. Mod Pathol. 2018;31(1):39–55. doi: 10.1038/modpathol.2017.130. [DOI] [PubMed] [Google Scholar]
- 83.Thomas CM, Asa SL, Ezzat S, Sawka AM, Goldstein D. Diagnosis and pathologic characteristics of medullary thyroid carcinoma-review of current guidelines. Curr Oncol. 2019;26(5):338–344. doi: 10.3747/co.26.5539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Nelkin B. Recent advances in the biology and therapy of medullary thyroid carcinoma. Res. 2017;6:2184. doi: 10.12688/f1000research.12645.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zhao Z, Yin XD, Zhang XH, Li ZW, Wang DW. Comparison of pediatric and adult medullary thyroid carcinoma based on SEER program. Sci Rep. 2020;10(1):13310. doi: 10.1038/s41598-020-70439-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wells SA, Jr, Asa SL, Dralle H, Elisei R, Evans DB, Gagel RF, et al. Revised American Thyroid Association guidelines for the management of medullary thyroid carcinoma. Thyroid. 2015;25(6):567–610. doi: 10.1089/thy.2014.0335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Cai S, Deng H, Chen Y, Wu X, Guan X. Treatment of medullary thyroid carcinoma with apatinib: a case report and literature review. Med (Baltimore). 2017;96(50):e8704. doi: 10.1097/MD.0000000000008704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ozaki T, Nagashima K, Kusakabe T, Kakudo K, Kimura S. Development of thyroid gland and ultimobranchial body cyst is independent of p63. Lab Invest. 2011;91(1):138–146. doi: 10.1038/labinvest.2010.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Preto A, Cameselle-Teijeiro J, Moldes-Boullosa J, Soares P, Cameselle-Teijeiro JF, Silva P, et al. Telomerase expression and proliferative activity suggest a stem cell role for thyroid solid cell nests. Mod Pathol. 2004;17(7):819–826. doi: 10.1038/modpathol.3800124. [DOI] [PubMed] [Google Scholar]
- 90.Rios Moreno MJ, Galera-Ruiz H, De Miguel M, Lopez MI, Illanes M, Galera-Davidson H. Inmunohistochemical profile of solid cell nest of thyroid gland. Endocr Pathol. 2011;22(1):35–39. doi: 10.1007/s12022-010-9145-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.de Graaf P, van der Linde EM, Rosier P, Izeta A, Sievert KD, Bosch J, et al. Systematic review to compare urothelium differentiation with urethral epithelium differentiation in fetal development, as a basis for tissue engineering of the Male Urethra. Tissue Eng Part B. 2017;23(3):257–267. doi: 10.1089/ten.TEB.2016.0352. [DOI] [PubMed] [Google Scholar]
- 92.Hazard JB. The C cells (parafollicular cells) of the thyroid gland and medullary thyroid carcinoma. A Rev Am J Pathol. 1977;88(1):213–250. [PMC free article] [PubMed] [Google Scholar]
- 93.Wenig BM. Atlas of Head and Neck Pathology. Elsevier Health Sciences; 2015.
- 94.<C CELLS AJP-1981.pdf>.
- 95.Burke JF, Chen H, Gosain A. Parathyroid conditions in childhood. Semin Pediatr Surg. 2014;23(2):66–70. doi: 10.1053/j.sempedsurg.2014.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Peissig K, Condie BG, Manley NR. Embryology of the parathyroid glands. Endocrinol Metab Clin North Am. 2018;47(4):733–742. doi: 10.1016/j.ecl.2018.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Gordon RJ, Levine MA. Genetic disorders of parathyroid development and function. Endocrinol Metab Clin North Am. 2018;47(4):809–823. doi: 10.1016/j.ecl.2018.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Stokes VJ, Nielsen MF, Hannan FM, Thakker RV. Hypercalcemic disorders in children. J Bone Miner Res. 2017;32(11):2157–2170. doi: 10.1002/jbmr.3296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Black BK, Ackerman LV. Tumors of the parathyroid; a review of twenty-three cases. Cancer. 1950;3(3):415–444. doi: 10.1002/1097-0142(1950)3:3<415::aid-cncr2820030304>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 100.Busaidy NL, Jimenez C, Habra MA, Schultz PN, El-Naggar AK, Clayman GL, et al. Parathyroid carcinoma: a 22-year experience. Head Neck. 2004;26(8):716–726. doi: 10.1002/hed.20049. [DOI] [PubMed] [Google Scholar]
- 101.Fiedler AG, Rossi C, Gingalewski CA. Parathyroid carcinoma in a child: an unusual case of an ectopically located malignant parathyroid gland with tumor invading the thymus. J Pediatr Surg. 2009;44(8):1649–1652. doi: 10.1016/j.jpedsurg.2009.04.024. [DOI] [PubMed] [Google Scholar]
- 102.Fujimoto Y, Obara T, Ito Y, Kanazawa K, Aiyoshi Y, Nobori M. Surgical treatment of ten cases of parathyroid carcinoma: importance of an initial en bloc tumor resection. World J Surg. 1984;8(3):392–400. doi: 10.1007/BF01655086. [DOI] [PubMed] [Google Scholar]
- 103.Hamill J, Maoate K, Beasley SW, Corbett R, Evans J. Familial parathyroid carcinoma in a child. J Paediatr Child Health. 2002;38(3):314–317. doi: 10.1046/j.1440-1754.2002.00802.x. [DOI] [PubMed] [Google Scholar]
- 104.Herrera-Hernandez AA, Aranda-Valderrama P, Diaz-Perez JA, Herrera LP. Intrathyroidal parathyroid carcinoma in a pediatric patient. Pediatr Surg Int. 2011;27(12):1361–1365. doi: 10.1007/s00383-011-2904-6. [DOI] [PubMed] [Google Scholar]
- 105.Kim HK, Oh YL, Kim SH, Lee DY, Kang HC, Lee JI, et al. Parafibromin immunohistochemical staining to differentiate parathyroid carcinoma from parathyroid adenoma. Head Neck. 2012;34(2):201–206. doi: 10.1002/hed.21716. [DOI] [PubMed] [Google Scholar]
- 106.Korpi-Hyovalti E, Cranston T, Ryhanen E, Arola J, Aittomaki K, Sane T, et al. CDC73 intragenic deletion in familial primary hyperparathyroidism associated with parathyroid carcinoma. J Clin Endocrinol Metab. 2014;99(9):3044–3048. doi: 10.1210/jc.2014-1481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.McHenry CR, Rosen IB, Walfish PG, Cooter N. Parathyroid crisis of unusual features in a child. Cancer. 1993;71(5):1923–1927. doi: 10.1002/1097-0142(19930301)71:5<1923::aid-cncr2820710531>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 108.Meier DE, Snyder WH, 3rd, Dickson BA, Margraf LR, Guzzetta PC., Jr Parathyroid carcinoma in a child. J Pediatr Surg. 1999;34(4):606–608. doi: 10.1016/s0022-3468(99)90084-2. [DOI] [PubMed] [Google Scholar]
- 109.Niramitmahapanya S, Deerochanawong C, Sarinnapakorn V, Sunthornthepvarakul T, Pingsuthiwong S, Athipan P, et al. Somatic HRPT2 mutation (Arg234X) of parathyroid carcinoma associated with slipped capital femoral epiphysis: a first case report. J Med Assoc Thai. 2016;99(Suppl 2):S201–S205. [PubMed] [Google Scholar]
- 110.Rahman MM, Karim SS, Joarder AI, Mubin S, Abir MM, Morshed MS. Parathyroid carcinoma in a 10 years old female child. Mymensingh Med J. 2015;24(3):619–623. [PubMed] [Google Scholar]
- 111.Righi A, Dimosthenous K, Mize J. Mediastinal parathyroid carcinoma with tumor implants in a child: a unique occurrence. Int J Surg Pathol. 2008;16(4):458–460. doi: 10.1177/1066896908315821. [DOI] [PubMed] [Google Scholar]
- 112.Schantz A, Castleman B. Parathyroid carcinoma: A study of 70 cases. Cancer. 1973;31(3):600–605. doi: 10.1002/1097-0142(197303)31:3<600::AID-CNCR2820310316>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 113.Serrano-Gonzalez M, Shay S, Austin J, Maceri DR, Pitukcheewanont P. A germline mutation of HRPT2/CDC73 (70 G>T) in an adolescent female with parathyroid carcinoma: first case report and a review of the literature. J Pediatr Endocrinol Metab. 2016;29(9):1005–1012. doi: 10.1515/jpem-2016-0109. [DOI] [PubMed] [Google Scholar]
- 114.Vinodh M, Rajeshwari A. Parathyroid carcinoma presenting as genu valgum. Indian Pediatr. 2012;49(2):156. doi: 10.1007/s13312-012-0014-8. [DOI] [PubMed] [Google Scholar]
- 115.Young TO, Saltzstein EC, Boman DA. Parathyroid carcinoma in a child: unusual presentation with seizures. J Pediatr Surg. 1984;19(2):194–196. doi: 10.1016/s0022-3468(84)80448-0. [DOI] [PubMed] [Google Scholar]
- 116.Zivaljevic VR, Jovanovic MD, Djordjevic MS, Diklic AD, Paunovic IR. Case report of parathyroid carcinoma in a pediatric patient. Int J Pediatr Otorhinolaryngol. 2019;124:120–123. doi: 10.1016/j.ijporl.2019.06.003. [DOI] [PubMed] [Google Scholar]
- 117.Kelly TG, Shattuck TM, Reyes-Mugica M, Stewart AF, Simonds WF, Udelsman R, et al. Surveillance for early detection of aggressive parathyroid disease: carcinoma and atypical adenoma in familial isolated hyperparathyroidism associated with a germline HRPT2 mutation. J Bone Miner Res. 2006;21(10):1666–1671. doi: 10.1359/jbmr.060702. [DOI] [PubMed] [Google Scholar]
- 118.Rosai J, DeLellis R, Carcangiu M, Frable W, Tallina G. Tumors of the thyroid and parathyroid glands. 4th ed. AFIP Atlas of Tumor Pathology. ARP Press; 2014