Abstract
Most guidelines and cardiovascular outcome trials (CVOTs) focus on secondary prevention of cardiovascular disease (CVD) in type 2 diabetes mellitus (T2DM). Patients with T2DM without established CVD (eCVD) also form a critical cohort, for whom primary prevention with timely pharmacological and non-pharmacological interventions can effectively prevent or delay the onset of CVD. Sodium-glucose co-transporter 2 inhibitors (SGLT2i) have demonstrated a promising role for primary prevention of CVD in CVOTs and real-world studies. The 2019 American College of Cardiology/American Heart Association guidelines on primary prevention of CVD recommend SGLT2i as one of the add-on treatment options to metformin for adults with T2DM and glycated hemoglobin >7% who have cardiovascular (CV) risk factors. The outcomes with maximal response to SGLT2i use in primary prevention are hospitalization for heart failure and chronic kidney disease. The cardiorenal benefits with SGLT2i are attributed to pleiotropic effects on CV risk factors, and interference with glucose and sodium handling in kidneys, independent of their glycemic benefits. Results therefore support a role for SGLT2i not only in patients with T2DM and eCVD but also in patients with T2DM without eCVD. This review examines the evidence for potential role of SGLT2i for primary prevention of CVD in T2DM.
Keywords: SGLT2i, Primary prevention, Type 2 diabetes mellitus, Cardiovascular disease
1. Introduction
Type 2 diabetes mellitus (T2DM) is a significant risk factor for cardiovascular diseases (CVDs), including angina, myocardial infarction (MI), heart failure (HF), and stroke [[1], [2], [3]]. Patients with T2DM are at more than 50% increased risk of developing CVD, MI, HF, and stroke compared with people without diabetes [3]. A recent systematic review of 57 studies globally showed that CVD, atherosclerosis, coronary heart disease, and HF affected 32.2%, 29.1%, 21.2%, and 14.9% of people with T2DM [4]. Heart failure remains a common initial diagnosis in T2DM with a high 5-year mortality risk of 50% [5]. In addition, CVD is associated with reduction in health-related quality of life (HRQoL) in patients with T2DM [6].
According to the International Diabetes Federation, the estimated global cost of T2DM along with its cardiovascular (CV) complications was US $727 billion in the year 2017, which is projected to rise to US $845 billion by 2045 [7]. With such a substantial clinical and economic burden, it is essential to prevent the development and reduce the progression of CVD. This was reflected in the latest American Diabetes Association/European Association for the Study of Diabetes (ADA/EASD) consensus report [8].
The development of CVD in T2DM is largely preventable by addressing the modifiable risk factors (smoking, sedentary behavior, high blood pressure [BP], hyperglycemia, raised lipid levels, and obesity) through pharmacological and nonpharmacological approaches [9,10]. The recent ADA guidelines recommend that CV risk factors be assessed at least annually in people with T2DM, and to achieve the treatment targets for these risk factors to reduce CVD risk [11]. A large Swedish registry reported a high risk of hospitalization for HF (HHF: hazard ratio [HR] 1.45, 95% confidence interval [CI] 1.34 to 1.57) despite the achievement of target levels for multiple modifiable CV risk factors [12].
In addition to the effective management of established CVD risk factors, the cardiovascular outcomes trials (CVOTs) over the last decade showed that some newer glucose-lowering drugs (GLD), sodium-glucose co-transporter-2 inhibitors (SGLT2i), and glucagon-like peptide-1 receptor agonists (GLP1-RA) also have additional CVD benefits beyond their glucose-lowering effects, which caused a major a shift in guideline recommendations [8].
However, the guidelines including the latest ADA/EASD consensus statement and majority of the CVOTs focus on patients with T2DM with established CVD (eCVD), which are not the majority of people with T2DM [8,[13], [14], [15], [16]]. The T2DM population without eCVD form a critical cohort for primary prevention in clinical practice, in whom timely pharmacological and nonpharmacological interventions can effectively prevent or delay the onset of CVD. This in turn can reduce the clinical, economic, and HRQoL burden of T2DM [17,18]. This is recognized by a paradigm shift in the recent 2019 American College of Cardiology/American Heart Association (ACC/AHA) guidelines on primary prevention of CVD, which recommended to consider SGLT2i or GLP1- RA as an add-on treatment to metformin and healthier lifestyle for adults with T2DM and glycated hemoglobin (HbA1c) >7% (64 mmol/mol) who have CV risk factors in addition to T2DM [19]. This primary prevention group may comprise a heterogeneous spectrum of patients with varying levels of CV risk (low, moderate, high), and may even include undiagnosed patients with atherosclerotic CVD (ASCVD), making CVD primary prevention a very important aspect when treating patients with T2DM. This is especially important in young patients with T2DM who are at a higher lifetime risk of CVD [20].
This narrative review aims to examine the potential role for SGLT2i in the primary prevention of CVD in patients with T2DM. We used key words such as “SGLT2i,” “cardiovascular disease in type 2 diabetes mellitus,” “SGLT2i in type 2 diabetes mellitus,” “primary prevention of cardiovascular disease in type 2 diabetes mellitus,” “cardiovascular risk in type 2 diabetes mellitus” in the PubMed database to search for relevant data from CVOTs and real-world studies.
2. Defining CV risk in T2DM population
2.1. Perspectives from guidelines and trials
So far, there has been no uniform method for stratifying CV risk in patients with T2DM. Guidelines and trials differ in their definitions. Table 1 highlights the recent important guidelines, consensus statements, and review articles [8,19,[21], [22], [23], [24], [25]] that stratify CV risk in T2DM and their recommendations regarding primary prevention with SGLT2i in T2DM (if any).
Table 1.
Recommendations for stratifying CV risk and primary prevention in T2DM.
| Source | CV risk stratification methodology | Primary prevention recommendations for SGLT2i |
|---|---|---|
| 2019 ADA/EASD consensus report [8] |
T2DM without eCVD with indicators of high risk
|
No specific recommendation separately for primary prevention Recommendations mention that level of evidence for benefits is greatest for SGLT2ia in patients with and without eCVD but with •HFrEF (EF <45%) or •CKD (eGFR 30 to ≤60 mL/min/1.73m2 or UACR >30 mg/g, particularly UACR >300 mg/g |
| 2019 ESC/EASD guidelines on diabetes, prediabetes, and CVD [21] |
Very high CV risk •T2DM + established CVD or •other target organ damageb or •≥3 major risk factorsc High CV risk
|
SGLT2i (empagliflozin, canagliflozin, dapagliflozin) reduce CV events in patients with T2DM who are at high CV riskd |
| 2019 ACC/AHA guideline on the primary prevention of CVD [19] | CV risk stratification is not specific for patients with T2DM (guidelines focused on primary prevention measures for atherosclerotic CVD) | For adults with T2DM and additional atherosclerotic CVD risk factors, it may be reasonable to initiate a SGLT2i to improve glycemic control and reduce CVD risk (class IIB), if glycemic control is not achieved despite lifestyle modification and metformin |
| 2018 ADA cardiovascular disease and risk management guidelines [22] |
Increased risk
|
No recommendation for primary preventione For patients with eCVD, add a second agent with evidence of cardiovascular risk reduction such as SGLT2i |
| 2016 European guidelines on CVD prevention in clinical practice [23] |
|
No recommendation for primary preventione In patients with T2DM and CVD, the use of SGLT2i should be considered early in the course of the disease to reduce CV and total mortality. |
| 2019 Bashier A et al (consensus recommendations for management of patients with T2DM and CVD) [24] |
Patients at high risk for CVD (multiple risk factors for CVD)
|
Recommend starting SGLT2i |
| 2019 Giugliano et al (primary versus secondary cardiorenal prevention in type 2 diabetes: Which newer anti-hyperglycemic drug matters?) [25] |
Very high CV risk
|
In primary prevention, SGLT2i reduce risk of hospitalization for heart failure and progression of kidney disease in patients with T2DM. |
ACC/AHA: American College of Cardiology/American Heart Association, ADA: American Diabetes Association, BP: blood pressure, CAD: coronary artery disease, CV: cardiovascular, CKD: chronic kidney disease, CVD: cardiovascular disease, eCVD: established cardiovascular disease, eGFR: estimated glomerular filtration rate, EF: ejection fraction, ESC: European Society of Cardiology, EASD: European Association for the Study of Diabetes, GLP-1: glucagon-like peptide 1, HFrEF: heart failure with reduced ejection fraction, HTN: hypertension, LDL-C: low-density lipoprotein cholesterol, MACE: major adverse cardiovascular events, SGLT2i: sodium-glucose co-transporter 2 inhibitors, T2DM: type 2 diabetes mellitus, UACR: urinary albumin creatinine ratio.
To reduce risk of MACE, GLP-1 receptor agonists can also be considered in patients with T2DM without established CVD with indicators of high risk, specifically, patients aged 55 years or older with coronary, carotid, or lower extremity artery stenosis >50%, left ventricular hypertrophy, eGFR <60 mL/min/1.73 m2 or albuminuria.
Proteinuria, renal impairment defined as eGFR <30 mL/min/1.73 m2,left ventricular hypertrophy or retinopathy.
Age, hypertension, dyslipidemia, smoking, obesity.
Empagliflozin, canagliflozin, and dapagliflozin reduce CV events in patients with DM and CVD, or in those who are at very high/high CV risk.
No evidence for primary prevention with SGLT2i at this point of time.
Proven CVD benefits means the agent has a label indication of reducing the CVD events. For SGLT2 inhibitors evidence-based preference is empagliflozin > canagliflozin. SGLT2 inhibitors vary in regard to eGFR pre-requisites for a continued use.
Dapagliflozin: preferred option for patients with eGFR 60 mL/min/1.73 m2.
There are several online calculators available for stratifying CV risk in patients with T2DM such as the ASCVD risk calculator (www.cvriskcalculator.com) proposed by ACC/AHA, UKPDS Risk Engine© based on UKPDS (United Kingdom Prospective Diabetes Study) trial (software package: https://www.dtu.ox.ac.uk/riskengine/download.php), and the Australian stroke foundation (http://www.cvdcheck.org.au/calculator/) calculator [17]. Using these CV risk stratification tools, clinicians can identify those patients with T2DM who would benefit from an early modification of CV risk factors, and if needed an intensive treatment and risk management strategy. However, CV risk calculators are considered relevant to the population from which the data are derived to prepare the algorithms. The World Health Organization/International Society of Hypertension (WHO/ISH) risk prediction charts, the ACC/AHA pooled cohort equations mainly derive their algorithms based on data drawn from the western population [26]. Therefore, these algorithms may underestimate the CV risk in countries like India, given the higher prevalence and earlier CVD onset [27,28]. A recent review by Gaikwad et al. predicting the applicability of such calculators suggests nonconventional measures such as carotid intima-media thickness, high-sensitivity C-reactive protein, coronary artery calcium for a more robust CV risk estimation in the Indian population [29].
2.2. CV risk definitions in SGLT2i CVOTs
The CVOTs with SGLT2i have likewise used different definitions to stratify CV risk in T2DM. The recent CVOTs, namely CANagliflozin cardioVascular Assessment Study (CANVAS)-program, Canagliflozin and Renal Endpoints in Diabetes with Established Nephropathy Clinical Evaluation (CREDENCE), and Dapagliflozin Effect on Cardiovascular Events–Thrombolysis in Myocardial Infarction 58 (DECLARE-TIMI 58), defined a list of risk factors and conditions to stratify participants in two categories: “at CV risk without eCVD” or in other words the primary prevention group and “with eCVD” or in other words secondary prevention group (Table 2) [[30], [31], [32]]. The primary prevention group were further categorized as “high CV risk” if they had ≥2 of the risk factors for the development of CVD and those who had <2 risk factors were considered to be “low or moderate CV risk.” The EMPA-REG OUTCOME [33] trial had <1% of the study population without eCVD similar to the recently completed VERTIS-CV [34].CVOT with ertugliflozin that also included only individuals with T2DM and eCVD, and had no primary prevention representation.
Table 2.
Cardiovascular risk characteristics in primary prevention T2DM groups in SGLT2i CVOT.
| CVOT/SGLT2i | Population characteristics in primary prevention group | %/n of patients in primary prevention group |
|---|---|---|
| DECLARE-TIMI 58 [32]/Dapagliflozin | Adults with T2DM (HbA1c ≥6.5%) and no known CVD and ≥2 CV risk factors in addition to T2DM, defined as:
|
59.4%, 10186 |
| CANVAS program [30]/Canagliflozin | Adults with T2DM (HbA1c: ≥7.0% to ≤10.5%) and the following risk factors:
|
34%, 3486 |
| CREDENCE [31]/Canagliflozina | Adults aged ≥30 years with T2DM (HbA1c ≥6.5% to ≤12.0%) and the following:
|
49.6%, 2181 |
ASCVD: atherosclerotic cardiovascular disease, CKD-EPI: Chronic Kidney Disease Epidemiology Collaboration equation, CV: cardiovascular, CVD: cardiovascular disease, CVOT: cardiovascular outcome trials, DBP: diastolic blood pressure, eGFR: estimated glomerular filtration rate, HbA1c: glycated hemoglobin, HDL-C: high-density lipoprotein cholesterol, LDL-C: low density lipoprotein-cholesterol, SBP: systolic blood pressure, SGLT2i: sodium-glucose co-transporter 2 inhibitor, T2DM: type 2 diabetes mellitus, UACR: urinary albumin:creatinine ratio.
In CREDENCE trial, the inclusion criteria for patients with CVD and patients at CV risk were not separately listed.
Overall, the EMPA-REG OUTCOME [33] trial majorly (>99%) included patients with eCVD (defined as presence of ≥1 of the following: history of MI/stroke, unstable angina, occlusive peripheral artery disease, evidence of single/multivessel coronary artery disease with history of revascularization), whereas those without eCVD were represented mainly in DECLARE-TIMI 58 [32] (10,186) but also in the CANVAS-program [30] and CREDENCE [31] (total number of patients around 15,856 patients) (Table 2).
Thus, there is no concurrence amongst guidelines or trials about the precise stratification of CV risk associated with T2DM; however, they are unanimous in suggesting that the risk stratification is an important step in formulating therapeutic strategies for every individual with T2DM.
3. Evidence for primary prevention with SGLT2i
3.1. SGLT2i cardiovascular outcome trials
All 4-landmark SGLT2i CVOT trials (EMPA-REG OUTCOME, DECLARE-TIMI 58, CANVAS-program, and CREDENCE) have shown significant reduction in 3-point major adverse cardiovascular events (3P-MACE: composite of CV death, nonfatal MI, or nonfatal stroke). These benefits were mainly seen in those with eCVD, except in CREDENCE where patients with multiple risk factors also had 3P-MACE reduction (Table 3). These results were also reflected in multiple meta-analyses. A meta-analysis of CVOTs (included only CANVAS-program and DECLARE-TIMI 58) showed SGLT2 benefits on 3P-MACE only in T2DM with eCVD [35]. Similarly, the Zelniker meta-analysis (included EMPA-REG OUTCOME, DECLARE-TIMI 58, CANVAS-program) demonstrated a reduced incidence of 3P-MACE by 11% (HR 0.89, 95% CI 0.83 to 0.96, p = 0.0014) only in eCVD (HR 0.86, 95% CI 0.80 to 0.93) but not in the primary prevention cohort (HR 1.00, 0.87 to 1.16, p for interaction = 0.0501) [36]. However, a recent large meta-analysis of 30 CVOTs (n = 225,305) of GLDs in T2DM demonstrated a risk reduction for MACE that was consistent among patients with (HR 0.87, 95% CI 0.83 to 0.92) and without eCVD (HR 0.92, 95% CI 0.83 to 1.02), among trials that assessed SGLT2i, GLP1-RA, or intensive lifestyle interventions [37].
Table 3.
Summary of SGLT2i CVOT results across the CV risk spectrum.
| Trials |
EMPA-REG OUTCOME [33,38,42,67] |
DECLARE-TIMI 58 [32] |
CANVAS-program [30,43] |
CREDENCE [31] |
|---|---|---|---|---|
| Intervention | Empagliflozin/placebo | Dapagliflozin/placebo | Canagliflozin/placebo | Canagliflozin/placebo |
| Primary outcomes | ||||
| 3-point MACE/Composite outcome (overall) | 0.86 (0.74–0.99) | 0.93 (0.84–1.03) | 0.86 (0.75–0.97) | 0.80 (0.67–0.95)a |
| eCVD | 0.86 (0.74–0.99) | 0.90 (0.79–1.02) | 0.82 (0.72–0.95) | 0.85 (0.69–1.06) |
| Multiple CVRF | NA | 1.01 (0.86–1.20) | 0.98 (0.74–1.30) | 0.68 (0.49–0.94) |
| Hx HF | NA | 1.01 (0.81–1.27) | 0.80 (0.61–1.05) | NA |
| No Hx HF | NA | 0.92 (0.82–1.02) | 0.87 (0.75–1.00) | NA |
| Key secondary outcomes | ||||
| CV death (overall) | 0.62 (0.49–0.77) | 0.98 (0.82–1.17) | 0.87 (0.72–1.06) | 0.78 (0.61–1.00) |
| eCVD | 0.62 (0.49–0.77) | 0.94 (0.76–1.18) | 0.86 (0.70–1.06) | 0.79 (0.58–1.07) |
| Multiple CVRF | NA | 1.06 (0.79–1.42) | 0.93 (0.60–1.43) | 0.75 (0.48–1.16) |
| Hx HF | 0.71 (0.43–1.16) | 1.01 (0.73–1.39) | 0.72 (0.51–1.02) | NA |
| No Hx HF | 0.60 (0.47–0.77) | 0.97 (0.78–1.20) | 0.95 (0.76–1.20) | NA |
| Fatal/nonfatal MI (overall) | 0.87 (0.70–1.09) | 0.89 (0.77–1.01) | 0.85 (0.69–1.05) | 0.86 (0.64–1.16) |
| eCVD | 0.87 (0.70–1.09) | 0.87 (0.74–1.02) | 0.79 (0.63–0.99) | 0.93 (0.66–1.32) |
| Multiple CVRF | NA | 0.94 (0.73–1.21) | 1.21 (0.73–2.00) | 0.70 (0.39–1.23) |
| Hx HF | NA | 0.85 (0.61–1.18) | 1.11 (0.65–1.89) | NA |
| No Hx HF | NA | 0.89 (0.77–1.04) | 0.86 (0.69–1.06) | NA |
| Fatal/nonfatal stroke (overall) | 1.18 (0.89–1.56) | 1.01 (0.84–1.21) | 0.90 (0.71–1.15) | 0.76 (0.55–1.22) |
| eCVD | 1.18 (0.89–1.56) | 0.97 (0.76–1.22) | 0.88 (0.67–1.16) | 0.87 (0.58–1.31) |
| Multiple CVRF | NA | 1.09 (0.82–1.45) | 0.97 (0.59–1.61) | 0.60 (0.34–1.08) |
| Hx HF | 1.48 (0.63–3.48) | 1.21 (0.77–1.91) | 0.84 (0.51–1.39) | NA |
| No Hx HF | 1.14 (0.85–1.53) | 0.98 (0.80–1.20) | 0.88 (0.68–1.14) | NA |
| HHF (overall) | 0.65 (0.50–0.85) | 0.73 (0.61–0.88) | 0.67 (0.52–0.87) | 0.61 (0.47–0.80) |
| eCVD | 0.65 (0.50–0.85) | 0.78 (0.63–0.97) | 0.68 (0.51–0.90) | 0.61 (0.44–0.85) |
| Multiple CVRF | NA | 0.64 (0.46–0.88) | 0.64 (0.35–1.15) | 0.61 (0.39–0.96) |
| Hx HF | 0.75 (0.48–1.19) | 0.73 (0.55–0.96) | 0.51 (0.33–0.78) | NA |
| No Hx HF | 0.59 (0.43–0.82) | 0.73 (0.58–0.92) | 0.79 (0.57–1.09) | NA |
| All-cause mortality (overall) | 0.68 (0.57–0.82) | 0.93 (0.82–1.04) | 0.87 (0.74–1.01) | 0.83 (0.68–1.02) |
| eCVD | 0.68 (0.57–0.82) | 0.92 (0.79–1.08) | 0.89 (0.75–1.07) | 0.79 (0.61–1.02) |
| Multiple CVRF | NA | 0.94 (0.78–1.12) | 0.79 (0.58–1.07) | 0.89 (0.63–1.26) |
| Hx HF | 0.79 (0.52–1.20) | 0.87 (0.68–1.12) | 0.70 (0.51–0.96) | NA |
| No Hx HF | 0.66 (0.51–0.81) | 0.94 (0.82–1.07) | 0.93 (0.78–1.11) | NA |
| Renal composite (overall) | 0.61 (0.53–0.70)b | 0.76 (0.67–0.87) | 0.60 (0.47–0.77) | 0.72 (0.54–0.97) |
| eCVD | NA | 0.79 (0.66–0.94) | 0.59 (0.44–0.79) | 0.83 (0.54–1.27) |
| Multiple CVRF | NA | 0.74 (0.60–0.91) | 0.63 (0.39–1.02) | 0.65 (0.43–0.97) |
| Hx HF | 0.78 (0.39–1.53) | 0.58 (0.36–0.92) | 0.67 (0.30–1.51) | NA |
| No Hx HF | 0.51 (0.37–0.70) | 0.52 (0.41–0.66) | 0.52 (0.37–0.72) | NA |
CI: confidence interval, CV: cardiovascular, CVD: cardiovascular disease, CVOTs: cardiovascular outcome trials, CVRF: cardiovascular risk factors, eCVD: established cardiovascular disease, eGFR: estimated glomerular filtration rate, HHF: hospitalization for heart failure, HR: hazard ratio, Hx HF: history of heart failure, MACE: major adverse cardiovascular events, MI: myocardial infarction, NA: not analyzed, No Hx HF: no history of heart failure, SGLT2i: sodium-glucose co-transporter 2 inhibitors, T2DM: type 2 diabetes mellitus.
All values presented as (HR [95% CI]). Values in bold represent overall population, and other values represent the respective subgroup population.
3-point MACE: composite of CV death, nonfatal MI, or nonfatal stroke.
Renal composite: CANVAS-program, % reduction in the eGFR, need for renal-replacement therapy, or death from renal causes, CREDENCE, dialysis, kidney transplantation, or renal death. DECARE-TIMI 58, ≥40% decrease in eGFR to <60 mL/min/1.73 m2, new end-stage renal disease, or death from renal or cardiovascular causes.
Primary outcome for CREDENCE, doubling of serum creatinine, end-stage kidney disease, renal death, or cardiovascular death.
Worsening nephropathy defined as new onset of UACR >300 mg/g creatinine or a doubling of the serum creatinine level and an estimated glomerular filtration rate of <45 mL/min/1.73 m2,the need for continuous renal-replacement therapy, or death from renal disease in EMPA-REG OUTCOME.
A very significant reduction was seen for the HHF endpoint in all 4 CVOTs; with robust primary prevention protection being demonstrated in the DECLARE-TIMI 58 and CREDENCE trials (Table 3). Furthermore, EMPA-REG OUTCOME [38] and DECLARE-TIMI 58 demonstrated significant reduction in risk of HHF in patients without HF at baseline; EMPA-REG OUTCOME also demonstrated a reduction in the risk of all-cause mortality in the same subgroup. The Zelniker meta-analysis supported this finding by demonstrating a significant primary prevention benefit for the composite of CV death or HHF (HR 0.84, 95% CI 0.69 to 1.01) in patients with T2DM and multiple risk factors. The results for the composite of CV death or HHF were similar when comparing patients with a history of HF (HR 0.71, 95% CI 0.61 to 0.84) versus those without HF (HR 0.79, 95% CI 0.71 to 0.88) at baseline, signifying a compelling HF primary prevention role for SGLT2i [36]. The recent Arnott et al. meta-analysis of SGLT2i CVOTs also demonstrated significant evidence of primary prevention for HHF (HR 0.63, 95% CI 0.50 to 0.80), and composite of CV death or HHF (HR 0.81, 95% CI 0.69 to 0.96) [39].
A posthoc analysis of the placebo arm of EXSCEL study assessed the impact of dapagliflozin and other SGLT2i on CV and renal outcomes. In participants without prior CVD, the adjusted Cox model showed with SGLT2i use, a significantly lower risk of MACE (HR 0.11, 95% CI 0.02 to 0.74, p = 0.02) and all-cause mortality (HR 0.10, 95% CI 0.01 to 0.80, p = 0.03) compared with no SGLT2i, whereas the effect was not significant in patients having prior CVD (MACE: HR 0.95, 95% CI 0.56 to 1.62, p = 0.86, all-cause mortality: HR 0.67, 95% CI 0.34 to 1.33, p = 0.26) [40].
Thus, a reduction in HHF is a consistent finding in all CVOTs with similar benefits in patients with and without eCVD at baseline (Table 3). The proportion of patients with HF at baseline was low in all the major CVOTs (10%–14.4%), implying SGLT2i were tested in a large cohort with no HF at baseline. Hence, the robust reduction in HHF in the CVOTs indicate a prime role for SGLT2i in the primary prevention of HF in T2DM.
The EMPA-REG OUTCOME cohort had very low representation (<1%) of the primary prevention cohort (without eCVD). However, a meta-analysis of data from seven empagliflozin randomized Phase III trials excluding EMPA-REG OUTCOME trial (empagliflozin: n = 2770, placebo: n = 1502), which studied a patient cohort with low/medium CV risk, demonstrated CV protection for the primary endpoint of 4P-MACE (HR 0.59, 95% CI 0.36 to 0.95); the secondary endpoint of 3P-MACE (HR 0.66, 95% CI 0.39 to 1.12) missed significance because of smaller number of events. Limitations included different treatment durations between studies, relatively short-term studies, and very few events in the low/medium CV risk group [41].
In addition to CV risk reduction, SGLT2i have also shown strong and consistent favorable impact on the development of chronic kidney disease (CKD) irrespective of the baseline CVD status. The CANVAS-program (HR 0.63, 95% 95% CI 0.39 to 1.02), CREDENCE (HR 0.65, 95% CI 0.43 to 0.97), and DECLARE-TIMI 58 (HR 0.51, CI 0.37–0.69) demonstrated robust primary prevention for renal outcomes (Table 3) [[30], [31], [32]]. In the subset of patients without HF at baseline EMPA-REG OUTCOME [42], DECLARE-TIMI 58, and CANVAS-program [43] showed significant renoprotection (Table 3). This was supported by the Zelniker meta-analysis with an overall 45% (HR 0.55, 95% CI: 0.48 to 0.64) reduction in renal composite (renal worsening, end-stage renal disease, or renal death); results being similar in patients with multiple risk factors (no eCVD; HR 0.54, 95% CI 0.42 to 0.71) and those with eCVD (HR 0.56, 95% CI 0.47 to 0.67). Moreover, the results were seen across a broad range of renal function and urine albumin-creatinine levels [36]. In addition, SGLT2i as a class, along with dapagliflozin, had a favorable effect on eGFR in patients with preserved eGFR and normo-albuminuria at baseline, suggesting a primary prevention benefit in the posthoc analysis of EXSCEL study [39].
3.2. Real-world studies with SGLT2i
Since CVOTs are conducted in controlled conditions with a highly selective population, many people potentially eligible for SGLT2i may have been excluded. The real-world studies, on the contrary, are conducted in clinical practice settings, which provide information on the clinical safety, effectiveness, as well as comparative effectiveness of a medication with other treatments, and provide clinicians with information closely related to the patients they see on a daily basis [44]. Several real-world studies have assessed SGLT2i in a broader population group with a wide range of age, ethnicities, CV risk, comorbidities, and concomitant medications.
The CVD-REAL [45] study sought an answer to the question whether SGLT2i impacted death and HF based on the presence or absence of CVD at the time of initiation when compared with other GLDs. About 87% of patients had no eCVD, and 97% of patients had no HF at baseline, forming a large primary prevention cohort. Compared with other GLDs, the prescription of SGLT-2i was associated with lower risk of death (eCVD: HR 0.56, 95% CI 0.44–0.70, no known CVD: HR 0.56, CI 0.50–0.63), HF (eCVD: HR 0.72, 95% CI 0.63–0.82, no known CVD: HR 0.61, CI 0.48–0.78), and composite of HF or death (eCVD: HR 0.63, 95% CI 0.57–0.70, no known CVD: HR 0.56, CI 0.50–0.62), thus establishing mortality and HF benefits for SGLT2i in primary prevention [46].
CVD-Real 2 [47] included a diverse cohort of patients from the Asia Pacific, Middle East, and the North American areas. About 73% of patients had no eCVD at baseline. All-cause death was significantly reduced in subgroups of patients with (HR 0.70, 95% CI 0.60 to 0.80) and without eCVD (HR 0.57 95% CI: 0.43 to 0.75) at baseline. For the outcomes of HHF (event rate [ER] 3.73 vs. 0.60), composite of HHF or death (ER 5.31 vs. 1.23), MI (ER 1.15 vs. 0.30), and stroke (ER 3.73 vs. 0.74), the event rates in patients with eCVD were higher compared with patients without CVD. CVD-Real Nordic [48] studied patients from the Nordic countries and results showed differences were similar for the 75% of individuals with no CVD at baseline versus those with eCVD for CV mortality (HR 0.55, 95% CI 0.34 to 0.90 vs. HR 0.60, 95% CI 0.42 to 0.85), and for MACE (HR 0.90, 95% CI 0.76 to 1.07 vs. HR 0.70, 95% CI 0.59 to 0.83). Additional subgroup analysis of CVD-REAL Nordic study [49] in the cohort receiving dapagliflozin, showed a greater risk reduction in MACE (HR 0.79, 95% CI 0.67 to 0.94; p = 0.006), HHF (HR 0.62, 95% CI 0.50 to 0.77; p < 0.001), and all-cause mortality (HR 0.44, 95% CI 0.33 to 0.60; p < 0.001) versus dipeptidyl peptidase-4 inhibitors, regardless of the CVD status. This analysis was conducted as dapagliflozin accounted for 94% of all SGLT2i prescriptions.
In another population-based, retrospective open cohort matched controlled study conducted in the United Kingdom using the Health Improvement Network (THIN) database, 80% of the patients had no eCVD at baseline [50]. A subgroup analysis was conducted in individuals without eCVD (defined as the absence of all CVDs at baseline: MI, ischemic heart disease, stroke, transient ischemic attack, and HF). In this population, the patients who received dapagliflozin experienced significantly lower risk of all-cause mortality (adjusted incidence rate ratio [aIRR] 0.44, 95% CI 0.25 to 0.78, p = 0.002) as compared with matched controls (matched for age, sex, body mass index, T2DM duration, and smoking) receiving other GLD therapy. However, the risk of incident CVD did not reach significance, (aIRR 0.89, 95% CI: 0.61–1.30, p = 0.55). EMPRISE [51] (EMPagliflozin comparative effectIveness and SafEty) study examined data to analyze outcomes in T2DM patients recently initiated on empagliflozin or sitagliptin; 75% of the patients had no eCVD at baseline. The incident rates of HHF were similar in patients with (HHF-specific HR 0.55, 95% CI 0.27 to 1.10, HHF-broad HR 0.60, 95% CI 0.44 to 0.83) and without CVD history (HHF-specific HR 0.41, 95% CI 0.10–1.68, HHF-broad HR 0.40, 95% CI 0.22 to 0.73). This study defined HHF-specific as an HF discharge diagnosis in the primary position, and HHF-broad as an HF discharge diagnosis at any position.
Overall, the results from all the real-world studies are in congruence with the CVOTs, with significant risk reduction in the primary and secondary outcomes such as all-cause mortality, HHF, and CV death. These significant results were seen in patients without eCVD, raising the possibility of a role for SGLT2i in primary prevention. Moreover, consistent results in multiple sensitivity analyses and across many countries globally support such a role.
4. SGLT2 inhibitors: mechanisms of cardiovascular and renal benefits
The SGLT2i reduce hyperglycemia by promoting urinary glucose excretion due to their kidney-specific action. This unique mechanism of action is independent of pancreatic β-cell function or degree of insulin resistance, and allows SGLT2i to be used at any stage of T2DM and in combination with any of the GLDs including insulin [18,52,53]. Hypoglycemia, a potential CV risk factor [54], is low with SGLT2i use, mainly because the SGLT2 inhibition is self-limiting when blood glucose concentrations drop to levels at which hypoglycemic symptoms develop (<72 mg/dL in most patients with T2DM) with the uninhibited SGLT2 and SGLT1 receptors reabsorbing almost all filtered glucose [55].
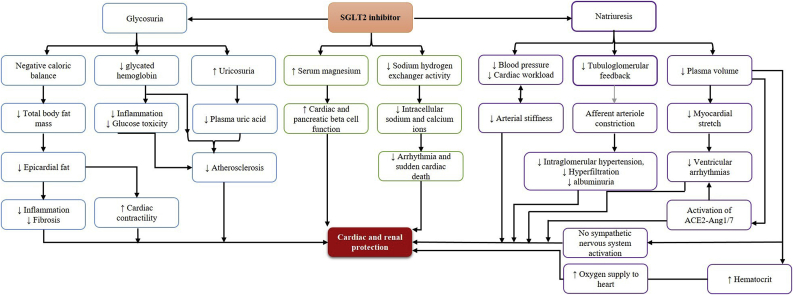
The SGLT2i offer the potential to reduce CVD through multiple interlinked pleiotropic benefits, although the exact or most important mechanism remains uncertain (Fig. 1) [56,57]. Multiple trials including the large CVOTs have demonstrated favorable changes in many CV risk factors (Table 4) including body weight (−0.88 to −3.3 kg), systolic BP (SBP, −2.3 to −5.4 mmHg), and serum high-density lipoprotein cholesterol (0.05–6.8 mmol/L) [[30], [31], [32], [33],[58], [59], [60], [61], [62]]. The reduction in BP is not associated with a reactive increase in heart rate, unlike with GLP1-RA and occurs consistently irrespective of the CVD status [63,64]. Further, the efficiency of cardiac metabolism is enhanced by an increase in ketone levels providing more energy at a lower oxygen cost, thereby improving cardiac pump function [65]. The CVOTs have also revealed that HF outcomes may be the most sensitive to SGLT2i treatment. One mechanism suggested in an experimental HF model is the inhibition of a Na+/H+exchanger-1 isoform in the myocardium, preventing an adverse increase in sodium and calcium levels [64] (Fig. 1).
Figure 1.
Mechanisms of SGLT2i for lowering CV risk in T2DM.
ACE-2-Ang 1/7: angiotensin-converting enzyme 2-angiotensin 1-7, CV: cardiovascular, SGLT2i: sodium-glucose co-transporter 2 inhibitor, T2DM: type 2 diabetes mellitus.
Table 4.
Summary of SGLT2i effects on CV risk factors.
| Study | Intervention | Treatment (mean change from baseline) – Placebo (mean change from baseline) |
|||
|---|---|---|---|---|---|
| Body weight (kg) | SBP (mmHg) | DBP (mmHg) | Other outcomes | ||
| EMPAGLIFLOZIN | |||||
| EMPA-REG OUTCOME [33] | E10 or E25 vs. Placebo |
Reduction in body weight, waist circumference, SBP/DBP, uric acid, and increase in both LDL-C and HDL-C observed with E10 and E25 vs. placebo | |||
| Gupta et al. [58] | E10 or E25 vs. Placebo |
E10: −1.41 (−2.51, −0.31; p = 0.0125); E25: −1.50 (−2.54, −0.46; p = 0.0051) |
E10: −3.3 (−9.8, −3.2; p = 0.3161); E25: −3.8 (−9.9, −2.4; p = 0.2313) |
E10: −1.0 (−4.9, −2.9; p = 0.4115); E25: −1.6 (−5.3, −2.0; p = 0.3780) |
NA |
| Roden et al. [59] | E10 or E25 vs. Placebo |
E10: −1.93 (−2.41, −1.45; p < 0.0001); E25: −2.15 (−2.63, −1.67; p < 0.0001) |
E10:–2.6 (−4.9, −0.4; p = 0.0231); E25: −3.4 (−5.7, −1.2; p = 0.0028) |
E10: −0.6 (−1.9, 0.8; p = 0.3987) E25: −1.5 (−2.8, −0.1; p = 0.0296) |
Change in waist circumference (cm): E10: −1.6 (−2.3, −0.8; p < 0.0001) E25: −1.6 (−2.4, −0.9; p < 0.0001)A |
| CANAGLIFLOZIN | |||||
| CANVAS program [30,60] | C100 vs. Placebo |
C100: −1.60 (−1.70, −1.51; p < 0.001) | C100: −3.93 (−4.30, −3.56; p < 0.001) | C100: −1.39 (−1.61, −1.17; p < 0.001) | Change in serum HDL-C (mmol/L): C100: 0.05 (0.05, 0.06) Change in serum LDL-C (mmol/L): C100: 0.12 (0.09, 0.15) |
| CREDENCE [31] | C100 or C300 vs. Placebo |
Overall mean change in C100/C300: −0.88 (−1.69, −0.07) | Overall mean change in C100/C300: −2.38 (−4.64, −0.11) | Overall mean change in C100/C300: −1.44 (−2.80, −0.09) | NA |
| Stenlöf K et al [61] | C100 or C300 vs. Placebo |
C100: −1.9 (−2.9, −1.6; p < 0.001) C300: −2.9 (−4.0, −2.6; p < 0.001) |
C100: −3.7 (−5.9, −1.6; p < 0.001); C300 −5.4 (−7.6, −3.3; p < 0.001) |
C100: −1.6 (−2.9, −0.2); C300: −2.0 (−3.4, −0.7) |
Change in serum LDL-C (mmol/L): C100: 2.0 (−3.2, 7.1) C300: 6.1 (0.9, 11.3) Change in serum HDL-C (mmol/L): C100: 6.8 (2.9, 10.6; p < 0.001) C300: 6.1 (2.3, 9.9; p < 0.01) Change in serum triglyceride (mmol/L): C100: −5.4 (−14.9, 4.1; p = NS) C300: −10.2 (−19.6, −0.7; p = NS) |
| DAPAGLIFLOZIN | |||||
| DECLARE-TIMI 58 [32]∗ | D10 vs. Placebo | D10: −1.8 (−2.0, −1.7) | D10: −2.7 (−3.0, −2.4) | D10: −0.7 (−0.9, −0.6) | Positive renal effect such as natriuretic effect, improved tubular glomerular feedback, vascular compliance, and endothelial function with dapagliflozin |
| Ferrannini et al. [62] | D2.5 or D5 or D10 vs. Placebo | D2.5: −3.3 ± 0.5, D5: −2.8 ± 0.5, D10: −3.2 ± 0.5 (p = NS vs. placebo for all) |
D2.5: −4.6 ± 1.8 D5: −2.3 ± 1.9 D10: −3.6 ± 1.9 (p = NS vs. placebo for all) |
D2.5: −2.8 ± 1.1 D5: −1.7 ± 1.1 D10: −2.0 ± 1.9 (p = NS vs. placebo for all) |
Change in serum uric acid (μmol/L): D2.5: −39.3 ± 6.0; D5: −50.6 ± 6.1, D10: −51.7 ± 5.8 Change in serum HDL-C (mmol/L): Overall placebo-subtracted adjusted mean (SE) change from baseline for dapagliflozin: (0.02 [0.07] to 0.17 [0.08] mmol/L) |
C100: canagliflozin 100 mg, C300: canagliflozin 300 mg, D2.5: dapagliflozin 2.5 mg, D5: dapagliflozin: 5 mg, D10: dapagliflozin 10 mg, DBP: diastolic blood pressure, E10: empagliflozin 10 mg, E25: empagliflozin 25 mg, HDL-C: high-density lipoprotein cholesterol, LDL-C: low-density lipoprotein cholesterol, NA: not available, NS: nonsignificant, SE: standard error, SBP: systolic blood pressure.
∗Values presented as least square mean difference between the comparator arms.
Positive renal benefits of SGLT2i are also multifactorial. A key mechanism is restoration of the tubuloglomerular feedback, which then regulates the glomerular filtration rate favorably. This in turn alleviates intrarenal hypoxia, oxidative stress, and the subsequent inflammatory effects [66]. There is also a reduction in intraglomerular pressure and albuminuria, a known CV risk factor [67]. Improvement in renal function is also reflected by enhanced erythropoietin and hematocrit levels, which then boosts myocardial and renal oxygenation [68]. The urinary excretion of uric acid by SGLT2i action lowers plasma uric acid levels, ensuring lowering of yet another surrogate CV risk marker [69]. Thus in many ways, renal protection [70] translates into CV risk reduction.
5. Implications for clinical practice
Most individuals with T2DM have their first clinical contact with a primary care physician. It is essential for physicians to be aware of the potential for CV complications in T2DM and make management of CV risk a top priority, with a focus on reducing potential CVD risk even in the absence of eCVD [71]. SGLT2i form a novel therapeutic option for addition as a first-line option after metformin right from the early stages of T2DM to de-escalate this CV risk. The CVOT and real-world data support a role for SGLT2i in a wide spectrum of CV risk, extending from low, moderate, high risk to those with eCVD in people with T2DM, especially for the endpoints of CV death or HHF. Prevention of HF is extremely important especially in early onset T2DM, considering there is almost a 5-fold increased risk of HF in this younger age group (≤40 years) [20]. Additionally, the benefits with SGLT2i across the broad range of renal function becomes important while selecting treatment options with comorbid T2DM and CKD, both being associated with increased CV risk. The numbers needed to treat in CREDENCE for the overall CV outcomes ranged from 36 to 53 in the primary prevention group, and 21 to 44 in the secondary prevention group [31].
The minimal risk of hypoglycemia with significant reduction in CV complications like HF and the ease of use have contributed to endocrinologists and cardiologists adopting SGLT2i very early into their clinical practice, thus benefiting their patients [72]. Like all the other GLDs, SGLT2i are associated with class-specific adverse events such as – increased risk of genital tract infections, bone fractures, amputations, and volume depletion-related adverse events [73]. However, bone fractures were observed majorly in individuals with osteoporosis or those with high bone fracture risk and attributed to disordered calcium and phosphate homeostasis affecting bone metabolism with increased parathyroid hormone and decreased 1,25-dihydroxy vitamin D levels [74]. Although the CANVAS-program [30] observed an increased risk of toe amputation with canagliflozin, none of the real-world studies could confirm this finding [[45], [46], [47],75]. The SGLT2i, when compared with other GLDs are considered to be cost-effective because of their potent glycemic and extra-glycemic effects and resultant improved quality of life [20,72,76]. Even when the cost of treatment-related adverse events were added to calculate the economic burden, the SGLT2i were still considered economical in comparison to other GLDs [77].
6. Conclusion
Individuals with T2DM without eCVD, form an important cohort for the primary prevention of CVD. The SGLT2i—through CVOTs and real-world studies—have shown a significant risk reduction in some of the CV outcomes, regardless of the underlying CVD status, allowing a mitigation of the overall disease burden. Primary prevention by SGLT2i holds the greatest promise for reducing HHF and CKD in patients with T2DM [23]. The guideline focus is now dynamically shifting from exclusive SGLT2i recommendation in eCVD, to a broader more inclusive population with no eCVD at baseline, but with CV risk. Further studies are needed in this specific primary prevention cohort of T2DM to crystalize the impact of SGLT2i.
Funding
AstraZeneca Pharma India Limited has assisted to prepare the document and funded towards publication.
Acknowledgments
The authors would like to thank AstraZeneca Pharma India Ltd., Bangalore for medical writing support in collaboration with Dr. Anita Bhat, M.B.B.S, D.T.C.D, from Covance Scientific Services & Solutions Pvt. Ltd., in accordance with GPP3 guidelines.
References
- 1.Sarwar N., Gao P., Seshasai S.R.K., Gobin R., Kaptoge S., EDi Angelantonio. Emerging Risk Factors Collaboration. Diabetes mellitus, fasting blood glucose concentration, and risk of vascular disease: a collaborative meta-analysis of 102 prospective studies. Lancet. 2010;375:2215–2222. doi: 10.1016/S0140-6736(10)60484-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Peters S.A., Huxley R.R., Woodward M. Diabetes as risk factor for incident coronary heart disease in women compared with men: a systematic review and meta-analysis of 64 cohorts including 858,507 individuals and 28,203 coronary events. Diabetologia. 2014;57:1542–1551. doi: 10.1007/s00125-014-3260-6. [DOI] [PubMed] [Google Scholar]
- 3.Shah A.D., Langenberg C., Rapsomaniki E., Denaxas S., Pujades-Rodriguez M., Gale C.P. Type 2 diabetes and incidence of a wide range of cardiovascular diseases: a cohort study in 1.9 million people. Lancet. 2015;385(Suppl.1):S86. doi: 10.1016/S0140-6736(15)60401-9. [DOI] [PubMed] [Google Scholar]
- 4.Einarson T.R., Acs A., Ludwig C., Panton U.H. Prevalence of cardiovascular disease in type 2 diabetes: a systematic literature review of scientific evidence from across the world in 2007-2017. Cardiovasc Diabetol. 2018;17:83. doi: 10.1186/s12933-018-0728-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Greene S.J., Butler J. Primary prevention of heart failure in patients with type 2 diabetes mellitus. Circulation. 2019;139:152–154. doi: 10.1161/CIRCULATIONAHA.118.037599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Briggs A.H., Bhatt D.L., Scirica B.M., Raz I., Johnston K.M., Szabo S.M. Health-related quality-of-life implications of cardiovascular events in individuals with type 2 diabetes mellitus: a subanalysis from the Saxagliptin Assessment of Vascular Outcomes Recorded in Patients with Diabetes Mellitus (SAVOR)-TIMI 53 trial. Diabetes Res Clin Pract. 2017;130:24–33. doi: 10.1016/j.diabres.2016.12.019. [DOI] [PubMed] [Google Scholar]
- 7.IDF diabetes Atlas. ninth ed. International Diabetes Federation; Brussels, Belgium: 2019. http://www.diabetesatlas.org Available from: [Google Scholar]
- 8.Buse J.B., Wexler D.J., Tsapas A., Rossing P., Mingrone G., Mathieu C. 2019 update to: management of hyperglycaemia in type 2 diabetes, 2018. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD) Diabetologia. 2020;63:221–228. doi: 10.1007/s00125-019-05039-w. [DOI] [PubMed] [Google Scholar]
- 9.Gaede P., Lund-Andersen H., Parving H.-H., Pedersen O. Effect of a multifactorial intervention on mortality in type 2 diabetes. N Engl J Med. 2008;358:580–591. doi: 10.1056/NEJMoa0706245. [DOI] [PubMed] [Google Scholar]
- 10.Singh P., Subramanian A., Adderley N., Gokhale K., Singhal R., Bellary S. Impact of bariatric surgery on cardiovascular outcomes and mortality: a population-based cohort study. Br J Surg. 2020 Mar;107(4):432–442. doi: 10.1002/bjs.11433. [DOI] [PubMed] [Google Scholar]
- 11.American Diabetes Association. 10 Cardiovascular disease and risk management: standards of medical care in Diabetes— 2020. Diabetes Care. 2020;43(Suppl 1):S111–S134. doi: 10.2337/dc20-S010. [DOI] [PubMed] [Google Scholar]
- 12.Rawshani A., Rawshani A., Franzen S., Sattar N., Eliasson B., Svensson A.-M. Risk factors, mortality, and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2018;379:633–644. doi: 10.1056/NEJMoa1800256. [DOI] [PubMed] [Google Scholar]
- 13.Chamberlain J.J., Johnson E.L., Leal S., Rhinehart A.S., Shubrook J.H., Peterson L. Cardiovascular disease and risk management: review of the American diabetes association standards of medical care in diabetes 2018. Ann Intern Med. 2018;168:640–650. doi: 10.7326/M18-0222. [DOI] [PubMed] [Google Scholar]
- 14.Davies M.J., D’Alessio D.A., Fradkin J., Kernan W.N., Mathieu C., Mingrone G., Management of Hyperglycemia in Type 2 Diabetes A consensus report by the American diabetes association (ADA) and the European association for the study of diabetes (EASD) Diabetes Care. 2018;41(12):2669–2701. doi: 10.2337/dci18-0033. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.World Health Organization . World Health Organization; 2014. ‎. Prevention of cardiovascular disease: guidelines for assessment and management of total cardiovascular risk.https://apps.who.int/iris/handle/10665/43685 [Google Scholar]
- 16.American diabetes association- position statement. 8. Cardiovascular disease and risk management. Diabetes Care. 2016;38(Supplement 1):S49–S57. doi: 10.2337/dc15-S011. [DOI] [PubMed] [Google Scholar]
- 17.National Vascular Disease Prevention Alliance . 2012. Guidelines for the management of absolute cardiovascular disease risk. [Google Scholar]
- 18.Brunton S.A. The potential role of sodium glucose co-transporter 2 inhibitors in the early treatment of type 2 diabetes mellitus. Int J Clin Pract. 2015;69(10):1071–1087. doi: 10.1111/ijcp.12675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Arnett D.K., Blumenthal R.S., Albert M.A., Buroker A.B., Goldberger Z.D., Hahn E.J. ACC/AHA guideline on the primary prevention of cardiovascular disease: a report of the American College of Cardiology/American heart association task force on clinical practice guidelines. Circulation. 2019;140(11):e649–e650. doi: 10.1161/CIR.0000000000000678. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sattar N., Rawshani R., Franzen S. Age at diagnosis of type 2 diabetes mellitus and associations with cardiovascular and mortality risks findings from the Swedish national diabetes registry. Circulation. 2019;139:2228–2237. doi: 10.1161/CIRCULATIONAHA.118.037885. [DOI] [PubMed] [Google Scholar]
- 21.Cosentino F., Grant P.J., Aboyans V., Bailey C.J., Ceriello A., Delgado V. ESC Guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD: the Task Force for diabetes, pre-diabetes, and cardiovascular diseases of the European Society of Cardiology (ESC) and the European Association for the Study of Diabetes (EASD) Eur Heart J. 2019:1–69. doi: 10.1093/eurheartj/ehz486. 2019. [DOI] [PubMed] [Google Scholar]
- 22.American Diabetes Association 9. Cardiovascular disease and risk management: standards of medical care in diabetes - 2018. Diabetes Care. 2018;41(Suppl. 1):S86–S104. doi: 10.2337/dc18-S009. [DOI] [PubMed] [Google Scholar]
- 23.Piepoli M.F., Hoes A.W., Agewall S., Albus C., Brotons C., Catapano A.L. 2016 European guidelines on cardiovascular disease prevention in clinical practice the sixth joint task force of the European society of Cardiology and other societies on cardiovascular disease prevention in clinical practice (constituted by representatives of 10 societies and by invited experts) Eur Heart J. 2016;37:2315–2381. doi: 10.1093/eurheartj/ehw106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bashier A., Hussain A.B., Abdelgadir E., Alawadi F., Sabbour H., Chilton R. Consensus recommendations for management of patients with type 2 diabetes mellitus and cardiovascular diseases. Diabetol Metab Syndrome. 2019;11:80. doi: 10.1186/s13098-019-0476-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Giugliano D., Ceriello A., Nicola L.D., Perrone-Filardi P., Cosentino F., Esposito K. Primary versus secondary cardiorenal prevention in type 2 diabetes: which newer anti-hyperglycaemic drug matters? Diabetes Obes Metabol. 2020;22:149–157. doi: 10.1111/dom.13881. [DOI] [PubMed] [Google Scholar]
- 26.Ravi R., Kasliwal R., Mahansaria K., Bansal M. Chapter 10 cardiovascular risk algorithms and their applicability to Indians. Prev Cardiol. 2017:75–84. [Google Scholar]
- 27.Bansal M., Shrivastava S., Mehrotra R., Agarwal V., Kasliwal R.R. Low Framingham risk score despite high prevalence of metabolic syndrome in asymptomatic North-Indian population. J Assoc Phys India. 2009;57:17–22. [PubMed] [Google Scholar]
- 28.Kanjilal S., Rao V.S., Mukherjee M., Natesha B.K., Renuka K.S., Sibi K. Application of cardiovascular disease risk prediction models and the relevance of novel biomarkers to risk stratifi cation in Asian Indians. Vasc Health Risk Manag. 2008;4:199–211. doi: 10.2147/vhrm.2008.04.01.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gaikwad A., Khan Y. Evaluation of discordance between 10 year cardiovascular risk scores in Indian patients presenting with myocardial infarction. Cardiol Cardiovasc Med. 2019;3:360–368. [Google Scholar]
- 30.Mahaffey K.W., Neal B., Perkovic V. Canagliflozin for primary and secondary prevention of cardiovascular events: results from the CANVAS program (canagliflozin cardiovascular assessment study) Circulation. 2018;137(4):323–334. doi: 10.1161/CIRCULATIONAHA.117.032038. doi: 10.1161/CIRCULATIONAHA.117.032038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mahaffey K.W., Jardine M.J., Bompoint S., Cannon C.P., Neal B., Heerspink H.J.L. Canagliflozin and cardiovascular and renal outcomes in type 2 diabetes mellitus and chronic kidney disease in primary and secondary cardiovascular prevention groups. Circulation. 2019;140:739–750. doi: 10.1161/CIRCULATIONAHA.119.042007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wiviott S.D., Raz I., Bonaca M.P. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl Med. 2019;380:347–357. doi: 10.1056/NEJMoa1812389. [DOI] [PubMed] [Google Scholar]
- 33.Zinman B., Wanner C., Lachin J.M., Fitchett D., Bluhmki E., Hantel S. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373:2117–2128. doi: 10.1056/NEJMoa1504720. [DOI] [PubMed] [Google Scholar]
- 34.Cannon C.P., McGuire D.K., Pratley R., Dagogo-Jack S., Mancuso J., Huyck S. Design and baseline characteristics of the eValuation of ERTugliflozin effIcacy and Safety CardioVascular outcomes trial (VERTIS-CV) Am Heart J. 2018;206:11–23. doi: 10.1016/j.ahj.2018.08.016. [DOI] [PubMed] [Google Scholar]
- 35.Giugliano D., Maiorino M.I., Bellastella G., Chiodini P., Esposito K. Glycemic control, preexisting cardiovascular disease, and risk of major cardiovascular events in patients with type 2 diabetes mellitus: systematic review with meta-analysis of cardiovascular outcome trials and intensive glucose control trials. J Am Heart Assoc. 2019;8 doi: 10.1161/JAHA.119.012356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zelniker T.A., Wiviott S.D., Raz I., Im K., Goodrich E.L., Bonaca M.P. SGLT2 inhibitors for primary and secondary prevention of cardiovascular and renal outcomes in type 2 diabetes:a systematic review and meta-analysis of cardiovascularoutcome trials. Lancet. 2019;393:31–39. doi: 10.1016/s0140-6736(18)32590-x. [DOI] [PubMed] [Google Scholar]
- 37.Ghosh-Swaby O.R., Goodman S.G., Leiter L.A., Cheng A., Connelly K.A., Fitchett D. Glucose-lowering drugs or strategies, atherosclerotic cardiovascular events, and heart failure in people with or at risk of type 2 diabetes: an updated systematic review and meta-analysis of randomised cardiovascular outcome trials. Lancet Diabetes Endocrinol. 2020;8:418–435. doi: 10.1016/S2213-8587(20)30038-3. [DOI] [PubMed] [Google Scholar]
- 38.Fitchett D., Zinman B., Wanner C., Lachin J.M., Hantel S., Salsali A. Heart failure outcomes with empagliflozin in patients with type 2 diabetes at high cardiovascular risk: results of the EMPA-REG OUTCOMEw trial. Eur Heart J. 2016;37:1526–1534. doi: 10.1093/eurheartj/ehv728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Arnott C., Li Q., Kang A., Neuen B., Bompoint S., Lam C.S.P. Sodium-glucose cotransporter 2 inhibition for the prevention of cardiovascular events in patients with type 2 diabetes mellitus: a systematic review and meta-analysis. J Am Heart Assoc. 2020;9 doi: 10.1161/JAHA.119.014908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Clegg L.E., Heerspink H.J.L., Penland R.C., Tang W., Boulton D.W., Bachina S. Reduction of cardiovascular risk and improved estimated glomerular filtration rate by SGLT2 inhibitors, including dapagliflozin, is consistent across the class: an analysis of the placebo arm of EXSCEL. Diabetes Care. 2019;42:318–326. doi: 10.2337/dc18-1871. [DOI] [PubMed] [Google Scholar]
- 41.Salsali A., Kim G., Woerle H.J., Broedl U.C., Hantel S. Cardiovascular safety of empagliflozin in patients with type 2 diabetes: a meta-analysis of data from randomized placebo-controlled trials. Diabetes Obes Metabol. 2016;18:1034–1040. doi: 10.1111/dom.12734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Butler J., Zannad F., Fitchett D., Zinman B., Koitka-Weber A., von Eynatten M. Empagliflozin improves kidney outcomes in patients with or without heart failure insights from the EMPA-REG OUTCOME trial. Circ Heart Fail. 2019;12 doi: 10.1161/CIRCHEARTFAILURE.118.005875. [DOI] [PubMed] [Google Scholar]
- 43.Radholm K., Figtree G., Perkovic V., Solomon S.D., Mahaffey K.W., Zeeuw Dde. Canagliflozin and heart failure in type 2 diabetes mellitus: results from the CANVAS program. Circulation. 2018;138:458–468. doi: 10.1161/CIRCULATIONAHA.118.034222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Blonde L., Khunti K., Harris S.B., Meizinger C., Skolnik N.S. Interpretation and impact of real-world clinical data for the practicing clinician. Adv Ther. 2018;35:1763–1774. doi: 10.1007/s12325-018-0805-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kosiborod M., Cavender M.A., Fu A.Z., Wilding J.P., Khunti K. Lower risk of heart failure and death in patients initiated on sodium-glucose cotransporter-2 inhibitors versus other glucose-lowering drugs: the CVD-REAL study (comparative effectiveness of cardiovascular outcomes in new users of sodium-glucose cotransporter-2 inhibitors) Circulation. 2017;136(3):249–259. doi: 10.1161/CIRCULATIONAHA.117.029190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cavender M.A., Norhammar A., Birkeland K.I. CVD-REAL investigators and study group. SGLT-2 inhibitors and CardiovascularRisk: an analysis of CVD-REAL. J Am Coll Cardiol. 2018 Jun 5;71(22):2497–2506. doi: 10.1016/j.jacc.2018.01.085. [DOI] [PubMed] [Google Scholar]
- 47.Kosiborod M., Lam C.S.P., Kohsaka S. Cardiovascular events associated with SGLT-2 inhibitors versus other glucose-lowering drugs: the CVD-REAL 2 study. J Am Coll Cardiol. 2018;71(23):2628–2639. doi: 10.1016/j.jacc.2018.03.009. [DOI] [PubMed] [Google Scholar]
- 48.Birkeland K.I., Jørgensen M.E., Carstensen B., Persson F< Gulseth H.L., Thuresson M. Cardiovascular mortality and morbidity in patients with type 2 diabetes following initiation of sodium-glucose co-transporter-2 inhibitors versus other glucose-lowering drugs (CVD-REAL Nordic): a multinational observational analysis. Lancet Diabetes Endocrinol. 2017;5(9):709–717. doi: 10.1016/S2213-8587(17)30258-9. [DOI] [PubMed] [Google Scholar]
- 49.Persson F., Nyström T., Jørgensen M.E., Carstensen B., Gulseth H.L., Thuresson M. Dapagliflozin is associated with lower risk of cardiovascular events and all-cause mortality in people with type 2 diabetes (CVD-REAL Nordic) when compared with dipeptidyl peptidase-4 inhibitor therapy: a multinational observational study. Diabetes ObesMetab. 2018;20(2):344–351. doi: 10.1111/dom.13077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Toulis K.A., Willis B.H., Marshall T., Kumarendran B., Gokhale K., Ghosh S. All-cause mortality in patients with diabetes under treatment with dapagliflozin: a population-based, open-cohort study in the health improvement Network database. J Clin Endocrinol Metab. 2017;102:1719–1725. doi: 10.1210/jc.2016-3446. [DOI] [PubMed] [Google Scholar]
- 51.Patorno E., Pawar A., Franklin J.M., Najafzadeh M., Déruaz-Luyet A., Brodovicz K.G. Empagliflozin and the risk of heart failure hospitalization in routine clinical care. Circulation. 2019;139(25):2822–2830. doi: 10.1161/CIRCULATIONAHA.118.039177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Garber A.J., Abrahamson M.J., Barzilay J.I., Blonde L., Bloomgarden Z.T., Bush M.A. American Association of Clinical Endocrinologists’ comprehensive diabetes management algorithm 2013 consensus statement. Endocr Pract. 2013;19(Suppl 2) doi: 10.4158/EP13176.CS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Inzucchi S.E., Bergenstal R.M., Buse J.B., Diamant M., Ferrannini E., Nauck M. Management of hyperglycemia in type 2 diabetes, 2015: a patient-centered approach: update to a position statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care. 2015;38:140–149. doi: 10.2337/dc14-2441. [DOI] [PubMed] [Google Scholar]
- 54.The International Hypoglycaemia Study Group Hypoglycaemia, cardiovascular disease, and mortality in diabetes: epidemiology, pathogenesis, and management. Lancet Diabetes and Endocrinol. 2019;7:385–396. doi: 10.1016/S2213-8587(18)30315-2. [DOI] [PubMed] [Google Scholar]
- 55.Tahrani Abd A., Barnett A.H., Bailey C.J. SGLT inhibitors in management of diabetes. Lancet Diabetes Endocrinol. 2013;2:140–151. doi: 10.1016/S2213-8587(13)70050-0. [DOI] [PubMed] [Google Scholar]
- 56.Staels B. Cardiovascular protection by sodium glucose cotransporter 2 inhibitors: potential mechanisms. Am J Med. 2017;130:S30–S39. doi: 10.1016/j.amjmed.2017.04.009. [DOI] [PubMed] [Google Scholar]
- 57.Ali A., Bain S., Hicks D., Jones P.N., Patel D.C., Evans M. SGLT2 inhibitors: cardiovascular benefits beyond HbA1c—Translating evidence into practice. Diabetes Ther. 2019;10:1595–1622. doi: 10.1007/s13300-019-0657-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gupta S., Shaikh S., Joshi P., Bhure S., Suvarna V. Long-term efficacy and safety of empagliflozin monotherapy in drug-naive patients with type 2 diabetes in Indian subgroup: results from a 76-week extension trial of phase III, double-blind, randomized study. Indian J Endocrinol Metab. 2017;21:286–292. doi: 10.4103/ijem.IJEM_517_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Roden M., Weng J., Eilbracht J., Delafont B., Kim G., Woerle H.J. Empagliflozin monotherapy with sitagliptin as an active comparator in patients with type 2 diabetes: a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Diabetes Endocrinol. 2013;13:208–219. doi: 10.1016/S2213-8587(13)70084-6. [DOI] [PubMed] [Google Scholar]
- 60.Neal B., Perkovic V., Mahaffey K.W. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med. 2017;377:644–657. doi: 10.1056/NEJMoa1611925. [DOI] [PubMed] [Google Scholar]
- 61.Stenlöf K., Cefalu W.T., Kim K.-A., Alba M., Usiskin K., Tong C. Efficacy and safety of canagliflozin monotherapy in patients with type 2 diabetes mellitus inadequately controlled with diet and exercise. Diabetes ObesMetab. 2013;15(4):372–382. doi: 10.1111/dom.12054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ferrannini E., Ramos S.J., Salsali A., Tang W., List J.F. Dapagliflozin monotherapy in type 2 diabetic patients with inadequate glycemic control by diet and exercise A randomized, double-blind, placebo-controlled, phase 3 trial. Diabetes Care. 2010;33(10):2217–2224. doi: 10.2337/dc10-0612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kalra S., Ghosh S., Aamir A.H., Ahmed MdT., Amin M.F., Bajaj S. Safe and pragmatic use of sodium-glucose co-transporter 2 inhibitors in type 2 diabetes mellitus: south Asian Federation of Endocrine Societies consensus statement. Indian J Endocrinol Metab. 2017;21(1):210–230. doi: 10.4103/2230-8210.196029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Georgianos P.I., Agarwal R. Ambulatory blood pressure reduction with SGLT-2 inhibitors: dose-response meta-analysis and comparative evaluation with low-dose hydrochlorothiazide. Diabetes Care. 2019;42(4):693–700. doi: 10.2337/dc18-2207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Verma S., McMurray J.J.V. SGLT2 inhibitors and mechanisms of cardiovascular benefit: a state-of-the-art review. Diabetologia. 2018;61:2108–2117. doi: 10.1007/s00125-018-4670-7. [DOI] [PubMed] [Google Scholar]
- 66.Rastogi A., Bhansali A. SGLT2 inhibitors through the windows of EMPA-REG and CANVAS trials: a review. Diabetes Ther. 2017;8:1245–1251. doi: 10.1007/s13300-017-0320-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Heerspink H.J., Perkins B.A., Fitchett D.H., Husain M., Cherney D.Z.I. Sodium glucose cotransporter 2 inhibitors in the treatment of diabetes mellitus: cardiovascular and kidney effects, potential mechanisms, and clinical applications. Circulation. 2016;134:752–772. doi: 10.1161/CIRCULATIONAHA.116.021887. [DOI] [PubMed] [Google Scholar]
- 68.Abdelgadir E., Rashid F., Bashier A., Ali R. SGLT-2 inhibitors and cardiovascular protection: lessons and gaps in understanding the current outcome trials and possible benefits of combining SGLT-2 inhibitors with GLP-1 agonists. J Clin Med Res. 2018;10(8):615–625. doi: 10.14740/jocmr3467w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wanner C., Inzucchi S.E., Lachin J.M., Fitchett D., von Eynatten M., Mattheus M. Empagliflozin and progression of kidney disease in type 2 diabetes. N Engl J Med. 2016;375:323–334. doi: 10.1056/NEJMoa1515920. [DOI] [PubMed] [Google Scholar]
- 70.Gilbert R.E. Sodium-glucose linked transporter-2 inhibitors: potential for renoprotection beyond blood glucose lowering? Kidney. Int. 2014;86:693–700. doi: 10.1038/ki.2013.451. [DOI] [PubMed] [Google Scholar]
- 71.Pradhan A., Vohra S., Vishwakarma P., Sethi R. Review on sodium-glucose cotransporter 2 inhibitor (SGLT2i) in diabetes mellitus and heart failure. J Fam Med Prim Care. 2019;8:1855–1862. doi: 10.4103/jfmpc.jfmpc_232_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kalra S. Sodium-glucose Co-Transporter-2 (SGLT2) inhibitors: a review of their basic and clinical pharmacology. Diabetes Ther. 2014;5(2):355–366. doi: 10.1007/s13300-014-0089-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Singh A.K., Unnikrishnan A.G., Zargar A.H., Kumar A., Das A.K., Saboo B. Evidence-based consensus on positioning of SGLT2i in type 2 diabetes mellitus in Indians. Diabetes Ther. 2019;10:393–428. doi: 10.1007/s13300-019-0562-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ye Y., Zhao C., Liang J., Yang Y., Yu M., Qu X. Effect of sodium-glucose Co-transporter 2 inhibitors on bone metabolism and fracture risk. Front Pharmacol. 2018;9:1517. doi: 10.3389/fphar.2018.01517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Toulis K.A., Bilezikian J.P., Thomas G.N., Hanif W., Kotsa K., Thayakaran R. Initiation of dapagliflozin and treatment-emergent fractures. Diabetes ObesMetab. 2018;20:1070–1074. doi: 10.1111/dom.13176. [DOI] [PubMed] [Google Scholar]
- 76.Hong D., Si L., Jiang M., Shao H., Ming W.-K., Zhao Y. Cost effectiveness of sodium-glucose cotransporter-2 (SGLT2) inhibitors, glucagon-like peptide-1 (GLP-1) receptor agonists, and dipeptidyl peptidase-4 (DPP-4) inhibitors: a systematic review. Pharmacoeconomics. 2019;37:777–818. doi: 10.1007/s40273-019-00774-9. [DOI] [PubMed] [Google Scholar]
- 77.Pawaskar M., Bilir S.P., Kowal S., Gonzalez C., Rajpathak S., Davies G. Cost-effectiveness of DPP-4 inhibitor and SGLT2 inhibitor combination therapy for type 2 diabetes. Am J Manag Care. 2019;25(5):231–238. [PubMed] [Google Scholar]