Abstract
Background
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is responsible for the current coronavirus disease 2019 (COVID-19). The main organ affected in this infection is the lung and the virus uses the angiotensin-converting enzyme 2 (ACE2) as a receptor to enter the target cells. In this context, a controversy raised regarding the use of renin-angiotensin system (RAAS) blockers, as these drugs might increase ACE2 expression in some tissues and potentially increase the risk for SARS-CoV-2 infection. This is specially concerning in diabetic patients as diabetes is a risk factor for COVID-19.
Methods
12-week old diabetic mice (db/db) were treated with ramipril, or vehicle control for 8 weeks. Non-diabetic db/m mice were included as controls. ACE2 expression and activity were studied in lung, kidney and heart of these animals.
Results
Kidney ACE2 activity was increased in the db/db mice as compared to the db/m (143.2% ± 23% vs 100% ± 22.3%, p = 0.004), whereas ramipril had no significant effect. In the lung, no differences were found in ACE2 when comparing db/db mice to db/m and ramipril also had no significant effect. In the heart, diabetes decreased ACE2 activity (83% ± 16.8%, vs 100% ± 23.1% p = 0.02), and ramipril increased ACE2 significantly (83% ± 16.8% vs 98.2% ± 15%, p = 0.04).
Conclusions
In a mouse model of type 2 diabetes, ramipril had no significant effect on ACE2 activity in either kidneys or in the lungs. Therefore, it is unlikely that RAAS blockers or at least angiotensin-converting enzyme inhibitors increase the risk of SARS-CoV-2 infection through increasing ACE2.
Keywords: ACE2, COVID-19, Diabetes, RAAS blockade, Lung
1. Introduction
The pandemic caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) that began in December 2019 in Wuhan, China, has led to a worldwide sanitary crisis. The lung is the main affected organ in coronavirus disease-19 (COVID-19). SARS-CoV-2 uses the transmembrane protein angiotensin-converting enzyme 2 (ACE2) as one of the receptors to enter the target cells (Hoffmann et al., 2020), a feature shared with SARS-CoV (the virus that caused the SARS epidemic in 2002) (Li et al., 2003). ACE2 is a carboxypeptidase that transforms angiotensin-I (Ang-I) and angiotensin-II (Ang-II) into angiotensin-1-9 (Ang-1-9) and angiotensin-1-7 (Ang-1-7), respectively. This enzyme is part of the alternative pathway of the renin-angiotensin-aldosterone system (RAAS). Its activation has vasodilator effects per se and regulates in a negative way the classical arm of the RAAS (vasoconstrictor) (Obukhov et al., 2020; Romero et al., 2015). ACE2 is widely expressed in the proximal tubular cells of the kidney (Lely et al., 2004). In a lesser extent it is also expressed in the lung (in pneumocytes type 2) (Hamming et al., 2004; Serfozo et al., 2020) and the heart (in cardiomyocytes) (Oudit et al., 2009).
The insights provided by the first SARS-CoV pandemic in 2002, revealed that SARS-CoV infection induced a decrease in ACE2 expression in the lung. In acute lung injury, ACE2 deficiency has been related with enhanced tissue damage, which may be ascribed to an overactivation of the vasoconstrictor arm of the RAAS (ACE/Ang-II/Angiotensin-II type 1 receptor axis) (Kuba et al., 2006). Therefore, the RAAS seems to play an important role in the severity of lung injury related to SARS-CoV-2 infection. Further, deregulation of the RAAS is classically seen in diabetes and changes in ACE2 expression have been identified in the kidney and the heart of diabetic patients (Reich et al., 2008). Meta-analysis data have revealed that SARS-CoV-2 infected patients do not have a higher prevalence of diabetes, although diabetic patients show an increased risk to suffer severe forms of SARS-CoV-2 infection and worse outcomes (Fadini et al., 2020). Approximately a 40% of type 2 diabetic patients are hypertensive (“Hypertension in diabetes study (HDS): I. Prevalence of hypertension in newly presenting type 2 diabetic patients and the association with risk factors for cardiovascular and diabetic complications,” 1993) and are therefore treated with RAAS blockers: angiotensin-converting enzyme inhibitors (ACEis) or angiotensin-II type 1 receptor blockers (ARBs). In addition, these drugs are also widely used to treat non-diabetic hypertension and proteinuria in several renal diseases (García-Carro et al., 2019).
During the first wave of the COVID-19 pandemic a controversy raised regarding the use of RAAS blockers as some studies in experimental models have shown that the treatment with this drugs increased ACE2 expression in cardiac tissue (Ferrario et al., 2005; Ishiyama et al., 2004; Ocaranza et al., 2006) and in the renal vasculature (Soler et al., 2009). Due to this preclinical evidence it has been suggested that the treatment with RAAS blockers could increase the susceptibility to SARS-CoV-2 infection creating a paradox as increased ACE2 levels are normally considered tissue-protective (Aleksova et al., 2020; Sparks et al., 2020; Wang et al., 2020). Despite the results of the aforementioned studies (Ferrario et al., 2005; Ishiyama et al., 2004; Ocaranza et al., 2006; Soler et al., 2009) there is not enough evidence to affirm that these drugs promote ACE2 expression as other authors using similar experimental models have not observed ACE2 modulation induced by RAAS blockers (Burchill et al., 2012; Burrell et al., 2005). Furthermore, the effects of RAAS blockers upon lung ACE2 modulation have been scarcely evaluated. In a recent report, Wysocki and collaborators described ACE2 modulation in kidney and lung of mice (C57BLKS/J) treated with RAAS blockers (telmisatan or captopril) (Wysocki et al., 2020). Here we study the effects of an ACEi (ramipril) on kidney, lung and heart ACE2 in a mouse model of type 2 diabetes.
2. Materials and methods
2.1. Animal procedures
Eight-week-old male leptin receptor deficient diabetic mice (db/db) and the non-diabetic heterozygote littermates (db/+) were purchased from Charles River (BKS.Cg-Dock7m+/+LeprdbJ. Strain Code: 607). The mice were housed in groups of 4 per cage as maximum with ad libitum access to regular mice Chow and water under a 12 h light:12 h dark cycle. After 4 weeks (12-week-old mice) a group of db/db (n = 7) were treated with ramipril (8 mg/kg/day) diluted in drinking water (20 mg/L) during 8 weeks. Vehicle db/db (n = 7) and db/m (n = 7) were used as controls. Weight and blood glucose were monitored biweekly and blood pressure before and after the treatment (see supplemental methods). After the treatment period, the animals were sacrificed under sodium pentobarbital anesthesia. Mice were then perfused with cold PBS 1x and whole blood was obtained by cardiac puncture. The blood samples were collected in tubes with clotting activator gel (41.1378.005, Sarstedt, Germany) to obtain the serum. Afterwards kidney, heart and lung were removed, snap-frozen in liquid nitrogen and maintained at −80 °C until use. Additionally, a portion of kidney and heart was fixed in 10% formalin and paraffin embedded for histological analyses.
The experimental protocol was approved by the Ethical Committees of Animal Experimentation of the Vall d’Hebrón Research Institute (47.18 CEEA). All procedures were conducted according to the guide of the Generalitat de Catalunya in the framework of the European Council Directives for the protection and care of the animals used for research and other scientific purposes (2010/63/EU).
2.2. ACE2 analysis
ACE2 gene and protein expression as well as ACE2 activity were analyzed in kidney, lung and heart extracts. In serum only ACE2 activity was assessed. ACE2 gene expression was measured by RT-qPCR and the ΔΔCT method was applied for relative quantification using the Hypoxanthine Phosphoribosyltransferase 1 (HPRT) as housekeeping gene. ACE2 protein levels were assessed by Western blot and ACE2 activity was determined via a fluorescent enzymatic assay using an ACE2-quenched fluorogenic substrate (Mca-Ala-Pro-Lys (Dnp)-OH; Enzo LifeSciences) as described previously (Riera et al., 2016). Additionally, in the kidney and in the heart we detected ACE2 by immunohistochemistry. The methodology is detailed in the supplemental methods.
2.3. Statistical analyses
Normal distribution was checked by comparing our data with the predicted normal distribution by quantil-quantil (Q-Q) plot and confirmed with normality tests (Shapiro-Wilk and/or Kolmogorov-Smirnov). The groups were compared (db/m vs db/db and db/db vs db/db + Ramipril) by Welch t-tests when normality was met or by Mann-Whitney test when the data followed a non-normal distribution. Data are always expressed as mean ± standard deviation (SD). The p-values are detailed to estimate the confidence of the comparisons and statistical significance was considered when p ≤ 0.05. All statistical analyses and graphs were performed with GraphPad Prism Version 8.2.1.
3. Results
3.1. Ramipril decreases blood pressure in db/db mice
The final aims of the present study were to assess (1) how the diabetic context modulates renal, pulmonary and cardiac ACE2 (SARS-CoV-2 receptor to infect target cells), and (2) whether the ACEi treatment could modify the ACE2 behavior in diabetes. To this end, 12-week-old diabetic mice (db/db) were treated with ramipril (8 mg/kg/day), an ACEi, during 8 weeks. Vehicle db/db and non-diabetic mice (db/m) were included as controls. As expected, all diabetic mice, both the treated with ramipril and the vehicle, showed hyperglycemia and increased weight at the end of the experiment as compared to db/m mice (Table 1 ). In our experimental setting, the db/db mice depicted decreased systolic and diastolic blood pressure as compared to control db/m mice (see Table 1). As expected, Ramipril administration for 8 weeks significantly decreased systolic blood pressure when compared to the vehicle db/db and/or the db/m group (Table 1) demonstrating that ramipril treatment was effective.
Table 1.
Weight, blood glucose levels, blood pressure (systolic and diastolic) and heart rate of the db/m, the db/db and the db/db treated with ramipril after the follow-up period. Data are expressed as mean ± SD.
| db/m | db/db | db/db + Ramipril | ANOVA or Kruskal-Wallis test | db/m vs db/db |
db/m vs db/db + Ramipril |
db/db vs db/db + Ramipril |
Statistical Test | |
|---|---|---|---|---|---|---|---|---|
| Weight (g) | 28.46 ± 2.11 | 41.36 ± 6.03 | 40.39 ± 4.96 | p = 0.0003 | p = 0.0009 | p = 0.0004 | p = 0.75 | Welch's t-test |
| Glycemia (mg/dl) | 150 ± 22.54 | 509.4 ± 92.33 | 542.6 ± 95.23 | p < 0.0001 | p = 0.0006 | p = 0.0006 | p = 0.12 | Mann-Whitney test |
| Systolic Blood Pressure (mmHg) | 101 ± 6 | 94 ± 6 | 82 ± 7 | p < 0.0001 | p = 0.03 | p = 0.0001 | p = 0.004 | Welch's t-test |
| Diastolic Blood Pressure (mmHg) | 78 ± 5 | 71 ± 8 | 64 ± 10 | p = 0.013 | p = 0.09 | p = 0.008 | p = 0.15 | Welch's t-test |
| Heart Rate (bpm) | 555 ± 59 | 523 ± 50 | 551 ± 34 | p = 0.43 | p = 0.29 | p = 0.90 | p = 0.24 | Welch's t-test |
3.2. ACE2 is increased in the kidney of the db/db mice that was not modified by ramipril
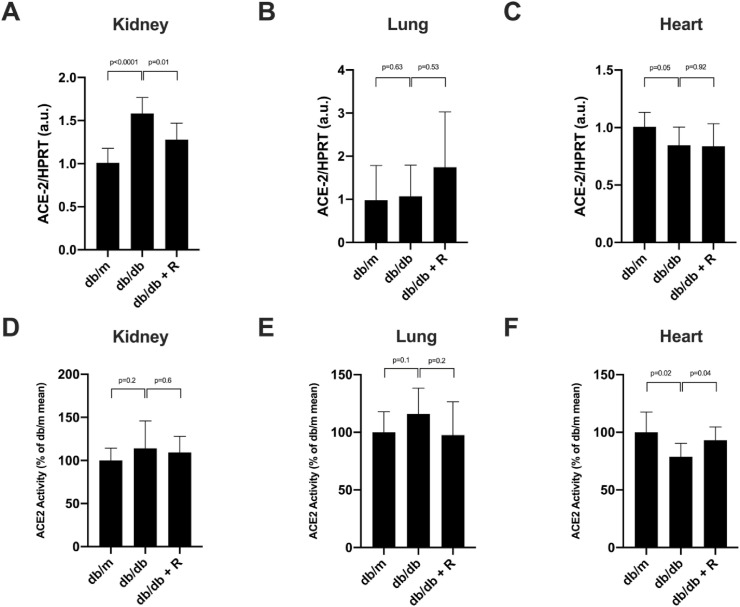
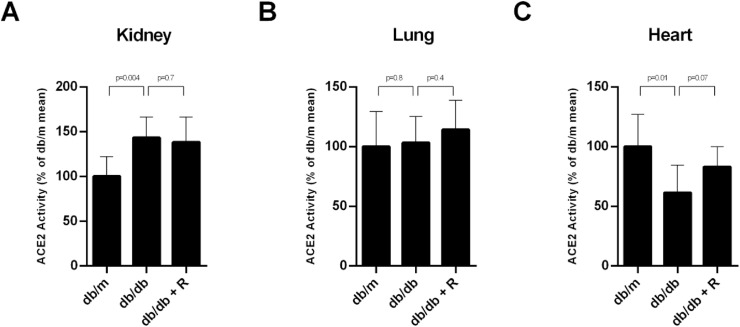
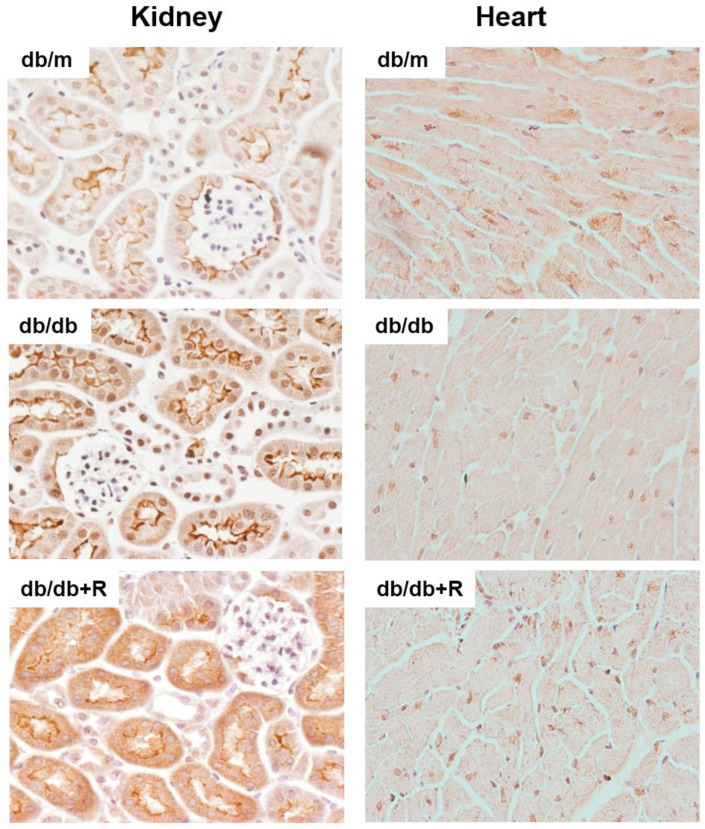
We studied the modulation of ACE2 in the kidney from the db/m, the vehicle db/db and the db/db mice treated with ramipril using two approaches: (1) expression of the ACE2 gene by qPCR and (2) ACE2 activity assay using a fluorescent substrate. The vehicle db/db mice showed increased renal ACE2 gene expression when compared to the db/m (1.011 ± 0.16 vs 1582 ± 0.18, p < 0.0001), this effect was reversed with ramipril administration (1.280 ± 0.18, p = 0.01) (Fig. 1 , A). Renal ACE2 activity (100% ± 14.2% vs 114% ± 31.9%, p = 0.2) also tended to increase in the vehicle db/db, however ramipril did not modify its activity (114% ± 31.9% vs 109.3% ± 18.6%) (Fig. 1D)). In membrane-enriched protein extracts, it became evident that the db/db animals had increased ACE2 activity as compared with the db/m (100% ± 22.3% vs 143.2% ± 23.1%, p = 0.004) and, again, ramipril did not modify ACE2 activity in kidney membrane-enriched extracts (Fig. 2 A). In the kidney tissue sections, ACE2 expression was localized in the brush border of the tubular cells and the staining was more prominent in the db/db when compared to the db/m (Fig. 3 ). We noted no major changes in ACE2 distribution in the kidney of ramipril treated animals when compared to the untreated db/db (Fig. 3).
Fig. 1.
ACE2 gene expression and activity in kidney (A and D, respectively), lung (B and E, respectively) and heart (C and F, respectively) of the db/m, db/db and db/db treated with ramipril mice. ACE2 mRNA significantly increased in the kidney (A) and decreased in the heart (B) of the db/db when compared to the db/m. Ramipril treatment restored ACE2 mRNA in the kidney (A) and had no effect on ACE2 gene expression in the lung and in the heart (panels B and C, respectively). Ramipril treatment did not affect ACE2 activity in the kidney and in the lung of the db/db (panel D and E, respectively). In the heart (F), ACE2 activity was significantly decreased in the db/db (vs db/m) and ramipril normalized ACE2 activity. The data are represented as mean ± SD (n = 7) and significance is considered when p ≤ 0.05. The ACE2 activity obtained in kidney (A), lung (B) and heart (C) expressed in RFU/mg/h is shown in supplemental Figure S1.
Fig. 2.
ACE2 activity in membrane-enriched extracts of kidney (A), lung (B) and heart (C) of the db/m, db/db and db/db treated with ramipril mice. ACE2 activity increased in kidney (A) and decreased in heart (C) of the db/db when compared with db/m. There were no differences between the db/m and the db/db regarding ACE2 activity in lung membrane-enriched extracts (B). Ramipril had no effect in the kidney (A) and in the lung (B) and tended to normalize ACE2 activity in the heart (C). The data are represented as mean ± SD (n = 7) and significance is considered when p ≤ 0.05. The ACE2 activity obtained in kidney (A), lung (B) and heart (C) expressed in RFU/mg/h is shown in supplemental Figure S2.
Fig. 3.
ACE2 localization in kidney and heart tissue of the db/m, db/db and db/db treated with ramipril mice. ACE2 was immunodetected in kidney and heart sections. In the kidney, ACE2 is located at the brush border of the tubular cells with a more prominent staining in db/db than in db/m. Ramipril did not induce major localization changes. ACE2 in heart showed a diffuse distribution with increased ACE2 staining in some areas. The staining intensity seemed to decrease in the db/db when compared to the db/m.
3.3. Ramipril did not modify pulmonary ACE2 in the db/db mice
In the lung, we performed the same approach as in the kidney to study ACE2. We found no differences between the db/m and the vehicle db/db regarding the ACE2 gene expression (Fig. 1, B) mainly related to the high variability in ACE2 mRNA expression (0.97 ± 0.8 vs 1.07 ± 0.71, p = 0.63). We were not able to find differences among the lung and ACE2 activity (nor in total protein extracts or in membrane-enriched extracts) (Figs. 1E and 2B, and Supplemental Figure 1 and 2, respectively). Furthermore, in the lung of the db/db, ramipril treatment did not induce changes in ACE2 gene expression and activity (Fig. 1B and E, Fig. 2B and Supplemental Figure 1 and 2).
3.4. ACE2 is decreased in the heart of the db/db and ramipril normalizes ACE2 levels
We also analyzed ACE2 in the heart. In cardiac tissue, ACE2 significantly decreased in the db/db vs the db/m attending to both, the mRNA levels (1.01 ± 0.12 vs 0.84 ± 0.16, p = 0.05) (Fig. 1C) and ACE2 activity (100% ± 23.1% vs 83% ± 16.8%, p = 0.02) (Fig. 1F). In membrane-enriched extracts ACE2 activity also significantly decreased in db/db when compared to db/m (Fig. 2C). Ramipril treatment significantly restored ACE2 activity to the observed in the control db/m mice (83% ± 16.8% vs 98.2% ± 15%, p = 0.04) (Fig. 1E). However, ramipril was not able to modify ACE2 gene expression in the heart (Fig. 1C). Further, we assessed ACE2 distribution in cardiac tissue where ACE2 staining showed a diffuse distribution with increased ACE2 staining in some areas. We noted that the intensity of the staining was milder in db/db when compared to db/m. Ramipril seemed to restore staining intensity (Fig. 3).
3.5. Serum ACE2 activity is increased in the db/db and it was not modified by ramipril
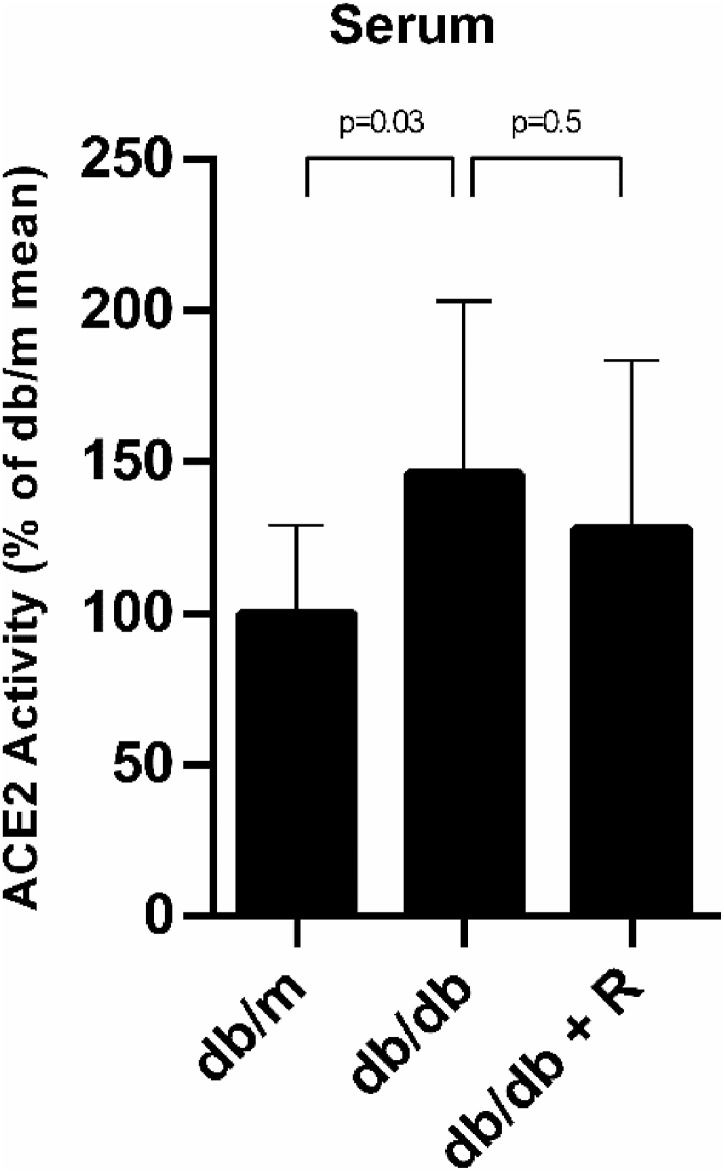
Finally, we measured ACE2 activity in serum samples of the db/m, db/db and db/db treated with ramipril. Serum ACE2 activity was significantly increased in the db/db when compared to the db/m (99.9% ± 29.3% vs 146% ± 57.2%, p = 0.03) and ramipril did not mitigate this effect (146% ± 57.2% vs 128% ± 55.7%, p = 0.5) (Fig. 4 ).
Fig. 4.
ACE2 activity is increased in serum of the db/db and Ramipril has no effect. Serum ACE2 activity of the db/m, db/db and db/db treated with Ramipril. Serum ACE2 activity was increased in the db/db when compared to the db/m but ramipril had no effect. Data are represented as mean ± SD (n = 7) and significance is considered when p ≤ 0.05. The ACE2 activity obtained in serum expressed in RFU/μL/h is shown in supplemental Figure S3.
4. Discussion
The current pandemic caused by SARS-CoV-2 has arisen a controversy related to the use of RAAS blockers in COVID-19 patients. Despite the demonstrated benefits of the use of ACEi or ARBs in cardiovascular patients (Fegan et al., 2000; Romero et al., 2015) it has been suggested that these drugs may increase the expression of ACE2, the receptor for SARS-CoV-2, and hence increase the risk and severity of COVID-19 (Aleksova et al., 2020; Sparks et al., 2020; Wang et al., 2020).
With the present study we aimed to shed light upon this controversy and to this end we analyzed ACE2 in kidney, lung, heart and serum samples of a diabetic mice model (db/db) treated with ramipril during 8 weeks. We used a diabetic model to determine the effect of RAAS blockade as the diabetic milieu is per se a risk factor for poor prognosis in COVID-19 patients (Fadini et al., 2020; Williamson et al., 2020). We found increased kidney ACE2 gene expression and activity in membrane extracts in the db/db mice (Figs. 1A and 2A, respectively). Previous studies have also demonstrated tubular renal ACE2 increase in the db/db diabetic mice (Wysocki et al., 2006; Ye et al, 2004, 2006) as well as in other diabetic mouse models (Riera et al., 2016; Wysocki et al., 2006). Ramipril was able to revert the increase of ACE2 at the gene expression level, suggesting that its administration was protective against the deleterious effect of diabetes within the kidney. Our findings in the kidney contrast with the results published in the recent report of Wysocki and collaborators (Wysocki et al., 2020) as they described that the RAAS blockade in C57BLKS/J mice promotes a decrease of ACE2 expression together with internalization of the protein. In our study using the db/db mice model, we did not find that ramipril reduced ACE2 activity in the kidney (Figs. 1D and 2A) and we neither observed protein internalization (Fig. 3). Even so, we found that ramipril treatment induced a decrease of ACE2 gene expression in db/db (Fig. 1A). Gene and protein expression may not correlate due to different timings between protein synthesis and degradation, but the results obtained in ACE2 gene expression (Fig. 1A) suggest that RAAS blockade would promote decrease of ACE2 in the kidney as suggested by Wysocki and collaborators (Wysocki et al., 2020). Our approach, unlike the study of Wysocki and cols.(Wysocki et al., 2020), mimicked the patients with diabetes that usually receive RAAS blockers to delay the progression of diabetic nephropathy (García-Carro et al., 2019). In line, a recent work of Batchu et al. has clearly shown in kidney and lungs of comorbid diabetes mice (aging, high fat diet and streptozotocin-induced diabetes) that ACE2 is upregulated, however RAAS blockade was not able to modify it (Batchu et al., 2020). Thus, at least for the kidney, our results do not sustain the idea that the RAAS blockade would favor ACE2 overexpression. In human, two independent studies described that tubular ACE2 gene and protein expression were decreased in diabetic nephropathy as compared to healthy individuals (Mizuiri et al., 2008; Reich et al., 2008), although another author was not able to find any differences (Lely et al., 2004). Regarding the effect of RAAS blockade in human, there is some evidence that it affects ACE2 expression in the kidney (Lely et al., 2004; Reich et al., 2008).
We also analyzed ACE2 in lung and heart tissues where ACE2 presence is much lower than in the kidney (Supplemental Figure S1 and S2). Regarding the lung, we obtained high interindividual variability in ACE2 gene expression (Fig. 1) that might be in part explained by the fact that ACE2 expression in the lung is very low and restricted to pneumocytes type 2 (Ziegler et al., 2020). We obtained sound results when ACE2 was estimated by enzyme activity measurement. Lung ACE2 activity was similar between the control db/m and the diabetic db/db and no differences were seen in the db/db treated with ramipril as compared to the vehicle db/db (Figs. 1E and 2B). Although ACE2 in lung has not been widely studied in animal models, it has been reported that the NOD mice and their non-diabetic littermates have similar ACE2 activity in the lung (Roca-Ho et al., 2017). Oppositely, streptozotocin and high-fat diet induced diabetic mice showed increased lung ACE2 activity as compared to non-diabetic mice (Batchu et al., 2020). Regarding the effect of the RAAS blockade on pulmonary ACE2, our results are consistent with the reports of Wysocki et al. and of Batchu et al. as they neither found differences in lung ACE2 activity when the mice were treated with ARBs or ACEi (Batchu et al., 2020; Wysocki et al., 2020). In human, a study in 1051 patients revealed that lung ACE2 gene expression was downregulated by long-term ACEi treatment whereas it was not altered by ARBs (Milne et al., 2020), although no data regarding ACE2 protein levels or activity were available.
Cardiac ACE2 gene expression and activity was significantly decreased in the db/db mice when compared to the db/m (Fig. 1C and F, respectively). In this case, the treatment with ramipril increased ACE2 activity (Fig. 1F). These results suggest that in the db/db model the diabetic profile promotes a decrease of cardiac ACE2 that is restored under RAAS blockade. In db/db diabetic mice, the decrease of cardiac ACE2 seems to happen over time as younger animals (8 week-old) do not show decreased ACE2 activity in the heart (Ye et al., 2004). Cardiac ACE2 is also decreased in streptozotocin induced-diabetic rats (Qiao et al., 2015; Shin et al., 2017) although other diabetic mouse models show rise of heart ACE2 when compared to their non-diabetic littermates (Patel et al., 2012; Roca-Ho et al., 2017). Even so, in vitro experiments suggest that glucose can directly downregulate ACE2 in cardiac vascular smooth muscle cells (Lavrentyev and Malik, 2009). ACE2 modulation in the heart as well as the effect of the RAAS blockade has been mostly studied in myocardial infarction (MI) both in rat models and in human. In a MI rat model, Ocaranza and collaborators showed that heart ACE2 activity was decreased in MI and the RAAS blockade with enalapril reversed it (Ocaranza et al., 2006), similarly to what happens in our study. However, other studies performed in similar MI rat models have not reached the same conclusions (Burrell et al., 2005; Ishiyama et al., 2004). In humans, ACE2 seems to be increased in the heart after MI (Burrell et al., 2005) and in idiopathic and ischemic cardiomyopathy (Goulter et al., 2004). To our knowledge, the effect of RAAS blockade on cardiac ACE2 expression has not been studied in human.
Finally, we analyzed ACE2 activity in serum. ACE2 can be found both in cell membranes of the tissue cells and soluble (sACE2); which origins from shedding of the membrane bound ACE2 by the ADAM metallopeptidase domain 17 (ADAM17) (Lambert et al., 2005). ACE2 only works as a receptor for SARS-CoV-2 at the organ level but it has been suggested that sACE2 could diminish the infection capacity of the virus by acting as a decoy (Batlle et al., 2020; Monteil et al., 2020; Wysocki et al., 2021), although the low sACE2 blood levels would hardly produce this beneficial effect. Even so, it is interesting to know if serum ACE2 levels are modulated (or not) in diabetes or by RAAS blockers as it could be a signal of variations in tissue ACE2. In this sense, it has been postulated that ADAM17 could play an important counteracting role by competing for ACE2 cleavage with the serine-protease Transmembrane Serine Protease 2 (TMPRSS2), a fundamental protease for SARS-CoV-2 internalization (Palau et al., 2020). We found that serum ACE2 was increased in the db/db when compared to the db/m and ramipril did not revert this effect (Fig. 3). Other studies have also described rise of blood ACE2 in diabetic mouse models (Riera et al., 2016; Roca-Ho et al., 2017; Wysocki et al., 2013) and in type 1 diabetic patients (Soro-Paavonen et al., 2012) although the effect of RAAS blockade has not been widely studied. In this regard in human the evidence is not homogenous. In the study of Soro-Paavonen and cols. (Soro-Paavonen et al., 2012), ACEi therapy increased ACE2 blood levels in both male and female patients and ARB treatment only in woman. In contrast, in a recent preprint a slight decrease of blood ACE2 was noted with ARB treatment but ACEi induced no change (Emilsson et al., 2020).
Due to the above mentioned discrepancies regarding ACE2 modulation among different diabetic animal models and in humans it is difficult to obtain a definitive answer for the question: “Does the RAAS blockade induce overexpression of ACE2?“. In an overall overview, attending to our data, and to evidence from other authors, the impression is that the diabetic context in most cases modifies ACE2 expression both in kidney and in heart (down- or upregulates, depending on the model or specie) and, that RAAS blockade: (1) decreases or does not modify kidney ACE2 (both in experimental models and in human), (2) does not modify lung ACE2 (although more evidence is needed here), (3) promotes ACE2 expression in heart (in most experimental models) and (4) has variable effects on blood ACE2 levels (depending on the cohort and the type of RAAS blocker). Hence, the made plane premise “The RAAS blockade induces ACE2 overexpression and, consequently, increases the risk of SARS-CoV-2 infection” does not stay true for the heart, the kidney and, more important, not for the lung. Although, this assumption is a moot point as it provides only a simplistic vision of the regulation of ACE2 expression and does not take into account that ACE2 is protective in most contexts (Batlle et al., 2012; Oudit et al., 2010; Zhong et al., 2010). It has been demonstrated that ACE2 is beneficial in experimental models of lung injury induced by H7N9 flu virus (Yang et al., 2015) or LPS (Ye and Liu, 2020). In a mice model with lung injury, SARS-CoV infection decreased lung ACE2 expression but a treatment with losartan ameliorated the lung injury and ACE2 expression increased at the same time (Kuba et al., 2005) suggesting that ACE2 was beneficial to resolve the viral infection. Moreover, the inflammatory context such as the cytokine storm that happens in a subset of COVID-19 patients (Mehta et al., 2020) can also regulate ACE2 expression although it is unclear in which direction (de Lang et al., 2006; Wu et al., 2020). In any case, both upregulation and downregulation of ACE2 could potentially be detrimental during SARS-CoV-2 infection suggesting that ACE2 levels should be in an equilibrium range (South et al., 2020).
In conclusion, here we demonstrate in a diabetic mice model that ACE inhibition only produces ACE2 upregulation in the diabetic heart, where ACE2 is decreased secondary to diabetes. This does not happen in the kidney or in the lung reinforcing the idea that in most tissues the RAAS blockade does not increase ACE2 expression levels. Even the results we obtained in the heart, it is discussable whether the treatment with ARB or ACEi confers a real risk for increased COVID-19 infection and worsened its prognosis. RAAS blockade has clear cardiovascular benefits (Fegan et al., 2000; Romero et al., 2015), while it is unlikely that low levels of ACE2 are beneficial for COVID-19 resolution.
Author's contribution
Conceptualization, M.J.S., C.J.C and A.V.; methodology, A.V., C.J.C, B.B. P.D.B. M.M.vdB.; Formal analysis, A.V., C.J.C., M.M. vdB., and M.J,S; Investigation, A.V., C.J.C., B.B., P.D.B., M.M.vdB. and M.J.S.; resources, M.J.S., D.S., C.G.C.; writing—original draft preparation A.V., C.J.C, and M.J.S; writing—review and editing, M.J.S. and D.S.; supervision, C.JC. D.S. and M.J.S.; project administration, M.J.S; funding acquisition, D.S. and M.J.S. All authors have read and agreed to the published version of the manuscript.
Funding
FONDO DE INVESTIGACIÓN SANITARIA-FEDER, ISCIII, PI17/00257, and REDINREN, RD16/0009/0030.
Declaration of competing interest
A.V. reports personal fees and non-financial support from Mundipharma, outside this work. M.J.S. reports personal fees from NovoNordisk, Jansen, Mundipharma, AstraZeneca, Esteve, Fresenius, Ingelheim Lilly, Vifor, ICU and grants and personal fees from Boehringer during the conduct of the study. C.G.C. has received travel and congress fees support from Astra-Zeneca, Esteve, NovoNordisk, Boehringer- Ingelheim Lilly, Astellas, Otsuka, Novartis and Baxter. C.G.C has given scientific lectures and participated in advisory boards organized by Astra-Zeneca, Boehringer-Ingelheim Lilly, Mundipharma and NovoNordisk. C.J.C. reports travel support from Travere Therapeutics, outside this work. The results presented in this paper have not been published previously in whole or part, except in abstract format.
Acknowledgments
The authors are current recipients of research grants from the FONDO DE INVESTIGACIÓN SANITARIA-FEDER, ISCIII, PI17/00257, and REDINREN, RD16/0009/0030. A.V. performed this work for the basis of his thesis at the Department de Medicina of Universitat Autònoma de Barcelona (UAB).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.mce.2021.111263.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- Aleksova A., Ferro F., Gagno G., Cappelletto C., Santon D., Rossi M., Ippolito G., Zumla A., Beltrami A.P., Sinagra G. COVID-19 and renin-angiotensin system inhibition: role of angiotensin converting enzyme 2 (ACE2) - is there any scientific evidence for controversy? J. Intern. Med. 2020;288(4):410–421. doi: 10.1111/joim.13101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batchu S.N., Kaur H., Yerra V.G., Advani S.L., Kabir M.G., Liu Y., Klein T., Advani A. Lung and kidney ACE2 and TMPRSS2 in renin angiotensin system blocker treated comorbid diabetic mice mimicking host factors that have been linked to severe COVID-19. Diabetes. 2020;70(3):759–771. doi: 10.2337/db20-0765. [DOI] [PubMed] [Google Scholar]
- Batlle D., Wysocki J., Satchell K. Soluble angiotensin-converting enzyme 2: a potential approach for coronavirus infection therapy? Clin. Sci. (Lond.) 2020;134(5):543–545. doi: 10.1042/CS20200163. [DOI] [PubMed] [Google Scholar]
- Batlle D., Wysocki J., Soler M.J., Ranganath K. Angiotensin-converting enzyme 2: enhancing the degradation of angiotensin II as a potential therapy for diabetic nephropathy. Kidney Int. 2012;81(6):520–528. doi: 10.1038/ki.2011.381. [DOI] [PubMed] [Google Scholar]
- Burchill L.J., Velkoska E., Dean R.G., Griggs K., Patel S.K., Burrell L.M. Combination renin-angiotensin system blockade and angiotensin-converting enzyme 2 in experimental myocardial infarction: implications for future therapeutic directions. Clin. Sci. 2012;123:649–658. doi: 10.1042/CS20120162. [DOI] [PubMed] [Google Scholar]
- Burrell L.M., Risvanis J., Kubota E., Dean R.G., MacDonald P.S., Lu S., Tikellis C., Grant S.L., Lew R.A., Smith A.I., Cooper M.E., Johnston C.I. Myocardial infarction increases ACE2 expression in rat and humans. Eur. Heart J. 2005;26:369–375. doi: 10.1093/eurheartj/ehi114. [DOI] [PubMed] [Google Scholar]
- de Lang A., Osterhaus A.D.M.E., Haagmans B.L. Interferon-γ and interleukin-4 downregulate expression of the SARS coronavirus receptor ACE2 in Vero E6 cells. Virology. 2006;353:474–481. doi: 10.1016/j.virol.2006.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emilsson V., Gudmundsson E.F., Aspelund T., Jonsson B.G., Gudjonsson A., Launer L.J., Jennings L.L., Gudmundsdottir V., Gudnason V. Antihypertensive medication uses and serum ACE2 levels: ACEIs/ARBs treatment does not raise serum levels of ACE2. medRxiv Prepr. Serv. Heal. Sci. 2020 doi: 10.1101/2020.05.21.20108738. [DOI] [Google Scholar]
- Fadini G.P., Morieri M.L., Longato E., Avogaro A. Prevalence and impact of diabetes among people infected with SARS-CoV-2. J. Endocrinol. Invest. 2020;43(6):867–869. doi: 10.1007/s40618-020-01236-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fegan G., Ward D., Clarke L., MacLeod K., Hattersley A. The HOPE study and diabetes. Heart outcomes prevention evaluation. Lancet. 2000;355(9210):1182–1183. doi: 10.1016/S0140-6736(05)72259-5. [DOI] [PubMed] [Google Scholar]
- Ferrario C.M., Jessup J., Chappell M.C., Averill D.B., Brosnihan K.B., Tallant E.A., Diz D.I., Gallagher P.E. Effect of angiotensin-converting enzyme inhibition and angiotensin II receptor blockers on cardiac angiotensin-converting enzyme 2. Circulation. 2005;111:2605–2610. doi: 10.1161/CIRCULATIONAHA.104.510461. [DOI] [PubMed] [Google Scholar]
- García-Carro C., Vergara A., Agraz I., Jacobs-Cachá C., Espinel E., Seron D., Soler M.J. The new era for reno-cardiovascular treatment in type 2 diabetes. J. Clin. Med. 2019;8:864. doi: 10.3390/jcm8060864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goulter A.B., Goddard M.J., Allen J.C., Clark K.L. ACE2 gene expression is up-regulated in the human failing heart. BMC Med. 2004;2 doi: 10.1186/1741-7015-2-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamming I., Timens W., Bulthuis M.L.C., Lely A.T., Navis G.J., van Goor H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J. Pathol. 2004;203:631–637. doi: 10.1002/path.1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann M., Kleine-Weber H., Schroeder S., Krüger N., Herrler T., Erichsen S., Schiergens T.S., Herrler G., Wu N.H., Nitsche A., Müller M.A., Drosten C., Pöhlmann S. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181(2):271–280. doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hypertension in diabetes study (HDS) I. Prevalence of hypertension in newly presenting type 2 diabetic patients and the association with risk factors for cardiovascular and diabetic complications. J. Hypertens. 1993;11:309–317. doi: 10.1097/00004872-199303000-00012. [DOI] [PubMed] [Google Scholar]
- Ishiyama Y., Gallagher P.E., Averill D.B., Tallant E.A., Brosnihan K.B., Ferrario C.M. Upregulation of angiotensin-converting enzyme 2 after myocardial infarction by blockade of angiotensin II receptors. Hypertension. 2004;43:970–976. doi: 10.1161/01.HYP.0000124667.34652.1a. [DOI] [PubMed] [Google Scholar]
- Kuba K., Imai Y., Penninger J.M. Angiotensin-converting enzyme 2 in lung diseases. Curr. Opin. Pharmacol. 2006;6(3):271–276. doi: 10.1016/j.coph.2006.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuba K., Imai Y., Rao S., Gao H., Guo F., Guan B., Huan Y., Yang P., Zhang Y., Deng W., Bao L., Zhang B., Liu G., Wang Z., Chappell M., Liu Y., Zheng D., Leibbrandt A., Wada T., Slutsky A.S., Liu D., Qin C., Jiang C., Penninger J.M. A crucial role of angiotensin converting enzyme 2 (ACE2) in SARS coronavirus-induced lung injury. Nat. Med. 2005;11:875–879. doi: 10.1038/nm1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert D.W., Yarski M., Warner F.J., Thornhill P., Parkin E.T., Smith A.I., Hooper N.M., Turner A.J. Tumor necrosis factor-α convertase (ADAM17) mediates regulated ectodomain shedding of the severe-acute respiratory syndrome-coronavirus (SARS-CoV) receptor, angiotensin-converting enzyme-2 (ACE2) J. Biol. Chem. 2005;280:30113–30119. doi: 10.1074/jbc.M505111200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavrentyev E.N., Malik K.U. High glucose-induced Nox1-derived superoxides downregulate PKC-βII, which subsequently decreases ACE2 expression and ANG(1-7) formation in rat VSMCs. Am. J. Physiol. Heart Circ. Physiol. 2009;296 doi: 10.1152/ajpheart.00239.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lely A.T., Hamming I., van Goor H., Navis G.J. Renal ACE2 expression in human kidney disease. J. Pathol. 2004;204:587–593. doi: 10.1002/path.1670. [DOI] [PubMed] [Google Scholar]
- Li W., Moore M.J., Vasllieva N., Sui J., Wong S.K., Berne M.A., Somasundaran M., Sullivan J.L., Luzuriaga K., Greeneugh T.C., Choe H., Farzan M. Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature. 2003;426:450–454. doi: 10.1038/nature02145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta P., McAuley D.F., Brown M., Sanchez E., Tattersall R.S., Manson J.J., HLH Across Speciality Collaboration, UK COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395:1033–1034. doi: 10.1016/S0140-6736(20)30628-0. (London, England) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milne S., Yang C.X., Timens W., Bossé Y., Sin D.D. SARS-CoV-2 receptor ACE2 gene expression and RAAS inhibitors. Lancet Respir. Med. 2020;8(6):e50–e51. doi: 10.1016/S2213-2600(20)30224-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizuiri S., Hemmi H., Arita M., Ohashi Y., Tanaka Y., Miyagi M., Sakai K., Ishikawa Y., Shibuya K., Hase H., Aikawa A. Expression of ACE and ACE2 in individuals with diabetic kidney disease and healthy controls. Am. J. Kidney Dis. 2008;51:613–623. doi: 10.1053/j.ajkd.2007.11.022. [DOI] [PubMed] [Google Scholar]
- Monteil V., Kwon H., Prado P., Astrid H., Wimmer R.A., Stahl M., Leopoldi A., Garreta E., Hurtado del Pozo C., Prosper F., Romero J.P., Wirnsberger G., Zhang H., Slutsky A.S., Conder R., Montserrat N., Mirazimi A., Penninger J.M. Inhibition of SARS-CoV-2 infections in engineered human tissues using clinical-grade soluble human ACE2. Cell. 2020;181(4):905–913. doi: 10.1016/j.cell.2020.04.004. [e-pub ahe. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obukhov A.G., Stevens B.R., Prasad R., Calzi S.L., Boulton M.E., Raizada M.K., Oudit G.Y., Grant M.B. SARS-CoV-2 infections and ACE2: clinical outcomes linked with increased morbidity and mortality in individuals with diabetes. Diabetes. 2020;69(9):1875–1886. doi: 10.2337/dbi20-0019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ocaranza M.P., Godoy I., Jalil J.E., Varas M., Collantes P., Pinto M., Roman M., Ramirez C., Copaja M., Diaz-Araya G., Castro P., Lavandero S. Enalapril attenuates downregulation of Angiotensin-converting enzyme 2 in the late phase of ventricular dysfunction in myocardial infarcted rat. Hypertension (Dallas) 2006;48:572–578. doi: 10.1161/01.HYP.0000237862.94083.45. (Dallas, Tex. 1979) [DOI] [PubMed] [Google Scholar]
- Oudit G.Y., Kassiri Z., Jiang C., Liu P.P., Poutanen S.M., Penninger J.M., Butany J. SARS-coronavirus modulation of myocardial ACE2 expression and inflammation in patients with SARS. Eur. J. Clin. Invest. 2009;39:618–625. doi: 10.1111/j.1365-2362.2009.02153.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oudit G.Y., Liu G.C., Zhong J.C., Basu R., Chow F.L., Zhou J., Loibner H., Janzek E., Schuster M., Penninger J.M., Herzenberg A.M., Kassiri Z., Scholey J.W. Human recombinant ACE2 reduces the progression of diabetic nephropathy. Diabetes. 2010;59:529–538. doi: 10.2337/db09-1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palau V., Riera M., Soler M.J. ADAM17 inhibition may exert a protective effect on COVID-19. Nephrol. Dial. Transplant. 2020;35:1071–1072. doi: 10.1093/ndt/gfaa093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel V.B., Bodiga S., Basu R., Das S.K., Wang W., Wang Z., Lo J., Grant M.B., Zhong J., Kassiri Z., Oudit G.Y. Loss of angiotensin-converting enzyme-2 exacerbates diabetic cardiovascular complications and leads to systolic and vascular dysfunction: a critical role of the angiotensin II/AT1 receptor axis. Circ. Res. 2012;110:1322–1335. doi: 10.1161/CIRCRESAHA.112.268029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiao W., Wang C., Chen B., Zhang F., Liu Y., Lu Q., Guo H., Yan C., Sun H., Hu G., Yin X. Ibuprofen attenuates cardiac fibrosis in streptozotocin-induced diabetic rats. Cardiol. 2015;131:97–106. doi: 10.1159/000375362. [DOI] [PubMed] [Google Scholar]
- Reich H.N., Oudit G.Y., Penninger J.M., Scholey J.W., Herzenberg A.M. Decreased glomerular and tubular expression of ACE2 in patients with type 2 diabetes and kidney disease. Kidney Int. 2008;74:1610–1616. doi: 10.1038/ki.2008.497. [DOI] [PubMed] [Google Scholar]
- Riera M., Anguiano L., Clotet S., Roca-Ho H., Rebull M., Pascual J., Soler M.J. Paricalcitol modulates ACE2 shedding and renal ADAM17 in NOD mice beyond proteinuria. Am. J. Physiol. Physiol. 2016;310:F534–F546. doi: 10.1152/ajprenal.00082.2015. [DOI] [PubMed] [Google Scholar]
- Roca-Ho H., Riera M., Palau V., Pascual J., Soler M.J. Characterization of ACE and ACE2 expression within different organs of the NOD mouse. Int. J. Mol. Sci. 2017;18 doi: 10.3390/ijms18030563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero C.A., Orias M., Weir M.R. Novel RAAS agonists and antagonists: clinical applications and controversies. Nat. Rev. Endocrinol. 2015;11(4):242–252. doi: 10.1038/nrendo.2015.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serfozo P., Wysocki J., Gulua G., Schulze A., Ye M., Liu P., Jin J., Bader M., Myöhänen T., García-Horsman J.A., Batlle D. Ang II (angiotensin II) conversion to angiotensin-(1-7) in the circulation is POP (Prolyloligopeptidase)-Dependent and ACE2 (Angiotensin-Converting enzyme 2)-independent. Hypertension (Dallas) 2020;75:173–182. doi: 10.1161/HYPERTENSIONAHA.119.14071. (Dallas, Tex. 1979) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin Y.H., Min J.J., Lee J.H., Kim E.H., Kim G.E., Kim M.H., Lee J.J., Ahn H.J. The effect of fluvastatin on cardiac fibrosis and angiotensin-converting enzyme-2 expression in glucose-controlled diabetic rat hearts. Heart Ves. 2017;32:618–627. doi: 10.1007/s00380-016-0936-5. [DOI] [PubMed] [Google Scholar]
- Soler M.J., Ye M., Wysocki J., William J., Lloveras J., Batlle D. Localization of ACE2 in the renal vasculature: amplification by angiotensin II type 1 receptor blockade using telmisartan. Am. J. Physiol. Ren. Physiol. 2009;296 doi: 10.1152/ajprenal.90488.2008. [DOI] [PubMed] [Google Scholar]
- Soro-Paavonen A., Gordin D., Forsblom C., Rosengard-Barlund M., Waden J., Thorn L., Sandholm N., Thomas M.C., Groop P.H. Circulating ACE2 activity is increased in patients with type 1 diabetes and vascular complications. J. Hypertens. 2012;30:375–383. doi: 10.1097/HJH.0b013e32834f04b6. [DOI] [PubMed] [Google Scholar]
- South A.M., Tomlinson L., Edmonston D., Hiremath S., Sparks M.A. Controversies of renin–angiotensin system inhibition during the COVID-19 pandemic. Nat. Rev. Nephrol. 2020;16:305–307. doi: 10.1038/s41581-020-0279-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparks M.A., South A., Welling P., Luther J.M., Cohen J., Byrd J.B., Burrell L.M., Batlle D., Tomlinson L., Bhalla V., Rheault M.N., Soler M.J., Swaminathan S., Hiremath S. Sound science before quick judgement regarding RAS blockade in COVID-19. Clin. J. Am. Soc. Nephrol. 2020;15(5):714–716. doi: 10.2215/CJN.03530320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J.J., Edin M.L., Zeldin D.C., Li C., Wang D.W., Chen C. Good or bad: application of RAAS inhibitors in COVID-19 patients with cardiovascular comorbidities. Pharmacol. Ther. 2020;215(107628) doi: 10.1016/j.pharmthera.2020.107628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson E.J., Walker A.J., Bhaskaran K., Bacon S., Bates C., Morton C.E., Curtis H.J., Mehrkar A., Evans D., Inglesby P., Cockburn J., McDonald H.I., MacKenna B., Tomlinson L., Douglas I.J., Rentsch C.T., Mathur R., Wong A.Y.S., Grieve R., Harrison D., Forbes H., Schultze A., Croker R., Parry J., Hester F., Harper S., Perera R., Evans S.J.W., Smeeth L., Goldacre B. OpenSAFELY: factors associated with COVID-19 death in 17 million patients. Nature. 2020;584 doi: 10.1038/s41586-020-2521-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J., Deng W., Li S., Yang X. Advances in research on ACE2 as a receptor for 2019-nCoV. Cell. Mol. Life Sci. 2020;1:1. doi: 10.1007/s00018-020-03611-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wysocki J., Garcia-Halpin L., Ye M., Maier C., Sowers K., Burns K.D., Batlle D. Regulation of urinary ACE2 in diabetic mice. Am. J. Physiol. Physiol. 2013;305:F600–F611. doi: 10.1152/ajprenal.00600.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wysocki J., Lores E., Ye M., Soler M.J., Batlle D. Kidney and lung ACE2 expression after an ACE inhibitor or an ang II receptor blocker: implications for COVID-19. J. Am. Soc. Nephrol. ASN. 2020;31(9):1941–1943. doi: 10.1681/ASN.2020050667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wysocki J., Ye M., Hassler L., Gupta A.K., Wang Y., Nicoleascu V., Randall G., Wertheim J.A., Batlle D. A novel soluble ACE2 variant with prolonged duration of action neutralizes SARS-CoV-2 infection in human kidney organoids. J. Am. Soc. Nephrol. ASN. 2021;32(4):795–803. doi: 10.1681/ASN.2020101537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wysocki J., Ye M., Soler M.J., Gurley S.B., Xiao H.D., Bernstein K.E., Coffman T.M., Chen S., Batlle D. ACE and ACE2 activity in diabetic mice. Diabetes. 2006;55:2132–2139. doi: 10.2337/db06-0033. [DOI] [PubMed] [Google Scholar]
- Yang P., Gu H., Zhao Z., Wang W., Cao B., Lai C., Yang X., Zhang L., Duan Y., Zhang S., Chen W., Zhen W., Cai M., Penninger J.M., Jiang C., Wang X. Angiotensin-converting enzyme 2 (ACE2) mediates influenza H7N9 virus-induced acute lung injury. Sci. Rep. 2015;4:7027. doi: 10.1038/srep07027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye M., Wysocki J., Naaz P., Salabat M.R., LaPointe M.S., Batlle D. Increased ACE 2 and decreased ACE protein in renal tubules from diabetic mice: a renoprotective combination? Hypertension. 2004;43:1120–1125. doi: 10.1161/01.HYP.0000126192.27644.76. [DOI] [PubMed] [Google Scholar]
- Ye M., Wysocki J., William J., Soler M.J., Cokic I., Batlle D. Glomerular localization and expression of Angiotensin-converting enzyme 2 and Angiotensin-converting enzyme: implications for albuminuria in diabetes. J. Am. Soc. Nephrol. 2006;17:3067–3075. doi: 10.1681/ASN.2006050423. [DOI] [PubMed] [Google Scholar]
- Ye R., Liu Z. ACE2 exhibits protective effects against LPS-induced acute lung injury in mice by inhibiting the LPS-TLR4 pathway. Exp. Mol. Pathol. 2020;113 doi: 10.1016/j.yexmp.2019.104350. [DOI] [PubMed] [Google Scholar]
- Zhong J., Basu R., Guo D., Chow F.L., Byrns S., Schuster M., Loibner H., Wang X.H., Penninger J.M., Kassiri Z., Oudit G.Y. Angiotensin-converting enzyme 2 suppresses pathological hypertrophy, myocardial fibrosis, and cardiac dysfunction. Circulation. 2010;122:717–728. doi: 10.1161/CIRCULATIONAHA.110.955369. [DOI] [PubMed] [Google Scholar]
- Ziegler C.G.K., Allon S.J., Nyquist S.K., Mbano I.M., Miao V.N., Tzouanas C.N., Cao Y., Yousif A.S., Bals J., Hauser B.M., Feldman J., Muus C., Wadsworth M.H., Kazer S.W., Hughes T.K., Doran B., Gatter G.J., Vukovic M., Taliaferro F., Mead B.E., Guo Z., Wang J.P., Gras D., Plaisant M., Ansari M., Angelidis I., Adler H., Sucre J.M.S., Taylor C.J., Lin B., Waghray A., Mitsialis V., Dwyer D.F., Buchheit K.M., Boyce J.A., Barrett N.A., Laidlaw T.M., Carroll S.L., Colonna L., Tkachev V., Peterson C.W., Yu A., Zheng H.B., Gideon H.P., Winchell C.G., Lin P.L., Bingle C.D., Snapper S.B., Kropski J.A., Theis F.J., Schiller H.B., Zaragosi L.E., Barbry P., Leslie A., Kiem H.P., Flynn J.A.L., Fortune S.M., Berger B., Finberg R.W., Kean L.S., Garber M., Schmidt A.G., Lingwood D., Shalek A.K., Ordovas-Montanes J., Banovich N., Brazma A., Desai T., Duong T.E., Eickelberg O., Falk C., Farzan M., Glass I., Haniffa M., Horvath P., Hung D., Kaminski N., Krasnow M., Kuhnemund M., Lafyatis R., Lee H., Leroy S., Linnarson S., Lundeberg J., Meyer K., Misharin A., Nawijn M., Nikolic M.Z., Pe’er D., Powell J., Quake S., Rajagopal J., Tata P.R., Rawlins E.L., Regev A., Reyfman P.A., Rojas M., Rosen O., Saeb-Parsy K., Samakovlis C., Schiller H., Schultze J.L., Seibold M.A., Shepherd D., Spence J., Spira A., Sun X., Teichmann S., Theis F., Tsankov A., van den Berge M., von Papen M., Whitsett J., Xavier R., Xu Y., Zhang K. SARS-CoV-2 receptor ACE2 is an interferon-stimulated gene in human airway epithelial cells and is detected in specific cell subsets across tissues. Cell. 2020;181 doi: 10.1016/j.cell.2020.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.