Abstract
Aim
The formation of a secondary liver is expected in ectopic transplants in liver therapy. It is reported that the transplantation of hepatocyte sheets constitutes one of the techniques used to form a secondary liver. Accordingly, we established a subcutaneous transplant for hepatocyte/fibroblast sheets in previous studies. In this development study with hepatocyte/fibroblast sheets, we evaluated the differences in transplantation sites to promote the maturation of transplanted tissue in a liver injury model.
Methods
A cocultured hepatocyte sheet of fibroblasts (TIG-118 cells) and human hepatocytes (PXB cells) was prepared on a temperature-responsive culture dish. The prepared cocultured hepatocyte sheet was either transplanted subcutaneously or on the liver surface of a persistent liver injury model (cDNA-uPA/SCID mouse: uPA mouse), and was evaluated by the human albumin concentration in mouse blood. As a control group, hepatocyte cell sheets were used that were transplanted to both areas and compared.
Results
Although the cocultured hepatocyte sheet led to functional improvements in the early stages of culture in subcutaneous transplantation, these did not last in the long-term after transplantation. Although coculture effects were not observed in the liver surface transplantation case, long-term functional expressions in mono- and cocultured sheets in the case of liver surface transplantation were exhibited compared with subcutaneous administration.
Conclusion
These results suggest that sustained stimulation of liver regenerationvaries depending on the transplant site and is largely involved in the maturation of hepatocyte tissue.
Keywords: Hepatocyte sheet, Transprantaiton site, Co-culture
1. Introduction
The liver is an organ that performs many functions, and the hepatocyte is an important cell source for regenerative therapy. Recently, the transplantation of hepatocytes and/or hepatic tissue is expected to resolve donor shortage problems [1]. It is reported that hepatocyte sheets can be used as hepatocyte transplants [2,3]. However, it is known that the hepatocyte properties are dependent on material supply, such as oxygen and/or growth factors, and on cell–cell interactions [4,5]. Thus, numerous techniques have been reported to control the culture environment that surrounds hepatocytes in vitro [[6], [7], [8]]. This concept is important in the transplanted hepatocyte sheets because the oxygen supply attributed to angiogenesis from the recipient is important for the engraftment and maturity of the cell sheet.
Various techniques have been recently used to control the oxygen supply to hepatocyte tissues in culture. Takebe et al. reported the generation of blood-vessel-like structures in hepatic tissue to supply oxygen by combining the blood vessels of the recipient and hepatic tissue [9]. We previously reported that hepatic sheets with fibroblasts were used as scaffolds to induce angiogenesis based on the secretion of various growth factors from fibroblasts [10]. Therefore, many reports have been performed on the development of tissue engineering technology that induces angiogenesis in the transplanted tissue. Conversely, Ohashi et al. reported a technique for the subcutaneous addition of various growth factors to induce angiogenesis and aid the transplantation of hepatocytes [11]. This technique focused on the control of the environment of the transplantation site used for the hepatocyte sheet. In recent years, the hepatocyte sheet was transplanted to various tissues and organs to maintain the oxygen supply [12,13]. However, the differences of the transplant sites and their effects on cell sheet properties have not been sufficiently evaluated.
In this study, we focused on the differences of oxygen supply depending on the transplant site. The original hepatocyte sheet was transplanted subcutaneously and on the liver surface of a chronic liver disease mouse model, and the effect of the supply of the liver regeneration stimulating factor on the hepatocyte sheet performance was evaluated based on the differences of the transplant sites.
2. Materials and methods
2.1. TIG-118 cell culture
Normal human fibroblast TIG-118 cells (JCRB0535) were provided by Health Science Research Resources (Osaka, Japan). TIG-118 cells were cultured as a uniform monolayer in a 55 cm2 tissue culture dish that contained 10 mL minimum essential medium (Gibco) supplemented with 10% fetal bovine serum (FBS), 100 U/mL penicillin, and 100 mg/mL streptomycin, and 29.2 mg/mL glutamine. TIG-118 cells grown to confluence in the tissue culture dish were trypsinized (0.25% trypsin, Wako, Japan) and resuspended in culture medium. The culture medium was changed every other day. The cells were cultured in a humidified atmosphere of 5% CO2 at 37 °C.
2.2. Cocultured hepatic sheet consisted of PXB and TIG-118 cells
Fibroblasts that had been cryopreserved at 1 × 106 cells were thawed and seeded evenly in 6-well temperature-responsive culture dishes. After 4 days of culture, it was confirmed that the cells became confluent. Human hepatocytes (PXB cells, PhoenixBio Co. Ltd.) [14,15] isolated from chimeric mice with murine hepatocytes (PXB mice) were seeded on fibroblasts. As a control experiment, the temperature-responsive culture dish was coated with FBS the day before, and PXB cell-seeded conditions were established. The seeding density of the PXB cells was 1 × 106 cells per well.
2.3. Transplant of cell sheets in cDNA-uPA/SCID mouse
All experimental procedures were approved by the Animal Care and Use Committee of PhoenixBio Co., Ltd. Before transplantation, an analgesic agent (butorphanol tartrate) was subcutaneously administered to a chronic liver injury model (cDNA-uPA/SCID mouse: males, 2–4 weeks old). Subcutaneous transplantations of cell sheets were performed in the presence of isoflurane anesthesia into the cDNA-uPA/SCID mouse [10]. Additionally, the abdomen of the host mouse was opened upward, the liver surface was peeled off with a cotton swab with scratches or tweezers, and the cell sheet was transplanted after hemostasis under isoflurane anesthesia (Fig. 1). After the treatment, it was confirmed that hemostasis was achieved. Two weeks after the transplantation, blood was collected every week for 12 weeks, and was stored at −30 °C until serum component analysis was performed.
Fig. 1.
Schematic of transplantation of PXB cells sheet or PXB/TIG118 cells sheet in cDNA-uPA/SCID mouse on subcutaneous space and liver surface.
2.4. Albumin and aminotransferase (AST) assays
Human albumin concentration in each uPA mouse was evaluated as an index of cell function. The concentration of albumin in the culture medium was determined by the enzyme-linked immunosorbent assay (Human Albumin ELISA Quantitation Kit, Bethyl Laboratories, Montgomery, TX, USA). Human aspartate aminotransferase (hAST) concentration was evaluated as an index of liver damage. The concentrations of hAST in the serum of uPA mouse was measured by the SRL company.
2.5. Immunohistochemistry
Each tissue in in cDNA-uPA/SCID were fixed with 4%paraformaldehyde phosphate buffer solution (Wako Pure Chemi-cal). Fixed samples were embedded in paraffin, cut into 5 mm cross-sections. For immunostaining, sections were heated in citrate buffer (pH 6.0) using a microwave for antigen retrieval, incubated in 3% hydrogen peroxide solution for 10 min to quench endogenous peroxidase activity-blocking system (Dako Japan, Kyoto), and then blocked in Tris-buffered saline (TBS) containing 5% bovine serum albumin (BSA) for 15 min at room temperature. Blocked sections were incubated overnight at 4 °C in TBS, and the following antibodies: rabbit anti-human albumin (hALB) (abcam; 1:8000) and rabbit anti-human alpha 1-antitrypsin (hA1AT) (Sigma–Aldrich; 1: 500). Sections were visualized using the Dako liquid DAB substrate chromogen system (Dako Japan). Bright-field images were captured using an optical microscope (BX53; Olympus, Tokyo).
2.6. Statistical analysis
Data are presented as mean ± standard deviation. All experiments were performed at least three times and showed reliable reproducibility. Statistical differences of cell number, albumin, glucose, and lactate assays were evaluated by a repeated-measures analysis of variance. Statistical analyses were performed using 2-way ANOVA. P-value of <0.05 was considered statistically significant.
3. Results
3.1. Formation of PXB cell sheets
The PXB cells in FBS-coated dished exhibited a spreading morphology on the first day of culture (Fig. 2). When both cell sheets were peeled off, PXB cells alone did not show cell contractility. Conversely, cell sheets in the cocultured sheet exhibited strong contractility and formed a relatively small cell sheet. Comparing the cross-sections of these cell sheets, PXB/TIG-118 cells had a thicker tissue than the PXB sheet that was only formed by single cells. The presence of fibroblast scaffolds greatly influenced the formation of hepatocyte sheets.
Fig. 2.
Morphology of hepatocytes at different stages of the culture and tissue morphology based on sheet formation. Scale bar: 100 μm.
3.2. Hepatic function of PXB cell sheets
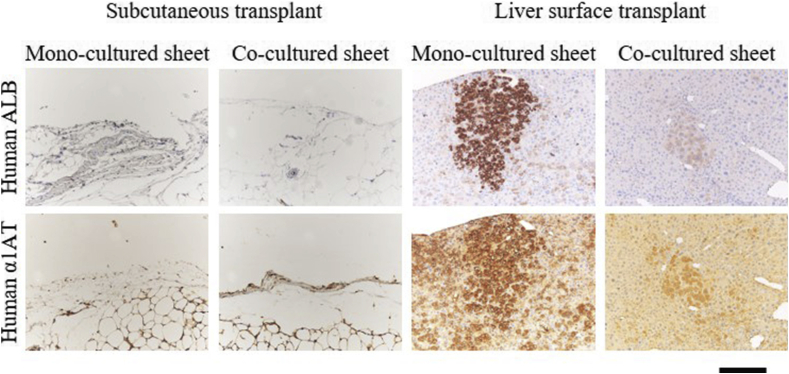
To analyze the improvement of liver function in the cell sheets, body weight gain ratio, human aspartate aminotransferase (hAST) and the blood albumin concentrations were compared in the mice that were transplanted with each sheet. Although there was no significant difference in changes in weight gain ratio and hAST concentrations in blood (Fig. 3A and B), albumin levels changed depending on the conditions. In the case of subcutaneous transplantation, a drastic decrease in functional expression was observed two weeks after transplantation on the sheet that only contained PXB. The cell sheet cocultured with TIG-118 was tampered with for periods up to 6 weeks after transplantation, but a decrease in functional expression was confirmed after 7 weeks. Conversely, in the case of the surface transplantation of the liver, a constant functional expression was maintained for 12 weeks after transplantation, regardless of the presence or absence of TIG-118 cells (Fig. 4). Although they were not present on subcutaneous space, human hepatocyte-positive cells could be seen on the liver surface on 12 weeks of transplantation (Fig. 5).
Fig. 3.
Effect for uPA mouse by transplant of each hepatocyte sheet. Change in (A) body weight gain ratio, (B) concentration of human aspartate aminotransferase (AST). in PXB cells sheet transplanted in the liver surface (open circles), PXB/TIG118 cells sheet transplanted in the liver surface (closed circles), PXB cells sheet transplanted in the subcutaneous (open squares), and PXB/TIG118 cells sheet transplanted in the subcutaneous (closed squares) conditions. Error bars represent the SD.
Fig. 4.
Human albumin concentration after transplant of each hepatocyte cells sheets in PXB cells sheet transplanted in the liver surface (open circles), PXB/TIG118 cells sheet transplanted in the liver surface (closed circles), PXB cells sheet transplanted in the subcutaneous (open squares), and PXB/TIG118 cells sheet transplanted in the subcutaneous (closed squares) conditions. Error bars represent the SD. ∗P < 0.05 (two-way ANOVA).
Fig. 5.
Engraftment of each hepatocyte sheet into the Subcutaneous and liver surface of uPA mice at 12weeks after transplantation. Human hepatocyte engraftment was evaluated by immunostaining of human albumin (Human ALB) and human α1-antitrypsin (Human A1AT). Scale bar: 200 μm.
These results suggested that the influence from the transplant site was very large and the coculture effect was almost ineffective in the liver surface compared with the subcutaneous transplant.
4. Discussion
Hepatocyte sheet transplantation is expected to be an effective treatment for various liver disease patients [2,3]. However, the environment surrounding the cells affects their function in the cases of cells with high-oxygen demand, such as hepatocytes [6,7]. In this study, we evaluated the effect of the differences of the transplant environment on the function for the cocultured hepatocyte sheet that was transplanted to each site.
Although there were a few damages in hepatocyte transplantation, there was almost no effect on growth owing to the damage after transplantation (Fig. 3A, B). Conversely, the albumin concentration in blood was dependent on the transplant site, and the significance of this effect was confirmed in liver transplantation cases (Fig. 4). Those growth factors such as HGF and IL-6 were secreted from various non-parenchymal cells in the chronic liver damage [16,17], and it is suggested that the hepatocyte sheets in liver transplantation were stimulated by those cytokines to promote hepatic function expression. In other words, the subcutaneous site has a poor capacity to supply substances from blood vessels, and hepatocyte sheet in subcutaneous transplantation can not constitute an environment in which hepatocytes work sufficiently. In other words, the subcutaneous site has a poor capacity to supply substances from blood vessels, and hepatocyte sheet in subcutaneous transplantation can not constitute an environment in which hepatocytes work sufficiently. In fact, subcutaneous transplantation requires the induction of angiogenesis by growth factors, such as bFGF and VEGF [11]. We found that cocultured hepatocyte sheets have angiogenic potential [10], and the difference in this capacity may cause differences in functional expressions under the subcutaneous with poor supply.
Conversely, the hepatocyte sheet on the liver surface maintained functional response on the long-term. It was suggested that the hepatocyte sheet transplanted on the liver surface was supplied with factors necessary for the maintenance of liver function and cell engraftment by the intestinal blood flow that is rich in oxygen and growth factors. In fact, it has been reported that cell sheets have enhanced engraftment capacity in tissues with abundant blood flow, such as the liver. It was reported that the pancreatic β-cell sheet maintains high functionality when transplanted in the liver surface [18]. However, the effects in co-culture conditions were not evident for hepatocyte sheets in the liver surface transplant. In addition, the mono-culture sheet engrafted better than co-culture sheets (Fig. 5). It is considered that the factor that supports cell–cell interaction functions at the transplant site where the substance supply is poor, such as subcutaneous tissue, but the supply of substances from the host animal is more involved than the effects of cell–cell interactions. The detailed mechanism is unknown, but hepatocytes alone may have a higher affinity for liver surface transplantation. However, the effects of co-culture with other cells were not observed in liver surface transplantations. It is considered that the factor responsible for the support of cell–cell interaction functions at transplant site where the supply of substance is poor, such as subcutaneous tissue (Fig. 4). However, the supply of substances from the host animal is more involved than the effects of cell–cell interactions. It is known that the various substances are provided for transplanted hepatocytes when liver regeneration occurs [19,20]. Furthermore, it is considered that sufficient liver regeneration-related factors were supplied even in the hepatocyte sheets because liver regeneration always occurs in uPA mice with chronic liver damage (Supporting Fig. 1).
Thus, it was suggested that the biological environment surrounding the cell sheet is involved considerably in the performance of the transplanted tissue, and that an organ with an enhanced substance supply, such as the liver surface, becomes an effective scaffold for cell tissue transplantation. However, only specific functional expressions such as albumin have been evaluated in this study. It is necessary to evaluate coagulation factor secretion and the drug metabolic ability in the future. In this study, we have a special transplant environment wherein chronic liver regeneration occurs. In addition, of the fact that the mice were immunodeficient did not facilitate the applicability of the technique and its translation to the clinic. However, it is expected that it will be applied to hepatocyte transplantation therapy for patients with liver diseases given that it is feasible to use laparoscopic surgery in liver surface transplantation.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Peer review under responsibility of the Japanese Society for Regenerative Medicine.
Supplementary data related to this article can be found at https://doi.org/10.1016/j.reth.2021.02.004.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
References
- 1.Pareja E., Gomez-Lechon M.J., Cortes M., Bonora-Centelles A., Castell J.V., Mir J. Human hepatocyte transplantation in patients with hepatic failure awaiting a graft. Eur Surg Res. 2013;50:273–281. doi: 10.1159/000351332. [DOI] [PubMed] [Google Scholar]
- 2.Yang J., Yamato M., Kohno C., Nishimoto A., Sekine H., Fukai F. Cell sheet engineering: recreating tissues without biodegradable scaffolds. Biomaterials. 2005;26:6415–6422. doi: 10.1016/j.biomaterials.2005.04.061. [DOI] [PubMed] [Google Scholar]
- 3.Itaba N., Matsumi Y., Okinaka K., Ashla A., Kono Y., Osaki M. Human mesenchymal stem cellengineered hepatic cell sheets accelerate liver regeneration in mice. Sci Rep. 2015;5 doi: 10.1038/srep16169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hamon M., Hanada S., Fujii T., Sakai Y. Direct oxygen supply with poly-dimethylsiloxane (PDMS) membranes induces a spontaneous organization of thick heterogeneous liver tissues from rat fetal liver cells in vitro. Cell Transplant. 2012;21:401–410. doi: 10.3727/096368911X605303. [DOI] [PubMed] [Google Scholar]
- 5.Kim K., Ohashi K., Utoh R., Kano K., Okano T. Preserved liver-specific functions of hepatocytes in 3D co-culture with endothelial cell sheets. Biomaterials. 2012;33:1406–1413. doi: 10.1016/j.biomaterials.2011.10.084. [DOI] [PubMed] [Google Scholar]
- 6.Anada T., Fukuda J., Sai Y., Suzuki O. An oxygen-permeable spheroid culture system for the prevention of central hypoxia and necrosis of spheroids. Biomaterials. 2012;33:8430–8441. doi: 10.1016/j.biomaterials.2012.08.040. [DOI] [PubMed] [Google Scholar]
- 7.Sakai Y., Hattori K., Yanagawa F., Sugiura S., Kanamori T., Nakazawa K. Detachably assembled microfluidic device for perfusion culture and post-culture analysis of a spheroid array. Biotechnol J. 2014;9:971–979. doi: 10.1002/biot.201300559. [DOI] [PubMed] [Google Scholar]
- 8.Tsuda Y., Kikuchi A., Yamato M., Chen G., Okano T. Heterotypic cell interactions on a dually patterned surface. Biochem Biophys Res Commun. 2006;348:937–944. doi: 10.1016/j.bbrc.2006.07.138. [DOI] [PubMed] [Google Scholar]
- 9.Takebe T., Zhang R., Koike H., Kimura H., Yoshizawa E., Enomura M. Generation of a vascularized and functional human liver from an iPSC-derived organ bud transplant. Nat Protoc. 2014;9:396–409. doi: 10.1038/nprot.2014.020. [DOI] [PubMed] [Google Scholar]
- 10.Sakai Y., Yamanouchi K., Ohashi K., Koike M., Utoh R., Hasegawa H. Vascularized subcutaneous human liver tissue from engineered hepatocyte/fibroblast sheets in mice. Biomaterials. 2015;65:66–75. doi: 10.1016/j.biomaterials.2015.06.046. [DOI] [PubMed] [Google Scholar]
- 11.Yokoyama T., Ohashi K., Kuge H., Kanehiro H., Iwata H., Yamato M. In vivo engineering of metabolically active hepatic tissues in a neovascularized subcutaneous cavity. Am J Transplant. 2006;6:50–59. doi: 10.1111/j.1600-6143.2005.01155.x. [DOI] [PubMed] [Google Scholar]
- 12.Ohashi K., Marion P.L., Nakai H., Meuse L., Cullen J.M., Bordier B.B. Sustained survival of human hepatocytes in mice: a model of in vivo infection with human hepatitis B and hepatitis delta viruses. Nat Med. 2000;6:327–331. doi: 10.1038/73187. [DOI] [PubMed] [Google Scholar]
- 13.Katsuda T., Teratani T., Ochiya T., Sakai Y. Transplantation of a fetal liver cell-loaded hyaluronic acid sponge onto the mesentery recovers a Wilson's disease model rat. J Biochem. 2010;148:281–288. doi: 10.1093/jb/mvq063. [DOI] [PubMed] [Google Scholar]
- 14.Sato Y., Yamada H., Iwasaki K., Tateno C., Yokoi T., Yoshizato K. Human hepatocytes can repopulate mouse liver: histopathology of the liver in human hepatocyte-transplanted chimeric mice and toxicologic responses to acetaminophen. Toxicol Pathol. 2008;36:581–591. doi: 10.1177/0192623308318212. [DOI] [PubMed] [Google Scholar]
- 15.Watari R., Kakiki M., Yamasaki C., Ishida Y., Tateno C., Kuroda Y. Prediction of human hepatic clearance for cytochrome P450 substrates via a new culture method using the collagen vitrigel membrane chamber and fresh hepatocytes isolated from liver humanized mice. Biol Pharm Bull. 2019;42:348–353. doi: 10.1248/bpb.b18-00582. [DOI] [PubMed] [Google Scholar]
- 16.Zimmers T.A., McKillop I.M., Yoo J.Y., Koniaris L.G. Massive liver growth in mice induced by systemic interleukin 6 administration. Hepatology. 2003:326–334. doi: 10.1053/jhep.2003.50318. [DOI] [PubMed] [Google Scholar]
- 17.Nair V.D., Olanow C.W. Differential modulation of akt/glycogen synthase kinase-3β Pathway regulates apoptotic and cytoprotective signaling responses. J Biol Chem. 2008;283:15469–15478. doi: 10.1074/jbc.M707238200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fujita I., Utoh R., Yamamoto M., Okano T., Yamato M. The liver surface as a favorable site for islet cell sheet transplantation in type 1 diabetes model mice. Regenerative Therapy. 2018;8:65–72. doi: 10.1016/j.reth.2018.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zimmers T.A., McKillop I.H., Pierce R.H., Yoo J., Koniaris L.G. Massive liver growth in mice induced by systemic interleukin 6 administration. Hepatology. 2003;38:326–334. doi: 10.1053/jhep.2003.50318. [DOI] [PubMed] [Google Scholar]
- 20.Pennisi P.A., Kopchick J.J., Thorgeirsson S., LeRoith D., Yakar S. Role of growth hormone (GH) in liver regeneration. Endocrinology. 2004;145:4748–4755. doi: 10.1210/en.2004-0655. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.