Abstract
Background:
Bloodstream infections (BSIs) occur in 20-45% of inpatient autologous and allogeneic hematopoietic cell transplant (HCT) patients. Daily bathing with the antiseptic chlorhexidine gluconate (CHG) has been shown to reduce the incidence of BSIs in critically ill patients although very few studies include HCT patients or have evaluated the impact of compliance on effectiveness.
Methods:
We conducted a prospective cohort study with historical controls to assess the impact of CHG bathing on the rate of BSIs and gut microbiota composition among adults undergoing inpatient HCT at the Duke University Medical Center. We present one year of data without CHG bathing (2016) and two years of data when CHG was used on the HCT unit (2017-2018). Because not all patients adhered to CHG, patients were grouped into four categories by rate of daily CHG usage: High (>75%), Medium (50-75%), Low (1-49%), and None (0%).
Results:
Among 192 patients, univariate trend analysis demonstrated that increased CHG usage was associated with decreased incidence of clinically-significant BSI, defined as any BSI requiring treatment by the medical team (High-8% BSI, Medium-15.2%, Low-15.6%, No CHG-30.3%, p=0.003), laboratory-confirmed BSI (LCBI, p=0.03), central line-associated BSI (CLABSI, p=0.04), and mucosal barrier injury BSI (MBI-LCBI, p=0.002), Multivariate analysis confirmed a significant effect of CHG bathing on clinically-significant BSI (p=0.023) and mucosal barrier injury BSI (MBI-LCBI, p=0.007), without consistently impacting gut microbial diversity. Benefits of CHG bathing were most pronounced with >75% daily usage, and there were no adverse effects attributable to CHG.
Conclusions:
Adherence to daily CHG bathing significantly decreases the rate of bloodstream infection following HCT.
Introduction
Over 20,000 patients undergo hematopoietic cell transplantation (HCT) in the United States each year1,2. While HCT has great therapeutic potential, intensive chemotherapy conditioning regimens and variability in time to stem cell engraftment result in a period of pancytopenia and immunosuppression that increases risk for infection2–5. Bloodstream infections (BSIs) occur in 20-45% of hospitalized autologous and allogeneic transplant patients, resulting in prolonged hospitalization and an increased risk of non-relapse mortality6–11. Many of these infections result from the entry of skin microbial flora into the bloodstream through a disruption of the skin barrier, such as the commonly-placed central venous catheter12.
One method of infection prevention is daily cutaneous application of chlorhexidine gluconate (CHG), an antiseptic with potent activity against gram-positive and gram-negative bacteria13,14. There are mixed data regarding the efficacy of daily CHG bathing in reducing the rates of vancomycin-resistant Enterococcus (VRE) rectal colonization, Clostridioides difficile infection, and BSI12,15–17. In some critically-ill populations, CHG has been shown to reduce skin microbial burden of gram-positive organisms and overall BSI rate 12–14, 18–22. In a study of non-critically ill patients, the ABATE multicenter trial found that daily CHG application was not more efficacious than regular bathing in reducing BSIs although subgroup analysis did demonstrate BSI reduction in patients with indwelling medical devices who used CHG23. Of note, these studies did not measure patient adherence to CHG application, and thus they could not account for the effect of varying levels of compliance on clinical outcomes.
Although the HCT population has unique transplant-related characteristics including periods of profound neutropenia and frequent rash development, few CHG studies include HCT patients. Moreover, while reported side effects of CHG bathing, such as rash and nerve irritation, are rare, they carry a greater significance in HCT patients as they could mimic symptoms of acute graft-versus-host disease (GVHD) of the skin and trigger a change in medical management24,25.
To better understand the effect of CHG on the rate of BSIs in HCT patients and the influence of CHG adherence on outcomes, we performed a prospective cohort study of patients admitted to our institution’s HCT unit while undergoing transplantation (including pre-transplant conditioning, stem cell infusion, and count recovery). We included patients from a historical cohort admitted during the year immediately prior to the institution of CHG use and compared these to patients admitted the two years following the adoption of CHG bathing in the HCT unit. We also evaluated the rate of new VRE rectal colonization, C. difficile infection, cutaneous GVHD and potential toxicities such as rash requiring treatment. In a subset of patients, we evaluated changes in the gut microbiota associated with CHG use. We hypothesized that CHG would be well-tolerated and that BSI rates would decrease with CHG use.
Methods
Study Design
We conducted a prospective cohort study with additional retrospective controls to assess the impact of daily CHG bathing in reducing the rate of BSIs in HCT patients at the Duke University Medical Center (DUMC). DUMC is a 957-bed, tertiary teaching hospital in Durham, NC. The HCT ward contains sixteen beds and cares for patients undergoing autologous and allogeneic hematopoietic cell transplantation.
On January 2, 2017, as part of an initiative of the infection prevention group, all patients on the HCT unit began receiving once-daily bathing with 2% chlorhexidine-impregnated cloths beginning on day of admission (Sage Products Inc, Cary, IL). Each CHG bath was recorded in a nursing flowsheet in the patient’s electronic medical record. Nurses educated patients on proper CHG bathing technique and performed the bath for patients unable to bathe themselves. CHG bathing was performed according to the manufacturer’s instructions with a total of six cloths used to clean the neck, torso, back, groin, arms, and legs26. Patients were also instructed to clean not only around their central line dressings but also to include the 6 inches of tubing most proximal to their skin. CHG was not applied to the face in order to avoid mucosal irritation. Standard central line care included a weekly dressing change using CHG antiseptic every 7 days; all central lines also used a CHG-infused gel pad covering the line exit site. With the exception of central lines placed at other institutions prior to transplant, lines for autologous recipients were installed prior to mobilization, within 30 days prior to HCT, and removed before discharging home after engraftment. For allogeneic recipients, lines were installed the day before admission and removed generally 3 months after transplant at discharge from the transplant service.
We collected CHG compliance and clinical outcomes data for all patients admitted to the unit between January 1, 2016 and December 31, 2018 for pre-transplant conditioning, stem cell infusion, and count recovery. This provides one year of data with no CHG bathing (2016) and two years of data after CHG bathing was instituted, though with variable adherence (2017-2018). Clinical practices were consistent throughout this period with the exception of a transition to the use of levofloxacin instead of ciprofloxacin for antibiotic prophylaxis and the addition of letermovir for CMV prophylaxis in allogeneic HCT recipients in the spring of 2018. A small subset of patients with fluoroquinolone allergies received prophylaxis with other antibiotics, primarily amoxicillin-clavulanate. Otherwise, standard prophylaxis included a fluoroquinolone starting on the day of admission and continuing until engraftment and antifungal prophylaxis with fluconazole in autologous HCT recipients and voriconazole or posaconazole in allogeneic HCT starting on the day of transplant and continuing through day +75. All patients received Pneumocystis jirovecii prophylaxis with trimethoprim-sulfamethoxazole during pre-transplant conditioning and then again on day+30 for a year or until off immunosuppression. Acyclovir was provided for antiviral prophylaxis on the day of admission for all patients undergoing HCT with the addition of letermovir on day +5 for CMV IgG+ allogeneic HCT recipients or donors.
Patients were included in this study if they were admitted for pre-transplant conditioning and inpatient monitoring for allogeneic or autologous HCT. Patients were excluded if they were febrile or had a known infection upon admission, or if they were admitted for reasons other than pre-transplant conditioning, stem cell infusion, or count recovery. Patients were tracked from admission until unit discharge, transfer to a different unit, or first bloodstream infection. In the case of transfer, we monitored whether a patient developed an infection within 48 hours of transfer. All data were abstracted through manual chart review, automated chart review using the Duke Enterprise Data Unified Content Explorer, or from the Duke Adult Blood and Marrow Transplant database. This study was approved of as a quality improvement project and met quality improvement exemption by the Duke University Health System Institutional Review Board.
Study Outcomes and Definitions
The primary outcome was bloodstream infection (BSI) incidence per day of hospitalization. Every BSI was characterized as belonging to one or more of the following categories: laboratory-confirmed bloodstream infection (LCBI), central line-associated bloodstream infection (CLABSI), mucosal barrier injury laboratory-confirmed bloodstream infection (MBI-LCBI), or clinically-significant BSI.
Laboratory-confirmed bloodstream infections were determined using 2018 CDC National Healthcare Safety Network (NHSN) definitions27. LCBI was defined as infection with a known pathogen identified in at least one blood culture or as infection with a common commensal isolated from two or more independently drawn blood specimens with at least one associated symptom of fever (>38°C), chills, or hypotension. CLABSI was defined as occurrence of an LCBI when an indwelling catheter was in place or had been removed in the prior 48 hours27. MBI-LCBI was defined as an LCBI due to a typically enteric organism associated with mucosal barrier injury27.
The variable “clinically-significant BSI” was created by the investigators to include all cases of BSI that led to medically significant changes in management, such as the initiation of broad-spectrum antibiotics. This encompassed both laboratory-confirmed BSIs and BSIs deemed significant by the treatment team but that did not meet CDC/NHSN criteria. For example, patients that were febrile (>38°C), hypotensive, and treated with antibiotics, but with only one positive culture of a common skin commensal such as Staphylococcus epidermidis were deemed to have a clinically-significant BSI. While CLABSI, LCBI, and MBI-LCBI designations are valuable for hospital epidemiology and infection control purposes, clinically-significant BSI were defined a priori as an important clinical outcome impacting clinical practice and the use of medical interventions.
Secondary outcomes included new vancomycin-resistant Enterococcus (VRE) rectal colonization, days to engraftment, incidence of febrile neutropenia, C. difficile infection, and rash requiring treatment, such as a drug-related rash or cutaneous GVHD. Methicillin-resistant Staphylococcus aureus colonization was not reported as this is not routinely screened on the unit.
Engraftment was defined as the first of three consecutive days with an absolute neutrophil count (ANC) of greater than 500 cells/μL. Febrile neutropenia was defined as fever (>38°C) occurring in a patient known to have an ANC less than 500 cells/μL28. C. difficile infection was defined as a positive C. difficile PCR in the setting of diarrhea. All pertinent infections were included from hospital admission to the date of discharge. New VRE rectal colonization was defined when a patient with a negative VRE rectal swab on admission later screened positive for VRE16. VRE rectal swabs were performed on all patients during admission and then once weekly while in the HCT unit. The variable “rash requiring treatment” included patients prescribed topical corticosteroids or antimicrobials used to treat rashes.
Because not all patients consistently bathed with CHG, they were grouped into four levels of adherence: High (>75%), Medium (50-75%), Low (1-49%), and None (0%). CHG adherence was determined by dividing the number of days a patient received at least one recorded CHG bath over the total number of days that patient spent in the HCT unit until discharge from the unit, transfer to another unit, death, or first infection. CHG bathing was not instituted in the HCT unit until January 2017, therefore, all patients transplanted in 2016 belong to the No CHG group and serve as historical controls. These usage categories were designated at the start of the study.
Statistical Analysis
Baseline characteristics and clinical outcomes between the four CHG usage groups were compared via ANOVA for continuous variables, Chi-square test or Cochran-Armitage linear trend test for categorical variables. Cumulative incidence function curves were generated based on the incidence of BSI within each category (LCBI, CLABSI, MBI-LCBI, or clinically-significant BSI) in one of two ways: 1) from transplant day −13 to discharge, or 2) by hospital day starting from the day of admission until discharge. Both transplant day and hospital day were used to separately calculate incidence curves because the risk of BSI is a factor of both the time from transplant and the duration of neutropenia as well as the duration of hospitalization. Incidence curves were compared via Gray’s Test with discharge, transfer, and death considered to be competing risks. Multivariate analysis using the Fine-Grey subdistribution hazard model was conducted to compare time to BSI for each BSI category, accounting for the effects of CHG usage/adherence, antibiotic prophylaxis regimen, and type of transplant on BSI incidence. Patients without BSI were censored at transplant day 30 as almost all uninfected patients had been discharged by then. All patients were assumed to be uninfected prior to admission if they were admitted after transplant day −13, and this was confirmed for accuracy via chart review to check for infections from transplant day −13 to admission date. A significance threshold of 2-sided alpha=0.05 was used for all analyses. All analyses were conducted using SAS version 9.4 (SAS Institute, Cary, NC).
Gut Microbiome Analysis
Stool samples from patients were collected as part of a separate institutional review board-approved biorepository starting from hospital admission to 30 days after transplant. Stools were grouped into 3 time points (days 0-7, 8-14, and 15-30 relative to transplant) and aliquots were frozen at −80°C. DNA extraction was performed by bead-beating, isolating nucleic acids using phenol-chloroform extraction, followed by PCR amplification of the genomic 16S ribosomal-RNA gene V4-V5 variable region and sequencing on the Illumina MiSeq platform as previously described29. Sequences were quality filtered and OTUs identified using the ‘dada2’, ‘phangorn’, and ‘DECIPHER’ packages in R. Alpha diversity was calculated using Shannon diversity and performed with the ‘phyloseq’ package. Multidimensional scaling using Bray-Curtis distance was utilized to evaluate community-level taxonomic differences between groups. Mixed effects modeling was implemented with the ‘lme4’ and ‘lmerTest’ packages, with supplementary Bayesian mixed effects modeling implemented via the ‘rstanarm’ and ‘rstan’ packages. Linear mixed effects modeling of Shannon diversity took into account patient demographics, study group, and days relative to transplant, using patient identity and sample batch as random effects.
Results
Demographic and transplant characteristics were relatively well-matched between chlorhexidine gluconate usage groups
We evaluated 192 patients hospitalized for pre-transplant conditioning, stem cell infusion, and count recovery, including 118 (62%) allogeneic transplants and 74 (38%) autologous transplants (Table 1a). Of these, 25 (13%) had high, 33 (17%) medium, 45 (23%) low, and 89 (46%) no CHG usage. Demographic and transplant characteristics were evenly matched between the CHG usage groups with the exception that patients in the High CHG usage group were more likely to receive levofloxacin instead of ciprofloxacin or other antibiotics for prophylaxis (p=0.003). Allogeneic transplant patients in the Low CHG group tended to receive T-cell depletion therapy more often than other groups (High CHG 20%, Medium CHG 16.7%, Low CHG 50%, No CHG 32.7%) although this did not meet the threshold for significance (p=0.07, Table 1b).
Table 1a.
Demographics and baseline characteristics by chlorhexidine gluconate usage
| High (>75%) N=25 |
Medium (50-75%) N=33 |
Low (<50%) N=45 |
None (0%) N=89 |
P-value1 | |
|---|---|---|---|---|---|
| Median Age (IQR) | 56 (47 - 59) | 58 (52 - 63) | 58 (48 - 62) | 54 (45 - 62) | 0.26 |
| Gender, Female | 11 (44%) | 13 (39.4%) | 19 (42.2%) | 34 (38.2%) | 0.94 |
| Race | 0.41 | ||||
| White | 17 (68%) | 18 (54.5%) | 30 (66.7%) | 65 (73%) | · |
| Black | 7 (28%) | 13 (39.4%) | 12 (26.7%) | 23 (25.8%) | · |
| Other | 1 (4%) | 2 (6.1%) | 3 (6.7%) | 1 (1.1%) | · |
| Hispanic | 0 (0%) | 1 (3%) | 2 (4.4%) | 1 (1.1%) | 0.45 |
| Disease | 0.42 | ||||
| Leukemia | 6 (24%) | 10 (30.3%) | 16 (35.6%) | 29 (32.6%) | · |
| Lymphoma | 8 (32%) | 17 (51.5%) | 16 (35.6%) | 43 (48.3%) | · |
| MDS2 | 7 (28%) | 4 (12.1%) | 8 (17.8%) | 12 (13.5%) | · |
| Other | 4 (16%) | 2 (6.1%) | 5 (11.1%) | 5 (5.6%) | · |
| Disease Status | 0.79 | ||||
| First Complete Remission | 7 (28%) | 12 (36.4%) | 16 (35.6%) | 33 (37.1%) | · |
| Second or Greater CR3 | 1 (4%) | 5 (15.2%) | 5 (11.1%) | 9 (10.1%) | · |
| Partial Remission | 5 (20%) | 7 (21.2%) | 7 (15.6%) | 23 (25.8%) | · |
| Stable | 8 (32%) | 6 (18.2%) | 11 (24.4%) | 13 (14.6%) | · |
| Other | 4 (16%) | 3 (9.1%) | 6 (13.3%) | 11 (12.4%) | · |
| Days from Diagnosis to Transplant (IQR) | 237 (166 - 835) | 288 (170 - 652) | 325.5 (152.5 - 1302.5) | 252 (170 - 764) | 0.20 |
| Days from CR1 to Transplant (IQR) | 35 (7 - 78) | 20 (17 - 23) | 23 (17 - 36) | 17 (12 - 27) | |
| Transplant Type | 0.75 | ||||
| Allogeneic | 15 (60%) | 18 (54.5%) | 30 (66.7%) | 55 (61.8%) | · |
| Karnofsky Performance Status | 0.17 | ||||
| 80 or below | 13 (52%) | 11 (33.3%) | 26 (57.8%) | 40 (44.9%) | · |
| 90-100 | 12 (48%) | 22 (66.7%) | 19 (42.2%) | 49 (55.1%) | · |
| ICU Transfer | 2 (8%) | 3 (9.1%) | 4 (8.9%) | 9 (10.1%) | 0.99 |
| Hospitalization Days (IQR) | 23 (17 - 27) | 22 (17 - 30) | 25 (19 - 31) | 25 (19 - 32) | 0.54 |
| WBCs on Admission4 (IQR) | 4.9 (3.3 - 6.2) | 3.8 (3.1 - 5) | 4.2 (2.1 - 5.7) | 4.9 (3.7 - 6.7) | 0.33 |
| ALC on Admission5 (IQR) | 2250 (1250 - 2318) | 3246 (3246 - 3246) | 1039 (428 - 1634) | 799 (700.5 - 1374) | 0.24 |
| Antibiotic Prophylaxis | 0.0003 | ||||
| Ciprofloxacin | 13 (52%) | 20 (60.6%) | 32 (71.1%) | 68 (76.4%) | · |
| Levofloxacin | 12 (48%) | 10 (30.3%) | 9 (20%) | 7 (7.9%) | · |
| Other6 | 0 (0%) | 3 (9.1%) | 4 (8.9%) | 14 (15.7%) | · |
| Any Infection 30 Days Prior to Transplant | 3 (12%) | 1 (3%) | 3 (6.7%) | 8 (9%) | 0.59 |
P-values obtained via ANOVA or chi-square test.
MDS: Myelodysplastic Syndrome.
CR: Complete Remission.
WBC: White blood cell.
ALC: Absolute lymphocyte count.
Other: Non-fluoroquinolone antibiotic prophylaxis.
Table 1b.
Allogeneic-specific baseline characteristics
| High (>75%) N=15 |
Medium (50-75%) N=18 |
Low (<50%) N=30 |
None (0%) N=55 |
P-value1 | |
|---|---|---|---|---|---|
| Stem Cell Source | 0.12 | ||||
| Peripheral Blood | 9 (60%) | 15 (83.3%) | 24 (80%) | 36 (65.5%) | · |
| Bone Marrow | 4 (26.7%) | 0 (0%) | 5 (16.7%) | 7 (12.7%) | · |
| Umbilical Cord Blood | 2 (13.3%) | 3 (16.7%) | 1 (3.3%) | 12 (21.8%) | · |
| HLA Matching/ Donor Type | 0.25 | ||||
| MRD2 | 5 (33.3%) | 6 (33.3%) | 16 (53.3%) | 14 (25.5%) | · |
| MUD3 | 8 (53.3%) | 8 (44.4%) | 10 (33.3%) | 24 (43.6%) | · |
| MMRD4 | 0 (0%) | 1 (5.6%) | 3 (10%) | 5 (9.1%) | · |
| Mismatched UCB5 | 2 (13.3%) | 3 (16.7%) | 1 (3.3%) | 12 (21.8%) | · |
| Myeloablative Conditioning | 14 (93.3%) | 17 (94.4%) | 28 (93.3%) | 53 (96.4%) | 0.92 |
| T-Cell Serotherapy6 | 3 (20%) | 3 (16.7%) | 15 (50%) | 18 (32.7%) | 0.07 |
| GVHD7 Prophylaxis | 0.72 | ||||
| CNI/MMF8 | 4 (26.7%) | 3 (16.7%) | 2 (6.7%) | 12 (21.8%) | · |
| CNI/MTX9 | 10 (66.7%) | 14 (77.8%) | 23 (76.7%) | 36 (65.5%) | · |
| PTCy10 | 1 (6.7%) | 1 (5.6%) | 3 (10%) | 5 (9.1%) | · |
| Other | 0 (0%) | 0 (0%) | 2 (6.7%) | 2 (3.6%) | · |
P-values obtained via chi-square test.
MUD: Matched Unrelated Donor.
MRD: Matched Related Donor.
MMRD: Mismatched Related Donor.
UCB: Umbilical Cord Blood.
T-Cell Serotherapy: Alemtuzumab or Anti-Thymocyte Globulin.
GVHD: Graft-Versus-Host Disease.
CNI/MMF: Calcineurin Inhibitor/ Mycophenolate mofetil.
CNI/MTX: Calcineurin Inhibitor/ Methotrexate.
PTCy: Post-transplant Cyclophosphamide.
Patients admitted the transplant unit demonstrate increasing chlorhexidine gluconate adherence over time
In order to assess CHG usage, we documented CHG adherence for each patient throughout their hospitalization on the HCT unit from January 2016 to December 2018 (Figure 1). CHG compliance gradually increased over time starting from January 2017 when CHG bathing began. By 2018, all patients were using CHG during their stay in the HCT unit with varying degrees of adherence. For example, in Quarter 4 of 2018, a total of 13 patients in the study were transplanted in the HCT unit: one patient received CHG for more than 50% of their admission whereas twelve received CHG baths during >75% of their days on the unit.
Figure 1. Chlorhexidine gluconate bathing adherence over time.
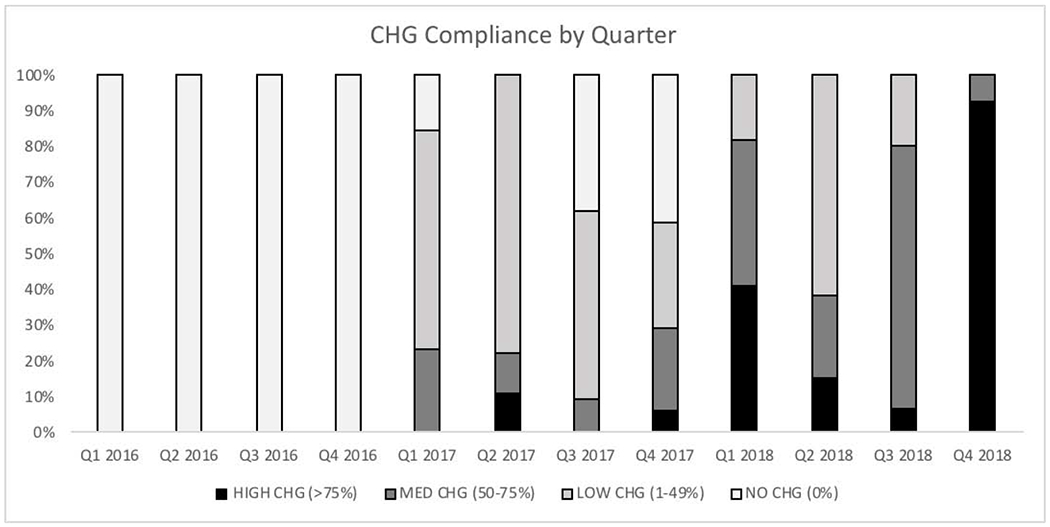
The percentage of patients belonging to each CHG group by quarter is shown here. The first quarter of each year starts in January. Patients were assigned to a quarter by their date of admission to the HCT unit. CHG compliance increased starting from the initiation of CHG bathing in January 2017.
Increased chlorhexidine gluconate adherence is associated with decreased incidence of BSI
In our study, we hypothesized that higher levels of CHG adherence would lead to decreased BSI incidence. Univariate analysis demonstrates that increased CHG usage was significantly associated with decreased incidence of clinically-significant BSI (p=0.003), LCBI (p=0.03), CLABSI (p=0.04), and MBI-LCBI (p=0.002) with the most significant reductions in BSI incidence seen in the High CHG usage group (Table 2). Only 2 (8%) patients with High CHG usage developed a clinically-significant BSI whereas this was seen in 27 (30.3%) patients that did not receive CHG. No High CHG usage patients developed an MBI-LCBI whereas 16 (18%) of those in the No CHG group developed an MBI-LCBI.
Table 2.
Incidence of hospital-acquired bloodstream infections by chlorhexidine gluconate usage
| High (>75%) N=25 |
Medium (50-75%) N=33 |
Low (<50%) N=45 |
None (0%) N=89 |
P-value1 | |
|---|---|---|---|---|---|
| Clinically-Significant BSI2 | |||||
| No. of Infections (%) | 2 (8%) | 5 (15.2%) | 7 (15.6%) | 27 (30.3%) | 0.003 |
| Incidence Rate (no./1000 patient-days) | 3.58 | 6.44 | 6.67 | 12.70 | |
| LCBI3 | |||||
| No. of Infections (%) | 1 (4%) | 5 (15.2%) | 6 (13.3%) | 20 (22.5%) | 0.03 |
| Incidence Rate (no./1000 patient-days) | 1.79 | 6.44 | 5.72 | 9.28 | |
| CLABSI4 | |||||
| No. of Infections (%) | 1 (4%) | 5 (15.2%) | 6 (13.3%) | 19 (21.3%) | 0.040 |
| Incidence Rate (no./1000 patient-days) | 1.79 | 6.44 | 5.72 | 9.30 | |
| MBI-LCBI5 | |||||
| No. of Infections (%) | 0 (0%) | 1 (3%) | 4 (8.9%) | 16 (18%) | 0.002 |
| Incidence Rate (no./1000 patient-days) | 0.00 | 1.29 | 3.81 | 7.81 | |
P-values obtained via the Cochran-Armitage linear trend test.
Clinically-Significant BSI: clinically-significant bloodstream infection.
LCBI: laboratory-confirmed bloodstream infection.
CLABSI: central line-associated bloodstream infection.
MBI-LCBI: mucosal barrier injury laboratory-confirmed bloodstream infection.
Cumulative incidence function curves showing BSI incidence relative to transplant day demonstrate that the risk of acquiring a clinically-significantly BSI was dose-dependent: BSI risk was lowest for patients in the High CHG group (Figure 2) and increased with decreasing CHG usage (p=0.02). High CHG usage was also associated with a lower risk of MBI-LCBI (p=0.03). Though a similar trend was seen for time to LCBI and CLABSI, these comparisons were not statistically significant. These results were replicated using BSI incidence relative to the day of hospitalization (Supplementary Figure S1). Of note, BSIs were observed prior to transplant in 2 patients in the NO CHG group (Figure 2). Although the existence of BSIs before Day 0 is unusual, these patients were already neutropenic from their disease or prior therapy. In order to determine whether the 2016 cohort differed from the 2017-2018 cohort, we evaluated only patients transplanted after January 2017, after CHG use was instituted, and observed similar results (Supplementary Figure S2). We also compared BSI risk among allogeneic and autologous HCT recipients and found that results were also replicated for allogeneic but not autologous transplantation (Supplementary Figures S3 and S4). Although, BSI risk did not differ between CHG usage groups for autologous HCT recipients, the High CHG group had the lowest rate of BSIs with significance likely limited by sample size.
Figure 2. Cumulative incidence of bloodstream infections based on days relative to transplant.
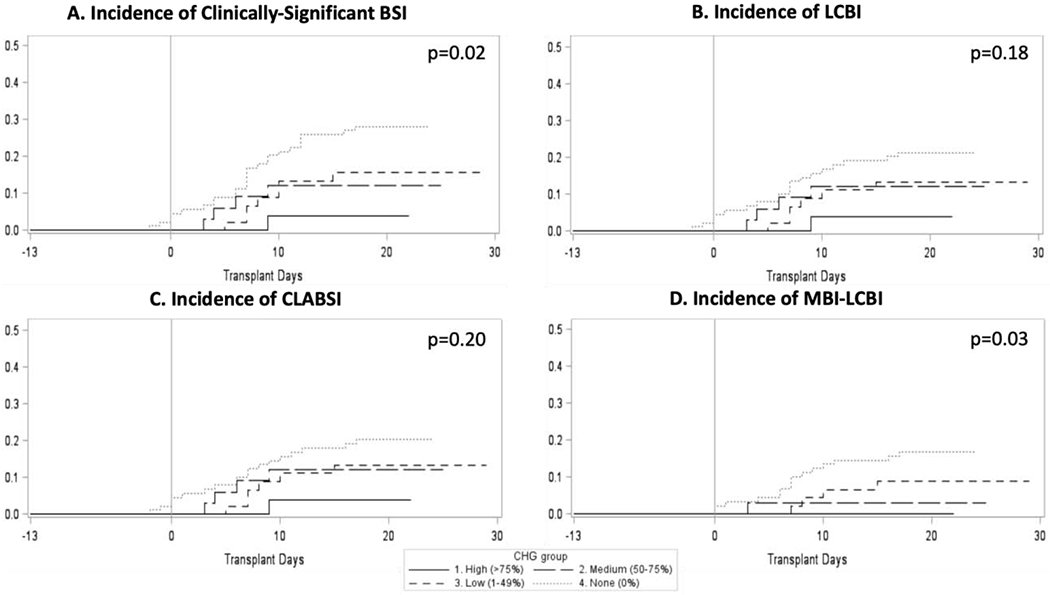
Cumulative incidence curves are plotted for clinically-significant BSIs (A, Gray’s Test p=0.02), LCBI (B, Gray’s Test p=0.18), CLABSI (C, Gray’s Test p=0.20), and MBI-LCBI (D, Gray’s Test p=0.03). Transplant day 0 corresponds to the day of stem cell infusion. Patients with greater CHG adherence experienced significantly reduced incidence of clinically significant BSI and MBI-LCBI.
We used multivariate analyses in order to compare time to BSI for each BSI category, accounting for the effects of CHG usage, antibiotic prophylaxis regimen (ciprofloxacin vs. levofloxacin vs. other), and type of transplant (allogeneic vs. autologous) on BSI incidence. We conducted separate analysis based on both time relative to transplant day and time relative to hospital day. For all BSI categories, antibiotic prophylaxis was not shown to significantly affect time to BSI (Table 3 and Supplementary Table S1). Relative to transplant day, CHG usage significantly affected time to clinically-significant BSI (p=0.023) and MBI-LCBI (p=0.007) but not for other BSI categories (Table 3). This finding was replicated when time to infection was based on hospital day (Table S1).
Table 3.
Multivariate analysis of time to bloodstream infection via a transplant day-based model
| Variable by Category of BSI | P-value1 |
|---|---|
| Clinically-Significant BSI | |
| CHG | 0.023 |
| Donor Type2 | 0.014 |
| Antibiotic Prophylaxis3 | 0.062 |
| LCBI | |
| CHG | 0.101 |
| Donor Type2 | 0.044 |
| Antibiotic Prophylaxis3 | 0.109 |
| CLABSI | |
| CHG | 0.131 |
| Donor Type2 | 0.058 |
| Antibiotic Prophylaxis3 | 0.096 |
| MBI-LCBI | |
| CHG | 0.007 |
| Donor Type2 | 0.011 |
| Antibiotic Prophylaxis3 | 0.059 |
P-values obtained via multivariate analysis using the Fine-Grey sub-distributional hazard regression model analyzing time to infection from transplant day −13. Discharge, transfer, and death are considered competing risks, and CHG is treated as a continuous variable.
Donor Type: Allogeneic vs. Autologous Transplant.
Antibiotic Prophylaxis Regimen: Ciprofloxacin vs. Levofloxacin vs. Other.
Gram-positive organisms were responsible for the majority of clinically-significant bloodstream infections in all CHG usage groups (Table 4). Viridans streptococci (9 out of 27 infections, 33.3%) and coagulase-negative staphylococci (8 out of 27 infections, 29.6%) were the causal organism in the majority of BSIs in the No CHG usage group. Only two other coagulase-negative staphylococci infections were identified, both for patients in the Low CHG group (2 out of 7 infections, 28.6%). No viridans streptococci infections were noted in any patients using CHG.
Table 4.
Pathogens found in clinically-significant bloodstream infections
| High (>75%) N=25 |
Medium (50-75%) N=33 |
Low (<50%) N=45 |
None (0%) N=89 |
|
|---|---|---|---|---|
| Total # BSI | 2 | 5 | 7 | 27 |
| Methicillin-Sensitive Staphylococcus aureus | 1 | 1 | 1 | |
| Methicillin-Resistant Staphylococcus aureus | 2 | |||
| Coagulase-negative Staphylococci | 2 | 8 | ||
| Viridans Streptococci | 9 | |||
| Vancomycin-Resistant Enterococcus | 2 | 3 | ||
| Escherichia | 1 | 1 | 1 | |
| Enterobacter | 2 | |||
| Candida | 2 | |||
| Polymicrobial | 1 | 1 | 1 | |
| Other | 1 | 1 |
Chlorhexidine gluconate use is safe and well-tolerated
In order to determine whether CHG was safe and well-tolerated, we first investigated the incidence of rash for each CHG usage group. No significant difference was found in the incidence of rashes requiring treatment between CHG usage groups (Table 5). While the High CHG group had a higher incidence of rash compared to the other groups (56% vs. 32.6-39.4% in the other groups) this was not statistically significant (p=0.20). In all patients, the most common identifiable cause for rash was noted to be a medication reaction unrelated to CHG use, and these rashes resolved after withholding the offending drug (Table 6). Importantly, the medical team treating the patients did not attribute any rash among the study patients to CHG usage.
Table 5.
Additional clinical outcomes by chlorhexidine gluconate usage
| High (>75%) N=25 |
Medium (50-75%) N=33 |
Low (<50%) N=45 |
None (0%) N=89 |
P-value1 | |
|---|---|---|---|---|---|
| Median Days to Engraftment (IQR) | 14.5 (12 - 19) | 13 (12 - 16) | 14 (12 - 18) | 13 (12 - 19) | 0.21 |
| Febrile Neutropenia, no. (%) | 16 (64%) | 26 (78.8%) | 31 (68.9%) | 64 (71.9%) | 0.64 |
| C. difficile Infection, no. (%) | 3 (12%) | 3 (9.1%) | 6 (13.3%) | 7 (7.9%) | 0.42 |
| Rash Requiring Treatment, no. (%) | 14 (56%) | 13 (39.4%) | 16 (35.6%) | 29 (32.6%) | 0.20 |
P-values obtained via the ordinal t-test for continuous variables or the Cochran-Armitage linear trend test for categorical variables.
Table 6.
Causes of rash in patients receiving chlorhexidine gluconate
| High (>75%) N=25 |
Medium (50-75%) N=33 |
Low (<50%) N=45 |
|
|---|---|---|---|
| Total No. Rash | 14 (56%) | 13 (39.4%) | 16 (35.6%) |
| Medication-Related | 4 | 3 | 2 |
| Engraftment Syndrome | 1 | 3 | 1 |
| Folliculitis | 2 | 2 | 3 |
| Dermatitis | 0 | 1 | 1 |
| GVHD1 | 0 | 1 | 1 |
| Infection | 0 | 1 | 1 |
| Other | 4 | 0 | 0 |
| Unexplained | 3 | 2 | 7 |
GVHD: Graft-versus-Host Disease
In addition to the incidence of rash, we examined the associations between CHG usage and other clinical outcomes including VRE colonization, time to stem cell engraftment, febrile neutropenia, and C. difficile infection (Table 5). Among patients who had negative VRE rectal swabs on admission, there was a significant trend toward lower incidence of new VRE colonization with increasing CHG usage (High 13.6%, Medium 3.1%, Low 19.1%, None 25.3%, p=0.02). No significant difference was found between CHG usage groups and median days to stem cell engraftment, incidence of febrile neutropenia, or C. difficile infection (Table 5).
Gut microbiota composition was similar between CHG usage groups
Given the association between CHG use and MBI-LCBI infections noted above, we investigated whether CHG use impacted gut microbial diversity and composition using 205 stool samples from 105 patients who had separately consented to our biorepository (N=37 stool samples in group 1, N=44 in group 2, N=55 in group 3, N=69 in group 4). Shannon diversity was measured for each sample and compared between CHG usage groups at each time point (Figure 3) using linear mixed models (Supplementary Tables S2 and S3). Although the High CHG group demonstrated higher diversity at days 15-30 compared to group 4 (p=0.032), no consistent differences in gut microbial diversity were observed between CHG usage groups (Figure 3 and Supplementary Tables S2 and S3).
Figure 3. Shannon diversity of the gut microbiome across chlorhexidine gluconate usage groups and stool collection time points.
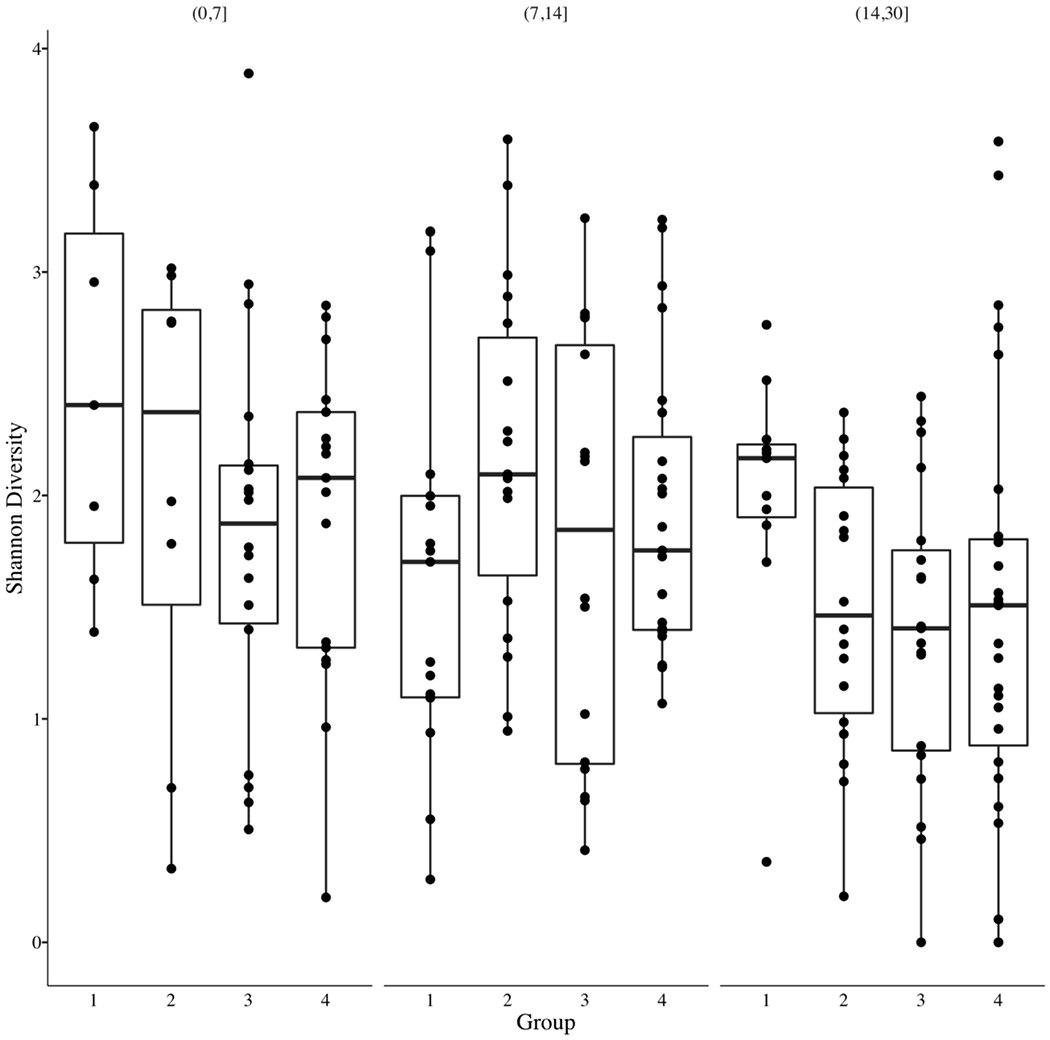
Stool samples were obtained for a subset of patients in the study (n=105 patients, 205 samples) and subjected to 16S rRNA gene sequencing. CHG usage groups are designated by number (High CHG=1, Medium CHG=2, Low CHG=3, No CHG=4). Group 1 (High CHG) diversity was higher at days 15-30 compared to Group 4 (p=0.032) although Shannon diversity was not consistently different between patients based on CHG usage.
In order to investigate whether CHG use impacted gut microbiome taxonomic composition, we performed Bray-Curtis non-metric multidimensional scaling (NMDS), dividing patients into two groups based on higher (High and Medium CHG) and lower (Low and No CHG) CHG use (Figure 4). An evaluation of the bacterial family in highest relative abundance within each sample (Figure 4) demonstrates a tendency for members of the Enterococcaceae family to dominate gut microbial composition in patients who used CHG less frequently, with domination defined by abundance relative to other taxa and not by absolute threshold. This finding is in line with new VRE rectal colonization and VRE bacteremia (Table 4) occurring more frequently in patients with decreased CHG use as noted above. In contrast, the Enterobacteriaceae family to which most gram-negative enteric pathogens belong appears to be rarely dominant relative to other taxa, irrespective of CHG use (Figure 4).
Figure 4. Bray-Curtis non-metric multidimensional scaling comparing community-level taxonomic composition between patients with higher and lower levels of chlorhexidine gluconate use.
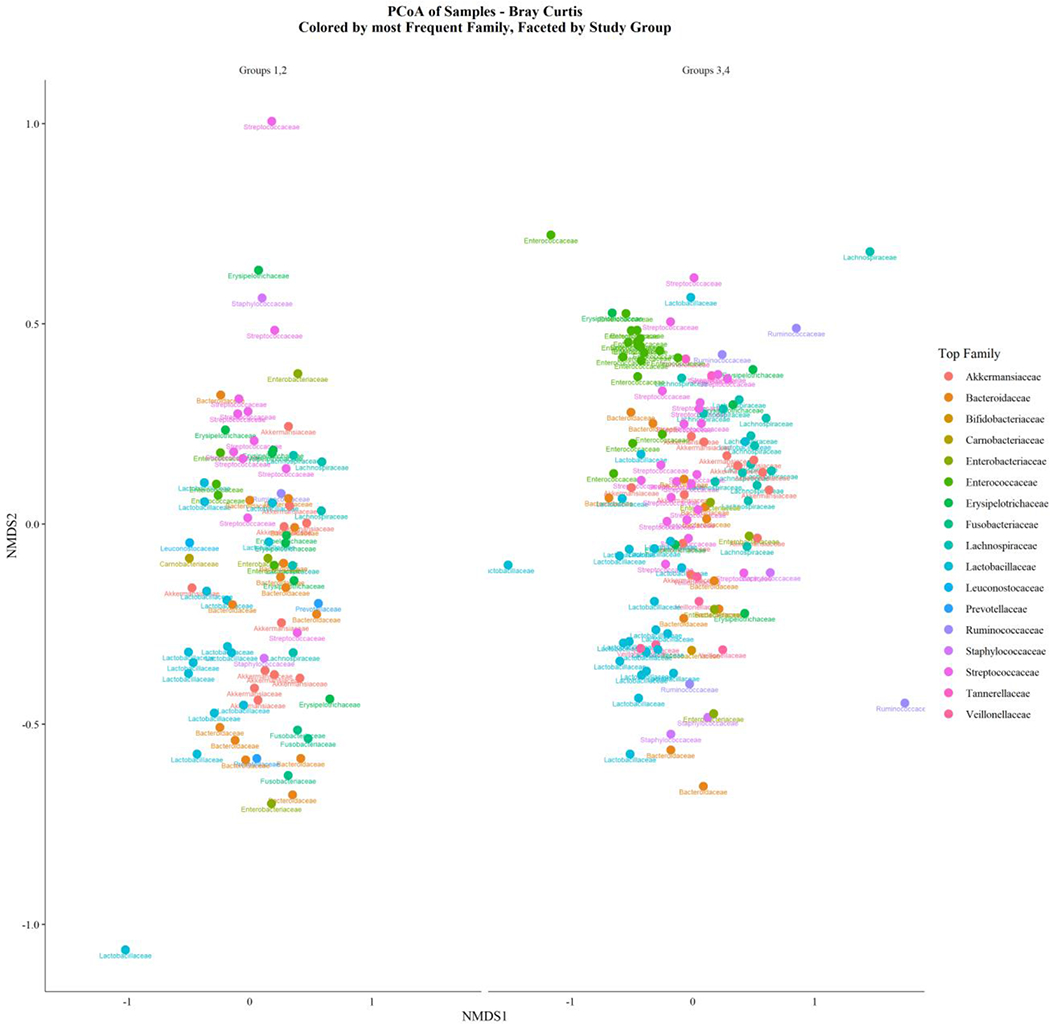
Bray-Curtis non-metric multidimensional scaling was performed to examine community level differences in taxonomic composition between patients grouped by CHG usage at the family level. Dots represent single stool samples and are colored based on the most abundant microbial family relative to other families. Groups 1 and 2 represent High and Medium CHG usage groups respectively, groups 3 and 4 refer to Low and No CHG usage groups. In patients with lower CHG use (groups 3 and 4, right), domination relative to other taxa was observed more frequently for members of the Enterococcaceae family (in green) compared to patients with higher CHG usage (groups 1 and 2, left). In contrast, no differences in the frequency of Enterobacteriaceae dominance were observed based on CHG usage.
Discussion
In our cohort of adults undergoing HCT, adherence to daily CHG bathing was associated with significantly lower incidence of clinically-significant bloodstream infection, LCBI, CLABSI, MBI-LCBI, and new VRE rectal colonization. Additionally, in those who underwent CHG bathing, there were no reports of rash requiring treatment attributable to CHG use and no association with C. difficile infection. Significant effects of CHG bathing were also seen when comparing cumulative incidence function curves examining time to BSI, specifically for clinically-significant BSI and MBI-LCBI. Multivariate analyses comparing time to BSI for each BSI category similarly demonstrated a significant effect of CHG on clinically-significant BSI and MBI-LCBI. The strongest impact of CHG on BSI incidence was seen with >75% adherence.
This reduction in bloodstream infection was driven by a decrease in coagulase-negative staphylococcus (CoNS) BSI and mucosal barrier injury-associated organisms. CHG bathing has been shown to reduce CoNS skin colonization, and this is thought to drive the decreased incidence of CoNS bacteremia12,19,30. Our results demonstrate a reduction in CoNS BSI from 30% of total BSIs in the No CHG usage cohort to 0% in the Medium and High CHG usage cohorts (Table 4).
We believe the surprising statistically significant reduction in MBI-LCBI observed with increased CHG use may be due in part to the assumption that MBI-LCBI organisms are always found in the gut, when in reality they may be originating from the environment and causing infection through the skin or nares in some hospitalized patients. Our study highlights the imperfect nature of the MBI classification system, e.g. CHG use could reduce general environmental exposure to Candida and other similarly classified organisms found in both the gut and the environment. In addition, topical CHG may have distant effects on the oral and gastrointestinal (GI) mucosa through a “gut-skin” axis, mediated by the immune system. A “gut-skin” axis mediated by immune and neural regulation is known to exist between the microbial communities of both regions, and disruption of the skin microbiota can affect local and systemic immune responses31–33. In our study, BSIs due to viridans streptococci, an oral and GI commensal bacteria, were only observed in patients that did not receive CHG. Viridans streptococci are known to translocate into the bloodstream in HCT patients with prior chemotherapy-induced GI mucosal damage34. The reduction of viridans streptococci infections and significant reduction of MBI-LCBI observed with CHG usage might suggest an interaction between the topical application of CHG and the dynamics of the immune system, GI mucosa, and gut microbiota. One may argue, however, that those patients who did not receive CHG baths were also those who received ciprofloxacin instead of levofloxacin in 2016, prior to the change in prophylaxis practices. While levofloxacin does have more streptococcal coverage than ciprofloxacin, this reduction in viridians streptococcal infection was observed in patients regardless of antibiotic prophylaxis regimen (data not shown).
Two published studies have analyzed CHG application in HCT patients. Mendes, et al. performed a single-institution pre-post study in Brazil and found a reduction of VRE colonization and VRE infection after the introduction of CHG bathing without a corresponding decrease in the rate of all-cause hospital-acquired infection35. Although the Mendes, et al. study did not find a reduction in BSI incidence, it spanned a nine-year period in which treatment algorithms, patient populations, and microorganism patterns might have changed. Furthermore, they did not account for varying levels of CHG adherence. Similarly, our study found that increased CHG usage led to a significant reduction in VRE rectal colonization and a trend towards lower rates of observed VRE BSIs (Table 4). We also observed differences in gut microbial composition between patients based on CHG use, with more frequent Enterococcaceae dominance found in those patients who used CHG less often. While CHG use was not associated with consistent changes in gut microbial diversity or dominance of gram-negative enteric pathogens in this study, it may serve to potentially decrease Enterococcaceae and VRE dominance in the gut. This finding will require further evaluation in larger prospective randomized studies however, given that stools were obtained from a smaller subset of patients in a retrospective manner.
Climo, et al. also examined CHG use in patients undergoing HCT12. The majority of those enrolled in the study were from a medical or surgical intensive care unit, however, and no subset analysis of HCT patients was presented. In both studies investigating CHG use in HCT recipients, any patient admitted to the HCT unit was enrolled. In contrast, our study was limited to only those who were admitted for conditioning and transplant, as this was the time in which patients are most vulnerable to infection. As a result, we have compelling evidence that patients admitted to the hospital for allogeneic or autologous stem cell transplantation benefit from daily application of CHG and that CHG use is safe and well-tolerated. Although we found a decreased incidence of BSI without an increase in rashes or C. difficile infection, these findings will need to be validated at other centers to confirm benefit.
Prior studies analyzing CHG compliance in the hospital suggest that the biggest barrier to daily CHG bathing adherence is a lack of knowledge by patients and providers regarding the effectiveness of CHG and that education and workflow improvements, such as standardized bathing procedures, checklists, and electronic medical record documentation increase CHG compliance36–40. This was observed in our HCT unit (Figure 1), as CHG bathing adherence increased over time as nurses educated fellow providers and patients about the effectiveness of CHG by sharing preliminary BSI data from the unit. We believe that similar measures to increase CHG use will allow other hospitals to effectively adopt CHG as a daily strategy for decreasing BSI incidence.
Our study is limited by the single center design and use of historical controls. Additionally, we did not determine whether CHG use increased the incidence of infections from multi-drug resistant organisms. We benefit, however, in being the first to investigate the impact of adherence on clinical outcomes, and to include only patients admitted for transplantation. Future analysis of the skin and gut microbiota during the peri-transplant period will help determine the effect of CHG on skin microbial composition and the potential interaction with the gut microbiome. These findings support the incorporation of daily CHG bathing into the care regimen for hospitalized adults undergoing HCT.
Supplementary Material
Highlights.
CHG baths are associated with decreased bloodstream infections in HCT recipients.
Benefits of CHG baths were most pronounced in patients with more than 75% usage.
CHG use was well-tolerated and not associated with an increase in adverse effects.
CHG use did not significantly alter gut microbiota diversity.
Acknowledgments
The authors are grateful for the assistance provided by the blood and marrow transplant inpatient staff at the Duke University Medical Center in Durham, NC.
Funding
This work was supported in part by the American Society of Hematology HONORS Award and the Duke University School of Medicine Eugene A. Stead Medical Student Research Fellowship awarded to author V.K.G. J.U.P. reports funding from NHLBI NIH Award K08HL143189, the MSKCC Cancer Center Core Grant NCI P30 CA008748. This research was supported by the Parker Institute for Cancer Immunotherapy at Memorial Sloan Kettering Cancer Center; J.U.P is a member of the Parker Institute for Cancer Immunotherapy. A.D.S reports funding from NCATS NIH Award KL2TR001115 and the American Society of Hematology Scholar Award.
Disclosure of Conflicts of Interest
Authors report no significant conflicts of interest. Jonathan U. Peled reports research funding, intellectual property fees, and travel reimbursement from Seres Therapeutics and consulting fees from DaVolterra. He has filed intellectual property applications related to the microbiome (reference numbers #62/843,849, #62/977,908, and #15/756,845). All authors have submitted the ICJME Form for Disclosure of Potential Conflicts of Interest.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Vinay K. Giri, Duke University School of Medicine, Durham, NC, USA, Stanford Department of Internal Medicine, Stanford, CA, USA.
Kristin G. Kegerreis, Division of Hematologic Malignancies and Cellular Therapy, Duke University Medical Center, Durham, NC, USA.
Yi Ren, Duke Cancer Institute Biostatistics Shared Resources, Duke University Medical Center, Durham, NC, USA.
Lauren M. Bohannon, Division of Hematologic Malignancies and Cellular Therapy, Duke University Medical Center, Durham, NC, USA.
Erica Lobaugh-Jin, Division of Infectious Diseases, Duke University Medical Center, Durham, NC, USA.
Julia A. Messina, Division of Infectious Diseases, Duke University Medical Center, Durham, NC, USA.
Anita Matthews, Division of Hematologic Malignancies and Cellular Therapy, Duke University Medical Center, Durham, NC, USA.
Yvonne M. Mowery, Department of Radiation Oncology, Duke University Medical Center, Durham, NC, USA.
Elizabeth Sito, Division of Hematologic Malignancies and Cellular Therapy, Duke University Medical Center, Durham, NC, USA.
Martha Lassiter, Division of Hematologic Malignancies and Cellular Therapy, Duke University Medical Center, Durham, NC, USA.
Jennifer L. Saullo, Division of Infectious Diseases, Duke University Medical Center, Durham, NC, USA.
Sin-Ho Jung, Department of Biostatistics and Bioinformatics, Duke University Medical Center, Durham, NC, USA.
Li Ma, Department of Statistical Science, Duke University, Durham, NC, USA.
Morris Greenberg, Department of Statistical Science, Duke University, Durham, NC, USA.
Tessa M. Andermann, Division of Infectious Diseases, University of North Carolina at Chapel Hill, Chapel Hill, NC, USA.
Marcel R.M. van den Brink, Department of Medicine, Weill Cornell Medical College & Department of Immunology, Adult Bone Marrow Transplantation Service, Memorial Sloan Kettering Cancer Center, New York, NY, USA.
Jonathan U. Peled, Adult Bone Marrow Transplantation Service, Department of Medicine, Memorial Sloan Kettering Cancer Center, Weill Cornell Medical College, New York, NY, USA.
Antonio L.C. Gomes, Department of Immunology, Sloan Kettering Institute, Memorial Sloan Kettering Cancer Center, New York, NY, USA.
Taewoong Choi, Division of Hematologic Malignancies and Cellular Therapy, Duke University Medical Center, Durham, NC, USA.
Cristina J. Gasparetto, Division of Hematologic Malignancies and Cellular Therapy, Duke University Medical Center, Durham, NC, USA.
Mitchell E. Horwitz, Division of Hematologic Malignancies and Cellular Therapy, Duke University Medical Center, Durham, NC, USA.
Gwynn D. Long, Division of Hematologic Malignancies and Cellular Therapy, Duke University Medical Center, Durham, NC, USA.
Richard D. Lopez, Division of Hematologic Malignancies and Cellular Therapy, Duke University Medical Center, Durham, NC, USA.
David A. Rizzieri, Division of Hematologic Malignancies and Cellular Therapy, Duke University Medical Center, Durham, NC, USA.
Stefanie Sarantopoulos, Division of Hematologic Malignancies and Cellular Therapy, Duke University Medical Center, Durham, NC, USA.
Nelson J. Chao, Division of Hematologic Malignancies and Cellular Therapy, Duke University Medical Center, Durham, NC, USA.
Deborah H. Allen, Duke University Health System Nursing, Durham, NC, USA.
Anthony D. Sung, Division of Hematologic Malignancies and Cellular Therapy, Duke University Medical Center, Durham, NC, USA.
References
- 1.D’Souza AFC. Current uses and outcomes of hematopoietic cell transplantation (HCT): CIBMTR Summary Slides. 2017. [Google Scholar]
- 2.Majhail NS, Farnia SH, Carpenter PA, et al. Indications for Autologous and Allogeneic Hematopoietic Cell Transplantation: Guidelines from the American Society for Blood and Marrow Transplantation. Biol Blood Marrow Transplant. 2015. ;21(11):1863–1869. doi: 10.1016/j.bbmt.2015.07.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Miceli MH, Churay T, Braun T, Kauffman CA, Couriel DR. Risk Factors and Outcomes of Invasive Fungal Infections in Allogeneic Hematopoietic Cell Transplant Recipients. Mycopathologia. 2017;182(5-6):495–504. doi: 10.1007/s11046-017-0115-y [DOI] [PubMed] [Google Scholar]
- 4.Slade M, Goldsmith S, Romee R, et al. Epidemiology of infections following haploidentical peripheral blood hematopoietic cell transplantation. Transpl Infect Dis. 2017;19(1):e12629. doi: 10.1111/tid.12629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yamagishi Y, Konuma T, Miwa Y, et al. Risk factors and survival impact of readmission after single-unit cord blood transplantation for adults. Int J Hematol. 2019;109(1):115–124. doi: 10.1007/s12185-018-2539-7 [DOI] [PubMed] [Google Scholar]
- 6.Liu C-Y, Lai Y-C, Huang L-J, et al. Impact of bloodstream infections on outcome and the influence of prophylactic oral antibiotic regimens in allogeneic hematopoietic SCT recipients. Bone Marrow Transplant. 2011;46(9):1231–1239. doi: 10.1038/bmt.2010.286 [DOI] [PubMed] [Google Scholar]
- 7.Perales M-A, Bonafede M, Cai Q, et al. Real-World Economic Burden Associated with Transplantation-Related Complications. Biol Blood Marrow Transplant. 2017;23(10):1788–1794. doi: 10.1016/j.bbmt.2017.06.017 [DOI] [PubMed] [Google Scholar]
- 8.Stuart J, Szydlo R, Apperley JF, Todd J, Salooja N. Mortality and Morbidity in 15-Year Survivors of Stem Cell Transplants for Haematological Malignancy. Blood. 2015;126(23):4348–4348. doi: 10.1182/blood.V126.23.4348.4348 [DOI] [Google Scholar]
- 9.Dandoy CE, Haslam D, Lane A, et al. Healthcare Burden, Risk Factors, and Outcomes of Mucosal Barrier Injury Laboratory-Confirmed Bloodstream Infections after Stem Cell Transplantation. Biol Blood Marrow Transplant J Am Soc Blood Marrow Transplant. 2016;22(9):1671–1677. doi: 10.1016/j.bbmt.2016.06.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hong J, Moon SM, Ahn HK, et al. Comparison of characteristics of bacterial bloodstream infection between adult patients with allogeneic and autologous hematopoietic stem cell transplantation. Biol Blood Marrow Transplant J Am Soc Blood Marrow Transplant. 2013;19(6):994–999. doi: 10.1016/j.bbmt.2013.03.019 [DOI] [PubMed] [Google Scholar]
- 11.Dandoy CE, Ardura MI, Papanicolaou GA, Auletta JJ. Bacterial bloodstream infections in the allogeneic hematopoietic cell transplant patient: new considerations for a persistent nemesis. Bone Marrow Transplant. 2017;52(8):1091–1106. doi: 10.1038/bmt.2017.14 [DOI] [PubMed] [Google Scholar]
- 12.Climo MW, Yokoe DS, Warren DK, et al. Effect of daily chlorhexidine bathing on hospital-acquired infection. N Engl J Med. 2013;368(6):533–542. doi: 10.1056/NEJMoa1113849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Climo MW, Sepkowitz KA, Zuccotti G, et al. The effect of daily bathing with chlorhexidine on the acquisition of methicillin-resistant Staphylococcus aureus, vancomycin-resistant Enterococcus, and healthcare-associated bloodstream infections: results of a quasi-experimental multicenter trial. Crit Care Med. 2009;37(6):1858–1865. doi: 10.1097/CCM.0b013e31819ffe6d [DOI] [PubMed] [Google Scholar]
- 14.Donskey CJ, Deshpande A. Effect of chlorhexidine bathing in preventing infections and reducing skin burden and environmental contamination: A review of the literature. Am J Infect Control. 2016;44(5 Suppl):e17–21. doi: 10.1016/j.ajic.2016.02.024 [DOI] [PubMed] [Google Scholar]
- 15.Kassakian SZ, Mermel LA, Jefferson JA, Parenteau SL, Machan JT. Impact of chlorhexidine bathing on hospital-acquired infections among general medical patients. Infect Control Hosp Epidemiol. 2011;32(3):238–243. doi: 10.1086/658334 [DOI] [PubMed] [Google Scholar]
- 16.Bass P, Karki S, Rhodes D, et al. Impact of chlorhexidine-impregnated washcloths on reducing incidence of vancomycin-resistant enterococci colonization in hematology-oncology patients. Am J Infect Control. 2013;41(4):345–348. doi: 10.1016/j.ajic.2012.04.324 [DOI] [PubMed] [Google Scholar]
- 17.Noto MJ, Domenico HJ, Byrne DW, et al. Chlorhexidine bathing and health care-associated infections: a randomized clinical trial. JAMA. 2015;313(4):369–378. doi: 10.1001/jama.2014.18400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Milstone AM, Elward A, Song X, et al. Daily chlorhexidine bathing to reduce bacteraemia in critically ill children: a multicentre, cluster-randomised, crossover trial. The Lancet. 2013;381(9872):1099–1106. doi: 10.1016/S0140-6736(12)61687-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bleasdale SC, Trick WE, Gonzalez IM, Lyles RD, Hayden MK, Weinstein RA. Effectiveness of chlorhexidine bathing to reduce catheter-associated bloodstream infections in medical intensive care unit patients. Arch Intern Med. 2007;167(19):2073–2079. doi: 10.1001/archinte.167.19.2073 [DOI] [PubMed] [Google Scholar]
- 20.Nerandzic MM, Donskey CJ. Induced sporicidal activity of chlorhexidine against Clostridium difficile spores under altered physical and chemical conditions. PloS One. 2015;10(4):e0123809. doi: 10.1371/journal.pone.0123809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kates AE, Zimbric ML, Mitchell K, Skarlupka J, Safdar N. The impact of chlorhexidine gluconate on the skin microbiota of children and adults: A pilot study. Am J Infect Control. 2019;47(8):1014–1016. doi: 10.1016/j.ajic.2019.01.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tien K-L, Sheng W-H, Shieh S-C, et al. Chlorhexidine Bathing to Prevent Central Line-Associated Bloodstream Infections in Hematology Units: A Prospective, Controlled Cohort Study. Clin Infect Dis Off Publ Infect Dis Soc Am. 2020;71(3):556–563. doi: 10.1093/cid/ciz874 [DOI] [PubMed] [Google Scholar]
- 23.Huang SS, Septimus E, Kleinman K, et al. Chlorhexidine versus routine bathing to prevent multidrug-resistant organisms and all-cause bloodstream infections in general medical and surgical units (ABATE Infection trial): a cluster-randomised trial. Lancet Lonci Engl. 2019;393(10177):1205–1215. doi: 10.1016/S0140-6736(18)32593-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Abdallah C Perioperative chlorhexidine allergy: Is it serious? J Anaesthesiol Clin Pharmacol. 2015;31(2):152–154. doi: 10.4103/0970-9185.155140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sviggum HP, Jacob AK, Arendt KW, Mauermann ML, Horlocker TT, Hebl JR. Neurologic complications after chlorhexidine antisepsis for spinal anesthesia. Reg Anesth Pain Med. 2012;37(2):139–144. doi: 10.1097/AAP.0b013e318244179a [DOI] [PubMed] [Google Scholar]
- 26.Dixon JM, Carver RL. Daily chlorohexidine gluconate bathing with impregnated cloths results in statistically significant reduction in central line-associated bloodstream infections. Am J Infect Control. 2010;38(10):817–821. doi: 10.1016/j.ajic.2010.06.005 [DOI] [PubMed] [Google Scholar]
- 27.CDC. CDC/NHSN Surveillance Definitions for Specific Types of Infections 2018.
- 28.Patel K, West H (Jack). Febrile Neutropenia. JAMA Oncol. 2017;3(12):1751. doi: 10.1001/jamaoncol.2017.1114 [DOI] [PubMed] [Google Scholar]
- 29.Peled JU, Gomes ALC, Devlin SM, et al. Microbiota as Predictor of Mortality in Allogeneic Hematopoietic-Cell Transplantation. N Engl J Med. 2020;382(9):822–834. doi: 10.1056/NEJMoa1900623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cassir N, Papazian L, Fournier P-E, Raoult D, La Scola B. Insights into bacterial colonization of intensive care patients’ skin: the effect of chlorhexidine daily bathing. Eur J Clin Microbiol Infect Dis Off Publ Eur Soc Clin Microbiol. 2015;34(5):999–1004. doi: 10.1007/s10096-015-2316-y [DOI] [PubMed] [Google Scholar]
- 31.Naik S, Bouladoux N, Wilhelm C, et al. Compartmentalized control of skin immunity by resident commensals. Science. 2012;337(6098):1115–1119. doi: 10.1126/science.1225152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Park YJ, Lee HK. The Role of Skin and Orogenital Microbiota in Protective Immunity and Chronic Immune-Mediated Inflammatory Disease. Front Immunol. 2017;8:1955. doi: 10.3389/fimmu.2017.01955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Salem I, Ramser A, Isham N, Ghannoum MA. The Gut Microbiome as a Major Regulator of the Gut-Skin Axis. Front Microbiol. 2018;9:1459. doi: 10.3389/fmicb.2018.01459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tunkel AR, Sepkowitz KA. Infections caused by viridans streptococci in patients with neutropenia. Clin Infect Dis Off Publ Infect Dis Soc Am. 2002;34(11):1524–1529. doi: 10.1086/340402 [DOI] [PubMed] [Google Scholar]
- 35.Mendes ET, Ranzani OT, Marchi AP, et al. Chlorhexidine bathing for the prevention of colonization and infection with multidrug-resistant microorganisms in a hematopoietic stem cell transplantation unit over a 9-year period: Impact on chlorhexidine susceptibility. Medicine (Baltimore). 2016;95(46):e5271. doi: 10.1097/MD.0000000000005271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Abbas S, Sastry S. Chlorhexidine: Patient Bathing and Infection Prevention. Curr Infect Dis Rep. 2016;18(8):25. doi: 10.1007/s11908-016-0532-y [DOI] [PubMed] [Google Scholar]
- 37.Caya T, Knobloch MJ, Musuuza J, Wilhelmson E, Safdar N. Patient perceptions of chlorhexidine bathing: A pilot study using the health belief model. Am J Infect Control. 2019;47(1):18–22. doi: 10.1016/j.ajic.2018.07.010 [DOI] [PubMed] [Google Scholar]
- 38.Kettelhut V, Van Schooneveld T, McClay J, Fruhling A, Dempsey K. The utility of electronic health record-based hygiene notes for chlorhexidine bathing practice evaluation. J Infect Prev. 2017; 18(2):72–77. doi: 10.1177/1757177416667288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Musuuza JS, Roberts TJ, Carayon P, Safdar N. Assessing the sustainability of daily chlorhexidine bathing in the intensive care unit of a Veteran’s Hospital by examining nurses’ perspectives and experiences. BMC Infect Dis. 2017;17(1):75. doi: 10.1186/s12879-017-2180-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vanhoozer G, Lovern Bs I, Masroor N, et al. Chlorhexidine gluconate bathing: Patient perceptions, practices, and barriers at a tertiary care center. Am J Infect Control. 2019;47(3):349–350. doi: 10.1016/j.ajic.2018.08.002 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


