Abstract
Background
Previous studies, both in vitro and in vivo, have established that apatinib has anti-tumor properties. However, insufficient empirical evidence of the efficacy and safety of apatinib has been published for bone and soft tissue sarcoma, the reported results differing widely. Here, we conducted a meta-analysis to assess the efficacy and toxicity of apatinib for the treatment of bone and soft tissue sarcoma.
Methods
Pubmed, Medline, Web of Science, ScienceDirect, Ovid, Embase, Cochrane Library, Scopus, Vip (China), Cnki (China), Wanfang (China), and CBM (China) databases and literature from conferences were searched for studies of apatinib for the treatment of bone and soft tissue sarcomas, published from the inception of each database to Sep 1, 2020, without language restrictions. Primary outcomes were efficacy and toxicity of apatinib for the treatment of bone and soft tissue sarcoma, including treatment response, progression-free survival (PFS), and the incidence of adverse events. After extraction of data and methodological quality evaluation, random or fixed-effects models, as appropriate, were selected to calculate pooled effect estimates using R software (Version 3.4.1).
Results
A total of 21 studies with 827 participants were included in the present meta-analysis. The mean MINORS score was 10.48 ± 1.75 (range: 7-13), indicating evidence of moderate quality. Pooled outcomes indicated that overall response rate (ORR) and disease control rate (DCR) were 23.85% (95% CI: 18.47%-30.21%) and 79.16% (95% CI: 73.78%-83.68%), respectively. Median PFS ranged from 3.5 to 13.1 months, with a mean of 7.08 ± 2.98 months. Furthermore, the rates of PFS (PFR) after 1, 6, and 12 months were 99.31%, 44.90%, and 14.31%, respectively. Drug-related toxicity appears to be common in patients administered apatinib, for which hand-foot syndrome (41.13%), hypertension (36.15%), and fatigue (20.52%) ranked the top three most common adverse events. However, the incidence of grade 3-4 adverse events was relatively low and manageable.
Conclusions
Based on the best evidence currently available, apatinib demonstrates promising clinical efficacy and an acceptable safety profile for the treatment of advanced bone and soft tissue sarcoma, although additional high-quality clinical studies are required to further define its properties and toxicity.
Keywords: apatinib, osteosarcoma, soft tissue sarcoma, advanced, meta-analysis
Introduction
Bone and soft tissue sarcomas (STS) are rare malignant tumors originating from mesenchymal tissues that are present at all ages (1). They comprise more than 50 subtypes with different histological features and clinical behavior (2). Complete tumor resection with a sufficient surgical-margin is the most effective primary therapy for bone sarcoma and STS, alone or in combination with radiotherapy and/or chemotherapy. Although significant improvements in multidisciplinary approaches have prolonged the survival of patients with bone sarcoma or STS, it displays a poor response and unfavorable prognosis for advanced, refractory, metastatic, or relapsed sarcomas (3–5).
Tumor angiogenesis plays a crucial role in the initiation of tumors, and their progression, invasion, and metastasis (6, 7). Antiangiogenic therapies represent a promising anticancer treatment strategy, already extensively used for the treatment of solid tumors, aiming to inhibit tumor angiogenesis by targeting the key signaling pathways in which it is implicated (8–10).
Tumor-derived angiogenic factors have been identified as members of the vascular endothelial growth factor (VEGF) family, participating in the process of tumor angiogenesis via interaction with the corresponding receptor (VEGFR), suggesting that disruption of the VEGF/VEGFR signaling pathway could suppress the growth of tumors (11). A number of VEGFR inhibitors (e.g. sorafenib, axitinib, pazopanib, etc.) have been developed following two decades of laboratory research and clinical trials, demonstrating clinical effectiveness in a variety of cancers (12–15). Unfortunately, the anticipated anti-tumorigenic properties have not been observed in advanced bone and soft tissue sarcomas, compared with other malignancies (16, 17).
Apatinib (YN968D1) is a small molecule tyrosine kinase inhibitor (TKI) which selectively inhibits VEGFR2, thereby blocking the VEGF/VEGFR signaling pathway, and inhibiting tumor angiogenesis (18). It was approved by the Chinese FDA in December 2014 for the clinical treatment of advanced gastric cancer based on significant improvement in overall survival (OS) and progression-free survival (PFS) in a phase III clinical trial (19, 20). Over the past few years, apatinib has demonstrated broad-spectrum antitumor effects in numerous trials for multiple malignancies, including lung cancer, breast cancer, and hepatoma, etc. (21–24). Apatinib has also been shown to display anti-angiogenic properties in advanced sarcoma, and its clinical efficacy in bone and soft tissue sarcoma has also been recognized (25–27). However, due to the low incidence of bone and soft tissue sarcoma, published studies are often single-center, retrospective, non-randomized, and non-controlled, combined with a limited sample size. Therefore, strong supportive evidence of clinical efficacy and safety is lacking for apatinib for the treatment of bone and soft tissue sarcoma.
Materials and Methods
Sources of Data and Search for Studies
Pubmed, Embase, Medline, Web of Science, ScienceDirect, Ovid, Cochrane library, Vip (China), CNKI (China), Wanfang (China), and CBM (China) databases were searched to identify all relevant articles up to September 1, 2020 in all languages. Additional relevant literature was also searched, including from the most important conferences (American Society of Clinical Oncology, European Society of Medical Oncology, and Chinese Society of Clinical Oncology) and dissertations with available data were also retrieved. Finally, the reference lists of articles selected for the present review were checked for additional articles.
The literature retrieval strategy combined two parts, adapted for each specific database: 1) diagnosis: “sarcoma” OR “osteosarcoma” OR “soft tissue sarcoma” OR “bone sarcoma”; 2) agent name: “apatinib” OR “apatinib mesylate” OR “YN968D1”. The two parts were combined with “AND” using Boolean logic.
Study Selection
Studies fulfilling the following criteria were included: 1) Enrolled patients with histologically confirmed bone or soft tissue sarcoma; 2) Patients treated with oral apatinib at a daily dose of 250-750 mg; 3) Clinical efficiency outcomes were reported as tumor response, including complete response (CR), partial response (PR), stable disease (SD), and progressive disease (PD); 4) Adverse events (AEs) related to the treatment of all grades of tumor were reported.
Studies not adhering to the inclusion criteria were excluded, in addition to the following exclusion criteria: 1) Studies published elsewhere; 2) Animal experiments, laboratory research, reviews, meta-analyses or letters; 3) Articles with no accessible valid data; 4) Inclusion of fewer than 20 cases; 5) Participants received concomitant medication (e.g. chemotherapeutic agents) or surgery during the period of apatinib administration.
To avoid selection bias, particular studies were included or excluded where the two independent investigators agreed. A third reviewer resolved disagreements between them by discussion and formation of a consensus. The equations should be inserted in editable format from the equation editor.
Evaluation of the Quality of Literature
The Methodological Index for Non-randomized Studies (MINORS) (28) was used to assess the quality of the literature included in the review, based on the following 12 items: stated aim of the study; inclusion of consecutive patients; prospective collection of data; an endpoint appropriate to the aim of the study; unbiased evaluation of endpoints; follow-up period appropriate to the major endpoint; loss to follow up not exceeding 5%; prospective calculation of sample size; control group having the gold standard intervention; contemporary groups; baseline equivalence of groups; and statistical analyses adapted to study design. Both non-comparative and comparative studies were assessed using the first 8 items, while only comparative studies were also evaluated using the remaining 4. The individual categories described above scored 0-2 points, so the quality score of an article ranged from 0 to 24 points.
Data Extraction
Data were independently extracted by two investigators using a standardized Microsoft Excel spreadsheet, then fully reviewed by a third reviewer. The extracted information included: 1) basic information of article: first author, publication year, range of study years, and study type; 2) baseline characteristics of the enrolled patients: sample size, age, gender, histopathological diagnosis, disease state, and previous treatment; 3) apatinib administration: usage and dosage; 4) efficacy outcomes: complete response (CR), partial response (PR), stable disease (SD), progressive disease (PD), objective response rate (ORR, defined as CR+PR), disease control rate (DCR, defined CR+PR+SD), and progression-free survival rate (PFR); 5) incidence and grade of all AEs related to the administration of apatinib. Tables should be inserted at the end of the manuscript.
Statistics Analysis
Dichotomous outcomes (CR, PR, SD, PD, ORR, DCR, PFR, and AEs) were reported as counts and proportions. Statistical analysis was conducted using the “meta” package of R statistical software (version 3.6.2, MathSoft, Massachusetts). Inter-study heterogeneity was evaluated using both Q tet and I2 tests, where p <0.1 and I2 >50% denoted heterogeneity. A fixed-effects model was used for pooled analysis where inter-study heterogeneity existed, otherwise, a random-effects model was adopted. Sensitivity analysis was conducted (where more than 9 studies were included) to evaluate the stability and credibility of the pooled results by exclusion of any single study from the meta-analysis one-by-one. Subgroup analysis was performed in terms of tumor type for bone sarcoma versus soft tissue sarcoma (STS). Additionally, Egger’s regression test was performed for the evaluation of publication bias and small study bias.
Results
Study Selection
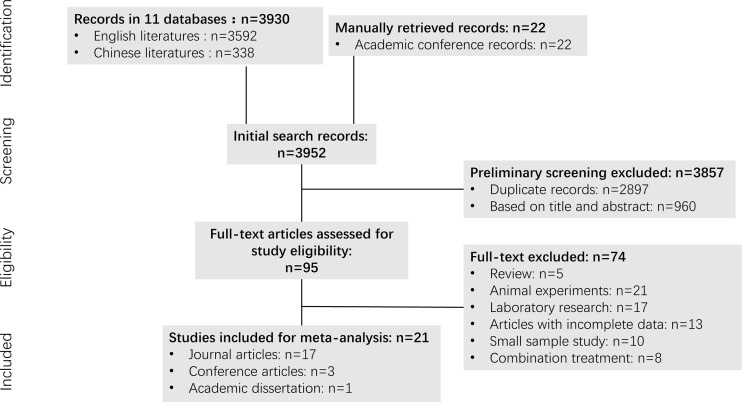
A flowchart of the study retrieval process is shown in Figure 1 . A total of 3,952 potential studies were initially identified from the initial search of the aforementioned databases prior to screening the titles and abstracts, of which 2,897 were duplicates. After removal of the duplicates and screening of the titles and abstracts, a total of 95 of the initial records remained, the full-text documents of which were assessed, from which 74 were excluded. Those included: 1) Review articles (N=5); 2) Animal experiments (N=21); 3) Laboratory research (N=17); 4) Articles with incomplete data (N=13); 5) Studies with a small sample size (N=10); and 6) Combination treatment (N=8). Ultimately, 21 studies (29–49) were selected for the meta-analysis that included 827 patients, of which 17 were journal articles, 3 were conference articles and one was a dissertation.
Figure 1.
The flowchart of literature selection on meta-analysis of apatinib for bone and soft tissue sarcoma.
Main Characteristics of Selected Studies
The principal characteristics of the included articles are displayed in Table 1 . The age of the enrolled patients ranged from 3 to 83 years with a gender ratio (female: male) of 1:1.47. Of the included studies, 7 investigated bone sarcoma patients, 5 studied STS patients, while the remaining 9 evaluated both. Apatinib was used in refractory bone sarcoma and STS, which possessed at least one of the following conditions: 1) distant metastasis (lungs or another organ); 2) postoperative recurrence; 3) a tumor that was unresectable; 4) failure of radiotherapy or chemotherapy. The initial dose of apatinib used in these studies was 425-750mg/d, except for children, for which an initial dose of 250 mg/d was used. The mean MINORS quality scores of the articles included in the analysis was 10.48 ± 1.75 (range: 7-13).
Table 1.
The main characteristics of included studies.
| Study ID | Study period | Sample Size | Gender | Age (range) | Diagnosis | Disease State | Treatment | Study Type | Quality Score |
| (female/male) | |||||||||
| Li et al. (29) | 2015.8-2017.3 | 23 | NA | NA | Bone and soft tissue sarcomas | Recurrence; Metastasis; Radiotherapy and chemotherapy failure |
500mg/d, po, qd | Journal articles | 10 |
| Yu et al. (30) | 2015.7-2016.11 | 26 | 8/18 | 54.5 | Osteosarcoma | Metastasis; Chemotherapy failure |
500mg/d, po, qd or 250mg/d, po, bid |
Conference articles | 10 |
| Zhou et al. (31) | 2015.5-2016.11 | 34 | NA | NA | Osteosarcoma | Advanced sarcoma; Lung metastasis (18 cases) |
250mg/d (5cases); 425mg/d (3 cases); 500mg/d (26 cases); dosage adjustment (8 cases) | Conference articles | 8 |
| Yang et al. (32) | 2015.9-2018.2 | 64 | NA | NA | Bone and soft tissue sarcomas | Stage IV; Chemotherapy failure |
500mg/d, po, qd | Conference articles | 8 |
| Zhu et al. (33) | 2015.9-2016.8 | 31 | 16/15 | 49.00 (3-71) |
Bone and soft tissue sarcoma | Stage IV; Metastasis; Relapse |
425mg/d, po, qd; 9 for temporary discontinuation; 7 for reduction | Journal articles | 10 |
| Lv et al. (34) | 2015.5-2017.5 | 28 | 10/18 | 54.5 | Soft tissue sarcoma | Stage IV; Chemotherapy failure |
500mg/d, po, qd | Journal articles | 7 |
| Xie et al. (35) | 2015.6-2016.12 | 44 | NA | NA | Bone and soft tissue sarcoma | Unresectable; Advanced sarcoma; Metastatic | 750mg (BSA>1.5); 500mg (BSA<1.5); 250mg (age<10) | Journal articles | 12 |
| Kang (36) | 2017.6-2018.9 | 21 | 9/12 | 38.3 (15-77) |
Bone and soft tissue sarcoma | Advanced sarcoma; Unresectable; Relapsed; Metastatic |
500mg/d, po, qd | Academic dissertation | 11 |
| Xie et al. (37) | 2016.3-2017.7 | 37 | 11/26 | 23.4 (16-62) |
Osteosarcoma | Unresectable; Metastatic Advanced sarcoma |
750mg/d, po, qd for 31 cases, 500mg/d, po, qd for 6 cases |
Journal articles | 13 |
| Zuo et al. (38) | 2016.1-2018.5 | 31 | 17/14 | 15-71 | Soft tissue sarcoma | Advanced sarcoma | 500mg/d, po, qd, for 10 cases; 425mg/d, po, qd, for 19 cases; 250mg/d, po, qd, for 2 cases |
Journal articles | 9 |
| Tian et al. (39) | 2016.1-2017.8 | 27 | 11/16 | 22.22 | Osteosarcoma | Chemotherapy failure; Unresectable lesions or distant metastasis | 750mg/d, po, qd, for 11 cases; 500mg/d, po, qd, for 16 cases |
Journal articles | 11 |
| Liao et al. (40) | 2015.9-2018.2 | 64 | 31/33 | 42.16 (11-83) |
Bone and soft tissue sarcoma | Stage IV; Chemotherapy failure |
500mg/d, po, qd | Journal articles | 11 |
| Yao et al. (41) | 2017.5-2018.7 | 45 | 16/29 | 43.02 (13-72) |
Bone and soft tissue sarcoma | Chemotherapy failure; Unresectable lesions or distant metastasis | 500mg/d, po, qd for 39 cases, 250 mg, po, qd for 6 cases |
Journal articles | 12 |
| He et al. (42) | 2016.9-2018.3 | 22 | NA | NA | Bone and soft tissue sarcoma | Stage IV | 500mg/d, po, qd | Journal articles | 8 |
| Gong et al. (43) | 2015.1-2018.7 | 27 | 12/15 | 30.20 (8-71) |
Soft tissue sarcoma | Unresectable lesions or distant metastasis | 500mg/d, po, qd, for 26 adults; 250mg/d, po, qd, for 1 child | Journal articles | 9 |
| Liu et al. (44) | 2015.3-2018.12 | 105 | 33/72 | 33.00 (18-58) |
Osteosarcoma | Chemotherapy failure; metastasis | 500mg/d, po, qd, for 57 patients; 750mg/d, po, qd, for 48 patients | Journal articles | 11 |
| Liu et al. (45) | 2015.9-2018.2 | 42 | 23/19 | 47.67 (14-83) |
Soft tissue sarcoma | Stage IV; Chemotherapy failure | 500mg/d, po, qd | Journal articles | 12 |
| Wang et al. (46) | 2016.3-2019.10 | 21 | 11/10 | 31.50 (8-71) |
Synovial sarcoma | Chemotherapy failure | 500mg/d, po, qd, for 20 adults; 250mg/d, po, qd, for 1 child | Journal articles | 12 |
| Tian et al. (47) | 2016.5-2019.2 | 68 | 31/37 | 35.88 | Bone and soft tissue sarcoma | Chemotherapy failure; Lung metastasis | 500mg/d, po, qd | Journal articles | 12 |
| Xie et al. (48) | 2009.10-2019.11 | 33 | 9/24 | 41.00 (17-72) |
Chondrosarcoma | Unresectable | 500mg/d, po, qd | Journal articles | 13 |
| Liao et al. (49) | 2015.9-2019.12 | 34 | 11/23 | 35.24 (11-73) |
Osteogenic sarcoma | Stage IV | 500mg/d, po, qd | Journal articles | 11 |
NA, not available; BSA, body surface area; po, peros (oral administration); qd, quaque die (once a day); bid, bis in die (twice a day).
Clinical Efficacy Response
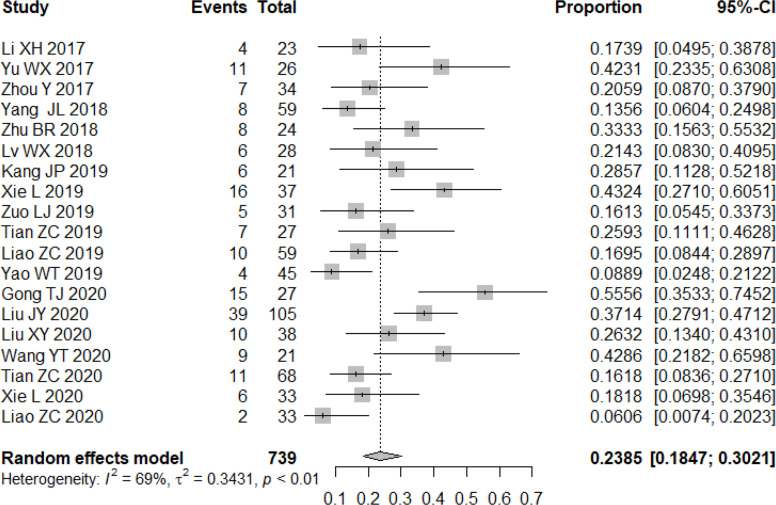
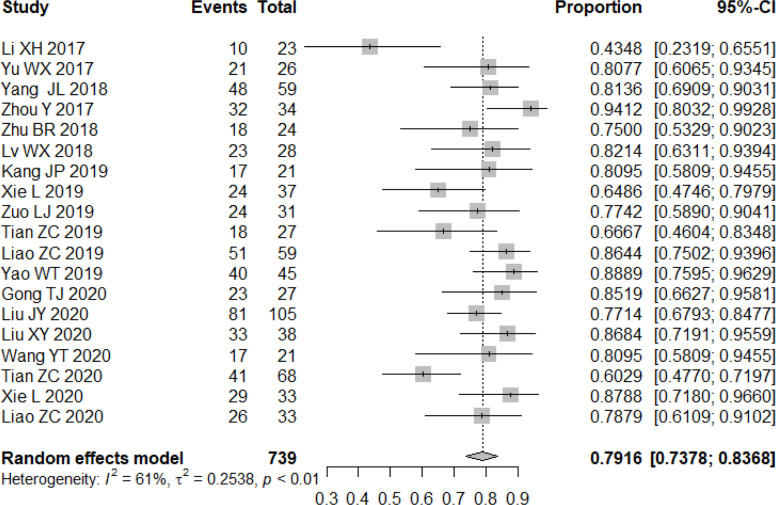
All studies included in the analysis reported the efficacy response of apatinib for bone and soft tissue sarcoma. A random-effects or fixed-effects model was selected for the meta-analysis depending on the calculated heterogeneity of data in the statistical analysis. Pooled results indicated that the ORR of apatinib for the treatment of bone and soft tissue sarcoma was 23.85% (95% confidence interval (CI): 18.47%-30.21%; heterogeneity: I2 = 69%, p<0.01) ( Figure 2 ), while DCR was 79.16% (95% CI: 73.78%-83.68%; heterogeneity: I2 = 61%, p<0.01) ( Figure 3 ). Pooled CR, PR, SD, and PD rate were 0.04% (95% CI: 0.00%-0.64%), 24.20% (95% CI: 18.67%-30.74%), 52.71% (95% CI: 44.26%-61.00%), and 21.98% (95% CI: 17.30%-27.93%), respectively. In summary, the majority of patients benefited from treatment with apatinib where efficacy for PR or SD was considered.
Figure 2.
Forest plot showing the ORR of apatinib in the treatment of bone and soft tissue sarcomas.
Figure 3.
Forest plot showing the DCR of apatinib in the treatment of bone and soft tissue sarcomas.
PFS
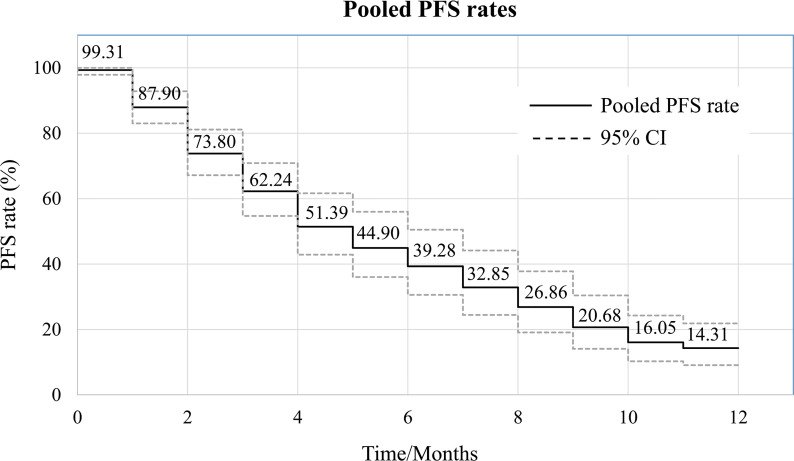
Of the 21 studies included in the analysis, 15 reported PFS for all patients after administration of apatinib. Median PFS ranged from 3.5 to 13.1 months, with a mean of 7.08 ± 2.98 months. Pooled PFR at various time points was calculated using the single rate meta-analysis method, from which a meta-pooled PFS curve was generated ( Figure 4 ). The results demonstrated that pooled PFRs in patients with bone and soft tissue sarcomas after 1, 6, and 12 months were 99.31% (95% CI: 97.87%-99.96%), 44.90% (95% CI: 36.03%-55.96%), and 14.31% (95% CI: 9.08% to 21.84%), respectively.
Figure 4.
Pooled survival curve regarding progression-free survival (PFS) (each PFR was synthesized using meta-analysis of single rate).
Incidence of Adverse Events
Adverse events were reported in 19 studies, involving 756 patients. The incidence of adverse events was extracted to systematically evaluate the safety profile of apatinib treatment ( Table 2 ). The severity of adverse events was graded using the National Institute-Common Terminology Criteria (CTCAE). The meta-analysis indicated that the three most commonly reported adverse events were hand-foot syndrome, hypertension, and fatigue, with an incidence of 41.13% (95% CI: 33.02%-49.49%), 36.15% (95% CI: 28.00%-44.73%), and 20.52% (95% CI: 13.14%-27.89%), respectively. Diarrhea, anorexia, and proteinuria were also relatively common, their incidence exceeding 10%. Furthermore, the incidence of grade 3-4 adverse events was significantly lower, of which hypertension (3.19%, 95% CI: 1.73%-4.64%) and hand-foot syndrome (2.71%, 95% CI: 1.36%-4.07%) were the two most common. It should be noted that none of the reported adverse events resulted in permanent morbidity, and they were alleviated by withdrawal, dose adjustment, or symptomatic treatment.
Table 2.
The pooled incidence of adverse events.
| Adverse event | Pooled Incidence (%) | 95%CI | Heterogeneity | Publication bias (p-value)* | |
|---|---|---|---|---|---|
| I2 (%) | p-value | ||||
| All grades | |||||
| Hand-foot syndrome | 41.13 | 33.02-49.49 | 81 | <0.01 | 0.532 |
| Hypertension | 36.15 | 28.00-44.73 | 83 | <0.01 | 0.466 |
| Fatigue | 20.52 | 13.14-27.89 | 90 | <0.01 | <0.01 |
| Diarrhea | 19.70 | 13.80-26.28 | 73 | <0.01 | 0.179 |
| Anorexia | 16.16 | 10.22-22.10 | 90 | <0.01 | <0.01 |
| Proteinuria | 13.13 | 6.14-21.97 | 90 | <0.01 | 0.175 |
| Mucositis | 6.23 | 2.36-11.42 | 80 | <0.01 | 0.045 |
| Rash | 5.46 | 0.94-12.45 | 89 | <0.01 | 0.615 |
| Hair hypopigmentation | 5.09 | 0.20-13.91 | 93 | <0.01 | 0.855 |
| Pneumothorax | 5.05 | 1.71-9.60 | 78 | <0.01 | 0.126 |
| Weight loss | 4.61 | 0.37-11.83 | 91 | <0.01 | 0.119 |
| Myelosuppression | 4.42 | 1.24-9.42 | 86 | <0.01 | 0.111 |
| Pain | 4.21 | 0.61-9.89 | 86 | <0.01 | <0.01 |
| Wound healing | 2.36 | 0.42-5.31 | 68 | <0.01 | 0.185 |
| Nausea | 1.80 | 0.00-5.66 | 82 | <0.01 | 0.043 |
| Elevated bilirubin | 1.01 | 0.01-2.95 | 52 | <0.01 | 0.460 |
| Elevated transaminase | 0.82 | 0.01-1.97 | 45 | 0.02 | 0.201 |
| Hypertriglyceridemia | 0.61 | 0.00-2.81 | 70 | <0.01 | 0.127 |
| Thrombocytopenia | 0.56 | 0.00-2.14 | 50 | <0.01 | 0.133 |
| Hematuresis | 0.40 | 0.00-1.33 | 32 | 0.10 | 0.048 |
| Coagulation disorder | 0.13 | 0.00-0.86 | 34 | 0.08 | 0.032 |
| Headache | 0.08 | 0.00-0.73 | 14 | 0.29 | 0.021 |
| Hoarseness | 0.00 | 0.00-0.39 | 0 | 0.99 | <0.01 |
| Grade 3-4 | |||||
| Hypertension | 3.19 | 1.73-4.64 | 45 | 0.01 | 0.524 |
| Hand-foot syndrome | 2.71 | 1.36-4.07 | 38 | 0.05 | 0.162 |
| Pneumothorax | 1.38 | 0.17-3.30 | 52 | <0.01 | 0.987 |
| Diarrhea | 1.32 | 0.43-2.53 | 0 | 0.62 | 0.451 |
| Proteinuria | 1.13 | 0.31-2.29 | 32 | 0.08 | 0.032 |
| Anorexia | 1.10 | 0.02-3.14 | 61 | <0.01 | 0.963 |
| Fatigue | 0.93 | 0.19-2.03 | 0 | 0.68 | 0.009 |
| Wound healing | 0.66 | 0.00-2.46 | 61 | <0.01 | 0.394 |
| Rash | 0.18 | 0.00-0.91 | 0 | 0.53 | 0.110 |
| Thrombocytopenia | 0.05 | 0.00-0.63 | 0 | 0.88 | 0.077 |
| Leukocytopenia | 0.02 | 0.00-0.50 | 0 | 0.93 | 0.068 |
| Anemia | 0.01 | 0.00-0.48 | 0 | 0.99 | <0.01 |
| Mucositis | 0.00 | 0.00-0.45 | 0 | 1.00 | 0.125 |
| Neutropenia | 0.00 | 0.00-0.38 | 0 | 0.97 | 0.035 |
| Coagulation disorder | 0.00 | 0.00-0.32 | 0 | 1.00 | <0.01 |
*Egger regression test for publication bias.
Subgroup analysis (Bone Sarcoma vs. STS)
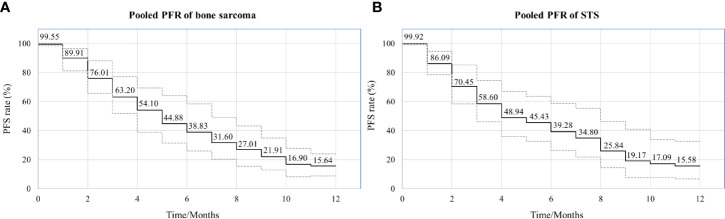
Both bone sarcoma and STS were included in the present meta-analysis, differing in their histological origins. Subgroup analysis was performed based on the category of tumor (bone sarcoma vs. STS). Eleven articles referred specifically to bone sarcoma and 8 to STS in the 21 articles included in the meta-analysis. The results of subgroup analysis indicated that the ORR and DCR of apatinib for bone sarcoma were 22.62% (95% CI: 12.19%-33.04%) and 76.94% (95% CI: 68.58%-83.61%), respectively, while for STS they were 29.03% (95% CI: 20.53%-41.06%) and 79.94% (95% CI: 68.94%-89.02%), respectively. Similarly, comparable values for pooled PFS were obtained in the two subgroups, with 6-month rates of PFS of 44.88% (95% CI: 31.45%-64.06%) and 45.43% (95% CI: 32.52%-63.46%) for the bone sarcoma and STS groups, respectively, and 12-month rates of PFS of 15.64% (95% CI: 8.75%-23.87%) and 15.58% (95% CI: 6.62%-32.46%) ( Figure 5 ). The incidence of adverse events was similar for the two subgroups. Details of the most common adverse events, such as hand-foot syndrome and hypertension, are listed in Table 3 .
Figure 5.
Pooled estimates of PFS between the two subgroups. (A) PFS curve in bone sarcoma; (B) PFS curve in STS.
Table 3.
The results of subgroup analysis (bone sarcoma vs. STS).
| Bone sarcoma | STS | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| N | Pooled rate (%) | 95%CI | Heterogeneity | N | Pooled rate (%) | 95%CI | Heterogeneity | |||
| I2 (%) | p-value | I2 (%) | p-value | |||||||
| Efficacy response | ||||||||||
| CR | 367 | 0.00 | 0.00-0.58 | 0 | 0.99 | 239 | 0.26 | 0.00-1.95 | 0 | 0.93 |
| PR | 367 | 21.60 | 12.62-32.04 | 78 | <0.01 | 239 | 28.48 | 20.36-39.83 | 62 | 0.01 |
| SD | 367 | 51.40 | 37.49-65.09 | 83 | <0.01 | 239 | 54.53 | 48.41-61.43 | 45 | 0.08 |
| PD | 367 | 25.11 | 17.76-35.52 | 71 | <.0.01 | 239 | 20.10 | 10.92-31.01 | 71 | <0.01 |
| ORR | 367 | 22.62 | 12.19-33.04 | 87 | <0.01 | 239 | 29.03 | 20.53-41.06 | 66 | <0.01 |
| DCR | 367 | 76.94 | 68.58-83.61 | 61 | 0.02 | 239 | 79.94 | 68.94-89.02 | 74 | <0.01 |
| Adverse event | ||||||||||
| All grades | ||||||||||
| HFS | 315 | 42.17 | 20.41-65.56 | 94 | <0.01 | 44.08 | 32.67-59.48 | 63 | 0.03 | |
| Hypertension | 315 | 28.75 | 13.35-43.98 | 94 | <0.01 | 35.99 | 15.99-58.71 | 87 | <0.01 | |
| Grade 3-4 | ||||||||||
| HFS | 315 | 5.01 | 1.36-8.66 | 51 | 0.05 | 188 | 4.27 | 1.46-8.07 | 48 | 0.09 |
| Hypertension | 315 | 2.79 | 0.67-4.90 | 49 | 0.06 | 188 | 4.23 | 0.00-8.89 | 58 | 0.04 |
CR, complete response; PR, partial response; SD, stable disease; PD, progressive disease; ORR, objective response rate; DCR, diease control rate; HFS, hand-foot syndrome.
Sensitivity Analysis
Sensitivity analysis was evaluated to assess the stability of the results and explore potential sources of heterogeneity. By excluding studies one-by-one, the recalculated results indicated that no substantial change in pooled ORR (maximum deviation: 5.41%), pooled DCR (maximum deviation: 1.38%), or pooled rate of PFS (maximum deviation: 0.26%, 4.94%, and 9.36% for 1, 6, and 12 months, respectively) was observed. The reanalyzed pooled incidence of predominant adverse events (incidence >10%) deviated within a range of 5.79% to 13.94%. Notably, the lower the incidence, the greater the deviation of the results, possibly due to the small sample size effect, that is, the majority of the studies included in the pooled analysis exhibited an incidence of 0. From this, we deduce that the pooled results of this meta-analysis were stable and reliable ( Table 4 ).
Table 4.
Sensitivity analysis of the main pooled results.
| Pooled results (%) | Sensitivity analysis (%) | Maximum deviation (%) | ||
|---|---|---|---|---|
| Min | Max | |||
| Efficacy response rate | ||||
| CR* | 0.04 | 0.01 | 0.06 | 75.00 |
| PR | 24.20 | 22.97 | 25.59 | 5.74 |
| SD | 52.71 | 51.45 | 54.68 | 3.74 |
| PD | 21.98 | 20.92 | 22.82 | 4.82 |
| ORR | 23.85 | 22.56 | 25.12 | 5.41 |
| DCR | 79.16 | 78.08 | 80.25 | 1.38 |
| PFS rate | ||||
| 1-month | 99.31 | 99.18 | 99.57 | 0.26 |
| 6-month | 44.90 | 42.68 | 46.59 | 4.94 |
| 12-month | 14.31 | 12.97 | 15.62 | 9.36 |
| The incidence of adverse events (All grades) | ||||
| HFS | 41.13 | 38.75 | 42.45 | 5.79 |
| Hypertension | 36.15 | 33.71 | 38.05 | 6.75 |
| Fatigue | 20.52 | 18.64 | 22.00 | 9.16 |
| Diarrhea | 19.70 | 18.30 | 20.49 | 7.11 |
| Anorexia | 16.16 | 14.67 | 17.60 | 9.22 |
| Proteinuria | 13.13 | 11.30 | 14.60 | 13.94 |
CR, complete response; PR, partial response; SD, stable disease; PD, progressive disease; ORR, objective response rate; DCR, diease control rate; HFS, hand-foot syndrome.
*An obvious deviation was detected because most of the included studies for pooled analysis had an incidence of 0.
Publication Bias
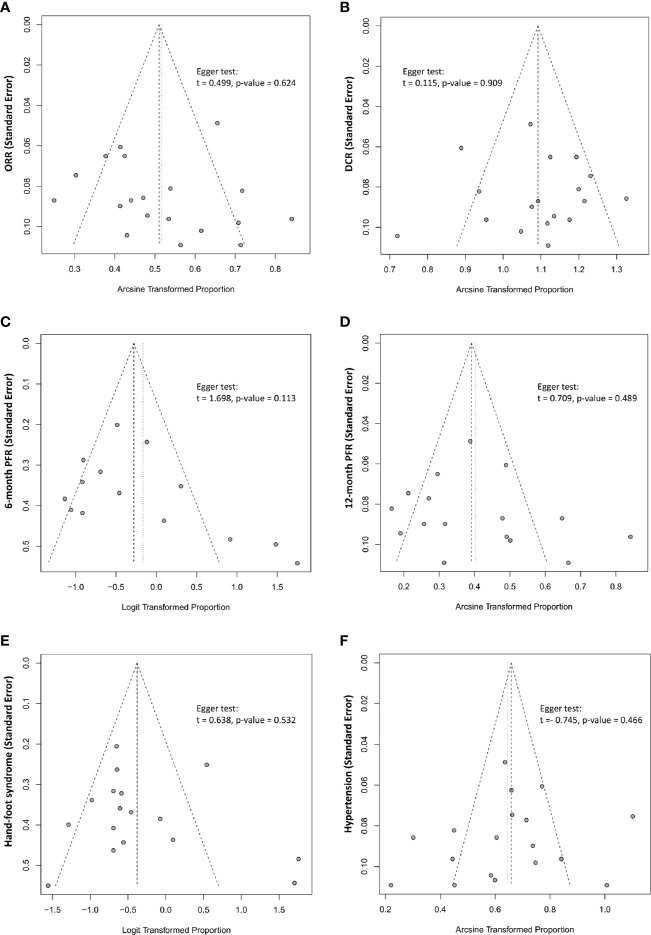
Tests for publication bias were performed on the primary outcome measures (ORR, DCR, 6-month PFS rate, 12-month PFS rate, and incidence of adverse events) in the present meta-analysis. Publication bias was assessed using funnel plots and an Egger’s regression test, finding no publication bias in the present meta-analysis ( Figure 6 ).
Figure 6.
The funnel plot and Egger linear regression test assessing publication bias. (A) ORR (p=0.624); (B) DCR (p=0.909); (C) 6-month PFR (p=0.113); (D) 12-month PFR (0.489); (E) AE-Hand-foot syndrome (0.532); (F) AE-Hypertension (p=0.466).
Discussion
Despite the development of chemotherapeutic agents, surgical techniques, and multi-modal treatments, long-term survival remains stagnant and poor for patients with advanced, refractory, metastatic, or relapsed bone and soft tissue sarcoma. Specifically, median overall survival (OS) was found to be less than 11 months and 12.5 months for advanced bone sarcomas and advanced STS, respectively (5). The 5-year OS rate is less than 30% and 45%, respectively. Because of the recognition of the critical role of tyrosine kinase in tumor angiogenesis, studies have focused on the development of TKIs as target-specific treatments in anti-tumor therapy by interfering with the VEGF/VEGFR signaling pathway. At least 20 TKIs (sorafenib, sunitinib, cediranib, pazopanib, regorafenib, imatinib, etc.) have been approved as anti-tumor agents by the FDA, some exhibiting promising efficacy with complications that are tolerable for the treatment of advanced diseases, such as gastric cancer (GC), hepatocellular carcinoma (HCC), non-small-cell lung carcinoma (NSCLC), colorectal cancer (CRC), and ovarian cancer (OC), etc.
Multiple preclinical studies and clinical trials have indicated that apatinib may act as a novel and promising VEGFR2-TKI for use in the treatment of bone and soft tissue sarcoma (18, 22, 23, 27). Its antitumor properties include vascular normalization, tumor regression (50), reversal of multidrug resistance (18), tumor microenvironment (TME) optimization (51), and enhancement of antitumor immunity (52). There are inconsistencies in clinical outcomes and complications in the reported studies, however. Combining the clinical studies using a meta-analysis indicated that pooled ORR and DCR for the treatment of bone and soft tissue sarcoma using apatinib was 23.85% (95% CI: 18.47%-30.21%) and 79.16% (95% CI: 73.78%-83.68%), respectively. Such efficacy is comparable or even superior to other TKIs, including sorafenib, pazopanib, sunitinib, regorafenib, and anlotinib, although no direct comparative studies have yet been conducted ( Table 5 ) (16, 53–70). It is noteworthy that pazopanib has been approved by the US FDA for the treatment of advanced STS as a second or third-line therapy. In a phase III clinical trial of pazopanib for advanced STS (PALETTE), the ORR and DCR were 5.69% (14/246) and 76.83% (189/246), respectively, but 10.57% (13/123) and 38.21% (47/123) in a real-world study (65). Therefore, for this comparison, apatinib represents among the most promising anti-angiogenic agents available for use as the preferable treatment option for advanced bone and soft tissue sarcoma.
Table 5.
The main anti-angiogenic TKIs for bone and soft tissue sarcoma.
| Compound | Targets | Refs | Histological subtypes | Sample size (n) | Clinical outcomes* | |||
|---|---|---|---|---|---|---|---|---|
| ORR | DCR | PFS (mo) | PFR-6 | |||||
| Apatinib | Mainly VEGFR2 | Pooled results of the present meta-analysis | Multiple STSs | 827 | 23.85% | 79.16% | 7.08 ± 2.98 | 44.90% |
| Sorafenib | VEGFR2, VEGFR3, PDGFR-α, PDGFR-β, c-kit, RET, RAF, FLT-3, FGFR-1 | Chevreau et al. (53) Grignani et al. (16) von Mehren et al. (54) Pacey et al. (55) Santoro et al. (56) |
EHE Osteosarcoma Multiple STSs Multiple STSs Multiple STSs |
15 35 38 21 101 |
13.33% 14.29% 0% 14.29% 14.47% |
84.62% 48.57% 37.84% 57.14% 47.36% |
6.0 4.0 3.0 / 4.2 |
38.46% 17% 32% 0% 35% |
| Sunitinib | VEGFR1, VEGFR2, VEGFR3, PDGFR-α, PDGFR-β, c-kit, RET, FLT-3, FGFR-1 | George et al. (57) Jo et al. (58) |
Multiple STSs AF |
48 19 |
2.08% 26.32% |
22.92% 68.42% |
1.8 / |
14.5% / |
| Cediranib | VEGFR1, VEGFR2, PDGFR-α, PDGFR-β, c-kit, FLT-3 | Kummar et al. (59) Judson et al. (60) |
ASPS ASPS |
46 32 |
34.88% 19.35% |
95.35% / |
/ 10.1 |
83.72% 59.38% |
| Pazopanib | VEGFR1, VEGFR2, VEGFR3, PDGFR-α, PDGFR-β, c-kit, FGFR-1 | Martin-Broto et al. (61) Samuels et al. (62) Sleijfer et al. (63) Stacchiotti et al. (64) van der Graaf et al. (65) |
SFT Liposarcoma Liposarcoma; Leiomyosarcoma; Synovialosarcoma; Others EMC Non-adipocytic STS |
36 41 19; 42; 38; 43 26 246 |
6% 2% 0%; 2.38%; 13.16%; 6.98% 18.18% 5.69% |
28.57% 43.9% / / / / 90.9% 72.36% |
5.6 4.4 2.6; 3.0; 5.3; 3.0 19.4 4.6 |
/ 39% / / / / / 25.61% |
| Regorafenib | VEGFR1, VEGFR2, VEGFR3, PDGFR-β, c-kit, RET, RAF | Mir et al. (66) Duffaud et al. (67) Davis et al. (68) |
Liposarcoma; leiomyosarcoma; Synovial sarcoma; Others Osteosarcoma Osteosarcoma |
20; 28; 13; 28 26 22 |
0%; 0% 7.69%; 10.71% 7.69% 13.6% |
45%; 85.71%; 84.61%; 75% 75% 65.38% / |
1.1; 3.7; 5.6; 2.9 4.1 3.6 |
20%; 21%; 38%; 43% 34.62% 30% |
| Axitinib | VEGFR1, VEGFR2, VEGFR3, PDGFR-α, PDGFR-β, c-kit | Stacchiotti et al. (69) | SFT | 17 | 5.9% | 88.2% | 9.4 | M-SFT:69.2% D-SFT:25.0% |
| Anlotinib | VEGFR1, VEGFR2, VEGFR-3, PDGFR-α, PDGFR-β, c-kit, FGFR-1 | Chi et al. (70) | Multiple STSs | 166 | 12.65% | 74% | 5.6 | 30.72% |
ORR, objective response rate; DCR, diease control rate; PFS, progression-free survival; PFR, PFS rate; EHE, epithelioid hemangioendothelioma; AF, Aggressive fibromatosis; ASPS, alveolar soft tissue part sarcoma; SFT, solitary fibrous tumour; EMC, extraskeletal myxoid chondrosarcoma; D-SFT, ‘dedifferentiated’ SFT; M-SFT, ‘malignant’ SFT.
*The clinical efficacy response was assessed based on RECIST criteria.
The current data indicate that the median PFS for patients that have received apatinib averaged 7.08 ± 2.98 mos (range: 3.5-13.1 mos), considered favorable in comparison with other TKI monotherapies (Sorafenib: 3.0-6.0 mos; Sunitinib: 1.8 mos; Pazopanib: 3.0-5.6 mos; Regorafenib: 1.1-5.6 mos; Anlotinib: 5.6 mos), although there is a lack of direct pairwise comparisons. Similarly, the pooled 6-month PFR (44.90%) for apatinib was also higher than other TKIs (17-43%) ( Table 5 ). However, it should be noted that the pooled efficacy response of apatinib in the present meta-analysis was lower than that of other TKIs in specific histological sarcoma subtypes. In a phase II clinical trial of cediranib (30 mg) for patients with metastatic or nonresectable alveolar soft part sarcoma (ASPS), the efficacy response was encouraging, with the ORR and DCR found to be 34.88% and 95.35%, respectively, with a 6-month PFR of 83.72% (59). Furthermore, a number of studies also confirmed an enhanced anti-tumor response for ASPS, achieving a PFS in excess of 10 months (60). These inconsistencies in clinical results of different sarcoma subgroups may be partially due to tumor heterogeneity, especially tumor vascularity which has been shown to be closely associated with the effectiveness of anti-VEGFR therapy. Apatinib also achieved better clinical outcomes when treating vascular-rich sarcomas such as ASPS (71). To further explore the therapeutic efficacy of apatinib in distinct sarcoma subtypes, a subgroup meta-analysis was conducted for two separate subgroups: bone sarcoma and STS. Regrettably, no substantial difference was identified in the present analysis. Therefore, subsequent research strategies should focus on a single sarcoma and conduct head-to-head randomized controlled clinical trials to identify the influence of histological type on the effectiveness of antiangiogenic therapy.
In terms of toxicity, adverse events appear to be common in patients that have been administered apatinib, and are, to a certain extent, the most common reason for drug withdrawal. Hand-foot syndrome (41.13%), hypertension (36.15%), and fatigue (20.52%) are the three most common adverse reactions. Comparatively, the incidence of grade 3-4 adverse events was relatively low, with the most frequently reported events being hypertension (3.19%) and hand-foot syndrome (2.71%). The pooled drug-related toxicity obtained in this meta-analysis is consistent with previous experience of apatinib in other refractory malignancies, such as gastric cancer, non-small cell lung carcinoma, and breast cancer (20, 21, 23, 72). However, notably, all such toxicities reported in this study were controllable, and able to be reduced by the adjustment of dose, withdrawal or symptomatic treatment. It has been reported that the toxicity of apatinib is positively correlated with the dose and duration of medication. But this conclusion needs to be further verified for bone and soft tissue sarcoma.
We acknowledge that the study has limitations. Firstly, there was a lack of high-level evidence from randomized clinical trials that had a large number of patients, hence influencing the overall outcomes of the study. Additionally, there was considerable heterogeneity across the subtypes of bone and soft tissue sarcomas. Although subgroup analysis was conducted to better understand the differences in efficacy or safety of apatinib between bone sarcoma and STS, no positive results were obtained. Further research into specific histological subtypes of sarcomas is required for a complete analysis of tumor heterogeneity. Lastly, the study only included a review of apatinib monotherapy in clinical applications, however, apatinib combination therapy (e.g. apatinib plus chemotherapy, or apatinib plus targeted therapy) is also common, and their clinical efficacy and safety also require confirmation in additional clinical trials.
Conclusion
Using the best evidence currently available, the results of the present meta-analysis indicate that apatinib has a beneficial influence in bone and soft tissue sarcoma, with favorable ORR, DCR, PFS, and PFR. Manifestations of toxicity are common during the administration of apatinib, although the majority are grade 1-2 and largely manageable by clinical intervention. Apatinib may thus benefit sarcoma patients, with favorable efficacy and an acceptable safety profile, although further high-quality clinical studies are required to further define its activity and toxicity in specific single subtype sarcoma.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding authors.
Author Contributions
ZL: Literature retrieval, data extraction, and article writing. MH and KL: Literature retrieval, data extraction, and literature quality evaluation. ML and JL: Data verification and literature quality evaluation. HZ: Statistical analysis. ZW: Study design and quality supervision. YL: Study design, statistical analysis, and manuscript modification. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- 1. Jo VY, Doyle LA. Refinements in Sarcoma Classification in the Current 2013 World Health Organization Classification of Tumours of Soft Tissue and Bone. Surg Oncol Clin N Am (2016) 25(4):621–43. 10.1016/j.soc.2016.05.001 [DOI] [PubMed] [Google Scholar]
- 2. Ferrari A, Dirksen U, Bielack S. Sarcomas of Soft Tissue and Bone. Prog Tumor Res (2016) 43:128–41. 10.1159/000447083 [DOI] [PubMed] [Google Scholar]
- 3. van Maldegem AM, Bhosale A, Gelderblom HJ, Hogendoorn PC, Hassan AB. Comprehensive analysis of published phase I/II clinical trials between 1990-2010 in osteosarcoma and Ewing sarcoma confirms limited outcomes and need for translational investment. Clin Sarcoma Res (2012) 2(1):5. 10.1186/2045-3329-2-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lagmay JP, Krailo MD, Dang H, Kim A, Hawkins DS, Beaty OR, et al. Outcome of Patients With Recurrent Osteosarcoma Enrolled in Seven Phase II Trials Through Children’s Cancer Group, Pediatric Oncology Group, and Children’s Oncology Group: Learning From the Past to Move Forward. J Clin Oncol (2016) 34(25):3031–8. 10.1200/JCO.2015.65.5381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Nakamura T, Tsukushi S, Asanuma K, Katagiri H, Ikuta K, Nagano A, et al. The clinical outcome of eribulin treatment in Japanese patients with advanced soft tissue sarcoma: a Tokai Musculoskeletal Oncology Consortium study. Clin Exp Metastasis (2019) 36(4):343–50. 10.1007/s10585-019-09980-3 [DOI] [PubMed] [Google Scholar]
- 6. Folkman J. Role of angiogenesis in tumor growth and metastasis. Semin Oncol (2002) 29(6 Suppl 16):15–8. 10.1053/sonc.2002.37263 [DOI] [PubMed] [Google Scholar]
- 7. Ollauri-Ibáñez C, Núñez-Gómez E, Egido-Turrión C, Silva-Sousa L, Díaz-Rodríguez E, Rodríguez-Barbero A, et al. Continuous endoglin (CD105) overexpression disrupts angiogenesis and facilitates tumor cell metastasis. Angiogenesis (2020) 23(2):231–47. 10.1007/s10456-019-09703-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Rust R, Gantner C, Schwab ME. Pro- and antiangiogenic therapies: current status and clinical implications. FASEB J (2019) 33(1):34–48. 10.1096/fj.201800640RR [DOI] [PubMed] [Google Scholar]
- 9. Askoxylakis V, Arvanitis CD, Wong C, Ferraro GB, Jain RK. Emerging strategies for delivering antiangiogenic therapies to primary and metastatic brain tumors. Adv Drug Deliv Rev (2017) 119:159–74. 10.1016/j.addr.2017.06.011 [DOI] [PubMed] [Google Scholar]
- 10. Moserle L, Jiménez-Valerio G, Casanovas O. Antiangiogenic therapies: going beyond their limits. Cancer Discov (2014) 4(1):31–41. 10.1158/2159-8290.CD-13-0199 [DOI] [PubMed] [Google Scholar]
- 11. Ellis LM, Hicklin DJ. VEGF-targeted therapy: mechanisms of anti-tumour activity. Nat Rev Cancer (2008) 8(8):579–91. 10.1038/nrc2403 [DOI] [PubMed] [Google Scholar]
- 12. Atkins MB, Plimack ER, Puzanov I, Fishman MN, McDermott DF, Cho DC, et al. Axitinib in combination with pembrolizumab in patients with advanced renal cell cancer: a non-randomised, open-label, dose-finding, and dose-expansion phase 1b trial. Lancet Oncol (2018) 19(3):405–15. 10.1016/S1470-2045(18)30081-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wrighton KH. Desmoid tumours stalled by sorafenib. Nat Rev Clin Oncol (2019) 16(4):209. 10.1038/s41571-019-0172-x [DOI] [PubMed] [Google Scholar]
- 14. Cortes JE, Kim DW, Pinilla-Ibarz J, le Coutre PD, Paquette R, Chuah C, et al. Ponatinib efficacy and safety in Philadelphia chromosome-positive leukemia: final 5-year results of the phase 2 PACE trial. Blood (2018) 132(4):393–404. 10.1182/blood-2016-09-739086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hellerstedt BA, Vogelzang NJ, Kluger HM, Yasenchak CA, Aftab DT, Ramies DA, et al. Results of a Phase II Placebo-controlled Randomized Discontinuation Trial of Cabozantinib in Patients with Non-small-cell Lung Carcinoma. Clin Lung Cancer (2019) 20(2):74–81. 10.1016/j.cllc.2018.10.006 [DOI] [PubMed] [Google Scholar]
- 16. Grignani G, Palmerini E, Dileo P, Asaftei SD, D’Ambrosio L, Pignochino Y, et al. A phase II trial of sorafenib in relapsed and unresectable high-grade osteosarcoma after failure of standard multimodal therapy: an Italian Sarcoma Group study. Ann Oncol (2012) 23(2):508–16. 10.1093/annonc/mdr151 [DOI] [PubMed] [Google Scholar]
- 17. Kim A, Widemann BC, Krailo M, Jayaprakash N, Fox E, Weigel B, et al. Phase 2 trial of sorafenib in children and young adults with refractory solid tumors: A report from the Children’s Oncology Group. Pediatr Blood Cancer (2015) 62(9):1562–6. 10.1002/pbc.25548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Mi YJ, Liang YJ, Huang HB, Zhao HY, Wu CP, Wang F, et al. Apatinib (YN968D1) reverses multidrug resistance by inhibiting the efflux function of multiple ATP-binding cassette transporters. Cancer Res (2010) 70(20):7981–91. 10.1158/0008-5472.CAN-10-0111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Mahipal A, Choi M, Kim R. Second-Line Treatment of Advanced Gastric Cancer: Where Do We Stand? J Natl Compr Canc Netw (2015) 13(10):1281–91, 1292. 10.6004/jnccn.2015.0154 [DOI] [PubMed] [Google Scholar]
- 20. Cheng H, Sun A, Guo Q, Zhang Y. Efficacy and safety of apatinib combined with chemotherapy for the treatment of advanced gastric cancer in the Chinese population: a systematic review and meta-analysis. Drug Des Devel Ther (2018) 12:2173–83. 10.2147/DDDT.S170678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Li F, Zhu T, Cao B, Wang J, Liang L. Apatinib enhances antitumour activity of EGFR-TKIs in non-small cell lung cancer with EGFR-TKI resistance. Eur J Cancer (2017) 84:184–92. 10.1016/j.ejca.2017.07.037 [DOI] [PubMed] [Google Scholar]
- 22. Xue JM, Astère M, Zhong MX, Lin H, Shen J, Zhu YX. Efficacy and safety of apatinib treatment for gastric cancer, hepatocellular carcinoma and non-small cell lung cancer: a meta-analysis. Onco Targets Ther (2018) 11:6119–28. 10.2147/OTT.S172717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Scott LJ. Apatinib: A Review in Advanced Gastric Cancer and Other Advanced Cancers. Drugs (2018) 78(7):747–58. 10.1007/s40265-018-0903-9 [DOI] [PubMed] [Google Scholar]
- 24. Liu J, Liu Q, Li Y, Li Q, Su F, Yao H, et al. Efficacy and safety of camrelizumab combined with apatinib in advanced triple-negative breast cancer: an open-label phase II trial. J Immunother Cancer (2020) 8(1):e000696. 10.1136/jitc-2020-000696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Liu K, Ren T, Huang Y, Sun K, Bao X, Wang S, et al. Apatinib promotes autophagy and apoptosis through VEGFR2/STAT3/BCL-2 signaling in osteosarcoma. Cell Death Dis (2017) 8(8):e3015. 10.1038/cddis.2017.422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Zhang D, Zhang C, Guo Q. A Case Report of Primary Pulmonary Synovial Sarcoma with Postoperative Multiple Metastases Treated with Apatinib. Zhongguo Fei Ai Za Zhi (2018) 21(11):880–4. 10.3779/j.issn.1009-3419.2018.11.11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Yang L, Liu L, Han B, Han W, Zhao M. Apatinib treatment for KIT- and KDR-amplified angiosarcoma: a case report. BMC Cancer (2018) 18(1):618. 10.1186/s12885-018-4523-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Slim K, Nini E, Forestier D, Kwiatkowski F, Panis Y, Chipponi J. Methodological index for non-randomized studies (minors): development and validation of a new instrument. Anz J Surg (2003) 73(9):712–6. 10.1046/j.1445-2197.2003.02748.x [DOI] [PubMed] [Google Scholar]
- 29. Li X, Yu X. Short term efficacy of apatinib in the treatment of recurrent and metastatic sarcoma. China Health Care Nutr (2017) 27(28):100. 10.3969/j.issn.1004-7484.2017.28.124 [DOI] [Google Scholar]
- 30. Yu WX, Yu XC, Wu SJ, Tang LN, Zheng SE, Zhou Y. Apatinib for patients with unresectable high-grade osteosarcoma progressing after standard chemotherapy: A multi-center retrospectively study. J Clin Oncol (2017) 35(suppl 15):11031. 10.1200/JCO.2017.35.15_suppl.11031 [DOI] [Google Scholar]
- 31. Zhou Y, Tang F, Min L, Zhang W, Shi R, Luo Y. A preliminary study of apatinib in chinese patients with osteosarcoma. J Clin Oncol (2017) 35(suppl 15):e22500. 10.1200/JCO.2017.35.15_suppl.e22500 [DOI] [Google Scholar]
- 32. Yang J, Yang Y. Efficacy and Safety of Apatinib in Stage Four Bone and Soft Tissue Sarcomas. Yang J, Yang Y, editors. Chicago, USA: American Association for Cancer Research (AACR) (2018). [Google Scholar]
- 33. Zhu B, Li J, Xie Q, Diao L, Gai L, Yang W. Efficacy and safety of apatinib monotherapy in advanced bone and soft tissue sarcoma: an observational study. Cancer Biol Ther (2018) 19(3):198–204. 10.1080/15384047.2017.1416275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Lv WX, Yuan MQ, Shi Z, Zhao YZ, Yang YS, Zhong HJ. Efficacy and Safety of Apatinib in the Treatment of 28 Patients with Advanced Refractory Soft Tissue Sarcomas. J Chin Oncol (2018) 24(6):592–6. 10.11735/j.issn.1671-170X.2018.06.B012 [DOI] [Google Scholar]
- 35. Xie L, Guo W, Wang Y, Yan T, Ji T, Xu J. Apatinib for advanced sarcoma: results from multiple institutions’ off-label use in China. BMC Cancer (2018) 18(1):396. 10.1186/s12885-018-4303-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kang JP. Clinical application of apatinib in the treatment of advanced bone and soft tissue sarcoma. Kunming: Kunming Medical University; (2019). [Google Scholar]
- 37. Xie L, Xu J, Sun X, Tang X, Yan T, Yang R, et al. Apatinib for Advanced Osteosarcoma after Failure of Standard Multimodal Therapy: An Open Label Phase II Clinical Trial. Oncologist (2019) 24(7). 10.1634/theoncologist.2018-0542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Zuo LJ, Liu WL, Li R, Wang YH, Yang YQ, Liu Z, et al. The clinical efficacy and safety of apatinib in the treatment of advanced soft tissue sarcoma. Oncol Progress (2019) 17(4):427–30. 10.11877/j.issn.1672-1535.2019.17.04.15 [DOI] [Google Scholar]
- 39. Tian Z, Liu H, Zhang F, Li L, Du X, Li C, et al. Retrospective review of the activity and safety of apatinib and anlotinib in patients with advanced osteosarcoma and soft tissue sarcoma. Invest New Drug (2020) 38(5):1559–69. 10.1007/s10637-020-00912-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Liao Z, Li F, Zhang C, Zhu L, Shi Y, Zhao G, et al. Phase II trial of VEGFR2 inhibitor apatinib for metastatic sarcoma: focus on efficacy and safety. Exp Mol Med (2019) 51(3):1–11. 10.1038/s12276-019-0221-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Weitao Y, Fangxing W, Qiqing C, Jiaqiang W. Efficacy and safety of apatinib in advanced sarcoma. Anti Cancer Drug (2019) 30(7):749–56. 10.1097/CAD.0000000000000778 [DOI] [PubMed] [Google Scholar]
- 42. He Y, Yang H, Sun XW, Zhang Y, Huang MJ, Gong YL, et al. The efficacy and safety analysis of apatinib in advanced sarcoma. J North Sichuan Med College (2020) 35(2):211–5. 10.3969/j.issn.1005-3697.2020.02.09 [DOI] [Google Scholar]
- 43. Gong TJ, He M, Wang YT, Min L, Zhou Y, Luo Y, et al. Efficacy and safety of apatinib in treating soft tissue sarcoma:a retrospective analysis of twenty-seven cases. Chin J Bone Joint (2020) 9(5):341–7. 10.3969/j.issn.2095-252X.2020.05.005 [DOI] [Google Scholar]
- 44. Liu JY, Zhu BR, Wang YD, Sun X. The efficacy and safety of Apatinib mesylate in the treatment of metastatic osteosarcoma patients who progressed after standard therapy and the VEGFR2 gene polymorphism analysis. Int J Clin Oncol (2020) 25(6):1195–205. 10.1007/s10147-020-01644-7 [DOI] [PubMed] [Google Scholar]
- 45. Liu XY, Xu J, Li F, Liao ZC, Ren ZW, Zhu L, et al. Efficacy and safety of the VEGFR2 inhibitor Apatinib for metastatic soft tissue sarcoma: Chinese cohort data from NCT03121846. BioMed Pharmacother (2020) 122:109587. 10.1016/j.biopha.2019.109587 [DOI] [PubMed] [Google Scholar]
- 46. Wang Y, Lu M, Zhou Y, Zhou S, Yu X, Tang F, et al. The Efficacy and Safety of Apatinib in Advanced Synovial Sarcoma: A Case Series of Twenty-One Patients in One Single Institution. Cancer Manag Res (2020) 12:5255–64. 10.2147/CMAR.S254296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Tian Z, Gu Z, Wang X, Liu Z, Yao W, Wang J, et al. Efficacy and safety of apatinib in treatment of osteosarcoma after failed standard multimodal therapy. Medicine (2019) 98(19):e15650. 10.1097/MD.0000000000015650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Xie L, Xu J, Sun X, Liu K, Li X, He F, et al. Apatinib for Treatment of Inoperable Metastatic or Locally Advanced Chondrosarcoma: What We Can Learn About the Biological Behavior of Chondrosarcoma from a Two-Center Study. Cancer Manag Res (2020) 12:3513–25. 10.2147/CMAR.S253201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Liao Z, Li T, Zhang C, Liu X, Xing R, Teng S, et al. Clinical study of apatinib in the treatment of stage IV osteogenic sarcoma after failure of chemotherapy. Cancer Biol Med (2020) 17(2):501–12. 10.20892/j.issn.2095-3941.2019.0397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Lin C, Wang S, Xie W, Zheng R, Gan Y, Chang J. Apatinib inhibits cellular invasion and migration by fusion kinase KIF5B-RET via suppressing RET/Src signaling pathway. Oncotarget (2016) 7(37):59236–44. 10.18632/oncotarget.10985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Zhao S, Ren S, Jiang T, Zhu B, Li X, Zhao C, et al. Low-Dose Apatinib Optimizes Tumor Microenvironment and Potentiates Antitumor Effect of PD-1/PD-L1 Blockade in Lung Cancer. Cancer Immunol Res (2019) 7(4):630–43. 10.1158/2326-6066.CIR-17-0640 [DOI] [PubMed] [Google Scholar]
- 52. Yang Y, Wang C, Sun H, Jiang Z, Zhang Y, Pan Z. Apatinib prevents natural killer cell dysfunction to enhance the efficacy of anti-PD-1 immunotherapy in hepatocellular carcinoma. Cancer Gene Ther (2020) 28(1–2):89–97. 10.1038/s41417-020-0186-7 [DOI] [PubMed] [Google Scholar]
- 53. Chevreau C, Le Cesne A, Ray-Coquard I, Italiano A, Cioffi A, Isambert N, et al. Sorafenib in patients with progressive epithelioid hemangioendothelioma. Cancer Am Cancer Soc (2013) 119(14):2639–44. 10.1002/cncr.28109 [DOI] [PubMed] [Google Scholar]
- 54. von Mehren M, Rankin C, Goldblum JR, Demetri GD, Bramwell V, Ryan CW, et al. Phase 2 Southwest Oncology Group-directed intergroup trial (S0505) of sorafenib in advanced soft tissue sarcomas. Cancer Am Cancer Soc (2012) 118(3):770–6. 10.1002/cncr.26334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Pacey S, Ratain MJ, Flaherty KT, Kaye SB, Cupit L, Rowinsky EK, et al. Efficacy and safety of sorafenib in a subset of patients with advanced soft tissue sarcoma from a Phase II randomized discontinuation trial. Invest New Drug (2011) 29(3):481–8. 10.1007/s10637-009-9367-9 [DOI] [PubMed] [Google Scholar]
- 56. Santoro A, Comandone A, Basso U, Soto Parra H, De Sanctis R, Stroppa E, et al. Phase II prospective study with sorafenib in advanced soft tissue sarcomas after anthracycline-based therapy. Ann Oncol (2013) 24(4):1093–8. 10.1093/annonc/mds607 [DOI] [PubMed] [Google Scholar]
- 57. George S, Merriam P, Maki RG, Annick DVDA, Yap JT, Akhurst T, et al. Multicenter Phase II Trial of Sunitinib in the Treatment of Nongastrointestinal Stromal Tumor Sarcomas. J Clin Oncol (2009) 27(19):3154–60. 10.1200/JCO.2008.20.9890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Jo J, Hong YS, Kim K, Lee J, Lee J, Park YS, et al. A prospective multicenter phase II study of sunitinib in patients with advanced aggressive fibromatosis. Invest New Drug (2014) 32(2):369–76. 10.1007/s10637-013-0059-0 [DOI] [PubMed] [Google Scholar]
- 59. Kummar S, Allen D, Monks A, Polley EC, Hose CD, Ivy SP, et al. Cediranib for Metastatic Alveolar Soft Part Sarcoma. J Clin Oncol (2013) 31(18):2296–302. 10.1200/JCO.2012.47.4288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Judson I, Morden JP, Kilburn L, Leahy M, Benson C, Bhadri V, et al. Cediranib in patients with alveolar soft-part sarcoma (CASPS): a double-blind, placebo-controlled, randomised, phase 2 trial. Lancet Oncol (2019) 20(7):1023–34. 10.1016/S1470-2045(19)30215-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Martin-Broto J, Stacchiotti S, Lopez-Pousa A, Redondo A, Bernabeu D, de Alava E, et al. Pazopanib for treatment of advanced malignant and dedifferentiated solitary fibrous tumour: a multicentre, single-arm, phase 2 trial. Lancet Oncol (2019) 20(1):134–44. 10.1016/S1470-2045(18)30676-4 [DOI] [PubMed] [Google Scholar]
- 62. Samuels BL, Chawla SP, Somaiah N, Staddon AP, Skubitz KM, Milhem MM, et al. Results of a prospective phase 2 study of pazopanib in patients with advanced intermediate-grade or high-grade liposarcoma. Cancer Am Cancer Soc (2017) 123(23):4640–7. 10.1002/cncr.30926 [DOI] [PubMed] [Google Scholar]
- 63. Sleijfer S, Ray-Coquard I, Papai Z, Le Cesne A, Scurr M, Schöffski P, et al. Pazopanib, a Multikinase Angiogenesis Inhibitor, in Patients With Relapsed or Refractory Advanced Soft Tissue Sarcoma: A Phase II Study From the European Organisation for Research and Treatment of Cancer–Soft Tissue and Bone Sarcoma Group (EORTC Study 62043). J Clin Oncol (2009) 27(19):3126–32. 10.1200/JCO.2008.21.3223 [DOI] [PubMed] [Google Scholar]
- 64. Stacchiotti S, Ferrari S, Redondo A, Hindi N, Palmerini E, Vaz Salgado MA, et al. Pazopanib for treatment of advanced extraskeletal myxoid chondrosarcoma: a multicentre, single-arm, phase 2 trial. Lancet Oncol (2019) 20(9):1252–62. 10.1016/S1470-2045(19)30319-5 [DOI] [PubMed] [Google Scholar]
- 65. van der Graaf WT, Blay J, Chawla SP, Kim D, Bui-Nguyen B, Casali PG, et al. Pazopanib for metastatic soft-tissue sarcoma (PALETTE): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet (British edition) (2012) 379(9829):1879–86. 10.1016/S0140-6736(12)60651-5 [DOI] [PubMed] [Google Scholar]
- 66. Mir O, Brodowicz T, Italiano A, Wallet J, Blay J, Bertucci F, et al. Safety and efficacy of regorafenib in patients with advanced soft tissue sarcoma (REGOSARC): a randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Oncol (2016) 17(12):1732–42. 10.1016/S1470-2045(16)30507-1 [DOI] [PubMed] [Google Scholar]
- 67. Duffaud F, Mir O, Boudou-Rouquette P, Piperno-Neumann S, Penel N, Bompas E, et al. Efficacy and safety of regorafenib in adult patients with metastatic osteosarcoma: a non-comparative, randomised, double-blind, placebo-controlled, phase 2 study. Lancet Oncol (2019) 20(1):120–33. 10.1016/S1470-2045(18)30742-3 [DOI] [PubMed] [Google Scholar]
- 68. Davis LE, Bolejack V, Ryan CW, Ganjoo KN, Loggers ET, Chawla S, et al. Randomized Double-Blind Phase II Study of Regorafenib in Patients With Metastatic Osteosarcoma. J Clin Oncol (2019) 37(16):1424–31. 10.1200/JCO.18.02374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Stacchiotti S, Simeone N, Lo Vullo S, Morosi C, Greco FG, Gronchi A, et al. Activity of axitinib in progressive advanced solitary fibrous tumour: Results from an exploratory, investigator-driven phase 2 clinical study. Eur J Cancer (2019) 106:225–33. 10.1016/j.ejca.2018.10.024 [DOI] [PubMed] [Google Scholar]
- 70. Chi Y, Fang Z, Hong X, Yao Y, Sun P, Wang G, et al. Safety and Efficacy of Anlotinib, a Multikinase Angiogenesis Inhibitor, in Patients with Refractory Metastatic Soft-Tissue Sarcoma. Clin Cancer Res (2018) 24(21):5233–8. 10.1158/1078-0432.CCR-17-3766 [DOI] [PubMed] [Google Scholar]
- 71. Zhou Y, Tang F, Wang Y, Min L, Luo Y, Zhang W, et al. Advanced alveolar soft part sarcoma responds to apatinib. Oncotarget (2017) 8(30):50314–22. 10.18632/oncotarget.18599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Hu X, Cao J, Hu W, Wu C, Pan Y, Cai L, et al. Multicenter phase II study of apatinib in non-triple-negative metastatic breast cancer. BMC Cancer (2014) 14:820. 10.1186/1471-2407-14-820 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding authors.