Abstract
Background
Myocardial bridge (MB) is the most common inborn coronary artery variant, in which a portion of myocardium overlies a major epicardial coronary artery segment. Myocardial bridge has been for long considered a benign condition, although it has been shown to cause effort-related ischaemia.
Case summary
We present the case of a 17-year-old female patient experiencing chest pain during physical activity. Since her symptoms became unbearable, electrocardiogram and echocardiography were performed together with a coronary computed tomography scan, revealing an MB on proximal-mid left anterior descending artery. In order to unequivocally unmask the ischaemic burden lent by MB, the patient underwent coronary angiography and physiological invasive test: instantaneous wave-free ratio (iFR) and fractional flow reserve (FFR) were calculated, both at baseline and after dobutamine infusion (5 µg/kg/min). At baseline, iFR value was borderline (= 0.89), whereas after dobutamine infusion and increase in the heart rate, the patient suffered chest pain. This symptom was associated with a decrease in the iFR value up to 0.77. Consistently, when FFR was performed, a value of 0.92 was observed at baseline, while after inotrope infusion the FFR reached the haemodynamic significance (= 0.79). Therefore, a medical treatment with bisoprolol was started.
Discussion
Our clinical case shows the importance of a comprehensive non-invasive and invasive assessment of MB in young patients experiencing chest pain, with significant limitation in the daily life. The coronary functional indexes allow to detect the presence of MB-derived ischaemia, thus guiding the decision to undertake a medical/surgical therapy.
Keywords: Case report, Chest pain, Myocardial bridge, Ischaemic heart disease, Functional intracoronary assessment
Learning points
Myocardial bridge (MB) is a rare but possible cause of ischaemia, especially in young patients without significant atherosclerotic burden. Therefore, it should be systematically ruled out in special population of individuals.
A multidimensional approach, including invasive physiological assessment of MB is crucial to evaluate the clinical relevance of this variant, thus guiding the most appropriate therapeutic pathway (medical/surgical).
An accurate diagnostic assessment and therapeutic optimization of MB might have a fundamental impact on symptoms and quality of life.
Primary Specialties involved other than cardiology
Sports Medicine, Cardiac Surgery, Radiology, Pneumology.
Introduction
Myocardial bridge (MB) is the most common inborn coronary artery variant in which a portion of myocardium overlies a major epicardial coronary artery segment (tunnelled artery). Myocardial bridge is a condition that in the majority of cases (70–98%) involves the left anterior descending artery (LAD).1 Myocardial bridges have been documented from 40% to 80% of autopsy reports and from 1.5% to 16% of invasive angiographic series.2 Thus, giving the wide range reported in the literature, the true prevalence of MB is uncertain, especially in special population of patients (i.e. athletes3). Myocardial bridge can be classified into profound (>2 mm) and superficial (≤2 mm) according to the depth of the tunnelled segment beneath the epicardial surface.4 The clinical relevance of an MB is heterogeneous, being usually an accidental finding in asymptomatic patients, while rarely is the cause of effort-related chest pain or ventricular arrhythmias. The correct diagnosis of an MB, followed by the clinical assessment and the risk stratification is particularly appropriate when dealing with professional and semi-professional athletes, exposed to maximum psychophysical stress during their performances.
We present a case of a young female patient with MB, who reported chest pain during training on exertion.
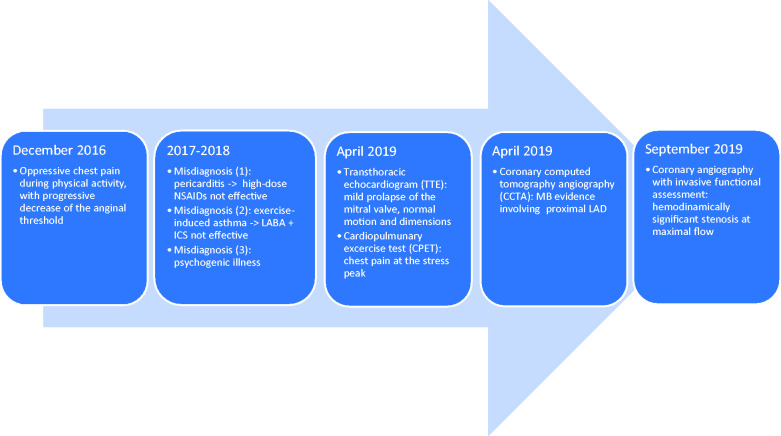
Timeline
Case presentation
A 17-year-old Caucasian female patient presented oppressive chest pain and dyspnoea during physical activity.
The onset of symptoms occurred in December 2016, when the patient started practicing competitive basketball in youth tournament, involving a substantial increase in workload. Since then, for a period of 24 months, the patient underwent several clinical and instrumental exams there were not considered conclusive.
Therefore, the patient was referred to another centre with a suspicion of pericarditis and subsequently exercise-induced asthma. Unfortunately, both treatment with high-dose non-steroidal anti-inflammatory drugs and therapy with long-acting beta-agonist and steroids did not induce any clinical and symptomatic improvement. Therefore, a psychogenic aetiology was also considered.
Progressively, the chest pain threshold decreased and pain appeared during low-intensity effort. So, in April 2019, she was referred to the Sports Medicine Unit at our institution.
The baseline electrocardiogram (ECG) documented sinus bradycardia (56 b.p.m.) without atrioventricular conduction irregularity nor repolarization abnormalities (Figure 1).
Figure 1.
Baseline electrocardiogram.
A 24-hour ECG monitoring evidenced uncommon sinus tachycardia phases, two supraventricular extrasystoles, one ventricular extrasystole but no anomaly of ST-segment, despite the patient experienced chest pain during physical activity (Figure 2).
Figure 2.
Twenty-four-hour electrocardiogram monitoring.
A transthoracic echocardiogram showed normal systolic and diastolic function, normal origins of coronary arteries, normal size of atrial and ventricular chambers, with mild prolapse of the anterior leaflet of the mitral valve (Figure 3A–C).
Figure 3.
Transthoracic echocardiogram: normal origins of coronary arteries (A), normal size of atrial and ventricular chambers (B), normal systolic and diastolic functions (C).
During cardiopulmonary exercise test, chest pain and dyspnoea arose at 60 W load [Heart rate (HR 123 b.p.m.)], forcing her to stop at 120 W work load (HR 171 b.p.m.). Concurrently, the oxygen consumption was blunted as peak Vo2 amounted to 27.1 mL pro kg (82% of theoretical), thus representing an impairment in aerobic exercise capacity. Mild repolarization abnormalities to ECG were recorded during this phase (Figure 4A–C).
Figure 4.
Cardiopulmonary exercise test: baseline electrocardiogram recording (A), peak event electrocardiogram recording (B) and VO2 trend (C).
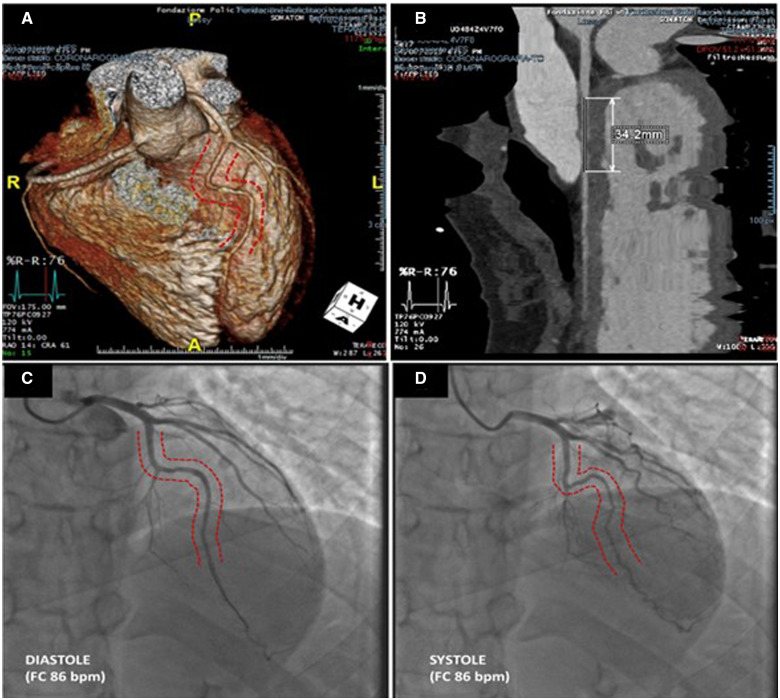
Considering the mismatch between symptoms and non-invasive diagnostic findings, a coronary computed tomography angiography (CCTA) was performed and revealed a segmental (34 mm) intra-septal course anomaly of the proximal-mid portion of LAD, mainly arranged along the epicardial portion of interventricular septum, with maximum depth of 17 mm (Figure 5).
Figure 5.
Coronary computed tomography scan showing the tunnelled arterial segment (A and B), compared with coronary angiography documenting the milking effect (C and D).
Therefore, in order to verify whether the computed tomography scan findings could be responsible for symptoms and exercise limitation, she was hospitalized for the invasive functional assessment of the MB.
The coronary angiography confirmed the MB on LAD. As such, the patient underwent functional intracoronary evaluation through pressure guidewire, with instantaneous wave-free ratio (iFR) assessment both at baseline and after dobutamine infusion (5 µg/kg/min). Fractional flow reverse (FFR) at rest and after dobutamine and atropine intravenous infusion was also recorded.
At baseline (HR 86 b.p.m.), iFR value was 0.89 (Figure 6A) (normal range: iFR ≥0.90), whereas after 5 min of dobutamine infusion it was 0.82 (HR 111 b.p.m.) and the patient experienced chest pain (Figure 6B). After 9 min, iFR values reached 0.77 (HR 140 b.p.m.) with persistence of angor (Figure 6C). Thus, the functional assessment at maximal flow and higher HR was indicative of haemodynamic significance of MB.
Figure 6.
Functional intracoronary evaluation: At baseline, instantaneous wave-free ratio value was 0.89 (A), reaching 0.82 after 5 min of dobutamine infusion (B) and 0.77 after 9 min at hyperaemic flow (C). Similarly, fractional flow reserve after dobutamine and atropine infusion acquired haemodynamic significance (= 0.79) (D). At the end, an instantaneous wave-free ratio pullback was performed and instantaneous wave-free ratio value was 0.53 (E).
Similarly, FFR at rest was 0.92 (normal range: FFR > 0.80), while after dobutamine and atropine infusion it was 0.79 with patient reporting angor (Figure 6D). Moreover, the iFR pullback demonstrated a remarkable drop upstream the MB, so that iFR value was 0.53 (Figure 6E).
In conclusion, the patient was discharged from the hospital in good general conditions and a choice was made to proceed with a medical treatment strategy with low-dose β-blockers (i.e. bisoprolol 1.25 mg qd). The compliance to therapy was optimal and the patient did not suffer from any side effect. To date, at 12 months of follow-up visit, the patient reports a partial clinical benefit, as the increase in anginal threshold allows her to carry out daily activities, though being forced to detraining.
Recently, an accurate cardiosurgical assessment was carried out to evaluate the feasibility of surgical unroofing of the MB. The severity of MB phenotype, in terms of length and depth, implies a complex unroofing procedure. However, it is reasonable to assume that surgical treatment is the only option that may allow our patient to grab the basketball again.
Discussion
Myocardial bridge has been considered for long a benign condition, mostly found incidentally in asymptomatic patients, but nowadays its clinical relevance is still matter of debate. The milking effect, consisting in the compression of the tunnelled artery, occurs only in systole, when the myocardial perfusion is limited (15%). Nevertheless, patients who have MB as their only cardiac abnormality may present symptomatic or silent myocardial ischaemia, along with acute coronary syndrome5 due to coronary spasm,6,7 thrombosis,8 and coronary dissection,9 which are all sporadic complications of the MB.
The fundamental moment of coronary perfusion is diastole. Therefore, exercise-induced ischaemia in the presence of an MB cannot be explained only by the narrowing of the vessel during systole. Both quantitative coronary angiography and intracoronary Doppler flow studies proved that vessel compression extends into diastole, thus affecting coronary perfusion. This extension was correlated with a localized phasic coronary artery spasm persisting during diastole, since relaxing time of vascular smooth muscle oversteps the duration of diastole. Additionally, the sympathetic drive during exercise leads to a delayed relaxation that further impairs early diastolic flow. Moreover, ischaemia can also be mediated by the so-called ‘branch steal’ mechanism, induced by a ‘Venturi’ effect triggered by vessel compression, particularly in presence of side branches originating within the MB.10,11
When estimating how threatening is an MB, many intrinsic and extrinsic characteristics of the MB have to be taken into consideration. Among the intrinsic features, the clinical relevance of MB is proportional to the depth and the length of the tunnelled arterial segment, together with vessel tortuosity and the presence of side branches; extrinsic risk factors may also unmask ischaemia in patients with MB, such as tachycardia, atherosclerotic plaque, left ventricle hypertrophy, and susceptibility to coronary spasm. As for tachycardia, increasing myocardial contractility over the involved segment of LAD reduces diastolic flow. At last, systolic kinking of the coronary artery may cause intimal damage to the endothelium, especially at high heart rates. This event could produce platelet aggregation and vasospasm, resulting in an acute coronary syndrome.12 Furthermore, the unfortunate eventuality of an atherosclerotic plaque located proximally to the initial part of the MB may induce complications, such as thrombosis, vasospasm, and coronary dissection.6,8,9
The detection of MB is accomplished by means of different diagnostic modalities (both invasive and non-invasive) because of the lack of a well-defined gold standard methodology: to date, physiological invasive assessment is considered superior to the anatomical assessment to evaluate the ischaemic threshold of MB.11 In a prospective study by Tarantini et al.,13 20 patients have been subjected to functional intracoronary evaluation with FFR and iFR at rest and after dobutamine infusion. This study revealed that the use of iFR is more frequently associated with MB-related myocardial ischaemia than FFR. The superiority of diastolic indices (iFR) over full cycle indices (FFR) in the evaluation of ischaemic burden reflects the aforementioned decisive involvement of diastole in the pathophysiology of MB.
In a retrospective study including 120 patients with MB on LAD and 41 controls, Zhou et al.14 have shown that the assessment of FFR derived from CCTA might be a helpful tool to unveil any MB-related ischaemia. In this cohort, MB patients (both those with deep and superficial MB)3 exhibited lower FFR values than controls and abnormal FFR values were associated with angina in the MB cohort.
Normally, asymptomatic patients receive no therapy, even if antiplatelet therapy could be considered given the abnormal rheology, predisposing to plaque development and progression. On the other hand, in symptomatic patients, β-blockers are considered as first-line therapy,15 while calcium-channel blockers may be added to reduce vasospasm, thus preventing hypoperfusion. Alternatively, percutaneous coronary intervention (PCI), myotomy, or coronary artery bypass grafting (CABG) can be considered for those individuals who do not respond to medical treatment. Stent implantation in symptomatic patients with MB can mitigate the peak intracoronary systolic pressure and the vessel compression, normalizing the flow and abolishing symptoms. Yet, this approach is limited to those patients who are refractory to optimal medical treatment and who are not suitable for surgery, since PCI has been associated with higher rate of adverse events related to stent fracture and in-stent restenosis.16 With regard to CABG, it is indicated for patients with extensive (>25 mm) or deep (>5 mm) myocardial bridging or when the tunnelled coronary segment is unlikely to be completely decompressed in diastole.17 Furthermore, the supra-arterial myotomy can be considered a valid alternative in a group of symptomatic patients.18
The guidelines on treatment of athletes are scarce. Up-to-date American guidelines propose that asymptomatic athletes with MB and no evidence of ischaemia during maximal effort stress testing may take part on all competitive sports.19 The Italian guidelines for sports admissibility suggest restricting physical activity for those cases with MB longer than 10 mm and deeper than 3 mm, or with evidence of myocardial ischaemia. In these individuals, a treatment with β-blockers or calcium-channel blockers is recommended, even though it may significantly affect the everyday life. Finally, the 2020 ESC guidelines on sports cardiology and exercise in patients with cardiovascular disease recommend in patients with MB and evidence of ischaemia to avoid participation in competitive sports and to be advised regarding leisure-time activities.20 Therefore, it is crucial to document the presence of ischaemia during the stress test and rely on a multidimensional judgement to guide the optimal treatment plan.
Our case report emphasizes the importance of this integrated approach in which invasive diagnostic tools and surgical therapeutic options could be considered in symptomatic patients, limited in sports and leisure activities, with evidence of exercise-induced ischaemia. Yet, a shared decision-making is critical, involving different specialists and requiring a well-informed discussion with the patients and the caregivers. In accordance with the current recommendations for athletes, given the risk augmentation for adverse events in symptomatic ischaemic athlete with MB, detraining was prescribed for our patient, allowing only mild, non-agonistic exercise. Further guidelines on treatment strategy are lacking and eagerly awaited. Nevertheless, the choice to return to full participation in competitive sports, passing through a cardiac surgical procedure, could be considered in selected cases: a careful evaluation considering technical aspects, clinical presentation, and patient expectations should be carried out, balancing the impact on one hand of the surgical procedure and on the other hand of the cessation of the agonistic sport activities on the physical, mental, and social well-being of the athlete.
In our case, the patient was not a professional athlete, but the severity of MB-related ischaemia, unequivocally demonstrated by invasive physiological assessment by means of iFR/FFR, was affecting her daily activities. The young age, the clinical presentation, the former exclusion of non-cardiac diagnosis induced the clinical team to start medical therapy, excluding the surgical option, initially. The primary goal of multidisciplinary follow-up was to improve her quality of life by restoring the ability to face the routine activities and this mission was accomplished by low-dose of β-blockers (i.e. bisoprolol).
A possible limitation of the characterization of the case could be regarded the lack of information on the microvascular function. However, the ESC guidelines20 do not recommend any systematic assessment of the microvascular function in patients with MB. Even considering this population of patients similar to INOCA patients, the same ESC guidelines acknowledge the role of PET and RM stress test in the evaluation of the microvascular compartment, but do not offer any specific recommendation regarding the relevance of these diagnostic exams in terms of risk stratification and therapeutic implications in patients with MB.
Myocardial bridge can be disabling as it worsens the quality of life, especially for young patients. An early detection of this congenital condition is crucial, and an invasive functional assessment of the ischaemic burden should be considered to evaluate the need for medical or surgical therapy.
Lead author biography

Michela Cammarano is a Sport Medicine Physician, focusing on the evaluation and treatment of professional, competitive, and non-competitive athletes with heart diseases.
Supplementary material
Supplementary material is available at European Heart Journal - Case Reports online.
Supplementary Material
Acknowledgements
We are deeply thankful to the Radiology Department of Fondazione Policlinico Universitario Agostino Gemelli IRCCS (Rome, Italy) for their contribution to this case.
Slide sets: A fully edited slide set detailing this case and suitable for local presentation is available online as Supplementary data.
Consent: The authors confirm that written consent for submission and publication of this case report including images and associated text has been obtained from the patient in line with COPE guidance.
Conflict of interest: none declared.
Funding: none declared.
References
- 1. Ciçek D, Kalay N, Müderrisoğlu H.. Incidence, clinical characteristics, and 4-year follow-up of patients with isolated myocardial bridge: a retrospective, single-center, epidemiologic, coronary arteriographic follow-up study in southern Turkey. Cardiovasc Revasc Med 2011;12:25–28. [DOI] [PubMed] [Google Scholar]
- 2. Corban MT, Hung OY, Eshtehardi P, Rasoul-Arzrumly E, McDaniel M, Mekonnen G. et al. Myocardial bridging: contemporary understanding of pathophysiology with implications for diagnostic and therapeutic strategies. J Am Coll Cardiol 2014;63:2346–2355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Pelliccia A, Adami PE, Quattrini F, Squeo MR, Caselli S, Verdile L. et al. Are Olympic athletes free from cardiovascular disease? Systematic investigation in 2352 participants from Athens 2004 to Sochi 2014. Br J Sports Med 2017;51:238–243. [DOI] [PubMed] [Google Scholar]
- 4. Forsdahl SH, Rogers IS, Schnittger I, Tanaka S, Kimura T, Pargaonkar VS. et al. Myocardial bridges on coronary computed tomography angiography-correlation with intravascular ultrasound and fractional flow reserve. Circ J 2017;81:1894–1900. [DOI] [PubMed] [Google Scholar]
- 5. Wang Y, Lv B, Chen J, Zhang Y, Luo F, Lu N. et al. Intramural coronary arterial course is associated with coronary arterial stenosis and prognosis of major cardiac events. Arterioscler Thromb Vasc Biol 2013;33:439–444. [DOI] [PubMed] [Google Scholar]
- 6. Kim JW, Park CG, Suh SY, Choi CU, Kim EJ, Rha SW. et al. Comparison of frequency of coronary spasm in Korean patients with versus without myocardial bridging. Am J Cardiol 2007;100:1083–1086. [DOI] [PubMed] [Google Scholar]
- 7. Porto I, D'Amario D, Paraggio L, Crea F.. Coronary stenosis as an innocent bystander in acute coronary syndrome. Circ J 2016;80:535–537. [DOI] [PubMed] [Google Scholar]
- 8. Ishikawa Y, Akasaka Y, Suzuki K, Fujiwara M, Ogawa T, Yamazaki K. et al. Anatomic properties of myocardial bridge predisposing to myocardial infarction. Circulation 2009;120:376–383. [DOI] [PubMed] [Google Scholar]
- 9. Wu S, Liu W, Zhou Y.. Spontaneous coronary artery dissection in the presence of myocardial bridge causing myocardial infarction: an insight into mechanism. Int J Cardiol 2016;206:77–78. [DOI] [PubMed] [Google Scholar]
- 10. Tarantini G, Migliore F, Cademartiri F, Fraccaro C, Iliceto S.. Left anterior descending artery myocardial bridging: a clinical approach. J Am Coll Cardiol 2016;68:2887–2899. [DOI] [PubMed] [Google Scholar]
- 11. Gould KL, Johnson NP.. Myocardial bridges: lessons in clinical coronary pathophysiology. JACC Cardiovasc Imaging 2015;8:705–709. [DOI] [PubMed] [Google Scholar]
- 12. Maseri A, Chierchia S.. Coronary artery spasm: demonstration, definition, diagnosis, and consequences. Prog Cardiovasc Dis 1982;25:169–192. [DOI] [PubMed] [Google Scholar]
- 13. Tarantini G, Barioli A, Nai Fovino L, Fraccaro C, Masiero G, Iliceto S. et al. Unmasking myocardial bridge-related ischemia by intracoronary functional evaluation. Circ Cardiovasc Interv 2018;11:e006247. [DOI] [PubMed] [Google Scholar]
- 14. Zhou F, Tang CX, Schoepf UJ, Tesche C, Bauer MJ, Jacobs BE. et al. Fractional flow reserve derived from CCTA may have a prognostic role in myocardial bridging. Eur Radiol 2019;29:3017–3026. [DOI] [PubMed] [Google Scholar]
- 15. Schwarz ER, Klues HG, Vom Dahl J, Klein I, Krebs W, Hanrath P.. Functional, angiographic and intracoronary Doppler flow characteristics in symptomatic patients with myocardial bridging: effect of short-term intravenous beta-blocker medication. J Am Coll Cardiol 1996;27:1637–1645. [DOI] [PubMed] [Google Scholar]
- 16. Tsujita K, Maehara A, Mintz GS, Doi H, Kubo T, Castellanos C. et al. Impact of myocardial bridge on clinical outcome after coronary stent placement. Am J Cardiol 2009;103:1344–1348. [DOI] [PubMed] [Google Scholar]
- 17. Attaran S, Moscarelli M, Athanasiou T, Anderson J.. Is coronary artery bypass grafting an acceptable alternative to myotomy for the treatment of myocardial bridging? Interact Cardiovasc Thorac Surg 2013;16:347–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Boyd JH, Pargaonkar VS, Scoville DH, Rogers IS, Kimura T, Tanaka S. et al. Surgical unroofing of hemodynamically significant left anterior descending myocardial bridges. Ann Thorac Surg 2017;103:1443–1450. [DOI] [PubMed] [Google Scholar]
- 19. Thompson PD, Myerburg RJ, Levine BD, Udelson JE, Kovacs RJ.. Eligibility and disqualification recommendations for competitive athletes with cardiovascular abnormalities: Task Force 8: coronary artery disease: a scientific statement from the American Heart Association and American College of Cardiology. J Am Coll Cardiol 2015;66:2406–2411. [DOI] [PubMed] [Google Scholar]
- 20. Pelliccia A, Sharma S, Gati S, Bäck M, Börjesson M, Caselli S. et al. 2020 ESC Guidelines on sports cardiology and exercise in patients with cardiovascular disease. Eur Heart J 2021;42:17–96. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.