Abstract
The most important question and concern in these circumstances of COVID-19 epidemic outspread is when will the pandemic end? Vaccination is the only solution to restore life to normalcy in the fastest and safest possible manner. Therefore, we have carried out a predictive analysis for realistic timescale estimates for overcoming the epidemic considering vaccination rate effect on the dynamics of COVID-19 control. In particular we discuss the worst affected large countries like India, Brazil and USA for estimating effect of vaccination rate in expediting the end of the COVID-19 epidemic. We analytically simulated the dynamic evolution of active cases of these countries in the last nine months using the modified SIR model and then included the effect of vaccination to forecast the proliferation dynamics. We hence obtained the transmission parameters, the variation in the reproduction numbers and the impact of the different values of the vaccination shots in the expected curves of active cases in the coming times to predicted the timescales of the end of the epidemic.
Keywords: COVID-19, SIR model, Vaccination, Numerical and analytical modeling of epidemic, Immunology, Reproduction number
1. Introduction
The state of emergency and the extent to which the novel corona virus has affected the mankind is unparalleled in human history (Tobin et al., n.d.). Like severe acute respiratory syndrome coronavirus (SARS-CoV) and Middle East respiratory syndrome coronavirus (MERS-CoV), a new noble-coronavirus outbreaks the respiratory disease in the current year 2020 (Perlman, 2020; Choi et al., 2018). This new coronavirus is named as “COVID-19” by the World Health Organization (WHO), and the International Committee on Taxonomy of Viruses called it Severe Acute Respiratory Syndrome coronavirus 2 (SARS-CoV-2) (Wang et al., 2020; Liang, 2020). The first patient died with pneumonia caused by the new coronavirus in Hubei province, China, in December 2019 (Wang et al., 2020; Lin et al., 2020), and it has progressively spread all over the world since then. By 30th November 2020, 61 million of people have been infected, in which 43.5 million have recovered (WorldOMeter, 2020). Though 1.4 million, 2.3% of total infected people have died from complications arising from COVID-19 related infection (WorldOMeter, 2020). Moreover, this disease has severely affected the world economy and plunged the world into a global recession (Ozili and Arun, 2020). The rapid outbreak of COVID-19 in the world happened due to interhuman transmission (Gabutti et al., 2020; Li et al., 2020) owing to the large Reproduction factor associated with this epidemic. As a preventive measure various control measures were proposed and implemented like social/physical distancing, frequent hand wash, sanitization, and using mask to inhibit the transmission further (Gabutti et al., 2020). Also, most of the countries has imposed lockdown to impart social distancing to control the transmission, like Italy, China, Spain, India, etc. (Zeynep, 2020; Giordano et al., 2020; Krishnakumar and Rana, 2020). Even though lockdown is not the remedial solution of the COVID-19 but still in order to ramp up the health facilities to cope up the upsurge of new infected cases it became an absolute necessity to implement extreme lockdowns despite the heavy loss in economy of the developed as well as the developing countries. There was a prompt response from scientists, doctors, health experts to look for the detection methods, treatment and prevention strategies to combat the disease effectively. Further, COVID-19 has motivated the researchers working in various fields to apply numerical, analytical, statistics analysis to study its transmission rate, reproduction number, and the effect of control measures on the transmission rates. Such studies are of immense help to equip and formulate COVID combat strategies by inhibition and control methods, helps to forecast how much time it would take to eradicate the disease. The transmission of COVID-19 has been studied by SIR (susceptible–infected–recovered) model which was first proposed and investigated by Kermack and McKendrick (Kermack and Mckendrick, 1927). The SIR model has only three compartments. One more compartment of asymptomatic or exposed population has been added in the modified SIR model which is named as SEIR model i.e. Susceptible, Exposed, Infectious, and Removed (SEIR). Huwen Wang et al. predicted the COVID cases in Wuhan, China by using SEIR model (Wang et al., 2020). Qianying Lin et al. studied the outbreak of COVID in China considering individual behavior and governmental actions using SEIR model (Lin et al., 2020). Kabir et al. presented an epidemic compartment model for covid-19 (Islam et al., 2020), and an evolutionary game theory modelling to study the dynamics of economic shutdown and shield immunity in the COVID (Kabir and Tanimoto, 2020). Z. Ceylan developed an Auto-Regressive Integrated Moving Average (ARIMA) models to predict the epidemiological trend of COVID-19 prevalence of Italy, Spain, and France (Zeynep, 2020). V.Volpert et al. proposed the quarantine model and studied the case of South Korea, China, and Italy (Volpert et al., 2020).
A dynamical analysis of infection spread when vaccination is introduced in the general population is also very important for Planning and Management at the local as well as global level. It is well agreed that herd immunity is not the ultimate solution for seeking respite from this menace, the only solution to restore life back to normalcy in the fastest and safest possible manner is through speedy administration of Vaccine through a massive immunological drive. Various modeling studies have been done to study the role of vaccination on the spread of infectious diseases. In 2011, T.K. Kara and A.Batabyal studied the existence of stability in the disease free and endemic equilibrium, and also discussed the vaccine induced reproduction number to investigate the consequences of providing vaccination (Kara and Batabyal, 2011). Farrington analyzed the impact of vaccination program on the transmission potential of the infection in large populations (Farrington, 2003). Shulgin et al. studied the pulse vaccination with simple SIR epidemic model and showed that it is possible to eradicate epidemic with pulse vaccination (Shulgin et al., 1998). D. Y. Logunov et al. developed a heterologous COVID-19 vaccine, and studied the safety and immunogenicity of two formulations (Logunov et al., 2020). Currently, 11 vaccine companies out of 48 are in the phase 3 trial stage on humans. Some companies are Moderna and Johnson & Johnson, Gamaleya Research Institute and Vector Institute, BioNTech, Bharat Biotech, Murdoch Children's Research Institute are from America, Russia, Germany, India and Australia respectively. Some Chinese companies like CanSino Biologics, Beijing Institute of Biological Products, Wuhan Institute of Biological Products, and Sinovac Biotec got approval for the limited use of the vaccine in China or UAE in the phase 3 stage. Thus, we hope by the starting of 2021, the effective vaccine of COVID-19 would be available in the market after the approval (The New York Times, 2020).
In case of an infectious disease there is a stage when the person becomes infected but is still not infectious. This time gap from catching infection to becoming infectious is known as the incubation period (Ma and Yingcun Xia, 2009). This phenomenon can be incorporated in SIR model by two approaches. First incorporating it as a delay effect known as delayed SIR model (Maleewong, 2020), or by introducing another compartment known as exposed class in the popular SEIR model (Ma and Yingcun Xia, 2009). It was shown that for low incubation time the dynamics of epidemic is almost similar in both cases (Maleewong, 2020). In this paper, we have investigated the impact of providing different values of vaccination rate to the progression of active cases of COVID-19. In this work the standard SIR model has been used instead of SEIR model as it is comparatively simple. For delayed SIR model and SEIR model, the incubation period gives rise slight shift and changes in the active and cumulative curves but the overall dynamics practically remain unchanged specially for epidemic like COVID -19. Since the incubation period of COVID-19 is 5-6 days (WHO) which is very small as compared to the time scale of epidemic which spans a year or so (Vilnius University Press, 2011). The SIR model has been modified to include the time dependent transmission coefficients mathematically; along with this a term for vaccinations also added to the modified SIR model coupled nonlinear equations. The two forms of transmission parameter (Chakravarty et al., n.d.) have been chosen based on the real time available data for COVID-19 progression from Feb-Nov 2020. In particular the major large populated countries like India, Brazil and America that are most severely affected are analyzed and studied in-depth. Also, the variation in the reproduction number in the last 9 months (Feb-Nov 2020) have been studied and discussed and further the effect of vaccination on reproduction number is outlined. In addition to this, the effect of different values of vaccination shots in the progression of active cases of COVID-19 in these countries has been delineated. Furthermore, we have predicted the possible timescales for the end of the epidemic for different values of vaccination rates. A nonlinear dynamics analysis of infection spread and control after the Vaccination drive is administered is of great interest for Planning and Management of various socio-economic activities like opening of schools, Malls, Industries, Travel for restoring life back to normalcy in various countries.
2. Theoretical model
SIR (susceptible–infected–recovered) epidemic model is a very popular and effective model to understand the dynamics of the spread of any epidemic disease (Chowell et al., 2016). The SIR model can be easily modified to include the vaccination term, if V s is the vaccination shots per day then the coupled set of equations of SIR model can be modified (Chowell et al., 2016) as follows-
| (1) |
| (2) |
| (3) |
here, the first equation is the susceptibility rate equation with S(t) is the susceptible population at time t, the second and the third equations describe the infection and recovery rate respectively, with I(t) and R p(t) are the infected and recovered population at time t. In these equations, β is the transmission rate, γ 0 is the recovery rate, and N T is the total population in any region. Here, we are assuming that the new births and deaths due to ageing, accidents, non-epidemic diseases, etc. are negligible. Then the total population N T is always constant, so we have
| (4) |
| (5) |
Eqs. (1), (2), (3) can be reduced to the normalized form by using s(t) = S(t)/N T, i(t) = I(t)/N T, r p(t) = R p(t)/N T, and v = V s/N T, and it can be written as
| (6) |
| (7) |
| (8) |
The transmission parameter, β is a dynamic parameter and dependent over the infected and recovered fraction of people at any time t. Thus, we have taken two different forms of β as discussed in paper (Chakravarty et al., n.d.), i.e.
| (9a) |
| (9b) |
here, the 9(a) form of β is suitably applied when the epidemic prevention is strict and inhibitive, and the 9(b) is applicable when the preventive measures are less restrictive that allows selective movements depending on the active infection reported in the region (Chakravarty et al., n.d.). The first term β 0 is the effective infection transmission (EIT) parameter of the epidemic, this term may change depending on the restriction imposed by the government and implementation of social distancing and the second term of β is the additional response term (RT) exhibited by the people on increase of cumulative (i(t) + r p(t)) and active cases i(t) following in response of the government restricted measures. The response term has two parameters, c and m in 9(a) and 9(b). c indicates the strength of infection inhibition or control, and the larger value implies better disease control. m represents the sluggishness or promptness of response, and a smaller value of m implies a broader peak around maximum infection and vice versa.
From Eq. (7), we obtain that the infection increases exponentially at the early epidemic growth phase if we assume that the susceptible population is equal to the total population of any city, region, or country (S(0) ≈ N T) at time t = t 0, then we have
| (10) |
Thus, from the above expression, we found that the number of secondary cases generated per primary case depends on the ratio of β 0 and γ 0, which is called a reproduction number R 0, and is given by (Chowell et al., 2016).
| (11) |
Thus, the secondary cases increase if R 0 is greater than 1 and it decays when it is less than 1. Hence the R 0 is the special parameter to study the transmission of infection in any area. But the number of susceptible people decreases with time due to the increase in infection, and the effective reproduction number over time R t, is given by the product of R 0 and the proportion of susceptible individuals in the population (Chowell et al., 2016).
| (12) |
| (13) |
The above expression shows that the effective reproduction number R t is a function of susceptible population, recovered population, infected population, effective transmission parameter, recovery rate, strength of infection inhibition or control and promptness of response parameter. When the strength of infection control measure is large then R t reduces. Thus, R t describes the dynamics of the transmission of infection in the population. If R t > 1, then the number of infections increases and when R t < 1, then it decays (Volpert et al., 2020). Also, the disease will not spread if R 0 is less than 1 (Kwang et al., 2013).
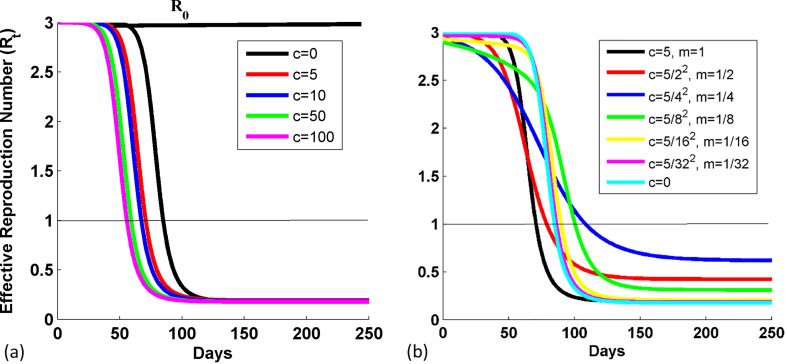
The variation in R t for different values of c is shown in Fig. 1(a) when m is 1, for the case when the transmission parameter is β(t) = β 0 − c(i(t) + r p(t))m. The black solid straight line at 3 shows the R 0 curve which remains constant due to its dependency over the effective infection transmission and recovery parameter. We noticed the vertical stretch in the R t curves for different c values (infection inhibition strength parameter), and it maintains the slope as m is same for all curves. R t varies from 2.991 to 2.844, 2.715, 2.04, and 1.614 for the respective c values 0, 5, 10, 50, and 100 at t = 50 days. R t decreases from 0.3323 to 0.1896 as c changes from 0 to 100 when t is 100. Thus, as the value of c i.e., the infection inhibition strength parameter increases, the value of response term increases, and hence R t decreases (see expression (13)). R t reaches first to 0.2 for the higher inhibition strength parameter. It means the epidemic eradicates faster for higher value of inhibition strength parameter. Fig. 1(b) has been plotted to show the variation in R t with days for different sets of response term, c and m. Initially, the steepness of the curve decreases with m (promptness of response parameter) and then increases as m reaches near to 0 value. Thus, the promptness of response decides the steepness or rate of decrease of R t curve.
Fig. 1.
Variation in the effective reproduction number with days, (a) for different values of c when m is 1, and (b) for different sets of response term, c and m, in the case when transmission parameter is β(t) = β0 − c(i(t) + rp(t))m. The values of c's are reduced by c/m2 for the respective m values. Other parameters are: NT=1 crore, γ0=0.1, β0=0.3, and i0 = 1/NT.
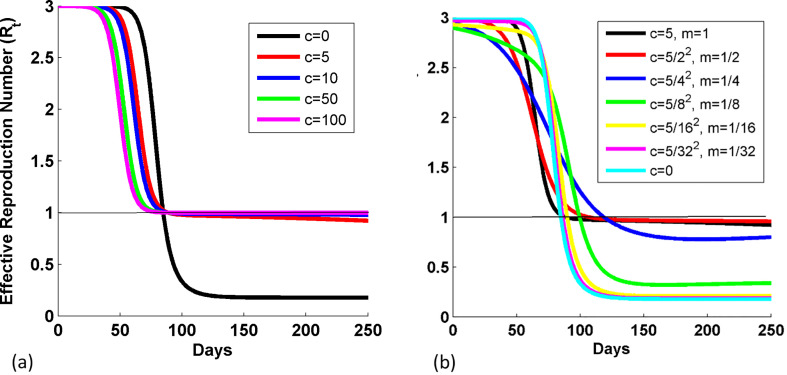
We also plotted the variation in R t for different values of c when m is 1, and for different sets of response term in Fig. 2(a) and (b), when the transmission parameter is β(t) = β 0 − ci(t)m. We see that the lower part of the curve shifts upward for nonzero values of c, when m is 1 (see Fig. 2(a)), and as m decreases, the lower part of the curve shifts downward (see Fig. 2(b)). It means that when infection inhibition strength parameter is zero, then R t reduces to 0.2, whereas it lies near to 1, when infection inhibition strength parameter is non zero. As the promptness of response decreases, the R t tends to shift downward. The epidemic eradicates faster for the case either when c is 0 or when m or c tend towards 0.
Fig. 2.
Variation in the effective reproduction number with days, (a) for different values of c when m is 1, and (b) for different sets of response term, c and m, in the case when transmission parameter is β(t) = β0 − ci(t)m. The values of c's are reduced by c/m2 for the respective m values. Other parameters are: NT=1 crore, γ0=0.1, β0=0.3, and i0 = 1/NT.
3. Results and discussion
In current scenario, the active cases of COVID-19 are highest in the countries like USA, India and Brazil. So, we have fitted and analytically simulated the active cases of these countries, and found the transmission parameters, and hence studied the variation in the reproduction numbers. This analysis is extremely crucial for understanding, government and societal response for disease control. The vaccination applied to a country will definitely gradually make a progressive control over the epidemic. However, the timescales to bring the situation under control or eradication of the epidemic completely will rely on the vaccination rate superimposed over the response parameters, b, c and m. Therefore, a quantitative estimate for a realistic determination of disease eradication requires estimation of the current values of b, c and m parameters from the available data of COVID-19 progression and then superimposing vaccination rates on the coupled nonlinear differential equations mentioned above.
3.1. The growth rate of COVID-19 in countries
3.1.1. Brazil
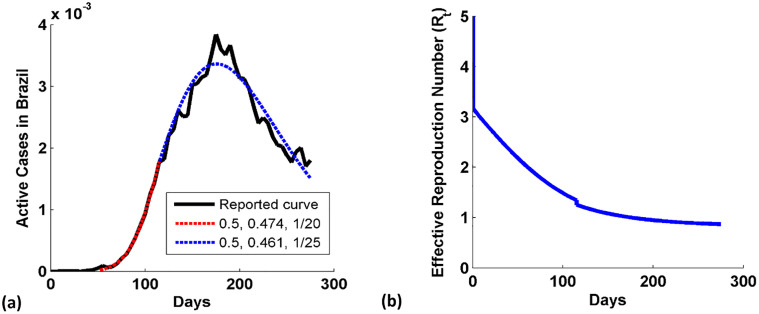
The evolution in active cases of COVID-19 in Brazil since Feb 15, 2020, is shown in Fig. 3(a) when only 0 cases were reported (WorldOMeter, 2020). It shows that the reported data (solid black curve) till Nov 17, 2020, is well simulated by the numerical model (dashed red and blue curves). Here, the form of infection transmission parameter which matched well with the reported data is β(t) = β 0 − c(i(t) + r p(t))m. The average value of effective infection transmission (EIT) parameter is taken for a period of 9 months for the curve, β 0 = 0.5. This infers that the government had taken strict actions to control the initial increase in cases and to restrain the transmission. In figure, we see that the fitting of active case curve is done with the two sets of transmission parameter. The second blue curve ((c, m) is (0.461, 1/25)) has lowered the maxima in comparison to the red curve ((c, m) is (0.471, 1/20)). It infers that both the government and people are able to control the growth of COVID-19.
Fig. 3.
(a) COVID-19 Active cases in Brazil since Feb 152,020. The reported data and model data show good coincidence. (b) Variation in the effective reproduction number (Rt) with days in Brazil.
To understand the transmission of COVID-19, it is necessary to study the dynamics of reproduction number with days, so we plotted R t in Fig. 3(b). Initially, R t is maximum and it is equal to 5 due to R 0 (β 0/γ 0) at 0th day. R t starts decreasing due to decrease in the number of susceptible populations as shown in Eq. (13). We see that the second set of transmission parameters (blue dashed curve of Fig. 3(a)) has shifted the R t curve down due to change in the strength of infection inhibiting parameter. The slope of the R t curve is reduced due to decrease in the promptness of response parameter from 1/20 to 1/25. It means that the people are actively responding to control the disease. Figure shows that the R t is reduced to 1 at nearly 176th day (9th August 2020) when the active case is maximum and thereafter it continues to reduce slowly. The R t is 0.8699 at 275th day of study. So, it is inferred that the epidemic in Brazil is under control as R t is less than 1.
3.1.2. USA
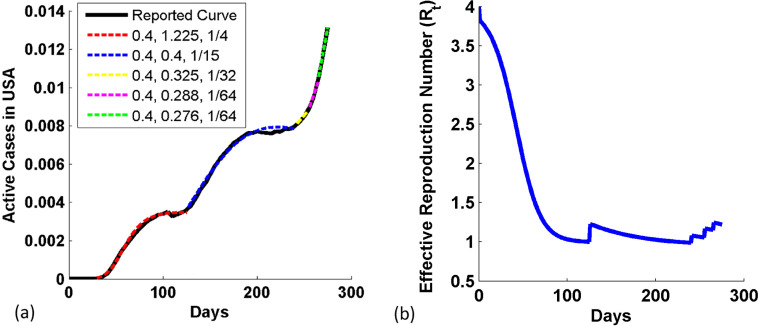
In USA, 12 cases of COVID-19 were reported on Feb 152,020. The Fig. 4(a) shows the progression in Active cases of COVID-19 in USA since Feb 152,020 (WorldOMeter, 2020). The active case first increases and get steady for small period after 95th day, i.e. from 20th May to 14th June, and thereafter again it started increasing at 120th day due to relaxation in restrictions imposed (stay at home) in different states. The second rise in COVID-19 also get steady after 190th day to 240th day and again rising again thereafter. The figure shows that the reported data is fitted by using 5 simulated curves with the form of the infection transmission parameter, β(t) = β 0 − ci(t)m implying the socio/economic activities are still allowed to function amidst strict restrictions and therefore a flattening of active cases is expected. This can be predicted that the USA government is attentive and has taken continuous steps in last 9 months to control the pandemic while easing out economic activities vigilantly. So, we took an average value of the effective infection transmission (EIT) parameter of the curve β 0 as 0.4, with the change in the response parameter value (c, m) from (1.225, 1/4) to (0.4, 1/15), (0.325, 1/32), (0.288, 1/64), and finally to (0.276, 1/64).
Fig. 4.
(a) COVID-19 Active cases in USA since Feb 152,020. The reported data and model data show good coincidence. (b) Variation in the effective reproduction number with days in USA.
Fig. 4(b) shows the variation in the effective reproduction number with days. Rt is equal to R 0 and it is maximum at t = 0 day. R t decreases at the faster rate as the value of the infection inhibition strength parameter is 1.225 and reduced to 1.001 at 125th days due to good governance by the American government. We observe a sharp hike in R t at 126th day due to a large drop in the infection inhibition strength parameter from 1.225 to 0.4, and also the slope is reduced as the promptness response parameter reduces from ¼ to 1/15. This change in R t is mainly due to the impact of releasing the restriction by the government. The figure shows that the R t is reduced to 1 at nearly 224th day (26th September 2020), thereafter it continues to reduce to 0.9878 till 240th day. Again R t becomes greater than 1 after 241st day. R t is 1.22 on 275th day of study. It infers that the epidemic in USA is rising. So, people have to become more attentive towards the COVID-19, so that they control the rise in active cases.
3.1.3. India
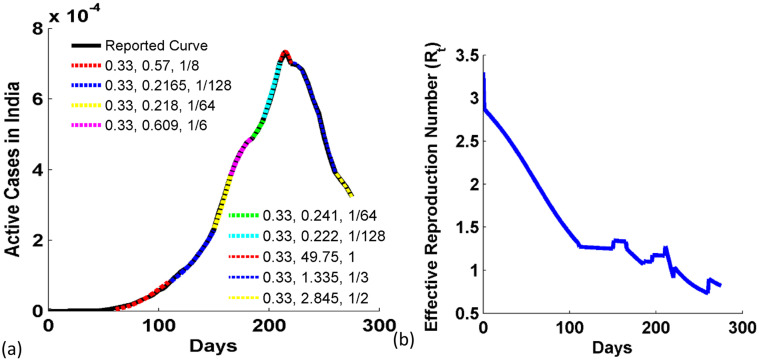
The Fig. 5(a) shows the progression in Active cases of COVID -19 in India since Feb 152,020, when 0 active cases were reported (WorldOMeter, 2020). It shows that the active cases increase slowly initially in India in comparison to the other two countries, Brazil and America. This is mainly due to the strict central lockdown imposed by the government, in which all services, educational institutes, transportation were closed. But we observe that the growth in the active cases does not follow any symmetric trend due to different phases of unlocking by the Indian government. So, the reported data is simulated with nine sets of transmission parameter taking an average value of effective infection transmission parameter as β 0= 0.33. The analytical form of the infection transmission parameter is β(t) = β 0 − c(i(t) + r p(t))m. The active case is maximum at the 215th day (i.e. 17th September 2020).
Fig. 5.
(a) COVID-19 Active cases in India since Feb 152,020. The reported data and model data show good coincidence. (b) Variation in the effective reproduction number with days in India.
In Fig. 5(b), we study the dynamic of reproduction number with days. It shows that R t is maximum at day 0 according to Eq. (13). R t reduces to 1.272 from 2.872 (day 1) in 111 days due to the promptness strength parameter as 1/8. It shows that the first phase of lockdown is very effective to control the transmission. But in the case of second transmission parameter set, the promptness strength parameter is reduced to 1/128, and thus the slope of the R t curve is reduced. Similarly, the slope of the next part is low due 1/64 value of promptness strength parameter. So, we see ups and downs or rise and fall due to different values of promptness strength parameter. It infers that R t does not follow the same trend of reduction due to different phases of lockdown. Also, the rise and fall in R t of India shows the sporadic, inconsistent and unpredictable responses. R t is reduced nearly to 1 at approx. 218th day (20th September 2020) and again it becomes greater than 1 at 221st day and again reduces back below to 1 near 223rd day. R t is 0.828 at 275th day of study. It shows that the epidemic is under control in India.
3.2. The impact of vaccination in active case of COVID-19
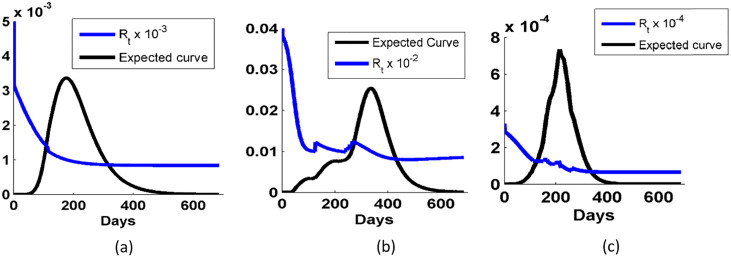
In the above section, we analytically simulated the reported data of COVID-19 of countries, Brazil, USA and India. We have seen that the effective reproduction number for Brazil and India is reduced to near to 1. But in the case of USA, the effective reproduction curve is increasing due to increase in the active cases. So, to have a better look over the transmission of COVID-19, we study the expected curves of active case and the effective reproduction number till the end of the next year 2021 in Fig. 6 . We found that the expected rate of decrease in the active case of India (Fig. 6(c)) is highest than that of USA (Fig. 6(b)) and Brazil (Fig. 6(a)) (denoted by black solidcurve). When we study the expected dynamic of reproduction number (blue solid curve), we found that after 275 days, R t continue to decrease slowly. It shows that the COVID-19 is now under control in these countries which are most affected. But in most of the countries like South Korea, Israel, the second spike of COVID-19 were reported after being controlled. Thus, vaccination is the only solution to completely eradicate the disease from the world.
Fig. 6.
Variation in the expected active cases and reproduction number of COVID-19 in counties: (a) Brazil, (b) USA, and (c) India. The blue solid curve corresponds to the expected active case and blue solid curve correspond to the reproduction number.
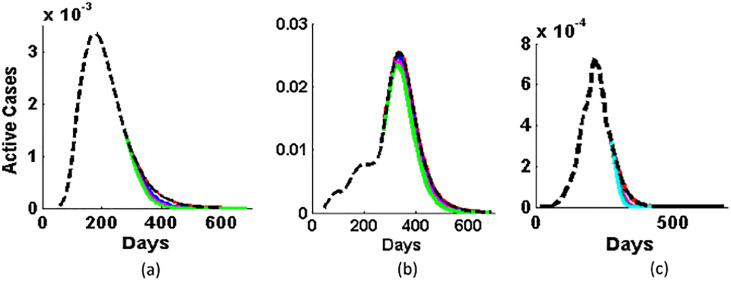
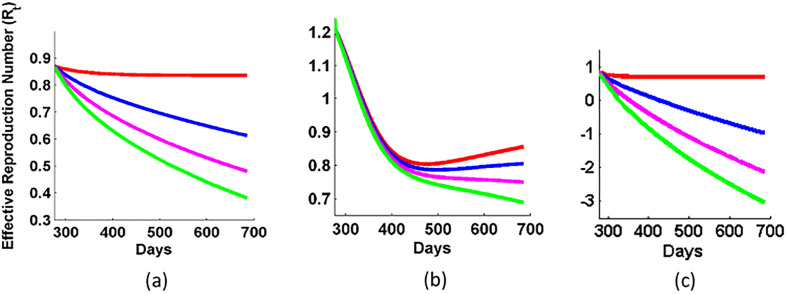
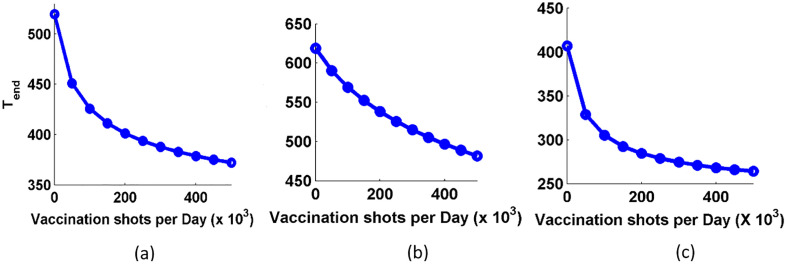
So, we study the impact of different values of vaccination shots in the expected curve of COVID-19. For this, we have used the Eqs. (6), (7), (8), and the vaccination shots (V s) given per day are 0, 0.5 lakh, 1 lakh, and 1.5 lakh and it is shown in Fig. 7 . When the V s is zero, the respective curve (red solid curve) just lies over the expected curve. The figure shows that as we increase the value of V s, the decreasing rate of active cases becomes faster in all the three cases. Also, we plotted the variation in the respective dynamics of reproduction number for different values of vaccination shots in Fig. 8 . We observe that the R t is lowered at faster rate for higher values of vaccination shots. Thus, the epidemic would be under control or eradicate faster if we increase the values of R t. In Fig. 9 , we study the approximated days (T end) for different values of vaccination shots when the active case is reduced to 1% of the maximum active cases of the respective countries. T end decreases with the increase in the vaccination shots. So, T end is inversely proportional to the value of the vaccination shots given per day. When we compare, the Fig. 9(a) and (c) of Brazil and India respectively, Brazil needs higher value of V s than India. When we see the curve of USA, we found that USA needs even higher value of V s to get control over COVID-19 due to its highest number of active cases and an approach of dynamic disease control with relaxation rather than inhibitive disease eradication approach.
Fig. 7.
Variation in the expected Active cases (black dashed curve) for different values of vaccination shots, 0 (solid red curve), 0.5 lakh (solid blue curve), 1 lakh (solid magenta curve), and 1.5 lakh (solid green curve) of countries: (a) Brazil, (b) USA, and (c) India.
Fig. 8.
Variation in the reproduction number for different values of vaccination shots for countries: (a) Brazil, (b) USA, and (c) India. The different values of vaccination shots are: 0 (solid red curve), 0.5 lakh (solid blue curve), 1 lakh (solid magenta curve), and 1.5 lakh (solid green curve).
Fig. 9.
Tend versus vaccination shots for countries: (a) Brazil, (b) USA, and (c) India.
4. Conclusions
In conclusion, we have predicted the possible timescales for the end of the epidemic for different values of vaccination rates. We have done the predictive analysis of vaccination for COVID-19 in India, Brazil and the USA. This analysis of infection spread and control after the Vaccination drive is administered is of great interest for Planning and Management of various socio-economic activities like opening of schools, Malls, Industries, Travel for restoring life back to normalcy in various countries.
-
1.
It is found that we require more than one sets of transmission parameter to fit the active cases of Brazil due to change in the response of the people while lockdown and on releasing the restrictions. While the active case of India is fitted by using nine sets of response term which shows the different phases of lockdown and unlock by the Indian government, and the active case of USA is fitted by using five sets of response term. We have taken an average of the effective infection transmission parameter for the last nine months.
-
2.
We have discussed the variation in the effective reproduction term (R t) with respect to the response term of the transmission parameter. We found that R t reduces at the faster rate for higher values of effective infection transmission parameter (c) whereas the rate decreases as the promptness response parameter decreases.
-
3.
It is found that COVID-19 is under the control in all the three countries as the expected effective reproduction term (R t) is less than 1.
-
4.
We found that the USA require very large number of vaccination shots to eradicate COVID-19 than India and Brazil due to its highest number of active cases as well as disease control regime with dynamic and lenient response.
-
5.
Even with an active vaccination administration it may take a year or so for overcoming COVID epidemic thus social distancing, hygiene practices and restrictions on educational institutions and travel may have to be continued in the year 2021 as well.
Declaration of competing interest
None.
References
- Chakravarty U., Chaturvedi D., Joshi M.P. A simple numerical and analytical analysis of COVID-19 progression, infection inhibition and control in various countries. medRxiv. 2020 doi: 10.1101/2020.08.11.20173203. preprint. [DOI] [Google Scholar]
- Choi S., Jung E., Choi B.Y., Hur Y.J., Ki M. High reproduction number of middle east respiratory syndrome coronavirus in nosocomial outbreaks: mathematical modelling in Saudi Arabia and South Korea. Journal of Hospital Infection. 2018;99(2):162–168. doi: 10.1016/j.jhin.2017.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chowell G., Sattenspiel L., Bansal S., Viboud C. Mathematical models to characterize early epidemic growth: a review. Phys Life Rev. 2016;18:66–97. doi: 10.1016/j.plrev.2016.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrington C.P. On vaccine efficacy and reproduction numbers. Math. Biosci. 2003;185:89–109. doi: 10.1016/s0025-5564(03)00061-0. [DOI] [PubMed] [Google Scholar]
- Gabutti G., d’Anchera E., Sandri F., Savio M., Stefanati A. Coronavirus: update related to the current outbreak of COVID-19. Infect. Dis. Ther. 2020;9:241–253. doi: 10.1007/s40121-020-00295-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giordano G., et al. Modelling the COVID-19 epidemic and implementation of population-wide interventions in Italy. Nat. Med. 2020;26:855–860. doi: 10.1038/s41591-020-0883-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Md. S. Islam, J. I. Ira, K. M. A. Kabir, and Md. Kamrujjaman, COVID-19 Epidemic Compartments Model and Bangladesh, doi: 10.20944/preprints202004. 0193.v1 (2020).
- Kabir K.M.A., Tanimoto J. Evolutionary game theory modelling to represent the behavioral dynamics of economic shutdowns and shield immunity in the COVID-19 pandemic. Royal Society Open Science. 2020;7:201095. doi: 10.1098/rsos.201095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kara T.K., Batabyal A. Stability analysis and optimal control of an SIR epidemic model with vaccination. BioSystems. 2011;104:127–135. doi: 10.1016/j.biosystems.2011.02.001. [DOI] [PubMed] [Google Scholar]
- Kermack W., Mckendrick A. Contribution to the mathematical theory of epidemics (part 1) Proc. R. Soc. Lond. Ser. A. 1927;115:700–721. [Google Scholar]
- Krishnakumar B., Rana S. COVID 19 in INDIA: Strategies to combat from combination threat of life and livelihood. Journal of Microbiology, Immunology and Infection. 2020;53(3):389–391. doi: 10.1016/j.jmii.2020.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwang K., Lin Z., Zhangb Q. An SIR epidemic model with free boundary. Nonlinear Analysis: Real World Applications. 2013;14(5):1992–2001. [Google Scholar]
- Li Y., Zhang R., Zhao J., Molina M.J. Understanding transmission and intervention for the COVID-19 pandemic in the United States. Sci. Total Environ. 2020;748:141560. doi: 10.1016/j.scitotenv.2020.141560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang K. Mathematical model of infection kinetics and its analysis for COVID-19, SARS and MERS. Infection, Genetics and Evolution. 2020;8:104306. doi: 10.1016/j.meegid.2020.104306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Q., et al. A conceptual model for the outbreak of coronavirus disease 2019 (COVID-19) in Wuhan, China with individual reaction and governmental action. Int. J. Infect. Dis. 2020;93:211–216. doi: 10.1016/j.ijid.2020.02.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logunov D.Y., et al. Safety and immunogenicity of an rAd26 and rAd5 vector-based heterologous prime-boost COVID-19 vaccine in two formulations: two open, non-randomized phase ½ studies from Russia. Lancet. 2020;396(887–97) doi: 10.1016/S0140-6736(20)31866-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Stefan, Yingcun Xia Mathematical Understanding of Infectious Disease Dynamics. 2009 [Google Scholar]
- Maleewong M. [DOI]
- Ozili P., Arun T. MPRA Paper 99317. University Library of Munich; Germany: 2020. Spillover of COVID-19: Impact on the Global Economy. [Google Scholar]
- Perlman S. Another decade, another coronavirus. N. Engl. J. Med. 2020;380(760–762) doi: 10.1056/NEJMe2001126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shulgin B., et al. Pulse vaccination strategy in the SIR epidemic model. Bull. Math. Biol. 1998;60:1123–1148. doi: 10.1006/S0092-8240(98)90005-2. [DOI] [PubMed] [Google Scholar]
- The New York Times. 2020. https://www.nytimes.com/interactive/2020/science/coronavirus-vaccine-tracker.html
- Tobin N.H., Campbell A.J.P., Zerr D.M., Melvin A.J. “Life-threatening viral diseases and their treatment”, chapter 95. Immunity and Infection. 2020;6:1324–1335. [Google Scholar]
- Volpert V., Banerjee M., Petrovskii S. On a quarantine model of coronavirus infection and data analysis. Math. Model. Nat. Phenom. 2020;15 [Google Scholar]
- Wang H., et al. Phase-adjusted estimation of the number of coronavirus disease 2019 cases in Wuhan, China. Cell Discov. 2020;6:10. doi: 10.1038/s41421-020-0148-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WorldOMeter 2020. https://www.worldometers.info/coronavirus/ URL.
- Zeynep C. Estimation of COVID-19 prevalence in Italy, Spain, and France. Sci. Total Environ. 2020;729:138817. doi: 10.1016/j.scitotenv.2020.138817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- https://www.who.int/emergencies/diseases/novel-coronavirus-2019/question-and-answers-hub/q-a-detail/coronavirus-disease-covid-19
- Vilnius University Press, Nonlinear Analysis: Modeling and Control, Vol. 16, No. 2, 181–190, (2011).