Abstract
Background
Cytokines seen in severe coronavirus disease 2019 (COVID-19) are associated with proliferation, differentiation, and survival of plasma cells. Plasma cells are not routinely found in peripheral blood, though may produce virus-neutralizing antibodies in COVID-19 later in the course of an infection.
Methods
Using the Johns Hopkins COVID-19 Precision Medicine Analytics Platform Registry, we identified hospitalized adult patients with confirmed severe acute respiratory coronavirus 2 (SARS-CoV-2) infection and stratified by presence of plasma cells and World Health Organization (WHO) disease severity. To identify plasma cells, we employed a sensitive flow cytometric screening method for highly fluorescent lymphocytes and confirmed these microscopically. Cox regression models were used to evaluate time to death and time to clinical improvement by the presence of plasma cells in patients with severe disease.
Results
Of 2301 hospitalized patients with confirmed infection, 371 had plasma cells identified. Patients with plasma cells were more likely to have severe disease, though 86.6% developed plasma cells after onset of severe disease. In patients with severe disease, after adjusting for age, sex, body mass index, race, and other covariates associated with disease severity, patients with plasma cells had a reduced hazard of death (adjusted hazard ratio: 0.57; 95% confidence interval: 0.38-0.87; P value: .008). There was no significant association with the presence of plasma cells and time to clinical improvement.
Conclusions
Patients with severe disease who have detectable plasma cells in the peripheral blood have improved mortality despite adjusting for known covariates associated with disease severity in COVID-19. Further investigation is warranted to understand the role of plasma cells in the immune response to COVID-19.
Keywords: Convalescent plasma, COVID-19, Mortality, Plasma cells, plasmacytosis
Clinical Significance.
-
•
Patients with severe coronavirus disease 2019 (COVID-19) who have detectable plasma cells in the peripheral blood have a reduced hazard of death, suggesting that plasma cells may play a critical role in the immune response to COVID-19.
-
•
Further evaluation of patients with peripheral plasma cells in COVID-19 may provide insight into novel pathophysiology and therapeutic avenues.
Alt-text: Unlabelled box
Introduction
Since the emergence of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in Wuhan, China, in December 2019, a number of laboratory findings have been associated with severe disease and death, including lymphopenia and elevated levels of inflammatory markers.1 These observations suggest a viral-induced systemic hyperinflammatory state driving lung injury and acute respiratory distress syndrome (ARDS).2 , 3 However, the key mediators driving this response are still under investigation. Importantly, the immune response to coronavirus disease 2019 (COVID-19) varies over time in a given patient.4 Initially, innate immune cells, including macrophages and neutrophils, are prominent, driving high levels of proinflammatory cytokines to target the virus. Within several days of infection, the adaptive immune response, including T and B lymphocytes, begins to activate. Dysregulation of the innate response may be a major contributor to pathologic inflammation and morbidity.5
Elevated levels of interleukin (IL)-6, IL-2R, and IL-10 and tumor necrosis factor-alpha (TNFα) correlate with increased COVID-19 disease severity.2 , 3 , 6 Interestingly, these same cytokines are linked to the proliferation, differentiation, and survival of plasma cells.7 , 8 Immunologically, plasma cells are responsible for synthesizing antibodies and have been identified as possibly producing virus-neutralizing antibodies in COVID-19.9 , 10 Not only have plasma cells been noted in bronchoalveolar lavage (BAL) fluid11 and gross lung pathology12 in COVID-19, but plasmablast expansion and extrafollicular B-cell activation has also been described in patients who are critically ill.13 Interestingly, plasmablast frequency in patients with severe COVID-19 did not correlate with age, days since symptom onset, comorbidities, or levels of receptor binding domain-specific immunoglobulin M (IgM) or immunoglobulin G (IgG).13 In a study of 11 patients, B-cell populations did not correlate with cytokine levels.14 Increasing our understanding of cytokines and immune cells throughout the clinical course of COVID-19 is critical because this could lead to potential therapeutic insights for both targeting dysregulated immune responses and promoting “protective” ones.15
This is the first multicenter retrospective study to describe hospitalized patients with confirmed SARS-CoV-2 infection with plasma cells on complete blood count with differential by disease severity and mortality. By using a blood cell counter widely available in clinical laboratories we have a sensitive tool to detect an important mediator in the disease process of COVID-19 that may also be useful as a marker of disease resolution.
Methods
Using the Johns Hopkins COVID-19 Precision Medicine Analytics Platform Registry (JH-CROWN), we identified 2540 patients with confirmed SARS-CoV-2 infection by real-time reverse transcriptase-polymerase chain reaction (RT-PCR) who were hospitalized at our 5-hospital health system between March 3 and August 29, 2020. We excluded patients who were discharged or deceased within 24 hours from admission. Patients with a past medical history of multiple myeloma or plasma cell leukemia were excluded from analysis. This study was approved by the institutional review board at Johns Hopkins Hospital (IRB00249226).
We used routinely available clinical hematology analyzers (Sysmex XN analyzers, Sysmex Corp.) to measure the immune status of patents with COVID-19. These analyzers can differentiate leukocyte subsets by flow cytometry-based forward scatter, side scatter, and RNA content. Antibody-synthesizing lymphoplasmacytoid B cells and plasma cells have high fluorescence intensity above that of normal lymphocytes and can be detected using this method.16 , 17 To confirm that these cells were plasma cells, blood samples that were flagged for high fluorescent lymphocytes had blood smears prepared and stained with Wright stain. The slides were scanned with a Cellavision scanner and results reviewed by a hematology technician and hematologist, who were both blinded concerning clinical information.
We stratified patients by disease severity using the World Health Organization (WHO) ordinal disease scale.18 We performed Cox regression analyses to evaluate time to death and time to clinical improvement in severely ill patients (ie, those requiring high-flow nasal cannula, noninvasive ventilation, mechanical ventilation, extracorporeal membrane oxygenation [ECMO], or with multisystem organ failure) with and without plasma cells after adjusting for age, sex, body mass index, race, as well as other covariates known to be associated with severe disease as described elsewhere.19 , 20 Other covariates included oxygen saturation-fraction of inspired oxygen (SpO2/FiO2) ratio, respiratory rate, blood pressure, heart rate, temperate, white blood cell count, absolute lymphocyte count, hemoglobin, albumin, glomerular filtration rate, alanine transaminase, D-dimer, ferritin, C-reactive protein, Charlson Comorbidity Index, and do not resuscitate/do no intubate (DNR/DNI) status.19 , 20 Cox regression analyses included all events that occurred up to 28 days after the first day of severe disease. Time to clinical improvement was determined by a 2-point decrease in World Health Organization score or discharge from the hospital.18 Statistical analyses were conducted using R software.21
Results
A total of 2540 patients were admitted to the Johns Hopkins Health System with COVID-19 during the study period. After excluding 224 patients who died or were discharged within 24 hours of admission and 15 patients with past diagnoses of multiple myeloma or plasma cell leukemia, 2301 remained. Of these, 371 were noted to have plasma cells on complete blood count (CBC) with differential at any point during their hospitalization. Although 198 of the 371 patients with plasma cells progressed to severe disease, 3 patients died without ever being classified as having severe disease and, thus, were not included in the Cox regression analyses. Moreover, only 27 patients developed plasma cells before the onset of severe disease. Of the patients with severe disease, 79 received corticosteroids, and 31 of these developed plasma cells prior to being treated with corticosteroids.
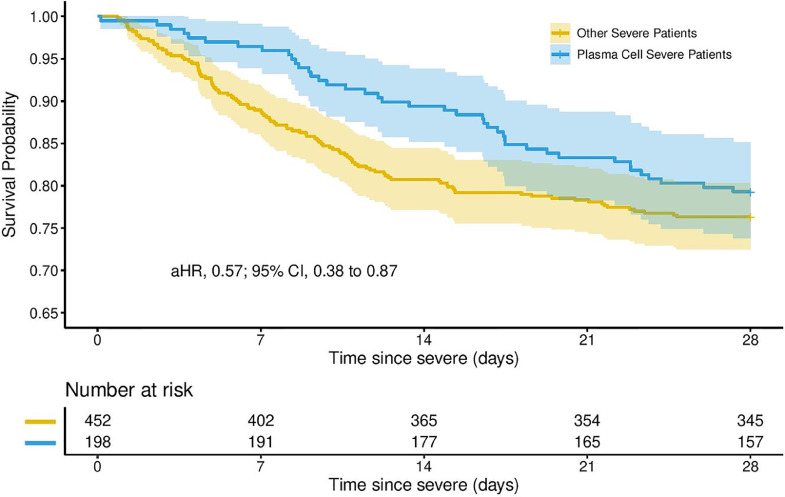
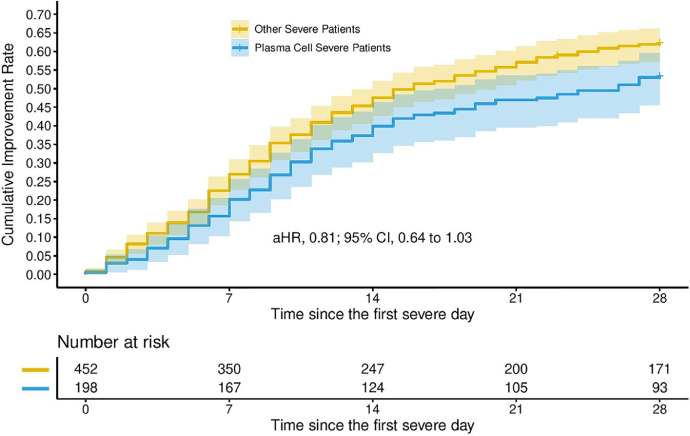
The 28-day mortality for patients with severe disease who had plasma cells was 20.7% (41 deaths) versus 23.7% (107 deaths) for patients without plasma cells. Overall mortality by date of data extraction for patients with plasma cells who had severe disease or died without reaching severe disease classification was 27.7% (56 deaths) versus 38.0% (202 deaths) for patients without plasma cells who had severe disease or died. Cox regression models evaluating time to death in the 198 patients with severe disease showed that, after adjusting for age, sex, body mass index, race, and other covariates associated with severe disease, the presence of plasma cells was significantly associated with a reduced hazard of death (adjusted hazard ratio: 0.57; 95% confidence interval: 0.38-0.87; P value: .008; Figure 1 ). After adjusting for the same covariates, there was no significant association with the presence of plasma cells and time to clinical improvement (Figure 2 ).
Figure 1.
Survival probability by presence of peripheral plasma cell in patients with severe coronavirus disease 2019 (COVID-19).
Figure 2.
Time to clinical improvement by presence of plasma cells in patients with severe coronavirus disease 2019 (COVID-19).
Discussion
Our study suggests that patients with severe COVID-19 who have circulating plasma cells were less likely to die. Plasma cells appeared after progression to severe disease in most patients and, thus, will not serve as an early marker for severe disease. However, the association between plasma cells in severe COVID-19 and the increased likelihood of survival suggests that plasma cells may play a crucial role in the immune response to COVID-19.
Plasma cells likely secrete neutralizing antibodies in COVID-19.9 , 10 Therefore, investigating the titer of COVID-19 antibodies in recovered patients who had circulating plasma cells would give insight into whether these cells correlate with a more robust or prolonged antibody response.22 If patients with circulating plasma cells have more neutralizing antibodies, this could serve as a marker to identify potential donors for convalescent plasma. Because transfusion of convalescent plasma with higher anti-SARS-CoV-2 immunoglobulin G antibody levels is associated with a lower risk of death, it is critical to identify donors who may have higher antibody titers.23 Interestingly, in patients with dengue fever, another syndrome associated with a positive-stranded RNA virus, plasmablast response is similarly associated with disease severity regardless of viral load; however, there was no correlation between neutralizing antibody titer and plasmablast response.24 This suggests that immunomodulatory factors promoting viral clearance and immunity are multifactorial.
Although our study is the first multicenter retrospective study following hospitalized patients with COVID-19 with confirmed plasma cells in the peripheral blood there are several limitations.
Although patients had CBCs monitored regularly during their hospital admission, the frequency of the CBC with differential was provider dependent. Our study suggests an association between the presence of plasma cells and the resolution of severe disease, but we are unable to assess for a causal link with our current data set.
Conclusion
Continued research on circulating plasma cells in patients with COVID-19 can better elucidate the immunologic response to SARS-CoV-2 and help identify factors associated with recovery from severe disease.
Acknowledgments
The data used were part of the JHCROWN: The COVID PMAP Registry, which is based on the contribution of many patients and clinicians and funded by Hopkins in Health, the Johns Hopkins Precision Medicine Program.
Footnotes
Funding: This work was supported by funding from Hopkins in Health, the Johns Hopkins Precision Medicine Program through JH-CROWN and the COVID-19 Administrative Supplement for the HHS Region 3 Treatment Center from the Office of the Assistant Secretary for Preparedness and Response (to BTG, KW, and YX). The funders were not in involved in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Conflicts of Interest: BTG is a member of the Food and Drug Administration Pulmonary and Asthma Drug Advisory Committee and a consultant for Janssen Research and Development, LLC. These arrangements have been reviewed and approved by Johns Hopkins University in accordance with its conflict-of-interest policies. MB, EM, KW, TK, YX report none.
Authorship: All authors had access to the data and a role in writing this manuscript.
References
- 1.Zhou P, Yang XL, Wang XG, et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579(7798):270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Conti P, Ronconi G, Caraffa A, et al. Induction of pro-inflammatory cytokines (IL-1 and IL-6) and lung inflammation by Coronavirus-19 (COVI-19 or SARS-CoV-2): anti-inflammatory strategies. J Biol Regul Homeost Agents. 2020;34(2):327–331. doi: 10.23812/CONTI-E. [DOI] [PubMed] [Google Scholar]
- 3.Chen G, Wu D, Guo W, et al. Clinical and immunologic features in severe and moderate coronavirus disease 2019. J Clin Invest. 2020;130(5):2620–2629. doi: 10.1172/JCI137244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Buckley LF, Wohlford GF, Ting C, et al. Role for anti-cytokine therapies in severe coronavirus disease 2019. Crit Care Explor. 2020;2(8):e0178. doi: 10.1097/CCE.0000000000000178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Merad M, Martin JC. Pathological inflammation in patients with COVID-19: a key role for monocytes and macrophages. Nat Rev Immunol. 2020;20(6):355–362. doi: 10.1038/s41577-020-0331-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang W, Zhao Y, Zhang F, et al. The use of anti-inflammatory drugs in the treatment of people with severe coronavirus disease 2019 (COVID-19): The experience of clinical immunologists from China. Clin Immunol. 2020;214 doi: 10.1016/j.clim.2020.108393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jego G, Bataille R, Pellat-Deceunynck CP. Interleukin-6 is a growth factor for nonmalignant human plasmablasts. Blood. 2001;97(6):1817–1822. doi: 10.1182/blood.v97.6.1817. [DOI] [PubMed] [Google Scholar]
- 8.Zhang J, Yan X, Li Y, Gao R, Wang P, Mo W. Reactive plasmacytosis mimicking multiple myeloma associated with SFTS virus infection: a report of two cases and literature review. BMC Infect Dis. 2018;18:528. doi: 10.1186/s12879-018-3431-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wen W, Su W, Tang H, Le W, Zhang X, Zheng Y. Immune cell profiling of COVID-19 patients in the recovery stage by single-cell sequencing. Cell Discov. 2020;6(1):31. doi: 10.1038/s41421-020-0168-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhu L, Yang P, Zhao Y, et al. Single-cell sequencing of peripheral blood mononuclear cells reveals distinct immune response landscapes of COVID-19 and influenza patients. Immunity. 2020;53:685–696.e3. doi: 10.1016/j.immuni.2020.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Voiriot G, Fajac A, Gibelin A, Parrot A, Fartoukh M. Alveolar lymphocytosis with plasmacytosis in severe COVID-19. Respir Med Res. 2020;7 doi: 10.1016/j.resmer.2020.100784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cai Y, Hao Z, Gao Y, et al. COVID-19 in the perioperative period of lung resection: a brief report from a single thoracic surgery department in Wuhan, China. J Thorac Oncol. 2020;15:1065–1072. doi: 10.1016/j.jtho.2020.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kuri-Cervantes L, Pampena MB, Meng W, et al. Comprehensive mapping of immune perturbations associated with severe COVID-19. Sci Immunol. 2020;5(49):eabd7114. doi: 10.1126/sciimmunol.abd7114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.De Biasi S, Lo Tartaro D, Meschiari M, et al. Expansion of plasmablasts and loss of memory B cells in peripheral blood from COVID-19 patients with pneumonia. Eur J Immunol. 2020;50:1283–1294. doi: 10.1002/eji.202048838. [DOI] [PubMed] [Google Scholar]
- 15.Mangalmurti N, Hunter CA. Cytokine storms: understanding COVID-19. Immunity. 2020;53:19–25. doi: 10.1016/j.immuni.2020.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Linssen J, Jennissen V, Hildmann J, et al. Identification and quantification of high fluorescence-stained lymphocytes as antibody synthesizing/secreting cells using the automated routine hematology analyzer XE-2100. Cytometry B Clin Cytom. 2007;72:157–166. doi: 10.1002/cyto.b.20150. [DOI] [PubMed] [Google Scholar]
- 17.Yip CY, Yap ES, Mel SD, et al. Temporal changes in immune blood cell parameters in COVID-19 infection and recovery from severe infection. Br J Haematol. 2020;190:33–36. doi: 10.1111/bjh.16847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.World Health Organization (WHO) 2020. WHO R&D Blueprint: Novel Coronavirus COVID-19 Therapeutic Trial Synopsis. https://www.who.int/blueprint/priority-diseases/key-action/COVID-19_Treatment_Trial_Design_Master_Protocol_synopsis_Final_18022020.pdf. [Google Scholar]
- 19.Garibaldi BT, Fiksel J, Muschelli J, et al. Patient Trajectories Among Persons Hospitalized for COVID-19. Ann Intern Med. 2021;174:33–41. doi: 10.7326/M20-3905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Garibaldi BT, Wang K, Robinson M, et al. Effectiveness of remdesivir with and without dexamethasone in hospitalized patients with COVID-19 [pre-print]. MedRxiv. https://www.medrxiv.org/content/10.1101/2020.11.19.20234153v1.full.
- 21.R Foundation for Statistical Computing. The R Project for Statistical Computing. Accessed at: www.r-project.org/on. Accessed May 17, 2020.
- 22.Alter G, Seder R. Vol. 383. 2020. The power of antibody-based surveillance; pp. 1782–1784. (N Engl J Med). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Joyner MJ, Carter RE, Senefeld JW, et al. Vol. 384. 2021. Convalescent plasma antibody levels and the risk of death from COVID-19; pp. 1015–1027. (N Engl J Med). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Garcia-Bates TM, Cordeiro MT, Nascimento EJ, et al. Association between magnitude of the virus-specific plasmablast response and disease severity in dengue patients. J Immunol. 2013;190(1):80–87. doi: 10.4049/jimmunol.1103350. [DOI] [PMC free article] [PubMed] [Google Scholar]