Abstract
The primary objective of this study was to investigate if alternative time-temperature carcass chilling combinations resulted in lower microbial (TVC, Enterobacteriaceae, Lactic Acid Bacteria, Pseudomonas spp. And Brochothrix thermosphacta) counts and, if achieved, would reduced levels remain throughout the beef chain. Physicochemical (temperature, pH, water activity) characteristics were also recorded. A secondary objective was to investigate the effect of primal maturation periods (2 versus 5 weeks) on the sensory properties of steaks by a trained panel for colour, odour, tenderness, and flavour. While microbial populations reduced by over 1 log10 cfu/cm2 by fast carcass chilling, these reductions were lost due to cross contamination in the boning hall and cutting room. The pH and water activity remained stable throughout the study and there was no significant difference for colour or sensory characteristics in retail steaks from the different treatment groups. It was concluded that there was no improvement to the microbial shelf-life of retail steaks from modified chilled carcasses or in the sensory shelf-life of primals which were aged for an extended period.
Keywords: Beef, Shelf-life, Carcass, Primals, Vacuum skin packaged steaks
Graphical abstract
Highlights
-
•
Alternative carcass chilling regimes achieved lower bacterial counts.
-
•
Cross-contamination during boning and cutting negates chilling gains.
-
•
Longer maturation did not enhance beef sensory properties.
1. Introduction
Food spoilage is a complex process, particularly for meat as a combination of biological and chemical activities may interact and render the product unappealing and/or unacceptable for human consumption (Gram et al., 2002). Fresh meat is highly perishable due to its high water activity (aw >0.99) and abundance of nutrients which support bacterial growth (Woraprayote et al., 2016). To avoid meat spoiling rapidly, physical parameters such as temperature, pH and aw need to be regulated to minimise microbial growth on beef throughout production and distribution.
The main groups of bacteria responsible for the spoilage of meat are Enterobacteriaceae, lactic acid bacteria (LAB), Pseudomonas spp., and Brochothrix thermosphacta (Hungaro et al., 2016). Pseudomonas spp. Is responsible for meat spoilage stored under aerobic conditions (Nychas et al., 2008) because of their high affinity for oxygen, fast growth rate and ability to grow at low temperatures (Gill and Newton, 1977). Pseudomonas spp. contributes to off-odours and slime by exhausting glucose and lactate in the meat and metabolising nitrogenous compounds such as amino acids (Nychas et al., 2007). Cold tolerant species of Enterobacteriaceae, such as Hafnia alvei, Serratia liquefaciens and Pantoea agglomerans are commonly present in aerobic conditions and may contribute to the spoilage of meat, especially if there is temperature abuse (Nychas et al., 2008; Wang et al., 2017). This spoilage is characterised by unpleasant odours and greening (Mills et al., 2014). LAB and B. thermosphacta are oxygen tolerant, but are not major contributors to the spoilage of carcasses (Reid et al., 2017a). However, carcass contamination by these bacteria is important, as once carcasses are deboned into primals and placed in anaerobic vacuum packs (VP), LAB and B. thermosphacta become the dominant spoilage organisms (Russo et al., 2006; Stanborough et al., 2017). VP primals and steaks allow for the growth of these bacteria as they are facultative anaerobes, capable of growth in the absence of oxygen and are not inhibited by carbon dioxide (Mills et al., 2014). LAB spoilage organisms are responsible for off-flavours, off-odours, discolouration, slime and the formation of bulging of packs (Samelis et al., 2000). Both LAB and B. thermosphacta cause souring rather than putrefaction (Nychas et al., 2008), with B. thermosphacta spoilage characterised by pungent cheesy or diary odours (Mills et al., 2014).
The shelf life of fresh meat can be significantly reduced by microbial growth if the storage temperature is incorrect (Casaburi et al., 2015). High carcass surface temperatures can result in a shorter shelf life, due to the increased growth of spoilage bacteria. It is essential for carcasses to be cooled rapidly preventing bacterial growth, however the rate of chilling must not be too rapid to avoid cold shortening of the meat (EFSA, 2014). Cold shortening occurs when muscles are chilled too quickly, before the onset of rigor mortis, resulting in a toughening of the meat (Savell et al., 2005). Typically, the quality of meat is maintained to a high standard by following temperature profiles regularly used, which ensures the core temperature does not decrease below 10 °C in the first 10 h of chilling thereby preventing cold shortening (EFSA, 2014). Despite the risk of cold shortening, the meat industry may apply fast/rapid/blast chilling of carcasses as it reduces weight loss and microbial populations on the surface of carcasses. Beef carcasses are usually chilled for 24–96 h before being moved to a boning hall where they are cut into primary pieces called primals, which are vacuum packed and valuable cuts such as striploin and silverside primals are allowed to mature for 3–6 weeks under anaerobic conditions (EFSA, 2016). This aging process is common practice in the meat industry to improve beef tenderness and palatability (Nair et al., 2019). Finally, steaks are prepared from matured primals and packaged using vacuum skin packaging (VSP). The process allows the packaging film to conform exactly to the profile of the product (Ščetar et al., 2010), and provides anaerobic conditions inside the package, resulting in a shelf-life extension (Stella et al., 2018). Nevertheless, physicochemical factors (temperature, pH and water activity) may alter bacterial growth levels of meat throughout the food chain, which in turn will alter organoleptic factors such as colour, odour and taste, which are important factors when considering product shelf-life. All of these aspects have to be taken into consideration in terms of achievable shelf life and product quality.
The objective of this study was to characterise the microbiology of beef throughout the beef chain from carcass chilling, through primal storage to retail steaks. Different chill regimes (conventional vs. mild blast chilling) and primal storage periods (two weeks vs. five weeks) were used and their impact on the microbiology, physicochemical and sensory characteristics of beef investigated.
2. Materials and methods
2.1. Experimental design
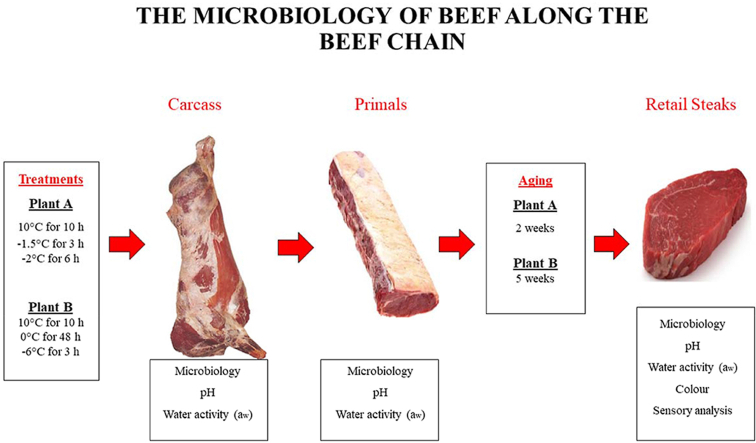
The experiment was conducted in two different slaughter plants (Plant A and Plant B) based in the Republic of Ireland. The design consisted of a comparison between five different chilling treatments. The control treatment represented the standard chill regime practiced in beef processing facilities throughout the Republic of Ireland (10 °C for 10 h followed by 0 °C for 38 h with low fan speed throughout) and was used as a baseline in both Plant A and Plant B, Treatment 1 (−1.5 °C for 3 h followed by 0 °C for 45 h with wind speed 3.5 m/s throughout storage), Treatment 2 (−2 °C for 6 h followed by 0 °C for 42 h with wind speed of 3.3 m/s throughout), Treatment 3 (0 °C for 48 h with wind speed between 1.5-2.5 m/s throughout) and Treatment 4 (−6 °C for 3 h followed by 0 °C for 45 h with wind speed of 6 m/s throughout). In Plant A, Treatment 1 and Treatment 2 were used as alternative chill regimes while in Plant B, Treatment 3 and Treatment 4 were applied. A total of 24 carcasses were used in this study (12 carcasses per plant) and, after dressing and evisceration, carcasses were centrally split into left and right sides (24 sides per plant) and randomly assigned to treatment groups (8 sides per treatment group). Once carcasses were in the chiller, the pH, aw and microbiology of the carcasses were monitored at t = 0 (immediately prior to the fans being switched on), t = 1 (24 h in storage) and t = 2 (48 h in storage). The ambient temperature was recorded every 5 min using an Easylog data logger (Lascar). The surface and core temperature of one carcass side in each treatment group (n = 6) were monitored every 10 min throughout storage using T-175 data loggers (Eurolec Instrumentation Ltd.).
After 48 h storage, carcasses were removed from allocated chills and moved to the boning hall for further processing within both plants. Striploins (n = 24) and silversides (n = 36) were boned from each carcass, vacuum packed (BB2050U bags, CryoVac, Sealed Air Ltd) and stored under commercial conditions within both plants. Striploin primals were matured in the slaughter plant until further processing where they would be then sliced into retail steaks. Silverside primals were sampled immediately after boning out (t = 2) and every 7 days thereafter where they were transported under refrigerated conditions to the laboratory based at Teagasc Food Research Centre, (Ashtown, Dublin 15) and tested for microbiology, pH and aw. Air temperature of the commercial chiller was recorded in 5 min intervals using an Easylog data logger (Lascer) which was placed in cardboard boxes alongside the primals. As Plant A and Plant B both matured primals for different periods of time (Plant A for two weeks and Plant B for five weeks) before primals were processed into sub-primals and retail cuts, potential differences in microbial growth during the different storage periods could be monitored.
Following maturation, striploin primals were transported to the cutting room and cut vertically into retail ready steaks (2.54 cm thick). Cutting started at the anterior end of the striploin, trimming 0.5 inches from either end of the loin to remove subcutaneous fat and any additional muscles. Steaks on the outside of the loin were used for microbial testing, whilst steaks coming from the core of the striploin were used for sensory analysis. Steaks were then vacuum skin packed (VSP, D15 3 Vacuum Skin Tray, Quinn Packaging, Ballyconnell, Cavan) and labelled accordingly. All samples were transported under refrigeration conditions to the Meat Industry Development Unit (MIDU) in Teagasc Food Research Centre, Ashtown, Dublin, where the steaks were placed into retail display cabinets (Capital Galaxy G14 S/S Multidesk, Cross Refrigeration, Ireland). Ambient temperature in retail display cabinets were recorded every 5 min using an Easylog data logger (Lascar). Three steaks representing each treatment (n = 18) were taken every three days, Plant A; (t = 23, 26, 29, 32, 35, 38, 41 days) and Plant B; (t = 39, 42, 45, 48, 51, 54, 57, 60, 63, 66 days) during retail storage and analysed.
2.2. Microbial analysis
Carcasses: Within both plants, carcass sides were sampled at times t = 0, 1 and 2 days using the sampling procedure described in EC Decision 2001/471/EC. Briefly a 10 cm × 10 cm cellulose acetate sponge pre-soaked in 10 ml maximum recovery diluent (MRD) in a sterile bag (Envirostik kit, Technical Service Consultants Ltd. UK) was used. Samples (neck, brisket, flank and rump) were obtained by inverting the bag to expose the sterile sponge and rubbing the sponge 5 times horizontally and 5 times vertically over the target area (100 cm2); alternative sides of the same sponge were used for 2 sites. On completion of swabbing the loaded sponge was withdrawn into the reverted bags and 2 sponges pooled (4 sampling sites on each carcass side; Total 400 cm2). All samples were stored and transported to the laboratory under chilled conditions (0–4 °C) until processing.
In the laboratory 40 ml of MRD (Oxoid, Basingstoke, Hampshire, UK (CM0733)) were added to each pair of pooled swabs and pulsified for 30 s (Pulsifier PUL100E, Microgen Bioproducts Ltd, Surrey, UK), serially diluted 1:10 in 9 ml MRD and plated in duplicate. Total viable counts (TVC) were enumerated using 3M Aerobic Petrifilm™ (Trafalgar Scientific, Leicester, UK (SB01)); and incubated at 30 °C for 72 h. Total Enterobacteriaceae Counts (TEC) was enumerated using 3M Enterobacteriaceae Petrifilm™ (Trafalgar Scientific, SB05); and incubated at 37 °C for 24 h. Lactic Acid Bacteria (LAB) were enumerated using 3M Lactic Acid Bacteria Petrifilm™ (Trafalgar Scientific, 6462); and incubated at 30 °C for 72 h. Pseudomonas spp. was enumerated on Pseudomonas Agar Base (Oxoid, CM0559) containing Cetrimide Fucidin Cephalosporin (CFC) selective supplement (Oxoid, SR103) and incubated at 30 °C for 48 h. Brochothrix thermosphacta was enumerated onto Streptomycin-thallous acetate-actidione agar base (Oxoid, CM0881) containing STAA selective supplement (Oxoid, SR0151) which was incubated at 25 °C for 48 h.
Primals: Silverside primals were sampled according to the procedure described in EC Decision 2001/471/EC and ISO microbiological methods. A 200 cm2 area of the sample swabbed using a sterile sampling sponge (Envirostik kit, Technical Service Consultants Ltd. UK), which was pre-soaked in 10 ml MRD in a sterile bag. Samples were obtained by rubbing the sponge 5 times horizontally and 5 times vertically over the target area. 40 ml of MRD (Oxoid, Basingstoke, Hampshire, UK) was then added to each sample and pulsified for 30 s (Pulsifier PUL100E, Microgen Bioproducts Ltd, Surrey, UK). Serial dilutions were prepared in MRD and plated in duplicate onto the appropriate agar as described previously.
Steaks: Steaks were aseptically removed from vacuum skin packs (VSP) using a sterile forceps and placed into a sterile Separator 400 Blender Bag (Grade Products Ltd, Leicestershire, UK) and 100 ml of MRD (Oxoid, Basingstoke, Hampshire, UK) was added to each sample. Samples were shaken and massaged for 2 min (Kaur et al., 2017). Serial dilutions were prepared in MRD and plated in duplicate onto the appropriate agar as previously described.
2.3. Surface pH measurements
Carcasses: The surface pH from each carcass side from each treatment group was monitored at t = 0 and 1 day using a Eutech pH 150 knife probe (Thermo Scientific, USA). The electrode was calibrated with pH 4, 7 and 10 standards immediately before use and cleaned with a probe wipe (Klipspringer, Ipswich, UK) between measurements to avoid cross contamination.
Primals: The surface pH of each vacuum packed beef primal (silverside) was recorded after the packaging was aseptically opened in a laminar flow unit using a Eutech pH 150 knife probe. The electrode was calibrated as previously described.
Steaks: The surface pH of each steak was recorded in a laminar flow unit and the pH probe was calibrated and cleaned as stated above.
2.4. Surface aw measurements
Carcasses: Water activity (aw) from each of the 48 carcass sides was recorded at time t = 0, 1 and 2 days by excising an area of 5 cm2 from the striploin using a 25 mm cork borer (VWR, Blanchardstown, Dublin 15), sterile scalpel and forceps. Samples were placed in separate sterile plastic Aqualab cups (Labcell, Basingstoke, UK), sealed and immediately transported back to the laboratory. Water activity values (aw) were measured using an Aqualab Pre water activity meter (Labcell). The machine was calibrated before use using a saturated solution of potassium chloride (0.50 mol/kg KCL, aw = 0.984 ± 0.003 at 20 °C).
Primals: A 5 cm2 excision sample of each primal was taken using a 25 mm cork borer, sterile scalpel and forceps in a laminar flow unit as described above.
Steaks: As above, a 5 cm2 excision sample of each steak was taken as previously outlined.
2.5. Instrumental colour analysis
Colour measurements of steaks were taken every 3 day at each time point for each plant using a Hunter Lab UltraScan Pro spectrophotometer (Hunter Associated Laboratory., Inc., Reston, VA). The instrument was standardized using a light trap and white tile that was covered with a sample of the packaging film to eliminate any packaging effect as outlined in AMSA (AMSA, 2012). Using a 78 mm diameter aperture and illuminant D65 with 10° observer, triplicate measurements of CIE L∗ (lightness), a∗ (redness) and b∗ (yellowness) were determined on each steak within the vacuum packages in different locations avoiding intramuscular fat and connective tissue and averaged. Chroma (C∗= (a∗2+b∗2)1/2) and Hue (tan−1(b∗/a∗)) values were also calculated (Van Rooyen et al., 2018).
2.6. Sensory analysis
Three steaks from each treatment within each plant (n = 18) were removed from the retail units on the particular days outlined in section 2.1 (above) and placed in frozen storage at −18 °C for sensory analysis. Descriptive sensory analysis was conducted in four trials using a trained panel (n = 7 Plant A; n = 8 Plant B) located in the Sensory Laboratory at Teagasc Food Research Centre, Ashtown within 6 months of storage. Samples were prepared and cooked following the guidelines outlined in AMSA (AMSA, 2016). Samples were scored for off-odour, tenderness, off-flavour and overall beef flavour. Odour and off-flavour were assessed on a 5-point scale; 1 = no off odour/flavour detected to 5 = a rancid odour/flavour – Not acceptable. Tenderness was determined from the resistance of the meat to molar teeth on the first bite through and for the first two chews. The score ranged from 1 = impossible to break down in the mouth to 10 = melt in the mouth, easy to break down. The overall beef flavour was also assessed on a 10-point scale; 1 = no beef flavour at all to 10 = extremely high level of beef flavour that lingers in the aftertaste.
2.7. Statistical analysis
A one-way analysis of variance (ANOVA) was carried out to evaluate the effect of primal chilling times on steaks colour and sensory characteristics. When statistical differences were detected, Tukey’s post-hoc comparison test was used to measure differences between means. Differences were considered significant at the 5% (P ≤ 0.05) level. The statistical package used was GraphPad Prism 7.02 (Graphpad Software Incorporated, San Diego, California, USA).
3. Results
3.1. Microbial analysis
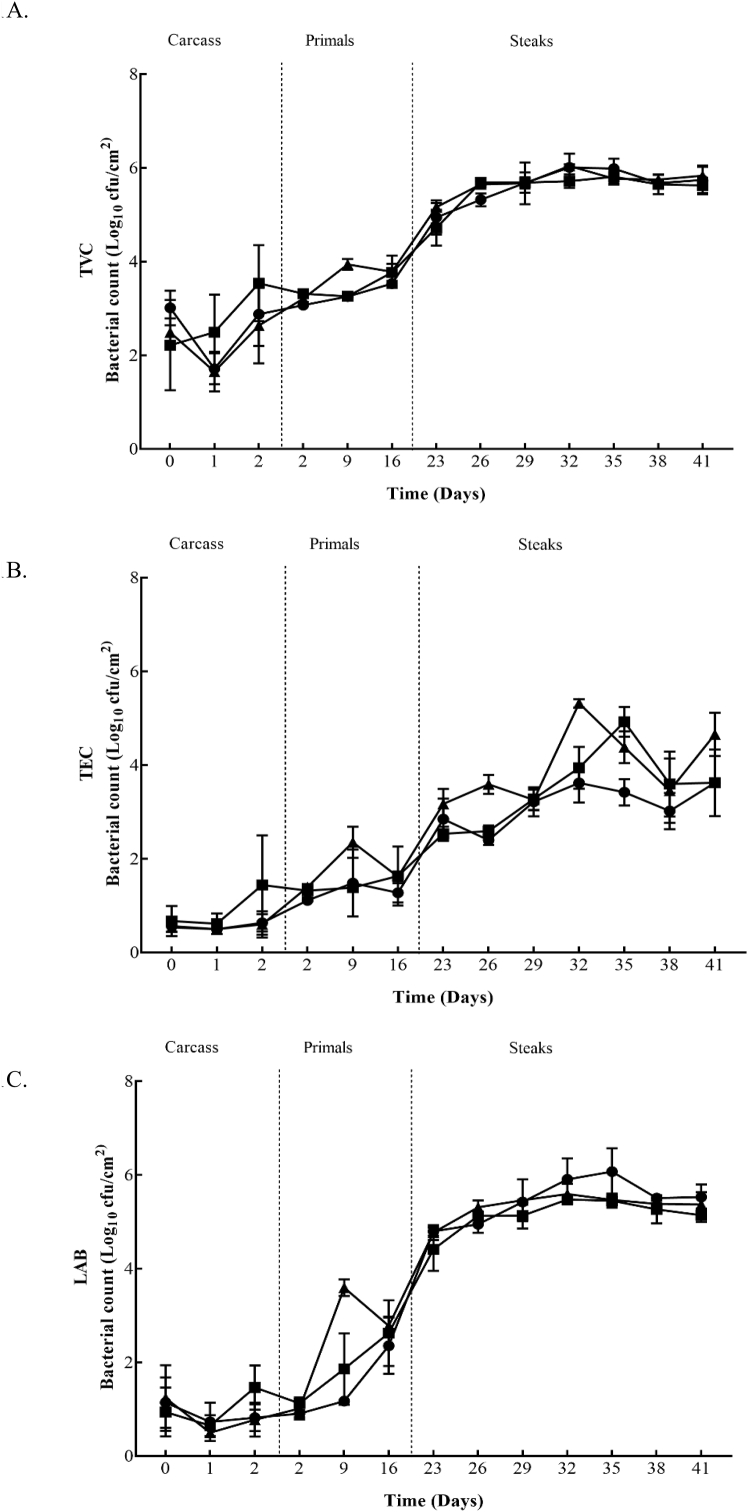
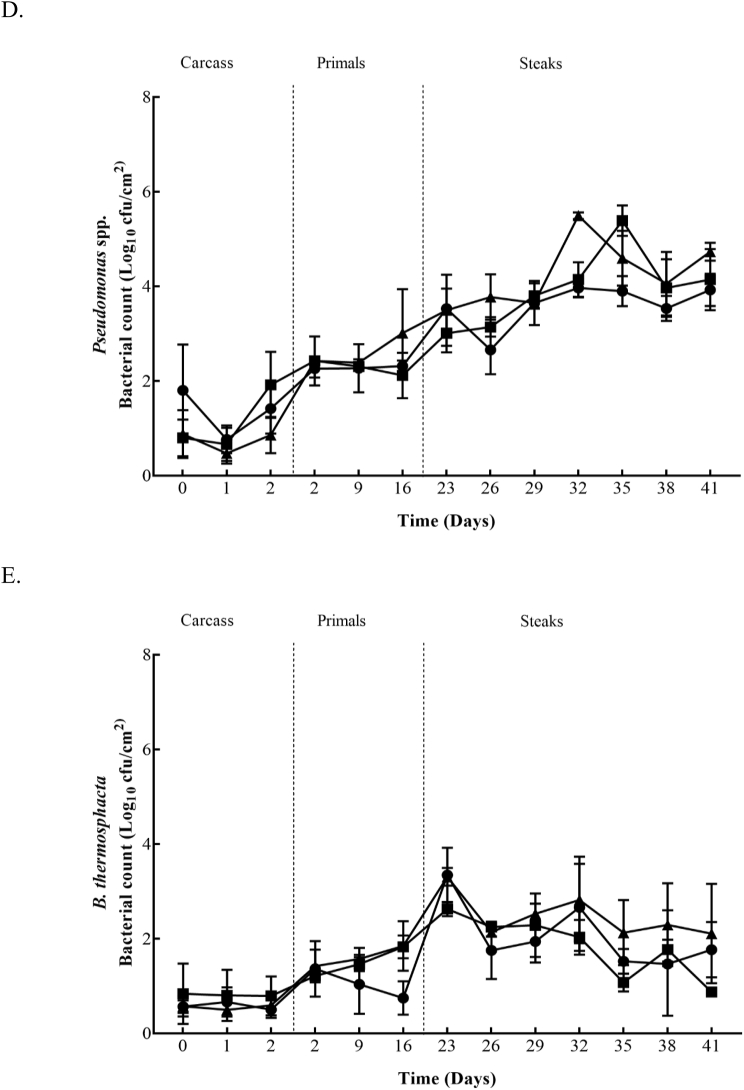
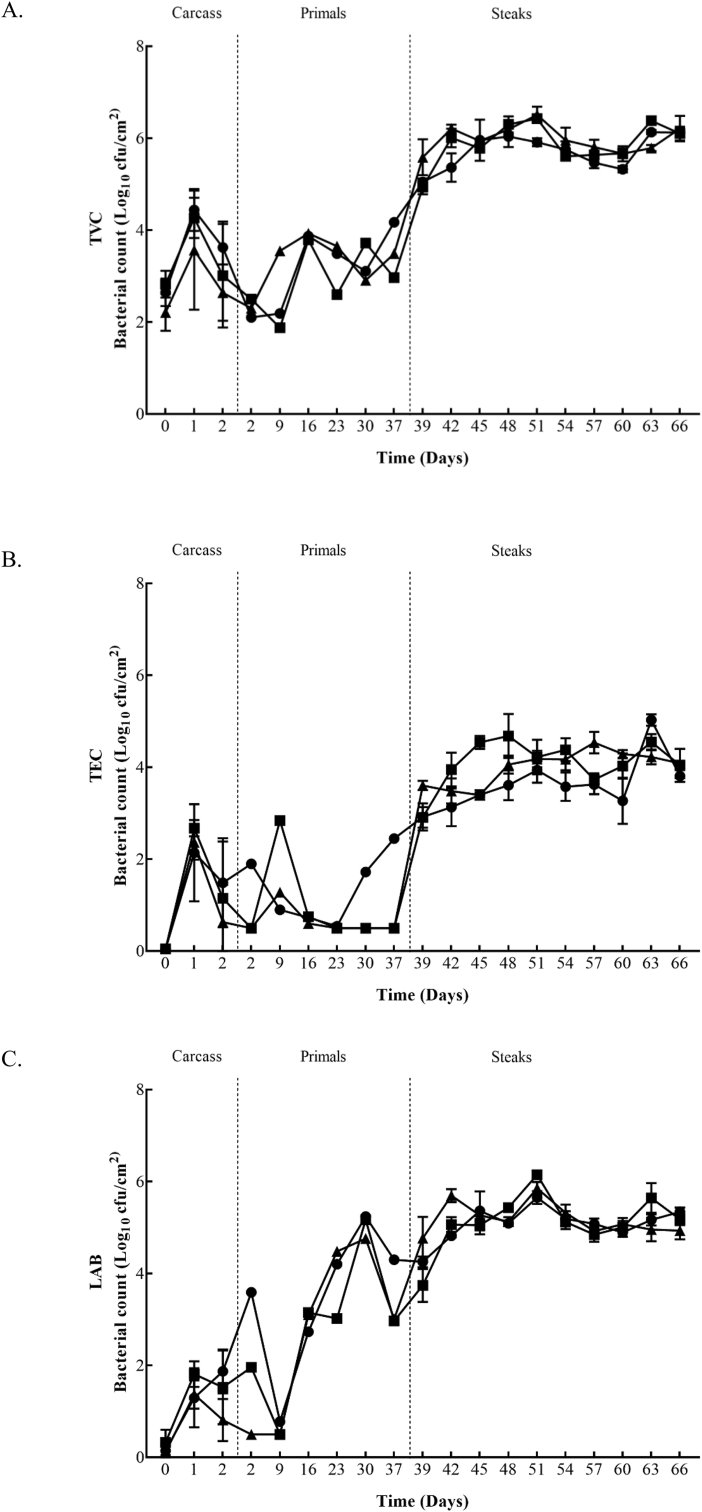
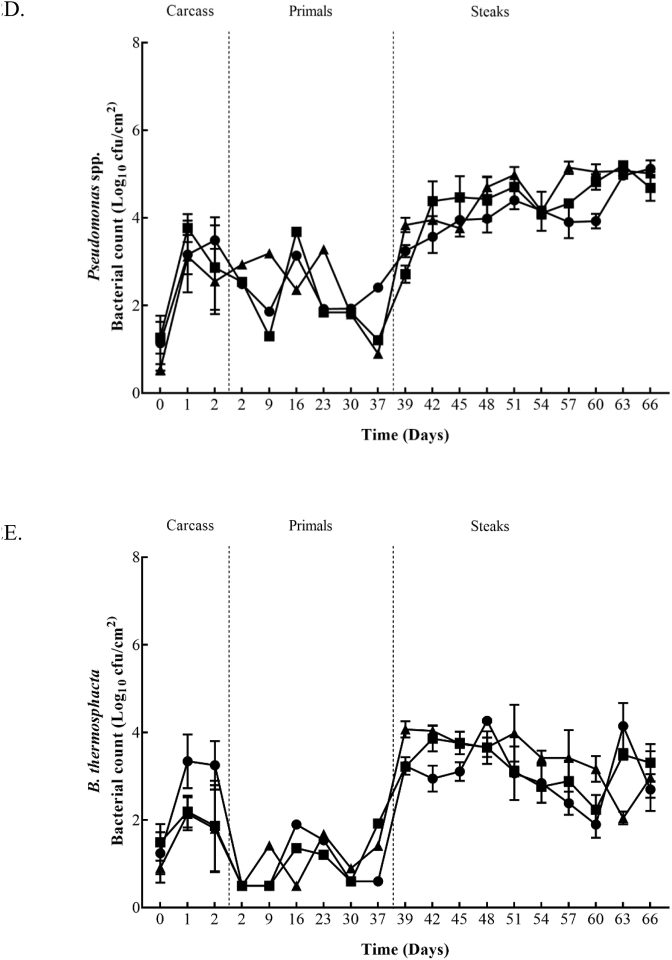
TVC, TEC, LAB, Pseudomomas spp., and B. thermosphacta counts from the surface of beef carcasses, primals and VSP steaks are shown in Fig. 1 and Fig. 2. The figures show the bacterial growth from the beginning to the end of storage in both plants (Plant A and B). In Plant A the initial TVC carcass counts ranged from 2.2 to 3.0 log10 cfu/cm2, decreased to 1.7 to 2.5 log10 cfu/cm2 after 24 h and increased to 2.6 to 3.5 log10 cfu/cm2 after 48 h. After 24 h and 48 h significant (P ≤ 0.05) differences between the Control and the fast chilled carcasses from Treatment 2 were recorded. Initial TEC, LAB, and Pseudomomas spp. counts ranged from 0.3 to 0.4 log10 cfu/cm2, 0.8 to 1.2 log10 cfu/cm2 and 0.8 to 0.9 log10 cfu/cm2, respectively. After 48 h, these counts were similar or increased to 0.4 to 1.4 log10 cfu/cm2, 0.7 to 1.5 log10 cfu/cm2 and 0.9 to 1.9 log10 cfu/cm2. TEC and LAB counts recorded a significant (P ≤ 0.05) difference between the Control and fast chilling Treatments 1 and 2 after 48 h. Similar to TVC, Pseudomomas spp. had significantly (P ≤ 0.05) lower counts for carcasses from Treatment 2 compared to the Control after 48 h. Plant B had initial carcass counts from 2.2 to 2.8 log10 cfu/cm2 (TVC), 0.1 log10 cfu/cm2 (TEC), 0.1 to 0.3 log10 cfu/cm2 (LAB), 0.5 to 1.3 log10 cfu/cm2 (Pseudomonas spp.) and 0.9 to 1.5 log10 cfu/cm2 (B. thermosphacta). After 24 h, TVC, TEC, LAB, Pseudomomas spp., and B. thermosphacta counts continued to increase, regardless of the chilling regime applied, and ranged from 3.6 to 4.4 log10 cfu/cm2, 2.1 to 2.7 log10 cfu/cm2, 1.3 to 1.8 log10 cfu/cm2, 3.1 to 3.8 log10 cfu/cm2 and 2.1 to 3.3 log10 cfu/cm2, respectively. At 24 h, B. thermosphacta counts were significantly (P ≤ 0.05) higher on carcasses chilled using Treatment 3 which continued up to 48 h in comparison to the other treatments. Also, after 48 h significantly (P ≤ 0.05) lower LAB counts were obtained for carcasses from Treatment 4 compared to carcasses from the Control and Treatment 3.
Fig. 1.
Bacterial counts; 1A (TVC), 1B (TEC), 1C (LAB), 1D (Pseudomonas spp.), and 1E (B.thermophacta) within Plant A for Control (■), Treatment 1 (●) and Treatment 2 (▲) chill regimes on beef carcasses, beef primals and vacuum skin packaged steaks. Each data point and the error bar show the mean ± the standard deviation.
Fig. 2.
Bacterial counts 2A (TVC), 2B (TEC), 2C (LAB), 2D (Pseudomonas spp.), and 2E (B.thermophacta) within Plant B for Control (■), Treatment 3 (●) and Treatment 4 (▲) chill regimes on beef carcasses, beef primals and vacuum skin packaged steaks. Each data point and the error bar show the mean ± the standard deviation.
Within Plant A, the mean counts of all microbial spoilage groups monitored on primals were similar for each of the three carcass treatment groups over the 16 days at each time-point, except for a significant increase (P ≤ 0.05) obtained on day 9 for LAB populations on primals derived from carcasses subject to chilling Treatment 2. In Plant B similar microbial counts were obtained on the primals throughout the first 36 days in commercial storage, regardless of the carcass chilling treatment. However, significantly higher (P ≤ 0.05) counts were observed on the final day of sampling (day 37) for TVC, TEC, LAB and Pseudomonas spp. from silverside primals that came from carcasses subjected to the Treatment 3 chilling regime.
Initial TVC, TEC, LAB, Pseudomonas spp., and B. thermosphacta counts for striploin steaks in Plant A ranged from 4.7 to 5.2 log10 cfu/cm2, 2.5 to 3.2 log10 cfu/cm2, 4.4 to 4.8 log10 cfu/cm2, 3.0 to 3.5 log10 cfu/cm2 and 2.6 to 3.3 log10 cfu/cm2, respectively. Within Plant A, highest counts throughout sampling (day 23–41) were achieved from TVC and LAB, reaching 6 log10 cfu/cm2. TEC and Pseudomonas spp. counts continued to increase overtime, while B. thermosphacta counts started to decrease gradually throughout the study. From day 26, no significant difference (P ≤ 0.05) was recorded for TVC’s from the three chilling regimes. On days 26 and 32, TEC and Pseudomonas spp. Achieved significantly (P ≤ 0.05) higher counts on steaks from carcass Treatment 2 and on day 35, significantly (P ≤ 0.05) lower counts were recorded for steaks from carcass Treatment 1 compared to the other treatments. Treatment 1 steaks achieved significantly (P ≤ 0.05) higher LAB counts on day 35. On the final day of sampling (day 41) steaks from Treatment 2 had significantly (P ≤ 0.05) higher TEC and B. thermosphacta counts. Plant B had initial counts ranging from 4.9 to 5.6 log10 cfu/cm2 (TVC), 2.9 to 3.6 log10 cfu/cm2 (TEC), 3.7 to 4.8 log10 cfu/cm2 (LAB), 2.7 to 3.8 log10 cfu/cm2 (Pseudomonas spp), and 3.2 to 4.1 log10 cfu/cm2 (B. thermosphacta). Throughout sampling (day 39–66) the highest counts were obtained for TVC (6.5 log10 cfu/cm2) and LAB (6.1 log10 cfu/cm2). TEC continued to increase until day 48, which was maintained until the end of sampling. Pseudomonas spp. counts also continued to increase overtime, however on day 54 counts from all three treatment groups slightly decreased before steadily growing until day 66. Overtime B. thermosphacta counts started to decrease gradually, but an increase on day 63 was recorded on Control and Treatment 3 steaks. On the first day of sampling (day 39) significantly (P ≤ 0.05) higher counts were recovered on steaks from Treatment 4 compared to the other treatments. TEC (day 57), Pseudomonas spp., (day 57) and B. thermosphacta (day 51 and 60) also recorded significantly (P ≤ 0.05) higher counts on steaks coming from Treatment 4. Whereas, significantly (P ≤ 0.05) lower B. thermosphacta counts were recovered on day 63 for steaks from Treatment 4. Steaks from Treatment 3 maintained significant (P ≤ 0.05) lower counts for all microorganisms on day 42, in comparison to the other treatments. Control steaks had significantly (P ≤ 0.05) higher TEC and Pseudomonas spp. counts on day 45, and again on day 48 for TEC. LAB recorded significantly (P ≤ 0.05) higher counts for Control steaks on day 63.
3.2. Physicochemical analysis
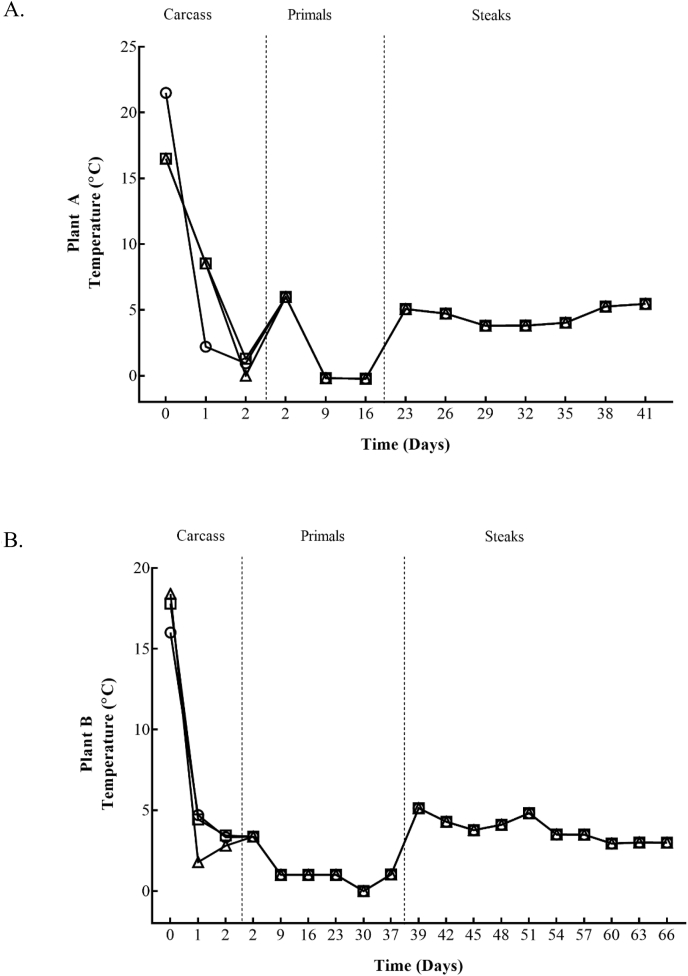
The mean carcass surface temperature profiles for the 2 days in the chillers are shown in Fig. 3 (Plant A and Plant B). The mean carcass surface temperature upon entry into the different chillers varied within plants, 18.9 °C–27.9 °C in Plant A and 16.0 °C–18.4 °C in Plant B. These decreased to below 10 °C after approximately 4–8 h in both plants. As expected, setting the chillers to achieve 0 °C as quickly as possible significantly (P ≤ 0.05) dropped the carcass surface temperature much faster, between 2–4 h within both plants. The average ambient temperature in the chiller after 2 days of carcass storage was higher for carcasses kept within Control treatments in both Plant A and Plant B, 5.7 °C and 4.8 °C, respectively. Similar ambient temperatures were recorded in both plants for fast chilled carcasses, ranging from 1.8 – 3.8 °C after the 2 days. The average ambient temperature of vacuum packed primals were stored at an average temperature of 1.4 °C for the 16 days storage in Plant A and at 1.2 °C for the 37 days storage in Plant B. Considerable increases and decreases of the ambient temperatures were recorded in the retail cabinets used to store the VSP steaks, and these varied depending on the locations (front or back) of the cabinet. Nevertheless, an average ambient temperature of 4.6 °C was achieved for steaks stored for 18 days taken from Plant A and 3.8 °C for steaks stored for 27 days from Plant B.
Fig. 3.
3A; Carcass surface temperatures recorded within both Plants. Plant A:Control; conventional chill (10 °C for 10 h) [□]), Treatment 1 (−1.5 °C for 3 h) [○]) and Treatment 2 (−2 °C for 6 h) [△)]. Plant B: Control; conventional chill (10 °C for 10 h) [□]), Treatment 3 (0 °C for 48 h) [○]) and Treatment 4 (−6 °C for 3 h) [△)].
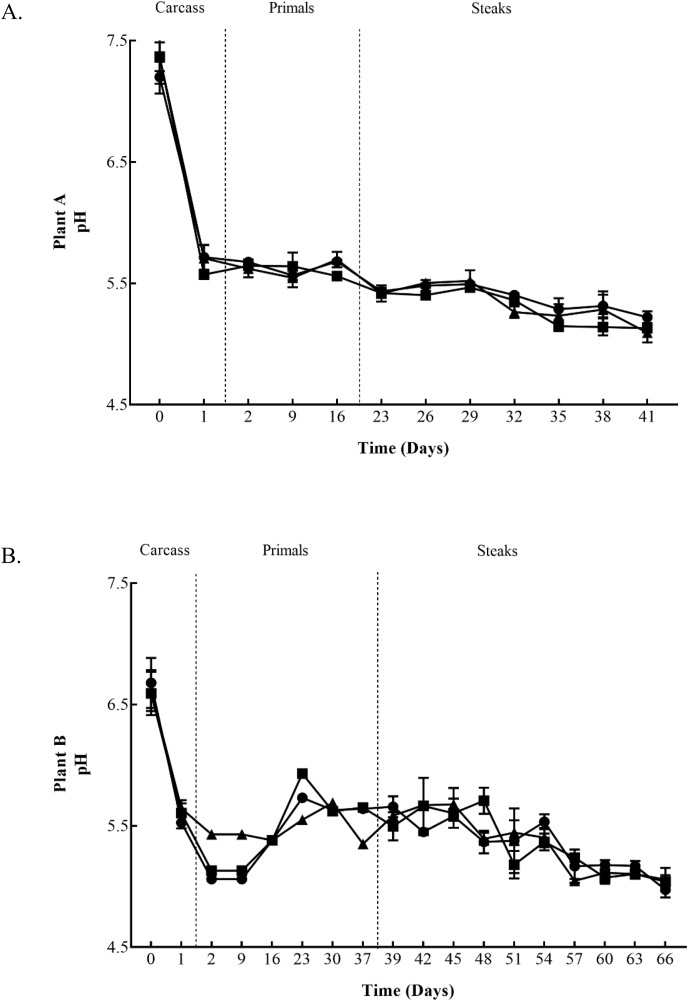
Mean carcass pH values decreased from 7.4 to 5.6 in Plant A and 6.7 to 5.5 in Plant B after 1 day in chilled storage conditions. Silverside primals maintained pH values between 5.5 – 5.7 throughout the 16 days of storage, as did primals from Plant B, displaying values of between 5.1 – 5.9 over the 37 days in storage. Finally, the pH values of VSP retail steaks continued to drop throughout storage, from 5.4 (Day 23) to 5.1–5.2 (Day 41) in Plant A and decreased from 5.5 – 5.7 (Day 39) to 5.0–5.1 (Day 66) in Plant B shown in Fig. 4.
Fig. 4.
pH values within Plant A (4A) for Control (■), Treatment 1 (●) and Treatment 2 (▲)and Plant B (4B) for Control (■), Treatment 3 (●) and Treatment 4 (▲) chill regimes on beef carcasses, beef primals and vacuum skin packaged steaks. Each data point and the error bar show the mean ± the standard deviation.
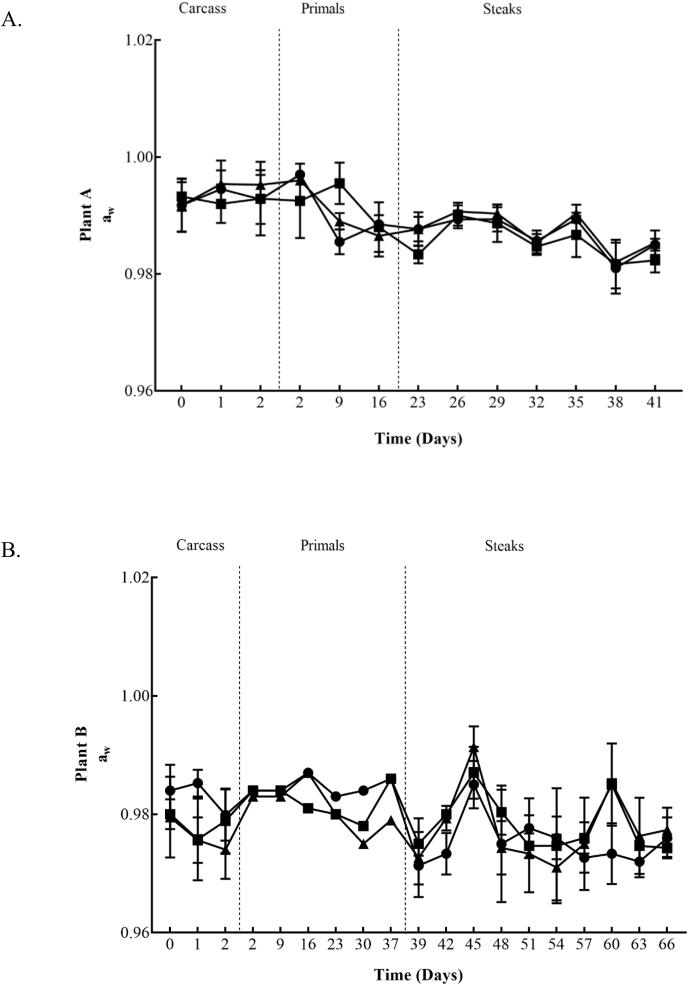
The aw values remained stable during carcass chilling ranging from 0.98 to 0.99 within both plants. Similar values were achieved for both plants throughout primal and VSP steak storage, ranging from 0.97 to 0.99 (Fig. 5).
Fig. 5.
Water activity (aw) values within Plant A (5A) for Control (■), Treatment 1 (●) and Treatment 2 (▲) and Plant B (5B) for Control (■), Treatment 3 (●) and Treatment 4 (▲) chill regimes on beef carcasses, beef primals and vacuum skin packaged steaks. Each data point and the error bar show the mean of ± the standard deviation.
3.3. Organoleptic analysis
Instrumental colour characteristics such as lightness (L∗), redness (a∗), yellowness (b∗), intensity (C∗) and colourfulness (hue angle) were calculated for the VSP steaks during retail storage (Table 1 and Table 2). Within Plant A, there was a significant difference (P ≤ 0.05) on day 26 between carcass Treatment 1 and 2 for lightness (L∗), and on day 32, Treatment 2 had a larger a∗ value and lower hue angle indicating the steaks did not have the same surface redness as the Control and Treatment 1 steaks. Steaks from Plant B showed more fluctuated values and significant differences throughout the storage period. The Control treatments appeared to be lighter then Treatment 3 and 4, but overtime steaks from the treated groups, especially Treatment 4 was significantly (P ≤ 0.05) lighter in colour. Also, initially Treatment 3 and 4 appeared to be redder (a∗), and less yellow (b∗) than steaks from the Control treatment. Eventually Control steaks appear redder and coincide with b∗ values at the later stages of storage. One of the major patterns within both plants was that the hue angles decreased over time indicating browning and discoloration had occurred in all treatment groups.
Table 1.
Mean measurements of CIE L∗ (lightness), a∗ (redness), b∗ (yellowness), Chroma (C) and Hue recorded from each vacuum skin packaged steaks from three different carcass chilling (Control (C), Treatment 1 (T1) and Treatment 2 (T2)) treatments in Plant A.
| Storage time (Days) |
|||||||
|---|---|---|---|---|---|---|---|
| 23a | 26 | 29 | 32 | 35 | 38 | 41 | |
| L∗ | |||||||
| C | 39.4 ± 1.6 | 41.6AB ± 0.6 | 40.4 ± 1.3 | 43.9 ± 1.4 | 42.3 ± 0.7 | 40.0 ± 1.9 | 44.6 ± 1.3 |
| T1 | 39.6 ± 1.8 | 38.9A ± 0.5 | 43.2 ± 0.7 | 44.7 ± 0.8 | 43.3 ± 0.1 | 40.0 ± 1.2 | 44.8 ± 0.5 |
| T2 | 38.4 ± 3.2 | 44.9B ± 1.3 | 42.6 ± 1.5 | 40.0 ± 0.7 | 43.1 ± 3.0 | 36.6 ± 0.3 | 41.1 ± 1.3 |
| a∗ | |||||||
| C | 9.8 ± 0.6 | 9.5 ± 0.3 | 9.5 ± 0.3 | 9.7A ± 0.6 | 10.8 ± 0.5 | 10.2 ± 0.9 | 10.9 ± 0.7 |
| T1 | 9.5 ± 0.6 | 9.7 ± 0.2 | 8.9 ± 0.4 | 9.6A ± 0.2 | 10.6 ± 0.2 | 9.8 ± 0.2 | 11.3 ± 0.4 |
| T2 | 9.7 ± 0.4 | 8.2 ± 0.7 | 9.0 ± 0.5 | 12.4B ± 0.6 | 10.3 ± 0.9 | 11.1 ± 0.1 | 12.4 ± 0.5 |
| b∗ | |||||||
| C | 10.2 ± 0.9 | 9.6 ± 0.1 | 8.9 ± 0.5 | 10.2 ± 0.1 | 11.1 ± 0.2 | 10.0 ± 0.2 | 10.3 ± 0.5 |
| T1 | 9.6 ± 0.4 | 8.7 ± 0.1 | 9.6 ± 0.2 | 10.6 ± 0.2 | 10.5 ± 0.2 | 10.7 ± 0.4 | 11.2 ± 0.1 |
| T2 | 9.4 ± 1.6 | 10.0 ± 0.0 | 9.9 ± 0.4 | 9.1 ± 0.1 | 10.2 ± 0.8 | 9.2 ± 0.5 | 9.8 ± 0.8 |
| Chroma | |||||||
| C | 14.2 ± 0.5 | 13.5 ± 0.1 | 13.0 ± 0.4 | 14.0 ± 0.3 | 15.5 ± 0.3 | 14.3 ± 0.7 | 15.0 ± 0.4 |
| T1 | 13.5 ± 0.4 | 13.0 ± 0.2 | 13.0 ± 0.3 | 14.3 ± 0.2 | 14.9 ± 0.3 | 14.5 ± 0.4 | 15.9 ± 0.2 |
| T2 | 13.6 ± 0.9 | 12.9 ± 0.4 | 13.4 ± 0.2 | 15.3 ± 0.5 | 14.6 ± 0.2 | 14.4 ± 0.3 | 15.8 ± 0.6 |
| Hue | |||||||
| C | 45.9 ± 3.7 | 45.2AB ± 1.2 | 43.2 ± 1.8 | 46.5A ± 1.9 | 45.7 ± 1.7 | 44.6 ± 2.4 | 43.6 ± 2.9 |
| T1 | 45.2 ± 2.5 | 41.9A ± 1.0 | 47.0 ± 1.5 | 47.7A ± 0.9 | 44.5 ± 0.5 | 47.4 ± 0.9 | 44.8 ± 1.1 |
| T2 | 43.3 ± 5.9 | 51.0B ± 2.5 | 47.7 ± 2.7 | 36.5B ± 1.4 | 44.7 ± 4.9 | 39.6 ± 1.5 | 38.3 ± 2.5 |
The values represent the average counts ± standard error of the mean (SEM). Numbers that have different letters in the same column (A,B) indicate statistical significant (P ≤ 0.05) between treatments within Plant A at each time point. Numbers in the same column that are not followed by letters indicate no significance found.
Table 2.
Mean measurements of CIE L∗ (lightness), a∗ (redness), b∗ (yellowness), Chroma (C) and Hue values recorded from vacuum skin packaged steaks from three different carcass chilling (Control (C), Treatment 3 (T3) and Treatment 4 (T4)) treatments in Plant B.
| Storage time (Days) |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 39a | 42 | 45 | 48 | 51 | 54 | 57 | 60 | 63 | 66 | |
| L∗ | ||||||||||
| C | 43.3A ± 0.7 | 44.1A ± 0.4 | 44.4A ± 0.3 | 37.7 ± 0.2 | 45.6A ± 0.5 | 46.6A ± 0.4 | 39.1A ± 0.3 | 37.4A ± 0.4 | 40.5 ± 0.6 | 40.8A ± 0.1 |
| T3 | 37.7B ± 0.5 | 40.6B ± 0.9 | 37.7B ± 0.3 | 39.6 ± 0.4 | 44.4A ± 1.5 | 39.6B ± 0.3 | 38.4A ± 0.4 | 38.4AB ± 0.3 | 42.3 ± 0.2 | 44.2B ± 0.6 |
| T4 | 40.6C ± 0.2 | 35.0C ± 0.3 | 34.9C ± 0.2 | 39.6 ± 1.1 | 40.9B ± 0.7 | 40.6B ± 1.1 | 42.7B ± 0.8 | 39.5B ± 0.2 | 41.8 ± 0.4 | 43.2B ± 0.5 |
| a∗ | ||||||||||
| C | 8.6 ± 0.1 | 9.2A ± 0.2 | 9.3A ± 0.0 | 10.5 ± 0.2 | 9.5 ± 0.1 | 9.3A ± 0.1 | 11.0A ± 0.1 | 11.7A ± 0.3 | 9.7A ± 0.5 | 11.1A ± 0.3 |
| T3 | 9.3 ± 0.2 | 9.7AB ± 0.1 | 10.3B ± 0.2 | 9.9 ± 0.3 | 9.0 ± 0.8 | 10.9B ± 0.4 | 10.7A ± 0.3 | 9.9B ± 0.0 | 8.6B ± 0.1 | 10.4A ± 0.1 |
| T4 | 8.9 ± 0.1 | 10.2B ± 0.2 | 10.2AB ± 0.1 | 10.1 ± 0.1 | 9.7 ± 0.3 | 9.9A ± 0.4 | 9.4B ± 0.0 | 9.5B ± 0.1 | 8.6B ± 0.1 | 9.3B ± 0.7 |
| b∗ | ||||||||||
| C | 10.8A ± 0.2 | 10.1A ± 0.3 | 10.4A ± 0.1 | 8.5 ± 0.3 | 9.8A ± 0.1 | 10.3A ± 0.1 | 8.0 ± 0.0 | 9.1 ± 0.2 | 10.2A ± 0.2 | 9.7AB ± 0.2 |
| T3 | 8.6B ± 0.1 | 9.4A ± 0.4 | 8.6B ± 0.2 | 8.8 ± 0.2 | 10.3A ± 0.6 | 8.3B ± 0.2 | 7.9 ± 0.1 | 9.2 ± 0.1 | 9.0B ± 0.2 | 10.5A ± 0.3 |
| T4 | 9.1C ± 0.1 | 7.2B ± 0.3 | 7.4C ± 0.0 | 8.2 ± 0.4 | 8.4B ± 0.2 | 8.1B ± 0.3 | 8.7 ± 0.2 | 9.0 ± 0.2 | 9.6AB ± 0.2 | 9.5B ± 0.3 |
| Chroma | ||||||||||
| C | 13.8A ± 0.1 | 13.6A ± 0.3 | 13.9A ± 0.0 | 13.5 ± 0.3 | 13.6AB ± 0.0 | 13.9A ± 0.0 | 13.6 ± 0.1 | 14.8A ± 0.3 | 14.1A ± 0.4 | 14.8A ± 0.3 |
| T3 | 12.7B ± 0.1 | 13.5A ± 0.2 | 13.4AB ± 0.3 | 13.3 ± 0.4 | 13.7A ± 0.2 | 13.8A ± 0.5 | 13.3 ± 0.2 | 13.5B ± 0.1 | 12.5B ± 0.2 | 14.8A ± 0.2 |
| T4 | 13.2AB ± 0.1 | 12.5B ± 0.3 | 12.6B ± 0.0 | 13.0 ± 0.3 | 12.9B ± 0.1 | 12.9B ± 0.2 | 12.8 ± 0.1 | 13.1B ± 0.0 | 12.9B ± 0.2 | 13.3B ± 0.7 |
| Hue | ||||||||||
| C | 51.4A ± 0.7 | 47.6A ± 0.9 | 48.2A ± 0.2 | 38.9 ± 0.5 | 45.9A ± 0.6 | 47.9A ± 0.6 | 36.1A ± 0.1 | 37.8A ± 0.6 | 46.7 ± 1.2 | 41.2A ± 0.4 |
| T3 | 42.7B ± 0.1 | 43.9A ± 1.3 | 39.8B ± 0.1 | 41.9 ± 0.1 | 48.7A ± 4.1 | 37.4B ± 0.5 | 36.6A ± 1.1 | 42.9B ± 0.4 | 46.5 ± 0.5 | 45.3B ± 0.7 |
| T4 | 47.6A ± 0.4 | 35.2B ± 0.9 | 36.0C ± 0.4 | 39.2 ± 1.1 | 40.7B ± 1.3 | 39.2B ± 2.0 | 42.5B ± 0.5 | 43.4B ± 0.9 | 48.3 ± 0.6 | 45.5B ± 1.2 |
The values represent the average counts ± standard error of the mean (SEM). Numbers that have different letters in the same column (A,B,C) indicate statistical significant (P ≤ 0.05) between treatments within Plant B at each time point. Numbers in the same column that are not followed by letters indicate no significance found.
When beef quality was assessed by a trained sensory panel, steaks from each treatment group scored highly for tenderness and beef flavour within both plants, indicating that neither the different chill parameters or the alternative maturation times used in this study had a negative impact on these attributes. Steaks remained in the acceptable range for all panellist’s, shown in Table 3, Table 4. There was no correlation between the chill or aging treatments used in this study and the sensory attributes of the retail steaks.
Table 3.
Mean sensory analysis completed by a trained panel (n = 7) of vacuum skin packaged steaks from Plant A treatments (Control (C), Treatment 1 (T1) and Treatment 2 (T2)). The sensory properties investigated off-odours, tenderness, off-flavours and overall beef flavour.
| Storage time (Days) |
|||||||
|---|---|---|---|---|---|---|---|
| 23a | 26 | 29 | 32 | 35 | 38 | 41 | |
| Off-odours | |||||||
| C | 1.0 ± 0.0 | 1.3 ± 0.3 | 1.0 ± 0.0 | 2.3A ± 0.5 | 2.1 ± 0.3 | 2.0 ± 0.3 | 2.3 ± 0.5 |
| T1 | 1.0 ± 0.0 | 1.0 ± 0.0 | 1.1 ± 0.1 | 1.3B ± 0.2 | 1.3 ± 0.2 | 1.4 ± 0.3 | 1.7 ± 0.4 |
| T2 | 1.3 ± 0.2 | 1.3 ± 0.2 | 1.6 ± 0.2 | 1.3B ± 0.2 | 1.9 ± 0.3 | 2.0 ± 0.4 | 2.3 ± 0.4 |
| Tenderness | |||||||
| C | 7.1A ± 0.2 | 7.3 ± 0.2 | 7.1 ± 0.1 | 7.3 ± 0.3 | 7.4AB ± 0.3 | 7.1 ± 0.3 | 6.9 ± 0.3 |
| T1 | 7.0AB ± 0.0 | 7.4 ± 0.2 | 7.1 ± 0.3 | 7.9 ± 0.3 | 6.9A ± 0.3 | 7.6 ± 0.2 | 7.1 ± 0.3 |
| T2 | 6.4B ± 0.3 | 7.6 ± 0.2 | 7.3 ± 0.4 | 7.9 ± 0.3 | 8.0B ± 0.3 | 7.7 ± 0.4 | 7.0 ± 0.5 |
| Off-flavours | |||||||
| C | 1.6 ± 0.4 | 1.7 ± 0.4 | 2.4 ± 0.4 | 3.1 ± 0.5 | 3.3 ± 0.4 | 3.1AB ± 0.6 | 3.4 ± 0.4 |
| T1 | 1.3 ± 0.2 | 2.0 ± 0.3 | 2.3 ± 0.4 | 2.9 ± 0.5 | 1.9 ± 0.4 | 2.0A ± 0.6 | 2.7 ± 0.5 |
| T2 | 2.0 ± 0.4 | 1.9 ± 0.5 | 2.4 ± 0.5 | 2.6 ± 0.5 | 2.3 ± 0.4 | 3.6B ± 0.5 | 3.0 ± 0.4 |
| Overall beef flavour | |||||||
| C | 6.6 ± 0.3 | 6.7 ± 0.3 | 6.6 ± 0.3 | 6.0 ± 0.5 | 5.6 ± 0.4 | 6.1 ± 0.6 | 6.0 ± 0.5 |
| T1 | 6.6 ± 0.2 | 6.6 ± 0.2 | 6.4 ± 0.3 | 6.3 ± 0.4 | 6.4 ± 0.4 | 6.3 ± 0.4 | 6.1 ± 0.5 |
| T2 | 6.0 ± 0.3 | 6.1 ± 0.3 | 6.1 ± 0.4 | 6.4 ± 0.3 | 6.6 ± 0.3 | 5.9 ± 0.6 | 5.6 ± 0.4 |
The values represent the average counts ± standard error of the mean (SEM). Numbers that have different letters in the same column (A,B) indicate statistical significant (P ≤ 0.05) between treatments within Plant A at each time point. Numbers in the same column that are not followed by letters indicate no significance found.
Table 4.
Mean sensory analysis completed by a trained panel (n = 8) of vacuum skin packaged steaks from Plant B treatments (Control (C), Treatment 3 (T3) and Treatment 4 (T4)). The sensory properties investigated off-odours, tenderness, off-flavours and overall beef flavour.
| Storage time (Days) |
|||||
|---|---|---|---|---|---|
| 39a | 42 | 45 | 48 | 51 | |
| Off-odours | |||||
| C | 1.1 ± 0.1 | 1.5 ± 0.3 | 1.8 ± 0.3 | 2.3A ± 0.3 | 1.5 ± 0.3 |
| T3 | 1.1 ± 0.1 | 1.5 ± 0.3 | 1.4 ± 0.2 | 1.3B ± 0.2 | 1.5 ± 0.3 |
| T4 | 1.5 ± 0.2 | 1.3 ± 0.2 | 1.1 ± 0.1 | 2.5A ± 0.1 | 1.6 ± 0.3 |
| Tenderness | |||||
| C | 6.4 ± 0.3 | 7.0 ± 0.3 | 7.5 ± 0.2 | 6.4 ± 0.4 | 7.1 ± 0.5 |
| T3 | 6.9 ± 0.2 | 6.9 ± 0.3 | 7.5 ± 0.3 | 6.1 ± 0.1 | 6.6 ± 0.3 |
| T4 | 6.3 ± 0.5 | 6.4 ± 0.3 | 7.0 ± 0.3 | 6.0 ± 0.4 | 6.5 ± 0.3 |
| Off-flavours | |||||
| C | 2.0 ± 0.2 | 2.1 ± 0.2 | 2.5 ± 0.3 | 3.5A ± 0.4 | 2.8 ± 0.5 |
| T3 | 1.9 ± 0.4 | 1.4 ± 0.3 | 2.1 ± 0.4 | 2.1B ± 0.3 | 2.5 ± 0.4 |
| T4 | 2.0 ± 0.3 | 2.1 ± 0.4 | 2.0 ± 0.4 | 3.5A ± 0.3 | 2.8 ± 0.5 |
| Overall beef flavour | |||||
| C | 6.6 ± 0.3 | 6.9 ± 0.4 | 6.8 ± 0.4 | 5.8 ± 0.3 | 6.6 ± 0.3 |
| T3 | 6.1 ± 0.3 | 6.4 ± 0.3 | 6.8 ± 0.3 | 6.1 ± 0.3 | 6.5 ± 0.3 |
| T4 | 6.5 ± 0.4 | 6.1 ± 0.4 | 6.5 ± 0.3 | 5.6 ± 0.3 | 6.4 ± 0.2 |
The values represent the average counts ± standard error of the mean (SEM). Numbers that have different letters in the same column (A,B) indicate statistical significant (P ≤ 0.05) between treatments within Plant B at each time point. Numbers in the same column that are not followed by letters indicate no significance found.
4. Discussion
This study reports the microbiology of beef along the beef chain from carcass chilling and primal storage under commercial conditions to vacuum packaged steaks under simulated retail display. For this study, alternative carcass chill regimes (conventional or faster chill) and extended primal storage (2 weeks or 5 weeks) were compared for their microbiological, physicochemical and organoleptic properties. The pre- and post-chill TVC, TEC, LAB, Pseudomonas spp., and B. thermosphacta counts obtained in this study were similar to those previously reported for Irish beef carcasses (Reid et al., 2017a; Murray et al., 2001), as were the fluctuations in counts during chilling (Lenahan et al., 2010). Faster chilling achieved significantly lower TVC counts (up to 1 log10 cfu/cm2), as was previously reported by Bowater (2001). However, this advantage was lost in the boning hall when the carcasses were cut into primals. Interestingly, the bacterial counts obtained on the primals were similar to those previously reported by Blixt and Borsch (2002) (Blixt and Borch, 2002) and Reid et al., 2017 (Reid et al., 2017a). Moreover, bacterial concentrations also increased in the cutting room when the primals were cut into retail steaks. This was not unexpected as previous studies have found that unclean cutting boards, cutting equipment and conveyor belts in boning and cutting rooms are a major source of bacterial contamination (McEvoy et al., 2004; Buncic et al., 2014). Both LAB and Pseudomonas counts increased in the vacuum packaged product (primals and steaks) while TEC and B. thermosphacta counts remained relatively low. LAB are psychrotrophic facultative anaerobes that grow readily in chilled vacuum packaged beef (Reid et al., 2017b). Pseudomonas spp. are also capable of growth at low temperatures and their growth in vacuum packaged beef was previously reported by Reid et al. (2017) (Reid et al., 2017a). In contrast Enterobacteriaceae do not grow in correctly chilled VP beef and rarely contribute to spoilage (Nychas et al., 1998), while B. thermosphacta are unable to compete against LAB in chill stored meat under anaerobic conditions (Russo et al., 2006; Sakala et al., 2002), which may explain the low levels of growth in VSP steaks.
The pH values obtained for beef carcasses and primals were similar to those previously reported (Reid et al., 2017a). For carcasses, the mean pH was between 5.5-5.7, which was expected (Reis and Rosenvold, 2014). Interestingly, the pH values of the silverside primals (pH 5.1–5.9) were similar regardless of maturation period (2 or 5 weeks). The slight decrease in the pH of steaks was attributed to LAB growth as has been previously reported (Hanna et al., 1983). Water activity (aw) influences microbial growth and the stability of the sensory attributes of food (Crowley et al., 2010; Lebert et al., 2005). The aw values remained above 0.97 at all stages of the beef chain which is enough to support microbial growth and similar to values previously reported (Reid et al., 2017a; Kinsella et al., 2006).
Most consumers purchasing beef steaks are initially influenced by the colour (Killinger et al., 2004; Souza et al., 2011), with a preference for bright red rather than purple or brown beef (Carpenter et al., 2001). The colour changes observed in the steaks during retail storage as measured by L∗ (lightness), a∗ (redness), b∗ (yellowness), Chroma (C) and Hue, were similar to those previously reported (Colle et al., 2016; King et al., 2012) which suggested that browning and discolouration had occurred over time. Odour, flavour and tenderness are also important sensory properties. As expected the odour and flavour of our steaks remained acceptable throughout the trial although a trend towards increased scores was observed towards the end of the storage period. This was most likely due to the action of spoilage bacteria, which would have eventually resulted in increased off-flavours, odours, discolouration, slime formation and ultimately the formation of CO2 and distorted packs (Samelis et al., 2000). Meat tenderness is influenced by the biochemical changes which occur, especially in the first 24 h post-mortem. Different carcass chilling methods may effect tenderness (15). The faster chilling regime of the carcasses (Treatment 4) was not sufficiently rapid enough to cause cold shortening. Moreover, while aging of beef improves tenderness (Johnston et al., 2001), 5 weeks primal storage instead of 2 has no impact on this important sensory parameter. Overall, there was no difference in the sensory properties (colour, odour, flavour and tenderness) of our steaks, regardless of chilling regime of the original carcasses or maturation period of the primals.
5. Conclusion
Faster carcass chilling decreases microbial counts on the carcasses but this advantage is lost due to cross-contamination of the primals and steaks during subsequent processing. Thus improved hygiene is required in boning and cutting rooms. The sensory properties of the steaks were not adversely affected by faster chilling of the original carcasses or improved by aging the primals for 5 instead of 2 weeks. This finding may allow for faster throughput in beef plants and improved logistics, especially when consumer demand is unpredictable.
Author contributions
Siobhán McSharry: Investigation, Formal analysis, Writing – original draft, Writing - review & editing. Leonard Koolman: Project administration, Experimental design, Investigation, Formal analysis, Writing - original draft, Writing - review & editing. Paul Whyte: Methodology, Supervision, Writing - review & editing. Declan Bolton: Conceptualization, Funding acquisition, Experimental design, Methodology, Project administration, Supervision, Writing - review & editing.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This work was funded by Meat Technology Ireland, a Technology Centre co-funded by Enterprise Ireland and a consortium of beef and sheepmeat processors and the Teagasc Walsh Scholarship scheme. The authors thank Dr. Emily Crofton and Dr. Cristina Botinestean for overseeing sensory analysis.
References
- AMSA . American Meat Science Association; 2012. Meat Color Measurement Guidelines; pp. 1–109. [Google Scholar]
- AMSA Research guidelines for cookery, sensory evaluation, and instrumental tenderness Measurements of meat. Am. Meat Sci. Assoc. 2016;(1.02):1–105. [Google Scholar]
- Blixt Y., Borch E. Comparison of shelf life of vacuum-packed pork and beef. Meat Sci. 2002;60(4):371–378. doi: 10.1016/s0309-1740(01)00145-0. [DOI] [PubMed] [Google Scholar]
- Bowater F.J. Rapid carcass chilling plants compared to conventional systems. Int. J. Refrig. 2001;1–6 [Google Scholar]
- Buncic S., Nychas G.J., Lee M.R.F., Koutsoumanis K., Hébraud M., Desvaux M., Chorianopoulos N., Bolton D., Blagojevic B., Antic D. Microbial pathogen control in the beef chain: recent research advances. Meat Sci. 2014;97(3):288–297. doi: 10.1016/j.meatsci.2013.04.040. [DOI] [PubMed] [Google Scholar]
- Carpenter C.E., Cornforth D.P., Whittier D. Consumer preferences for beef color and packaging did not affect eating satisfaction. Meat Sci. 2001;57(4):359–363. doi: 10.1016/S0309-1740(00)00111-X. [DOI] [PubMed] [Google Scholar]
- Casaburi A., Piombino P., Nychas G.J., Villani F., Ercolini D. Bacterial populations and the volatilome associated to meat spoilage. Food Microbiol. 2015;45:83–102. doi: 10.1016/j.fm.2014.02.002. [DOI] [PubMed] [Google Scholar]
- Colle M.J., Richard R.P., Killinger K.M., Bohlscheid J.C., Gray A.R., Loucks W.I., Day R.N., Cochran A.S., Nasados J.A., Doumit M.E. Influence of extended aging on beef quality characteristics and sensory perception of steaks from the biceps femoris and semimembranosus. Meat Sci. 2016;119:110–117. doi: 10.1016/j.meatsci.2016.04.028. [DOI] [PubMed] [Google Scholar]
- Crowley K.M., Prendergast D.M., Sheridan J.J., McDowell D.A. Survival of Pseudomonas fluorescens on beef carcass surfaces in a commercial abattoir. Meat Sci. 2010;85(3):550–554. doi: 10.1016/j.meatsci.2010.03.004. [DOI] [PubMed] [Google Scholar]
- EFSA Scientific Opinion on the public health risks related to the maintenance of the cold chain during storage and transport of meat. Part 1 (meat of domestic ungulates) EFSA J. 2014;12(3):3601. doi: 10.2903/j.efsa.2014.3783. [DOI] [Google Scholar]
- EFSA Growth of spoilage bacteria during storage and transport of meat. EFSA J. 2016;14(6):1–38. doi: 10.2903/j.efsa.2016.4523. [DOI] [Google Scholar]
- Gill C.O., Newton K.G. The development of aerobic spoilage flora on meat stored at chill temperatures. J. Appl. Bacteriol. 1977;43(2):189–195. doi: 10.1111/j.1365-2672.1977.tb00742.x. [DOI] [PubMed] [Google Scholar]
- Gram L., Ravn L., Rasch M., Bruhn J.B., Christensen A.B., Givskov M. Food spoilage—interactions between food spoilage bacteria. Int. J. Food Microbiol. 2002;78(1–2):79–97. doi: 10.1016/s0168-1605(02)00233-7. [DOI] [PubMed] [Google Scholar]
- Hanna M.O., Savell J.W., Smith G.C., Purser D.E., Gardner F.A., Vanderzant C. Effect of growth of individual meat bacteria on pH, color and odor of aseptically prepared vacuum-packaged round steaks. J. Food Protect. 1983;46(3):216–221. doi: 10.4315/0362-028X-46.3.216. [DOI] [PubMed] [Google Scholar]
- Hungaro H.M., Caturla M.Y.R., Horita C.N., Furtado M.M., Sant’Ana A.S. Blown pack spoilage in vacuum-packaged meat: a review on clostridia as causative agents, sources, detection methods, contributing factors and mitigation strategies. Trends Food Sci. Technol. 2016;52:123–138. doi: 10.1016/j.tifs.2016.04.010. [DOI] [Google Scholar]
- Johnston D.J., Reverter A., Robinson D.L., Ferguson D.M. Sources of variation in mechanical shear force measures of tenderness in beef from tropically adapted genotypes, effects of data editing and their implications for genetic parameter estimation. Aust. J. Exp. Agric. 2001;41(7):991–996. doi: 10.1071/EA00018. [DOI] [Google Scholar]
- Kaur M., Shang H., Tamplin M., Ross T., Bowman J.P. Culture-dependent and culture-independent assessment of spoilage community growth on VP lamb meat from packaging to past end of shelf-life. Food Microbiol. 2017;68:71–80. doi: 10.1016/j.fm.2017.06.015. [DOI] [PubMed] [Google Scholar]
- Killinger K.M., Calkins C.R., Umberger W.J., Feuz D.M., Eskridge K.M. Consumer visual preference and value for beef steaks differing in marbling level and color. J. Anim. Sci. 2004;82(11):3288–3293. doi: 10.2527/2004.82113288x. [DOI] [PubMed] [Google Scholar]
- King D.A., Shackelford S.D., Kalchayanand N., Wheeler T.L. Sampling and aging effects on beef longissimus color stability measurements. J. Anim. Sci. 2012;90(10):3596–3605. doi: 10.2527/jas.2011-4871. [DOI] [PubMed] [Google Scholar]
- Kinsella K., Sheridan J., Rowe T., Butler F., Delgado A., Quispe-Ramirez A., Blair I., McDowell D. Impact of a novel spray-chilling system on surface microflora, water activity and weight loss during beef carcass chilling. Food Microbiol. 2006;23(5):483–490. doi: 10.1016/j.fm.2005.05.013. [DOI] [PubMed] [Google Scholar]
- Lebert I., Baucour P., Lebert A., Daudin J.D. Assessment of bacterial growth on the surface of meat under common processing conditions by combining biological and physical models. J. Food Eng. 2005;68(1):89–98. doi: 10.1016/j.jfoodeng.2004.05.026. [DOI] [Google Scholar]
- Lenahan M., O’Brien S.B., Kinsella K., Sweeney T., Sheridan J.J. Assessment of lamb carcass hygiene before and after chilling at five Irish abattoirs. Food Contr. 2010;21(3):313–318. doi: 10.1016/j.foodcont.2009.06.011. [DOI] [Google Scholar]
- McEvoy J.M., Sheridan J.J., Blair I.S., McDowell D.A. Microbial contamination on beef in relation to hygiene assessment based on criteria used in EU Decision 2001/471/EC. Int. J. Food Microbiol. 2004;92(2):217–225. doi: 10.1016/j.ijfoodmicro.2003.09.010. [DOI] [PubMed] [Google Scholar]
- Mills J., Donnison A., Brightwell G. Factors affecting microbial spoilage and shelf-life of chilled vacuum-packed lamb transported to distant markets: a review. Meat Sci. 2014;98(1):71–80. doi: 10.1016/j.meatsci.2014.05.002. [DOI] [PubMed] [Google Scholar]
- Murray K.A., Gilmour A., Madden R.H. Microbiology quality of chilled beef carcasses in Northern Ireland: a baseline survey. J. Food Protect. 2001;64(4):498–502. doi: 10.4315/0362-028X-64.4.498. [DOI] [PubMed] [Google Scholar]
- Nair M.N., Canto A.C., Rentfrow G., Suman S.P. Muscle-specific effect of aging on beef tenderness. LWT. 2019;100:250–252. doi: 10.1016/j.lwt.2018.10.038. [DOI] [Google Scholar]
- Nychas G.J.E., Drosinos E., Board R.G. Chemical Changes in Stored Meat. In the Microbiology of Meat and Poultry. In: Board R.G., Davies A.R., editors. vols. 288–326. Blackie Academic and Professional; London: 1998. [Google Scholar]
- Nychas G.J.E., Marshall D.L., Sofos J.N. third ed. American Society of Microbiology; 2007. Meat, Poultry, and Seafood. In Food Microbiology: Fundamentals and Frontiers. [Google Scholar]
- Nychas G.J.E., Skandamis P.N., Tassou C.C., Koutsoumanis K.P. Meat spoilage during distribution. Meat Sci. 2008;78(1):77–89. doi: 10.1016/j.meatsci.2007.06.020. [DOI] [PubMed] [Google Scholar]
- Reid R., Fanning S., Whyte P., Kerry J., Lindqvist R., Yu Z.Y., Bolton D. The microbiology of beef carcasses and primals during chilling and commercial storage. Food Microbiol. 2017;61:50–57. doi: 10.1016/j.fm.2016.08.003. [DOI] [PubMed] [Google Scholar]
- Reid R., Fanning S., Whyte P., Kerry J., Bolton D. Comparison of hot versus cold boning of beef carcasses on bacterial growth and the risk of blown pack spoilage. Meat Sci. 2017;125:46–52. doi: 10.1016/j.meatsci.2016.11.012. [DOI] [PubMed] [Google Scholar]
- Reis M.M., Rosenvold K. Early on-line classification of beef carcasses based on ultimate pH by near infrared spectroscopy. Meat Sci. 2014;96(2):862–869. doi: 10.1016/j.meatsci.2013.10.016. [DOI] [PubMed] [Google Scholar]
- Russo F., Ercolini D., Mauriello G., Villani F. Behaviour of Brochothrix thermosphacta in the presence of other meat spoilage microbial group. Food Microbiol. 2006;23(8):797–802. doi: 10.1016/j.fm.2006.02.004. [DOI] [PubMed] [Google Scholar]
- Sakala R.M., Hayashidani H., Kato Y., Hirata T., Makino Y., Fukushima A., Yamada T., Kaneuchi C., Ogawa M. Change in the composition of the microflora on vacuum-packaged beef during chiller storage. Int. J. Food Microbiol. 2002;74(1–2):87–99. doi: 10.1016/S0168-1605(01)00732-2. [DOI] [PubMed] [Google Scholar]
- Samelis J., Kakouri A., Rementzis J. Selective effect of the product type and the packaging conditions on the species of lactic acid bacteria dominating the spoilage microbial association of cooked meats at 4 degrees C. Food Microbiol. 2000;17(3):329–340. doi: 10.1006/fmic.1999.0316. [DOI] [Google Scholar]
- Savell J.W., Mueller S.L., Baird B.E. The chilling of carcasses. Meat Sci. 2005;70(3):449–459. doi: 10.1016/j.meatsci.2004.06.027. [DOI] [PubMed] [Google Scholar]
- Ščetar M., Kurek M., Galić K. Trends in meat and meat products packaging–a review. Croat. J. Food Sci. Technol. 2010;2(1):32–48. [Google Scholar]
- Souza C.M., Boler D.D., Clark D.L., Kutzler L.W., Holmer S.F., Summerfield J.W., Cannon J.E., Smit N.R., McKeith F.K., Killefer J. The effects of high pressure processing on pork quality, palatability, and further processed products. Meat Sci. 2011;87(4):419–427. doi: 10.1016/j.meatsci.2010.11.023. [DOI] [PubMed] [Google Scholar]
- Stanborough T., Fegan N., Powell S.M., Tamplin M., Chandry P.S. Insight into the genome of Brochothrix thermosphacta, a problematic meat spoilage bacterium. Appl. Environ. Microbiol. 2017;83(5) doi: 10.1128/AEM.02786-16. e02786-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stella S., Bernardi C., Tirloni E. Influence of skin packaging on raw beef quality: a review. J. Food Qual. 2018:1–9. doi: 10.1155/2018/7464578. 2018. [DOI] [Google Scholar]
- Van Rooyen L.A., Allen P., O’Connor D.I. Effect of muscle type and CO-pretreatment combinations on the colour stability, protein oxidation and shelf-life of vacuum packaged beef steaks. Meat Sci. 2018;145:407–414. doi: 10.1016/j.meatsci.2018.07.021. [DOI] [PubMed] [Google Scholar]
- Wang H., Qi H., Dong Y., Li Y., Xu X., Zhou G. Characterization of attachment and biofilm formation by meat-borne Enterobacteriaceae strains associated with spoilage. LWT. 2017;86:399–407. doi: 10.1016/j.lwt.2017.08.025. [DOI] [Google Scholar]
- Woraprayote W., Malila Y., Sorapukdee S., Swetwiwathana A., Benjakul S., Visessanguan W. Bacteriocins from lactic acid bacteria and their applications in meat and meat products. Meat Sci. 2016;120:118–132. doi: 10.1016/j.meatsci.2016.04.004. [DOI] [PubMed] [Google Scholar]