Abstract
There is growing attention and effort focused on treating the root cause of sensorineural hearing loss rather than managing associated secondary characteristic features. With recent substantial advances in understanding sensorineural hearing-loss mechanisms, gene delivery has emerged as a promising strategy for the biological treatment of hearing loss associated with genetic dysfunction. There are several successful and promising proof-of-principle examples of transgene deliveries in animal models; however, there remains substantial further progress to be made in these avenues before realizing their clinical application in humans. Herein, we review different aspects of development, ongoing preclinical studies, and challenges to the clinical transition of transgene delivery of the inner ear toward the restoration of lost auditory and vestibular function.
Keywords: AAV, inner ear, sensorineural hearing loss
Graphical abstract

With recent substantial advances in understanding the mechanisms of sensorineural hearing loss (SNHL), gene therapy has emerged as a promising strategy for its treatment. There are several promising examples of gene therapy in animal models with SNHL; however, substantial advancements need to be made prior to their clinical application.
Introduction
Hearing loss (HL) is accompanied by substantial clinical implications affecting quality of life, including communication malfunction, abridged social interaction, seclusion, melancholy, diminished cognition, and dementia leading to poor quality of life.1 Two or three out of every 1,000 infants are diagnosed with clinically significant unilateral or bilateral HL. HL can be divided into conductive HL (CHL), which is caused by the issues of transferring sound waves anywhere along the pathway through the outer ear, tympanic membrane, or middle ear, or sensorineural HL (SNHL), which is caused by damage to the structures in the inner ear or auditory nerve, or a mixed CHL with SNHL form. Among all etiologies, SNHL is the most common type of HL, affecting ∼278 million individuals worldwide, among whom 1% are children. SNHL may be caused by an underlying disease, drug ototoxicity, noise exposure, aging, or genetic etiology leading to partial or complete loss of hair cells (HCs) or auditory neurons. Current statistical analysis of HL data suggests that 50% of congenital cases have a genetic etiology affecting ∼4,000 infants per year.2
The current treatment of SNHL involves the use of hearing aids or cochlear implants, which are both limited by their total amplification and resultant clarity, along with additional barriers to universal clinical benefit.3 Hearing aids worn in ear primarily amplify acoustic waves, whereas cochlear implants are surgically placed to directly access the cochlea via the round window (RW) or cochleostomy (CO), translating acoustic wave to electric signals that stimulate the auditory nerve and send signals to the brain for comprehension of sound. An implantation age-dependent learning curve is associated with hearing aids (2 weeks or less) and cochlear implants (6−12+ months) requiring special assistance or guidance of general audiologists or specially trained implant audiologists. Broad application of the cochlear implant is limited due the outcomes. Although cochlear implant technology has progressed rapidly over recent years, these implants cannot completely replace the function of the inner ear leading to partially restored hearing. These limitations have led to growing attention and effort focused on treating the root cause of hearing impairment rather than treating secondary characteristic features with a one-size-fits-all approach. With recent substantial advances in understanding SNHL’s molecular mechanisms, gene therapy has emerged as a promising strategy for restoring hearing with targeting to different inner-ear molecular pathologies.4
Gene delivery is a multifactorial process reliant on multiple simultaneous avenues of scientific advancement, including the genetic etiology of deafness to be treated, gene sequence to be used, vectors used for delivery, route of delivery, treatment time point, and cost to be incurred for its efficacy and successful translation to the clinic.5 Vectors used for gene therapy facilitate the transportation of DNA into cells, usually to be classified as non-viral, viral, and hybrid vectors. Non-viral methods can easily be scaled up for large production and possess low host immunogenicity; however, these methods suffer from low gene transfer efficiency compared to viral vectors.6,7 Currently, viral vectors dominate clinical trials in gene therapy due to higher transduction rates compared to non-viral methods. Unlike non-viral vectors, viruses (lytic or lysogenic) bind to host cells, use host replication machinery to replicate their genetic material, and reside in the host for an extended period before responding to a trigger.8,9 Commonly used viral vectors in gene therapy include herpes virus, vaccinia virus, retrovirus, adenovirus (AV), alphavirus, lentivirus, and adeno-associated virus (AAV). Retroviruses and lentiviruses are not commonly used due to the known risk of integration into the host genome that can disrupt gene function or lead to oncogene activation.10,11 Hybrid vectors are among the least explored in gene therapy. They are a combination of both viral and chemical vectors, which allows them to overcome their limitations when working independently. The hybrid vectors augment desirable features such as targeting ability, low immunogenicity, improved cytotoxicity, higher payload, and the ability to deliver more than one transgene. These hybrid vectors can evade the host immune system through masking the immunogenic epitopes present on viral vectors and have been reported to have higher transduction efficiency than viral or non-viral strategies.12, 13, 14 AAV is the most frequently investigated gene-delivery vehicle owing to its lack of pathogenicity, persistence, availability of various serotypes (which specify its host tissue/cell targeting), and low risk of insertion mutagenesis due to lower host DNA integration.15 This review will focus on the biology of AAV-based gene therapy in treating genetic deafness in humans.
AAV biology, types, and tropism
AAV biology
AAV, a replication-deficient virus from the family Parvoviridae genus Dependoparvovirus, is composed of an ∼26-nm-diameter icosahedral protein capsid containing ∼4.7 kb single-stranded DNA (ssDNA) genome (sense/antisense strand). AAV can replicate by co-infection with a helper virus (AV/herpes virus/vaccinia virus) or in certain hostile conditions, like severe stress to the cell or host due to UV/cytotoxic chemical treatment independent of the helper virus but in a limited fashion.16 The AAV ssDNA contains three genes: Cap (responsible for capsid synthesis by encoding 60 molecules of collinear capsid proteins [VP1, VP2, and VP3 (3:3:54)], identical in their C-terminal portion), Rep (controls viral replication by encoding Rep78, Rep68, Rep52, and Rep40), and AAP (supports virion assembly programmed through the Cap coding sequence using a different reading frame).17 Two T-shaped inverted terminal repeats (ITRs) flank the genome and function as the viral origin of replication and signal for packaging.18 Recombinant AAVs (rAAVs) are genetically engineered AAVs with a similar capsid sequence to wild-type AAV but lack AAV Rep and Cap genes, so the only viral DNA sequences retained in the vector genome are the two ITRs. Without Rep, rAAV does not efficiently integrate into the host genome, making it a non-pathogenic, non-replicative vector.
AAV transduction involves a cascade of simultaneously occurring events, including attachment, internalization, endosomal cytosolic processing, nucleus trafficking, viral uncoating, and integration into host cells (Figure 1). AAV vector attaches to the host membrane through specific surface receptors. In the case of AAV2, it primarily attaches to the heparan sulfate proteoglycan (HSP) receptor; however, a few co-receptors have also been identified, including hepatocyte growth factor receptor (HGFR), fibroblast growth factor receptor I (FGFR1), and ανβ5 integrin. Many binding receptors have been identified by overexpression/inhibition experiments for various AAV serotypes, including HSP (for AAV2, -3, and -6), N-linked sialic acids (for AAV1, -5, and -6), O-linked 2,3-sialic acid (for AAV4), N-terminal galactose (for AAV9), and 37/67 kDa laminin receptor (for AAV3, -8, and -9).19 Various serotypes recognize discrete cell receptors demonstrating diverse tissue/cell-type tropism profiles. Successful recognition of the surface receptor leads to AAV internalization via endocytosis in a receptor-mediated manner through clathrin-coated pits.20 AAV is likely to trek through the Rab5+ early endosomes, Rab7+ late endosomes, and Rab 11+ recycling endosomes before finally reaching the Golgi apparatus where endosome acidification takes place.21 After cytosolic trafficking and endosomal escape, AAV enters the nucleus, and ssDNA is converted to double-stranded (ds)DNA by either second-strand synthesis using host machinery or by annealing via Watson-Crick base pairing once “+” and “—” stranded genomes in separate virions reach the nucleus.22 Synthesized viral dsDNA undergoes circularization and concatemerization by intra-/inter-molecular recombination of ITRs, leading to stability of episomal viral DNA resulting in the expression of a gene of interest in cells after mitosis.
Figure 1.
Schematic representation of intracellular AAV transduction via a receptor-mediated pathway
See also Schultz and Chamberlain.19
AAV vectors used for inner-ear gene delivery
rAAV has been explored successively in a variety of genetic disorders such as hemophilia, retinitis pigmentosa, cystic fibrosis, San Filippo A, and the muscular dystrophies.23 AAVs, although endemic in humans, have not been related to any life-threatening disease in various preclinical studies conducted. They have been explored extensively in inner-ear in vivo preclinical studies (Table 1) in a variety of genetic defects.32, 33, 34 Twelve natural serotypes of AAV (1−12) have been characterized to date, having differential tropism and transduction potential in vasculature, retina, brain, muscle, liver, and lung. AAV1, -2, and -8 have been reported to transduce outer HCs (OHCs), whereas AAV1, -2, -3, -5, -7, -8, and -9 have been detected in the inner HCs (IHCs) of the inner ear.25 AAV3 has been demonstrated to infect IHCs selectively with high efficiency in the middle and basal cochlear regions when injected through the RW membrane (RWM).35 The supporting cells (SCs) of the organ of Corti in the inner ear are also reported to be transduced by AAV. Transduction of pillar cells has been reported by AAV1, -2, and -8; Claudius cells are transduced by AAV1, -2, -5, -7, and -8; and Deiters cells were positively infected by AAV1 and -2. AAV1−4, -7, and -8 have shown their efficiency in transducing the spiral limbus area, i.e., limbus, ganglion cells, and ligament.25 In order to improve transduction efficacy and tropism, extensive studies have been performed for pseudotyping, capsid engineering, or exosomes synthesis from naturally occurring AAV. AAV pseudotyping/hybrid AAV strategy utilizes the ITR genome of one AAV (the most commonly reported being AAV2) and capsid genome of another to tailor the tropism/efficacy. Six AAV2-based pseudotyped AAV2/1, -2/5, -2/7, -2/8, and -2/9 serotypes using cytomegalovirus (CMV) hybrids have been studied in guinea pig cochlea for their tropism and efficiency by CO via perilymph injection with AAV2/2 most efficient among all. Another study showed the safety and efficacy of AAV2/1 in utero cochlear gene transfer, transducing progenitor cells that transdifferentiate to IHC, OHC, and SC.36 AAV2/5, having a CMVEGFP cassette, showed specific tropism for the SC of the organ of Corti’s in both ex vivo mouse cochlear explants and in vivo studies in the adult guinea pig by scala media perfusion.37 Capsid engineering facilitated the rebuilding of ancestral sequences, and to date, nine functional ancestral AAVs have been synthesized. AAV2/Anc80L65, a novel designer AAV imputed from an ancestral sequence of AAV1, -2, -8, and -9, is a robust synthetic carrier reported for in vivo cochlear gene therapy.29 AAV2/Anc80L65 with a CMV-driven EGFP transgene cassette has been reported to show high efficiency with established safety in transducing IHC and OHC via RWM injection in C57BL/6 mice. Literature suggests that promoters, to some extent, drive specific AAV tropism; like the CMV-beta-globin hybrid promoter supports HC transduction, whereas the chicken β-actin (CBA) promoter drives SC transduction.21 Recent studies on AAV2.7m8 showed superior transduction efficiency to sensory cells (IHCs and OHCs), inner pillar cells, and inner phalangeal cells compared to Anc80L65.31 Further, more sophisticated approaches to tailor tropism and efficacy include the engagement of small bioactive molecules like peptides, ligands, bispecific antibodies, or biotin (interacts with both viral proteins and host surface) to the viral capsid to attain host targeting. For instance, AAV, i.e., designed with the CAG promoter and peptide “DGTLAVPFK,” has been demonstrated to cross a membrane-like structure leading to high transduction efficiency in HCs and SCs in C57BL/6 mice via RWM injection.34 Also, nanosized cell-secreted vesicles required for regular intercellular communication in AAV, known as exosomes, have demonstrated excellent transduction efficiency in both ex vivo and in vivo studies post-RWM or CO injection in lipoma HMGIC fusion partner-like 5 (Lhfpl5)/tetraspan membrane protein of HC stereocilia (Tmhs)−/− mutant mice, demonstrating partial recovery in hearing and balance dysfunction.38 Nevertheless, a detailed study on the possible side effects for long-term use of exosomes needs to be explored, as they constitute a variety of biomolecules including protein, RNA, and other nucleic acids. Artificial exosomes can act as an alternative for AAV packaging to avoid safety issues in clinics.
Table 1.
Tropism profile of commonly used AAVs for inner-ear transduction
| AAV subtype | Model | Promoter | In vitro/in vivo | Dose | Outcome |
Reference | ||
|---|---|---|---|---|---|---|---|---|
| Transduction IHC | Transduction OHC | Other transduced components | ||||||
| AAV2/1 | C57BL/6J | CBA | in vivo (P0−P2) | RWM (1 μL) 6 × 1012 genome copies (GC)/mL | 59% ± 2% | sporadic expression in the basal half of the cochlea | vestibular (hair cells [HCs] and SC) | 24 |
| CMV | in vivo (P0−P2) | RWM (1 μL) 4.5 × 1014 GC/mL | 70% ± 9% | sporadic expression in the basal half of the cochlea | vestibular (HCs and SC) | 24 | ||
| AAV1 | CD1 | CBA | in vivo (P1 –P2) | cochleostomy to scala media (∼0.2 μL) 1–8 × 1012 GC/mL | A - 5.6 ± 2.1 | A - 3.2 ± 2.1 | SC (B - 2.5 ± 2; M - 1.2 ± 1) | 25 |
| M - 13.6 ± 1.2 | M - 13.4 ± 1.83 | |||||||
| B - 16.5 ± 2.64 | B - 15.5 ± 1.9 | |||||||
| in vivo (6 weeks old) | cochleostomy to scala media (∼0.2 μL) 1–8 × 1012 GC/mL | A - 12.2 ± 2.3 | − | SC (B - 8.2 ± 2.8; M - 4.1 ± 1.4; A - 2 ± 1.2) | ||||
| M - 24.1 ± 6.2 | ||||||||
| B - 45.8 ± 7.3 | ||||||||
| AAV2 | CBA/J mice | CAG | in vivo (P0−P5) | posterior semicircular canal injection (1 μL); 5.69 × 1012 GC/mL | 43.6% ± 13.5% | 54.5% ± 12.7% | utricle HC - 32.4% ± 6.16% | 21 |
| inner pillar cell - 60.3% ± 7.96% | ||||||||
| inner phalangeal cell - no infection | ||||||||
| CD1 | CBA | in vivo (P1 –P2) | cochleostomy to scala media (∼0.2 μL) 1–8 × 1012 GC/mL | A - 4.6% | A - 3.1% | SC (B - 7.1%; M - 2.4%) | 25 | |
| M - 11.4% | M - 33.3% | |||||||
| B - 19.3% | B - 39% | |||||||
| CBA/CAJ | in vivo (6 weeks old) | A - 13.2% ± 2.1% | no transduction | SC (B - 13.6% ± 4.5%; M - 6% ± 1.4%; A - 1.6% ± 0.7%) | ||||
| M - 27.2% ± 4.5% | ||||||||
| B - 35.2% ± 6.3% | ||||||||
| AAV2 quadY-F | C57BL/6 | CMV | in vivo (P2) | RWM (2 μL) 1013 viral genomes (vg)/mL | 78% ± 6% | transduces OHC sporadically | transduces pillar cells sporadically | 26 |
| AAV5 | CD1 | CBA | in vivo (P1 –P2) | cochleostomy to scala media (∼0.2 μL) 1–8 × 1012 GC/mL | A - | − | SC (B - 2.2% ± 1%) | 25 |
| M - 11.2% ± 2.9% | ||||||||
| B - 28.1% ± 3.4% | ||||||||
| AAV6.2 | CD1 | CBA | in vivo (P1 –P2) | cochleostomy to scala media (∼0.2 μL) 1–8 × 1012 GC/mL | − | − | SC (B - 11.4% ± 2%) | 25 |
| CBA/CAJ | in vivo (6 weeks old) | cochleostomy to scala media (∼0.2 μL) 1–8 × 1012 GC/mL | A - 10.5 ± 1.5 | − | SC (B - 3.5 ± 1.3; M - 1.9 ± 1.1) | |||
| M - 18 ± 2.1 | ||||||||
| B - 28 ± 4.8 | ||||||||
| AAV7 | CD1 | CBA | in vivo (P1 –P2) | cochleostomy to scala media (∼0.2 μL) 1–8 × 1012 GC/mL | A - 3.1% ± 0.8% | − | SC (B - 3.6 ± 0.8; M - 2.6 ± 1.1) | 25 |
| M - 16.2 ± 2.6 | ||||||||
| B - 20.5% ± 2.5% | ||||||||
| AAV2/8 | CBA/J mice | CAG | in vivo (P0−P5) | posterior semicircular canal injection (1 μL); 1.10 × 1013 GC/mL | 86.0% ± 5.34% | 51.7% ± 5.95% | utricle HC - 93.3% ± 2.15% | 21 |
| inner pillar cell - 50.4% ± 7.49% | ||||||||
| inner phalangeal cell - no infection | ||||||||
| AAV8 | Institute of Cancer Research (ICR) mice | in vivo (P1) | RWM (0.6 μL) 1 × 1010 GC/mL | A - 98.94% ± 1.30% | no transduction | transduction in SC very low | 27 | |
| M - 76.83% ± 27.41% | 5.38% ± 0.63% | |||||||
| B - 73.91% ± 17.15% | ||||||||
| CD1 | CBA | in vivo (P1 –P2) | cochleostomy to scala media (∼0.2 μL) 1–8 × 1012 GC/mL | A - 6.1% ± 1.3% | A - 4.2 ± 0.9 | SC (B - 6.8% ± 1.5%; M - 4.1% ± 2%) | 25 | |
| M - 18.7% ± 1.7% | M - 14.2 ± 2.1 | |||||||
| B - 21% ± 2.5% | B - 15 ± 3.1 | |||||||
| CBA/CAJ | in vivo (6 weeks old) | cochleostomy to scala media (∼0.2 μL) 1–8 × 1012 GC/mL | A - 18.5 ± 1.8 | − | SC (B - 10.1 ± 3.9; M - 5.8 ± 2.1; A - 3.1 ± 0.9) | |||
| M - 22.2 ± 3.9 | ||||||||
| B - 51.2 ± 7.5 | ||||||||
| AAV8BP2 | CBA/J mice | CAG | in vivo (P0−P5) | posterior semicircular canal injection (1 μL); 1.10 × 1013 GC/mL | 55.7% ± 9.53% | 44.1% ± 7.94% | utricle HC - 34.2% ± 9.84% | 21 |
| no GFP expression in the inner pillar cells and inner phalangeal cells | ||||||||
| AAV9 | ICR mice | CAG | in vivo (P1) | RWM (0.6 μL) 1 × 1010 GC/mL | A - 98.41% ± 1.94% | 33.62% ± 13.72% | transduction in SC very low | 28 |
| M - 92.05% ± 5.06% | 11.10% ± 2.70% | |||||||
| B - 69.16% ± 20.17% | ||||||||
| CD1 | CBA | in vivo (P1 –P2) | cochleostomy to scala media (∼0.2 μL) 1–8 × 1012 GC/mL | A - 4.2% ± 0.9% | − | SC (B - 6.1% ± 1.5%; M - 3.2 ± 11) | 25 | |
| M - 16.2% ± 2.3% | ||||||||
| B - 21% ± 3.1% | ||||||||
| CBA/CAJ | in vivo (6 weeks old) | cochleostomy to scala media (∼0.2 μL) 1–8 × 1012 GC/mL | A - 9.1 ± 1.4 | − | SC (B - 6.2 ± 3.1; M - 4.1 ± 2.1; A - 2.1 ± 0.9) | |||
| M - 35.1 ± 3.2 | ||||||||
| B - 61.6 ± 8 | ||||||||
| AAVrh.10 | CD1 | CBA | in vivo (P1 –P2) | cochleostomy to scala media (∼0.2 μL) 1–8 × 1012 GC/mL | A - 8.1% ± 2% | − | SC (B - 5.2% ± 1.5%; M - 3.2% ± 0.8%) | 25 |
| M - 24% ± 4.7% | ||||||||
| B - 34% ± 5.7% | ||||||||
| AAVrh.43 | CD1 | CBA | in vivo (P1 –P2) | cochleostomy to scala media (∼0.2 μL) 1–8 × 1012 GC/mL | A - | − | SC (B - 12.1 ± 3.9; M - 8.9% ± 2.8%; A - 4.5% ± 1.7%) | 25 |
| M - 3 ± 1.1 | ||||||||
| B - 5.3% ± 2.1% | ||||||||
| AAV-PHP.eB | ICR mice | CAG | in vivo (P1) | RWM (0.6 μL) 1 × 1010 GC/mL | A - 100.00% ± 0.00% | A - 98.6% | SC - not done | 28 |
| M - 99.07% ± 1.13% | M - 96.2% | |||||||
| B - 100.00% ± 0.00% | B - 97.6% | |||||||
| AAV-DJ | ICR mice | CAG | in vivo (P1) | RWM (0.6 μL) 1 × 1010 GC/mL | no transduction | no transduction | SC - 52.51% ± 0.96% | 28 |
| AAV2/Anc80L65 | C57BL/6J | CMV | ex vivo (harvested P4) | 1010 GC/mL | 60%−100% | 60%−100% | utricle HC - 67.7% ± 2.46% | 29 |
| apical and base | apical and base | |||||||
| in vivo (P0−P2) | RWM (1 μL) 1.7 × 1012 GC/mL | 100% IHC (base) | 95% OHC (base) | human vestibular epithelia (HCs and SC) | ||||
| 83% HC in vestibular epithelia were transduced | ||||||||
| in vivo (embryonic otocyst < E12) | in utero (1 μL) 2.52 × 1012 GC/mL | 91%−97% | 84%−94% | vestibular HCs - 92.6% | 30 | |||
| spiral ganglionic neurons - 96.7% | ||||||||
| CBA/J mice | CAG | in vivo (P0−P5) | posterior semicircular canal injection (1 μL), 1.89 × 1013 GC/mL | 94.0% ± 3.63% | 67.0% ± 4.32% | utricle HC - 67.7% ± 2.46% | 21 | |
| inner pillar cell - 75.3% ± 4.94% | ||||||||
| inner phalangeal cell - no infection | ||||||||
| AAV2.7m8 | CBA/J mice | CAG | in vivo (P0−P5) | posterior semicircular canal injection (1 μL) 9.75 × 1012 GC/mL | Average (avg.) - 84.1% ± 5.66% | avg. - 83.1% ± 6.17% | utricle HC - 27.5% ± 9.65% | 21 |
| apex - 90.3% ± 8.98% | apex - 89.0% ± 9.53% | inner pillar cell - 86.1% ± 4.56% | ||||||
| middle - 84.6% ± 10.4% | middle - 85.2% ± 10.9% | inner phalangeal cell - 61.4% ± 9.30% | ||||||
| base - 77.5% ± 10.8% | base - 74.9% ± 12.2% | |||||||
| in vivo (1–6 months old) | posterior semicircular canal injection (1 μL) 9.75 × 1012 GC/mL | 84.5% ± 4.91% | 74.9% ± 6.53% | − | ||||
| AAV-ie | C57/B6 | CAG | in vivo (P2–P3) | RWM injection (1.5 μL) 1 × 1010 GC/mL | A - 100% | A - 95%–100% | SC (A - 81%; M - 77%; B - 62%) | 31 |
| RWM injection (2.0 μL) | M - 100% | M - 75%–80% | mouse utricle (utricular SCs - 93%; HC - 76%) | |||||
| B - 100% | B - 60%–70% | SGNs were also transduced | ||||||
One of the critical shortcomings of AAV is that the small cargo (4.8 kb) capacity and cargo sizes larger than 4.8 kb lead to instability of vector.39,40 However, genetic mutations in large genes affect a substantial number of patients in various age groups that could be treated by gene therapy, including cDNAs encoding cadherin-23 (CDH23; 10 kb), otoferlin (6 kb), myosin 15A (MYO15A; 10.6 kb), otogelin-like (7 kb), myosin 7A (MYO7A; 6.5 kb), protocadherin-15 (PCDH15; up to 5.9 kb), and otogelin (8.8 kb).41 Different strategies have been utilized to develop dual AAV vectors, including overlapping, trans-splicing, and hybrid AAV dual vectors.41, 42, 43 Overlapping dual AAV involves intentionally overlapping two specified sequences of demarcated fragments of the target transgene in two AAVs, and the joining of two transgenes to a single transgene occurs from a sequence of overlap.44,45 Dual-overlapping AAV has a capacity of 8−8.5 kb, as the overlapping segment length is limited by the size of the target cDNA, but it requires extensive background research to optimize the design of overlap regions for new therapies in order to avoid the unwanted transgene products.46 Another approach, “trans-splicing of the transgene,” utilizes splice sequences to split the target transgene sequence into two halves to be carried by two AAVs and then reassembled inside the host to generate the original transgene sequence. The concatemerized ITR structure of the transgene will be removed via native cellular mechanisms through transcription.47, 48, 49 Trans-splicing AAV dual-vector strategy resulted in superior transgene expression post-transduction compared to overlapping AAV dual vectors but requires additional foreign genetic material, efficient transcript processing, and dependency on the inefficient concatemerization process and runs the risk for potentially unwanted transgene products.42,50 The hybrid dual-vector strategy offers a solution to the concerns involved with techniques discussed above by combining overlap regions with splice donors/acceptors in a dual-vector transgene.50,51 The approach utilizes highly recombinogenic genes (like phage DNA) in addition to their splice sequence supporting the correct orientation of dual AAV vector two halves. Unlike in overlap strategy, customized DNA sequence designing is not required for each gene therapy once a universally suitable sequence has been optimized. However, the vector is still introducing foreign DNA into the cell, which may trigger an immunogenic response.42,44,50,52 Recently, the hybrid dual AAV has been utilized for the delivery of the Otof (cDNA ∼6 kb) gene in Otof−/− mutant mice leading to a reversal of the deafness phenotype.26,53
Transport barrier, targets, and delivery strategies for inner-ear gene delivery
The development of gene therapies for the inner ear is a challenging task requiring appropriate consideration of transport barriers for vector selection, sorting targets to maximize efficiency, characterizing the optimum therapeutic window for treatment, and identifying the best route and strategy for high-throughput delivery.
Transport barriers
The inner ear is a closed compartment disconnected from systemic circulation by the blood cochlea barrier/blood-labyrinthine barrier (BLB) and the middle ear by the RWM. These two complicating characteristic features of the inner ear make transportation of drugs or biological material to the organ of Corti and vestibular labyrinth difficult. The BLB shares similar properties to the blood-brain barrier and is composed of vascular endothelial cells with tight junctions. The BLB separates blood/perilymph, blood/endolymph, and endolymph/intra-strial fluid restricting flow toward or away from the inner ear. Once a therapeutic crosses the BLB via diffusion or injection, elevated concentrations may occur in the cochlea due to diminished distribution, thereby increasing the probability of toxicity. The BLB restricts the movement of high molecular weight molecules, although some drugs can cross if sufficiently lipophilic. Drugs or molecules can be transported across BLB using specific/nonspecific endocytosis, ion exchangers, transporters, or ion channels. For example, aminoglycosides, an ototoxic class of antibiotic, cross the strial and perilymphatic BLB readily through an unknown mechanism hypothesized to be via intracellular transport by saturable uptake kinetics. BLB permeability can also be altered by external factors including osmoregulators (glycerol, diuretics), inflammation, and acoustic trauma.54
The RWM is a semi-permeable, tri-membranous structure with its middle layer composed of connective tissue sandwiched between two epithelial layers connecting the inner ear to the middle ear.55 The permeability of RWM varies in both inter- and intraspecies depending on the thickness, size, and charge of the RWM, as well as the nature of the therapeutic to be applied. For instance, RWM thickness is ∼70 μm in humans, 10−14 μm in rodent chinchilla, 12 μm in rats, and 10−30 μm in guinea pigs.55 Studies have shown that a 1-μm microsphere can transverse through the RWM of a Chinchilla, but a 3-μm is not able to cross it; lower molecular weight molecules can cross, but higher molecular weight molecules do not readily cross through RWM, and cationic ferritin has been reported to cross the intact RWM, but anionic molecules fail to pass.55, 56, 57, 58, 59, 60 Additionally, the rate of cationic ferritin movement across the RWM was higher in rodents than in cats and primates due to RWM thickness variation.57 In addition to the BLB and RWM, the protective membranous labyrinth enclosing the cochlea provides structural limitations for access to the inner ear.
Targets
Inside the cochlea, therapeutics can act on sensory or non-sensory cells as a target for gene therapy.
Non-sensory cells
SCs, such as the otocyst-derived epithelium lining the scala media around the organ of Corti, serve as a frequent gene therapy target for genetic deafness (like the gap junction protein, beta 2 [GJB2], gene leading to HL due to the connexin 26 [Cx26] mutation), with budding applications in regenerative treatments.61, 62, 63 Regenerative therapies are based on the principle that HC loss from the vestibular or auditory sensory epithelium in non-mammalian vertebrates’ regenerates simultaneously from trans-differentiation of SC.64, 65, 66 Although the mammalian auditory system’s HCs do not regenerate, there are reports of limited regeneration ability in mammalian vestibular tissue by phenotypic conversion from SCs, particularly in early developmental stages.67,68 Many studies are currently exploring trans-differentiation induction by introducing sets of genes for forced expression, like trans-differentiation of adult mouse cochlear SCs by overexpression of the Atoh-1 transcription factor in vitro using transient MYC and NOTCH activities.69, 70, 71 Mechanotransduction of sound in cochlear HC depends on the electrochemical difference between cochlear fluid, i.e., perilymph and endolymph. The stria vascularis (SV), a highly vascularized epithelial tissue, is responsible for endolymph generation and maintenance in the scala media. Mutations in marginal cells can cause dysfunction of gap junctions affecting the endocochlear potential (EP) and apoptosis of HC, leading to hearing impairment, as in KCNQ1/KCNE1, pannexin, and others.72,73
Sensory cells
The IHCs are the true sensory cells that transmit impulses via the auditory nerve, whereas the OHCs facilitate both qualitative (by increasing selectivity) and quantitative amplification (by increasing sensitivity) of the signal. At birth, the human cochlea has 3,500 IHCs in one row and 12,000 OHCs in 3 rows. Mutation or degeneration of these sensory cells causes hearing impairment. IHC and OHC are the most studied target in genetic or environmental acquired diseases like Usher syndrome (USH)III, TMC1 (transmembrane channel-like 1) mutation, VGLUT3 (glutamate transporter-3 vesicular) mutation, noise-induced HL (NIHL), and ototoxicity due to drugs (like cisplatin, aminoglycosides, and others).74, 75, 76, 77, 78, 79 These auditory signals are transmitted to the brain via spiral ganglion neuron (SGN), with type I SGNs (90% of the total SGN population) connecting to IHCs and type II SGNs (5%–10%) connecting to OHCs. Brain-derived neurotrophic factors (BDNF) and neurotrophin-3 (NT-3) are expressed in HCs and SCs in the developing organ of Corti and are essential for normal function of SGNs.80,81 SGN degeneration is caused by disoriented synapse ribbons, damaged SGN cells, or underlying mutations in sensory cells, causing non-syndromic hearing deterioration.82
Time of treatment
Early intervention is the best strategy for the treatment of any hearing impairment, and it remains critically important for gene therapies of the inner ear by increasing the probability of rescuing both cell and organ functions. For instance, treatment of VGLUT3 mutation using AAV1 injection on postnatal days 1−2 (P1−P2) mice led to better HC transduction and auditory restoration as compared to a later time point, i.e., P10.75 Similar observations were found when AAV5-GJb2 was injected at P42 in GJb2 knockout (KO) mice or AAV2/Anc80L65-USH1c at P10−P12 in Ush1c KO mice. Once HL occurred, any treatment at a later time point was unable to rescue degeneration in rodent models, owing to a closed therapeutic window for treatment.83,84 As an additional concern regarding the timing of intervention, many genetic disorders can be hereditary, i.e., congenital or developed at a specific developmental period depending on the type of mutation. Rescuing hearing in cases of early/congenital onset (like SIXI, CHD7, and EYA1 mutations) is essential, as it adversely affects the development of other functions related to hearing, such as language, social, and cognitive function.85, 86, 87 A recent genetic breakthrough was reported in an OTOF−/− mouse model, wherein HL was either prevented or recovered by delivering a gene of interest both before and after the onset of HL, leading to the introduction of a new paradigm for interventions in mutation-specific treatments.26,53 However, it is still important to consider the notable difference in the developmental process of mice and human cochlea. In mice, the cochlea continues to develop after birth, maturing between P16 and P18, whereas the human cochlea matures before birth. This time discrepancy provides a larger therapeutic window for rescue studies in mice, whereas humans may require in utero gene therapy to treat congenital/developmental gene mutations.
Delivery routes
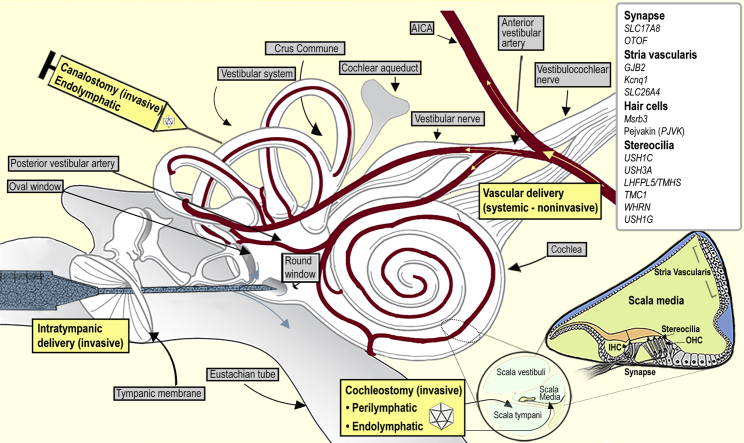
Various surgical strategies can be considered to safely deliver therapeutic agents to the cochlea, whereas not adversely affecting the native structure and functionality (Figure 2). The strategies applied for the peri-lymphatic or endolymphatic delivery of therapeutics include direct injection through the RWM (peri-lymphatic delivery),88, 89, 90 CO to the scala tympani (peri-lymphatic delivery),91,92 CO to the scala media (endolymphatic delivery),93,94 and semicircular canal canalostomy (endolymphatic delivery).95,96 The peri-lymphatic approach is comparatively safer and has been used clinically in cochlear implantation in humans.97 Endolymphatic delivery is comparatively more complex, leaving the inner ear vulnerable to damage of its innate structure/function, making it clinically unfeasible. However, there is ongoing research to establish safer delivery approaches to the endolymphatic space in murine models.94,98 The ultimate objective for successful clinical practice is to develop a non-invasive technique for delivering transgene to the inner-ear cells of interest. The different cochlear transgene delivery approaches explored are discussed below.
Figure 2.
Schematic demonstrating inner-ear gross anatomy, transport barriers, AAV delivery routes, and anatomical location of commonly addressed preclinical inner-ear mutation leading to hearing loss
See also Ahmed et al.82
Systemic route
A systemic injection is not a well-explored method for cochlear delivery due to higher probabilities of off-target delivery, unwanted side effects, toxicity, or BLB-restricted transportation. In rodents, including mice and rats, the BLB develops and matures even after birth until P14,99,100 providing a broader therapeutic window for studying hearing impairment during the developmental process. Shibata and colleagues101 injected rAAV2/9 intravenously via the systemic route in P1 wild-type mice and reported the successful gene transduction of IHC, vestibular HC, and the SGN. The transduction efficiency was dependent on dose, virus serotype, and the age of injection. Transduction was observed binaurally in HC along the whole length of the cochlea (i.e., from apex to base with 96% at the apical turn), and hearing ability was unaffected in treated mice up to 30 days of study. Further studies exploring different serotypes and their tropism profiles will be required to improve targeting for increased specificity and subside off-target effects. Other obstacles impacting efficacy include host immune response, neutralizing antibodies, and blood clearance of virus particles, requiring additional consideration before designing new vectors for systemic delivery.102,103
Intra-cochlear route
Intra-cochlear delivery transports viral vectors via the RWM or oval window (OW) to the scala tympani (perilymph) or scala media (endolymph), respectively. The OW connects to the inner ear from the middle ear via the stapes, and the delivery approach requires a transcanal or transmastoid microsurgical procedure for its access in humans. In rodents, the smaller size, shape of the bulla, and its relative anatomical position to the cochlea facilitate easier visualization of the RWM than the OW. The RWM is a tri-layered, membranous structure connecting the middle ear to the inner ear lying anatomically inferior and posterior to OW. Since the RWM is more easily accessible to various therapeutic approaches than the OW approach, it is more frequently explored for gene delivery in animal models. Thus far, perilymph delivery of AAV via RWM injection has been shown to partially rescue hearing with TMC1, TMC2, and USH1C mouse models.74,84 However, this approach has significant adverse effects, such as perilymphatic fluid leakage, virus transportation to the cerebellum, and cross-transfer to the contralateral inner ear through cochlear aqueduct, hematogenous, or systematic spread via temporal bone marrow.103,214 These adverse effects can result in further permanent hearing damage and life-threatening complications. The risk of perilymph leakage can be mitigated by plugging fascia to RWM perforations, but the outcome is unpredictable.104,105 Another unwanted effect observed in intra-cochlear administration is restricted viral distribution secondary to the low flow rate of cochlear fluid in adult mice. After RWM injection, a high local concentration of viral vectors was found with an efficiency gradient from base to apex due to slow distribution and subsequently, poor transduction leading to a high therapeutic concentration in the basal area but sub-therapeutic in the apical area.104
Canalostomy
Canalostomy, delivery of virus/biomolecules to the semicircular canal, has been applied in rodents to deliver AAV to the cochlea with an analogous process feasible in humans via transmastoid surgery. Thus far, multiple AAV serotypes have been delivered to the organ of Corti by canalostomy, resulting in successful transduction of IHCs and OHCs without adversely affecting native cochlear function.95,96 Of note, combinatorial treatment via RWM injection and semicircular canal fenestration (CF) led to higher transduction efficiency due to uniform AAV distribution provided by CF reducing intracochlear AAV gradient promoting the longitudinal flow of AAV throughout the cochlea.105 The superiority of the canalostomy over RWM/OW delivery needs to be studied in detail before translation to clinics in humans, as it is comparatively more invasive with potential complications similar to those experienced following superior canal dehiscence syndrome (SCDS) repair, including significant (albeit temporary) post-operative vertigo and risk of total HL due to leakage or loss of endolymphatic fluid.106
Trans-tympanic route
This approach has been extensively explored for the delivery of drugs and depends on the absorption or permeation of injected material from the middle ear to the inner ear through the intact RWM. There have been attempts to use gel foam for sustained delivery of intracochlear diffusion on an intact RWM for cochlear gene delivery, but it has failed to deliver significant transgenes to transduce HC, suggesting that the RWM is not permeable to rAAV.107 Another study explored rAAV transduction to the cochlea via RWM by using collagenase I or II, which increased efficiency compared to those untreated; however, the results were inferior to that achieved from direct injection through RWM and may damage the RWM structure.88 Cationic liposomes and a few viral vectors like AV can transduce through the RWM pathways similar to the diffusion of small drug molecules, yet transduction efficiency is low.107 Another study developed TAT dsRNA-binding domains (TAT-DRBDs) to enhance the delivery of short interfering RNA (siRNA) across the intact RWM in the chinchilla inner ear, demonstrating successful transfection of IHC, OHC, macula sacculi, macula utriculi, and crista ampullaris.108 Transtympanic strategy currently holds the advantages of non-invasiveness, widespread clinician familiarity with the technique, and shorter treatment time requiring only local anesthesia. At the same time, its application is curbed by restricted permeability, variability in RWM thickness (both inter- and intraspecies), as well as the non-significant fluid movement inside the cochlea.
Delivery strategies
Various pathologies and molecular mechanisms can cause hearing impairment, making it vital to develop a specific or combinatorial strategy to treat discrete disorders. The treatment strategy of inner-ear gene therapy for SNHL includes replacement, silencing, or editing of a target gene, which is chosen after considering the various factors (Table 2). Gene replacement is the most common treatment strategy used in inner-ear gene therapy to date, delivering a copy of the wild-type gene of interest to the inner ear. Gene replacement strategy is recommended for mutations leading to loss of function or variation in splicing, resulting in recessively inherited diseases, or in the case of haploinsufficiency, dominantly inherited diseases. Gene replacement strategy has been successfully applied in vivo in the HL mutant animal model of TMC1, Vglut3, Whirlin, GJB2, and Clarin-1 (CLRN1).61,74, 75, 76,115 For successful gene replacement treatment, there should be an appropriate treatment window, stable expression of the gene, or reintroduction at a different period to maintain function. However, mutations leading to the synthesis of misfolded or dysfunctional proteins with a dominant-negative effect cannot be treated efficiently using this strategy alone.
Table 2.
Mutation, therapeutic strategy, and outcomes of genetic mutation studied pre-clinically in inner ear
| Mutation, disease, chromosome location, and inheritance | Model and target organ | Therapeutic strategy (GC/mL) | Age of intervention | Outcomes |
Reference | |||
|---|---|---|---|---|---|---|---|---|
| Transduction IHC | Transduction OHC | Auditory or vestibular analysis | Longevity post-treatment | |||||
| VGLUT3 (12q21-q24) DFNA25 (AR) | knockout (KO) mice IHC | GR - VGLUT3 | P10 | ∼40% | none | ABR within 10 dB of wild-type (WT) threshold | degeneration post-7 week | 75 |
| AAV1 - CBA | P1−P3 | ∼100% | none | ABR within 10 dB of WT threshold | up to 9 months | |||
| RWM (0.6 μL−2.3 × 1013 GC/mL) (injected over 1−2 min) | ||||||||
| Usher syndrome (USH)1C | Ush1c c.216G > A knock-in mice | GR - harmonin a1 or b1 | P0−P1 | yes | yes | Partial rescue was observed at 22.6 kHz and little to none at 32 kHz. | From 6 weeks to 3 months of treatment, ∼10 dB ABR threshold shifts were observed in the low-frequency range and ∼30 dB in the high-frequency range, up to 6 months. | 84 |
| (11p15.1-p14) | IHC and OHC | AAV2/Anc80L65-CMV harmonin a - 4.1 × 1012 | Rescue of DPOAE thresholds was also evident at low frequencies. | |||||
| USH type 1 (AR) | harmonin b1 - 3.0 × 1012 GC/mL RWM (0.8−1 μl) (injected - 0.02 μL/min over 10 min) | Co-injection of harmonin b1 and harmonin a1 did not enhance recovery; harmonin b1 alone was enough to restore partial function. | ||||||
| P10−P12 | yes | yes | no improvement in ABR and DPOAE | − | ||||
| USH1C | Ush1c 216A knock-in mice IHC and OHC | GS - ASO-29 blocking 216A cryptic splicing | P3–P5 | − | − | no circling behavior in mice treated | starts degenerating 3 months post-treatment; significant degeneration on all frequencies on 6-month post-injection at P3−P5 | 109 |
| (11p15.1-p14) | intraperitoneal injection - 50 mg/kg body weight (body wt.) - twice a week for 2 weeks (4 doses) | rescue of low- and mid-frequency hearing comparable to control while higher frequency not rescued to same level | ||||||
| USH type 1 (AR) | P10−P12 | − | − | rescues vestibular function and partially rescues hearing | ||||
| P16 | − | − | vestibular function not rescued; circling behavior like untreated mice | |||||
| CLRN1 (3q25.1) USH type 3A syndrome (AR) | KO-TgAC1 (transgene Atoh1-enhancer-Clrn1) and KO mice IHC and OHC | GR - CLRN1-UTR | P1−P3 | almost all IHC | mosaic pattern in 3 OHC rows | in KO mice, no improvement | up to P150 | 76 |
| AAV2, AAV8 | treated KO-TgAC1 mice showed ABR 20−30 dB difference from WT, high frequency (>16 Hz) partially rescued | |||||||
| RWM injection (2 μL) | AAV2 and AAV8 produced similar results. | |||||||
| AAV2 (8.6 × 1012 vg/mL) | ||||||||
| AAV8 (3.4 × 1013 vg/mL) | ||||||||
| conditional KO - Clrn1ex4fl/fl Myo15-Cre+/– | GR - CLRN1- isoform 2 | P1−P3 | 90% | 20% | P22–P24 showed an almost complete rescue of hearing, at all frequencies tested. | Degeneration started progressively from P60 to P120. | 110 | |
| KO - Clrn1ex4–/– | AAV2/8 | KO mice no improvement | slow progressive degradation of DPOAEs after P20 | |||||
| RWM injection | ||||||||
| Lhfp15 (3p21.31) humans -DFNB67 mice - hurry-scurry deafness (AR) | Lhfpl5−/− KO mice IHC and OHC | GR - Lhfpl5 | P0−P1 | 72% ± 17% | 30% ± 5% | partial recovery of hearing thresholds at frequencies from 4 to 22 kHz | − | 34 |
| exo-AAV1 - CBA | Head tossing and circling were significantly decreased. | |||||||
| RWM injection (1– 1.2 μL) | ||||||||
| 2.7 × 109 GC/mL | ||||||||
| TMC1 (9q31-q21) DFNB7/11 (AR) | TMC1 KO IHC and OHC | GR - TMC1; AAV2/1 - CBA | P0−P2 | 59% ± 2% | sporadic expression in basal turn | partial recovery of hearing threshold | up to 60 days | 24 |
| RWM (1 μL−0.1 μL/min) | DPOAE no recovery | |||||||
| 2.4 × 1013 GC/mL | ||||||||
| GR - TMC2; AAV2/1 - CBA | P0−P2 | 59% ± 2% | sporadic expression in basal turn | partial recovery of hearing threshold | ||||
| RWM (1 μL−0.1 μL/min) | DPOAE no recovery | |||||||
| 1.8 × 1013 GC/mL | ||||||||
| GR - TMC1; AAV2/Anc80L65 - CMV; RWM injection (1 μL) | P1−P2 | approximately (approx.) 93% | approx. 93% | partial recovery at lower frequency; almost 30 dB higher than WT, whereas higher frequency little or no recovery | stable up to 12 weeks | 74 | ||
| 8.1 × 1014 GC/mL | improved DPOAE; no recovery when injected at P14, transduction reduced to 3%; breeding success improved survival rate | |||||||
| TMC1/TMC2 KO | GR - TMC1/TMC2; AAV2/Anc80L65 - CMV; RWM injection (1 μL) | P1 | approx. 93% | approx. 93% | restores vestibular function- treated mice showed visually evoked eye movements equivalent to wild | 74 | ||
| TMC1 - 8.1 × 1014 GC/mL | improved balance even when injected at P30 | |||||||
| TMC2 - 1.6 × 1014 GC/mL | ||||||||
| Baringo mice Tmc1 p.Y182C | GE - base editing | P0−P1 | IHC (41.7% in apex and 22.6% in base of cochlea) | OHC (8.3% in apex and 2.6% in base of cochlea) | 10% to 51% editing efficiency of Tmc1 mRNA | − | 111 | |
| dual AAV using Anc80L65 | restored sensory transduction in a substantial fraction (64% to 75%) of IHCs | |||||||
| BE3.9max-AID-N-terminal (NT; 6.11 × 1012 vg/mL) | 46% ± 6% HC survival at 4 weeks post-treatment | |||||||
| AAV2/Anc80-Cbh-GFP (9.7 × 1011 vg/mL) | ||||||||
| 1 μL of dual AAV | ||||||||
| TMC1 (9q31-q21) DFNA36 (AD) | Tmc1 Beethoven point mutation IHC | GR-TMC2; AAV2/1 - CBA | P0−P2 | 59% ± 2% | Sporadic expression in basal turn | no recovery | − | 24 |
| RWM (1 μL−0.1 μL/min) | ||||||||
| TMC2 - 1.8 × 1013 GC/mL | ||||||||
| GS - miRNA targeting Tmc1 c.1235T > A allele; AAV2/9 - CMV and mU6 | P0−P2 | 74% efficiency in the apical cochlear turn | very low expression | significant preservation of hearing at 8 and 16 kHz; 32 kHz no rescue | 8 kHz - 4−35-weeks post-injection 16 kHz - lost by 13-week post-injection | 89 | ||
| trans-RWM injections (injections (0.5 μL) at 1.59 × 1013 vg/mL | ||||||||
| GE - disrupt dominant mutation | P1 | − | − | At 24 weeks, injected mice exhibited normal or near-normal thresholds at 5–8 kHz. | up to 1 year post-injection | 112 | ||
| Anc80-AAV-CMV-SaCas9-KKH-U6-gRNA-4.2 | ||||||||
| 4.8 × 1014 GC mL–1 | ||||||||
| RWM injection 1 μL rate of 60 nL min–1 | ||||||||
| GS - miRNA targeting Tmc1 c.1235T > A allele; AAV2/9 - CMV and mU6 | P15−P16 | 100% (apex - 98.26% ± 0.54%, middle 100% ± 0.00%; base 100% ± 0.00%;) | very low expression | Hearing thresholds remained ∼50 dB better than in untreated. | degeneration after 8–12 weeks of age | 113 | ||
| Protective effect was not observed at 16 and 32 kHz. | ||||||||
| RWM + CF injection (1.0 μL) | P56–P60 | low expression | − | mild protective effect on hearing (∼30 dB better than untreated) | ||||
| 3.30 × 1013 vg/mL | P84–P90 | low expression | − | no effect | ||||
| Msrb3 (12q14.3) DFNB74 (AR) | MsrB3 KO | GR - MsrB3 | E12.5 | >90% | 83% | ABR-like WT at all frequencies | up to 4 weeks | 114 |
| AAV2/1 - CMV | Hearing threshold at higher frequency started degenerating at approx. 7 weeks of age. | |||||||
| in utero | ||||||||
| (0.6–1 μL) in otocyst | ||||||||
| 1.31 × 1013 vg/mL | ||||||||
| WHRN (9q32) DFNB31 or type 2 USH (AR) | whirler mouse (Whrnwi/wi) stereocilia IHC | GR - long isoform Whrn | P1−P5 | 10%−15% | no transduction observed | promoted IHC survival, restored stereocilia length | At P90, significant IHC loss was detected in treated mice. | 115 |
| AAV2/8 - CMV | no improvement in ABR threshold | |||||||
| RWM (10 injections (400–500 nL) at 40–50 nL/s) | ||||||||
| 5 × 109 GC/mL | ||||||||
| GR - Whrn long isoform | P1−P5 | apex - 71.7% ± 26.0% | apex - 10.4% ± 6.38%; middle - 8.64% ± 13.2%; | improves balance function | stable for 4 months | 116 | ||
| AAV2/8 - CMV | middle - 81.2% ± 15.3% | base - 3.21% ± 5.95% | Improvement in hearing was seen at all four. | |||||
| injection through posterior semi-circular canal (0.98 μL) | base 75.2% ± 17.6% | partial recovery of hearing thresholds at tested frequencies (4, 8, 16, and 32 kHz), with most of the hearing improvement at 8 kHz | ||||||
| 1 × 1013 GC/mL | ||||||||
| Kcnq1 (11p15.5-p15.4) Jervell and Lange-Nielsen (JNL) syndrome (AR) | Kcnq1 KO | GR - Kcnq1 | P0−P2 | − | − | 75% ± 5%, 71% ± 8%, and 61% ± 10% for marginal cells in the basal, middle, and apical turns | stable up to 18 weeks | 72 |
| stria vascularis | AAV1 - CB7 | ABR showed significant hearing preservation, ranging from 20 dB improvement to complete correction. | ||||||
| marginal cells | scala media injection | |||||||
| (0.5 μL) | ||||||||
| 5.0 × 1012 to 1.5 × 1013 GC/mL | ||||||||
| Pjvk (2q31.1-q31.3) DFNB59 (AR) | Pjvk KO impaired neural transmission | GR - pjvk | P3 | − | − | normal ABR latencies (interwave I−IV latencies) | − | 117 |
| AAV8-CB7 | partial improvement in ABR thresholds | |||||||
| RWM injection (2 μL) | Electrically evoked brainstem response (EEBR) wave-E IV amplitude was indifferent to controlled electrical stimulation. | |||||||
| 1013 GC/mL | ||||||||
| USH1G (17q25.1) USH (AR) | USH1G KO tip link of IHC, OHC, and vestibular HC | GR - sans | P2.5 | apex - 80%−85% | apex - 25%−30% | Partial restoration may be due to lower transduction of cochlear HC when compared to vestibular HCs. | degenerating at approx. 12 weeks post-injection | 118 |
| AAV8-CAG | middle - 50%−55% | middle - 20%−25% | ||||||
| RWM injection (2 μL) | base - 35%−40% | base - 20%−25% | ||||||
| 1.47 × 1013 GC/mL | ||||||||
| SLC26A4 (7q22.3) DFNB4 or thyroid goiter-associated SNHL | Slc26a4 - KO pendrin-deficient knock-in (Slc26a4tm1Dontuh/tm1Dontuh) mice. | GR - Slc26a4 AAV2/1-CMV | E12.5 | − | − | fails to restore vestibular function; restored hearing phenotype is unstable | unstable and degenerates within 3−11 weeks | 119 |
| in utero injection (0.6−1 μL) 1.08 × 1013 GC/mL | ||||||||
| GJB2 13q12 DFNB1 (AR) | conditional Cx26 KO mice (Foxg1-cCx26KO) non-sensory cells in the sensory epithelium, lateral wall, and spiral limbus | GR - Gjb2 | P0–P1 | basal - 44 ± 3 | Supporting cells and marginal cell were also transduced. | − | 120 | |
| AAV2/1 - CB7 | middle - 32 ± 4 | Outer sulcus cells showed 100% transduction. | ||||||
| scala media injection | apical - 13 ± 2 | partial morphology recovery ABR no recovery | ||||||
| GJB2 - 1.5 × 1013 GC/mL | ||||||||
| GJB2 GFP - 1.2 × 1012 GC/mL | ||||||||
| conditional KO (Cx26fl/flP0-Cre mice) non-sensory cells in the sensory epithelium, lateral wall, and spiral limbus | GR - Gjb2 | P0 | − | significant improvement in the ABR | AAV transduction lasted over 6 months | 83 | ||
| Thresholds were observed, but still it was 30–40 dB higher than WT thresholds. | ||||||||
| AAV1-CMV | P42 | − | no correction of ABR or cochlear morphology | − | ||||
| Perilymph injection through RWM | ||||||||
| 8.6 × 1011 GC/mL | ||||||||
| adult - 0.05 μL/min | ||||||||
| neonates - 0.02 μL/min | ||||||||
| for 10 min | ||||||||
| Otof (6 kb) 2p23.1 DFNB9 (AR) | otoferlin KO IHC and synaptic vesicle | GR - mini-Otof sequences | P1−P3 | 80.4% ± 2.3% | 29.5% ± 3.5% | did not restore normal synaptic exocytotic properties | − | 121 |
| AAV8-CB6 | ABR also was not rescued. | |||||||
| RWM injection (1 μL) | partially restores the fast exocytotic component | |||||||
| 3.21 × 1013 GC/mL | ||||||||
| GR - Otof using dual AAV | P6−P7 | dual AAV-trans-splicing 30% ± 4% | Fast exocytosis of the readily releasable pool of vesicles was fully recovered, and vesicle replenishment was restored to 35%–50% of WT controls. | − | 53 | |||
| AAV2/6 | dual-AAV-hybrid 19% ± 3% | partially rescued auditory function (ABR threshold improved from untreated, still significantly different from WT) | ||||||
| RWM injection | ||||||||
| AAV2/6-trans-splicing - 1.2 × 1010 vg/μL | ||||||||
| AAV2/6-hybrid - 1.38 × 1010 vg/μL | ||||||||
| GR - Otof using dual AAV | P10 | 64 ± 6 | none | substantial restoration of hearing thresholds in response to click and tone-burst stimuli (8, 16, and 32 kHz) in all of the treated mice; treated mice showed ABR within 10 dB of WT | tested until 30 weeks post-injection; within 10 dB of WT | 26 | ||
| AAV2 quadY-F capsid - CMV promoter | P17 | 82 ± 9 | none | substantial restoration of hearing thresholds in response to click and tone-burst stimuli (8, 16, and 32 kHz) in all of the treated mice; treated mice showed ABR within 10 dB of WT | Hearing thresholds in response to clicks remained unchanged for 20 weeks after injection. | |||
| RWM injection | P30 | 85% ± 7% | none | substantial restoration of hearing thresholds in response to click and tone-burst stimuli (8, 16, and 32 kHz) in all of the treated mice; treated mice showed ABR within 10 dB of WT | Hearing thresholds in response to clicks remained unchanged for 20 weeks after injection. | |||
| 2 μL - AAV2-Otof NT (6.3 × 1012 vg/mL) and AAV2-Otof C-terminal (CT; 4.5 × 1012 vg/mL) vector pair | mean ABR wave I amplitude reduced to about one-half of WT | |||||||
For dominant mutations leading to HL, gene silencing or gene editing can be used. Gene silencing “switches off” the expression of the mutant gene using antisense oligonucleotides (ASOs), microRNA (miRNA), or siRNA. Gene silencing can be performed at transcriptional or post-transcriptional levels. At the transcriptional level, gene silencing is achieved by CRISPR-Cas9 or engineered zinc finger nucleases (ZFNs). Post-transcriptional gene silencing is accomplished using ASO, siRNA, or miRNA. ASOs are a designed DNA, or RNA strand, which bind to specific mRNA-inhibiting translation/facilitate degradation by enzymes like RNase H. Another approach for silencing genes is RNA interference (RNAi) using complementary ds-siRNA or miRNA to target genes where the RNAi pathway is activated, leading to mRNA cleavage and gene knockdown. Gene silencing approaches have been used successfully in vivo in HL mutant animal models of USH1C, TMC1, and GJB2.109,113,122,123 Gene silencing results in transient transgene expression and requires transgene reintroduction at a predefined period; however, siRNA/ASO delivery via AAV vectors is thought to be a one-time treatment. Gene editing is more precise than gene silencing and requires agents like ZFN, CRISPR-Cas9, and transcription activator-like effector nuclease (TALEN), which avoid off-target effects, leading to the complete KO of a gene compared to RNAi strategy. Gene editing agents are based on designed nucleases that target genes of interest, guiding ssDNA or dsDNA to manipulate innate DNA repair machinery via non-homologs end joining (NHEJ) or homology-directed repair (HDR). CRISPR-Cas9 strategy is the most powerful and frequently used gene editing tool with simple design techniques and flexibility for tailoring to different applications. The DNA repair process occurs through the NHEJ pathway, which is prone to errors, and it may cause insertion or deletion of nucleotides leading to frameshift and truncated proteins. The CRISPR strategy has been applied in vivo in the TMC1 mutant model.124,125 Recently, an effort has been made toward designing the CRISPR nuclease with different protospacer adjacent motif (PAM) specificities, including reduced off-target activities, facilitating more precision in technique.
The most common causes of genetic HL arise from recessive point mutations that need correction rather than disruption (since two alleles carry the mutation instead of one allele in the case of a dominant mutation) to benefit patients. In this context, base editing has the potential to directly repair point mutations and provide therapeutic restoration of gene function in a recessive mutation causing HL. Recently Yeh et al.111 developed a base editing strategy to treat the recessive Tmc1 mutation that causes deafness. They were successful in reverting 51% of mutant TMC1 to the wild-type sequence, which resulted in the rescue of low-frequency hearing. This proof-of-concept data support further development of base editing to correct point mutations that cause inherited human diseases.
Additionally, it may serve as an alternative to gene replacement, where there are chances of unexpected adverse effects due to overexpression of transgene in vivo, or there is a requirement for re-administration of a transgene after a predetermined period. Although gene editing seems to be a one-time treatment with promising lasting therapeutic effects, there have been reports of potential off-target mutagenesis, genomic mutations, large deletions and rearrangements, on-site damage, and biallelic modification and genetic mosaicism in the treated organism.126 Gene editing requires rigorous exploration of the therapeutic temporal window for intervention in mice or humans and long-term safety assessment of editing agents delivered via viral vectors.
Preliminary studies with genetic HL
SNHL is a common disease in humans, with an incidence of 186 per 100,000 births in the United States.127 More than 50% of congenital SNHL cases are due to genetic etiology, and the vast majority of them are from non-syndromic causes. Genetic HL, depending on the type of mutation, can have different rates of progression. The deafness gene involved in HL often plays an important, irreplaceable role in inner-ear structure, development, or function. Preliminary studies of gene delivery to genetic HL have been performed successfully in many mutant rodent models mimicking human HL diseases (Table 2) and are reviewed as follows.
Vglut3 mutation
The SLC17A8 gene encoding VGLUT3 is responsible for DFNA25 (12q21-q24) in humans, which is characterized by an autosomal-dominant, high-frequency, and non-syndromic-progressive HL.128 Synaptic transmission at IHC auditory nerve terminals requires glutamate to transport excitatory amino acids into secretory synaptic vesicles by VGLUT1–3 (expressed in the IHC) before its exocytotic release.128, 129, 130, 131 Successful restoration/rescue of HL was reported by virally mediated gene replacement in VGLUT3 KO mice. This study was the first demonstration of successful inner-ear gene therapy for mammalian inner-ear defects.75 RWM injection of AAV1 delivering VGLUT3-GFP at P10 showed a 40% expression, whereas a similar dose between P1 and P3 resulted in 100% transduction of VGLUT3 in IHC. The majority of treated mice with the RWM injection had improved hearing on auditory brain stem response (ABR) testing, whereas synaptic morphology was partially improved. The study demonstrated the budding potential for gene therapy to rescue auditory function and to lead a new paradigm of motivated research for other genetic HL diseases.
USH1C mutation
USH, an autosomal-recessive sensory defect, is characterized by pre-pubertal progressive blindness, SNHL, and vestibular areflexia, which accounts for 3%–6% of congenital deafness affecting 16,000−20,000 people in the United States.132, 133, 134 USH has three clinical subtypes USH I−III, with USH I as the most common and severe form, characterized by profound deafness at birth and absence of vestibular function. The present treatment for Ush1 patients is cochlear implants. The genes associated with USH1 are MYO7A (USH1B, 11q13.5), USH1C (harmonin; 11p15.1-p14), CDH23 (USH1D, 10q21-q22), PCDH15 (USH1F, 10q11.2-q21), SANS (sans; USH 1G, 17q24-q25)18, and CIB2 (calcium and integrin-binding protein 2; USH1J, 15q25.1).135, 136, 137, 138, 139, 140, 141 USH1 proteins are important for the structure and morphogenesis of mechanosensory hair bundles, anatomically localized in the apex of HCs, and bind to harmonin lying in the core of the USH1 interactome. The harmonin gene contains 28 exons coding for ten alternate splicing forms, categorized according to protein domain composition into three subgroups: harmonin a, b, and c.135,136 Harmonin splice form “a” is anatomically localized in HC synapses, where it associates with calcium channels through a ubiquitin-dependent pathway and maintains synaptic transmission.141,142 Harmonin “b” is present in stereocilia tip links, forming a tertiary complex with myosin VIIa and Sans, playing an important role in sensory transduction of both auditory and vestibular HCs.143, 144, 145 For rescuing USH1C in the mouse model, gene silencing was used via ASO designed against 216A RNA to block 216A cryptic splicing. The ASO, when injected intraperitoneally in adult mutated mice, corrected splicing with augmented dose-dependent harmonin expression. Interestingly, no circling (improved vestibular function) was observed in mutated mice treated at P3, P5, P10, or P13, but when treated at P16, circling behavior was similar to those untreated ones. Further, the treatment also rescued hearing, as analyzed by measuring quantitatively using ABR thresholds. The single dose of ASO between P3 and P5 rescued hearing at lower frequencies, i.e., 8 and 16 kHz, but it was unable to improve thresholds at higher frequencies (32 kHz). Injecting at P10 mice had a significantly higher threshold compared to P3, P4, and P5, indicating a therapeutic window for treatment. The therapeutic effect exerted by ASO could be maintained for 3 months.109 However, the mechanism of systematically delivered ASO to cross BLB and transfect cells is still unknown. A more recent study used a gene replacement approach by delivering harmonin a or b to the inner ear of mutant mice using AAV2/ANC80 as a carrier via the RWM at the early postnatal stage. Interestingly, the delivery of harmonin b alone was enough to partially rescue both auditory and vestibular functions when compared to co-injection of harmonin a and b. The rescued hearing was significant at lower frequencies but absent in higher frequencies.84 However, to rescue the function of the basal region, harmonin c intervention may play an essential role. Alternatively, the basal region may be beyond the therapeutic window, given that development starts at the basal region by P1. If the latter is true, an embryonic injection may be more effective in rescuing the hearing at higher frequencies.36,146
USH3A mutation
USH3A is caused by a mutation in the CLRN1 gene, characterized by postlingual progressive HL and loss of vision accompanied by variable vestibular dysfunction.147,148 Progressive HL in human USH3 typically begins before 10 years of age, which worsens between 30 and 40 years.149,150 CLRN1is a tetraspan protein reported to be involved in hair bundle morphogenesis and tight clustering of presynaptic CaV1.3 channels required in the ribbon synapse of HC.151,152 The absence or degeneration of CLRN1 can lead to abnormal clustering of calcium channels, decreased exocytosis efficiency, and subsequent postsynaptic defects. Interestingly, AAV2/8 Clrn1 injection between P1 and P3 to KO-TgAC1 mice showed little to no effect in preserving HL. However, when Clrn1 was modified with the UTR sequence, the treated mutant mice showed improved HC structure and significantly better hearing than untreated mice. In contrast, KO mice did not show any improvement with either AAV2- or AAV8-Clrn1-UTR on injection between P1 and P3, since the onset of HC degeneration in this mutant model starts very early.213 These findings restate the need for gene therapy intervention before the onset of genetic degeneration, leading to permanent non-reversible damage to the structure of the organ of Corti.76 A recent study reported preservation of HC morphology using a single injection of AAV2/8 Clrn1 between P1 and P3 with Clrn1ex4fl/fl Myo15-Cre+/– mice, whereas Clrn1ex4–/– KO mice showed little or no improvement, indicating the potential of gene therapy as an alternative potential treatment in USH3A patients.76,110
LHFPL5/TMHS mutation
LHFPL5 (6p21.3) gene, also known as TMHS gene, is responsible for autosomal-recessive non-syndromic HL (ARNSHL) in humans (DFNB67) and hurry-scurry deafness in mice.153, 154, 155 TMHS is localized near the stereociliary tips, where it plays a vital role in maintaining tip-link assembly, mechanosensory transduction (MT) machinery, and regulating MT channels by interacting with tip-link component PCDH15 gene, as demonstrated via co-precipitation studies.156,157 A study showed in vivo gene delivery of Lhfpl5 in Lhfpl5−/− mice using AAV1 exosomes. AAV1 exosomes have greater transduction efficiency than conventional AAV1 vector. It has been reported to transduce both IHC and OHC efficiently. Exo AAV1 was transduced in Lhfpl5−/− mice through RWM injection at P0 or P1; treated mutant mice showed improved hearing and balance-related abnormal movements. However, the HL was not rescued completely, which may be due to a limited therapeutic window for the treatment of Lhfpl5. Lhfpl5 expression starts as early as embryonic day 16.5 (E16.5), and degeneration in KO mice is visible by P8.38 Although there is a partial recovery of hearing, the exosome-associated AAV strategy is an important forward step in strategies for inner-ear gene therapy.
TMC1 mutation
Recessive mutations in human TMC1 account for 4% to 8% of genetic deafness leading to DFNB7/11 congenital HL, whereas dominant mutations often lead to DFNA36 progressive HL.158,159 TMC1 and TMC2 are essential components of the MT channels (cationic channels with high Ca2+ permeability) that are located anatomically at the tip of the shorter stereocilia of HCs, which are responsible for transducing sound into electrical signals.160, 161, 162, 163 TMC2 is expressed early in postnatal development of the cochlea and replaced by TMC1 at the end of the postnatal first week.163,164 In humans, the onset of DFNA36 mutation-mediated HL occurs at 5−28 years old, and it develops profound HL at the age of 60, providing a greater temporal window for successful therapeutic intervention. The treatment could allow for rescuing the mid- to high-frequency hearing.163, 164, 165 Beethoven (Bth) mice with the p.M412K mutation are a good model for DFNA36, whereas TMC KO mutant mice are a good model for DFNB7/11.163,166,167 In a study by Askew et al.24, investigators tried to rescue HL in TMC1-KO and Bth mutant mice using gene replacement therapy by delivering wild-type TMC1 or TMC2 using AAV2/1 vector with CBA promoter via RWM injection. AAV2/1-TMC1 delivery at P0 and P2 to TMC1-KO mice reestablished mechanotransduction in IHC- but not OHC-treated mice and showed no improvement in distortion product otoacoustic emission (DPOAE; OHC), but ABR showed a partial recovery hearing threshold. Delivery of AAV2/1-TMC2 to Bth mice also preserved HL to the same extent as observed by ABR in the treated mice, but it did not recover startle responses, suggesting TMC1 and TMC2 can partially substitute each other.167
Another gene silencing study, using a single RWM injection (P0 and P2) of rAAV2/9 carrying artificial miRNA, inhibited the expression of the dominant allele carrying a single missense mutation in Bth mice. The treated Bth mice showed improved HC survival and delayed onset of HL progression up to 35 weeks, whereas untreated Bth mice are generally deaf by 17−21 weeks.89 The protective effect of miRNA on HC lasted for 35 weeks, which was considerably longer than the Vglut3 gene replacement therapy using AAV2/1, where function deteriorates by 6 weeks. These findings were also considerably longer than methionine sulfoxide reductase B3 (MsrB3) gene replacement therapy using rAAV2/1, which lasted 3 weeks.75,89,114 All studies performed above were at the neonatal or utero stage.
Yoshimura et al.113 demonstrated slowing of HL progression, protection of HC, and avert stereocilia degeneration through gene silencing using miRNA in the AAV2/9 vector using RWM injection with CF in mature Bth mice. Bth mice treated at P15−P16 showed their ABR threshold reduced by 50 dB over 20 weeks, P56−P60 by 30 dB, and P84−P90 with no reduction. Treatment at P15−P16 and P56−P60 showed a protected stereocilia bundle and IHC degeneration rate, corroborating with improved ABR results. However, treatment at P84−P90 did not show any improvement in auditory function, suggesting that the age of treated animals directly impacted therapy outcomes. The auditory threshold of miRNA-treated mice was higher than wild-type, indicating an incomplete rescue of function, which may require miRNA modification or ongoing, irreversible HC loss. This study suggested a therapeutic window between 8 and 12 weeks post-birth.113 Another study by Nist-Lund et al.74 showed significant restoration of auditory and vestibular function using AAV2/An80L65-TMC1/TMC2 with CMV promotor in the DFNB7/11 mouse model. Treated mutant mice showed restoration of sensory transduction in IHC and OHC, with improved ABR thresholds and DPOAEs, and were able to drive auditory behavior (i.e., startle response) in treated mice. The study reported the dependence of transduction rate on the mice’s age, which changes from 93% at P1 to 3% at P14, suggesting the efficiency reduces as the mice develop. For evaluating vestibular function, TMC2 is located in the vestibular organ, where it was injected at neonatal and mature stages. Significant recovery was observed in vestibular function in both the TMC2 mutant and TMC1/TMC2 double mutant mice post-TMC1 or -TMC2 injection, both in early and the mature stage mice. TMC1 and TMC2 double mutants are deaf with vestibular dysfunction and limited breeding efficiency, showing offspring with a lower survival rate and stunted growth. Post-treatment, approximately 80% of litters survived until P21, and their weights were almost equal to age-matched wild-type pups. TMC gene therapy improved hearing and balance and led to improved breeding success, survival, and growth rate, indicating that it may be appropriate for clinical transition in the treatment of recessive DFNB7/11 HL.74
In a recent study, György et al.112 screened 14 Cas9/guide RNA (gRNA) combinations for specific and efficient disruptions of a nucleotide substitution that caused the dominant-progressive HL, DFNA36. They also identified a PAM variant of Staphylococcus aureus Cas9 (SaCas9-KKH) that selectively and efficiently disrupted the mutant allele, but not the wild-type Tmc1/TMC1 allele, in Bth mice and a DFNA36 human cell line. AAV-mediated SaCas9-KKH delivery prevented deafness in Bth mice up to 1 year post-injection. Post-treatment mice showed robust preservation of thresholds at low frequencies (8 and 16 kHz) but less restoration at high frequencies (32 kHz). Analysis of current ClinVar entries revealed that ∼21% of dominant human mutations could be targeted using a similar approach with significant improvement over previous strategies, where hearing preservation was only modest and not sustained even at low frequencies.112 In another recent study, Yeh et al.111 endeavored a one-time base editing treatment strategy to permanently correct the pathogenic allele in the recessive Tmc1 mutation that causes deafness. With this strategy, they were successful in reverting 51% of mutant TMC1 to wild-type sequence, leading to rescue of low-frequency hearing.111 To prevent progressive HL, two recent studies documented the relationship between HC survival and stable hearing thresholds, suggesting that more than 75% HC survival is needed for stable hearing.74,168 In this study, it was also observed that 46% HC survival after 4 weeks was consistent with continued progressive HL. Although the study provides new insight into gene editing approaches as a treatment strategy for recessive mutations, it also introduces challenges that remain to be explored, including the exploration of the therapeutic temporal window for intervention in mice or humans and long-term safety assessment of editing agents delivered via viral vectors. Future studies may include improvements to viral capsids to increase transduction efficiency, promoters to decrease age-dependent transduction, miRNA, Cas9, PAM sequencing, improvement in base editor expression, intron-mediated splicing, and base editing efficiency to improve the extent of mutation silencing/editing without off-target reactions, and improvement in injection techniques to ensure homogeneous distribution.
Msrb3 mutation
Msrb3 deficiency of the human DFNB74 gene causes ARNSHL leading to congenital deafness.169 Msrb3 is expressed in HCs; its deficiency causes distortion of stereocilia bundle morphology and finally apoptosis of HC, causing HL.170 A study analyzed the treatment of Msrb3 mutant mice by delivering the Msrb3 gene in Msrb3 KO mice (Msrb3−/−) using rAAV2/1.114 Since deafness is congenital in Msrb3 KO mice, it was injected in utero to otocyst at E12.5, and treated mice showed HL recovery at P28. The morphology of stereocilia bundles in treated ears was similar to the control ears, and transduction efficiency was very high at P28 with >90% for IHC and >83% in OHC. Msrb3 mutant mice did not respond to click stimulus or tone burst, whereas the treated mice showed a normal threshold similar to the wild-type at all frequencies. The improved HL started degenerating at higher frequencies at 4 weeks post-treatment. The expression of Msrb3 was observed mainly in HC, whereas more widespread expression may be required for the maintenance of hearing in adult mice. Hence, for longevity, either a different AAV variant or re-administration of the same AAV can be explored. This study was the first report of in utero AAV delivery for gene therapy of congenital HL.24
GJB2 mutation
Mutations in GJB2, or Cx26, can lead to bilateral neurosensory ARNSHL (DFNB1) and autosomal-dominant HL (DFNA3) in humans.171,172 The GJB2 gene encrypts tetraspan transmembrane protein Cx26, a component of the epithelial gap junction channel facilitating the transportation of signaling molecules between neighboring cells.173,174 Cx26 is hypothesized to facilitate potassium (K+) recycling in the endolymphatic fluid to maintain the endolymph potential. The endolymph potential in mice appeared around P5 and reached its regular level by P18.175,176 Absence of Cx26 has been shown to lead to HC degeneration through inadequate K+ recycling leading to apoptosis of sensory, non-sensory, and SGN, causing progressing HL. Several strategies have been explored to restore Cx26 and rescue HL with partial success. Yu et al.120 used gene replacement via AAV2/1 delivery of GJB2 through the scala media in conditional Cx26 KO mice. GJB2 delivery reduced the degeneration of HC and SGNs; however, it did not lead to the rescue of HL. Failure to restore hearing may be due to poor transduction or the narrowing of the therapeutic window as the expression of Cx26 starts from E14.5, and the treatment in the present study started on P0−P1, i.e., beyond the developmental window, which may impair functional recovery adversely.120 The endolymph electrochemical environment is sensitive to physiological changes, as demonstrated in a previous study that used an injection volume of >8 nL in the scala media leading to swollen OHC and shrunken IHC due to a decrease in the endolymph potential. The injection of a Na+-rich buffer in the K+-rich endolymph might interrupt mechanotransduction in HC, which may be why HL did not improve in the study.177,178 A more recent study by Iizuka et al.83 explored the delivery of GJB2 to otic vesicle-specific Cx26 KO mice using AAV1 by injection into the perilymph through the RWM. The strategy showed reduced degeneration of cochlear structures and improved ABR thresholds in treated mice. The study also revealed that the tunnel of Corti failed to open in mutant mice, which usually opens by P10 in wild-type mice, indicating a developmental defect.83 Gene therapy for SGNs has also been explored in Cx26 conditional KO mice using AV to deliver BDNF via scala media or scala tympani. The delivery of BDNF via scala media or scala tympani can reduce degeneration of the SGNs in the cochlea base region with rescued neurons, demonstrating similar morphology to wild-type neurons.179 These studies advocate for using a combinatorial approach, i.e., gene and neurotrophic factors delivered by advanced viral gene therapy to rescue HL in Cx26 mutant models.
WHRN mutation
WHRN gene codes for whirlin, a putative PDZ scaffold protein. Depending on the type of allele and mutation, it can cause either ARNSHL DFNB31 or type 2 USH (retinitis pigmentosa and moderate SNHL without vestibular dysfunction) in humans.180,181 Whrn consists of 13 exons with two major splice variants: a long isoform (WHRN-L) that is encoded by exons 1–13 and composed of two PDZ domains at the N terminus followed by a proline-rich domain and a third PDZ at the C terminus and a short form (WHRN-S) that is encoded by exons 6–13, which lacks PDZ1 and PDZ2 of the N terminus.180,182,183 Whirlin protein is found in the ankle joint of stereocilia along with other Usher type II proteins USH2A, GPR98, and PDZD7 postnatally, whereas in the mature stage, it is present in tips of stereocilia of HC. Myosin-XVa interacts with whirlin, and it is required for its transportation to stereocilia tip. There has been little success in rescuing HL from the WHRN mutation by delivering the wild-type gene to mutant mice. WHRN−/− mice treated with AAV2/8-WHRN long isoform injected through the RWM restored normal stereocilia morphology, but improved auditory function was not observed in treated mice. The absence of HL recovery may be due to a low rate of infectivity (15% of IHC and no OHC was transduced), whirlin isoform type, or when AAV was injected at P0, the permanent damage to HC had already occurred.115 In a follow-up study of AAV2/8-WHRN, the long isoform was delivered through the posterior semicircular canal. Treated mice showed improved vestibular and auditory function with normal stereocilia morphology comparable to wild-type mice. Cochlear IHC showed 71.7%–81.2% transduction, whereas OHC transduction was not significant. Partial recovery may be due to the isoform type or lower transduction rate in OHC.116 The long isoform has been reported to restore stereocilia length in WHRN−/− mice.184 However, the short isoform of WHRN may have a critical role in auditory functioning that needs to be explored in the future.
Kcnq1 mutation
KCNQ1 is a subunit of a voltage-gated K+ channel, and its mutation leads to Jervell and Lange-Nielsen (JNL) syndrome in humans, characterized by congenital bilateral profound deafness and cardiac dysfunction. In the cochlea, KCNQ1 and KCNE1 play a pivotal role in the transportation of K+ into endolymph and maintaining the EP.185, 186, 187 KCNQ1 is primarily expressed in the SV in the apical membrane of marginal cells.187 Chang et al.72 explored gene replacement in JNL mutant mice using AAV2/1 and CBA promoter via a scala media injection at P0−P1. Endolymph delivery led to the expression of KCNQ1 in marginal cells of the SV, where it was primarily expressed in wild-type mice. It showed rescued HC morphology, restoration of spiral ganglion cells, and prevention of the collapse of Reissner’s membrane. Treated mice also showed a normal EP, and ABR showed significant hearing preservation, which remained until post-18 weeks treatment. Hearing thresholds began to increase from 18 to 30 weeks, suggesting that a one-time treatment for SV was not permanent. Future studies may require multiple injections over time or exploration of advanced AAV vectors to increase transduction efficiency and longevity.72
OTOF mutation
The mutation in OTOF (cDNA ∼6 kb) gene coding protein otoferlin leads to autosomal-recessive HL, DFNB9, in humans and constitutes 2%–8% of total cases of congenital HL.188 Otoferlin is a large 6 C2 domain protein indispensable for IHC exocytosis, vesicle replenishment of synaptic vesicles, and linkage of calcium channels and SNAREs (SNAP receptor, i.e., soluble NSF -N-ethylmaleimide-sensitive factor] attachment protein) protein.189,190 AAV has been used successfully in many gene replacement therapies for inner-ear gene mutation-related disorders, but AAV’s limited DNA packaging capacity of 4.7 kb makes it impossible to carry larger genes like otoferlin (cDNA ∼6 kb) whole. Tertrais et al.121 investigated the effect of delivering mini otoferlin using AAV2/8 in OTOF KO mice through RWM injection. Various C2 domain (mini otoferlin) combinations were explored, showing that Otof-C2-ACEF, among others, can partially restore readily releasable pool (RRP) exocytosis in OTOF KO mice. However, none of the compositions recovered sustained vesicle release components, and no rescue of HL was observed.121 Al-Moyed et al.53 made the first attempt to deliver large transgenes via dual AAV using a hybrid and trans-splicing approaches encoding cDNA fragments of the OTOF gene in otoferlin-deficient mice (Otof−/−). They observed a transfection efficiency of ∼75% for AAV2/6 GFP injected by RWM in Otof−/− mice at P6−P7. The study revealed a dual AAV2/6 transduction rate of 19% and 30% when treated with hybrid or trans-splicing dual vectors, respectively. The treated Otof−/− ears showed full-length mRNA and protein expression, as confirmed by western blot and PCR. The post-treatment number of synapses improved, yet it differed from the control mice, suggesting the injection period may be too late to rescue the synapse numbers. Further, ABR of treated Otof−/− mice showed partial recovery of auditory function, as it depends on the recombination event rather than the transduction process. Prior gene therapy research has reported that for normal auditory function, at least ∼70% IHC transduction is required, but in this study, a maximum transduction of only 30% was observed.191
In a recent study, Akil et al.26 reported an interesting observation of the reversal of the deafness phenotype in Otof−/− mice using the dual AAV approach. In this study, the AAV2 vector was modified to AAV2 quadY-F with a CMV promoter based on prior work, which demonstrated increased transduction efficiency in the retina.192 The virus injected at P2 through the RWM revealed 78% ± 6% transduction in the IHC post-2 weeks of treatment, revealing its potential as an agent for gene delivery to the inner ear. Otoferlin was divided into two split cDNA sequences containing a recombinogenic bridging sequence and packaged in two AAV vectors. Dual AAV was injected once through RWM of Otof−/− mice at P10 (before the onset of hearing), P17 (after the onset of hearing but IHC synapses still under maturation), and P30 (cochlea is matured). Post-P10 injection-treated mice displayed rescue of HL, and the ABR threshold did not vary significantly from control. Injection at P17 and P30 led to a higher transduction rate of 82% ± 9% and 85% ± 7%, respectively, compared to the P10-injected mice. ABR thresholds for P17- and P30-injected mice were similar to control mice post-3 weeks of treatment, and restoration was sustained until 20 weeks post-injection. The number of ribbons per IHC in transduced cells injected at P17 or P30 was higher than non-transduced cells, indicating that the gene therapy augmented the production of ribbons rather than limiting their degeneration. This local gene delivery not only rescued HL when delivered before the onset of hearing but also reversed HL in a sustained manner when delivered at post-hearing onset or maturation, suggesting a large therapeutic window for the treatment of DFNB9.26
Pejvakin (PJVK) mutations
The PJVK gene (2q31.1–q31.3) encodes for protein pejvakin in vertebrates, and it is involved in the oxidative stress-induced proliferation of peroxisomes (essential organelles in redox homeostasis of the auditory system), primarily due to neuronal defects. Mutations in this gene cause DFNB59, a recessive auditory neuropathy that causes non-progressive NIHL in humans.117,193 When murine pejvakin cDNA was transferred to PJVK KO mice using AAV8 by RWM injection at P3, the treated mice at P21 had normal ABR latencies (interwave I−IV latencies), and their electrically evoked brainstem response (EEBR) wave-E IV amplitude was indifferent to controlled electrical stimulation. AAV8 post-injection transduced primary cochlear ganglionic neurons (cochlear ganglion neurons) but not the HC, confirming the defect was of neuronal origin.117
USH1G mutations
USH1G encodes the sub-membrane scaffold protein SANS, which is anatomically localized at the stereocilia tip, an essential component of mechanotransduction and the sensory antenna of IHC.140,194 Emptoz et al.118 delivered SANS cDNA using AAV8 via RWM injection using a CAG promoter at P2.5. The transgene delivery in KO mice restored sans protein in the tip link of IHC, OHC, and vestibular HC, hence rescuing mechanotransduction and vestibular dysfunction and improving their hearing threshold. Partial HL restoration was observed in treated mice, which started degenerating approximately 12 weeks post-injection. Partial recovery may be due to lower transduction of cochlear HC compared to vestibular HCs in this study.118
SLC26A4 mutations
SLC26A4 gene encodes for protein pendrin, a Cl and HCO3 anion exchanger, which facilitates inner-ear fluid homeostasis.195,196 Its mutation accounts for the second-most predominant cause of genetic HL after GJB2 mutations, and it is associated with both vestibular aqueduct enlargement (EVA), causing non-syndromic HL (DFNB4), and thyroid goiter-associated SNHL (Pendred syndrome).197,198 Pendrin is expressed in the SV (spindle cells), cochlea (outer sulcus and spiral prominence cells), and vestibular labyrinth (transitional cells).199,200 Kim et al.119 delivered AAV2/1-CMV-Slc26a4 to the otocyst of KO Slc26a4Δ/Δ, a knock-in Slc26a4tm1Dontuh/tm1Dontuh mutant mice at E12.5. Post-treatment, transient expression of pendrin cDNA prevented membranous labyrinth enlargement and rescued HL. However, the recovery was unstable as degeneration in hearing was observed around 3−11 weeks of age. Also, the treatment failed to rescue otoconia development and restoration of vestibular function. Viral transduction was observed in the endolymphatic sac, but it failed to transduce cochlear and vestibular organs, which may be responsible for the study’s observation. An extended period or higher expression of pendrin in the endolymphatic sac may be essential to restore auditory and vestibular function, depending on the viral vector used and biology of mutation in the inner ear.119
Translating to clinics
The success achieved using gene therapy in animal models needs careful consideration for translation to the clinics as a therapeutic strategy for human application.
Safety and efficacy
Concerns related to the efficacy and safety of an approach is of utmost importance for clinical transition. As explored in various genetic studies, the efficacy depends on the route of administration, vector type, and volume of delivery vehicle administered, which must be analyzed in the human inner ear. Along with efficacy, safety data regarding the effects of overexpression or silencing a gene of interest and its pharmacology and toxicological parameters post-gene delivery are significant concerns. As discussed previously, there is a clinical window for the treatment of inner-ear anomalies using gene therapy, and it will be crucial to analyze the critical period for the best results along with other factors. Aside from expression profiles, longevity needs to be carefully assessed, since it has been observed in animal models that the effect is for a finite time period, which varies with vector type, the gene of interest, and route of administration.
The genetic similarity between mouse and human has led to various pre-clinical gene therapy studies in the mouse model. Genetically modified mice allow the development of almost any monogenetic disease model enabling the analysis of gene function or regulation and the underlying mechanisms of clinical diseases. Also, since the mice strains are highly inbred, they facilitate homogeneous conditions in which experiments can be easily reproduced, and statistical significance can be achieved, as evidenced by the large volume of literature using mice models. Additionally, they are small, relatively economical to maintain, and produce large litters with a short generation time. However, there are limitations to mouse models, as they may fail to fully imitate clinical signs and substantial pathologic hallmarks of human disease. Further, longitudinal studies are not possible because of their short lifespan.201, 202, 203, 204 Hence, large animal models such as non-human primates may complement the murine studies of human genetic diseases, as they have a longer lifespan, and their genetics and background genetic heterogeneity are more closely related to humans when compared to mice.205,206 Further, large animals can also address scaling up issues, since the size of their tissues and organs will be comparable, unlike mice, where there is a many-fold size difference. Additionally, owing to the longevity and size, it facilitates more samples from an individual for evaluating the safety and long-term efficacy of concerned therapy. Large animal models represent an important intermediatory step in the preclinical evaluation of human-directed gene transfer protocols.
Explored genetic strategy should meet the minimal criterion to be accepted by the US Food and Drug Administration (FDA; i.e., residual DNA quantity should not be ≤10 ng/dose or DNA size ≤200 bp set for biological drugs or cell substrate). Clinical translations can be supported by relevant in vitro studies in human tissue (i.e., cultured ex vivo inner-ear tissue), or organoids from human pluripotent cells can be a viable platform for smooth translation to clinics. In this context, two studies have confirmed targeting and transducing human vestibular hair using an AV vector with or without the encoded therapeutic gene.29,207 Another study reports the successful development of inner-ear organoids from human pluripotent stem cells containing functional HCs.201 Although genetic deafness studies are not possible with these models currently, they may provide valuable insight into vector targeting, gene/protein expression, localization, and toxicological data in human tissue in vivo.
Clinical trials
The transition from bench to bedside for AAV-mediated gene therapy took its first steps in 2008 when the efficacy of gene therapy was demonstrated to treat Leber congenital amaurosis. Three successful clinical trials were completed regarding the safety of a subretinal injection of retinal pigment epithelium-specific 65-kDa protein (RPE65)-expressing AAV vector for Leber congenital amaurosis.208, 209, 210, 211 These trials paved the way for the first FDA-approved gene therapy product in 2018, LUXTURNA (voretigene neparvovec-rzyl).212 To date, this AAV-mediated gene therapy remains one of two FDA-approved gene therapies alongside Zolgensma (ACXS-101), which was approved in 2019 for spinal muscular atrophy (SMA) treatment. Since its approval, there have been multiple clinical trials studying AAV-mediated gene therapy in the eye. However, there have only been two trials involving SNHL, which will be discussed in this review. The discrepancy between the progress of ocular and SNHL gene therapies has mostly been attributed to the earlier preclinical success and the increased accessibility of the eye for treatments relative to the cochlea. Based on the experience gained through ocular gene therapy, there has been a preemptive movement to define and categorize SNHL etiologies into four stages of cellular degeneration.5 The stages outline the level of cellular damage present in the inner ear, which may offer a standardized approach for researchers to categorize SNHL etiologies and preclinical studies to direct future clinical work.
ClinicalTrials.gov: NCT02132130 is the first clinical trial to date using AV gene therapy to treat severe-to-profound HL in patients with documented, non-fluctuating HL with intact vestibular functioning in their nonoperative ear. It assesses the safety, tolerability, and efficacy of intra-labyrinthine (IL) infusion of CGF166 (AV5 encoding human atonal transcription factor [Hath1]) directed by Novartis Pharmaceuticals. It is expected that forced ATOH1 expression in HL patients may transdifferentiate remaining SC to functional HC, leading to rescue of HL, as observed in non-mammalian vertebrates. The study was completed in December 2019, and information regarding analysis and outcomes is still awaited at the time of review. Another clinical trial, ClinicalTrials.gov: NCT03996824, is a prospective observational study focusing on the in vitro AAV transduction in human inner-ear cells collected during non-conservative surgeries for vestibular schwannoma. Immunostaining techniques will measure AAV transduction post-10 days of treatment. Currently, this study is recruiting patients with an anticipated end date in February 2022.
Challenges
With 10 years set between the first successful clinical ocular gene therapy trial in 2008 and its FDA approval in 2018, we do not expect the human application of cochlear gene therapy to be occurring anytime soon. Although gene therapy of monogenic disease using AAV has become feasible, the high cost and risks involved with AAV-based investigational new drugs (INDs) discourage investigators from transitioning to clinical trials. For instance, Glybera, the first approved gene therapy drug in the European Union, costs 1 million euros (US $1.2 million) per patient and is still the most expensive drug globally. Luxturna, which was launched in 2017 in the United States, costs $425,000 per eye treatment and has a similarly high price tag.211 Other than the high economic costs involved, it also deals with hurdles regarding the purification of AAV in large scale, removal of the empty capsid, and lack of quality-control techniques to avoid batch-to-batch variations of vector titer.
Conclusion and future directions
Exponential growth in clinical trials based on AAV vectors suggests that it is just the beginning of a new era in treating human ailments by manipulating viral vectors. Although several challenges still exist, advancement in gene regulation and gene editing will augment the specificity and efficacy of gene therapy in the future. Heterogeneity is the major challenge in genetic HL treatment, as several factors affect the efficacy of treatment like the therapeutic window, targets, targeting molecules, and protein function, which are still under discussion. Recent advancements in the development of synthetic AAV and sophisticated techniques like AAV capsid modification using targeting molecules (peptide) of interest can tailor their expression profile and increase the probability of wholesale clinical efficacy. Also, hybrid vectors like virosomes have been reported for their superior efficacy compared to their respective parent virus, and it may be interesting to study AAV virosomes modified with targeting molecules for transduction efficacy in the near future. With the consideration of the high economic costs involved with this research, there is an increasing interest from government-funding agencies, industry, private foundations, patients, and doctors. Companies like AGTC (Applied Genetic Technologies), Akouos, Rescue Hearing, Novartis, and Decibel Therapeutics are currently engaged in preclinical/clinical trials to treat HL. Likewise, Casebia Therapeutics is involved in preclinical testing of CRISPR-Cas9 for HL treatment. Despite the hurdles, there have been significant breakthroughs in the path of HL gene therapy, holding great potential for providing novel and effective treatment to patients for improving their quality of life.
Acknowledgments
This work was supported by the National Institute for Deafness and Communication Disorders (NIDCD; R01 DC014464 and R01 R01DC017144).
Author contributions
Conceptualization, K.B., B.W.O., and D.L.; investigation, K.B., C.G., and D.L.; writing – original draft, K.B. and C.G.; writing – review & editing, K.B., C.G., T.H., L.Y., and D.L.; visualization, C.G.; funding acquisition, B.W.O. and D.L.; supervision, D.L.
Declaration of interests
NIDCD had no involvement in study design; the collection, analysis, and interpretation of data; writing of this report; or the decision to submit this article for publication. The authors declare no competing interests.
References
- 1.Pichora-Fuller M.K., Mick P., Reed M. Hearing, Cognition, and Healthy Aging: Social and Public Health Implications of the Links between Age-Related Declines in Hearing and Cognition. Semin. Hear. 2015;36:122–139. doi: 10.1055/s-0035-1555116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kochhar A., Hildebrand M.S., Smith R.J.H. Clinical aspects of hereditary hearing loss. Genet. Med. 2007;9:393–408. doi: 10.1097/gim.0b013e3180980bd0. [DOI] [PubMed] [Google Scholar]
- 3.Parker M.A. Biotechnology in the treatment of sensorineural hearing loss: foundations and future of hair cell regeneration. J. Speech Lang. Hear. Res. 2011;54:1709–1731. doi: 10.1044/1092-4388(2011/10-0149). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Khera A.V., Kathiresan S. Genetics of coronary artery disease: discovery, biology and clinical translation. Nat. Rev. Genet. 2017;18:331–344. doi: 10.1038/nrg.2016.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang W., Kim S.M., Wang W., Cai C., Feng Y., Kong W., Lin X. Cochlear Gene Therapy for Sensorineural Hearing Loss: Current Status and Major Remaining Hurdles for Translational Success. Front. Mol. Neurosci. 2018;11:221. doi: 10.3389/fnmol.2018.00221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ren Y., Landegger L.D., Stankovic K.M. Gene Therapy for Human Sensorineural Hearing Loss. Front. Cell. Neurosci. 2019;13:323. doi: 10.3389/fncel.2019.00323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gigante A., Li M., Junghänel S., Hirschhäuser C., Knauer S., Schmuck C. Non-viral transfection vectors: are hybrid materials the way forward? MedChemComm. 2019;10:1692–1718. doi: 10.1039/c9md00275h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Howarth J.L., Lee Y.B., Uney J.B. Using viral vectors as gene transfer tools (Cell Biology and Toxicology Special Issue: ETCS-UK 1 day meeting on genetic manipulation of cells) Cell Biol. Toxicol. 2010;26:1–20. doi: 10.1007/s10565-009-9139-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Howard-Varona C., Hargreaves K.R., Abedon S.T., Sullivan M.B. Lysogeny in nature: mechanisms, impact and ecology of temperate phages. ISME J. 2017;11:1511–1520. doi: 10.1038/ismej.2017.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Anson D.S. The use of retroviral vectors for gene therapy-what are the risks? A review of retroviral pathogenesis and its relevance to retroviral vector-mediated gene delivery. Genet. Vaccines Ther. 2004;2:9. doi: 10.1186/1479-0556-2-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Escors D., Breckpot K. Lentiviral vectors in gene therapy: their current status and future potential. Arch. Immunol. Ther. Exp. (Warsz.) 2010;58:107–119. doi: 10.1007/s00005-010-0063-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hodgson C.P., Solaiman F. Virosomes: cationic liposomes enhance retroviral transduction. Nat. Biotechnol. 1996;14:339–342. doi: 10.1038/nbt0396-339. [DOI] [PubMed] [Google Scholar]
- 13.Mendez N., Herrera V., Zhang L., Hedjran F., Feuer R., Blair S.L., Trogler W.C., Reid T.R., Kummel A.C. Encapsulation of adenovirus serotype 5 in anionic lecithin liposomes using a bead-based immunoprecipitation technique enhances transfection efficiency. Biomaterials. 2014;35:9554–9561. doi: 10.1016/j.biomaterials.2014.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fein D.E., Limberis M.P., Maloney S.F., Heath J.M., Wilson J.M., Diamond S.L. Cationic lipid formulations alter the in vivo tropism of AAV2/9 vector in lung. Mol. Ther. 2009;17:2078–2087. doi: 10.1038/mt.2009.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lundstrom K. Viral Vectors in Gene Therapy. Diseases. 2018;6:42. doi: 10.3390/diseases6020042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zinn E., Vandenberghe L.H. Adeno-associated virus: fit to serve. Curr. Opin. Virol. 2014;8:90–97. doi: 10.1016/j.coviro.2014.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhou X., Muzyczka N. In vitro packaging of adeno-associated virus DNA. J. Virol. 1998;72:3241–3247. doi: 10.1128/jvi.72.4.3241-3247.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dubielzig R., King J.A., Weger S., Kern A., Kleinschmidt J.A. Adeno-associated virus type 2 protein interactions: formation of pre-encapsidation complexes. J. Virol. 1999;73:8989–8998. doi: 10.1128/jvi.73.11.8989-8998.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schultz B.R., Chamberlain J.S. Recombinant adeno-associated virus transduction and integration. Mol. Ther. 2008;16:1189–1199. doi: 10.1038/mt.2008.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Srivastava A. In vivo tissue-tropism of adeno-associated viral vectors. Curr. Opin. Virol. 2016;21:75–80. doi: 10.1016/j.coviro.2016.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gu X., Chai R., Guo L., Dong B., Li W., Shu Y., Huang X., Li H. Transduction of Adeno-Associated Virus Vectors Targeting Hair Cells and Supporting Cells in the Neonatal Mouse Cochlea. Front. Cell. Neurosci. 2019;13:8. doi: 10.3389/fncel.2019.00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xiao P.J., Samulski R.J. Cytoplasmic trafficking, endosomal escape, and perinuclear accumulation of adeno-associated virus type 2 particles are facilitated by microtubule network. J. Virol. 2012;86:10462–10473. doi: 10.1128/JVI.00935-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li C., Samulski R.J. Engineering adeno-associated virus vectors for gene therapy. Nat. Rev. Genet. 2020;21:255–272. doi: 10.1038/s41576-019-0205-4. [DOI] [PubMed] [Google Scholar]
- 24.Askew C., Rochat C., Pan B., Asai Y., Ahmed H., Child E., Schneider B.L., Aebischer P., Holt J.R. Tmcgene therapy restores auditory function in deaf mice. Sci. Transl. Med. 2015;7:295ra108–295ra291. doi: 10.1126/scitranslmed.aab1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shu Y., Tao Y., Wang Z., Tang Y., Li H., Dai P., Gao G., Chen Z.-Y. Identification of Adeno-Associated Viral Vectors That Target Neonatal and Adult Mammalian Inner Ear Cell Subtypes. Hum. Gene Ther. 2016;27:687–699. doi: 10.1089/hum.2016.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Akil O., Dyka F., Calvet C., Emptoz A., Lahlou G., Nouaille S., Boutet de Monvel J., Hardelin J.-P., Hauswirth W.W., Avan P. Dual AAV-mediated gene therapy restores hearing in a DFNB9 mouse model. Proc. Natl. Acad. Sci. USA. 2019;116:4496–4501. doi: 10.1073/pnas.1817537116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Koehler K.R., Nie J., Longworth-Mills E., Liu X.-P., Lee J., Holt J.R., Hashino E. Generation of inner ear organoids containing functional hair cells from human pluripotent stem cells. Nat. Biotechnol. 2017;35:583–589. doi: 10.1038/nbt.3840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hu X., Wang J., Yao X., Xiao Q., Xue Y., Wang S., Shi L., Shu Y., Li H., Yang H. Screened AAV variants permit efficient transduction access to supporting cells and hair cells. Cell Discov. 2019;5:49. doi: 10.1038/s41421-019-0115-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Landegger L.D., Pan B., Askew C., Wassmer S.J., Gluck S.D., Galvin A., Taylor R., Forge A., Stankovic K.M., Holt J.R., Vandenberghe L.H. A synthetic AAV vector enables safe and efficient gene transfer to the mammalian inner ear. Nat. Biotechnol. 2017;35:280–284. doi: 10.1038/nbt.3781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hu C.-J., Lu Y.-C., Tsai Y.-H., Cheng H.-Y., Takeda H., Huang C.-Y., Xiao R., Hsu C.-J., Tsai J.-W., Vandenberghe L.H. Efficient in Utero Gene Transfer to the Mammalian Inner Ears by the Synthetic Adeno-Associated Viral Vector Anc80L65. Mol. Ther. Methods Clin. Dev. 2020;18:493–500. doi: 10.1016/j.omtm.2020.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Isgrig K., McDougald D.S., Zhu J., Wang H.J., Bennett J., Chien W.W. AAV2.7m8 is a powerful viral vector for inner ear gene therapy. Nat. Commun. 2019;10:427. doi: 10.1038/s41467-018-08243-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Calcedo R., Wilson J.M. Humoral Immune Response to AAV. Front. Immunol. 2013;4:341. doi: 10.3389/fimmu.2013.00341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mingozzi F., High K.A. Immune responses to AAV vectors: overcoming barriers to successful gene therapy. Blood. 2013;122:23–36. doi: 10.1182/blood-2013-01-306647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tan F., Chu C., Qi J., Li W., You D., Li K., Chen X., Zhao W., Cheng C., Liu X. AAV-ie enables safe and efficient gene transfer to inner ear cells. Nat. Commun. 2019;10:3733. doi: 10.1038/s41467-019-11687-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu Y., Okada T., Sheykholeslami K., Shimazaki K., Nomoto T., Muramatsu S., Kanazawa T., Takeuchi K., Ajalli R., Mizukami H. Specific and efficient transduction of Cochlear inner hair cells with recombinant adeno-associated virus type 3 vector. Mol. Ther. 2005;12:725–733. doi: 10.1016/j.ymthe.2005.03.021. [DOI] [PubMed] [Google Scholar]
- 36.Bedrosian J.C., Gratton M.A., Brigande J.V., Tang W., Landau J., Bennett J. In vivo delivery of recombinant viruses to the fetal murine cochlea: transduction characteristics and long-term effects on auditory function. Mol. Ther. 2006;14:328–335. doi: 10.1016/j.ymthe.2006.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ballana E., Wang J., Venail F., Estivill X., Puel J.-L., Arbonès M.L., Bosch A. Efficient and specific transduction of cochlear supporting cells by adeno-associated virus serotype 5. Neurosci. Lett. 2008;442:134–139. doi: 10.1016/j.neulet.2008.06.060. [DOI] [PubMed] [Google Scholar]
- 38.György B., Sage C., Indzhykulian A.A., Scheffer D.I., Brisson A.R., Tan S., Wu X., Volak A., Mu D., Tamvakologos P.I. Rescue of Hearing by Gene Delivery to Inner-Ear Hair Cells Using Exosome-Associated AAV. Mol. Ther. 2017;25:379–391. doi: 10.1016/j.ymthe.2016.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wu Z., Yang H., Colosi P. Effect of genome size on AAV vector packaging. Mol. Ther. 2010;18:80–86. doi: 10.1038/mt.2009.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Grieger J.C., Samulski R.J. Packaging capacity of adeno-associated virus serotypes: impact of larger genomes on infectivity and postentry steps. J. Virol. 2005;79:9933–9944. doi: 10.1128/JVI.79.15.9933-9944.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Reisinger E. Dual-AAV delivery of large gene sequences to the inner ear. Hear. Res. 2020;394:107857. doi: 10.1016/j.heares.2019.107857. [DOI] [PubMed] [Google Scholar]
- 42.McClements M.E., MacLaren R.E. Adeno-associated virus (AAV) dual vector strategies for gene therapy encoding large transgenes. Yale J. Biol. Med. 2017;90:611–623. [PMC free article] [PubMed] [Google Scholar]
- 43.Chamberlain K., Riyad J.M., Weber T. Expressing Transgenes That Exceed the Packaging Capacity of Adeno-Associated Virus Capsids. Hum. Gene Ther. Methods. 2016;27:1–12. doi: 10.1089/hgtb.2015.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Trapani I., Colella P., Sommella A., Iodice C., Cesi G., de Simone S., Marrocco E., Rossi S., Giunti M., Palfi A. Effective delivery of large genes to the retina by dual AAV vectors. EMBO Mol. Med. 2014;6:194–211. doi: 10.1002/emmm.201302948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhang Y., Duan D. Novel mini-dystrophin gene dual adeno-associated virus vectors restore neuronal nitric oxide synthase expression at the sarcolemma. Hum. Gene Ther. 2012;23:98–103. doi: 10.1089/hum.2011.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lostal W., Kodippili K., Yue Y., Duan D. Full-length dystrophin reconstitution with adeno-associated viral vectors. Hum. Gene Ther. 2014;25:552–562. doi: 10.1089/hum.2013.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yan Z., Zak R., Zhang Y., Engelhardt J.F. Inverted terminal repeat sequences are important for intermolecular recombination and circularization of adeno-associated virus genomes. J. Virol. 2005;79:364–379. doi: 10.1128/JVI.79.1.364-379.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Duan D., Sharma P., Dudus L., Zhang Y., Sanlioglu S., Yan Z., Yue Y., Ye Y., Lester R., Yang J. Formation of adeno-associated virus circular genomes is differentially regulated by adenovirus E4 ORF6 and E2a gene expression. J. Virol. 1999;73:161–169. doi: 10.1128/jvi.73.1.161-169.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lai Y., Yue Y., Liu M., Ghosh A., Engelhardt J.F., Chamberlain J.S., Duan D. Efficient in vivo gene expression by trans-splicing adeno-associated viral vectors. Nat. Biotechnol. 2005;23:1435–1439. doi: 10.1038/nbt1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Duan D., Yue Y., Engelhardt J.F. Expanding AAV packaging capacity with trans-splicing or overlapping vectors: a quantitative comparison. Mol. Ther. 2001;4:383–391. doi: 10.1006/mthe.2001.0456. [DOI] [PubMed] [Google Scholar]
- 51.Ghosh A., Yue Y., Lai Y., Duan D. A hybrid vector system expands adeno-associated viral vector packaging capacity in a transgene-independent manner. Mol. Ther. 2008;16:124–130. doi: 10.1038/sj.mt.6300322. [DOI] [PubMed] [Google Scholar]
- 52.Pryadkina M., Lostal W., Bourg N., Charton K., Roudaut C., Hirsch M.L., Richard I. A comparison of AAV strategies distinguishes overlapping vectors for efficient systemic delivery of the 6.2 kb Dysferlin coding sequence. Mol. Ther. Methods Clin. Dev. 2015;2:15009. doi: 10.1038/mtm.2015.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Al-Moyed H., Cepeda A.P., Jung S., Moser T., Kügler S., Reisinger E. A dual-AAV approach restores fast exocytosis and partially rescues auditory function in deaf otoferlin knock-out mice. EMBO Mol. Med. 2019;11:e9396. doi: 10.15252/emmm.201809396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nyberg S., Abbott N.J., Shi X., Steyger P.S., Dabdoub A. Delivery of therapeutics to the inner ear: The challenge of the blood-labyrinth barrier. Sci. Transl. Med. 2019;11:eaao0935. doi: 10.1126/scitranslmed.aao0935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Goycoolea M.V., Lundman L. Round window membrane. Structure function and permeability: a review. Microsc. Res. Tech. 1997;36:201–211. doi: 10.1002/(SICI)1097-0029(19970201)36:3<201::AID-JEMT8>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 56.Carpenter A.M., Muchow D., Goycoolea M.V. Ultrastructural Studies of the Human Round Window Membrane. Arch. Otolaryngol. Head Neck Surg. 1989;115:585–590. doi: 10.1001/archotol.1989.01860290043012. [DOI] [PubMed] [Google Scholar]
- 57.Goycoolea M.V., Carpenter A.M., Muchow D. Ultrastructural Studies of the Round-Window Membrane of the Cat. Arch. Otolaryngol. Head Neck Surg. 1987;113:617–624. doi: 10.1001/archotol.1987.01860060043013. [DOI] [PubMed] [Google Scholar]
- 58.Nordang L., Linder B., Anniko M. Morphologic changes in round window membrane after topical hydrocortisone and dexamethasone treatment. Otol. Neurotol. 2003;24:339–343. doi: 10.1097/00129492-200303000-00034. [DOI] [PubMed] [Google Scholar]
- 59.Tanaka K., Motomura S. Permeability of the labyrinthine windows in guinea pigs. Arch. Otorhinolaryngol. 1981;233:67–73. doi: 10.1007/BF00464276. [DOI] [PubMed] [Google Scholar]
- 60.Mikulec A.A., Hartsock J.J., Salt A.N. Permeability of the round window membrane is influenced by the composition of applied drug solutions and by common surgical procedures. Otol. Neurotol. 2008;29:1020–1026. doi: 10.1097/MAO.0b013e31818658ea. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chan D.K., Schrijver I., Chang K.W. Connexin-26-associated deafness: phenotypic variability and progression of hearing loss. Genet. Med. 2010;12:174–181. doi: 10.1097/GIM.0b013e3181d0d42b. [DOI] [PubMed] [Google Scholar]
- 62.Franco B., Malgrange B. Concise Review: Regeneration in Mammalian Cochlea Hair Cells: Help from Supporting Cells Transdifferentiation. Stem Cells. 2017;35:551–556. doi: 10.1002/stem.2554. [DOI] [PubMed] [Google Scholar]
- 63.McGovern M.M., Randle M.R., Cuppini C.L., Graves K.A., Cox B.C. Multiple supporting cell subtypes are capable of spontaneous hair cell regeneration in the neonatal mouse cochlea. Development. 2019;146:dev171009. doi: 10.1242/dev.171009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Collado M.S., Burns J.C., Hu Z., Corwin J.T. Recent advances in hair cell regeneration research. Curr. Opin. Otolaryngol. Head Neck Surg. 2008;16:465–471. doi: 10.1097/MOO.0b013e32830f4ab5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Rubel E.W., Furrer S.A., Stone J.S. A brief history of hair cell regeneration research and speculations on the future. Hear. Res. 2013;297:42–51. doi: 10.1016/j.heares.2012.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Cafaro J., Lee G.S., Stone J.S. Atoh1 expression defines activated progenitors and differentiating hair cells during avian hair cell regeneration. Dev. Dyn. 2007;236:156–170. doi: 10.1002/dvdy.21023. [DOI] [PubMed] [Google Scholar]
- 67.Kawamoto K., Izumikawa M., Beyer L.A., Atkin G.M., Raphael Y. Spontaneous hair cell regeneration in the mouse utricle following gentamicin ototoxicity. Hear. Res. 2009;247:17–26. doi: 10.1016/j.heares.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lin V., Golub J.S., Nguyen T.B., Hume C.R., Oesterle E.C., Stone J.S. Inhibition Of Notch Activity Promotes Nonmitotic Regeneration of Hair Cells in the Adult Mouse Utricles. J. Neurosci. 2011;31:15329–15339. doi: 10.1523/JNEUROSCI.2057-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Minoda R., Izumikawa M., Kawamoto K., Zhang H., Raphael Y. Manipulating cell cycle regulation in the mature cochlea. Hear. Res. 2007;232:44–51. doi: 10.1016/j.heares.2007.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Walters B.J., Coak E., Dearman J., Bailey G., Yamashita T., Kuo B., Zuo J. In Vivo Interplay between p27Kip1, GATA3, ATOH1, and POU4F3 Converts Non-sensory Cells to Hair Cells in Adult Mice. Cell Rep. 2017;19:307–320. doi: 10.1016/j.celrep.2017.03.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Shu Y., Li W., Huang M., Quan Y.Z., Scheffer D., Tian C., Tao Y., Liu X., Hochedlinger K., Indzhykulian A.A. Renewed proliferation in adult mouse cochlea and regeneration of hair cells. Nat. Commun. 2019;10:5530. doi: 10.1038/s41467-019-13157-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Chang Q., Wang J., Li Q., Kim Y., Zhou B., Wang Y., Li H., Lin X. Virally mediated Kcnq1 gene replacement therapy in the immature scala media restores hearing in a mouse model of human Jervell and Lange-Nielsen deafness syndrome. EMBO Mol. Med. 2015;7:1077–1086. doi: 10.15252/emmm.201404929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Holt J.R., Corey D.P. Ion channel defects in hereditary hearing loss. Neuron. 1999;22:217–219. doi: 10.1016/s0896-6273(00)81083-1. [DOI] [PubMed] [Google Scholar]
- 74.Nist-Lund C.A., Pan B., Patterson A., Asai Y., Chen T., Zhou W., Zhu H., Romero S., Resnik J., Polley D.B. Improved TMC1 gene therapy restores hearing and balance in mice with genetic inner ear disorders. Nat. Commun. 2019;10:236. doi: 10.1038/s41467-018-08264-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Akil O., Seal R.P., Burke K., Wang C., Alemi A., During M., Edwards R.H., Lustig L.R. Restoration of hearing in the VGLUT3 knockout mouse using virally mediated gene therapy. Neuron. 2012;75:283–293. doi: 10.1016/j.neuron.2012.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Geng R., Omar A., Gopal S.R., Chen D.H.C., Stepanyan R., Basch M.L., Dinculescu A., Furness D.N., Saperstein D., Hauswirth W. Modeling and Preventing Progressive Hearing Loss in Usher Syndrome III. Sci. Rep. 2017;7:13480. doi: 10.1038/s41598-017-13620-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kurabi A., Keithley E.M., Housley G.D., Ryan A.F., Wong A.C.Y. Cellular mechanisms of noise-induced hearing loss. Hear. Res. 2017;349:129–137. doi: 10.1016/j.heares.2016.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Minasian L.M., Frazier A.L., Sung L., O’Mara A., Kelaghan J., Chang K.W., Krailo M., Pollock B.H., Reaman G., Freyer D.R. Prevention of cisplatin-induced hearing loss in children: Informing the design of future clinical trials. Cancer Med. 2018;7:2951–2959. doi: 10.1002/cam4.1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.O’Sullivan M.E., Perez A., Lin R., Sajjadi A., Ricci A.J., Cheng A.G. Towards the Prevention of Aminoglycoside-Related Hearing Loss. Front. Cell. Neurosci. 2017;11:325. doi: 10.3389/fncel.2017.00325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wan G., Gómez-Casati M.E., Gigliello A.R., Liberman M.C., Corfas G. Neurotrophin-3 regulates ribbon synapse density in the cochlea and induces synapse regeneration after acoustic trauma. eLife. 2014;3:e03564. doi: 10.7554/eLife.03564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wheeler E.F., Bothwell M., Schecterson L.C., Von Bartheld C.S. Expression of BDNF and NT-3 mRNA in hair cells of the organ of Corti: Quantitative analysis in developing rats. Hear. Res. 1994;73:46–56. doi: 10.1016/0378-5955(94)90281-x. [DOI] [PubMed] [Google Scholar]
- 82.Ahmed H., Shubina-Oleinik O., Holt J.R. Emerging Gene Therapies for Genetic Hearing Loss. J. Assoc. Res. Otolaryngol. 2017;18:649–670. doi: 10.1007/s10162-017-0634-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Iizuka T., Kamiya K., Gotoh S., Sugitani Y., Suzuki M., Noda T., Minowa O., Ikeda K. Perinatal Gjb2 gene transfer rescues hearing in a mouse model of hereditary deafness. Hum. Mol. Genet. 2015;24:3651–3661. doi: 10.1093/hmg/ddv109. [DOI] [PubMed] [Google Scholar]
- 84.Pan B., Askew C., Galvin A., Heman-Ackah S., Asai Y., Indzhykulian A.A., Jodelka F.M., Hastings M.L., Lentz J.J., Vandenberghe L.H. Gene therapy restores auditory and vestibular function in a mouse model of Usher syndrome type 1c. Nat. Biotechnol. 2017;35:264–272. doi: 10.1038/nbt.3801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zheng W. The role of Six1 in mammalian auditory system development. Development. 2003;130:3989–4000. doi: 10.1242/dev.00628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Yao H., Hill S.F., Skidmore J.M., Sperry E.D., Swiderski D.L., Sanchez G.J., Bartels C.F., Raphael Y., Scacheri P.C., Iwase S., Martin D.M. CHD7 represses the retinoic acid synthesis enzyme ALDH1A3 during inner ear development. JCI Insight. 2018;3:e97440. doi: 10.1172/jci.insight.97440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Abdelhak S., Kalatzis V., Heilig R., Compain S., Samson D., Vincent C., Weil D., Cruaud C., Sahly I., Leibovici M. A human homologue of the Drosophila eyes absent gene underlies branchio-oto-renal (BOR) syndrome and identifies a novel gene family. Nat. Genet. 1997;15:157–164. doi: 10.1038/ng0297-157. [DOI] [PubMed] [Google Scholar]
- 88.Wang H., Murphy R., Taaffe D., Yin S., Xia L., Hauswirth W.W., Bance M., Robertson G.S., Wang J. Efficient cochlear gene transfection in guinea-pigs with adeno-associated viral vectors by partial digestion of round window membrane. Gene Ther. 2012;19:255–263. doi: 10.1038/gt.2011.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Shibata S.B., Ranum P.T., Moteki H., Pan B., Goodwin A.T., Goodman S.S., Abbas P.J., Holt J.R., Smith R.J.H. RNA Interference Prevents Autosomal-Dominant Hearing Loss. Am. J. Hum. Genet. 2016;98:1101–1113. doi: 10.1016/j.ajhg.2016.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Aarnisalo A.A., Pietola L., Joensuu J., Isosomppi J., Aarnisalo P., Dinculescu A., Lewin A.S., Flannery J., Hauswirth W.W., Sankila E.M., Jero J. Anti-clarin-1 AAV-delivered ribozyme induced apoptosis in the mouse cochlea. Hear. Res. 2007;230:9–16. doi: 10.1016/j.heares.2007.03.004. [DOI] [PubMed] [Google Scholar]
- 91.Lalwani A.K., Walsh B.J., Reilly P.G., Muzyczka N., Mhatre A.N. Development of in vivo gene therapy for hearing disorders: introduction of adeno-associated virus into the cochlea of the guinea pig. Gene Ther. 1996;3:588–592. [PubMed] [Google Scholar]
- 92.Stöver T., Yagi M., Raphael Y. Cochlear gene transfer: round window versus cochleostomy inoculation. Hear. Res. 1999;136:124–130. doi: 10.1016/s0378-5955(99)00115-x. [DOI] [PubMed] [Google Scholar]
- 93.Ishimoto S., Kawamoto K., Kanzaki S., Raphael Y. Gene transfer into supporting cells of the organ of Corti. Hear. Res. 2002;173:187–197. doi: 10.1016/s0378-5955(02)00579-8. [DOI] [PubMed] [Google Scholar]
- 94.Kilpatrick L.A., Li Q., Yang J., Goddard J.C., Fekete D.M., Lang H. Adeno-associated virus-mediated gene delivery into the scala media of the normal and deafened adult mouse ear. Gene Ther. 2011;18:569–578. doi: 10.1038/gt.2010.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Okada H., Iizuka T., Mochizuki H., Nihira T., Kamiya K., Inoshita A., Kasagi H., Kasai M., Ikeda K. Gene transfer targeting mouse vestibule using adenovirus and adeno-associated virus vectors. Otol. Neurotol. 2012;33:655–659. doi: 10.1097/MAO.0b013e31825368d1. [DOI] [PubMed] [Google Scholar]
- 96.Suzuki J., Hashimoto K., Xiao R., Vandenberghe L.H., Liberman M.C. Cochlear gene therapy with ancestral AAV in adult mice: complete transduction of inner hair cells without cochlear dysfunction. Sci. Rep. 2017;7:45524. doi: 10.1038/srep45524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Gantz B.J., Tyler R.S. Cochlear implant comparisons. Am. J. Otol. 1985;(Suppl):92–98. [PubMed] [Google Scholar]
- 98.Wang Y., Sun Y., Chang Q., Ahmad S., Zhou B., Kim Y., Li H., Lin X. Early postnatal virus inoculation into the scala media achieved extensive expression of exogenous green fluorescent protein in the inner ear and preserved auditory brainstem response thresholds. J. Gene Med. 2013;15:123–133. doi: 10.1002/jgm.2701. [DOI] [PubMed] [Google Scholar]
- 99.Suzuki M., Yamasoba T., Kaga K. Development of the blood-labyrinth barrier in the rat. Hear. Res. 1998;116:107–112. doi: 10.1016/s0378-5955(97)00208-6. [DOI] [PubMed] [Google Scholar]
- 100.Suzuki M., Kaga K. Development of blood-labyrinth barrier in the semicircular canal ampulla of the rat. Hear. Res. 1999;129:27–34. doi: 10.1016/s0378-5955(98)00214-7. [DOI] [PubMed] [Google Scholar]
- 101.Shibata S.B., Yoshimura H., Ranum P.T., Goodwin A.T., Smith R.J.H. Intravenous rAAV2/9 injection for murine cochlear gene delivery. Sci. Rep. 2017;7:9609. doi: 10.1038/s41598-017-09805-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Duan D. Systemic delivery of adeno-associated viral vectors. Curr. Opin. Virol. 2016;21:16–25. doi: 10.1016/j.coviro.2016.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Stöver T., Yagi M., Raphael Y. Transduction of the contralateral ear after adenovirus-mediated cochlear gene transfer. Gene Ther. 2000;7:377–383. doi: 10.1038/sj.gt.3301108. [DOI] [PubMed] [Google Scholar]
- 104.De Paolis A., Watanabe H., Nelson J.T., Bikson M., Packer M., Cardoso L. Human cochlear hydrodynamics: A high-resolution μCT-based finite element study. J. Biomech. 2017;50:209–216. doi: 10.1016/j.jbiomech.2016.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Yoshimura H., Shibata S.B., Ranum P.T., Smith R.J.H. Enhanced viral-mediated cochlear gene delivery in adult mice by combining canal fenestration with round window membrane inoculation. Sci. Rep. 2018;8:2980. doi: 10.1038/s41598-018-21233-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Xie Y., Sharon J.D., Pross S.E., Abt N.B., Varma S., Della Santina C.C., Minor L.B., Carey J.P. Surgical Complications from Superior Canal Dehiscence Syndrome Repair: Two Decades of Experience. Otolaryngol. Head Neck Surg. 2017;157:273–280. doi: 10.1177/0194599817706491. [DOI] [PubMed] [Google Scholar]
- 107.Jero J., Mhatre A.N., Tseng C.J., Stern R.E., Coling D.E., Goldstein J.A., Hong K., Zheng W.W., Hoque A.T., Lalwani A.K. Cochlear gene delivery through an intact round window membrane in mouse. Hum. Gene Ther. 2001;12:539–548. doi: 10.1089/104303401300042465. [DOI] [PubMed] [Google Scholar]
- 108.Qi W., Ding D., Zhu H., Lu D., Wang Y., Ding J., Yan W., Jia M., Guo Y. Efficient siRNA transfection to the inner ear through the intact round window by a novel proteidic delivery technology in the chinchilla. Gene Ther. 2014;21:10–18. doi: 10.1038/gt.2013.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Lentz J.J., Jodelka F.M., Hinrich A.J., McCaffrey K.E., Farris H.E., Spalitta M.J., Bazan N.G., Duelli D.M., Rigo F., Hastings M.L. Rescue of hearing and vestibular function by antisense oligonucleotides in a mouse model of human deafness. Nat. Med. 2013;19:345–350. doi: 10.1038/nm.3106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Dulon D., Papal S., Patni P., Cortese M., Vincent P.F.Y., Tertrais M., Emptoz A., Tlili A., Bouleau Y., Michel V. Clarin-1 gene transfer rescues auditory synaptopathy in model of Usher syndrome. J. Clin. Invest. 2018;128:3382–3401. doi: 10.1172/JCI94351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Yeh W.-H., Shubina-Oleinik O., Levy J.M., Pan B., Newby G.A., Wornow M., Burt R., Chen J.C., Holt J.R., Liu D.R. In vivo base editing restores sensory transduction and transiently improves auditory function in a mouse model of recessive deafness. Sci. Transl. Med. 2020;12:eaay9101. doi: 10.1126/scitranslmed.aay9101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.György B., Nist-Lund C., Pan B., Asai Y., Karavitaki K.D., Kleinstiver B.P., Garcia S.P., Zaborowski M.P., Solanes P., Spataro S. Allele-specific gene editing prevents deafness in a model of dominant progressive hearing loss. Nat. Med. 2019;25:1123–1130. doi: 10.1038/s41591-019-0500-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Yoshimura H., Shibata S.B., Ranum P.T., Moteki H., Smith R.J.H. Targeted Allele Suppression Prevents Progressive Hearing Loss in the Mature Murine Model of Human TMC1 Deafness. Mol. Ther. 2019;27:681–690. doi: 10.1016/j.ymthe.2018.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Kim M.-A., Cho H.-J., Bae S.-H., Lee B., Oh S.-K., Kwon T.-J., Ryoo Z.-Y., Kim H.-Y., Cho J.-H., Kim U.-K., Lee K.Y. Methionine Sulfoxide Reductase B3-Targeted In Utero Gene Therapy Rescues Hearing Function in a Mouse Model of Congenital Sensorineural Hearing Loss. Antioxid. Redox Signal. 2016;24:590–602. doi: 10.1089/ars.2015.6442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Chien W.W., Isgrig K., Roy S., Belyantseva I.A., Drummond M.C., May L.A., Fitzgerald T.S., Friedman T.B., Cunningham L.L. Gene Therapy Restores Hair Cell Stereocilia Morphology in Inner Ears of Deaf Whirler Mice. Mol. Ther. 2016;24:17–25. doi: 10.1038/mt.2015.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Isgrig K., Shteamer J.W., Belyantseva I.A., Drummond M.C., Fitzgerald T.S., Vijayakumar S., Jones S.M., Griffith A.J., Friedman T.B., Cunningham L.L., Chien W.W. Gene Therapy Restores Balance and Auditory Functions in a Mouse Model of Usher Syndrome. Mol. Ther. 2017;25:780–791. doi: 10.1016/j.ymthe.2017.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Delmaghani S., Defourny J., Aghaie A., Beurg M., Dulon D., Thelen N., Perfettini I., Zelles T., Aller M., Meyer A. Hypervulnerability to Sound Exposure through Impaired Adaptive Proliferation of Peroxisomes. Cell. 2015;163:894–906. doi: 10.1016/j.cell.2015.10.023. [DOI] [PubMed] [Google Scholar]
- 118.Emptoz A., Michel V., Lelli A., Akil O., Boutet de Monvel J., Lahlou G., Meyer A., Dupont T., Nouaille S., Ey E. Local gene therapy durably restores vestibular function in a mouse model of Usher syndrome type 1G. Proc. Natl. Acad. Sci. USA. 2017;114:9695–9700. doi: 10.1073/pnas.1708894114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Kim M.A., Kim S.H., Ryu N., Ma J.H., Kim Y.R., Jung J., Hsu C.J., Choi J.Y., Lee K.Y., Wangemann P. Gene therapy for hereditary hearing loss by SLC26A4 mutations in mice reveals distinct functional roles of pendrin in normal hearing. Theranostics. 2019;9:7184–7199. doi: 10.7150/thno.38032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Yu Q., Wang Y., Chang Q., Wang J., Gong S., Li H., Lin X. Virally expressed connexin26 restores gap junction function in the cochlea of conditional Gjb2 knockout mice. Gene Ther. 2014;21:71–80. doi: 10.1038/gt.2013.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Tertrais M., Bouleau Y., Emptoz A., Belleudy S., Sutton R.B., Petit C., Safieddine S., Dulon D. Viral transfer of mini-otoferlins partially restores the fast component of exocytosis and uncovers ultrafast endocytosis in auditory hair cells of otoferlin knock-out mice. J. Neurosci. 2019;39:3394–3411. doi: 10.1523/JNEUROSCI.1550-18.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Maeda Y., Fukushima K., Nishizaki K., Smith R.J.H. In vitro and in vivo suppression of GJB2 expression by RNA interference. Hum. Mol. Genet. 2005;14:1641–1650. doi: 10.1093/hmg/ddi172. [DOI] [PubMed] [Google Scholar]
- 123.Maeda Y., Fukushima K., Kawasaki A., Nishizaki K., Smith R.J.H. Cochlear expression of a dominant-negative GJB2R75W construct delivered through the round window membrane in mice. Neurosci. Res. 2007;58:250–254. doi: 10.1016/j.neures.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 124.Gao X., Tao Y., Lamas V., Huang M., Yeh W.-H., Pan B., Hu Y.-J., Hu J.H., Thompson D.B., Shu Y. Treatment of autosomal dominant hearing loss by in vivo delivery of genome editing agents. Nature. 2018;553:217–221. doi: 10.1038/nature25164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Kang W., Sun Z., Zhao X., Wang X., Tao Y., Wu H. Gene editing based hearing impairment research and therapeutics. Neurosci. Lett. 2019;709:134326. doi: 10.1016/j.neulet.2019.134326. [DOI] [PubMed] [Google Scholar]
- 126.Davies B. The technical risks of human gene editing. Hum. Reprod. 2019;34:2104–2111. doi: 10.1093/humrep/dez162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Morton C.C., Nance W.E. Newborn hearing screening--a silent revolution. N. Engl. J. Med. 2006;354:2151–2164. doi: 10.1056/NEJMra050700. [DOI] [PubMed] [Google Scholar]
- 128.Fremeau R.T., Jr., Kam K., Qureshi T., Johnson J., Copenhagen D.R., Storm-Mathisen J., Chaudhry F.A., Nicoll R.A., Edwards R.H. Vesicular glutamate transporters 1 and 2 target to functionally distinct synaptic release sites. Science. 2004;304:1815–1819. doi: 10.1126/science.1097468. [DOI] [PubMed] [Google Scholar]
- 129.Takamori S., Malherbe P., Broger C., Jahn R. Molecular cloning and functional characterization of human vesicular glutamate transporter 3. EMBO Rep. 2002;3:798–803. doi: 10.1093/embo-reports/kvf159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Amilhon B., Lepicard E., Renoir T., Mongeau R., Popa D., Poirel O., Miot S., Gras C., Gardier A.M., Gallego J. VGLUT3 (vesicular glutamate transporter type 3) contribution to the regulation of serotonergic transmission and anxiety. J. Neurosci. 2010;30:2198–2210. doi: 10.1523/JNEUROSCI.5196-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Seal R.P., Akil O., Yi E., Weber C.M., Grant L., Yoo J., Clause A., Kandler K., Noebels J.L., Glowatzki E. Sensorineural deafness and seizures in mice lacking vesicular glutamate transporter 3. Neuron. 2008;57:263–275. doi: 10.1016/j.neuron.2007.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Boughman J.A., Vernon M., Shaver K.A. Usher syndrome: Definition and estimate of prevalence from two high-risk populations. J. Chronic Dis. 1983;36:595–603. doi: 10.1016/0021-9681(83)90147-9. [DOI] [PubMed] [Google Scholar]
- 133.Smith R.J., Berlin C.I., Hejtmancik J.F., Keats B.J., Kimberling W.J., Lewis R.A., Möller C.G., Pelias M.Z., Tranebjaerg L., Usher Syndrome Consortium Clinical diagnosis of the Usher syndromes. Am. J. Med. Genet. 1994;50:32–38. doi: 10.1002/ajmg.1320500107. [DOI] [PubMed] [Google Scholar]
- 134.Vernon M. Usher’s syndrome—deafness and progressive blindness. J. Chronic Dis. 1969;22:133–151. doi: 10.1016/0021-9681(69)90055-1. [DOI] [PubMed] [Google Scholar]
- 135.Bitner-Glindzicz M., Lindley K.J., Rutland P., Blaydon D., Smith V.V., Milla P.J., Hussain K., Furth-Lavi J., Cosgrove K.E., Shepherd R.M. A recessive contiguous gene deletion causing infantile hyperinsulinism, enteropathy and deafness identifies the Usher type 1C gene. Nat. Genet. 2000;26:56–60. doi: 10.1038/79178. [DOI] [PubMed] [Google Scholar]
- 136.Verpy E., Leibovici M., Zwaenepoel I., Liu X.-Z., Gal A., Salem N., Mansour A., Blanchard S., Kobayashi I., Keats B.J.B. A defect in harmonin, a PDZ domain-containing protein expressed in the inner ear sensory hair cells, underlies Usher syndrome type 1C. Nat. Genet. 2000;26:51–55. doi: 10.1038/79171. [DOI] [PubMed] [Google Scholar]
- 137.Bolz H., von Brederlow B., Ramírez A., Bryda E.C., Kutsche K., Nothwang H.G., Seeliger M., del C-Salcedó Cabrera M., Vila M.C., Molina O.P. Mutation of CDH23, encoding a new member of the cadherin gene family, causes Usher syndrome type 1D. Nat. Genet. 2001;27:108–112. doi: 10.1038/83667. [DOI] [PubMed] [Google Scholar]
- 138.Ahmed Z.M., Riazuddin S., Bernstein S.L., Ahmed Z., Khan S., Griffith A.J., Morell R.J., Friedman T.B., Riazuddin S., Wilcox E.R. Mutations of the protocadherin gene PCDH15 cause Usher syndrome type 1F. Am. J. Hum. Genet. 2001;69:25–34. doi: 10.1086/321277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Alagramam K.N., Murcia C.L., Kwon H.Y., Pawlowski K.S., Wright C.G., Woychik R.P. The mouse Ames waltzer hearing-loss mutant is caused by mutation of Pcdh15, a novel protocadherin gene. Nat. Genet. 2001;27:99–102. doi: 10.1038/83837. [DOI] [PubMed] [Google Scholar]
- 140.Weil D., El-Amraoui A., Masmoudi S., Mustapha M., Kikkawa Y., Lainé S., Delmaghani S., Adato A., Nadifi S., Zina Z.B. Usher syndrome type I G (USH1G) is caused by mutations in the gene encoding SANS, a protein that associates with the USH1C protein, harmonin. Hum. Mol. Genet. 2003;12:463–471. doi: 10.1093/hmg/ddg051. [DOI] [PubMed] [Google Scholar]
- 141.Riazuddin S., Belyantseva I.A., Giese A.P.J., Lee K., Indzhykulian A.A., Nandamuri S.P., Yousaf R., Sinha G.P., Lee S., Terrell D. Alterations of the CIB2 calcium- and integrin-binding protein cause Usher syndrome type 1J and nonsyndromic deafness DFNB48. Nat. Genet. 2012;44:1265–1271. doi: 10.1038/ng.2426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Gregory F.D., Bryan K.E., Pangršič T., Calin-Jageman I.E., Moser T., Lee A. Harmonin inhibits presynaptic Cav1.3 Ca2+ channels in mouse inner hair cells. Nat. Neurosci. 2011;14:1109–1111. doi: 10.1038/nn.2895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Boëda B., El-Amraoui A., Bahloul A., Goodyear R., Daviet L., Blanchard S., Perfettini I., Fath K.R., Shorte S., Reiners J. Myosin VIIa, harmonin and cadherin 23, three Usher I gene products that cooperate to shape the sensory hair cell bundle. EMBO J. 2002;21:6689–6699. doi: 10.1093/emboj/cdf689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Lefevre G., Michel V., Weil D., Lepelletier L., Bizard E., Wolfrum U., Hardelin J.P., Petit C. A core cochlear phenotype in USH1 mouse mutants implicates fibrous links of the hair bundle in its cohesion, orientation and differential growth. Development. 2008;135:1427–1437. doi: 10.1242/dev.012922. [DOI] [PubMed] [Google Scholar]
- 145.Michalski N., Michel V., Caberlotto E., Lefèvre G.M., van Aken A.F.J., Tinevez J.-Y., Bizard E., Houbron C., Weil D., Hardelin J.-P. Harmonin-b, an actin-binding scaffold protein, is involved in the adaptation of mechanoelectrical transduction by sensory hair cells. Pflugers Arch. 2009;459:115–130. doi: 10.1007/s00424-009-0711-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Wang L., Jiang H., Brigande J.V. Gene Transfer to the Developing Mouse Inner Ear by In Vivo Electroporation. J. Vis. Exp. 2012;30:3653. doi: 10.3791/3653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Adato A., Vreugde S., Joensuu T., Avidan N., Hamalainen R., Belenkiy O., Olender T., Bonne-Tamir B., Ben-Asher E., Espinos C. USH3A transcripts encode clarin-1, a four-transmembrane-domain protein with a possible role in sensory synapses. Eur. J. Hum. Genet. 2002;10:339–350. doi: 10.1038/sj.ejhg.5200831. [DOI] [PubMed] [Google Scholar]
- 148.Fields R.R., Zhou G., Huang D., Davis J.R., Möller C., Jacobson S.G., Kimberling W.J., Sumegi J. Usher Syndrome Type III: Revised Genomic Structure of the USH3 Gene and Identification of Novel Mutations. Am. J. Hum. Genet. 2002;71:607–617. doi: 10.1086/342098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Ness S.L., Ben-Yosef T., Bar-Lev A., Madeo A.C., Brewer C.C., Avraham K.B., Kornreich R., Desnick R.J., Willner J.P., Friedman T.B., Griffith A.J. Genetic homogeneity and phenotypic variability among Ashkenazi Jews with Usher syndrome type III. J. Med. Genet. 2003;40:767–772. doi: 10.1136/jmg.40.10.767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Ebermann I., Wilke R., Lauhoff T., Lübben D., Zrenner E., Bolz H.J. Two truncating USH3A mutations, including one novel, in a German family with Usher syndrome. Mol. Vis. 2007;13:1539–1547. [PubMed] [Google Scholar]
- 151.Geng R., Melki S., Chen D.H.C., Tian G., Furness D.N., Oshima-Takago T., Neef J., Moser T., Askew C., Horwitz G. The mechanosensory structure of the hair cell requires clarin-1, a protein encoded by Usher syndrome III causative gene. J. Neurosci. 2012;32:9485–9498. doi: 10.1523/JNEUROSCI.0311-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Gopal S.R., Chen D.H.C., Chou S.W., Zang J., Neuhauss S.C.F., Stepanyan R., McDermott B.M., Jr., Alagramam K.N. Zebrafish Models for the Mechanosensory Hair Cell Dysfunction in Usher Syndrome 3 Reveal That Clarin-1 Is an Essential Hair Bundle Protein. J. Neurosci. 2015;35:10188–10201. doi: 10.1523/JNEUROSCI.1096-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Shabbir M.I., Ahmed Z.M., Khan S.Y., Riazuddin S., Waryah A.M., Khan S.N., Camps R.D., Ghosh M., Kabra M., Belyantseva I.A. Mutations of human TMHS cause recessively inherited non-syndromic hearing loss. J. Med. Genet. 2006;43:634–640. doi: 10.1136/jmg.2005.039834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Longo-Guess C.M., Gagnon L.H., Cook S.A., Wu J., Zheng Q.Y., Johnson K.R. A missense mutation in the previously undescribed gene Tmhs underlies deafness in hurry-scurry (hscy) mice. Proc. Natl. Acad. Sci. USA. 2005;102:7894–7899. doi: 10.1073/pnas.0500760102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Liaqat K., Chiu I., Lee K., Chakchouk I., Andrade-Elizondo P.B., Santos-Cortez R.L.P., Hussain S., Nawaz S., Ansar M., Khan M.N. Novel missense and 3′-UTR splice site variants in LHFPL5 cause autosomal recessive nonsyndromic hearing impairment. J. Hum. Genet. 2018;63:1099–1107. doi: 10.1038/s10038-018-0502-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Xiong W., Grillet N., Elledge H.M., Wagner T.F., Zhao B., Johnson K.R., Kazmierczak P., Müller U. TMHS is an integral component of the mechanotransduction machinery of cochlear hair cells. Cell. 2012;151:1283–1295. doi: 10.1016/j.cell.2012.10.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Beurg M., Xiong W., Zhao B., Müller U., Fettiplace R. Subunit determination of the conductance of hair-cell mechanotransducer channels. Proc. Natl. Acad. Sci. USA. 2015;112:1589–1594. doi: 10.1073/pnas.1420906112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Kurima K., Peters L.M., Yang Y., Riazuddin S., Ahmed Z.M., Naz S., Arnaud D., Drury S., Mo J., Makishima T. Dominant and recessive deafness caused by mutations of a novel gene, TMC1, required for cochlear hair-cell function. Nat. Genet. 2002;30:277–284. doi: 10.1038/ng842. [DOI] [PubMed] [Google Scholar]
- 159.Hilgert N., Alasti F., Dieltjens N., Pawlik B., Wollnik B., Uyguner O., Delmaghani S., Weil D., Petit C., Danis E. Mutation analysis of TMC1 identifies four new mutations and suggests an additional deafness gene at loci DFNA36 and DFNB7/11. Clin. Genet. 2008;74:223–232. doi: 10.1111/j.1399-0004.2008.01053.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Beurg M., Barlow A., Furness D.N., Fettiplace R. A Tmc1 mutation reduces calcium permeability and expression of mechanoelectrical transduction channels in cochlear hair cells. Proc. Natl. Acad. Sci. USA. 2019;116:20743–20749. doi: 10.1073/pnas.1908058116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Mahendrasingam S., Furness D.N. Ultrastructural localization of the likely mechanoelectrical transduction channel protein, transmembrane-like channel 1 (TMC1) during development of cochlear hair cells. Sci. Rep. 2019;9:1274. doi: 10.1038/s41598-018-37563-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Kurima K., Ebrahim S., Pan B., Sedlacek M., Sengupta P., Millis B.A., Cui R., Nakanishi H., Fujikawa T., Kawashima Y. TMC1 and TMC2 Localize at the Site of Mechanotransduction in Mammalian Inner Ear Hair Cell Stereocilia. Cell Rep. 2015;12:1606–1617. doi: 10.1016/j.celrep.2015.07.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Kawashima Y., Géléoc G.S.G., Kurima K., Labay V., Lelli A., Asai Y., Makishima T., Wu D.K., Della Santina C.C., Holt J.R., Griffith A.J. Mechanotransduction in mouse inner ear hair cells requires transmembrane channel-like genes. J. Clin. Invest. 2011;121:4796–4809. doi: 10.1172/JCI60405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Beurg M., Cui R., Goldring A.C., Ebrahim S., Fettiplace R., Kachar B. Variable number of TMC1-dependent mechanotransducer channels underlie tonotopic conductance gradients in the cochlea. Nat. Commun. 2018;9:2185. doi: 10.1038/s41467-018-04589-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Marcotti W., Erven A., Johnson S.L., Steel K.P., Kros C.J. Tmc1 is necessary for normal functional maturation and survival of inner and outer hair cells in the mouse cochlea. J. Physiol. 2006;574:677–698. doi: 10.1113/jphysiol.2005.095661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Zhao Y., Wang D., Zong L., Zhao F., Guan L., Zhang P., Shi W., Lan L., Wang H., Li Q. A novel DFNA36 mutation in TMC1 orthologous to the Beethoven (Bth) mouse associated with autosomal dominant hearing loss in a Chinese family. PLoS ONE. 2014;9:e97064. doi: 10.1371/journal.pone.0097064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Vreugde S., Erven A., Kros C.J., Marcotti W., Fuchs H., Kurima K., Wilcox E.R., Friedman T.B., Griffith A.J., Balling R. Beethoven, a mouse model for dominant, progressive hearing loss DFNA36. Nat. Genet. 2002;30:257–258. doi: 10.1038/ng848. [DOI] [PubMed] [Google Scholar]
- 168.Imtiaz A., Maqsood A., Rehman A.U., Morell R.J., Holt J.R., Friedman T.B., Naz S. Recessive mutations of TMC1 associated with moderate to severe hearing loss. Neurogenetics. 2016;17:115–123. doi: 10.1007/s10048-016-0477-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Ahmed Z.M., Yousaf R., Lee B.C., Khan S.N., Lee S., Lee K., Husnain T., Rehman A.U., Bonneux S., Ansar M. Functional null mutations of MSRB3 encoding methionine sulfoxide reductase are associated with human deafness DFNB74. Am. J. Hum. Genet. 2011;88:19–29. doi: 10.1016/j.ajhg.2010.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Kwon T.J., Cho H.J., Kim U.K., Lee E., Oh S.K., Bok J., Bae Y.C., Yi J.K., Lee J.W., Ryoo Z.Y. Methionine sulfoxide reductase B3 deficiency causes hearing loss due to stereocilia degeneration and apoptotic cell death in cochlear hair cells. Hum. Mol. Genet. 2014;23:1591–1601. doi: 10.1093/hmg/ddt549. [DOI] [PubMed] [Google Scholar]
- 171.Kelley P.M., Harris D.J., Comer B.C., Askew J.W., Fowler T., Smith S.D., Kimberling W.J. Novel mutations in the connexin 26 gene (GJB2) that cause autosomal recessive (DFNB1) hearing loss. Am. J. Hum. Genet. 1998;62:792–799. doi: 10.1086/301807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Denoyelle F., Lina-Granade G., Plauchu H., Bruzzone R., Chaïb H., Lévi-Acobas F., Weil D., Petit C. Connexin 26 gene linked to a dominant deafness. Nature. 1998;393:319–320. doi: 10.1038/30639. [DOI] [PubMed] [Google Scholar]
- 173.Goodenough D.A., Paul D.L. Gap junctions. Cold Spring Harb. Perspect. Biol. 2009;1:a002576. doi: 10.1101/cshperspect.a002576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Wang B., Hu B., Yang S. Cell junction proteins within the cochlea: A review of recent research. J. Otol. 2015;10:131–135. doi: 10.1016/j.joto.2016.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Hibino H., Kurachi Y. Molecular and physiological bases of the K+ circulation in the mammalian inner ear. Physiology (Bethesda) 2006;21:336–345. doi: 10.1152/physiol.00023.2006. [DOI] [PubMed] [Google Scholar]
- 176.Sadanaga M., Morimitsu T. Development of endocochlear potential and its negative component in mouse cochlea. Hear Res. 1995;89:155–161. doi: 10.1016/0378-5955(95)00133-x. [DOI] [PubMed] [Google Scholar]
- 177.Salt A.N., Thalmann R., Marcus D.C., Bohne B.A. Direct measurement of longitudinal endolymph flow rate in the guinea pig cochlea. Hear Res. 1986;23:141–151. doi: 10.1016/0378-5955(86)90011-0. [DOI] [PubMed] [Google Scholar]
- 178.Konishi T., Kelsey E., Singleton G.T. Effects of chemical alteration in the endolymph on the cochlear potentials. Acta Otolaryngol. 1966;62:393–404. doi: 10.3109/00016486609119584. [DOI] [PubMed] [Google Scholar]
- 179.Takada Y., Beyer L.A., Swiderski D.L., O’Neal A.L., Prieskorn D.M., Shivatzki S., Avraham K.B., Raphael Y. Connexin 26 null mice exhibit spiral ganglion degeneration that can be blocked by BDNF gene therapy. Hear Res. 2014;309:124–135. doi: 10.1016/j.heares.2013.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Tlili A., Charfedine I., Lahmar I., Benzina Z., Mohamed B.A., Weil D., Idriss N., Drira M., Masmoudi S., Ayadi H. Identification of a novel frameshift mutation in the DFNB31/WHRN gene in a Tunisian consanguineous family with hereditary non-syndromic recessive hearing loss. Hum. Mutat. 2005;25:503. doi: 10.1002/humu.9333. [DOI] [PubMed] [Google Scholar]
- 181.Ebermann I., Scholl H.P.N., Charbel Issa P., Becirovic E., Lamprecht J., Jurklies B., Millán J.M., Aller E., Mitter D., Bolz H. A novel gene for Usher syndrome type 2: mutations in the long isoform of whirlin are associated with retinitis pigmentosa and sensorineural hearing loss. Hum. Genet. 2007;121:203–211. doi: 10.1007/s00439-006-0304-0. [DOI] [PubMed] [Google Scholar]
- 182.Mburu P., Mustapha M., Varela A., Weil D., El-Amraoui A., Holme R.H., Rump A., Hardisty R.E., Blanchard S., Coimbra R.S. Defects in whirlin, a PDZ domain molecule involved in stereocilia elongation, cause deafness in the whirler mouse and families with DFNB31. Nat. Genet. 2003;34:421–428. doi: 10.1038/ng1208. [DOI] [PubMed] [Google Scholar]
- 183.Mustapha M., Chouery E., Chardenoux S., Naboulsi M., Paronnaud J., Lemainque A., Mégarbané A., Loiselet J., Weil D., Lathrop M., Petit C. DFNB31, a recessive form of sensorineural hearing loss, maps to chromosome 9q32-34. Eur. J. Hum. Genet. 2002;10:210–212. doi: 10.1038/sj.ejhg.5200780. [DOI] [PubMed] [Google Scholar]
- 184.Belyantseva I.A., Boger E.T., Naz S., Frolenkov G.I., Sellers J.R., Ahmed Z.M., Griffith A.J., Friedman T.B. Myosin-XVa is required for tip localization of whirlin and differential elongation of hair-cell stereocilia. Nat. Cell Biol. 2005;7:148–156. doi: 10.1038/ncb1219. [DOI] [PubMed] [Google Scholar]
- 185.Jervell A., Lange-Nielsen F. Congenital deaf-mutism, functional heart disease with prolongation of the Q-T interval and sudden death. Am. Heart J. 1957;54:59–68. doi: 10.1016/0002-8703(57)90079-0. [DOI] [PubMed] [Google Scholar]
- 186.Lee M.P., Ravenel J.D., Hu R.-J., Lustig L.R., Tomaselli G., Berger R.D., Brandenburg S.A., Litzi T.J., Bunton T.E., Limb C. Targeted disruption of the Kvlqt1 gene causes deafness and gastric hyperplasia in mice. J. Clin. Invest. 2000;106:1447–1455. doi: 10.1172/JCI10897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Lang F., Vallon V., Knipper M., Wangemann P. Functional significance of channels and transporters expressed in the inner ear and kidney. Am. J. Physiol. Cell Physiol. 2007;293:C1187–C1208. doi: 10.1152/ajpcell.00024.2007. [DOI] [PubMed] [Google Scholar]
- 188.Yasunaga S., Grati M., Cohen-Salmon M., El-Amraoui A., Mustapha M., Salem N., El-Zir E., Loiselet J., Petit C. A mutation in OTOF, encoding otoferlin, a FER-1-like protein, causes DFNB9, a nonsyndromic form of deafness. Nat. Genet. 1999;21:363–369. doi: 10.1038/7693. [DOI] [PubMed] [Google Scholar]
- 189.Pangršič T., Lasarow L., Reuter K., Takago H., Schwander M., Riedel D., Frank T., Tarantino L.M., Bailey J.S., Strenzke N. Hearing requires otoferlin-dependent efficient replenishment of synaptic vesicles in hair cells. Nat. Neurosci. 2010;13:869–876. doi: 10.1038/nn.2578. [DOI] [PubMed] [Google Scholar]
- 190.Hams N., Padmanarayana M., Qiu W., Johnson C.P. Otoferlin is a multivalent calcium-sensitive scaffold linking SNAREs and calcium channels. Proc. Natl. Acad. Sci. USA. 2017;114:8023–8028. doi: 10.1073/pnas.1703240114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Wang J., Powers N.L., Hofstetter P., Trautwein P., Ding D., Salvi R. Effects of selective inner hair cell loss on auditory nerve fiber threshold, tuning and spontaneous and driven discharge rate. Hear Res. 1997;107:67–82. doi: 10.1016/s0378-5955(97)00020-8. [DOI] [PubMed] [Google Scholar]
- 192.Petrs-Silva H., Dinculescu A., Li Q., Deng W.-T., Pang J.-J., Min S.-H., Chiodo V., Neeley A.W., Govindasamy L., Bennett A. Novel Properties of Tyrosine-mutant AAV2 Vectors in the Mouse Retina. Mol. Ther. 2011;19:293–301. doi: 10.1038/mt.2010.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Delmaghani S., del Castillo F.J., Michel V., Leibovici M., Aghaie A., Ron U., Van Laer L., Ben-Tal N., Van Camp G., Weil D. Mutations in the gene encoding pejvakin, a newly identified protein of the afferent auditory pathway, cause DFNB59 auditory neuropathy. Nat. Genet. 2006;38:770–778. doi: 10.1038/ng1829. [DOI] [PubMed] [Google Scholar]
- 194.Yan J., Pan L., Chen X., Wu L., Zhang M. The structure of the harmonin/sans complex reveals an unexpected interaction mode of the two Usher syndrome proteins. Proc. Natl. Acad. Sci. USA. 2010;107:4040–4045. doi: 10.1073/pnas.0911385107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Kim H.-M., Wangemann P. Epithelial Cell Stretching and Luminal Acidification Lead to a Retarded Development of Stria Vascularis and Deafness in Mice Lacking Pendrin. PLoS One. 2011;6:e17949. doi: 10.1371/journal.pone.0017949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Wangemann P., Nakaya K., Wu T., Maganti R.J., Itza E.M., Sanneman J.D., Harbidge D.G., Billings S., Marcus D.C. Loss of cochlear HCO3- secretion causes deafness via endolymphatic acidification and inhibition of Ca2+ reabsorption in a Pendred syndrome mouse model. Am. J. Physiol. Renal Physiol. 2007;292:F1345–F1353. doi: 10.1152/ajprenal.00487.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Li X.C., Everett L.A., Lalwani A.K., Desmukh D., Friedman T.B., Green E.D., Wilcox E.R. A mutation in PDS causes non-syndromic recessive deafness. Nat. Genet. 1998;18:215–217. doi: 10.1038/ng0398-215. [DOI] [PubMed] [Google Scholar]
- 198.Everett L.A., Glaser B., Beck J.C., Idol J.R., Buchs A., Heyman M., Adawi F., Hazani E., Nassir E., Baxevanis A.D. Pendred syndrome is caused by mutations in a putative sulphate transporter gene (PDS) Nat. Genet. 1997;17:411–422. doi: 10.1038/ng1297-411. [DOI] [PubMed] [Google Scholar]
- 199.Wangemann P., Itza E.M., Albrecht B., Wu T., Jabba S.V., Maganti R.J., Lee J.H., Everett L.A., Wall S.M., Royaux I.E. Loss of KCNJ10 protein expression abolishes endocochlear potential and causes deafness in Pendred syndrome mouse model. BMC Med. 2004;2:30. doi: 10.1186/1741-7015-2-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Royaux I.E., Wall S.M., Karniski L.P., Everett L.A., Suzuki K., Knepper M.A., Green E.D. Pendrin, encoded by the Pendred syndrome gene, resides in the apical region of renal intercalated cells and mediates bicarbonate secretion. Proc. Natl. Acad. Sci. USA. 2001;98:4221–4226. doi: 10.1073/pnas.071516798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Gu W., Brooks M., Catalfamo J., Ray J., Ray K. Two distinct mutations cause severe hemophilia B in two unrelated canine pedigrees. Thromb. Haemost. 1999;82:1270–1275. [PubMed] [Google Scholar]
- 202.Ohshima T., Murray G.J., Swaim W.D., Longenecker G., Quirk J.M., Cardarelli C.O., Sugimoto Y., Pastan I., Gottesman M.M., Brady R.O. alpha-Galactosidase A deficient mice: A model of Fabry disease. Proc. Natl. Acad. Sci. USA. 1997;94:2540–2544. doi: 10.1073/pnas.94.6.2540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Phaneuf D., Wakamatsu N., Huang J.Q., Borowski A., Peterson A.C., Fortunato S.R., Ritter G., Igdoura S.A., Morales C.R., Benoit G. Dramatically Different Phenotypes in Mouse Models of Human Tay-Sachs and Sandhoff Diseases. Hum. Mol. Genet. 1996;5:1–14. doi: 10.1093/hmg/5.1.1. [DOI] [PubMed] [Google Scholar]
- 204.Casal M., Haskins M. Large animal models and gene therapy. Eur. J. Hum. Genet. 2006;14:266–272. doi: 10.1038/sj.ejhg.5201535. [DOI] [PubMed] [Google Scholar]
- 205.Abkowitz J.L., Persik M.T., Catlin S.N., Guttorp P. Simulation of hematopoiesis: implications for the gene therapy of lysosomal enzyme disorders. Acta Haematol. 1996;95:213–217. doi: 10.1159/000203880. [DOI] [PubMed] [Google Scholar]
- 206.Abkowitz J.L., Persik M.T., Shelton G.H., Ott R.L., Kiklevich J.V., Catlin S.N., Guttorp P. Behavior of hematopoietic stem cells in a large animal. Proc. Natl. Acad. Sci. USA. 1995;92:2031–2035. doi: 10.1073/pnas.92.6.2031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Kesser B.W., Hashisaki G.T., Fletcher K., Eppard H., Holt J.R. An in vitro model system to study gene therapy in the human inner ear. Gene Ther. 2007;14:1121–1131. doi: 10.1038/sj.gt.3302980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Hauswirth W.W., Aleman T.S., Kaushal S., Cideciyan A.V., Schwartz S.B., Wang L., Conlon T.J., Boye S.L., Flotte T.R., Byrne B.J. Treatment of Leber Congenital Amaurosis Due to RPE65 Mutations by Ocular Subretinal Injection of Adeno-Associated Virus Gene Vector: Short-Term Results of a Phase I Trial. Hum. Gene Ther. 2008;19:979–990. doi: 10.1089/hum.2008.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Bainbridge J.W.B., Smith A.J., Barker S.S., Robbie S., Henderson R., Balaggan K., Viswanathan A., Holder G.E., Stockman A., Tyler N. Effect of gene therapy on visual function in Leber’s congenital amaurosis. N. Engl. J. Med. 2008;358:2231–2239. doi: 10.1056/NEJMoa0802268. [DOI] [PubMed] [Google Scholar]
- 210.Cideciyan A.V., Aleman T.S., Boye S.L., Schwartz S.B., Kaushal S., Roman A.J., Pang J.J., Sumaroka A., Windsor E.A.M., Wilson J.M. Human gene therapy for RPE65 isomerase deficiency activates the retinoid cycle of vision but with slow rod kinetics. Proc. Natl. Acad. Sci. USA. 2008;105:15112–15117. doi: 10.1073/pnas.0807027105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Wang D., Tai P.W.L., Gao G. Adeno-associated virus vector as a platform for gene therapy delivery. Nat. Rev. Drug Discov. 2019;18:358–378. doi: 10.1038/s41573-019-0012-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Rodrigues G.A., Shalaev E., Karami T.K., Cunningham J., Slater N.K.H., Rivers H.M. Pharmaceutical Development of AAV-Based Gene Therapy Products for the Eye. Pharm. Res. 2019;36:29. doi: 10.1007/s11095-018-2554-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.György B., Meijer E.J., Ivanchenko M.V., Tenneson K., Emond F., Hanlon K.S., Indzhykulian A.A., Volak A., Karavitaki K.D., Tamvakologos P.I. Gene Transfer with AAV9-PHP.B Rescues Hearing in a Mouse Model of Usher Syndrome 3A and Transduces Hair Cells in a Non-human Primate. Mol. Ther. Methods Clin. Dev. 2018;13:1–13. doi: 10.1016/j.omtm.2018.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Kho S.T., Pettis R.M., Mhatre A.N., Lalwani A.K. Safety of adeno-associated virus as cochlear gene transfer vector: analysis of distant spread beyond injected cochleae. Mol. Ther. 2000;2:368–373. doi: 10.1006/mthe.2000.0129. [DOI] [PubMed] [Google Scholar]