Abstract
Objective
To investigate the efficacy and safety of MLC901 in vascular cognitive impairment no dementia (VCIND) patients.
Design
This was a multi‐center, double‐blind, randomized, placebo‐controlled pilot study.
Setting and participant
VCIND patients from hospitals in Singapore (67), Vietnam (19), and the Philippines (17) were recruited and followed‐up from March 2013 to April 2018.
Methods
The primary outcome was executive function as measured by the Verbal Fluency (VF) and 2‐part Color Trails Test (CTT). The mean difference in the scores between baseline and week 12, and baseline and week 24, was compared between MLC901 and placebo using a two‐sample t‐test.
Results
The trial randomized 103 subjects: MLC901 (n = 57) and placebo (n = 46). The mean age of participants was 68.3 ± 8.4 years and 38.8% were female. Improvement in executive function with MLC901 was not significantly better than placebo at week 12 (CTT1 mean difference [md] 3.8 seconds, 95% confidence interval [CI]: –9.0 to 16.5, CTT2 md 10.9 seconds, 95% CI: –0.2 to 22.0), and at week 24 (CTT1 md 2.8 seconds, 95% CI: –8.4 to 14.0, CTT2 md = 4.4 seconds, 95% CI: –8.2 to 16.9). Improvement in VF from baseline was not significantly different between MLC901 and placebo at weeks 12 and 24. There were no significant differences in adverse events (43.5% vs. 56.1%) or serious adverse events (13% vs. 22.8%) in placebo versus MLC901 groups. In post hoc exploratory analysis, the treatment effect of MLC901 on cognitive function appears more apparent in subjects with existing impairment in executive function: CTT2 (md 14.4 seconds [P = .05] and 9.9 seconds [P = .3] at week 12 and week 24, respectively).
Conclusions
Whilst MLC901 appears to be safe, there was no significant cognitive benefit from MLC901 in the study population. Post hoc hypotheses generating analyses suggest that VCIND patients with existing impairment in executive function may show benefit.
Keywords: clinical trial, executive function, MLC901, NEUROAID II, vascular cognitive impairment
1. INTRODUCTION
Vascular dementia (VaD), the second most common cause of dementia after Alzheimer's disease (AD), accounts for ≈20% of all dementia cases. 1 Vascular cognitive impairment (VCI) better describes the full spectrum of cognitive impairment due to cerebrovascular disease that spans mild vascular cognitive impairment no dementia (VCIND) to VaD. 2 VCI diagnosis requires demonstration of cognitive deficit by neuropsychological testing and presence of cerebrovascular disease. 3 , 4 The prevalence of VCIND is higher than VaD and nearly 50% of VCIND subjects convert to dementia within 5 years, 5 hence VCIND is an important target population for dementia prevention. 6 , 7 Moreover, a substantial proportion of non‐disabling stroke survivors are cognitively impaired compared to age‐ and education‐matched community dwelling controls. 8 Furthermore, those who have VCIND of moderate severity after stroke have an increased risk of incident dementia. 9
Neuroaid II (MLC901) is a Traditional Chinese medicine with nine herbal components (Radix Astragali, Radix salvia miltiorrhizae, Radix paeoniae rubra, Rhizoma chuanxiong, Radix angelicae sinensis, Carthamus tinctorius, Semen persica/Prunus persica, Radix polygalae, Rhizoma acori tatarinowii). MLC901 showed neurorestorative and neuroprotective properties in animal and cellular models of focal or global cerebral ischemia. 10 , 11 , 12 , 13 , 14 Additionally, MLC901 has been shown to have a pro‐neurogenesis effect resulting in an increase in the number of mature hippocampal neurons, which correlates well with reversal learning (which is highly dependent on cognitive flexibility) in the Morris water maze model in mice. 15 Clinical studies have demonstrated the safety of MLC901 and its precursor MLC601, and their possible effects on functional and neurological outcomes in stroke. 16 , 17 , 18 , 19 As MLC901 is a simplified version of MLC601 with similar pharmacological properties, it may potentially improve outcomes after stroke. Hence, MLC901 may be effective and safe in improving cognition through these mechanisms in patients with VCIND.
In VCI cognitive deficits in executive function are characteristic; information processing becomes slow, the ability to shift from one task to another is impaired, and there are deficits in the ability to hold and manipulate information. 20 Therefore, we hypothesized that patients with VCIND receiving MLC901 would improve on neuropsychological tests that assess executive function. Our primary objective was to evaluate the comparative change in executive function from baseline (BL) to 24 weeks in VCIND patients after treatment with either MLC901 or placebo. The secondary objectives were to evaluate comparative change from BL to 24 weeks with MLC901 and placebo on cognitive function, activities of daily living, behavior, and mood. Additionally, safety and tolerability of MLC901 was assessed throughout the study.
2. METHODS AND ASSESSMENTS
2.1. Trial design and methodology
NEURITES was a double‐blind, randomized, placebo‐controlled pilot study of NeuroAiDII (MLC901) in subjects with VCIND. 21
The trial recruited subjects from Singapore, the Philippines, and Vietnam. It was conducted as per the ICH‐GCP guidelines. Local ethics committee approval was obtained prior to commencing the study.
After informed consent, eligible subjects were randomized to receive either MLC901 or matched placebo at a dose of two capsules three times daily for 24 weeks in addition to standard post‐stroke care. (Standard post‐stroke care includes any concomitant medication that the subjects are administered for secondary stroke prevention.) Each capsule contains 400 mg MLC901/placebo.
Executive function was assessed by the 2‐part Color Trail Test (CTT) 22 and the Verbal Fluency (VF) 23 test. The CTT is composed of CTT‐1 (respondent uses a pencil to rapidly connect circles numbered 1 to 25 in sequence) and CTT‐2 (respondent rapidly connects numbered circles in sequence, but alternates between pink and yellow); the length of time to complete each test is recorded in seconds and is compared to normative data. The task is discontinued if the subject takes longer than 240 seconds to complete. The VF test evaluates an individual's ability to retrieve specific information within restricted search parameters, consisting of VF‐Animals and VF‐Food. Successful retrieval requires executive control over cognitive processes such as selective attention, selective inhibition, mental set shifting, internal response generation, and self‐monitoring. In our study we assessed category fluency, tested by asking the examinee to generate semantic category exemplars within 1 minute (animals and food).
As part of the secondary objectives, cognition was assessed by the Alzheimer's Disease Assessment Scale‐Cognitive Subscale (ADAS‐Cog), 24 Montreal Cognitive Assessment (MoCA), 25 and a cognitive battery (symbol digits modalities test, digital cancellation test, visual memory test, 26 frontal assessment battery 27 ). Activities of daily living was assessed by the Alzheimer's Disease Cooperative Study Activities of Daily Living (ADCS‐ADL) 28 , 29 scale for mild cognitive impairment, behavior by the Neuropsychiatric Inventory (NPI), 30 and mood by the Geriatric Depression Scale (GDS). 31 Safety was assessed by serious adverse events (SAEs) and adverse events (AEs). The details of the study rationale, design, and procedures are published in Chen et al.. 21
2.2. Study population
The inclusion criteria were: male or female aged 55 to 85 years, living with a caregiver, modified Rankin Score (mRS) < 3, diagnosis of cognitively impaired not demented (CIND) due to cerebrovascular disease, cognitive impairment documented by neuropsychological evaluation within 12 months of index stroke or transient ischemic attack (TIA), not demented according to Diagnostic and Statistical Manual of Mental Disorders 4th edition (DSM‐IV) criteria, and written informed consent signed by subject. CIND diagnosis was based on the Vascular Dementia Battery (VDB), 8 which assesses six cognitive domains: attention, language, verbal memory, visual memory, visuoconstruction, and visuomotor speed. Subjects who did not meet the DSM‐IV criteria but were impaired in one or more cognitive domains were classified as CIND.
The exclusion criteria were: advanced, severe, and unstable disease of any type that may interfere with the efficacy evaluations or put the subject at special risk; DSM‐IV current diagnosis of dementia or major depression (subjects were included if currently being treated on an antidepressant and clinically stable for 3 months); a disability that may prevent the subject from completing all study requirements (e.g., blindness, deafness, severe language difficulty); ingestion of any of the following treatments: an investigational drug in the past 4 weeks, or a drug or treatment known to cause major organ system toxicity during the past 4 weeks, or acetylcholinesterase inhibitors or memantine in the past 3 months.
RESEARCH IN CONTEXT
Systematic review: The authors screened the literature using Medline, PubMed, and meeting abstracts. Relevant clinical and nonclinical studies on MLC901 and its precursor, MLC601, on neurological diseases were reviewed and appropriately cited.
Interpretation: Vascular cognitive impairment no dementia (VCIND) patients were included in this study, with the aim to evaluate the effect of MLC901 in improving cognitive function (in particular executive function). MLC901 was found to be safe but did not show an overall effect on executive function; however, it may be beneficial to subjects with more severe VCIND at baseline.
Future directions: For future studies, more severely cognitively impaired VCIND patients should be included and should be followed up for longer duration, that is, >24 weeks. Our results would help to design, select, and characterize the type of subjects to be included in future clinical trials.
2.3. Randomization and blinding
All eligible subjects were randomly assigned to MLC901 or placebo at an allocation ratio of 1:1, according to a block randomization (block of 10) list prepared by Moleac Pte Limited. Randomization was stratified by BL mRS levels (mRS 0–1, mRS 2, and mRS 3) and study sites (five sites); in total there were 15 strata. MLC901 and placebo were provided as indistinguishable capsules to maintain blinding. An emergency envelope with randomization codes inside (one envelope per subject) was prepared and kept in a secured place at each study center. This was to be opened only in emergency cases for which the randomization code needed to be known prior to further treatment.
3. STATISTICS
3.1. Sample size
The primary outcome was mean change from BL to week 24 (W24) on executive function, as measured by CTT and VF. A previous study on VCIND showed a standard deviation (SD) of ≈2.5 for improvement in VF from BL to W24. 32 Hence, to detect a 2‐point difference in improvement in VF between MLC901 and placebo with a power of 90% and a significance level of 5%, a total sample size of 68 subjects was sufficient. This trial targeted to recruit 100 subjects to allow for 30% drop‐out. This enabled the same power to detect a difference in completion time of 20 seconds in CTT between MLC901 and placebo, based on an estimated SD of 27 seconds from the previous study. 32
3.2. Analysis
BL data including demographics were summarized by descriptive statistics and presented by treatment groups. Analysis of BL characteristics and efficacy data was performed with the intention‐to‐treat (ITT) population, and per‐protocol (PP) population was used for sensitivity analysis of primary outcomes. The PP population included subjects who had completed the study without major protocol deviations; subjects with no BL or W24 CTT/VF data and with a treatment compliance < 80% were excluded. Last observation carried forward (LOCF) method was used for imputing missing data.
3.3. Primary analysis
3.3.1. Primary efficacy outcomes
For CTT, descriptive statistics using the mean and SD were calculated for improvement in the CTT scores from BL to week 12 (W12) and W24 by treatment groups. For VF, both the individual scores for animals and food, and the sum of the two scores, descriptive statistics including the mean and SD, were calculated for improvement similar to CTT. The treatment effect of MCL901 was assessed by comparing the mean difference in the improvement between MLC901 and placebo using a two‐sample t test. To compare the treatment effect after adjustment for BL scores and other BL characteristics, we fitted multiple linear regression models for the primary outcomes (improvement in CTT and VF), adjusting for their respective base BL line scores, and other BL characteristics, including mRS (0 to 1 vs. 2 to 3), MoCA (≤ 15 vs. > 15), age, presence of hypertension, aspirin use, and previous neurological event.
3.3.2. Secondary efficacy endpoints
Mean change from BL was calculated by treatment group and their difference was determined.
3.4. Exploratory analysis
To explore if MLC901 would show better efficacy in subjects who have impaired executive function, subgroup analysis based on BL CTT scores and VF was done using two‐sample t test for comparison of primary outcomes between MLC901 and placebo. The CTT subgroups are:
Impaired at baseline–‐Subjects were included if they had CTT scores below the fifth percentile scores of the normative data (these scores have been adjusted for age and education) at BL. 22
Able to perform (overall)—This group included subjects from the overall (ITT) population who were able to perform the CTT within the maximum cut‐off time, that is, 240 seconds in at least one visit either at BL/W12/W24. (Subjects unable to perform CTT within 240 seconds in all three consecutive visits, that is, at BL, W12, and W24, are excluded from this group.)
Able to perform (Impaired)‐–Subjects who were impaired (CTT scores below fifth percentile) at BL as per the normative data but who are able to perform the CTT within 240 seconds in at least one visit either at BL/W12/W24 were included in this group. (Subjects unable to perform CTT within 240 seconds in all three consecutive visits, that is, at BL, W12, and W24, are excluded.)
In the absence of a widely accepted standard definition, the subgroups described above are based upon an ad hoc categorization, defining group of patients (1) with documented substantial executive function impairment at BL; (2) patients with or without impairment at BL, but excluding those patients who cannot be assessed quantitatively with the CTT test; and (3) patients substantially impaired at BL, after exclusion of those patients who cannot be assessed quantitatively with the CTT test.
The VF subgroups are based on a locally validated cut‐off score that uses two education groups: low (0 to 6 years of education) and high (7 to 12 years of education). 33
Impaired on the VFA = score < 7 and < 9 for low education and high education, respectively.
Impaired on the VFF = score < 9 and < 11 for low education and high education, respectively.
4. RESULTS
One hundred three subjects were recruited for the NEURITES study, randomized between MLC901 group (n = 57) and placebo group (n = 46). The CONSORT (Consolidated Standards of Reporting Trials) diagram for patient flow is in Figure 1.
FIGURE 1.
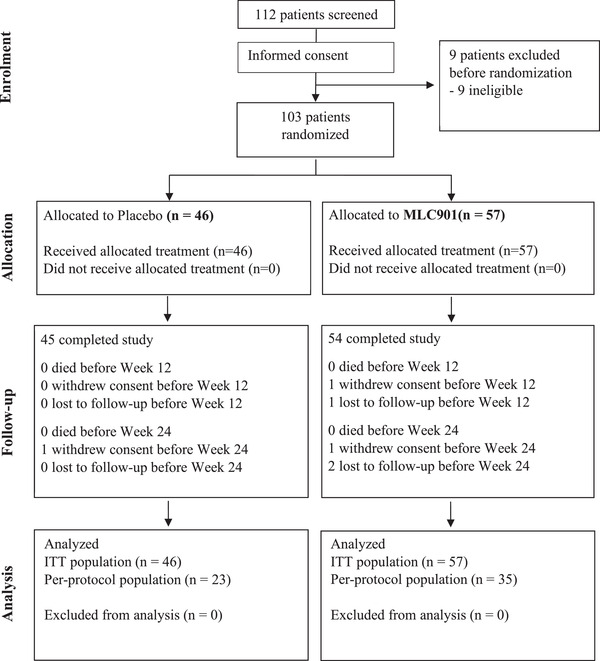
Consolidated Standards of Reporting Trials (CONSORT) flowchart
4.1. Baseline characteristics
The mean age was 68.3 ± 8.4 years, 38.8% subjects were female, and the major ethnic group was Chinese (50%). Most subjects (96%) had an ischemic stroke prior to enrolment into the study (details in Table 1). The treatment compliance rate of ≥ 80% was achieved in 61.4% in the MLC901 group compared to 50% in the placebo group. The median time from index stroke to randomization into the trial was 9.9 (interquartile range [IQR]: 12.8) months in the placebo and 7.2 (IQR: 13.7) months in the MLC901 group (P = .089). As per the protocol inclusion criteria, entry into the trial could in some cases have been more that 12 months after the index stroke or TIA (as long as a post stroke/TIA assessment documenting cognitive impairment was performed within 12 weeks of the index event). The BL CTT1 score was 113.6 ± 62.4 seconds in placebo and 128.2 ± 60.4 seconds in MLC901 groups (P = .2); for CTT2 the scores were 180.5 ± 56.8 seconds and 199.6 ± 50.2 seconds (P = .07); for VFA the scores were 10.2 ± 3.75 and 11.1 ± 4.03 (P = .25); and for VFF the scores were 10.8 ± 4.12 and 11.0 ± 3.94 (P = .84).
TABLE 1.
Baseline characteristics
| Placebo (n = 46) | MLC901 (n = 57) | |
|---|---|---|
| Characteristics | mean/SD/%) | mean/SD/%) |
| Age (year) | 67.2 (± 8.6) | 69.4 (± 8.2) |
| Female | 15 (32.6) | 25 (43.9) |
| Type of stroke (index stroke) | ||
| Ischemic | 45 (97.8) | 54 (94.7) |
| TIA | 1 (2.2) | 3 (5.3) |
| TOAST classification | ||
| LAA | 9 (19.6) | 14 (24.6) |
| CE | 9 (19.6) | 3 (5.3) |
| SAO | 25 (54.3) | 34 (59.6) |
| OC | 1 (2.2) | 2 (3.5) |
| UND | 1 (2.2) | 1 (1.8) |
| TIA | 1 (2.2) | 3 (5.3) |
| History of previous stroke | 11 (23.9) | 15 (26.3) |
| Ischaemic | 8 | 7 |
| Hemorrhagic | 1 | 0 |
| TIA | 1 | 1 |
| Unknown type of stroke | 1 | 7 |
| Medical history | ||
| Myocardial infarction | 5 (10.9) | 5 (8.8) |
| Angina | 5 (10.9) | 6 (10.5) |
| Hypertension | 38 (82.6) | 53 (93%) |
| DM | 20 (43.5) | 25 (43.9) |
| Hyperlipidemia | 38 (82.6) | 48 (84.2) |
| Peripheral vascular disease | 3 (6.5) | 1 (1.8) |
| Smoking history | 19 (41.3) | 18 (31.6) |
| Habitual alcohol drinking | 10 (21.7) | 15 (26.3) |
| Modified Rankin Scale | ||
| 0 | 0 | 2 (3.5) |
| 1 | 30 (65.2) | 33 (57.9) |
| 2 | 13 (28.3) | 14 (24.6) |
| 3 | 3 (6.5) | 8 (14.0) |
| Barthel index | 98.4 (± 5.8) | 96.1 (± 9.7) |
| MoCA | 18.0 (± 5.1) | 19.2 (± 5.1) |
| CIND severity level | ||
| Moderate | 25 (54.3) | 28 (49.1) |
| Mild | 21 (45.7) | 29 (50.9) |
| Ethnicity | ||
| Chinese | 28 (60.9) | 24 (42.1) |
| Malay | 1 (2.2) | 4 (7.0) |
| Indian | 2 (4.3) | 7 (12.3) |
| Filipino | 9 (19.6) | 10 (17.5) |
| Vietnamese | 6 (13.0) | 11 (19.3) |
| Other | 0 (0) | 1 (1.8) |
| Marital status | ||
| Never married | 4 (8.7) | 6 (10.5) |
| Married | 35 (76.1) | 39 (68.4) |
| Divorced/widowed/other | 7 (15.2) | 12 (21.1) |
| Right handedness | 45 (97.8) | 55 (96.5) |
| Living situation | ||
| Lives with partner/spouse | 26 (56.5) | 37 (64.9) |
| Lives with children/ relative/friend/group | 13 (28.3) | 18 (31.6) |
| Lives alone/other | 7 (15.2) | 2 (3.5) |
| Level of independence | ||
| Able to live independently | 33 (71.7) | 40 (70.2) |
| Require some assistance with complex activities | 10 (21.7) | 12 (21.1) |
| Require some assistance with basic activities | 3 (6.5) | 4 (7.0) |
| Completely dependent | 0 (0) | 1 (1.8) |
Abbreviations: CE, cardioembolism; CIND, cognitive impaired no dementia; DM, diabetes mellitus; LAA, large‐artery atherosclerosis; MoCA, Montreal Cognitive Assessment; OC, stroke of other determined cause; SAO, small‐artery occlusion; SD, standard deviation; TIA, Transient ischemic attack; TOAST, Trial of Org 10172 in Acute Stroke Treatment; UND, Stroke of undetermined cause.
4.2. Primary analysis
4.2.1. Primary efficacy outcomes
No statistically significant difference was observed between MLC901 and placebo for the CTT1 or CTT2 change from BL to W12 or W24. The mean difference in improvement of CTT1 between the MLC901 and placebo groups was 3.8 seconds at W12 (95% confidence interval [CI]: –9.0 to 16.5) and 2.8 seconds at W24 (95% CI: –8.4 to 14.0). The mean difference in improvement of CTT2 between the MLC901 and placebo groups was 10.9 seconds at W12 (95% CI: –0.2 to 22.0, P = .055) and 4.4 seconds at W24 (95% CI: –8.2 to 16.9). Some improvement in CTT1 and CTT2 from BL to W12 was observed in both MLC901 and placebo groups, with a somewhat greater (albeit not statistically significant) improvement in the MLC901 group (–10 seconds) than in the placebo group (–7 seconds). At W24, the CTT1 scores in placebo and MLC901 groups were 106.7 ± 57.9 and 118.5 ± 57.6, respectively (P = .3), while the CTT2 scores were 176.1 ± 58.9 and 190.8 ± 52.5 (P = .18).
Similarly, no statistically significant difference was observed between MLC901 and placebo for VF change from BL. Improvement of VF from BL was minor (< 1) and similar at W12 and W24 in both MLC901 and placebo groups (Figure 2). At W24, the VFA scores were 10.6 ± 3.46 and 10.9 ± 3.82 (P = .67) and the VFF scores 11.0 ± 4.22 and 11.5 ± 4.01 (P = .53) for placebo and MLC901, respectively.
FIGURE 2.
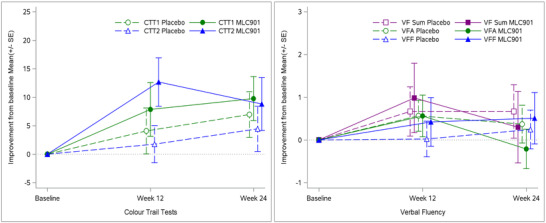
Comparisons of primary endpoints: Color Trails Test [CTT; CTT1, CTT2] and Verbal Fluency [VF; VF Animal (VFF), VF Food (VFA)]
Analysis of primary outcomes in the PP population indicated similar results as the ITT population described above. Regression analyses of the primary outcomes adjusting for BL characteristics showed consistent results with those from unadjusted analysis in terms of treatment effect. Improvements in CTT and VF were found to be significantly associated with corresponding BL scores (P < .0001 for each primary outcome) in both unadjusted and adjusted regressions, that is, subjects with worse BL scores generally had greater improvements.
4.2.2. Secondary efficacy outcomes
Performance of subjects on cognitive function, activities of daily living, and behaviors were unchanged from BL to W24 with no statistically significant difference between the placebo and MLC901 groups (Table 3).
TABLE 3.
Secondary endpoints –‐Summary of comparisons between MLC901 and placebo at BL and change from BL to week W24 (mean +/– SD)
| BL | Change from BL at W24 | Comparison | ||||
|---|---|---|---|---|---|---|
| Variable | Placebo | MLC901 | Placebo | MLC901 | Difference (CI) | P value |
| ADAS‐Cog | 26.3 ± 7.33 | 26.1 ± 7.6 | −0.53 ± 5.35 | −1.89 ± 6.54 | 1.36 (−1.03, 3.75) | .26 |
| MoCA | 18.0 ± 5.14 | 19.2 ± 5.06 | 1.17 ± 3.03 | 0.88 ± 2.61 | 0.30 (−0.80, 1.40) | .59 |
| Digit Cancellation Test | 18.0 ± 8.22 | 16.1 ± 6.72 | 0.76 ± 5.78 | 1.63 ± 4.83 | −0.87 (−2.94,1.20) | .41 |
| Clock Drawing Test | 3.61 ± 1.31 | 3.65 ± 1.41 | 0.28 ± 1.28 | 0.26 ± 1.04 | 0.02 (−0.43,0.47) | .93 |
| Picture Recall | ||||||
| Immediate | 4.17 ± 1.99 | 4.79 ± 1.88 | 0/09 ± 1.93 | 0.09 ± 1.49 | 0.00 (−0.67,0.99) | .99 |
| Delayed | 2.63 ±1.94 | 3.11 ± 1.89 | 0.80 ± 1.77 | 0.77 ± 1.75 | 0.03 (−0.66,0.72) | .93 |
| Delayed recognition | 7.98 ± 2.49 | 8.32 ± 1.97 | 0.37 ± 2.25 | 0.05 ± 1.87 | 0.31 (−0.49,1.12) | .44 |
| VMR | ||||||
| Immediate | 19.7 ± 8.69 | 19.5 ± 8.85 | 0.72 ± 5.62 | 2.16 ± 6.84 | −1.44 (−3.93,1.05) | .25 |
| Delayed | 9.9 ± 9.04 | 10.6 ± 9.23 | 2.62 ± 9.65 | 3.49 ± 8.73 | −0.87 (−4.49,2.75) | .63 |
| Delayed recognition | 1.75 ± 1.18 | 1.67 ± 1.23 | 0.20 ± 1.59 | 0.39 ± 1.37 | −0.18 (−0.77,0.40) | .54 |
| FAB | 12.8 ± 2.95 | 13.6 ± 2.96 | 0.70 ± 1.88 | 0.09 ± 2.18 | 0.61 (−0.20,1.42) | .14 |
| ADSC‐ADL | 38.3 ± 9.5 | 37.9 ± 10.1 | 0.6 ± 8.2 | 0.1 ± 7.4 | 0.5 (−2.8,3.8) | .75 |
| NPI | 3.84 ± 5.83 | 5.36 ± 8.55 | −0.42 ± 6.93 | −2.50 ± 5.33 | 2.08 (−0.52,4.68) | .12 |
| GDS | 3.67 ± 3.55 | 3.35 ± 2.83 | −0.09 ± 3.77 | −0.74 ± 2.63 | 0.65 (−0.60,1.90) | .31 |
Abbreviations: ADAS‐Cog, Alzheimer's Disease Assessment Scale‐Cognitive Subscale; ADCS‐ADL, Alzheimer's Disease Cooperative Study Activities of Daily Living; BL, baseline; CI, confidence interval; FAB, Frontal Assessment Battery; GDS, Geriatric Depression Scale; MoCA, Montreal Cognitive Assessment; NPI, Neuropsychiatry Inventory; VMR, Visual Memory Test; W24, week 24.
4.3. Exploratory analysis
4.3.1. CTT
The effect size of MLC901 in CTT1 appeared greater numerically (but remained statistically non‐significant) in the population impaired at BL compared to the overall population. For CTT2, better improvement in MLC901 than placebo was seen at W12 in ”Impaired at BL” (P = .05), ”Able to perform (overall)“ (P = .04) and ”Able to perform (impaired)“ subgroups (P = .03); however, these treatment effects were not statistically significant at W24 (Figure 3).
FIGURE 3.
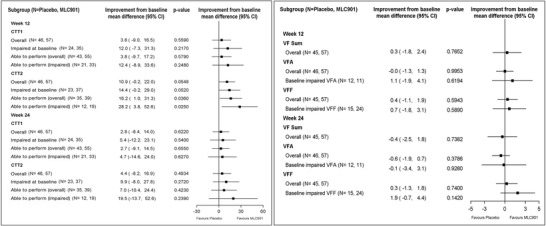
Forest plot showing subgroup analysis on Color Trails Test (CTT) and Verbal Fluency (VF)
4.3.2. VF
MLC901 did not appear to improve VFA (compared to placebo) in either the overall population or the subgroup of ”BL impaired VFA”. The improvement in the VFF from BL was better but statistically non‐significant in MLC901 than placebo groups at W24 in the subgroup of ”BL impaired VFF” (Figure 3).
In this study 42% of subjects were not impaired at each in CTT1 and CTT2 at BL. Similarly, 78% and 62% of subjects were not impaired on VFA and VFF, respectively.
4.4. Safety
There was no significant difference between the MLC901 and placebo group in terms of proportion of subjects experiencing AE or SAE. The number of subjects experiencing AE was 20 (43.5%) in placebo and 32 (56.1%) in MLC901 groups (P = .24). The number of subjects experiencing SAE was 6 (13.0%) in placebo and 13 (22.8%) in MLC901 groups (proportion difference = 9.8%, 95% CI: –5.7% to 24.0%, P = .31). There were six AEs that were deemed possibly related to MLC901: dry throat, dizziness, hematoma right thigh, vomiting, neuropathic pain (left face and arm), and sleepiness. The details of the AEs and SAEs are presented in Table 2.
TABLE 2.
Adverse events
| Placebo (N = 46) | MLC901 (N = 57) | |
|---|---|---|
| Number of patients experiencing AEs (including SAEs), n (%) | 20 (43%) | 32 (56%) |
| Number of AEs (including SAEs) | 34 | 43 |
| Severity | 24 (70%) | 26 (61%) |
| Mild | 8 (24%) | 10 (23%) |
| Moderate | 2 (6%) | 7 (16%) |
| Severe | ||
| Treatment related | ||
| Not related | 31 (91%) | 36 (84%) |
| Possibly related | 2 (6%) | 6 (14%) |
| Definitely related | 1 (3%) | 0 |
| Unknown | 0 | 1 (2%) |
| Action taken to study treatment | ||
| None | 31 (91%) | 34 (79%) |
| Discontinued permanently | 1 (3%) | 3 (7%) |
| Discontinued temporarily | 2 (6%) | 6 (14%) |
| Treatment given | ||
| Yes | 20 (59%) | 30 (70%) |
| No | 13 (38%) | 12 (28%) |
| Unknown | 1 (3%) | 1 (2%) |
| Outcome | ||
| Resolved, no sequelae | 23 (68%) | 30 (70%) |
| AE still present, no treatment | 3 (9%) | 2 (5%) |
| AE still present, being treated | 3 (9%) | 2 (5%) |
| Residual effects present, treated | 2 (6%) | 6 (13%) |
| Death | 0 | 1 (2%) |
| Unknown | 3 (9%) | 2 (5%) |
| Possible alternative explanation | ||
| Study procedure | 2 (6%) | 1 (2%) |
| Disease state | 3 (9%) | 6 (14%) |
| Concomitant medications | 1 (3%) | 2 (5%) |
| Concurrent illness | 3 (9%) | 5 (12%) |
| Other causes | 24 (71%) | 27 (63%) |
| Not applicable | 1 (3%) | 2 (5%) |
| Number of patients experiencing SAEs subjects, n (%) | 6 (13%) | 13 (23%) |
| Number of SAEs | 7 | 14 |
| SAE Criteria a | ||
| Death | 0 | 1 (7%) |
| Life threatening | 0 | 0 |
| Inpatient/prolonged hospitalization | 5 (71%) | 13 (93%) |
| Persistent disability/incapacity | 1 (14%) | 0 |
| Important medical event | 1 (14%) | 1 (7%) |
| Treatment related | ||
| Not related | 6 (86%) | 12 (86%) |
| Unlikely related | 1 (14%) | 1 (7%) |
| Possibly related | 0 | 1 (7%) |
| Action taken to study treatment | ||
| None | 5 (72%) | 9 (65%) |
| Interrupted temporarily | 1 (14%) | 3 (21%) |
| Discontinued/terminated study | 1 (14%) | 2 (14%) |
| Outcome | ||
| Complete recovery | 5 (72%) | 6 (43%) |
| Recovery with sequelae | 1 (14%) | 7 (50%) |
| Death | 0 | 1 (7%) |
| Unknown | 1 (14%) | 0 |
| Causality of SAE | ||
| Disease under study | 0 | 1 (7%) |
| Other illness | 4 (57%) | 4 (29%) |
| Concurrent medication / treatment | 1 (14%) | 1 (7%) |
| Others | 2 (29%) | 8 (57%) |
Notes: Number of patients in the study arm was the denominator for calculating proportion of patients experiencing AEs (SAEs); for the remaining proportions, the number of AEs (SAEs) was the denominator. .
Abbreviations: AE, adverse events; SAE, serious adverse event.
One SAE in the MLC901 arm satisfied two SAE criteria: inpatient/prolonged hospitalization, and important medical event.
5. DISCUSSION
NEURITES is a pilot randomized placebo‐controlled trial exploring the efficacy of MLC901 to improve post‐stroke executive function. There was no statistical difference between MLC901 and placebo for the primary and secondary outcomes. The sample size was based upon the need to have sufficient power to detect an anticipated 2‐point difference in the improvement in VF between MLC901 and placebo, but we observed only a difference < 1 point at 24 weeks, and the study did not have adequate power to detect such a difference. Furthermore, the the NEURITES trial included a large proportion (48%) of subjects with mild CIND, these patients performing relatively well with the executive function tests (VF and CTT), and therefore leaving only a small margin for improvement to observe the treatment effect of MLC901. It could be, and this hypothesis is to some extent supported by the multiple regression analysis, that there is a greater chance to be able to detect a treatment effect in patients who have worse BL scores, and, after this pilot study, we believe that future trials should be performed in more severely impaired patients. Indeed, this is further supported by the post hoc hypothesis‐generating exploratory analysis (i.e., subgroup analysis), suggesting that treatment effect might be enhanced in subjects more severely impaired. We recognize, however, that when using multiple subgroups, there is a risk of Type I error inflation, and false positive results. Yet, the purpose here was not an attempt to detect statistical significance within one or several subgroups when the overall primary results of the study are not significant. The objective was to assess whether the observation that treatment effect might be more likely in patients with more impairment at BL is just a chance finding in one particular subgroup, or whether it is seen consistently, independently of how precisely the subgroup is defined.
A person's cognition may fluctuate for many reasons such as, for example, tiredness, anxiety, medication, comorbidity. Cognitive fluctuations (CF) are prevalent in dementia. 34 , 35 , 36 , 37 In CF there is spontaneous alteration in cognition, attention, and arousal. We acknowledge that this is a limitation of using cognitive outcomes. An alternative approach could be to use CIND severity subgroups (mild/moderate). The executive function performance may have improved in both the groups as the Trail Making Test and VF are susceptible to practice effects. 38 , 39 , 40 Further study should investigate the role of practice effects, and the possible ”regression to the mean” phenomenon, leading to patients with more severe impairment at BL improving the most during the study. Spontaneous recovery in cognition has been observed in 35.9% of VCIND patients, 41 which is similar to the current results in which a significant effect observed at W12 was no longer observed at W24. The decline in cognition after stroke is slow; 7 out of 18 (39%) subjects who had VCIND (Cognitive Dementia Rating [CDR] = 0.5) at month 3 improved (CDR = 0) at 6 to 12 months and 78% of subjects had stable cognition at the end of 2 years. 42 Therefore, a longer follow‐up duration might be required, while the 24‐week study duration in NEURITES was probably too short to detect a significant treatment response. A larger sample size with 80 patients per arm is required to detect the observed difference of 19.5 seconds in CTT2 (SD ± 43.4 seconds) in patients who are impaired at BL but remain able to perform the test, while 62 patients per arm is required to detect the observed difference of 1.9 (SD ± 3.73) on VFF in patients with BL‐impaired VFF. The improvements in CTT and VF were found to be significantly associated with their corresponding BL values.
The safety of MLC901 was considered manageable in this study. There was no significant difference of AE and SAE rates between MLC901 and placebo. A total of six AEs was considered “possibly related” to administration of MLC901. Among these AEs, vomiting and dizziness have already been reported in other MLC901 studies. The other four AEs (hematoma right thigh, dry throat, neuropathic pain at left face and arm, sleepiness) appear more idiosyncratic in nature. Overall, with only 57 patients in the MLC901 group, the extent of the safety database from this trial is limited, but the general safety profile is similar to what has been observed in other MLC901 studies, and in post‐marketing experience with MLC901 obtained in > 30 countries.
We note six study limitations. (1) We could not achieve 100% follow‐up; treatment compliance rate ≥ 80% was observed only in 56% of subjects. Lost contact and refusal to attend visits were the main factors that contributed to < 100% follow‐up. The low compliance to the protocal may be due to dosing frequency or difficulty in taking two capsules 3 times a day especially with concomitant medications. (2) CIND at study entry was based on the VDB 8 whereas the trial primary endpoint was executive function as measured by CTT and VF; these differences might be considered while planning for future trials as the VDB does not measure executive functions such as cognitive flexibility and task shifting. (3) There was imbalance in the randomization, with more subjects in the treatment group (n = 57) than the placebo group (n = 46). The block size of 10 may have been too large in a study of 103 subjects with stratified randomization. The imbalance may be due to discarding/skipping some randomization numbers for several blocks due to drug expiry. (4) We did not capture the acute stroke or rehabilitation treatment that the patients received after the index stroke and prior to recruitment and this data should be collected in future studies. (5) Given the age range in this study, a substantial proportion of the older participants may have mixed neuropathology contributing to their cognitive impairment. The diagnosis of VCIND was based on clinical features and neuroimaging confirmation of stroke but no other biomarkers were used to detect other pathologies. (6) We also did not perform apolipoprotein E carrier status testing, which has been shown to influence post‐stroke cognitive impairment.
Our results suggest that improvements in CTT and VF were significantly associated with the corresponding BL values and most of the participants were not impaired. For future studies selection of a population with executive function impairment and a longer follow‐up duration is recommended.
6. CONCLUSIONS
MLC901 did not have a detectable effect in our study population. MLC901 might be beneficial to subjects with more severe VCIND at BL. MLC901 had an acceptable safety profile in this population, consistent with what is known from the experience obtained with MLC901 in a stroke patient population. A larger clinical trial with longer follow‐up duration in subjects with executive function impairment at BL could improve the possibility of detecting the treatment effects of MLC901. The results from this study could help to design and characterize the type of subjects to be included in future clinical trials.
CONFLICTS OF INTEREST
Grants were received by Christopher L. H. Chen from the National Medical Research Council of Singapore (NMRC/MOHIAFCAT1/0060/2016, NMRC/1288/2011 and NMRC/1096/2006) for research in MLC601 and MLC901. Christopher L. H. Chen and Narayanaswamy Venketasubramanian received grants from the CHIMES society for MLC601– and 901–related research and meetings for presenting the research data. Christopher L. H. Chen and Narayanaswamy Venketasubramanian also received honorarium from Moleac for being scientific advisory board members. Nguyen Trọng Hung, Simeon Marasigan, Nagaendran Kandiah, Deidre de Silva, Eddie Chong, Lu Qingshu received funding/grants for the trial and accommodation and transportation support for meetings from Moleac Pte Ltd. Chun Fan Lee has no conflict of interest. The NEURITES trial was supported by the Moleac Pte Ltd. Moleac provided the investigational product and grant for the study and conduct of the trial.
Chen CLH, Nguyen TH, Marasigan S, et al. NEURoaid II (MLC901) in cognitively Impaired not demenTEd patientS (NEURITES): A pilot double blind, placebo‐controlled randomized trial. Alzheimer's Dement. 2021;7:e12161. 10.1002/trc2.12161
ClinicalTrials.gov Identifier: NCT01847924
REFERENCES
- 1. Gorelick PB, Scuteri A, Black SE, et al. American heart association stroke council, council on epidemiology and prevention, council on cardiovascular nursing, council on cardiovascular radiology and intervention, and council on cardiovascular surgery and anesthesia. Vascular contributions to cognitive impairment and dementia: a statement for healthcare professionals from the American heart association/American stroke association. Stroke. 2011;42:2672‐2713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Van der Flier WM, Skoog I, Schneider JA, et al. Vascular cognitive impairment. Nat Rev Dis Primers. 2018;4:18003. [DOI] [PubMed] [Google Scholar]
- 3. Skrobot OA, Attems J, Esiri M, et al. Vascular cognitive impairment neuropathology guidelines (VCING): the contribution of cerebrovascular pathology to cognitive impairment. Brain. 2016;139:2957‐2969. [DOI] [PubMed] [Google Scholar]
- 4. Sachdev PS, Kalaria RN, O'Brien J, et al. Diagnostic criteria for vascular cognitive disorders: a VASCOG statement. Alzheimer Dis Assoc Disord. 2014;28:206‐218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Rockwood K, Wentzel C, Hachinski V, et al. Prevalence and outcomes of vascular cognitive impairment. Vascular cognitive impairment investigators of the canadian study of health and aging. Neurology. 2000;54:447‐451. [DOI] [PubMed] [Google Scholar]
- 6. Moorhouse P, Rockwood K. Vascular cognitive impairment: current concepts and clinical developments. Lancet Neurol. 2008;7:246‐255. [DOI] [PubMed] [Google Scholar]
- 7. Wentzel C, Rockwood K, MacKnight C, et al. Progression of impairment in subjects with vascular cognitive impairment without dementia. Neurology. 2001;57:714‐716. [DOI] [PubMed] [Google Scholar]
- 8. Tham W, Auchus AP, Thong M, et al. Progression of cognitive impairment after stroke: one year results from a longitudinal study of Singaporean stroke patients. J Neurol Sci. 2002;203‐204(C):49‐52. [DOI] [PubMed] [Google Scholar]
- 9. Narasimhalu K, Ang S, De Silva DA, et al. Severity of CIND and MCI predict incidence of dementia in an ischemic stroke cohort. Neurology. 2009;73:1866‐1872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Heurteaux C, Gandin C, Borsotto M, et al. Neuroprotective and neuroproliferative activities of NeuroAid (MLC601, MLC901), a Chinese medicine, in vitro and in vivo. Neuropharmacology. 2010;58:987‐1001. [DOI] [PubMed] [Google Scholar]
- 11. Quintard H, Borsotto M, Veyssiere J, et al. MLC901, a traditional Chinese medicine protects the brain against global ischemia. Neuropharmacology. 2011;61:622‐631. [DOI] [PubMed] [Google Scholar]
- 12. Moha Ou Maati H, Borsotto M, Chatelain F, et al. Activation of ATP‐sensitive potassium channels as an element of the neuroprotective effects of the Traditional Chinese Medicine MLC901 against oxygen glucose deprivation. Neuropharmacology. 2012;63:692‐700. [DOI] [PubMed] [Google Scholar]
- 13. Gandin C, Widmann C, Lazdunski M, Heurteaux C. MLC901 favors angiogenesis and associated recovery after ischemic stroke in mice. Cerebrovasc Dis. 2016;42:139‐154. [DOI] [PubMed] [Google Scholar]
- 14. Widmann C, Gandin C, Petit‐Paitel A, et al. The traditional chinese medicine MLC901 inhibits inflammation processes after focal cerebral ischemia. Sci Rep. 2018;8:18062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lorivel T, Gandin C, Veyssière J, et al. Positive effects of the traditional chinese medicine MLC901 in cognitive tasks. J Neurosci Res. 2015;93:1648‐1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kong KH, Wee SK, Ng CY, et al. A double blind, placebo‐controlled, randomized phase II pilot study to investigate the potential efficacy of the traditional Chinese medicine Neuroaid (MLC 601) in enhancing recovery after stroke (TIERS). Cerebrovasc Dis. 2009;28:514‐521. [DOI] [PubMed] [Google Scholar]
- 17. Hasan ZN, Al‐Mahdawi AM, Hasan HA, FI Al‐Saffar. Observing the outcome of using NeuroAid [MLC 601] on a sample of Iraqi stroke patients. Iraqi J Med Sci. 2012;10:255‐259. [Google Scholar]
- 18. Chen CL, Young SH, Gan HH, et al. Chinese medicine neuroaid efficacy on stroke recovery: a double‐blind, placebo‐controlled, randomized study. Stroke. 2013;44:2093‐2100. [DOI] [PubMed] [Google Scholar]
- 19. Venketasubramanian N, Young SH, Tay SS, et al. CHInese medicine NeuroAiD efficacy on stroke recovery ‐ Extension study (CHIMES‐E): a multicenter study of long‐term efficacy. Cerebrovasc Dis. 2015;39:309‐318. [DOI] [PubMed] [Google Scholar]
- 20. Nyenhuis DL, Gorelick PB, Geenen EJ, et al. Pattern of neuropsychological deficits in vascular cognitive impairment‐no dementia (vascular CIND). Clin Neuropsychol. 2004;18:41‐49. [DOI] [PubMed] [Google Scholar]
- 21. Chen CL, Ikram K, Angi Q, et al. The NeuroAiD II (MLC901) in vascular cognitive impairment study (NEURITES). Cerebrovasc Dis. 2013;35 Suppl 1:23‐29. [DOI] [PubMed] [Google Scholar]
- 22. D'Elia L, Satz P, Uchiyama CL, White T. Color Trails Test. Professional manual. Odessa (FL): Psychological Assessment Resources Inc.; 1996. [Google Scholar]
- 23. Shao Z, Janse E, Visser K, Meyer AS. What do verbal fluency tasks measure? Predictors of verbal fluency performance in older adults. Front Psychol. 2014;5:772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Rosen WG, Mohs RC, Davis KL. A new rating scale for Alzheimer's disease. Am J Psychiatry. 1984;141:1356‐1364. [DOI] [PubMed] [Google Scholar]
- 25. Nasreddine ZS, Phillips NA, Bedirian V, et al. The montreal cognitive assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53:695‐699. [DOI] [PubMed] [Google Scholar]
- 26. Yeo D, Gabriel C, Chen C, et al. Pilot validation of customized neuropsychological battery in elderly Singaporeans. Neurol J South East Asia. 1997;2:123. [Google Scholar]
- 27. Dubois B, Slachevsky A, Litvan I, Pillon BFAB. The FAB: a frontal assessment battery at bedside. Neurology. 2000;55:1621‐1626. [DOI] [PubMed] [Google Scholar]
- 28. Galasko D, Bennett D, Sano M, et al. An inventory to assess activities of daily living for clinical trials in Alzheimer's disease. Alzheimer disease and associated disorders. Alzheimer Dis Assoc Disord. 1997;11 Suppl 2:33‐39. [PubMed] [Google Scholar]
- 29. Galasko D, Bennett DA, Sano M, et al. ADCS prevention instrument project: assessment of instrumental activities of daily living for community‐dwelling elderly individuals in dementia prevention clinical trials. Alzheimer Dis Assoc Disord. 2006;20(4 Suppl 3):152‐169. [DOI] [PubMed] [Google Scholar]
- 30. Cummings JL, Mega M, Gray K, et al. The neuropsychiatric inventory: comprehensive assessment of psychopathology in dementia. Neurology. 1994;44:2308‐2308. [DOI] [PubMed] [Google Scholar]
- 31. Sheikh JI, Yesavage JA. Geriatric Depression Scale (GDS): recent evidence and development of a shorter version. Clin Gerontol. 1986;5:165‐173. [Google Scholar]
- 32. Narasimhalu K, Effendy S, Sim CH, et al. A randomized controlled trial of rivastigmine in patients with cognitive impairment no dementia because of cerebrovascular disease. Acta Neurol Scand. 2010;121:217‐224. [DOI] [PubMed] [Google Scholar]
- 33. Lezak M, Howieson D, Loring D. Neuropsychological Assessment. New York: Oxford University Press; 2004. [Google Scholar]
- 34. Román GC. Vascular dementia: distinguishing characteristics, treatment, and prevention. J Am Geriatr Soc. 2003;51(5 Suppl Dementia):296‐304. [DOI] [PubMed] [Google Scholar]
- 35. Sachdev PS, Brodat H, Looi JCL. Vascular dementia: diagnosis, management and possible prevention. Med J Aust. 1999;170:81‐85. [DOI] [PubMed] [Google Scholar]
- 36. Lee DR, Taylor JP, Thomas AJ. Assessment of cognitive fluctuation in dementia: a systematic review of the literature. Int J Geriatr Psychiatry. 2012;27:989‐998. [DOI] [PubMed] [Google Scholar]
- 37. Escandon A, Hammadi NA, Galvin JE. Effect of cognitive fluctuation on neuropsychological performance in aging and dementia. Neurology. 2010;74:210‐217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Durvasula RS, Satz P, Hinkin CH, et al. Does practice make perfect? Results of a six‐year longitudinal study with semi‐annual testings. Archives of Clinical Neuropsychology [Abstract]. 1996;11:386. [Google Scholar]
- 39. Dye OA. Effects of practice on Trail Making Test performance. Percept Mot Skills. 1979;48:206.450619 [Google Scholar]
- 40. Bartels C, Wegrzyn M, Wiedl A, et al. Practice effects in healthy adults: a longitudinal study on frequent repetitive cognitive testing. BMC Neurosci. 2010;11:118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Desmond DW, Moroney JT, Sano M, Stern Y. Recovery of cognitive function after stroke. Stroke. 1996;27:1798‐1803. [DOI] [PubMed] [Google Scholar]
- 42. der Ser T, Barba R, Morin MM, et al. Evolution of cognitive impairment after stroke and risk factors for delayed progression. Stroke. 2005;36:2670‐2675. [DOI] [PubMed] [Google Scholar]


