Abstract
Although participatory workplace improvement programs are known to provide favorable effects on high stress occupations like nursing, no studies have confirmed its effect using biomarkers. The aim of this study was to determine whether a participatory workplace improvement program would decrease stress-related symptoms as evaluated by biomarkers and self-reported stress among hospital nurses. Three actions to alleviate job stress, which were determined through focus group interviews and voting, were undertaken for two months. A total of 31 female Japanese nurses underwent measurement of inflammatory markers, autonomic nervous activity (ANA), and perceived job stress (PJS) at three-time points; before the program (T1), within a week after the completion of the program (T2), and three months after the program (T3). A series of inflammatory markers (Interferon-γ, Interleukin (IL)-6, and IL-12/23p40) decreased significantly at T2, and IL-12/23p40 and IL-15 significantly decreased at T3 compared to T1, while ANA and PJS remained unchanged. Our participatory program exerted beneficial effects in reducing inflammatory responses, but not for ANA and PJS. Further investigations with a better study design, i.e., a randomized controlled trial, and a larger sample size are warranted to determine what exerted beneficial effects on inflammatory markers and why other outcomes remained unchanged.
Keywords: Participatory workplace improvement program, Inflammatory markers, Nurses, Focus group interview, Voting, Job stress, Autonomic nervous activity
Introduction
Tasks of nurses are diverse; their main tasks are to provide patients’ medical care, assist daily activities of living, and consult with other medical professions, patients’ families, relatives, friends, and even more1, 2). Nursing is a profession with high responsibility along with long work hours and irregular shifts, but yet nurses are poorly treated2). They are often exposed to high quantitative/qualitative job demands3) and are therefore relatively at a higher risk of burnout4), anxiety5), emotional exhaustion6), and depression7). According to the white paper published by the Japanese Nursing Association in 20098), nearly one in 23 Japanese nurses are at risk of Karoshi (death by overwork) due to excessive workloads and shortage of nursing staff. A study on occupational stress among Japanese healthcare professions reported that nursing staff had significantly higher levels of quantitative and qualitative workloads compared to administrative/clerical employees at the same hospital, and nurses also reported not having sufficient support from supervisors and coworkers3). Thus, it is an urgent matter for Japanese nursing professions to improve such demanding work conditions with poor human relationships.
In the past ten years, organizational interventions to improve employees’ physical/mental health and work environment have been developed and implemented9,10,11,12,13,14,15,16,17). A systematic review regarding job stress interventions concluded that those targeting ‘organizations’ rather than ‘individuals’ are more beneficial because it exerted more favorable effects on workplace climate, absenteeism, and health behaviors12). One of those effective organizational interventions is an employee participatory program13, 14, 16), which encourages employees to actively participate in identifying workplace problems and find feasible actions/solutions. In a participatory program, the employees take a lead in planning the improvement at their workplaces. Thus, making an employee participatory program more flexible and acceptable than researcher-lead programs12). Indeed, an employee participatory intervention study of Japanese nurses revealed that the program increased coworker support and workplace goals16). Another participatory intervention study among health care workers including nurses reported significant increases in reward leading to a decrease of effort to reward imbalance scores18).
However, carrying out effective workplace interventions is often difficult because it demands every employee to be actively involved19). In addition to work demands, problems in the workplace vary by situations of the workplace; many complex problems exist, and the characteristics of individuals and the group are often diverse18, 19). Therefore, employees may not always be eager to be involved in the intervention programs, i.e., they may have other priorities that make them hesitate to participate in the intervention20). Furthermore, a potential criticism of these studies is that the evaluation of outcomes is mainly based on “self-reports” not validated by objective markers, for instance, biomarkers such as inflammatory markers and autonomic nervous activities, which may limit the generalizability and reproducibility of the results. Objective markers including the immune and autonomic nervous system indicators are known to reflect levels of stress and are helpful to evaluate stress objectively21,22,23,24,25,26,27,28,29).
In the present study, we examined the effect of an employee participatory program among university hospital nurses. The specific aim of this study was to investigate whether the workplace participatory program is effective in alleviating stress-related inflammatory markers, improving the balance of the autonomic nervous activities, and perceived stress-related outcomes. We hypothesized that after completion of the employee participatory program, we would observe a reduction of stress-related inflammatory markers, improved balance of the autonomic nervous activities, and reduced perceived job stress by the effects of the program itself. Because the intervention effect may be delayed or lasting over a longer period, we examined the baseline and two-time points after the participatory intervention program, that is, immediately after and three months after the intervention.
Subjects and Methods
Setting
The present study was conducted at a branch hospital of A university hospital with 150 beds in the southern part of Japan. The hospital had 144 nurses working under 19 clinical departments with seven units. The hospital is well-known for community-based nursing, palliative care, and orthopedics specialized in sports.
Design and procedure
Throughout the nursing departments, all nurses working at the hospital were asked to participate in this study (n=144, female ratio: 96.5%). Inclusion criteria were, 1) working at the hospital as a full-time nurse, 2) not being pregnant, and 3) not having a severe illness which may interfere with objective evaluations. A total of 36 nurses agreed to participate in this study. We excluded participants who became pregnant during the implementation of the program (n=1) and were absent from work due to illness or family issues at the time of evaluations (n=3). A male participant was excluded from the analysis because of possible sex differences in outcome measures (n=1). Finally, a total of 31 female nurses who participated in the whole process were submitted to the final analysis.
Ethics
The study was reviewed and approved by the ethical committee of the University of Occupational and Environmental Health (H29-049) and the ethical committee of the International University of Health and Welfare (18-ml-002). This study was registered on the University Hospital Medical Information Network Clinical Trials Registry (UMIN000039836). We informed potential participants about the study aim, procedure, and confidentiality policy for individual information, and participants filled out a consent form before enrollment. Participants who agreed to participate in blood sampling to measure inflammatory markers, autonomic nervous activity, and job stress-related questionnaire received a 1,000-yen gift card as a reward at each time of the measurement.
Outline of the participatory program
We carried out a participatory program referring to the procedure for the workplace improvement by the National Personnel Authority, Japan10) and several other reports conducted in Japan9, 11). The following procedure was undertaken. We first explained the idea of the participatory workplace improvement program to the nursing director/head nurses and then, held an information session to explain the program and our research (presentations, and handouts for those who could not attend the session) for all nurses in the hospital. After that, we asked every nurse at the hospital to fill out an anonymous questionnaire with two open-ended questions; ‘Please share us your best ideas to create ideal/pleasant workplaces’ and ‘What could you and other nurses do to create a better and ‘easy to work’ workplace in a common manner?’. We obtained more than 100 ideas from nurses. We also asked the nursing director/head nurses to recruit nurses who are willing to participate in the focus group interview (FGI) to discuss feasible ideas regarding time, cost, and effort, to improve the work environment/conditions. With reference to the ideas from the questionnaire, we conducted two 2-h FGIs to prioritize the action. Each FGI group consisted of five to eight nurses representing their units/departments and a total of 24 nurses participated in the FGIs. Three facilitators (TT, AN, and YO) assisted the FGIs throughout.
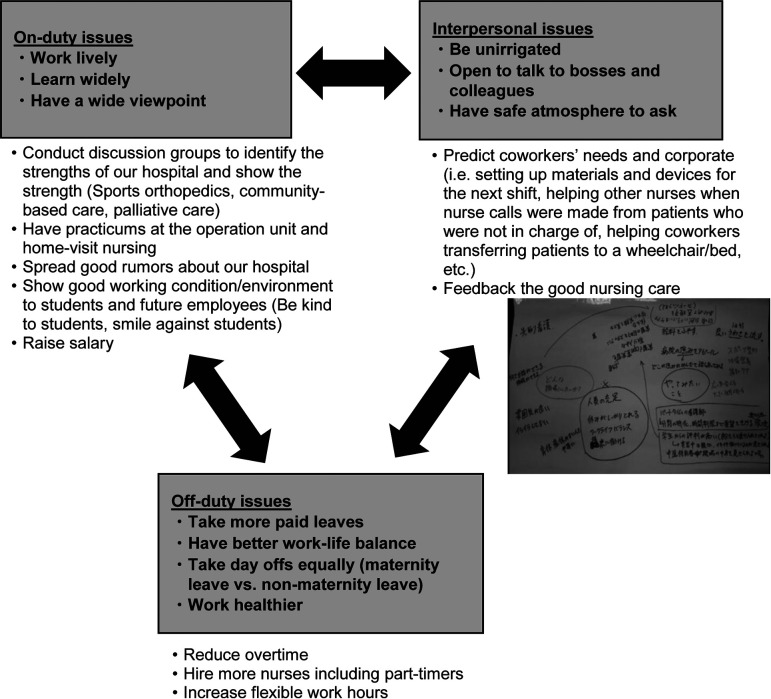
Next, based on the FGIs and discussion with facilitators, actions of the participatory program were narrowed-down to three ideas: a) taking turns to have no overtime, b) voluntarily predicting coworkers’ needs to smoothly carry out their tasks, and c) sending ‘Thank-you’ cards to coworkers to show appreciation. The idea that ‘voluntarily predicting coworkers’ needs’ was, for examples, to set up materials and devices for the next shift, to help other nurses when nurse-calls were made from patients who were not in charge of, to help coworkers transferring a patient to a wheelchair or a bed, etc. Finally, we held a vote to select one action from all the anonymous nurses to determine the participatory program; we obtained 43 votes for a), 24 votes for b), 15 votes for c), and two votes with blank. However, after the vote, head nurses pointed out that some units were already conducting the action a), so the nurses eventually decided to carry out all three actions. These three actions were facilitated by the nursing director/head nurses in each unit/department and implemented for two months continuously by each unit (Fig. 1).
Fig. 1.
Examples of obtained ideas from the focus group interviews. A group of interviewees raised ideas about on-duty, off-duty, and interpersonal issues.
Evaluation
This study employed three types of measurements; inflammatory markers, autonomic nervous activity, and self-administered questionnaire. Participants were asked to take blood sampling and evaluation of autonomic nervous activity, and to fill out the questionnaire at three time points; before the participatory program for baseline evaluation (T1), within a week after the end of the program to measure an immediate effect (T2), and three months after the end of the program (T3) to evaluate delayed/lasting effect (Fig. 2). Each evaluation point was scheduled for five days to secure participants working daytime shifts on the evaluation day. The data were collected between August 2017 and February 2018.
Fig. 2.
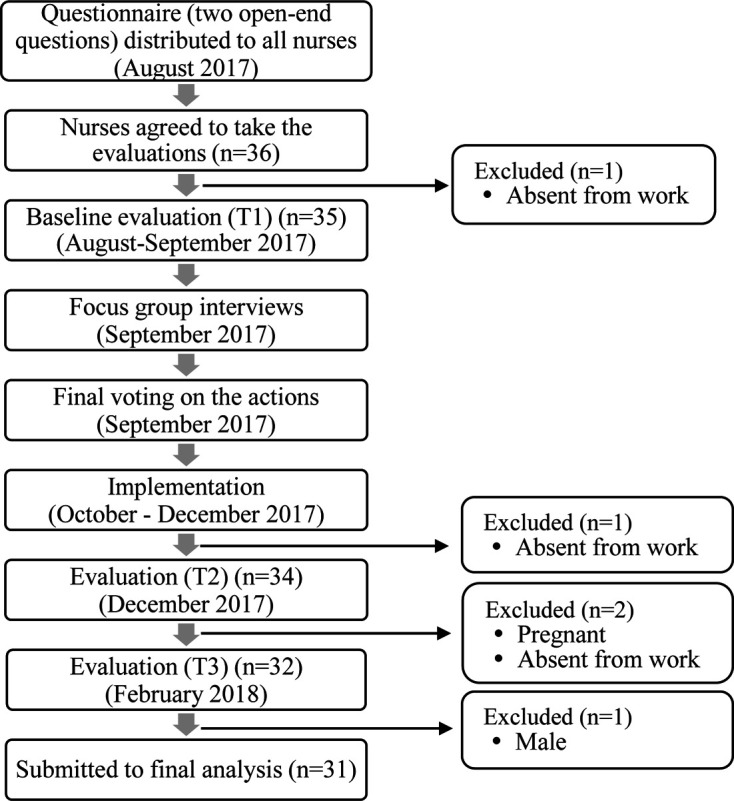
Flow chart of methods and study participants.
Objective evaluations
Inflammatory markers
A number of studies indicated that chronic psychosocial job stress elevates inflammatory markers such as C-reactive protein (CRP), interleukin (IL)-6, and tumor necrosis factor (TNF)-α22, 23, 25,26,27,28,29). These studies observed that workers with higher job stress (low organizational justice, increased job strain, or low social support) exhibited increased levels of CRP and IL-622, 23, 26) compared to the lower stress counterpart. To objectively evaluate chronic psychosocial job stress level, we measured traditional inflammatory markers, such as interferon (IFN)-γ, IL-6, TNF-α, and high-sensitivity C-reactive protein (hs-CRP), and as well as IL-12/23p40, IL-15, and IL-27 since measuring autonomic nervous balance may only reflect momentary stress levels but not the chronic one30). Because past studies confirmed an association between psychosocial factors at work including job stress and inflammatory markers22, 23, 25,26,27,28,29), we could expect a reduction of those inflammatory markers if the participatory program is effective.
A total of 8.5 ml of venous blood samples was collected to measure serum cytokines and hs-CRP. The blood samples of each participant were collected in gamma-rays sterilized polyethylene-terephthalate tubes containing serum separating gel and coagulation accelerant (silica particles) between 2 pm and 5 pm and stored in a cooler box (0–5°C). These samples were then transported to our laboratory twice in the afternoon by 4:30 pm and 7:30 pm (within two and half hours from blood collection). At the laboratory, the samples were centrifugalized with 2,400 rpm for ten minutes to extract 500 µl of the serum and deep-freezed (−20°C) until the analysis. The levels of IFN-γ, IL-6, TNF-α, IL-12/23p40, IL-15, IL-27, and hs-CRP were measured with Enzyme Immunoassay or Chemiluminescent Enzyme Immunoassay using MESOTM QuickPlex SQ 120 (Meso Scale Diagnostic, LCC, Rockville, MD, USA), which were measured by the analyzing company, Life Science Institute Medience Corporation, Japan. The minimum detectable level for IFN-γ, IL-6, TNF-α, IL-12/23p40, IL-15, IL-27, and hs-CRP was 0.2 pg/ml, 0.06 pg/ml, 0.04 pg/ml, 15.0 pg/ml, 2.0 pg/ml, 8 pg/ml, and 0.004 mg/dl, respectively, and the values lower than the measurement limit were divided by square root of two, “minimum detectable level/√2” for each inflammatory markers31). In this sample, there were 12 cases for hs-CRP, which exhibited below the minimal detectable limits. Three cases for IFN-γ and 14 cases for IL-6 also exhibited below the minimal detectable level, however, their values were detected from the calibration curve.
Autonomic nervous activity
Autonomic nervous activity is commonly assessed by measuring heart rate variability (HRV), which is the variation of time intervals of heartbeats (R-R intervals). Several studies reported that increased heart rate and decreased HRV, which reflects sympathetic nervous activity (SNA), are associated with high levels of job stress21, 24, 28, 32). The SNA and parasympathetic nervous activity (PNA) are known as antagonistic. Therefore, if a decrease of SNA and increase of PNA are observed simultaneously after the participatory program, we could expect a stress reduction effect by the program.
We measured the autonomic nervous activity by Silmee Bar Type Lite (Silmee, Tokyo Denki Kagaku (TDK), Tokyo, Japan), a non-invasive device, that can measure HRV within 15 min, as described elsewhere33). Briefly, Silmee is a small electrocardiograph device that is applied to the lower sternum of a subject, and HRV, pulse wave, and body motion were detected by the sensors. It automatically calculates HRV by the power spectral analysis and could measure three SNA parameters; low-frequency HRV/total frequency HRV (standing position), mean R-R interval / R-R interval per minute (standing position), and mean R-R interval (supine-stand position). Similarly, we could measure three PNA parameters; mean R-R interval (supine position), high-frequency HRV/total frequency HRV (supine position), and the standard deviation of R-R intervals (SDRR) (supine position). From these values, SNA/PNA ratios were calculated. All these measured values can be shown immediately by a radar chart on the tablet screen, which is connected by Bluetooth. We measured autonomic nervous activities in two rooms of the hospital with a dim light and quiet environment between 2 pm and 5 pm to adjust in-day fluctuation. Since Silmee detects current autonomic nervous activity, we used it to evaluate participants’ acute stress levels, as it is used in past studies34, 35).
Subjective evaluation
The self-administered questionnaire contained questions regarding psychosocial job stress, demographics, health, lifestyle, and work. In this study, we used perceived psychosocial job stress and psychosocial/physical stress reactions as measured by the Brief Job Stress Questionnaire36). Job satisfaction was measured by the NIOSH job satisfaction scale (translated in Japanese)37, 38), and depressive symptoms as measured by the Center for Epidemiologic Studies Depression Scale (CES-D)39). We also asked all nurses at the hospital to fill out anonymous questions whether they were voluntarily predicting coworkers’ needs and taking proactive actions before and after implementation by a 10-point Likert scale (0= not at all aware of/not taking any proactive action at all, …, 10= very much aware of/taking enough proactive actions).
Statistical analyses
To observe distributions of obtained values, we performed the Shapiro-Wilk test. If the Gaussian distributions were confirmed (p>0.05), we applied a one-way analysis of variance (ANOVA), and if the non-Gaussian distributions (p<0.05), we applied Friedman’s tests to compare differences among time points of evaluations (T1, T2, and T3). In this sample, the Gaussian distributions were confirmed on IL-15, mean R-R interval in the supine position, sympathetic nerve/parasympathetic nerve ratio, subjective quantitative workload, and physical stress response, and the rest was the non-Gaussian distributions. If there was a significant difference among three time points, we performed a pairwise comparison with the Bonferroni test.
Before comparing the intervention effects on outcome measures, we compared inflammatory markers, autonomic nervous activity markers, and sleep duration between daytime and 2-shift workers at baseline, because working under two different shifts may have an impact on these outcomes. As a result, we found no statistical differences between the groups, and thus we handled the data with shift and non-shift-workers together (Appendix 1–3).
To estimate the reliability of items on the questionnaire, Cronbach’s alphas were calculated as well. We confirmed that 30 out of 33 job stress scales measured in this study had acceptable level (>0.60) of Cronbach’s alphas. We analyzed data using IBM SPSS Statistics for Windows, version 25.0 (IBM Corp., Chicago, IL, USA), and the level of significance was set at p<0.05.
Results
Characteristics of participants
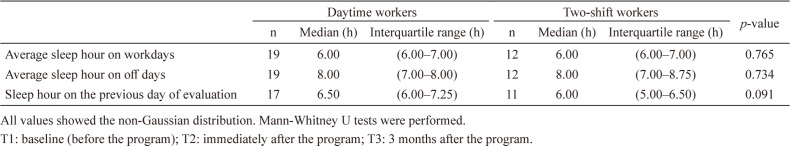
The baseline characteristics of female nurse participants who were submitted to the final analysis (n=31) are shown in Table 1. The median age of participants was 38.0 yr old and one-quarter of them were married. Except for the department G (nursing department), four to six nurses in each unit participated in this study. More than half of the participants worked for the day shift and the rest of them worked for 2-shifts with 16-hour night shift (16:30−9:30). They had the median overtime of 9.25 h in the previous month of the evaluation. Over 90 percent of participants had six or more hours of sleep on workdays. More than three-quarters of participants were under regular menstrual cycle.
Table 1. Baseline characteristics of female nurse participants (N=31).
| n | % | Median | Interquartile range | |
|---|---|---|---|---|
| Age | 38.0 | 25.0–42.0 | ||
| Marriage status | ||||
| Single | 22 | 71.0 | ||
| Married | 8 | 25.8 | ||
| Divorced | 1 | 3.2 | ||
| Number of years employed as a nurse | 7.0 | 3.0–20.0 | ||
| Number of participants by units and departments | ||||
| A (orthopaedics, gastrointestinal surgery, obstetrics and gynecology unit) | 5 | 16.1 | ||
| B (rheumatology, diabetic tract medicine, pulmonary medicine, cardiovascular medicine, and nephrology unit) | 4 | 12.9 | ||
| C (gastrointestinal medicine, palliative care, hematology, oncology, and urology unit) | 6 | 19.3 | ||
| D (operation department) | 5 | 16.1 | ||
| E (out-patient department) | 5 | 16.1 | ||
| F (home nursing department) | 5 | 16.1 | ||
| G (nursing department) | 1 | 3.2 | ||
| Work shift | ||||
| Daytime | 19 | 61.3 | ||
| 2-Shifts | 12 | 38.7 | ||
| Overtime (hours/last month) | 9.25 | 4.60–16.50 | ||
| Average sleep hours on work days | 6.00 | 6.00–7.00 | ||
| < 6 h | 3 | 9.7 | ||
| ≥ 6 h | 28 | 90.3 | ||
| Menstrual cycle | ||||
| Menstruation | 3 | 9.7 | ||
| Follicular phase | 7 | 22.6 | ||
| Luteal phase | 14 | 45.2 | ||
| Menopause | 4 | 12.9 | ||
| Other | 1 | 3.2 | ||
| Not ascertained | 2 | 6.5 |
Selected actions
Overall, four units/departments (orthopaedics, gastrointestinal surgery, obstetrics, and gynecology unit, rheumatology, diabetic tract medicine, pulmonary medicine, cardiovascular medicine, and nephrology unit, gastrointestinal medicine, palliative care, hematology, oncology, and urology unit, and operation department) in the hospital carried out the action ‘taking a turn to have no overtime’. The scores of voluntarily predicting coworkers’ needs and taking proactive actions were not significantly different before and after the implementation; the median for awareness of proactive actions before and after were both 7.0 (no change), and the median score for taking proactive actions before and after were both 6.0 (no change). In addition, nurses sent each other a total of 43 ‘Thank-you’ cards.
Inflammatory markers
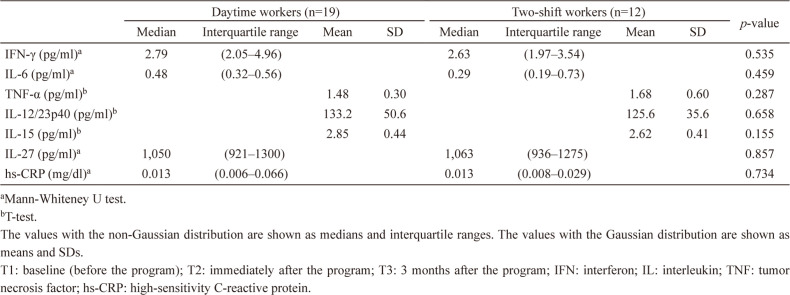
Table 2 shows medians, interquartile range, means, standard deviations (SD) of inflammatory markers at T1, T2, and T3, and statistical comparisons among the time points by either Friedman’s tests or one-way ANOVA. IFN-γ (p=0.003), IL-6 (p=0.047) and IL-12/23p40 (p=0.004) were significantly decreased at T2 compared to T1. Similarly, IL-12/23p40 (p=0.019) and IL-15 (p=0.015) were significantly decreased at T3 compared to T1.
Table 2. Differences in inflammatory markers at T1, T2, and T3.
| T1 | T2 | T3 | p-value | p-value(pair-wise)c | ||||||||||
| Median | Interquartile range | Mean | SD | Median | Interquartile range | Mean | SD | Median | Interquartile range | Mean | SD | |||
| IFN-γ (pg/ml)a | 2.76 | (2.05–4.91) | 2.15 | (1.31–3.48) | 2.68 | (1.86–3.92) | 0.004 | T1 vs. T2: 0.003, | ||||||
| T1 vs. T3: 0.432, | ||||||||||||||
| T2 vs. T3: 0.197 | ||||||||||||||
| IL-6 (pg/ml)a | 0.46 | (0.24–0.60) | 0.35 | (0.25–0.54) | 0.36 | (0.29–0.56) | 0.044 | T1 vs. T2: 0.047, | ||||||
| T1 vs. T3: 0.226, | ||||||||||||||
| T2 vs. T3: 1.000 | ||||||||||||||
| TNF-α (pg/ml)a | 1.46 | (1.25–1.82) | 1.42 | (1.10–1.68) | 1.33 | (1.20–1.62) | 0.244 | |||||||
| IL-12/23p40 (pg/ml)a | 124 | (98.7–154.0) | 102 | (83.4–127.0) | 115 | (87.1–137.0) | 0.003 | T1 vs. T2: 0.004, | ||||||
| T1 vs. T3: 0.019, | ||||||||||||||
| T2 vs. T3: 1.000 | ||||||||||||||
| IL-15 (pg/ml)b | 2.76 | 0.44 | 2.75 | 0.48 | 2.6 | 0.42 | 0.009 | T1 vs. T2: 1.000, | ||||||
| T1 vs. T3: 0.015, | ||||||||||||||
| T2 vs. T3: 0.058 | ||||||||||||||
| IL-27 (pg/ml)a | 1050 | (935–1300) | 973 | (886–1220) | 1090 | (887–1400) | 0.089 | |||||||
| hs-CRP (mg/dl)a | 0.013 | (0.007–0.032) | 0.013 | (0.006–0.031) | 0.013 | (0.008–0.042) | 0.304 | |||||||
aFriedman’s test.
bOne-way analysis of variance.
cBonferroni test.
The values with the non-Gaussian distribution are shown as medians and interquartile range. The values with the Gaussian distribution are shown as means and SDs.
T1: baseline (before the program); T2: immediately after the program; T3: 3 months after the program; IFN: interferon; IL: interleukin; TNF: tumor necrosis factor; hs-CRP: high-sensitivity C-reactive protein.
Autonomic nervous activity
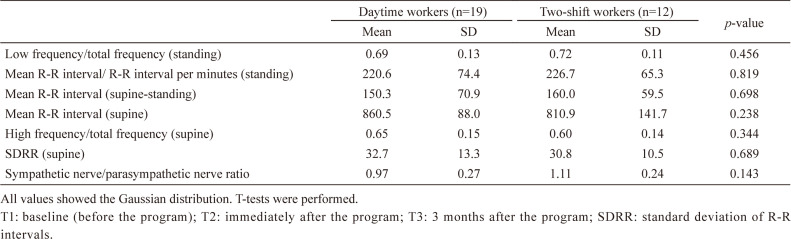
Table 3 shows medians, interquartile range, means, SD of autonomic nervous activity at T1, T2, and T3, and statistical comparisons among time points by either Friedman’s tests or one-way ANOVA. There were no significant differences among time points.
Table 3. Differences in autonomic nervous activity at T1, T2, and T3.
| T1 | T2 | T3 | p-value | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Median | Interquartile range | Mean | SD | Median | Interquartile range | Mean | SD | Median | Interquartile range | Mean | SD | ||
| Low frequency/total frequency (standing)a | 0.71 | (0.62–0.76) | 0.75 | (0.65–0.81) | 0.76 | (0.63–0.83) | 0.368 | ||||||
| Mean R-R interval/ R-R interval per minutes (standing)a | 223.4 | (172.7–273.0) | 236.4 | (179.4–261.6) | 225.9 | (180.2–263.0) | 0.542 | ||||||
| Mean R-R interval (supine-standing)a | 157.6 | (96.7–204.4) | 147.8 | (123.5–196.9) | 142.2 | (113.0–193.0) | 0.542 | ||||||
| Mean R-R interval (supine)b | 841.3 | 112.3 | 839.9 | 112.0 | 836.7 | 106.0 | 0.969 | ||||||
| High frequency/total frequency (supine)a | 0.62 | (0.53–0.71) | 0.65 | (0.50–0.77) | 0.68 | (0.54–0.74) | 0.798 | ||||||
| SDRR (supine)a | 29.2 | (23.7–41.7) | 32.7 | (24.8–40.5) | 32.8 | (24.7–42.6) | 0.968 | ||||||
| Sympathetic nerve/parasympathetic nerve ratiob | 1.02 | 0.26 | 1.08 | 0.28 | 1.05 | 0.25 | 0.286 | ||||||
aFriedman’s test.
bOne-way analysis of variance.
The values with the non-Gaussian distribution are shown as medians and interquartile range. The values with the Gaussian distribution are shown as means and SDs.
T1: baseline (before the program); T2: immediately after the program; T3: 3 months after the program; SDRR: standard deviation of R-R intervals.
Psychosocial job stress
Table 4 presents medians, interquartile range, means, SD of perceived psychosocial job stress, psychosocial and physical stress reactions, job satisfaction, and CES-D at T1, T2, and T3, and comparison among time points by either Friedman’s tests or one-way ANOVA. The physical stress response showed a significant difference among three-time points (p=0.048), however, with the pair-wise comparison, we did not observe any significant difference. CES-D score was marginally decreased at T2 (median=12.5) and T3 (median=12.0) compared to T1 (median=14.0) (p=0.077).
Table 4. Differences in evaluation of perceived job stress, job satisfaction, and depressive symptoms at T1, T2, and T3.
| Number of items | T1 | T2 | T3 | p-value | p-value(pair-wise)d | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Median | Interquartilerange | Mean | SD | α | Median | Interquartilerange | Mean | SD | α | Median | Interquartilerange | Mean | SD | α | ||||
| Psychosocial job stress | ||||||||||||||||||
| Quantitative workloadb, c | 3 | 8.7 | 1.6 | 0.73 | 8.9 | 1.6 | 0.68 | 8.8 | 1.9 | 0.61 | 0.618 | |||||||
| Qualitative workloada, c | 3 | 9 | (9.0–10.0) | 0.56 | 9 | (8.0–10.0) | 0.80 | 9 | (9.0–10.0) | 0.72 | 0.611 | |||||||
| Job controla | 3 | 7 | (6.0–9.0) | 0.61 | 7 | (6.0–8.0) | 0.72 | 7 | (6.0–8.0) | 0.67 | 0.946 | |||||||
| Interpersonal conflicta, c | 3 | 6 | (6.0–9.0) | 0.36 | 6 | (5.0–7.0) | 0.61 | 6 | (6.0–7.0) | 0.68 | 0.542 | |||||||
| Psychological and physical stress reactions | ||||||||||||||||||
| Vigora | 3 | 6 | (3.0–6.0) | 0.93 | 5 | (3.0–6.0) | 0.91 | 5 | (3.0–6.0) | 0.87 | 0.327 | |||||||
| Irritationa, c | 3 | 6 | (5.0–8.0) | 0.89 | 5 | (4.0–7.3) | 0.85 | 5 | (4.0–6.0) | 0.80 | 0.291 | |||||||
| Fatiguea, c | 3 | 7 | (6.0–9.0) | 0.90 | 7 | (5.0–9.0) | 0.92 | 6 | (5.0–9.0) | 0.93 | 0.192 | |||||||
| Anxietya, c | 3 | 5 | (4.0–7.0) | 0.79 | 5 | (3.0–7.0) | 0.82 | 4 | (3.0–6.0) | 0.24 | 0.209 | |||||||
| Physical stress responseb, c | 11 | 18.9 | 4.9 | 0.79 | 19 | 5.1 | 0.80 | 17.3 | 4.6 | 0.80 | 0.048 | T1 vs. T2: 1.000, | ||||||
| T1 vs. T3: 0.262, | ||||||||||||||||||
| T2 vs. T3: 0.071 | ||||||||||||||||||
| Job satisfactiona | 4 | 9 | (8.0–10.0) | 0.68 | 9 | (8.0–10.0) | 0.67 | 9 | (8.0–9.0) | 0.79 | 0.841 | |||||||
| CES-Da, c | 20 | 14 | (10.0–18.5) | 0.77 | 12.5 | (8.0–19.0) | 0.9 | 12 | (7.0–16.0) | 0.86 | 0.077 | |||||||
aFriedman’s test.
bOne-way analysis of variance.
cNegatively oriented.
dBonferroni test.
The values with the non-Gaussian distribution are shown as medians and interquartile range. The values with the Gaussian distribution are shown as means and SDs.
T1: baseline (before the program); T2: immediately after the program; T3: 3 months after the program; CES-D: Center for Epidemiologic Studies Depression Scale.
Discussion
The primary objective of this study was to determine whether a participatory workplace improvement program would decrease stress-related symptoms as evaluated by stress-related biomarkers, i.e., inflammatory markers and autonomic nervous activity, and self-reported stress as measured by the Brief Job Stress Questionnaire among hospital nurses. We evaluated its effect at baseline (T1), immediately after (T2), and three months after the end of the program (T3). As a result, we observed reductions in inflammatory markers at T2 and T3 compared to T1, although autonomic nervous activity and self-reported stress remained unchanged. The differences in inflammatory markers could not be explained by the difference in work shifts because we did not confirm statistical differences by work schedules. To our knowledge, this is one of the first studies to evaluate the effects of participatory intervention by introducing multiple biomarkers and self-reported stress at the same time.
Our initial hypothesis was that the current participatory program would exert beneficial effects on alleviating stress-related symptoms, i.e., a reduction of inflammatory markers, healthier autonomic nervous system balance, and a decrease of perceived job stress simultaneously. However, only a significant reduction in inflammatory markers was observed. There are several possible explanations for this unexpected finding. First, past studies have reported that participatory intervention is protective against the deterioration of mental health rather than improving it14, 40). According to a study by Tsutsumi et al., that tested the effects of participatory intervention aimed to improve mental health and job performance among blue-collar workers, participants allocated to the control group had poorer scores on mental health status after the 1 yr intervention period14). In contrast, participants allocated to the intervention group maintained the same level of mental health status, resulting in significant protective effects of the intervention. More recently, a study that examined the effects of participatory organizational-level workplace intervention aimed to reduce the level of unnecessary and unreasonable tasks for employees in pre-schools reported that the intervention group maintained the same level of unnecessary and unreasonable tasks after the 2 yr intervention period, whereas the control group yielded an increased level of those tasks40). Our results of maintaining a comparable level of self-reported stress before and after intervention are in accord with these results, although our finding needs to be validated by a better study design such as the randomized controlled trial.
Second, the intervention effect may not always appear at the same time when a different type of outcome is measured simultaneously41). According to a study which evaluated the effects of an art-based leadership development program, the intervention (art-based leadership) group exerted a protective effect on dehydroepiandrosterone-sulfate (DHEA-s) but not for the cortisol levels42). With regard to psychosocial factors in the same study, the intervention group had significant improvement in total mental health status, covert coping, and performance-based self-esteem scores than the conventional intervention (control) group, but scores on emotional exhaustion, sleep disturbances, and depressive symptoms did not show significant intervention effect. Similarly, a study by Uchiyama et al., which focused on improving the psychosocial workplace environment, reported that scores of coworker support and workplace goals significantly improved by intervention but not for mental health status after a 6-month intervention period16). Thus, the appearance of the intervention effect may differ by the timing of evaluation and the type of outcomes measured.
Third, because of the small sample size of this study, our analysis may have had less power to detect statistical significance. For example, we observed a borderline (p<0.10) significant decrease in depressive symptom (CES-D) scores before and after the intervention (Table 4); compared to CES-D score (median) of 14.0 at baseline, the CES-D score decreased to 12.5 immediately after the intervention and further decreased to 12.0 after the three-month from the intervention period. A borderline significant decrease in physical stress response score was also observed at T3 compared to T2. Further studies with a larger sample size are needed to confirm this speculation.
Multiple lines of evidence suggest that high job stress is associated with increased levels of inflammatory markers such as CRP, IL-6, and TNF-α25, 29). Such evidence supports that alleviating job stress may lead to a reduction in basal level of inflammatory markers. In this study, we found significant decreases of IFN-γ, IL-6, and IL-12/23p40 immediately after the termination of the intervention program and delayed decreases or lasting effect in IL-12/23p40 and IL-15 three months after the termination of the program. Although we cannot fully deny that the current finding was by chance or merely by seasonal/diurnal variation of inflammatory markers43,44,45), our result was in an expected direction that reduced job stress is associated with lower levels of inflammation in our body.
The results of our study may also be relevant to the findings that more regular/flexible schedules among nurses working under shift work are associated with healthier cardiovascular profile46). According to this quasi-experimental intervention study, which aimed to achieve regularity of shift schedules, i.e., fewer consecutive night shifts, more weekends off, and only 2 different types of shifts (day-evening or day-night), have led to an increase in high-density lipoprotein (HDL) cholesterol level and reduction of the total cholesterol and low-density lipoprotein (LDL) cholesterol levels after 6 months of intervention. Increasing regularity against shift work may have reduced job stress associated with shift work, and consequently lead to a healthier cardiovascular profile. Our results are partly in line with above results that reducing job stress is associated with healthier inflammatory/cardiovascular profiles.
Unlike inflammatory markers, we did not find significant changes in the measures of autonomic nervous activity. Several studies confirmed that acute psychological stress had a significant impact on HRV34, 35, 47), whereas some studies reported that chronic job stress does not associate with an increase of HRV48, 49). Because nurses are under high quantitative and qualitative workload in everyday settings, perceived chronic stress may be detected by inflammatory markers but not by autonomic nervous system activity50, 51).
Our study did not find significant decreases in subjective job stress measures as well. It could be construed that the amount of individual workload may have not been reduced by the intervention itself but those participating in the program may have felt increased job performance, organizational beliefs, role clarification, and social relationships as well as reducing illegitimate job tasks than before implementing intervention40, 41, 52). Such organization-focused measures may help understand the effects of intervention programs further.
Study strengths and limitations
The most obvious strength of this study was that we used stress-related biological measures, i.e., blood inflammatory markers and autonomic nervous activity simultaneously, to confirm the effects of the program. Given that most past studies examined the impact of workplace improvement intervention programs only by self-reported measures13, 14, 16, 53,54,55,56,57), it remained to be resolved whether intervention programs could exert beneficial effects on biological markers. In this way, we could prevent reporting bias as well as increase the validity of the results.
However, we must mention several limitations of this study. First, we had a small sample size (n=31), which may have hampered by reduced statistical power. With the small sample size at the initial point, we were not able to conduct a study setting a control group, i.e., applying a randomized controlled trial design. Thus, it leaves a question toward the generalization of the results. Second, because we placed the feasibility of intervention important, nurses carried out three actions, which made it difficult to identify which action had the strongest impact on biomarkers. Although we targeted only nurses at a single hospital, the hospital had multiple units/departments and we are not certain that those implementations were truly suitable for each unit/department. Also, we decided to conduct a 2-months intervention period through several discussions with the nursing representatives. A longer length of intervention may have given nurses more time to get used to the current intervention, leading to clearer differences in outcome measures. In addition, those actions were facilitated by head nurses in each unit/department, therefore, commitment to take these actions may vary by units/departments. Third, we could not confirm the tasks and demands that participants engaged in during the program. The operations and demands, i.e. number of patients, the severity of patients’ illness and disability, were more likely to be inconsistent over the program. In consideration of these limitations, we must carefully interpret that the decline of inflammatory markers may not be the direct effect of the participatory program as stated earlier. Finally, in the current study, participants were working under two-different shifts, i.e., daytime and 2-shift schedules. Although we measured objective markers when participants were under daytime schedule, we cannot conclusively deny that they were under different phase of diurnal rhythm affecting measured outcomes.
Conclusion
Although limitations need to be considered carefully when interpreting the results, we observed reducing effects of inflammatory markers after the participatory workplace improvement program targeting hospital nurses. This study suggests the importance of introducing objective measures to evaluate participatory workplace improvement program, in addition to subjective measures.
Conflicts of Interest
The authors do not have a conflict of interest to disclose.
Acknowledgments
We thank all nurses who participated in this study and made an effort to improve their workplace, and anyone who helped us carry out this study.
This study was supported by JSPS the Grant-in-Aid for Challenging Exploratory Research Grant (26671048), the Work-related Diseases Clinical Research Grant (160701-01, 170701-01, 180701-01) from the Ministry of Health, Labour and Welfare, Japan, and the Telecommunications Advancement Foundation, Japan.
Appendix 1
Differences in inflammatory markers at T1 between daytime and shift workers
Appendix 2
Differences in autonomic nervous activity at T1 between daytime and shift workers
Appendix 3
Differences in sleep hours at T1 between daytime and shift workers
References
- 1.American Nurses Association What is nursing? https://www.nursingworld.org/practice-policy/workforce/what-is-nursing/. Accessed August 16, 2020.
- 2.Japanese Nursing Association (2016) Nursing in Japan. https://www.nurse.or.jp/jna/english/pdf/nursing-in-japan2016.pdf. Accessed August 16, 2020.
- 3.Ito S, Fujita S, Seto K, Kitazawa T, Matsumoto K, Hasegawa T. (2014) Occupational stress among healthcare workers in Japan. Work 49, 225–34. [DOI] [PubMed] [Google Scholar]
- 4.Reith TP. (2018) Burnout in united states healthcare professionals: a narrative review. Cureus 10, e3681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jaracz M, Rosiak I, Bertrand-Bucińska A, Jaskulski M, Nieżurawska J, Borkowska A. (2017) Affective temperament, job stress and professional burnout in nurses and civil servants. PLoS One 12, e0176698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Molina-Praena J, Ramirez-Baena L, Gómez-Urquiza JL, Cañadas GR, De la Fuente EI, Cañadas-De la Fuente GA. (2018) Levels of burnout and risk factors in medical area nurses: a meta-analytic study. Int J Environ Res Public Health 15, 2800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Letvak S, Ruhm CJ, McCoy T. (2012) Depression in hospital-employed nurses. Clin Nurse Spec 26, 177–82. [DOI] [PubMed] [Google Scholar]
- 8.Japanese Nursing Association Emergency survey regarding overtime, night/shift work 2008. (in Japanese) https://www.nurse.or.jp/nursing/shuroanzen/jikan/kaeru.html. Accessed June 19, 2020.
- 9.Shimomitsu T, Kobayashi A, Nakahara T, Iwata N, Kawakami N, Tsutsumi A, Watanabe N, Ministry of Labour Health, Labour Sciences Research final report: Studies regarding improving mental health by workplace improvement (in Japanese) http://www.tmu-ph.ac/pdf/090716_03.pdf. Accessed June 9, 2020.
- 10.National Personnel Authority Japan. Procedure for workplace improvement 2016. (in Japanese) https://www.jinji.go.jp/kenkou_anzen/tebiki.pdf. Accessed July 20, 2017.
- 11.Yoshikawa T, Yoshikawa E, Tsuchiya M, Kobayashi Y, Shimazu A, Tsutsumi A, Odagiri Y, Kogi K, Kawakami N. (2013) Development of evidenced-based medicine guidelines for improving the workplace environment by means of primary job stress prevention. Jpn J Job Stress Res 20, 135–45. [Google Scholar]
- 12.Lamontagne AD, Keegel T, Louie AM, Ostry A, Landsbergis PA. (2007) A systematic review of the job-stress intervention evaluation literature, 1990–2005. Int J Occup Environ Health 13, 268–80. [DOI] [PubMed] [Google Scholar]
- 13.Kobayashi Y, Kaneyoshi A, Yokota A, Kawakami N. (2008) Effects of a worker participatory program for improving work environments on job stressors and mental health among workers: a controlled trial. J Occup Health 50, 455–70. [DOI] [PubMed] [Google Scholar]
- 14.Tsutsumi A, Nagami M, Yoshikawa T, Kogi K, Kawakami N. (2009) Participatory intervention for workplace improvements on mental health and job performance among blue-collar workers: a cluster randomized controlled trial. J Occup Environ Med 51, 554–63. [DOI] [PubMed] [Google Scholar]
- 15.Osatuke K, Leiter M, Belton L, Dyrenforth S, Ramsel D. (2013) Civility, respect and engagement at the workplace (CREW): a national organization development program at the department of veterans affairs. J Manage Policies Pract 1, 25–34. [Google Scholar]
- 16.Uchiyama A, Odagiri Y, Ohya Y, Takamiya T, Inoue S, Shimomitsu T. (2013) Effect on mental health of a participatory intervention to improve psychosocial work environment: a cluster randomized controlled trial among nurses. J Occup Health 55, 173–83. [DOI] [PubMed] [Google Scholar]
- 17.Magnavita N. (2018) Medical surveillance, continuous health promotion and a participatory intervention in a small company. Int J Environ Res Public Health 15, 662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lavoie-Tremblay M, Bourbonnais R, Viens C, Vézina M, Durand PJ, Rochette L. (2005) Improving the psychosocial work environment. J Adv Nurs 49, 655–64. [DOI] [PubMed] [Google Scholar]
- 19.Yoshikawa E. (2013) [Concept analysis of a participatory approach to occupational safety and health]. Sangyo Eiseigaku Zasshi 55, 45–52. [DOI] [PubMed] [Google Scholar]
- 20.Schneider A, Wehler M, Weigl M. (2019) Effects of work conditions on provider mental well-being and quality of care: a mixed-methods intervention study in the emergency department. BMC Emerg Med 19, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.de Looff PC, Cornet LJM, Embregts PJCM, Nijman HLI, Didden HCM. (2018) Associations of sympathetic and parasympathetic activity in job stress and burnout: a systematic review. PLoS One 13, e0205741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Elovainio M, Ferrie JE, Singh-Manoux A, Gimeno D, De Vogli R, Shipley M, Vahtera J, Brunner E, Marmot MG, Kivimäki M. (2010) Organisational justice and markers of inflammation: the Whitehall II study. Occup Environ Med 67, 78–83. [DOI] [PubMed] [Google Scholar]
- 23.Emeny R, Lacruz ME, Baumert J, Zierer A, von Eisenhart Rothe A, Autenrieth C, Herder C, Koenig W, Thorand B, Ladwig KH. (2012) Job strain associated CRP is mediated by leisure time physical activity: results from the MONICA/KORA study. Brain Behav Immun 26, 1077–84. [DOI] [PubMed] [Google Scholar]
- 24.Järvelin-Pasanen S, Sinikallio S, Tarvainen MP. (2018) Heart rate variability and occupational stress-systematic review. Ind Health 56, 500–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nakata A. (2012) Psychosocial job stress and immunity: a systematic review. Methods Mol Biol 934, 39–75. [DOI] [PubMed] [Google Scholar]
- 26.Nakata A, Irie M, Takahashi M. (2014) Source-specific social support and circulating inflammatory markers among white-collar employees. Ann Behav Med 47, 335–46. [DOI] [PubMed] [Google Scholar]
- 27.Segerstrom SC, Miller GE. (2004) Psychological stress and the human immune system: a meta-analytic study of 30 years of inquiry. Psychol Bull 130, 601–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Siegrist J, Li J. (2017) Work stress and altered biomarkers: a synthesis of findings based on the effort–reward imbalance model. Int J Environ Res Public Health 14, 1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Eguchi H, Watanabe K, Kawakami N, Ando E, Arima H, Asai Y, Inoue A, Inoue R, Iwanaga M, Imamura K, Kobayashi Y, Nishida N, Otsuka Y, Sakuraya A, Tsuno K, Shimazu A, Tsutsumi A. (2018) Psychosocial factors at work and inflammatory markers: protocol for a systematic review and meta-analysis. BMJ Open 8, e022612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Roos LE, Knight EL, Beauchamp KG, Berkman ET, Faraday K, Hyslop K, Fisher PA. (2017) Acute stress impairs inhibitory control based on individual differences in parasympathetic nervous system activity. Biol Psychol 125, 58–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hornung RW, Reed LD. (1990) Estimation of average concentration in the presence of nondetectable values. Appl Occup Environ Hyg 5, 46–51. [Google Scholar]
- 32.Collins SM, Karasek RA, Costas K. (2005) Job strain and autonomic indices of cardiovascular disease risk. Am J Ind Med 48, 182–93. [DOI] [PubMed] [Google Scholar]
- 33.Yanagihara N, Seki M, Nakano M, Hachisuga T, Goto Y. (2014) Inverse correlation between the standard deviation of R-R intervals in supine position and the simplified menopausal index in women with climacteric symptoms. Menopause 21, 669–72. [DOI] [PubMed] [Google Scholar]
- 34.Grantcharov PD, Boillat T, Elkabany S, Wac K, Rivas H. (2018) Acute mental stress and surgical performance. BJS Open 3, 119–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rodrigues S, Paiva JS, Dias D, Aleixo M, Filipe RM, Cunha JPS. (2018) Cognitive impact and psychophysiological effects of stress using a biomonitoring platform. Int J Environ Res Public Health 15, 1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shimomitsu T, Haratani T .(2000) Ministry of Labour Heisei 11th Health Labour Sciences Research report: Studies regarding improving mental health by workplace improvement; Reliability of the brief job stress questionnaire and the reference values, 126–38 (in Japanese) https://www.mhlw.go.jp/file/05-Shingikai-11201000-Roudoukijunkyoku-Soumuka/0000050919.pdf. Accessed June 25, 2020.
- 37.Hurrell JJJ, Jr, McLaney MA. (1988) Exposure to job stress—a new psychometric instrument. Scand J Work Environ Health 14Suppl 1, 27–8. [PubMed] [Google Scholar]
- 38.Nakata A, Takahashi M, Ikeda T, Haratani T, Hojou M, Araki S. (2007) Perceived job stress and sleep-related breathing disturbance in Japanese male workers. Soc Sci Med 64, 2520–32. [DOI] [PubMed] [Google Scholar]
- 39.Radloff LS. (1977) The CES-D scale: a self-report depression scale for research in the general population. Appl Psychol Meas 1, 385–401. [Google Scholar]
- 40.Framke E, Sørensen OH, Pedersen J, Rugulies R. (2018) Can illegitimate job tasks be reduced by a participatory organizational-level workplace intervention? Results of a cluster randomized controlled trial in Danish pre-schools. Scand J Work Environ Health 44, 219–23. [DOI] [PubMed] [Google Scholar]
- 41.Semmer NK. (2006) Job stress interventions and the organization of work. Scand J Work Environ Health 32, 515–27. [DOI] [PubMed] [Google Scholar]
- 42.Romanowska J, Larsson G, Eriksson M, Wikström BM, Westerlund H, Theorell T. (2011) Health effects on leaders and co-workers of an art-based leadership development program. Psychother Psychosom 80, 78–87. [DOI] [PubMed] [Google Scholar]
- 43.Itoh H, Mori I, Matsumoto Y, Maki S, Ogawa Y. (2012) Seasonal and inter-day variation in serum high-sensitivity C-reactive protein in Japanese male workers: a longitudinal study. Ind Health 50, 60–3. [DOI] [PubMed] [Google Scholar]
- 44.Sung KC. (2006) Seasonal variation of C-reactive protein in apparently healthy Koreans. Int J Cardiol 107, 338–42. [DOI] [PubMed] [Google Scholar]
- 45.Lange T, Dimitrov S, Born J. (2010) Effects of sleep and circadian rhythm on the human immune system. Ann N Y Acad Sci 1193, 48–59. [DOI] [PubMed] [Google Scholar]
- 46.Bøggild H, Jeppesen HJ. (2001) Intervention in shift scheduling and changes in biomarkers of heart disease in hospital wards. Scand J Work Environ Health 27, 87–96. [DOI] [PubMed] [Google Scholar]
- 47.Pulopulos MM, Vanderhasselt MA, De Raedt R. (2018) Association between changes in heart rate variability during the anticipation of a stressful situation and the stress-induced cortisol response. Psychoneuroendocrinology 94, 63–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lo EV, Wei YH, Hwang BF. (2020) Association between occupational burnout and heart rate variability: a pilot study in a high-tech company in Taiwan. Medicine (Baltimore) 99, e18630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kageyama T, Nishikido N, Kobayashi T, Kurokawa Y, Kaneko T, Kabuto M. (1998) Self-reported sleep quality, job stress, and daytime autonomic activities assessed in terms of short-term heart rate variability among male white-collar workers. Ind Health 36, 263–72. [DOI] [PubMed] [Google Scholar]
- 50.Asberg M, Nygren A, Leopardi R, Rylander G, Peterson U, Wilczek L, Källmén H, Ekstedt M, Akerstedt T, Lekander M, Ekman R. (2009) Novel biochemical markers of psychosocial stress in women. PLoS One 4, e3590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bellingrath S, Rohleder N, Kudielka BM. (2010) Healthy working school teachers with high effort-reward-imbalance and overcommitment show increased pro-inflammatory immune activity and a dampened innate immune defence. Brain Behav Immun 24, 1332–9. [DOI] [PubMed] [Google Scholar]
- 52.von Thiele Schwarz U, Lundmark R, Hasson H. (2016) The dynamic integrated evaluation model (DIEM): achieving sustainability in organizational intervention through a participatory evaluation approach. Stress Health 32, 285–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Takao S, Tsutsumi A, Nishiuchi K, Mineyama S, Kawakami N. (2006) Effects of the job stress education for supervisors on psychological distress and job performance among their immediate subordinates: a supervisor-based randomized controlled trial. J Occup Health 48, 494–503. [DOI] [PubMed] [Google Scholar]
- 54.Ruotsalainen JH, Verbeek JH, Mariné A, Serra C. (2015) Preventing occupational stress in healthcare workers. Cochrane Database Syst Rev 4, CD002892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Framke E, Sørensen OH, Pedersen J, Clausen T, Borg V, Rugulies R. (2019) Effect of a participatory organizational workplace intervention on workplace social capital: post-hoc results from a cluster randomized controlled trial. BMC Public Health 19, 693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Huijs JJJM, Houtman ILD, Taris TW, Blonk RWB. (2019) Effect of a participative action intervention program on reducing mental retirement. BMC Public Health 19, 194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bakhuys Roozeboom MC, Schelvis RMC, Houtman ILD, Wiezer NM, Bongers PM. (2020) Decreasing employees’ work stress by a participatory, organizational level work stress prevention approach: a multiple-case study in primary education. BMC Public Health 20, 676. [DOI] [PMC free article] [PubMed] [Google Scholar]