Abstract
The benefits of pre-transplant induction chemotherapy in light chain amyloidosis (AL), a low burden plasma cell (PC) neoplasm associated with multiorgan dysfunction, is debatable, although with the availability of bortezomib, this is increasingly pursued. We analyzed outcomes of AL patients undergoing autologous hematopoietic cell transplant between 2014 and 2018, reported to the Center for International Blood and Marrow Transplant Research database. Of 440 patients, 294 received bortezomib-based induction and 146 received no induction. Patients receiving induction had greater PC burden compared to no induction (PC 10% or more: 39% vs 11%, p <0.01). At 2-years, the induction group compared to no induction had lower relapse/progression [13(9–18)% vs 23(16–32)%, p 0.02], better progression-free survival (PFS) [82(77–87)% vs 69(61–77)%, p <0.01] and similar overall survival (OS) [92(88–95)% vs 89(84–94)%, p 0.22], which was confirmed on multivariate analysis. A subset analysis limited to patients <10% PC also showed superior relapse/progression (HR 0.43, 95% CI 0.24–0.78, p <0.01) and PFS (HR 0.43, 95% CI 0.26–0.72, p<0.01) for induction compared to no induction. Thus, we conclude that pre-transplant bortezomib-based induction was associated with improved relapse/progression and PFS in AL. Longer survival follow-up is warranted as OS was excellent in both cohorts at 2 years.
Introduction
Light chain (AL) amyloidosis is a plasma cell (PC) disorder characterized by insoluble fibrillary deposition in organs and tissues derived from clonal free light chains.1 Clinically, AL amyloidosis patients present with multi-organ dysfunction associated with high morbidity and early mortality, particularly when cardiac AL involvement is present.1, 2
Therapies for AL amyloidosis are almost entirely derived from the ones employed for multiple myeloma aiming at interrupting production of the amyloidogenic free light chain. The use of high dose melphalan with autologous hematopoietic cell transplantation (AHCT) performed as initial therapy, can yield long term clone control with subsequent stabilization and/or improvement of organ dysfunction.3, 4 While early experience with AHCT was hindered by relatively high toxicity and transplanted-related mortality (TRM),5 more recent registry data from the US have shown a remarkable reduction in TRM in recent years with excellent 5-year survival.6
While AHCT in AL amyloidosis has been traditionally performed without prior induction, the availability of bortezomib, the first-generation proteasome inhibitor found to be safe and rapidly efficacious in AL amyloidosis,7–9 has created a common practice of promptly initiating therapy with a bortezomib-based regimen even in patients who are transplant candidates.10–12 However, a valid concern has been that delaying transplant to allow induction therapy may result in potentially transplant-eligible patients becoming transplant ineligible.13, 14 Prior reports suggest that AL amyloid patients with a PC burden of >10% may benefit from induction.15 Bortezomib-based induction may improve outcomes by 1) rapidly lowering the toxic amyloidogenic light chain resulting in some degree of organ improvement and 2) by allowing exclusion of less fit patients by testing their ability to tolerate chemotherapy, thus, making transplant safer among those who undergo AHCT. We thus sought to study contemporaneous induction practices and the impact of bortezomib-based induction in AL amyloidosis patients who undergo AHCT in the US using the Center for International Blood and Marrow Transplant Research® (CIBMTR®) database.
Patients and Methods
Data Source
The CIBMTR is a research collaboration between The National Marrow Donor Program/Be The Match, Minneapolis, MN and the Medical College of Wisconsin, Milwaukee, WI. It comprises of a voluntary working group of more than 200 transplantation centers in the US. Participating centers are required to report all transplants consecutively; compliance is monitored by on-site audits and patients are followed longitudinally. Computerized checks for discrepancies, physicians’ review of submitted data, and on-site audits of participating centers ensure data quality. Studies conducted by the CIBMTR are performed in compliance with all applicable federal regulations pertaining to the protection of human research participants. Protected health information used in the performance of such research is collected and maintained in CIBMTR’s capacity as a Public Health Authority under the HIPAA Privacy Rule.
Patients
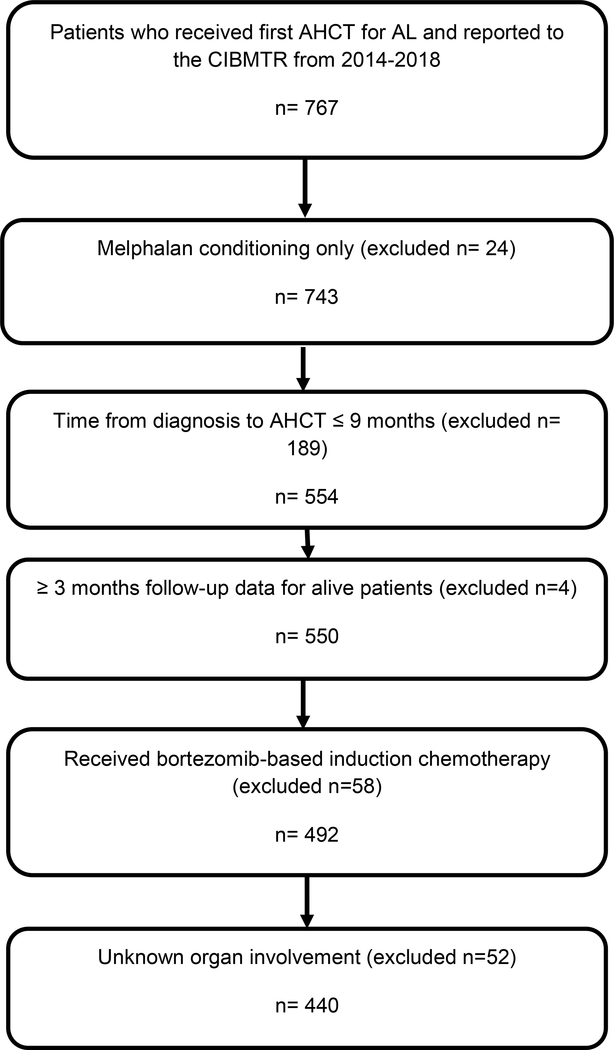
This study involved AL patients who received AHCT for AL in the United States between January 1, 2014 and December 31, 2018 from the CIBMTR database with comprehensive data reported. All patients who received AHCT within 9 months of their AL diagnosis with melphalan conditioning alone with organ involvement information were included. Patients with a concurrent diagnosis of symptomatic multiple myeloma were excluded. Patients who received induction with non-bortezomib therapy were excluded (Figure 1). We identified 440 patients meeting the study criteria. The overall completeness of follow-up at 2 years was 93%.
Figure 1.
CONSORT flow diagram of patient selection.
Definitions and Responses
Hematologic response was defined as the best hematologic response to transplant based on the 2004 uniform consensus criteria proposed at the 10th International Symposium on Amyloidosis.16 Hematologic relapse/progression was defined as the time to first evidence of laboratory recurrence or progression of amyloidosis based on the 2004 uniform consensus criteria. Hematologic progression-free survival (PFS) was defined as survival without progressive disease or relapse from complete response (CR). Progressive disease, relapse from CR and death in remission are considered events. Patients who are alive and in complete remission, partial response, no response, or stable disease are censored at time of last follow-up. Overall survival (OS) was defined as survival with death by any cause as an event. Surviving patients were censored at the time of last contact. Renal organ response was defined as the best renal response achieved after transplant. Renal response was defined as a 50% decrease (at least 0.5 g/day) of 24-hour urine protein with less than 25% decrease in renal function estimated by eGFR.
Statistical Analysis
Descriptive statistics were used to summarize patient-, disease-, and transplant-related characteristics. The t-test or Wilcoxon rank-sum test was used to compare continuous variables and the Pearson chi-square test or Fisher’s exact test was used to evaluate differences between proportions for categorical variables. The probability of PFS and OS were calculated using the Kaplan-Meier estimator while non-relapse mortality and relapse/progression were summarized using the cumulative incidence function. Comparison of survival and cumulative incidence curves was done using the log-rank test and Gray’s test, respectively. A multivariate model was fitted using the Cox proportional hazards regression model to identify prognostic factors associated with the above endpoints. A stepwise model building approach was adopted and variables that attained a p-value less than 5% were retained in the final model. Bortezomib induction was considered the main effect and kept in the model during the variable selection process. Other variables included in the multivariate model included age at transplant, sex, race, PC% at diagnosis, presence of t(11;14), cardiac involvement, liver involvement, renal involvement, number of organs involved, Karnofsky performance score (KPS) at transplant, HCT comorbidity index (HCT-CI) score, serum creatinine at transplant, serum albumin at transplant, melphalan dose, year of transplant, maintenance therapy, and AL transplant center volume. A subset multivariate analysis of patients with <10% bone marrow PC at diagnosis was conducted.
Results
A total of 440 patients underwent first AHCT for AL between 2014–2018 were eligible for analysis. Baseline characteristics are summarized in Table 1. Bortezomib-based induction therapy was administered to 294 patients and included combination with cyclophosphamide and dexamethasone in 82% (n=242); lenalidomide and dexamethasone in 10% (n=29); dexamethasone alone in 7% (n=22); thalidomide and dexamethasone (n=1). The majority (92%) received only one line of therapy prior to AHCT- 37% <3 months, 41% 3–6 months, 1% 6–9 months, and missing in 21%. No pre-AHCT induction therapy occurred in 146 patients. Median age, gender, race, KPS, and baseline end-organ involvement were similar in both groups. Patients with a baseline plasma cell percentage of ≥10% were more likely to receive induction therapy (39% vs 11%). Patients receiving induction therapy were less likely to receive a melphalan conditioning dose of 200 mg/m2 (42% vs 55%). At pre-AHCT, 71% of the induction group were reported to be in a partial response (PR) or better, with 14% CR. The majority of patients in this study did not receive maintenance, but a slightly higher number of patients in the induction group received post-transplant maintenance compared to no induction (20% vs 15%). The median follow-up for survivors was 24.6 months (range, 3–63 months) from the time of AHCT.
Table 1.
Characteristics of patients in the US who underwent first AHCT for light chain amyloidosis and reported to the CIBMTR between year 2014 and 2018
| Variable | Bortezomib Induction (n=294) | No Induction (n=146) |
|---|---|---|
| Number of centers | 65 | 37 |
| Median age, years, (range) | 61 (28–78) | 62 (24–77) |
| Male gender | 166 (56) | 82 (56) |
| Race | ||
| White | 250 (85) | 123 (84) |
| Black | 29 (10) | 19 (13) |
| Otherb | 9 (3) | 3 (2) |
| Unknown | 6 (2) | 1 (1) |
| Karnofsky score ≥ 90% | 128 (44) | 72 (49) |
| HCT-CI | ||
| 0 | 54 (18) | 37 (25) |
| 1 | 31 (11) | 19 (13) |
| 2 | 48 (16) | 19 (13) |
| 3+ | 161 (55) | 71 (49) |
| Disease-related | ||
| Cardiac involvement | ||
| Yes | 154 (52) | 71 (49) |
| No | 91 (31) | 58 (40) |
| Missing | 49 (17) | 17 (12) |
| Renal involvement | ||
| Yes | 207 (70) | 105 (72) |
| No | 19 (6) | 19 (13) |
| Missing | 68 (23) | 22 (15) |
| Liver involvement | ||
| Yes | 39 (13) | 15 (10) |
| No | 224 (76) | 127 (87) |
| Missing | 31 (11) | 4 (3) |
| Organ involvement | ||
| 1 | 115 (39) | 57 (39) |
| 2 | 108 (37) | 45 (31) |
| ≥3 | 71 (24) | 44 (30) |
| Serum creatinine at diagnosis ≥2 mg/dl | 37 (13) | 12 (8) |
| Serum albumin at diagnosis, g/dL <3.5 g/dl | 161 (55) | 93 (64) |
| Bone marrow plasma cells at diagnosis ≥10%c | 116 (39) | 16 (11) |
| t(11;14) abnormality present | ||
| No | 203 (69) | 89 (61) |
| Yes | 66 (23) | 35 (24) |
| Test not done/unknown | 24 (8) | 22 (15) |
| Transplant-related | ||
| Time (months) from diagnosis to AHCT, median (range) | 6 (1–9) | 3 (1–9) |
| Center experiencea | ||
| <4 AHCT | 161 (55) | 61(42) |
| ≥4 AHCT | 133 (45) | 85(58) |
| Melphalan Conditioning Dose | ||
| 100 mg/m2 | 40 (14) | 8 (5) |
| 140 mg/m2 | 89 (30) | 36 (25) |
| 180 mg/m2 | 42 (14) | 22 (15) |
| 200 mg/m2 | 123 (42) | 80 (55) |
| No. of CD34 cells infused (x10/kg), range | 3.78 (0.04–12.12) | 4.41 (0.08–12.71) |
| Year of transplant | ||
| 2014 | 7 (16) | 35 (24) |
| 2015 | 63 (21) | 31 (21) |
| 2016 | 77 (26) | 29 (20) |
| 2017 | 65 (22) | 28 (19) |
| 2018 | 42 (14) | 23 (16) |
| Maintenance therapy | ||
| Bortezomib-based | 20 (6) | 12 (8) |
| Lenalidomide-based | 25 (9) | 3 (2) |
| Bortezomib + Lenalidomide | 9 (3) | 6 (4) |
| Other | 7 (2) | 1 (1) |
| No maintenance | 210 (71) | 115 (79) |
| Missing | 23 (8) | 9 (6) |
| Median f/u of survivors | 24.6 (2.3–60.7) | 29.1 (3.4–62.8) |
Center experience defined as mean number of AHCT across 4 years from 2015–2018
Other race: Asian (n=8); Native American (n=2); More than one race (n=2)
AHCT, autologous hematopoietic cell transplantation; HCT-CI, Hematopoietic cell transplant comorbidity index
Post-AHCT response and outcomes
Table 2 shows post-AHCT responses. Day 100 post-AHCT hematologic overall response rate (ORR; ≥PR) in the bortezomib group was 64% with 18% CR and 46% PR. In the no induction group, ORR was 57% with 20% CR and 37% PR. Best hematologic response was ORR in 80% (36% CR, 44% PR) vs 73% (39% CR, 34% PR) in the bortezomib induction vs no induction group respectively.
Table 2.
Hematologic and Renal responses to HCT
| Bortezomib induction | No Induction | P Value | |
|---|---|---|---|
| Hematologic response at 100 days | 0.01a | ||
| CR | 53 (18) | 29 (20) | |
| PR | 136 (46) | 54 (37) | |
| NR/SD | 53 (18) | 43 (29) | |
| Prog | 5 (2) | 5 (3) | |
| Not evaluable* | 8 (3) | 6 (4) | |
| Missing | 39 (13) | 9 (6) | |
| Best hematologic response | 0.13a | ||
| CR | 107 (36) | 57 (39) | |
| PR | 128 (44) | 49 (34) | |
| NR/SD | 35 (12) | 25 (17) | |
| Prog | 2 (1) | 4 (3) | |
| Not evaluable* | 8 (3) | 6 (4) | |
| Missing | 14 (5) | 5 (3) | |
| Renal response at 100 days | 0.06a | ||
| CR | 50 (17) | 21 (14) | |
| NR/SD | 91 (31) | 61 (42) | |
| Prog | 9 (3) | 8 (5) | |
| Not evaluable* | 8 (3) | 6 (4) | |
| Missing | 136 (46) | 50 (34) | |
| Best renal response | 0.63a | ||
| CR | 99 (34) | 57 (39) | |
| NR/SD | 84 (29) | 40 (27) | |
| Prog | 5 (2) | 3 (2) | |
| Not evaluable* | 8 (3) | 6 (4) | |
| Missing | 98 (33) | 40 (27) |
Non-evaluable were patients who died in the first 100 days after transplant.
Renal responses at day 100 was available in 244 (56%) of patients with the bortezomib-induction cohort showing CR in 33%, stable disease (SD) in 60% and progression in 7% and CR in 14%, SD in 42%, and progression in 7% of the no induction cohort. Best renal response was available in 292 (66%) of patients with 34% CR, 29% SD and 2% progression in the bortezomib induction and 39% CR, 28% SD and 3% progression in the no induction groups.
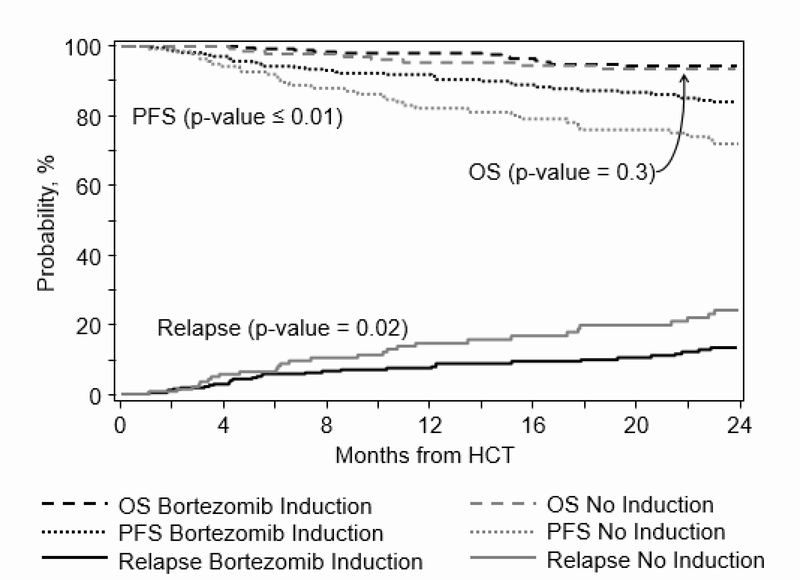
Transplant outcomes from time of AHCT are described (Table 3). Day 100 TRM was 2(1–4) % in bortezomib induction versus 3(1–7) % in the no induction groups (p 0.4). The 2-year cumulative incidence of relapse was 13(9–18) % in the bortezomib-induction cohort and 23(16–32) % in the no induction cohort (p=0.02) (Figure 2). Similarly, the 2-year probability of PFS was 82 (77–87) % and 69(61–77) % in these groups respectively (p<0.01) (Figure 2). There were no significant differences in the 2-year OS among cohorts, with an adjusted probability of 92(88–95) % and 89(84–94) %, respectively (p=0.5) (Figure 2).
Table 3.
Patient outcomes from time of AHCT
| Bortezomib induction (N = 294) | No Induction (N = 146) | ||||
|---|---|---|---|---|---|
| Outcomes | N | Prob (95% CI) | N | Prob (95% CI) | P-value |
| Transplant-related mortality | 288 | 145 | |||
| 100-day | 2 (1–4)% | 3 (1–7)% | 0.43 | ||
| Hematologic Relapse/Progression | 288 | 145 | 0.02 | ||
| 1-year | 8 (5–11)% | 15 (9–21)% | 0.04 | ||
| 2-year | 13 (9–18)% | 23 (16–32)% | 0.02 | ||
| Progression-free survival | 288 | 145 | <0.01 | ||
| 1-year | 90 (86–93)% | 79 (72–85)% | <0.01 | ||
| 2-year | 82 (77–87)% | 69 (61–77)% | 0.01 | ||
| Overall survival | 294 | 146 | 0.22 | ||
| 1-year | 95 (93–98)% | 91 (86–95)% | 0.13 | ||
| 2-year | 92 (88–95)% | 89 (84–94)% | 0.47 | ||
Figure 2.
Cumulative incidence of relapse and probability of progression-free survival and overall survival
Multivariate analysis of outcomes (Table 4)
Table 4.
Multivariable analysis evaluating outcomes following AHCT in patients with AL from time of transplant
| Effect | Hazard Ratio | 95%CI_L | 95%CI_U | P-value |
|---|---|---|---|---|
| Relapse/Progression | ||||
| Bortezomib Induction (Main Effect) | ||||
| No | 1.00 | <0.01 | ||
| Yes | 0.48 | 0.31 | 0.74 | <0.01 |
| Serum Creatinine Prior to AHCT | ||||
| <2 mg/dl | 1.00 | 0.04 | ||
| ≥2 mg/dl | 1.78 | 1.02 | 3.10 | 0.04 |
| Karnosky Score | ||||
| ≥90% | 1.00 | 0.02 | ||
| <90% | 1.97 | 1.24 | 3.11 | <0.01 |
| Melphalan Dose (mg/m2) | ||||
| 200 | 1.00 | 0.02 | ||
| 100 | 2.45 | 1.22 | 4.92 | 0.01 |
| 140 | 2.08 | 1.22 | 3.55 | <0.01 |
| 180 | 0.99 | 0.47 | 2.10 | 0.99 |
| Progression-free Survival | ||||
| Bortezomib Induction (Main Effect) | ||||
| No | 1.00 | <0.01 | ||
| Yes | 0.47 | 0.32 | 0.69 | <0.01 |
| Serum Creatinine Prior to AHCT | ||||
| <2 mg/dl | 1.00 | 0.04 | ||
| ≥2 mg/dl | 1.66 | 1.02 | 2.72 | 0.04 |
| Karnosky Score | ||||
| ≥90% | 1.00 | <0.01 | ||
| <90% | 2.13 | 1.41 | 3.21 | <0.01 |
| Melphalan Dose (mg/m2) | ||||
| 200 | 1.00 | <0.01 | ||
| 100 | 2.32 | 1.24 | 4.34 | <0.01 |
| 140 | 2.09 | 1.30 | 3.36 | <0.01 |
| 180 | 1.14 | 0.60 | 2.14 | 0.69 |
| Overall Survival | ||||
| Bortezomib Induction (Main Effect) | ||||
| No | 1.00 | 0.07 | ||
| Yes | 0.57 | 0.32 | 1.04 | 0.07 |
| Melphalan Dose (mg/m2) | ||||
| 200 | 1.00 | <0.01 | ||
| 100 | 3.93 | 1.65 | 9.42 | <0.01 |
| 140 | 3.01 | 1.49 | 6.09 | <0.01 |
| 180 | 1.05 | 0.34 | 3.23 | 0.93 |
AHCT, autologous hematopoietic cell transplantation
Relapse/progression:
Bortezomib induction was associated with significantly lower relapse/progression compared with no induction cohort (HR 0.48, 95% CI 0.31–0.74, p<0.01). Patients with a creatinine ≥2 mg/dl had significantly higher risk of relapse/progression compared with <2 mg/dl (HR 1.78, 95% CI 1.02–3.10, p=0.04). Similarly, patients with a KPS <90% had significantly higher relapse/progression compared with >90% (HR 1.97, 95% CI 1.24–3.11, p<0.01). Compared with those receiving Mel 200, patients who received Mel 100 and Mel 140 had significantly higher relapse/progression (p 0.01). There was no difference between Mel 180 and Mel 200.
PFS:
In multivariate analysis, variables significantly impacting PFS included bortezomib-based induction therapy, serum creatinine, KPS and melphalan conditioning dose. Use of bortezomib-based induction chemotherapy was associated with superior PFS compared with no induction therapy (HR 0.47, 95% CI 0.32–0.69, p<0.01). Patients with a creatinine ≥2 mg/dl had inferior PFS compared with <2 mg/dl (HR 1.66, 95% CI 1.02–2.72, p=0.04). Similarly, patients with a KPS <90% had inferior PFS compared with >90% (HR 2.13, 95% CI 1.41–3.21, p<0.01). Compared to Mel 200, Mel 100 and Mel 140 conditioning was associated with worse PFS (p 0.008) with Mel 180 having similar PFS.
OS:
Use of bortezomib-based induction demonstrated no significant difference in OS compared with no induction therapy (HR 0.57, 95% CI 0.32–1.04, p=0.07). Compared to Mel 200, use of lower doses of melphalan at 140 and below were associated with worse survival (p 0.002).
Additional analysis was done to assess outcomes in patients who did not receive maintenance therapy (N=325). This showed similar findings as the main analysis for bortezomib induction vs no induction with 2 year relapse/progression of 11 (7–17)% vs 22 (14–31)%, p-value 0.02, 2-year PFS 82 (76–88)% vs 68 (59–77)%, p-value 0.01, and 2-year OS 91 (87–95)% vs 88 (80–93)%, p-value 0.3, respectively.
Subset analysis of patients with <10% of bone marrow plasma cells at diagnosis (N=263)
We conducted a subset analysis of patients with <10% bone marrow plasma cells to understand the effect of bortezomib-based induction in this group. These results were similar to the main analysis and confirmed that bortezomib induction was associated with improved relapse/progression (HR 0.43, 95% CI 0.24–0.78, p 0.005) and PFS (HR 0.43, 95% CI 0.26–0.72, p 0.001) without an OS difference (HR 0.56, 95% CI 0.27–1.18, p 0.1). Detailed results are shown in Supplementary table 1.
Causes of death:
A total of 46 patients died during follow-up including 26 patients in the bortezomib induction cohort and 20 patients in the no induction cohort. Most patients died from AL (n=33; 72%). Other causes of death included infection (n=3; 7%); organ failure (n=3; 7%); respiratory failure (n=2; 4%); other causes including 1 peritonitis, 2 accident/suicide (n=3; 7%). The cause of death was unknown in 2 cases.
Discussion
In this registry study of AL amyloidosis patients receiving upfront AHCT in a contemporaneous period in the US, we make the following observations. 1) The use of bortezomib induction was higher in those with a higher clonal plasma cell burden; 2) Patients who received bortezomib-based induction appeared to benefit from lower relapse/progression and improved PFS compared to patients receiving AHCT without prior induction at 2 years post-AHCT; 3) Both groups had excellent 2-year OS with no difference observed by bortezomib induction at this short follow-up; 4) while bortezomib induction use was heavily determined by clonal burden, a subset analysis of patients with <10% PC showed a similar benefit of bortezomib-based induction on outcomes; and 5) use of high intensity melphalan conditioning at 180 mg/m2 or higher was associated with improved outcomes.
Multiple single center retrospective studies have shown the benefit of bortezomib-based induction therapy on AHCT outcomes in AL amyloidosis patients.15, 17–21 A randomized controlled trial of 56 patients from China also showed a benefit in 2-year outcomes with bortezomib-based induction followed by AHCT compared to AHCT alone,22 though the 2-year overall survival after AHCT alone arm in this study was much lower at 69.5% than that reported from the US in recent years.6 In a prospective study of 2 cycles of bortezomib induction prior to AHCT, 5 of 35 patients (14%) were unable to proceed to transplant owing to clinical deterioration during induction or mobilization.13 Thus, there is a valid concern that induction therapy may lead to loss of the ‘window of opportunity’ to transplant in AL amyloidosis patients. However, an alternate view of this is that induction therapy can serve as an initial test of fitness and allow the ‘selecting out’ of patients who may be unable to withstand the transplant procedure safely. In the multicenter HOVON 104 study,14 50 AL amyloidosis patients were treated with 4 cycles of bortezomib/dexamethasone induction; of these 15 (30%) did not proceed to transplant. However, among the 70% who underwent AHCT, TRM was 0.14 Historically, the use of AHCT in AL amyloidosis has been associated with high TRM compared to multiple myeloma5 and even in more recent years, with increasing experience and better patient selection has been as high as 5% at 100 days.6 Our current study confirms this hypothesis as the 100 day TRM is even lower at 2–3% compared to our prior CIBMTR registry analysis studying transplant outcomes prior to 2013.6
Our analysis shows that patients receiving bortezomib induction were more likely to have a higher plasma cell burden but also receive lower melphalan dosing. Patients with a higher PC disease burden at diagnosis were more likely to receive induction in order to achieve debulking of disease prior to AHCT consistent with data that patients with >10% PC burden have worse prognosis and the use of induction presumably was a clinical decision based on the higher clone size at diagnosis.23 To account for these practice differences, we performed a subset analysis in patients with <10% PC burden at diagnosis and found that bortezomib-based induction led to similar improvements in outcomes as the overall study.
Previous studies have demonstrated that use of full intensity (200 mg/m2) melphalan conditioning is associated with superior outcomes including disease responses and improved survival.6, 24 However, a reduction in melphalan dosing is often used to adjust for anticipated transplant associated morbidity.25, 26 Our data demonstrate that, consistent with previous reports, even in the era of bortezomib based induction, higher doses of melphalan remain important for survival outcomes including OS. We were indeed surprised to see that patients who received bortezomib-based induction were less likely to receive melphalan 200 mg/m2. Our data do not allow us to determine whether this was because patients developed toxicity to induction and were thus unable to receive full intensity melphalan or because a better hematologic disease response led to a choice of dose reduction by the transplant physician. Based on our analysis, even after adjusting for receipt of induction therapy, the use of higher intensity melphalan dose is associated with improved outcomes. Although we tested multiple covariates in our Cox proportional hazards models and looked for interactions, we acknowledge that the use of melphalan 200 mg/m2 can be confounded by other covariates at transplant such as creatinine, KPS, and other comorbidities.
No difference in OS was identified according to induction therapy use in this study with the short follow-up given that this study is restricted to a recent time period. Overall, AL amyloidosis patients in our study had an excellent 2-year survival after AHCT regardless of the use of induction therapy. A longer follow up at 5 or 10 years may be needed to discern the effect of induction therapy, if any, on OS.
Our study has several limitations, the main one being it is restricted to patients undergoing AHCT. Only 20–25% of patients with AL are felt to be eligible for AHCT at time of diagnosis.27 While some patients ineligible for AHCT at diagnosis may become eligible with the use of induction therapy and end-organ improvement,11, 12, 20 induction therapy itself may lead to toxicity and subsequent ineligibility to AHCT.13, 14 We are unable to parse through these scenarios as we do not have data on patients who did not receive transplant. In addition, while cardiac and renal involvement were balanced between the 2 groups, the severity of these, particularly cardiac, are unavailable which could influence the determination of AHCT eligibility and timing.28 We also did not analyze cardiac biomarkers (data unavailable) to determine the severity of involvement between the groups or measure cardiac response after transplant. Lastly, we were not able to use the 2012 response criteria for hematologic and organ response owing to the inconsistent availability of free light chains and cardiac biomarkers at the different timepoints.
In conclusion, this registry analysis proves the benefit of bortezomib-based induction in AL amyloidosis patients undergoing AHCT. This benefit was evident even in patients with low tumor burden. Longer follow up is needed to study the impact on overall survival given the excellent survival at 2 years regardless of induction use. Our data also highlight the importance of using full intensity melphalan conditioning in AL amyloidosis. We propose that with the availability of continued improvement in induction therapies with the combination of bortezomib with monoclonal antibodies, it is timely to study induction and transplant in a multicenter randomized clinical trial in AL amyloidosis patients.
Supplementary Material
Acknowledgements
The CIBMTR is supported primarily by Public Health Service U24CA076518 from the National Cancer Institute (NCI), the National Heart, Lung and Blood Institute (NHLBI) and the National Institute of Allergy and Infectious Diseases (NIAID); U24HL138660 from NHLBI and NCI; OT3HL147741, and U01HL128568 from the NHLBI; HHSH250201700006C, and HHSH250201700007C from the Health Resources and Services Administration (HRSA); and N00014-18-1-2850, N00014-18-1-2888, and N00014-20-1-2705 from the Office of Naval Research. Additional federal support is provided by P01CA111412, R01CA152108, R01CA215134, R01CA218285, R01CA231141, R01AI128775, R01HL126589, R01HL129472, R01HL130388, R01HL131731, U01AI069197, U01AI126612, and BARDA. Support is also provided by Be the Match Foundation, Boston Children’s Hospital, Dana Farber, St. Baldrick’s Foundation, Stanford University, the Medical College of Wisconsin the National Marrow Donor Program, and from the following commercial entities: AbbVie; Actinium Pharmaceuticals, Inc.; Adaptive Biotechnologies; Adienne SA; Allovir, Inc.; Amgen Inc.; Angiocrine Bioscience; Astellas Pharma US; AstraZeneca; Atara Biotherapeutics, Inc.; bluebird bio, Inc.; Bristol Myers Squibb Co.; Celgene Corp.; CSL Behring; CytoSen Therapeutics, Inc.; Daiichi Sankyo Co., Ltd.; Gamida-Cell, Ltd.; Genentech Inc; HistoGenetics, Inc.; Incyte Corporation; Janssen Biotech, Inc.; Jazz Pharmaceuticals, Inc.; Johnson & Johnson; Kiadis Pharma; Kite, a Gilead Company; Kyowa Kirin; Legend Biotech; Magenta Therapeutics; Mallinckrodt LLC; Merck & Company, Inc.; Merck Sharp & Dohme Corp.; Millennium, the Takeda Oncology Co.; Miltenyi Biotec, Inc.; Novartis Pharmaceuticals Corporation; Omeros Corporation; Oncoimmune, Inc.; Orca Biosystems, Inc.; Pfizer, Inc.; Pharmacyclics, LLC; Sanofi Genzyme; Shire; Sobi, Inc.; Stemcyte; Takeda Pharma; Terumo BCT; Viracor Eurofins; Vor Bio Pharma; Xenikos BV; The views expressed in this article do not reflect the official policy or position of the National Institute of Health, the Department of the Navy, the Department of Defense, Health Resources and Services Administration (HRSA) or any other agency of the U.S. Government.
CIBMTR supports accessibility of research in accord with the National Institutes of Health (NIH) Data Sharing Policy and the National Cancer Institute (NCI) Cancer Moonshot Public Access and Data Sharing Policy. The CIBMTR releases only de-identified datasets that comply with all relevant global regulations regarding privacy and confidentiality
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Merlini G AL amyloidosis: from molecular mechanisms to targeted therapies. Hematology / the Education Program of the American Society of Hematology. American Society of Hematology. Education Program 2017; 2017(1): 1–12. e-pub ahead of print 2017/12/10; doi: 10.1182/asheducation-2017.1.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gertz MA. Immunoglobulin light chain amyloidosis: 2020 update on diagnosis, prognosis, and treatment. American journal of hematology 2020; 95(7): 848–860. e-pub ahead of print 2020/04/09; doi: 10.1002/ajh.25819 [DOI] [PubMed] [Google Scholar]
- 3.Sidana S, Sidiqi MH, Dispenzieri A, Buadi FK, Lacy MQ, Muchtar E et al. Fifteen year overall survival rates after autologous stem cell transplantation for AL amyloidosis. American journal of hematology 2019; 94(9): 1020–1026. e-pub ahead of print 2019/06/30; doi: 10.1002/ajh.25566 [DOI] [PubMed] [Google Scholar]
- 4.Sanchorawala V, Sun F, Quillen K, Sloan JM, Berk JL, Seldin DC. Long-term outcome of patients with AL amyloidosis treated with high-dose melphalan and stem cell transplantation: 20-year experience. Blood 2015; 126(20): 2345–2347. e-pub ahead of print 2015/10/08; doi: 10.1182/blood-2015-08-662726 [DOI] [PubMed] [Google Scholar]
- 5.Jaccard A, Moreau P, Leblond V, Leleu X, Benboubker L, Hermine O et al. High-dose melphalan versus melphalan plus dexamethasone for AL amyloidosis. The New England journal of medicine 2007; 357(11): 1083–1093. doi: 10.1056/NEJMoa070484 [DOI] [PubMed] [Google Scholar]
- 6.D’Souza A, Dispenzieri A, Wirk B, Zhang MJ, Huang J, Gertz MA et al. Improved Outcomes After Autologous Hematopoietic Cell Transplantation for Light Chain Amyloidosis: A Center for International Blood and Marrow Transplant Research Study. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 2015; 33(32): 3741–3749. doi: 10.1200/JCO.2015.62.4015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kastritis E, Wechalekar AD, Dimopoulos MA, Merlini G, Hawkins PN, Perfetti V et al. Bortezomib with or without dexamethasone in primary systemic (light chain) amyloidosis. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 2010; 28(6): 1031–1037. doi: 10.1200/JCO.2009.23.8220 [DOI] [PubMed] [Google Scholar]
- 8.Reece DE, Hegenbart U, Sanchorawala V, Merlini G, Palladini G, Blade J et al. Efficacy and safety of once-weekly and twice-weekly bortezomib in patients with relapsed systemic AL amyloidosis: results of a phase 1/2 study. Blood 2011; 118(4): 865–873. doi: 10.1182/blood-2011-02-334227 [DOI] [PubMed] [Google Scholar]
- 9.Manwani R, Cohen O, Sharpley F, Mahmood S, Sachchithanantham S, Foard D et al. A prospective observational study of 915 patients with systemic AL amyloidosis treated with upfront bortezomib. Blood 2019. e-pub ahead of print 2019/10/04; doi: 10.1182/blood.2019000834 [DOI] [PubMed] [Google Scholar]
- 10.Venner CP, Lane T, Foard D, Rannigan L, Gibbs SD, Pinney JH et al. Cyclophosphamide, bortezomib, and dexamethasone therapy in AL amyloidosis is associated with high clonal response rates and prolonged progression-free survival. Blood 2012; 119(19): 4387–4390. doi: 10.1182/blood-2011-10-388462 [DOI] [PubMed] [Google Scholar]
- 11.Mikhael JR, Schuster SR, Jimenez-Zepeda VH, Bello N, Spong J, Reeder CB et al. Cyclophosphamide-bortezomib-dexamethasone (CyBorD) produces rapid and complete hematologic response in patients with AL amyloidosis. Blood 2012; 119(19): 4391–4394. doi: 10.1182/blood-2011-11-390930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cornell RF, Zhong X, Arce-Lara C, Atallah E, Blust L, Drobyski WR et al. Bortezomib-based induction for transplant ineligible AL amyloidosis and feasibility of later transplantation. Bone marrow transplantation 2015; 50(7): 914–917. e-pub ahead of print 2015/04/29; doi: 10.1038/bmt.2015.73 [DOI] [PubMed] [Google Scholar]
- 13.Sanchorawala V, Brauneis D, Shelton AC, Lo S, Sun F, Sloan JM et al. Induction Therapy with Bortezomib Followed by Bortezomib-High Dose Melphalan and Stem Cell Transplantation for Light Chain Amyloidosis: Results of a Prospective Clinical Trial. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation 2015; 21(8): 1445–1451. e-pub ahead of print 2015/04/11; doi: 10.1016/j.bbmt.2015.04.001 [DOI] [PubMed] [Google Scholar]
- 14.Minnema MC, Nasserinejad K, Hazenberg B, Hegenbart U, Vlummens P, Ypma PF et al. Bortezomib-based induction followed by stem cell transplantation in light chain amyloidosis: results of the multicenter HOVON 104 trial. Haematologica 2019; 104(11): 2274–2282. e-pub ahead of print 2019/03/30; doi: 10.3324/haematol.2018.213900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hwa YL, Kumar SK, Gertz MA, Lacy MQ, Buadi FK, Kourelis TV et al. Induction therapy pre-autologous stem cell transplantation in immunoglobulin light chain amyloidosis: a retrospective evaluation. American journal of hematology 2016; 91(10): 984–988. e-pub ahead of print 2016/06/25; doi: 10.1002/ajh.24453 [DOI] [PubMed] [Google Scholar]
- 16.Gertz MA, Comenzo R, Falk RH, Fermand J-P, Hazenberg BP, Hawkins PN et al. Definition of Organ Involvement and Treatment Response in Primary Systemic Amyloidosis (AL): A Consensus Opinion from the 10th International Symposium on Amyloid and Amyloidosis. Blood 2004; 104(11): 754–754. doi: 10.1182/blood.V104.11.754.754 [DOI] [PubMed] [Google Scholar]
- 17.Scott EC, Heitner SB, Dibb W, Meyers G, Smith SD, Abar F et al. Induction Bortezomib in AL Amyloidosis Followed By High Dose Melphalan and Autologous Stem Cell Transplantation: A Single Institution Retrospective Study. Clinical lymphoma, myeloma & leukemia 2014. doi: 10.1016/j.clml.2014.02.003 [DOI] [PubMed] [Google Scholar]
- 18.Cowan AJ, Klippel ZK, Stevenson PA, Hyun TS, Tuazon S, Becker PS et al. Pre-transplantation novel agent induction predicts progression-free survival for patients with immunoglobulin light-chain amyloidosis undergoing high-dose melphalan and autologous stem cell transplantation. Amyloid : the international journal of experimental and clinical investigation : the official journal of the International Society of Amyloidosis 2016; 23(4): 254–259. e-pub ahead of print 2016/11/24; doi: 10.1080/13506129.2016.1258356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Afrough A, Saliba RM, Hamdi A, Honhar M, Varma A, Cornelison AM et al. Impact of Induction Therapy on the Outcome of Immunoglobulin Light Chain Amyloidosis after Autologous Hematopoietic Stem Cell Transplantation. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation 2018; 24(11): 2197–2203. e-pub ahead of print 2018/07/18; doi: 10.1016/j.bbmt.2018.07.010 [DOI] [PubMed] [Google Scholar]
- 20.Hong S, Valent J, Rybicki L, Abounader D, Bolwell B, Dean R et al. Outcomes of autologous hematopoietic cell transplantation in primary amyloidosis after bortezomib-based induction therapy. Bone marrow transplantation 2016; 51(5): 732–734. e-pub ahead of print 2016/01/05; doi: 10.1038/bmt.2015.326 [DOI] [PubMed] [Google Scholar]
- 21.Mikhael J Turn off the Tap! The Need for Induction Therapy for AL Amyloidosis Before Transplantation. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation 2018; 24(11): e1–e2. e-pub ahead of print 2018/09/24; doi: 10.1016/j.bbmt.2018.09.019 [DOI] [PubMed] [Google Scholar]
- 22.Huang X, Wang Q, Chen W, Zeng C, Chen Z, Gong D et al. Induction therapy with bortezomib and dexamethasone followed by autologous stem cell transplantation versus autologous stem cell transplantation alone in the treatment of renal AL amyloidosis: a randomized controlled trial. BMC medicine 2014; 12: 2. doi: 10.1186/1741-7015-12-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kourelis TV, Kumar SK, Gertz MA, Lacy MQ, Buadi FK, Hayman SR et al. Coexistent multiple myeloma or increased bone marrow plasma cells define equally high-risk populations in patients with immunoglobulin light chain amyloidosis. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 2013; 31(34): 4319–4324. doi: 10.1200/JCO.2013.50.8499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tandon N, Muchtar E, Sidana S, Dispenzieri A, Lacy MQ, Dingli D et al. Revisiting conditioning dose in newly diagnosed light chain amyloidosis undergoing frontline autologous stem cell transplant: impact on response and survival. Bone marrow transplantation 2017; 52(8): 1126–1132. e-pub ahead of print 2017/04/11; doi: 10.1038/bmt.2017.68 [DOI] [PubMed] [Google Scholar]
- 25.Skinner M, Sanchorawala V, Seldin DC, Dember LM, Falk RH, Berk JL et al. High-dose melphalan and autologous stem-cell transplantation in patients with AL amyloidosis: an 8-year study. Annals of internal medicine 2004; 140(2): 85–93. e-pub ahead of print 2004/01/22; doi: 10.7326/0003-4819-140-2-200401200-00008 [DOI] [PubMed] [Google Scholar]
- 26.Gertz MA, Lacy MQ, Dispenzieri A, Ansell SM, Elliott MA, Gastineau DA et al. Risk-adjusted manipulation of melphalan dose before stem cell transplantation in patients with amyloidosis is associated with a lower response rate. Bone marrow transplantation 2004; 34(12): 1025–1031. doi: 10.1038/sj.bmt.1704691 [DOI] [PubMed] [Google Scholar]
- 27.Gertz MA, Lacy MQ, Dispenzieri A, Hayman SR, Kumar SK, Dingli D et al. Autologous stem cell transplant for immunoglobulin light chain amyloidosis: a status report. Leukemia & lymphoma 2010; 51(12): 2181–2187. e-pub ahead of print 2010/10/21; doi: 10.3109/10428194.2010.524329 [DOI] [PubMed] [Google Scholar]
- 28.Gertz MA, Lacy MQ, Dispenzieri A, Kumar SK, Dingli D, Leung N et al. Refinement in patient selection to reduce treatment-related mortality from autologous stem cell transplantation in amyloidosis. Bone marrow transplantation 2013; 48(4): 557–561. doi: 10.1038/bmt.2012.170 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.