Dear Editor,
The safety and efficacy of coronavirus disease 2019 (COVID-19) vaccine in older adults have been eagerly anticipated owing to the 23-fold greater risk of death due to COVID-19 in adults over 65 years of age [1]. Maheshi Ramasamy and colleagues [2] have recently presented the safety and immunogenicity of AZD1222 (previously ChAdOx1 nCoV-19), the COVID-19 vaccine candidate of AstraZeneca/Oxford, including adults aged 70 years and more. The vaccine seems to be better tolerated in older adults, exhibiting similar immunogenicity, irrespective of age, following the booster dose [2]. The inclusion of older adult populations in the vaccine trials help to understand how health status and frailty can influence vaccine efficacy, safety, immunogenicity, and reactogenicity in older adults [3]. The plan of AstraZeneca to expand their manufacturing capacity to 3 billion doses in 2021 is a major step in attaining 100% global vaccination coverage [4]. This correspondence aims to analyze the major COVID-19 vaccines developed by AstraZeneca/Oxford, Pfizer/BioNTech, and Moderna, in terms of safety, efficacy, cost, storage, and transportation.
1. Vaccine efficacy and safety
The replication-defective viral vector (adenovirus) vaccine of AstraZeneca/Oxford has exhibited an average efficacy of 70.4% [4,5], whereas that of the mRNA vaccine candidate developed by Pfizer/BioNTech (BNT162b2) was estimated at 95%, following the conclusion of phase III trial [6]. The latter team have already applied to US-FDA for emergency-use authorization [7,8]. Almost similar efficacy was also exhibited (94.1%) by mRNA-1273, the mRNA vaccine candidate developed by the US biotech company Moderna [9,10]. The efficacy of AstraZeneca/Oxford vaccine was evaluated in participants aged 18 years and older. The randomized controlled trial conducted across Brazil, UK, and South Africa, confirmed the safety and efficacy of AZD1222 against symptomatic COVID-19 [5]. The AstraZeneca/Oxford COVID-19 vaccine was found to possess similar immunogenicity across all age groups following the booster dose [2]. Similarly, the randomized controlled trial conducted to evaluate the efficacy of Pfizer/BioNTech vaccine (BNT162b2) enrolled 43,548 participants having 16 years of age or more [6]. The studies conducted among the younger and older adults have already established the immunogenicity and safety profile of the Pfizer/BioNTech vaccine [11,12]. The randomized controlled trial that confirmed the efficacy of Moderna vaccine (mRNA-1273) enrolled 30,420 participants aged 18 years and older [10]. Furthermore, the phase 2 trial that evaluated the safety and immunogenicity confirmed robust immune responses without any adverse effects in healthy adults vaccinated with the mRNA-1273 vaccine. The vaccine also exhibited similar immunogenicity in younger (18–55 years) and older (≥55 years) adults [13].
2. Storage and logistics
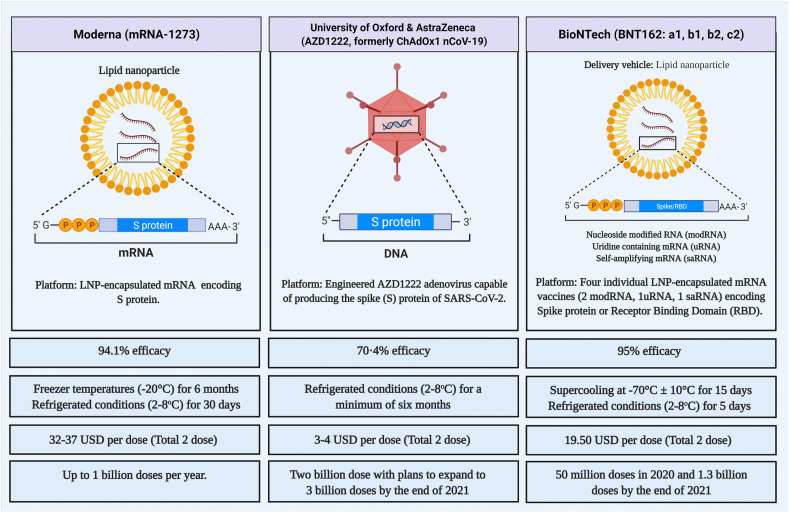
Although the average efficacy of AstraZeneca/Oxford COVID-19 vaccine candidate, AZD1222, is lower than those produced by Moderna and Pfizer/BioNTech, its recommended storage conditions are worth consideration (Fig. 1) [4,8,14]. Pfizer/BioNTech requires temperature-controlled shippers for transporting vaccines at the recommended storage temperature of −70 °C ± 10 °C for up to 15 days. After thawing, the vaccine can only be stored at refrigerated conditions (2–8 °C) for up to 5 days [8]. The mRNA vaccine candidate of Moderna can remain stable at refrigerated conditions (2–8 °C) for 30 days; it can be stored for 6 months at −20 °C [14]. However, AZD1222 nCoV-19 can be transported, stored, and distributed at refrigerated conditions (2–8 °C) for a minimum of six months using existing healthcare settings [4]. Pfizer, the manufacturer of BNT162b2, has recently submitted data to the FDA that supports the use of their vaccine under more flexible conditions. The proposed alternative temperature requirements for the transportation and storage (−25 °C to −15 °C for up to 2 weeks) will allow the undiluted vials to be stored without the need for ultra-low cold storage equipments [15].
Fig. 1.
Comparison of COVID-19 vaccine candidates manufactured by AstraZeneca/Oxford, Moderna, and Pfizer/BioNTech. The figure is created using Biorender.com.
3. Cost of vaccine per dose
Since AstraZeneca/Oxford used a less expensive technology for the production of AZD1222, the total price per dose is less; it is the cheapest, at just 3–4 USD per dose, compared to the rest (Moderna, 32–37 USD and Pfizer/BioNTech, 19.50 USD) [16]. Fig. 1 illustrates the comparison of COVID-19 vaccine candidates manufactured by AstraZeneca/Oxford, Moderna, and Pfizer/BioNTech in terms of efficacy, storage requirements, and cost.
4. Vaccination in low- and middle-income countries
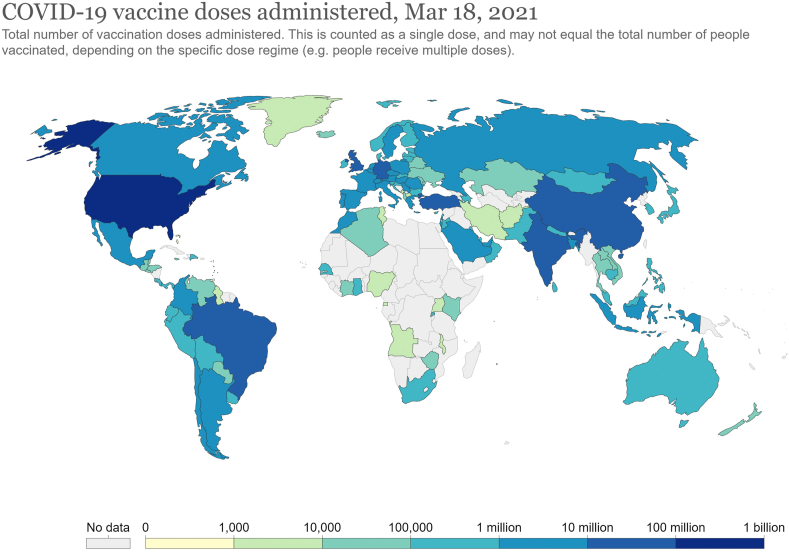
Following the reports of high efficacy, several countries, especially the wealthier ones, have already started pre-order/purchase of billions of COVID-19 vaccine doses triggering a “global vaccine race”. The UK government has already pre-ordered five million doses of mRNA-1273 vaccine from Moderna, this being its seventh deal with different pharmaceutical companies for securing early doses of the vaccine [9]. The total number of COVID-19 vaccine doses administered by different countries is illustrated in Fig. 2 (Updated on March 18, 2021). Among the countries, the USA occupies the top position with the administration of 118.31 million doses, followed by China (64.98 million), India (42.06 million), the US (28.27 million), and Brazil (13.34 million). However, in terms of the population that has received at least one dose, Israel tops the list with 59.6%, followed by UK (38.7%), Chile (29.1%), Bahrain (23.3%), and the USA (23.1%) [17]. Although India occupies the third position in terms of the total dose administered, it constitutes only about 2.5% of the total population [17].
Fig. 2.
Total number of COVID-19 vaccine dose administered as on March 18, 2021. Reproduced from Our World in Data (https://ourworldindata.org/covid-vaccinations) under CC BY license.
Several governments and research institutes have already invested both efforts and finances into developing vaccines that can protect their citizens from the virus. Although the process of vaccine development and evaluation seems to be rapid, very little is known about the logistics and implementation strategies required for vaccinating billions of people worldwide, especially in low- and middle-income countries. Success of a vaccine depends on the global vaccination coverage and its efficacy in inducing protective immunity. With limited access to advanced storage and logistic facilities, along with dearth of funds, attaining successful vaccine-induced heard immunity is obviously a challenge for low- and middle-income countries. COVAX is a global alliance initiated to provide such countries equitable access to effective and safe vaccines. However, they could only secure vaccines for 250 million people, much less than the actual requirement, thereby limiting the accessibility of poor countries to the vaccines [18]. COVAX intends to purchase two billion doses of COVID-19 vaccine by the end of 2021. This vaccine dose will only cover about 20% of the participating country's population. Therefore, the doses available with COVAX will be sufficient to vaccinate healthcare workers and the elderly [19].
Although Moderna and Pfizer have not announced the supply of vaccines to COVAX, AstraZeneca has already committed to providing vaccines to this cause [19]. The requirement of specific cold chain conditions for the storage and transport of vaccines manufactured by Moderna and Pfizer/BioNTech might prevent the low- and middle-income countries from using these vaccines through COVAX [19]. Achieving global vaccination coverage will be a challenge as it involves manufacturing billions of doses, coordinating storage, logistics, and equitable distribution of vaccines. Therefore, the low- and middle-income countries should focus on vaccinating the priority population (key workers/clinically vulnerable groups/elderly groups), since the available doses of vaccines will be insufficient to vaccinate the whole population.
5. Conclusion
The choice of vaccine for the immunization program depends on the availability, cost, efficacy, storage, and logistic requirements [20,21]. Despite their high efficacy, the recommended storage conditions of vaccines from Moderna and Pfizer/BioNTech make it difficult for the low- and middle-income countries to store, transport, and distribute them. On the contrary, AstraZeneca/Oxford offers an effective COVID-19 vaccine candidate for resource-constrained countries that is safe, immunogenic, cost-effective, and can be transported, stored, and distributed at refrigerated conditions. Furthermore, the governments have to establish policies on COVID-19 vaccination depending on their ability to procure vaccine doses. The low- and middle-income countries should focus on vaccinating the key workers/clinically vulnerable groups/elderly. This will enable them to protect the older adults who are at greater risk of death due to COVID-19. It is also recommended that the vaccination should be offered initially to the essential workers, especially frontline healthcare workers. Therefore, the low- and middle-income countries should attempt to use the available resources (vaccines) in the existing healthcare settings (storage and logistics) to the best.
Ethical approval
Not applicable.
Sources of funding
None to be stated.
Author contribution
All authors contributed equally - study concept or design, data collection, data analysis or interpretation, writing the paper.
Registration of Research Studies
Name of the registry: Not applicable.
Unique Identifying number or registration ID: Not applicable.
Hyperlink to your specific registration (must be publicly accessible and will be checked): Not applicable.
Guarantor
Dr. Khan Sharun, Division of Surgery, ICAR-Indian Veterinary Research Institute, Izatnagar, Bareilly-243 122, Uttar Pradesh, India. Email: sharunkhansk@gmail.com.
Dr. Kuldeep Dhama, Division of Pathology, ICAR-Indian Veterinary Research Institute, Izatnagar, Bareilly-243 122, Uttar Pradesh, India. Email: kdhama@rediffmail.com.
Consent
Not applicable.
Provenance and peer review
Not Commissioned, internally reviewed.
Declaration of competing interest
None to be stated.
Acknowledgments
All the authors acknowledge and thank their respective Institutes and Universities.
Contributor Information
Khan Sharun, Email: sharunkhansk@gmail.com.
Kuldeep Dhama, Email: kdhama@rediffmail.com.
References
- 1.Mueller A.L., McNamara M.S., Sinclair D.A. Why does COVID-19 disproportionately affect older people? Aging (Albany NY) 2020 May 29;12(10):9959–9981. doi: 10.18632/aging.103344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ramasamy M.N., Minassian A.M., Ewer K.J. Safety and immunogenicity of ChAdOx1 nCoV-19 vaccine administered in a prime-boost regimen in young and old adults (COV002): a single-blind, randomised, controlled, phase 2/3 trial. Lancet. 2020 Nov 18;(20):S0140–S6736. doi: 10.1016/S0140-6736(20)32466-1. 32466–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Andrew M.K., McElhaney J.E. Age and frailty in COVID-19 vaccine development. Lancet. 2020 Nov 18;(20):S0140–S6736. doi: 10.1016/S0140-6736(20)32481-8. 32481-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.AstraZeneca AZD1222 vaccine met primary efficacy endpoint in preventing COVID-19. 2020. https://www.astrazeneca.com/media-centre/press-releases/2020/azd1222hlr.html
- 5.Voysey M., Clemens S.A.C., Madhi S.A., Weckx L.Y., Folegatti P.M. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet. 2021 Jan 9;397(10269):99–111. doi: 10.1016/S0140-6736(20)32661-1. Epub 2020 Dec 8. Erratum in: Lancet. 2021 Jan 9;397(10269):98. PMID: 33306989; PMCID: PMC7723445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Polack F.P., Thomas S.J., Kitchin N., Absalon J., Gurtman A. Safety and efficacy of the BNT162b2 mRNA covid-19 vaccine. N. Engl. J. Med. 2020 Dec 31;383(27):2603–2615. doi: 10.1056/NEJMoa2034577. Epub 2020 Dec 10. PMID: 33301246; PMCID: PMC7745181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mahase E. Covid-19: Pfizer and BioNTech submit vaccine for US authorisation. BMJ. 2020:371. doi: 10.1136/bmj.m4552. Nov 20. m4552. [DOI] [PubMed] [Google Scholar]
- 8.Pfizer . 2020. Pfizer and BioNTech to Submit Emergency Use Authorization Request Today to the U.S. FDA for COVID-19 Vaccine.https://www.pfizer.com/news/press-release/press-release-detail/pfizer-and-biontech-submit-emergency-use-authorization Accessed on. [Google Scholar]
- 9.Mahase E. Covid-19: Moderna vaccine is nearly 95% effective, trial involving high risk and elderly people shows. BMJ. 2020;371:m4471. doi: 10.1136/bmj.m4471. [DOI] [Google Scholar]
- 10.Baden L.R., El Sahly H.M., Essink B., Kotloff K., Frey S. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N. Engl. J. Med. 2021 Feb 4;384(5):403–416. doi: 10.1056/NEJMoa2035389. Epub 2020 Dec 30. PMID: 33378609; PMCID: PMC7787219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Walsh E.E., Frenck R.W., Jr., Falsey A.R., Kitchin N., Absalon J. Safety and immunogenicity of two RNA-based covid-19 vaccine candidates. N. Engl. J. Med. 2020 Dec 17;383(25):2439–2450. doi: 10.1056/NEJMoa2027906. Epub 2020 Oct 14. PMID: 33053279; PMCID: PMC7583697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhu F., Li J., Hui A., Zhang X., Yang Y., Tang R., Ye H., Ji R., Lin M., Zhu Z., Türeci Ö. First report demonstrating the safety and immunogenicity of the SARS-COV-2 BNT162b1 mRNA vaccine in younger and older Chinese adults: a randomized, placebo-controlled, observer-blind Phase I study. Researchsquare. 2021 doi: 10.21203/rs.3.rs-137265/v1. (preprint) [DOI] [PubMed] [Google Scholar]
- 13.Chu L., McPhee R., Huang W., Bennett H., Pajon R., Nestorova B., Leav B. mRNA-1273 Study Group. A preliminary report of a randomized controlled phase 2 trial of the safety and immunogenicity of mRNA-1273 SARS-CoV-2 vaccine. Vaccine. 2021 Feb 9;(21):S0264–410X. doi: 10.1016/j.vaccine.2021.02.007. 00153–5. Epub ahead of print. PMID: 33707061; PMCID: PMC7871769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moderna Moderna announces longer shelf life for its COVID-19 vaccine candidate at refrigerated temperatures. 2020. https://investors.modernatx.com/news-releases/news-release-details/moderna-announces-longer-shelf-life-its-covid-19-vaccine Accessed on.
- 15.FDA Coronavirus (COVID-19au) Update: FDA Allows More Flexible Storage, Transportation Conditions for Pfizer-BioNTech COVID-19 Vaccine. 2021. https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-allows-more-flexible-storage-transportation-conditions-pfizer Accessed on.
- 16.Ries Julia. 2020. How Much Will it Cost to Get a COVID-19 Vaccine? November 29.https://www.healthline.com/health-news/how-much-will-it-cost-to-get-a-covid-19-vaccine [Google Scholar]
- 17.Our World in Data . 2021. What Share of the Population Has Received at Least One Dose of the COVID-19 Vaccine?https://ourworldindata.org/covid-vaccinations#what-share-of-the-population-has-received-at-least-one-dose-of-the-covid-19-vaccine Accessed on. [Google Scholar]
- 18.Editorial Nature. The COVID vaccine challenges that lie ahead. Nature. 2020 Nov;587(7835):522. doi: 10.1038/d41586-020-03334-w. [DOI] [PubMed] [Google Scholar]
- 19.Kim J.H., Marks F., Clemens J.D. Looking beyond COVID-19 vaccine phase 3 trials. Nat. Med. 2021 Feb;27(2):205–211. doi: 10.1038/s41591-021-01230-y. Epub 2021 Jan 19. PMID: 33469205. [DOI] [PubMed] [Google Scholar]
- 20.Guignard A., Praet N., Jusot V., Bakker M., Baril L. Introducing new vaccines in low- and middle-income countries: challenges and approaches. Expert Rev. Vaccines. 2019 Feb;18(2):119–131. doi: 10.1080/14760584.2019.1574224. Epub 2019 Feb 11. PMID: 30689472. [DOI] [PubMed] [Google Scholar]
- 21.Hardt K., Bonanni P., King S., Santos J.I., El-Hodhod M., Zimet G.D., Preiss S. Vaccine strategies: optimising outcomes. Vaccine. 2016 Dec 20;34(52):6691–6699. doi: 10.1016/j.vaccine.2016.10.078. Epub 2016 Nov 23. PMID: 27887796. [DOI] [PubMed] [Google Scholar]