Abstract
Craniofacial development, one of the most complex sequences of developmental events in embryology, features a uniquely transient, pluripotent stem cell-like population known as the neural crest (NC). Neural crest cells (NCCs) originate from the dorsal aspect of the neural tube and migrate along pre-determined routes into the developing branchial arches and frontonasal plate. The exceptional rates of proliferation and migration of NCCs enable their diverse contribution to a wide variety of craniofacial structures. Subsequent differentiation of these cells gives rise to cartilage, bones, and a number of mesenchymally-derived tissues. Deficiencies in any stage of differentiation can result in facial clefts and abnormalities associated with craniofacial syndromes. A small number of conserved signaling pathways are involved in controlling NC differentiation and craniofacial development. They are used in a reiterated fashion to help define precise temporospatial cell and tissue formation. Although many aspects of their cellular and molecular control have yet to be described, it is clear that together they form intricately integrated signaling networks required for spatial orientation and developmental stability and plasticity, which are hallmarks of craniofacial development. Mutations that affect the functions of these signaling pathways are often directly or indirectly identified in congenital syndromes. Clinical applications of NC-derived mesenchymal stem/progenitor cells, persistent into adulthood, hold great promise for tissue repair and regeneration. Realization of NCC potential for regenerative therapies motivates understanding of the intricacies of cell communication and differentiation that underlie the complexities of NC-derived tissues.
Keywords: Neural crest, Orofacial development, Bone, Cartilage, Signalling
Introduction
Craniofacial development is intricately linked to the biology of neural crest cells (NCCs). The neural crest (NC) is a highly migratory cell population with the ability to acquire a broad spectrum of cell fates. It is the ultimate driving force for the tremendous craniofacial variation seen in vertebrates. The dynamic nature of the NC should take center stage when considering normal development, congenital malformations, malignancies of the craniofacial complex, and when developing strategies for tissue repair. In this review, we present a concise NC-centric view of craniofacial development and highlight important biological principles that govern the formation of major craniofacial structures. A common theme throughout this review will be mechanisms that ensure appropriate development of multipotential NCCs, particularly in bones, cartilages, and teeth. We also provide examples of malformations resulting from perturbations in these mechanisms.
Neural Crest Contribution to Early Craniofacial Development
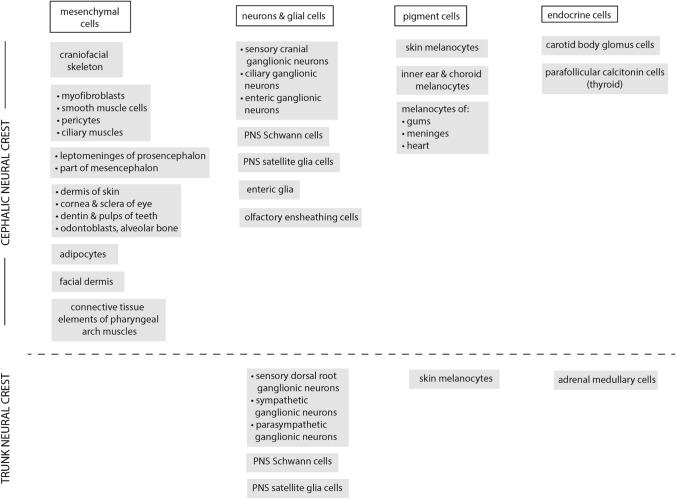
The NC is a transient pluripotent stem cell-like population that originates from the most dorsal aspect of the neural tube along the entire anteroposterior axis of the embryo (Fig. 1) [1]. Its ability for extensive migration and potential to give rise to a broad spectrum of cell and tissue types are its two most recognized characteristics (Fig. 2). It is for these reasons that the scientific community calls the NC the fourth germ layer [2].
Fig. 1.
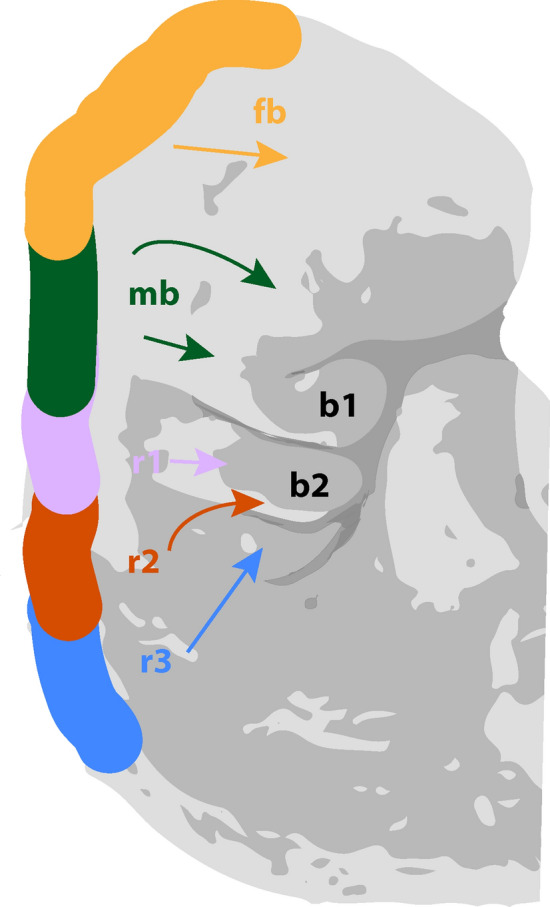
Migration pattern of cell populations from embryonic neural crest. Graphic representation of stage 12, week 4 human embryo. fb: forebrain; mb: midbrain; r1: rhombomere 1; r2: rhombomere 2; r3: rhombomere 3; b1: branchial arch 1; b2: branchial arch 2
Fig. 2.
Neural crest derivatives. Adapted from Sperber, 2018 [56] and Etchevers et al. [57]
NCCs are exclusive to vertebrates and essential for development of the head and face. Over the last 150 years, researchers have worked to elucidate the many facets of the NC given its central role for vertebrate development and evolution [3]. This combined knowledge forms the foundation of developmental and regenerative biology and has furthered understanding of human pathologies caused by altered NC development. These pathologies, called “neurocristopathies”, include multiple syndromes, many associated with craniofacial malformations as well as some types of cancer [2].
The development of the NC occurs in phases and some steps chronologically overlap. All of them are strictly coordinated by gene regulatory networks (GRN) comprised of signaling proteins, transcription factors, and epigenetic modifiers that ensure the correct differentiation of these multi-potential NCCs [2]. NC development occurs in a coordinated manner along the anterior-posterior axis with cranial segments developing first followed by more posterior segments (cranial, cardiac, trunk, vagal and sacral) [4]. Note that this principle applies to all steps of the NC’s development, but the timing has not been deeply studied with most studies restricted to the cranial NC [5].
The many different types of neurocristopathies can be divided according to the type of pathology (tumors, tumor syndromes, malformations, etc.), stage of NC development (NC induction and specification, NC migration, and NC differentiation), or by origin along the anterior-posterior axis (cranial, cardiac, trunk, sacral) [6].
Induction and Specification
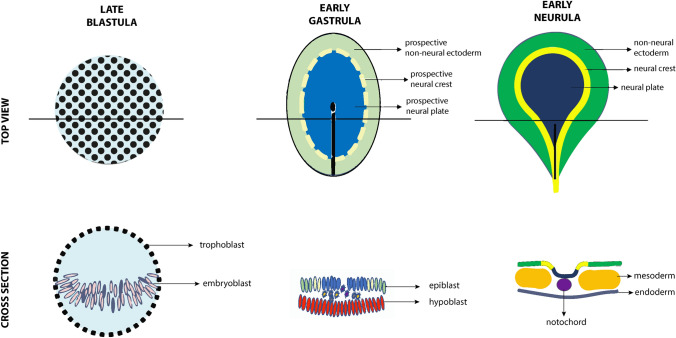
During late gastrulation, establishment of the prospective NC, between the neural and non-neural ectoderm, occurs in response to early extrinsic and late intrinsic signals [1, 2]. The exact moment of NC induction is the subject of debate. Given that some gene expression from the blastula stage continues into the gastrulation phase, it has been suggested that NC induction might precede gastrulation [7]. Thus, the pluripotency of NCCs may be maintained from the blastula-stage rather than a novel feature acquired at a later stage [8]. The observation that NCCs are able to generate both ectodermal and mesenchymal structures is compatible with the view that induction occurs during the pre-border stage, prior to the establishment of the neural plate, neuroectoderm, and mesoderm (border stage) (Fig. 3) [2, 7].
Fig. 3.
Establishment of neural crest population in early embryogenesis. Graphic representation of chick embryogenesis from late blastula to early neurula. Initially, cells of the embryoblast within the trophoblast are interspersed with no apparent specification. At the stage of the early gastrula, these cells form the epiblast and hypoblast, further segregated into the prospective non-neural ectoderm, neural crest, and neural plate. The onset of neurulation in the early neurula brings distinction of these already-designated cell populations into the non-neural ectoderm, neural crest, neural plate, mesoderm, and endoderm grouped around the notochord
Induction of most tissues requires crosstalk between two adjacent cell types. In the NC, this interaction could be facilitated by ectoderm-mesoderm and/or neural-non-neural ectoderm interactions [2]. Although such communication has been demonstrated in some species, a variety of interactions are hypothesized to induce NC development. One possibility is that signaling events during earlier stages (late blastula/early gastrula) in the epiblast lead to induction of the NC; however, it remains unknown whether additional mechanisms at later stages are also required. In summary, interactions between neural, non-neural ectoderm, and underlying mesoderm are important in development. NC induction requires an epithelial signal, but additional mechanisms may also be involved [7].
Delamination and Migration
After specification, the NCCs individualize and segregate from the surrounding tissue in a process referred to as delamination. This term is a misnomer as NCCs only undergo a physical separation from the originating cell population rather than from an opposing lamina [9]. Delamination typically involves molecular changes that promote the transition from an epithelial phenotype to a mesenchymal one, a process known as epithelial-to-mesenchymal transition (EMT) [2]. All NCCs undergo EMT, but this is not synchronized with delamination or migration [9]. The NCCs can delaminate before changing cell shape (i.e. undergoing EMT) or undergo EMT before delamination [9]. In order to separate from the surrounding tissues and subsequently migrate, the NCCs weaken cell-cell adhesion through the regulation of transmembrane proteins like cadherins and integrins, lose their apical-basal polarity, change their pattern of cellular proliferation, acquire polarized motility, form blebs and lamellipodia, produce more metalloproteinases and laminin, and degrade the basal lamina [9, 10]. This catalogue of cellular events occurs in parallel during the different steps of NC development and reflects its complexity.
After delamination, NCCs move toward their final destination, invading the space between the non-neural ectoderm and the surrounding mesoderm [2, 10]. Gradually, NCCs split into different streams until they reach their destination. Migration occurs from specific positions along the neural plate border to predetermined locations in the developing face, keeping an anteroposterior order and a dorsolateral and ventromedial direction. Additionally, migration can follow a collective manner or occur as a single cell movement depending on the species and region. For example, cranial migration occurs simultaneously for the whole segment while the trunk NC migrates in an anteroposterior order. These migratory paths are determined by positive and negative signals originating in surrounding regions and cells [10].
A variety of mechanisms have been suggested as regulators of the process of migration: contact inhibition of locomotion (CIL), co-attraction, mesodermal stiffening, chase and run, rear actomyosin contraction, tissue fluidity, and confinement [11]. CIL involves repolarization of the colliding cells followed by moving away from the site of cell–cell contact. As a consequence, the free-edges experience morphologic changes prompting reduction in intercellular tension [12]. While CIL tends to disperse cells, co-attraction tries to keep them together and balances CIL activity. This process of short-range chemotaxis is dependent on the complement factor C3a [13]. Mechanical signals such as stiffening of the underlying mesoderm seem to activate NC EMT and facilitate migration [14]. NCCs can also migrate toward molecules produced by other cells (chemotaxis) such as epithelial placode cells. Once the cells contact, they “run away” from each other, a process called “chase and run” [11]. Additionally, differences in actomyosin contraction between the poles of a cell cluster can generate changes in the direction of movement [15]. Another essential mechanism is the characteristic of the NC to behave like a fluid by constantly remodeling cell junctions. Finally, the ability to respond to repulsive signals prevents NCCs from invading non-NC tissue or the mixing of different streams [11]. All these mechanisms interplay with one another and are required for successful migration.
Differentiation
The establishment of NCC fates is a dynamic process influenced by many aspects including their origin along the anteroposterior axis, the surrounding tissues, and cellular interactions along their migration path. In this respect, NCCs provide an archetype of cellular plasticity [2]. Cells migrating from the cranial NC contribute to cartilage, bone, muscles, connective tissue, and nervous tissue of the face and part of the skull. Meanwhile, the vagal NC gives rise to connective tissue forming part of the heart outflow tract and to glia and neurons belonging to the enteric nervous system [5]. The trunk NC generates part of the enteric system, melanocytes, dorsal root ganglia, sensory neurons and adrenal gland [10]. With respect to the molecular regulation of these many differentiation events, there is a broad range of GRNs controlling respective terminal differentiation, a topic of many reviews [5, 16]. The broad range of neurocristopathies that have a NC development background reflect the complex developmental events required for NC development, the different tissues with NC contribution, and the range of physical locations [6]. A thorough understanding of their developmental origins allows better understanding these pathologies and a potential for more targeted interventions that take into account the biological underpinnings.
Early Facial Development: Pharyngeal Arches and Facial Prominences
Facial development requires coordinated establishment of the pharyngeal arches (PA). The PAs (five pairs in mammals) are bilateral outgrowths alongside the pharyngeal area and form around the fourth week of gestation. They are the direct result of the migration of the NCCs and reorganization of the pharyngeal endoderm and the non-neural ectoderm [17]. NCCs from the first to third rhombomeres are the first to reach the first pharyngeal arch (PA1), while other NCCs migrate in an anteroposterior direction to form the other four PAs [17]. PAs are transient structures that are formed by all four germ layers: the medial aspect is endoderm, the most external is ectoderm, and the central part is shared by mesoderm and mesenchyme derived from NCCs [18].
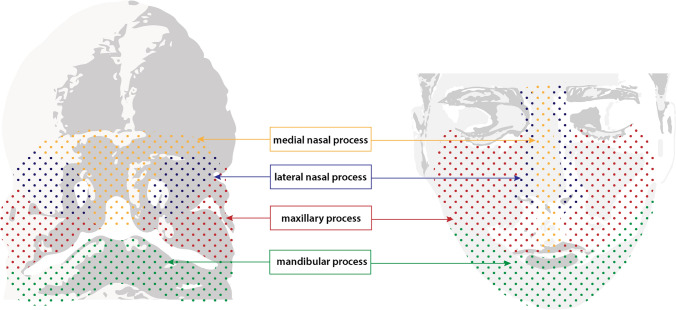
The high rate of proliferation and migration of NCCs contributes to the formation of small buds of tissue called the facial prominences (FP) [19]. The cells migrating rostrally toward the most anterior part of the brain in development establish the frontonasal prominence (FNP). Meanwhile, PA1 grows and develops into paired maxillary prominences (MxP) and mandibular prominences (MP). The FPs will converge to form the nose and the upper and lower jaws [2]. The FNP is split into medial and lateral prominences. The medial prominences form the nasal septum, midline of the nose, philtrum, premaxilla, and four incisors. The lateral prominences, located laterally to the nasal pits, give rise to the nasal turbinates and alae of the nose [2]. The upper lip (adjacent to the midline, the maxillary and palatine bones) is a product of the fusion of the MxP and FNP. Meanwhile the mandibular arch results from the fusion of the MPs [2] (Fig. 4).
Fig. 4.
Structures derived from facial prominences of the early embryo. Graphic representation of 5 week human embryo (left) with labelled facial prominences: medial nasal process (yellow), lateral nasal process (blue), maxillary process (red), and mandibular process (green). Structures derived from these processes are indicated in their respective colors on an adult face (right)
Palate Development
The palate provides an essential structural support for oral-nasal functions like breathing, speaking, and swallowing and acts as a barrier between the nasal and oral cavities [20]. It can be distinguished into primary and secondary processes, each with different embryological origins but common cellular components [20]. The primary process forms from the FNP, while the secondary process forms from outgrowths of the MxPs [21]. The primary palate includes the four upper incisors and extends to the foramen incisivum. The secondary palate consists of the bony palate anterior and the soft palate posterior [22]. The upper lip and primary palate complete formation prior to initiation of secondary palatogenesis [22].The main cellular contributors of both the primary and the secondary palate are mesenchyme derived from the NC and epithelium from the ectoderm [20, 23]. The primary process approaches and contacts the secondary palatal shelves then undergoes fusion, leading to the formation of the palate and resultant separation of the oral and nasal cavities. The development of the secondary palate can be divided into distinct synchronized phases. Initially, the palatal shelves grow vertically along either side of the tongue. They then elevate to a horizontal position through complex cellular reorganization, followed by rapid horizontal growth. The palatal shelves meet in the middle and begin to fuse. At this point, the central epithelium covering the palatal segments disintegrates and disappears allowing complete fusion of the shelves at the midline, followed by the ossification of the bony portion [23].
Two important mechanisms are involved in the face and palate formation: merging and fusion. Merging consists of proliferation and migration of mesenchyme, filling in the spaces between the two adjacent prominences. Fusion occurs when two structures covered by epithelium contact each other and form a bilayered epithelial seam which is later degraded to impart continuity to the mesenchyme. An example of merging is the connection at the midline of the two mandibular processes, in contrast to palate formation which is an example of fusion [21].
Diverse cellular phenomena occur during the palate's establishment: high rate of cellular proliferation, extracellular matrix production, transformation of epithelium to mesenchyme, and apoptosis. Each of these is highly regulated and driven by several signaling pathways. Like other craniofacial structures, palate formation involves complex and detailed cellular and molecular interactions that are not thoroughly understood. This complexity is evident in the wide variety and relatively high incidence of congenital defects involving palate formation such as cleft lip (with or without cleft palate) and isolated cleft palate, the genetic cause for which differs between each condition [24]. Knowledge of the molecular/cellular reason for cleft formation can help to predict the risk for developing other abnormalities associated with cleft repair, such as midfacial hypoplasia or excessive scarring.
Signaling Pathways
To understand NCC behavior throughout development, one must be familiar with some important developmental signaling pathways. We provide a brief overview of four common, evolutionarily conserved signaling pathways that are inseparable from NC biology: WNT, bone morphogenetic protein (BMP), fibroblast growth factor (FGF), and Hedgehog (HH) signaling. These pathways involve secreted ligands that show varying affinities for extracellular matrix molecules. Rather than being freely diffusible factors, ligand retention by the extracellular matrix or binding to activity-blocking secreted antagonists allows for precise spatio-temporal control of signaling activity. While often presented in isolation, all of these signaling pathways interact, either directly through providing input into intracellular signaling hubs or indirectly through cross-regulation of pathway members. Together they provide the majority of the precise instructions required for coordinated craniofacial development.
WNT Signaling
WNT signaling is associated with a multitude of cellular responses as diverse as cell polarity, energy metabolism, cell proliferation, differentiation, and stem cell maintenance. WNT signaling is involved in tissue formation, regeneration, and tumorigenesis. It is important for all aspects of the NC, from induction to terminal differentiation of NC-derived structures. Extracellular availability of WNT ligands is controlled by WNT antagonists. Signaling is initiated by one of the many WNT ligands binding to cognate receptors which can trigger a variety of intracellular cascades. Canonical WNT signaling involves the intracellular mediator β-catenin which controls transcriptional changes through binding of cofactors in the nucleus. Non-canonical WNT signaling is independent of β-catenin and controls cell polarity and movement. Interplay between β-catenin dependent/independent signaling is frequently observed. Most craniofacial structures show sustained WNT signaling activity [25]. With respect to bone, sub-ectodermal dermal and cranial bone progenitors exhibit a transient, possibly instructive, role for WNT signaling. In late stage embryonic development, WNT signaling is increasingly defined by differentiated cells; however, it has a dynamic expression pattern throughout development. This pathway has several roles throughout craniofacial development including interactions with other signaling cascades. Genomic variants in Robinow syndrome have been associated with aberrant non-canonical WNT/planar cell polarity signaling [26].
BMP Signaling
Bone morphogenetic proteins (BMPs) belong to the TGF-β family of signaling molecules. In Drosophila melanogaster, the BMP orthologues form two subgroups: Decapentaplegic or dpp (BMP2, 4 group) and the 60A group (BMP5-8 group). BMP signaling is highly regulated in the extracellular space, at the cell membrane level, and intracellularly. BMP pro-peptides help regulate their bioavailability, whereas a multitude of BMP antagonists control receptor binding. Following BMP ligand binding to heteromeric BMP Type I/II receptors, canonical signaling involves SMAD-dependent signal transduction to the nucleus. Non-SMAD signaling involves a variety of pathways regulating cell organization and polarity (Wang 2014). BMP signaling plays critical roles in NCCs and morphogenesis of the craniofacial skeleton, such as NC induction, migration, and coordination of cell proliferation and cell death through apoptosis. Additionally, it is critical for mandible, lip, and palate development, in Meckel’s cartilage, cranial bone formation, and sutures [27]. However, the name ‘bone morphogenetic protein’ is misleading, as these molecules control a myriad of cellular processes, much like WNTs [28]. BMP signaling is required for growth and patterning of the facial processes, and mutations to downstream targets or receptors result in midline and patterning defects associated with holoprosencephaly, craniosynostosis, cleft palate, and facial dysmorphism [27].
FGF Signaling
Fibroblastic growth factors (FGFs) are a family of secreted molecules with an affinity for heparin sulfate in the extracellular matrix. They signal through one of four conserved FGF receptors (FGFR). Tissue-specific expression of FGFR isoforms, receptor subtype-specific FGFs, and proteoglycans provide fine regulation of FGFR signaling [29]. Like the other signaling pathways, FGF signaling is tightly regulated within the cell by multiple regulatory layers and negative feedback loops which is vital for spatial and temporal control of signaling. This signaling cascade has multiple essential roles in skeletal development, specifically in bone progenitor proliferation, bone matrix growth, and mineralization. Mutations in FGFRs are associated with various syndromes including Crouzon, Apert, or chondrodysplasias [30].
HH Signaling
Hedgehog signaling (HH) is achieved by three vertebrate homologues of the Drosophila gene hedgehog: Indian Hedgehog (IHH), Desert Hedgehog, and Sonic Hedgehog (SHH). With respect to craniofacial development, SHH patterns the microenvironment of the first pharyngeal arch spatiotemporally and regulates neural crest cell kinetics. IHH has important functions in the regulation of cartilage differentiation by controlling chondrocyte hypertrophy. Overall, HH signaling has an important role in neural crest survival, production, and apoptosis in the first pharyngeal arch. It contributes to mandibular and maxillary jaw development by maintaining a critical mass of neural crest-derived cells in the first pharyngeal arch. Mutations in SHH most notably cause midline (e.g. holoprosencephaly) and eye defects [31]. HH signaling is known to interact with environmental factors like ethanol, vitamin deficiency, and tobacco smoke, making it an example of gene-environment interaction.
Signaling Crosstalk
During embryonic development, the control of shape and function often involves crosstalk between different tissue types and the above listed signaling pathways. This is exemplified by the recurrent requirement of BMP, WNT, FGF, and HH signaling during tooth development, where their reiterated use during epithelial-mesenchymal interactions controls tooth development from placode formation, bud invagination, enamel knot formation, crown mineralization, and, finally, root formation [32]. Tooth development thus provides a striking example of how precise spatio-temporal control and integration of multiple signals enables the formation of the hardest biological material known: enamel.
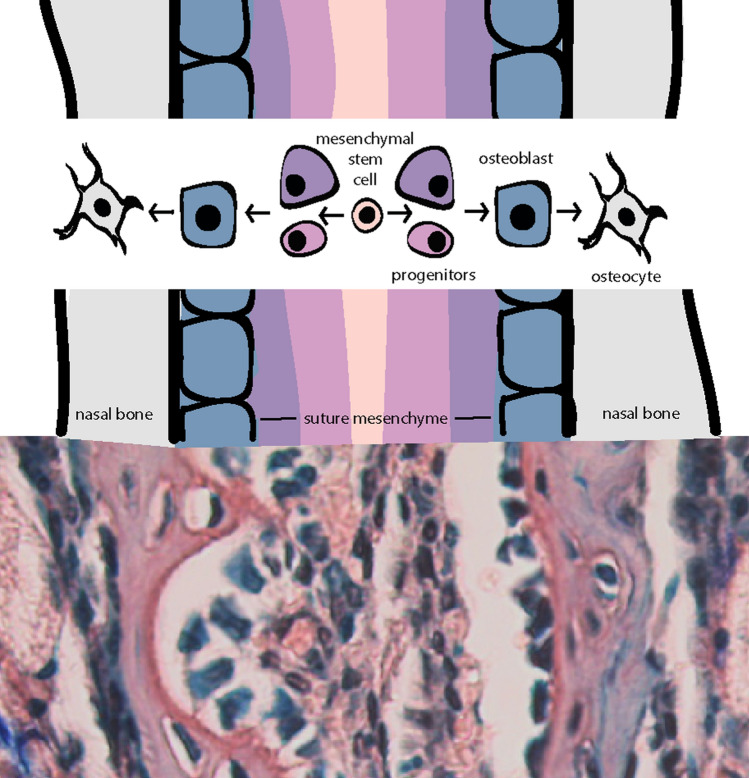
Cranial sutures provide another example of regulation of a complex craniofacial system with signaling crosstalk. The suture microenvironment reflects all stages of the intramembranous bone formation pathway, from mesenchymal stem cells to osteoblasts (Fig. 5). A regulatory cascade involving BMP and FGF signaling has been identified for the development and maintenance of sutures during skull expansion, as several gene mutations associated with craniosynostosis involve either FGF or BMP signaling. In contrast, WNT and HH signaling have been demonstrated to control mesenchymal stem cell niches.
Fig. 5.
Differentiation of cells within cranial sutures. Graphic representation (top) of cranial suture cell populations and their presumptive differentiation pathway. Note: the indicated intramembranous bone stage overtop each region of the suture is not the only composing cell type, however, would likely be found within. In reality, the suture mesenchyme is a heterogeneous population of cells ranging in differentiation from stem cells to committed osteoprogenitors. Corresponding orcein/methylene blue stain (bottom) of the internasal suture of a 4-week old mouse portrays suture mesenchyme in red/brown studded with blue nuclei between two layers of blue cuboidal osteoblasts lining the red/brown bone. Within the lacunae of the bone reside dark blue osteocytes
Bone is a highly specialized structure, the formation and maintenance of which require constant coordinated crosstalk between osteoblasts, osteocytes, and osteoclasts. BMP/WNT/FGF ligands not only control direct cell-cell signaling but are probably also stored in the bone matrix and released when bone is broken down, recruiting mesenchymal stem cells to sites of bone resorption.
The above examples not only illustrate crosstalk, but also the importance of local regulation of signaling. This needs to be considered when planning therapeutic approaches involving any of the described signaling molecules. The exquisite crosstalk, local action, and wide effects explain the often-unpredictable results and side effects associated with their application [33].
Signaling Centers
The development of many NC-derived structures is regulated by defined signaling centers which direct the overall organization of the developing structures. The enamel knot and frontonasal ectodermal zone (FEZ) are two examples of such craniofacial signaling centers.
Enamel knots are epithelial structures that form during tooth development to control cusp formation [34]. They express a multitude of signaling molecules to organize their surroundings. Epithelial-mesenchymal interactions and stimulation of proliferation in the nearby epithelium (but not in the knot itself) permanently change tooth morphology. The enamel knot illustrates the importance of local fine regulation of signaling to achieve both shape and function.
The FEZ controls jaw growth and patterning and regulates tissue interactions between the forebrain, neural crest, and stomodeal ectoderm. The molecular mechanisms mediating FEZ function rely on SHH, FGF, and BMP signaling, and its organization is dependent on the distribution of neural crest cells in a given species. The FEZ is a source of patterning information directing initial growth patterns for the frontonasal and subsequent medial nasal process. Involvement of SHH, FGF, and BMP signaling in midline specification/division in both the telencephalon and midface are evidenced by variable changes to the facial appearance in individuals with holoprosencephaly, ranging from singular median incisor to cyclopia [35].
Later in development, or in the adult, such signaling centers are less apparent but could be reflected in specialized niches regulating tissue homeostasis and repair, e.g. through provision of stem cell niches. A better understanding of maintenance of these niches is a prerequisite for future stem cell therapies.
Redundancy
The concept of redundancy between members of the same protein family is often considered but rarely tested. However, both BMP signaling (as regulators of tooth mineralization) and MSX1/2 (as transcriptional regulators) reveal surprising mechanisms of intricate control of the involved developmental processes.
Different BMPs are frequently expressed in similar domains, such as BMP2 and BMP7 in odontoblasts at the onset of mineralization. A spatiotemporal overlap in expression might suggest that these two proteins are redundant or act in synergy. However, loss of BMP2 leads to dentinogenesis imperfecta, a dentin anomaly, due to a failure of odontoblast polarization. In contrast, loss of BMP7 results in a delay of tooth mineralization by a couple of days resulting in wider teeth with extra cusps [36]. Thus, concomitant expression of signaling molecules does not automatically indicate redundancy.
Members of the MSX family of transcription factors play a major role in craniofacial development. Expression of MSX1 and MSX2 frequently overlap at sites of epithelial-mesenchymal interactions [37]. Although both show preference for similar DNA binding sites and have the ability to repress transcription, suggestive of functional redundancy, MSX1 is associated with both tooth agenesis and cleft palate, whereas MSX2 is associated with craniosynostosis.
Both examples illustrate the concept that factors from the same family may act in similar context, suggesting redundancy, but it is more likely that individual differences between family members enable precise coordination of the development of complex craniofacial structures.
Plasticity and Developmental Timing
NCCs are known for their plasticity and sensitivity to regulation by cellular interactions, both with each other and with non-NC tissues [38]. This property allows them to evolve and modulate in response to the environment they migrate through, unlike the pre-programmed Hox patterning of the neural tube. NCCs respond to contact with other cells and signaling molecules found in the tissues they pass through or avoid, which is essential for the generation of distinct craniofacial phenotypes without dramatic changes to the primordial pattern. Hence, their fate can range from neurons and glia, to cartilage and bone, connective tissue, and melanocytes.
Developmental timing is important; mistimed developmental events can have dramatic phenotypes. Correct timing is crucial for normal development of the secondary palate and cranial sutures. The sequence of secondary palate development including vertical growth, elevation, horizontal elongation, fusion, and breakdown of the midline epithelial seam are reliant on numerous temporally constrained proliferation and differentiation cues. Changes to individual cell morphology underlie cell polarization and tissue organization. Mistiming of processes like early tongue contraction will cause delayed palatal shelf elevation, resulting in subsequent failure of the shelves to meet and fuse in the rapidly expanding face.
Development and maintenance of patency in cranial sutures is similarly dependent on spatiotemporally regulated expression patterns of many genes including TWIST, MSX2, FGFR1/2/3, and FGF2. Loss, functional change, or mistiming can push the intricately regulated osteogenic differentiation off balance, leading to premature ossification (craniosynostosis) or delayed fusion (cleidocranial dysplasia).
The high degree of temporal regulation in the craniofacial complex is also evident from tooth development, which involves reciprocal epithelial-mesenchymal crosstalk reliant on the reiterated use of a limited number of signaling molecules. It is currently not well understood how cells at the various differentiation stages interpret seemingly identical signals for different outcomes. The precise spatio-temporal regulation of involved signaling events is a prerequisite. Cellular organization and epigenetic regulation must equally play critical roles. While mistiming of any stage of tooth development can have compounding effects on the final structure (i.e. abnormal teeth), tooth development also shows the power of self-contained developmental programs evidenced by rare, ectopically formed teeth.
The above examples illustrate how precise temporo-spatial regulation of cell differentiation is required for successful craniofacial development. In light of this complexity, it must be recognized that developmental processes are overall very robust, a reflection of the high-level under-appreciated molecular and cellular integration that underlie these developmental processes. Thus, although the plasticity of NC cells provides opportunities to use these cells for tissue regeneration or repair, the complex spatiotemporal requirements controlling cell differentiation are often not sufficiently considered and likely compromise overall success.
Bone Development
The NC is a major contributor to craniofacial bones and cartilages often affected by many congenital syndromes; hence bone and cartilage development deserve closer consideration.
Intramembranous ossification describes the process of osteoblast differentiation directly into bone without a cartilaginous intermediate [39]. This type of bone formation occurs within the membranous neurocranium and viscerocranium of the skull and part of the clavicle and is thought to be dependent on prior vascularization. Here, NC mesenchymal stem cells differentiate through various osteoprogenitor stages into mature osteoblasts which lay down an extracellular matrix (osteoid) that subsequently mineralizes, and end up surrounded by mineralized bone as osteocytes. Current research in osteoblast differentiation seeks to understand the ramifications genetic abnormalities have on osteoblast maturation and how this affects their ability to form and grow craniofacial bones. In addition, the detailed understanding of how signaling cascades interact with mechanical cues to regulate osteoblast differentiation is essential for designing successful treatments [40]. It is reasonable to assume that changes to the intrinsic genetic program of the bone-forming environment affect the success rate of various surgical procedures. A better foundational understanding on how to make, maintain, and repair bone will help to improve current clinical practices.
The vertebrate skull is formed by both neural crest and mesoderm. The anterior facial bones and calvaria are NC-derived, whereas posterior bones, such as parietal and occipital bones, are derived from mesoderm. As a result, the intervening sutures can have dual origin (e.g. coronal suture), and the relative position of a suture can indicate the growth potential of the flanking bones. During late embryonic development the skull vault is defined, and the sites of sutures become apparent [41]. Many congenital pathologies affecting craniofacial bones are based on patterning or intramembranous ossification defects.
The flat bones of the skull are joined by fibrous joints called sutures. Cranial sutures are populated by mesenchymal stem cells, which are often made up by NC and their progeny, leading to bone-forming osteoblasts. Sutures are a fascinating system for study of intramembranous bone growth as they represent the entire osteoblast differentiation pathway, with mesenchymal stem cells in the central suture mesenchyme and terminal osteoblasts lining the new bone surface (with more differentiated osteocytes within the mineralized matrix) [42]. Researchers can study perturbations to the sutures anatomically (in the case of craniosynostosis), histologically (looking at cellular components), biomechanically (studying tension, forces, and the extracellular matrix), and in vitro (cell signaling cascades). Suture malformations are often studied in the context of delayed or premature fusion. What is still lacking in the field of suture biology is a holistic interpretation of differing fields - combining anthropology, clinical findings, historical reports, anatomy, embryology, development, stem cell biology, physics, and beyond [43]. The pathways governing establishment, maintenance, and closure are still quite unclear. Similarly, the mechanisms of biomechanical interpretation and response remain only partly understood. These principles have clinical application in fields like otolaryngology, plastic surgery, and orthodontics especially.
Cartilage
Cartilage can be divided into three different groups: hyaline, fibro- and elastic cartilage. The type of cartilage is dependent on function and reflected by extracellular matrix composition. Hyaline cartilage is resilient, fibrocartilage is strong and dense, and elastic cartilage is flexible. Hyaline cartilage is found in the cranial base and nasal septum [44]. The temporomandibular joint is formed by fibrocartilage and the ear by elastic cartilage. Cranial neural crest cells give rise to all of these cartilaginous structures, except for the basioccipital aspect of the cranial base (Fig. 6).
Fig. 6.
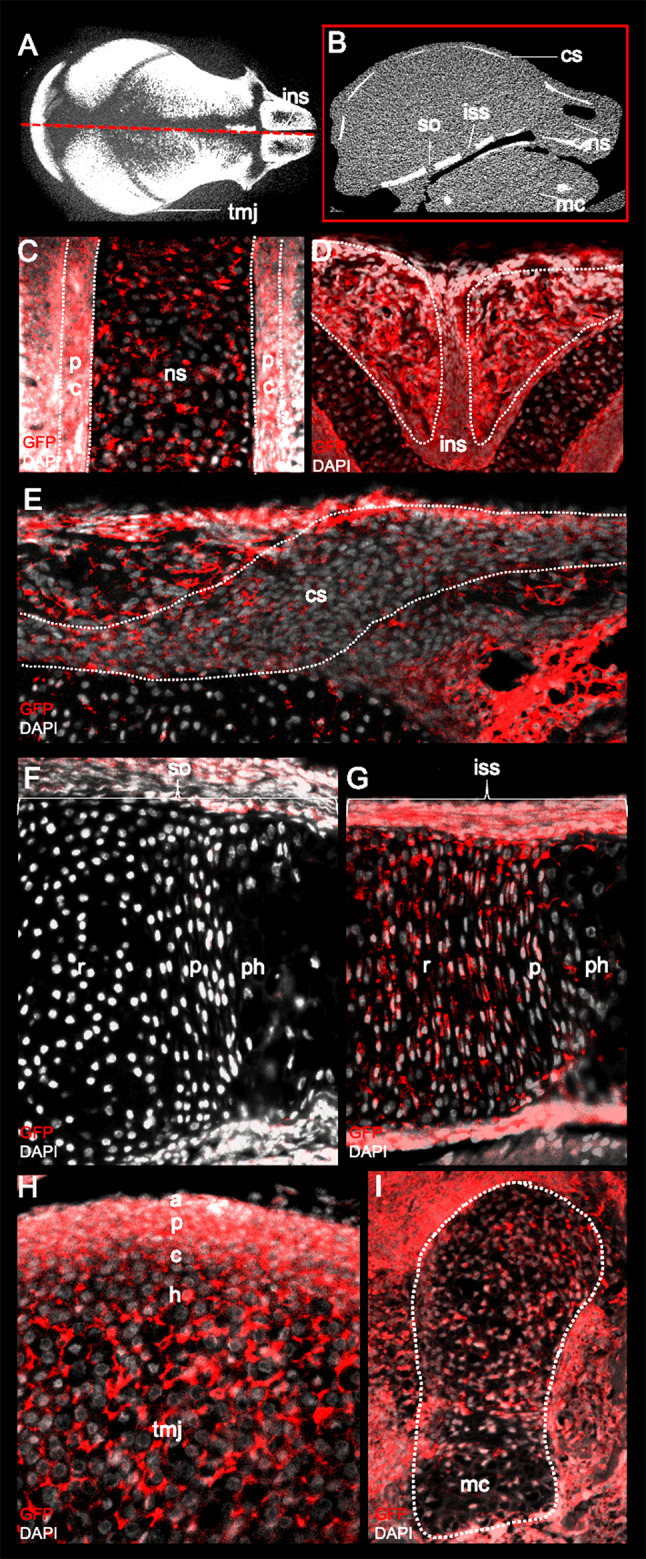
Identification of lineage-traced neural crest cells (NCCs) in the craniofacial complex using Green fluorescent protein (GFP) staining. a & b Annotation of various craniofacial structures on a midsagittal cross-section (red box) of a P0 micro-computed tomography mouse scan. Paraffin-embedded frontal section of the P0 mice skulls stained with GFP and counterstained with DAPI indicates the presence of NC-derived cells in the (c) cartilage and perichondrium (pc) of the nasal septum (ns) and the (d) internasal suture (ins). Sagittal sections of P0 skulls demonstrate the presence of NC-derived cells in the (e) periosteum and osteogenic front of the coronal suture. NCCs also contribute to the resting (r), proliferative (p), pre-hypertrophic (ph) zone of the (G) intersphenoidal (iss) and not (f) sphenooccipital (so) synchondroses of the cranial base. (h) The articular (a), proliferative (p), chondrogenic (c) and hypertrophic (h) layers of the temporomandibular joint (TMJ) are also neural crest-derived along with the (i) chondrocytes of the Meckel's cartilage (mc)
Hyaline cartilage is involved in the process of endochondral ossification whereby a cartilage intermediate forms the template for bone development [45]. In this process, mesenchymal stem cells differentiate into chondrocytes, mature into hypertrophic chondrocytes, and then transdifferentiate into bone-forming osteoblasts or are replaced by osteoblasts. In the craniofacial complex, the cranial base and the posterior aspect of the nasal septum, also known as the perpendicular plate of ethmoid, undergo endochondral ossification. Onset and completion of ossification is directly linked to growth requirements for each cartilage and therefore varies. Both the nasal septum and cranial base drive midfacial growth; altered midfacial growth can directly result from cartilage anomalies and might manifest as a deviated nasal septum or synchondroses in the cranial base [46, 47]. Reduced growth of nasal septum or anterior cranial base are associated with severe midfacial hypoplasia [47, 48]. However, it remains unresolved whether cartilage abnormalities give rise to midfacial hypoplasia, vice versa, or whether there is a reciprocal interaction. Assigning cause to consequence is often difficult due to the relations and complexity of craniofacial structures. Additionally, changes in progenitor cells themselves could affect their differentiation potential and predispose to growth and developmental abnormalities that only become apparent at later stages (e.g. midfacial hypoplasia). The consequence of such changes may be reduced success rates for corrective surgical interventions due to compromised potential of the cells themselves. Understanding the genetic underpinnings of craniofacial abnormalities thus becomes essential for personalized medicine.
Mandible Development
The mandible, or lower jaw, is a crucial component of the orofacial complex that not only assists with mastication but is essential for holistic craniofacial development. Meckel’s cartilage (MC), formed by the first pharyngeal arch, provides early structural support and acts as a template for mandibular bone development. MC develops posteriorly to anteriorly. It forms anterior to the symphysis of the mandible and the most posterior part gives rise to the malleus and incus. MC is primarily derived from cranial NCC [4]. Initiated by mesenchymal condensation, pre-chondroblasts differentiate into chondrocytes that later undergo hypertrophy.
Mandibular bone formation occurs via intramembranous ossification around MC, initiated by early vascularization rather than endochondral ossification (Fig. 7). Hypertrophic chondrocytes in MC are degraded by chondroclasts and replaced by intramembranous bone (Fig. 8). Some limited endochondral ossification has been described for the formation of the mental ossicles, restricted to the most anterior region. This view has been challenged recently following the observation that newly forming bone surrounding the MC expresses both type I and II collagen, suggestive of (limited) endochondral ossification [49]. A common mandibular defect is micrognathia/retrognathia, which is often associated with Pierre Robin sequence (PRS). PRS describes a developmental sequence of retrognathia, glossoptosis, and airway obstruction. The underlying palatal defect is commonly attributed to abnormal MC development and associated lack of genioglossus muscle support [50]. The various underlying cellular/molecular defects leading to abnormal MC development may explain why some children fail to recover from micrognathia when treated with non-invasive techniques and require surgical intervention such as distraction osteogenesis to stimulate mandibular growth [51].
Fig. 7.
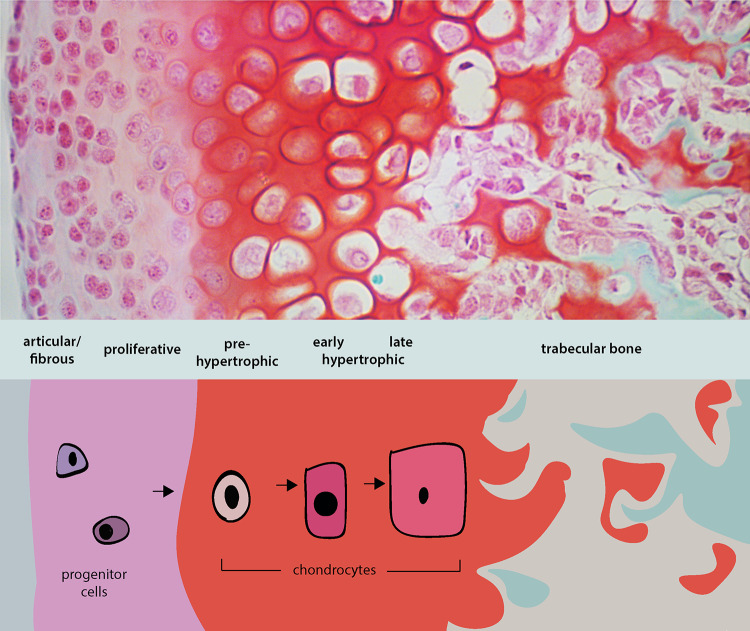
Illustration of the chondrocyte organization and differentiation during endochondral ossification. (Top panel) Safranin O staining (red) of paraffin-embedded temporomandibular joint (TMJ) section counterstained with fast green indicating the organization of the TMJ into articular (outermost/surface), proliferative, pre-hypertrophic, early and late hypertrophic chondrocytes followed by bone. (Bottom panel) Chondrocyte progenitors undergo differentiation into proliferative chondrocytes which then mature to become hypertrophic chondrocytes. The hypertrophic chondrocytes either undergo apoptosis or transdifferentiate into bone. This process of progenitors undergoing an intermediate cartilage phase to form bone is called endochondral ossification
Fig. 8.
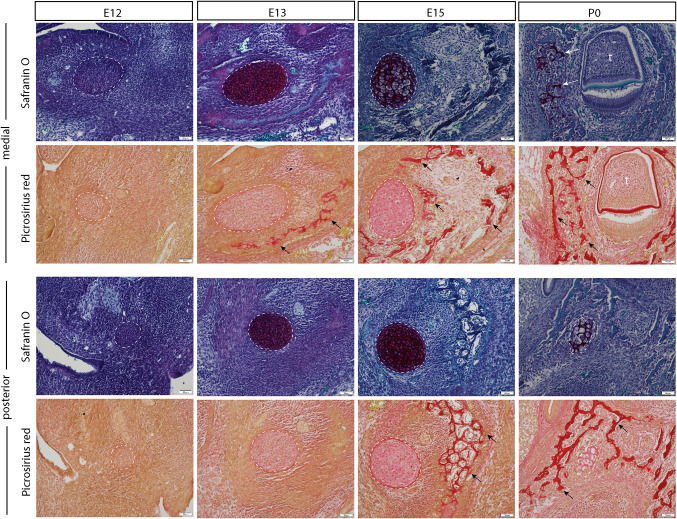
Cartilage and intramembranous bone formation throughout mandibular development. Paraffin histology demonstrating mandibular development via intramembranous bone formation throughout mouse development, from embryonic day 12 post-conception (E12) to postnatal day 0 (P0). As Meckel’s cartilage (deep orange on Safranin O stain, indicated by white dotted lines and arrows) is degraded, bone forms in the surrounding space (red stain on Picrosirius red stain, indicated by black arrows). Note that the stage of intramembranous bone formation differs along the length of the mandible, with the medial mandible ossifying sooner than the posterior aspect
Neural Crest for Tissue Regeneration
Tissue regeneration is a fast-evolving aspect of craniofacial research with strong emphasis on stem cell biology. The craniofacial complex is home to a number of stem cell niches thought to be involved in continuous tissue homeostasis and repair [52]. Most emphasis is on NC-derived mesenchymal stem cells (MSCs) that are able to form bone, cartilage, and mesenchymal tissue. Classically, MSCs have to fulfill three main criteria: they must adhere to plastic plates in vitro, express a specific subset of surface markers, and be able to differentiate into osteocytes, chondrocytes, and adipocytes (Fig. 9) [52]. MSCs are present in calvaria, maxillary and mandibular bones, and tooth-supporting tissue. Stem cell niches have been described for the periosteum, craniofacial bone marrow, TMJ, sutures, and periodontium. It is important to note that the niche in which craniofacial stem cells reside is expected to be different from those of other MSCs, such as in long bones. While stem cell-based regenerative therapies are often generalized to stem cells overall, craniofacial stem cell niches are located in specialized environments supporting very specific roles for tissue homeostasis/repair. It is thus critical to recognize that stem cells isolated for craniofacial repair from various locations will likely display some biological differences reflective of those environments.
Fig. 9.
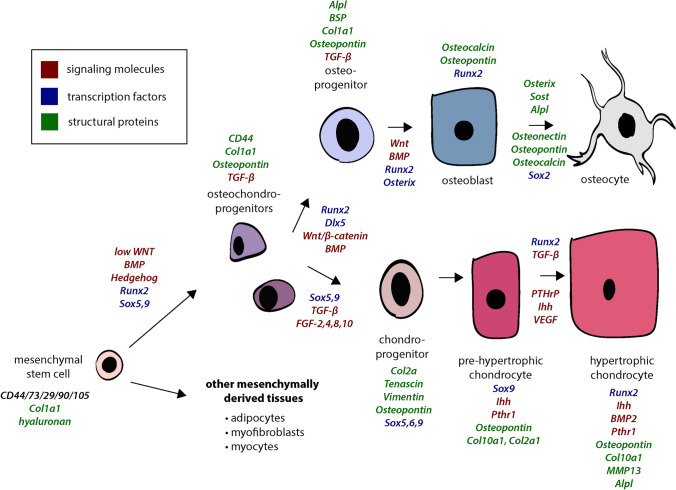
Differentiation of mesenchymal stem cells. Gene names listed in italics representing activity of signaling molecules, transcription factors, and structural proteins at each stage of the differentiation pathway for bone and cartilage. Red text references genes encoding for signaling molecules, blue text references genes encoding for transcription factors, and green text references genes encoding for structural proteins
A current application of regenerative stem cell therapy is temporomandibular joint (TMJ) cartilage engineering [53]. Regeneration of worn cartilage has been demonstrated using primary cells from the TMJ disc, articular chondrocytes, or costal chondrocytes. MSCs isolated from the synovium or synovial fluid of the TMJ are also promising new sources for regeneration. However, intrinsic cellular differences in differentiation/proliferation capacity and variable success indicate a poorly understood cellular heterogeneity. This is likely a reflection of the intricate fine regulation of stem cell niches, similarly to the intricate regulation of the development of the various craniofacial structures described earlier.
Regeneration of craniofacial bone using NC stem cells generally requires support from a scaffold on which the cells can differentiate [54]. Scaffolds can be polymeric, ceramic, metallic, or composed of composite materials. Each has specific benefits and disadvantages and must be optimized to suit their intended task. Ceramic scaffolds mimic hydroxyapatite found in bone, improving osteoblast adherence and proliferation, but lack the natural tissue-derived quality of a polymeric scaffold. Stem cells reside in niches in vivo, but in vitro are typically expanded on plastic surfaces. The advent of single-cell RNA sequencing has painted a much more complex picture of stem and progenitor cell populations. It is likely that the current criteria used do not allow accurate identification of stem cells. Thus, although NC is a multipotent embryonic cell lineage, it is reasonable to assume that the various adult stem cell niches identified in NC-derived tissues will display differences that affect stem cell properties, potential, and behavior. Identification of properties of stemness in craniofacial stem cells must be an important future direction. The paracrine function and secretome of stem cells are also not yet understood, nor is the influence of underlying support tissues for niche maintenance.
In closing, experimental embryology using state-of-the-art -omics approaches has been instrumental for casting light upon the intricate relationships and complexities of craniofacial development. NC as a multi-potent cell source takes center stage in craniofacial development. Knowledge of which genes affect various biologic processes has enabled rescue of craniofacial malformations associated with Treacher Collins syndrome by targeting the process, rather than the gene, in an experimental setting. Despite considerable advances, we have yet to uncover how reiterated employment of only a few signaling systems is interpreted within a spatiotemporal context to ensure appropriate, precise development of the coordinated craniofacial complex. However, the strong link between genetics, distinct biological processes, and craniofacial morphology has been made evident by recent advances in systematic 3D morphometric image analysis able to assist with syndrome diagnosis [55]
The use of NC-derived stem cells for tissue repair holds great promise. Realization of their potential, however, requires a more profound understanding of the intricacies of cell communication and cell differentiation that underlie the complexities of NC-derived tissues. Further advances in this direction will ultimately enable innovative and successful application of NC-derived stem cells in clinical settings.
Acknowledgements
This work was supported by Natural Science and Engineering Research Council of Canada (NSERC) RGPIN-2014-06311 and funds from the Alberta Dental Association & College (ADA&C) Chair in Oral Health Research to DG.
Compliance with Ethical Standards
Conflict of interest
The authors declare no conflict of interest.
Ethical Approval
Work involving animals presented in this review was approved by the Animal Use and Care Committee of the University of Alberta, protocol AUP1149, in accordance with guidelines of the Canadian Council of Animal Care.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Daniela Marta Roth, Email: dmroth@ualberta.ca.
Francy Bayona, Email: bayonaro@ualberta.ca.
Pranidhi Baddam, Email: pranidhi@ualberta.ca.
Daniel Graf, Email: dgraf@ualberta.ca.
References
- 1.Dooley CM, Wali N, Sealy IM, White RJ, Stemple DL, Collins JE, et al. The gene regulatory basis of genetic compensation during neural crest induction. PLOS Genet. Public Lib Sci. 2019;15:1008213. doi: 10.1371/journal.pgen.1008213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Saint-Jeannet J-P, editor. Neural Crest Induction and Differentiation [Internet]. Springer US; 2006 [cited 2020 Oct 26]. https://www.springer.com/gp/book/9780387351360
- 3.Donoghue PCJ, Graham A, Kelsh RN. The origin and evolution of the neural crest. Bioessays. 2008;30:530–41. doi: 10.1002/bies.20767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Le Douarin NM, Ziller C, Couly GF. Patterning of Neural Crest Derivatives in the Avian Embryo: in Vivo and in Vitro Studies. Developmental Biology. 1993;159:24–49. doi: 10.1006/dbio.1993.1219. [DOI] [PubMed] [Google Scholar]
- 5.Rothstein M, Bhattacharya D, Simoes-Costa M. The molecular basis of neural crest axial identity. Dev Biol. 2018;444(Suppl 1):S170–80. doi: 10.1016/j.ydbio.2018.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vega-Lopez GA, Cerrizuela S, Tribulo C, Aybar MJ. Neurocristopathies: New insights 150 years after the neural crest discovery. Developmental Biology [Internet]. 2018 [cited 2020 Dec 23];444:S110–43. http://www.sciencedirect.com/science/article/pii/S0012160617308382 [DOI] [PubMed]
- 7.Prasad MS, Charney RM, García-Castro MI. Specification and formation of the neural crest: perspectives on lineage segregation. Genesis. 2019;57:e23276. doi: 10.1002/dvg.23276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Buitrago-Delgado E, Nordin K, Rao A, Geary L, LaBonne C. Shared regulatory programs suggest retention of blastula-stage potential in neural crest cells. Science. Am Assoc Advancement Sci. 2015;348:1332–5. [DOI] [PMC free article] [PubMed]
- 9.Theveneau E, Mayor R. Neural crest delamination and migration: From epithelium-to-mesenchyme transition to collective cell migration. Dev Biol. 2012;366:34–54. doi: 10.1016/j.ydbio.2011.12.041. [DOI] [PubMed] [Google Scholar]
- 10.Szabó A, Mayor R. Mechanisms of neural crest migration. Ann Rev Genet. 2018;52:43–63. doi: 10.1146/annurev-genet-120417-031559. [DOI] [PubMed] [Google Scholar]
- 11.Shellard A, Mayor R. Integrating chemical and mechanical signals in neural crest cell migration. Curr Opin Genet Dev. 2019;57:16–24. doi: 10.1016/j.gde.2019.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Genuth MA, Allen CDC, Mikawa T, Weiner OD. Chick cranial neural crest cells use progressive polarity refinement, not contact inhibition of locomotion, to guide their migration. Dev Biol. 2018;444(Suppl 1):S252–61. doi: 10.1016/j.ydbio.2018.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Carmona-Fontaine C, Theveneau E, Tzekou A, Tada M, Woods M, Page KM, et al. Complement fragment C3a controls mutual cell attraction during collective cell migration. Dev Cell. 2011;21:1026–37. doi: 10.1016/j.devcel.2011.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Barriga EH, Franze K, Charras G, Mayor R. Tissue stiffening coordinates morphogenesis by triggering collective cell migration in vivo. Nature. Nat Publish Group. 2018;554:523–7. doi: 10.1038/nature25742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shellard A, Szabó A, Trepat X, Mayor R. Supracellular contraction at the rear of neural crest cell groups drives collective chemotaxis. Science. Am Assoc Adv Sci. 2018;362:339–43. [DOI] [PMC free article] [PubMed]
- 16.Hovland AS, Rothstein M, Simoes-Costa M. Network architecture and regulatory logic in neural crest development. WIREs Syst Biol Med. 2020;12:e1468. doi: 10.1002/wsbm.1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Frisdal A, Trainor PA. Development and evolution of the pharyngeal apparatus. Wiley Interdiscip Rev Dev Biol. 2014;3:403–18. doi: 10.1002/wdev.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Graham A, Okabe M, Quinlan R. The role of the endoderm in the development and evolution of the pharyngeal arches. J Anat. 2005;207:479–87. doi: 10.1111/j.1469-7580.2005.00472.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Feng W, Leach SM, Tipney H, Phang T, Geraci M, Spritz RA, et al. Spatial and temporal analysis of gene expression during growth and fusion of the mouse facial prominences. PLoS One. 2009;4:e8066. doi: 10.1371/journal.pone.0008066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu J, Chen J, Yuan D, Sun L, Fan Z, Wang S, et al. Dynamic mRNA Expression Analysis of the Secondary Palatal Morphogenesis in Miniature Pigs. Int J Mol Sci. Multidisc Digit Publish Inst. 2019;20:4284. [DOI] [PMC free article] [PubMed]
- 21.Danescu A, Mattson M, Dool C, Diewert VM, Richman JM. Analysis of human soft palate morphogenesis supports regional regulation of palatal fusion. J Anat. 2015;227:474–86. doi: 10.1111/joa.12365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tarr JT, Lambi AG, Bradley JP, Barbe MF, Popoff SN. Development of Normal and Cleft Palate: A Central Role for Connective Tissue Growth Factor (CTGF)/CCN2. J Dev Biol [Internet]. 2018 [cited 2020 Oct 25];6. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6162467/ [DOI] [PMC free article] [PubMed]
- 23.Paiva KBS, Maas CS, Santos PM dos, Granjeiro JM, Letra A. Extracellular Matrix Composition and Remodeling: Current Perspectives on Secondary Palate Formation, Cleft Lip/Palate, and Palatal Reconstruction. Front Cell Dev Biol [Internet]. Frontiers; 2019 [cited 2020 Oct 25];7. https://www.frontiersin.org/articles/10.3389/fcell.2019.00340/full [DOI] [PMC free article] [PubMed]
- 24.Lidral AC, Moreno LM. Progress toward discerning the genetics of cleft lip. Curr Opin Pediatr. 2005;17:731–9. doi: 10.1097/01.mop.0000185138.65820.7f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mani P, Jarrell A, Myers J, Atit R. Visualizing canonical Wnt signaling during mouse craniofacial development. Dev Dyn. 2010;239:354–63. doi: 10.1002/dvdy.22072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang C, Mazzeu JF, Eisfeldt J, Grochowski CM, White J, Akdemir ZC, et al. Novel pathogenic genomic variants leading to autosomal dominant and recessive Robinow syndrome. Am J Med Genet A. 2020 [DOI] [PMC free article] [PubMed]
- 27.Graf D, Malik Z, Hayano S, Mishina Y. Common mechanisms in development and disease: BMP signaling in craniofacial development. Cytokine Growth Factor Rev. 2016;27:129–39. doi: 10.1016/j.cytogfr.2015.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wagner DO, Sieber C, Bhushan R, Börgermann JH, Graf D, Knaus P. BMPs: from bone to body morphogenetic proteins. Sci Signal. 2010;3:mr1. [DOI] [PubMed]
- 29.Hatch NE. FGF signaling in craniofacial biological control and pathological craniofacial development. Criti RevTM Eukaryotic Gene Exp. 2010;20:295–311. doi: 10.1615/CritRevEukarGeneExpr.v20.i4.20. [DOI] [PubMed] [Google Scholar]
- 30.Neben CL, Roberts RR, Dipple KM, Merrill AE, Klein OD. Modeling craniofacial and skeletal congenital birth defects to advance therapies. Hum Mol Genet. 2016;25:R86–93. doi: 10.1093/hmg/ddw171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dworkin S, Boglev Y, Owens H, Goldie SJ. The Role of Sonic Hedgehog in Craniofacial Patterning, Morphogenesis and Cranial Neural Crest Survival. J Dev Biol [Internet]. 2016 [cited 2020 Aug 31];4. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5831778/ [DOI] [PMC free article] [PubMed]
- 32.Jussila M, Thesleff I. Signaling Networks Regulating Tooth Organogenesis and Regeneration, and the Specification of Dental Mesenchymal and Epithelial Cell Lineages. Cold Spring Harb Perspect Biol [Internet]. 2012 [cited 2020 Aug 31];4. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3312678/ [DOI] [PMC free article] [PubMed]
- 33.Dumic-Cule I, Peric M, Kucko L, Grgurevic L, Pecina M, Vukicevic S. Bone morphogenetic proteins in fracture repair. Int Orthop. 2018;42:2619–26. doi: 10.1007/s00264-018-4153-y. [DOI] [PubMed] [Google Scholar]
- 34.Thesleff I, Keränen S, Jernvall J. Enamel knots as signaling centers linking tooth morphogenesis and odontoblast differentiation. Adv Dent Res. 2001;15:14–8. doi: 10.1177/08959374010150010401. [DOI] [PubMed] [Google Scholar]
- 35.Petryk A, Graf D, Marcucio R. Holoprosencephaly: signalling interactions between the brain and the face, the environment and the genes, and the phenotypic variability in animal models and humans. Wiley Interdiscip Rev Dev Biol. 2015;4:17–32. doi: 10.1002/wdev.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Malik Z, Roth DM, Eaton F, Theodor JM, Graf D. Mesenchymal Bmp7 Controls Onset of Tooth Mineralization: A Novel Way to Regulate Molar Cusp Shape. Front Physiol [Internet]. Frontiers; 2020 [cited 2020 Jul 13];11. https://www.frontiersin.org/articles/10.3389/fphys.2020.00698/full [DOI] [PMC free article] [PubMed]
- 37.Alappat S, Zhang ZY, Chen YP. Msx homeobox gene family and craniofacial development. Cell Res. 2003;13:429–42. doi: 10.1038/sj.cr.7290185. [DOI] [PubMed] [Google Scholar]
- 38.Trainor PA. Specification and patterning of neural crest cells during craniofacial development. Brain Behav Evol. 2005;66:266–80. doi: 10.1159/000088130. [DOI] [PubMed] [Google Scholar]
- 39.Berendsen AD, Olsen BR. Bone development. Bone. 2015;80:14–8. doi: 10.1016/j.bone.2015.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Huang W, Yang S, Shao J, Li Y-P. Signaling and transcriptional regulation in osteoblast commitment and differentiation. Front Biosci. 2007;12:3068–92. doi: 10.2741/2296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Morriss-Kay GM, Wilkie AO. Growth of the normal skull vault and its alteration in craniosynostosis: insights from human genetics and experimental studies. J Anat. 2005;207:637–53. doi: 10.1111/j.1469-7580.2005.00475.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Opperman LA. Cranial sutures as intramembranous bone growth sites. Dev Dyn. 2000;219:472–85. doi: 10.1002/1097-0177(2000)9999:9999<::AID-DVDY1073>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 43.Di Ieva A, Bruner E, Davidson J, Pisano P, Haider T, Stone SS, et al. Cranial sutures: a multidisciplinary review. Childs Nerv Syst. 2013;29:893–905. doi: 10.1007/s00381-013-2061-4. [DOI] [PubMed] [Google Scholar]
- 44.Kim I-S, Lee M-Y, Lee K-I, Kim H-Y, Chung Y-J. Analysis of the development of the nasal septum according to age and gender using MRI. Clin Exp Otorhinolaryngol. 2008;1:29–34. doi: 10.3342/ceo.2008.1.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Prein C, Beier F. Chapter Two - ECM signaling in cartilage development and endochondral ossification. In: Olsen BR, editor. Current Topics in Developmental Biology [Internet]. Academic Press; 2019 [cited 2020 Oct 26]. p. 25–47. http://www.sciencedirect.com/science/article/pii/S0070215318300826 [DOI] [PubMed]
- 46.Baddam P, Bussolaro C-T, Flores-Mir C, Graf D. Nasal cavity structural anomalies among children at high risk of sleep-disordered breathing: an exploratory cone-beam computed tomography study. Am J Orthodont Dentofac Orthoped. 2020 [DOI] [PubMed]
- 47.Cha BK, Choi DS, Jang IS, Yook HT, Lee SY, Lee SS, et al. Aberrant growth of the anterior cranial base relevant to severe midface hypoplasia of Apert syndrome. Maxillofac Plastic Reconstruct Surg. 2018;40:40. doi: 10.1186/s40902-018-0179-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Moss ML, Bromberg BE, Song IC, Eisenman G. The passive role of nasal septal cartilage in mid-facial growth. Plast Reconstr Surg. 1968;41:536–42. doi: 10.1097/00006534-196806000-00004. [DOI] [PubMed] [Google Scholar]
- 49.Svandova E, Anthwal N, Tucker AS, Matalova E. Diverse Fate of an Enigmatic Structure: 200 Years of Meckel’s Cartilage. Front Cell Dev Biol [Internet]. Frontiers; 2020 [cited 2020 Oct 26];8. https://www.frontiersin.org/articles/10.3389/fcell.2020.00821/full [DOI] [PMC free article] [PubMed]
- 50.Kouskoura T, El Fersioui Y, Angelini M, Graf D, Katsaros C, Chiquet M. Dislocated Tongue Muscle Attachment and Cleft Palate Formation. Journal of Dental Research. 2016;95:453–9. doi: 10.1177/0022034515621869. [DOI] [PubMed] [Google Scholar]
- 51.Hammoudeh JA, Fahradyan A, Brady C, Tsuha M, Azadgoli B, Ward S, et al. Predictors of failure in infant mandibular distraction osteogenesis. J Oral Maxillofac Surg. 2018;76:1955–65. doi: 10.1016/j.joms.2018.03.008. [DOI] [PubMed] [Google Scholar]
- 52.Zhang G, Li Q, Yuan Q, Zhang S. Spatial distributions, characteristics, and applications of craniofacial stem cells. Stem Cells Int. 2020;2020:8868593. doi: 10.1155/2020/8868593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhang S, Yap AUJ, Toh WS. Stem cells for temporomandibular joint repair and regeneration. Stem Cell Rev and Rep. 2015;11:728–42. doi: 10.1007/s12015-015-9604-x. [DOI] [PubMed] [Google Scholar]
- 54.Ghassemi T, Shahroodi A, Ebrahimzadeh MH, Mousavian A, Movaffagh J, Moradi A. Current concepts in scaffolding for bone tissue engineering. Arch Bone Jt Surg. 2018;6:90–9. [PMC free article] [PubMed] [Google Scholar]
- 55.Hallgrímsson B, Aponte JD, Katz DC, Bannister JJ, Riccardi SL, Mahasuwan N, et al. Automated syndrome diagnosis by three-dimensional facial imaging. Genet Med. 2020;22:1682–93. doi: 10.1038/s41436-020-0845-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sperber GH, Sperber SM. Craniofacial Embryogenetics and Development [Internet]. [cited 2020 Oct 30]. https://pmphusa.com/book/craniofacial-embryogenetics-and-development-3e/
- 57.Etchevers HC, Dupin E, Le Douarin NM. The diverse neural crest: from embryology to human pathology. Development. 2019;146. [DOI] [PubMed]