Abstract
Background
Malnutrition among older people is one of the serious public health problem worldwide. Nutritional status and levels of nutrients of older patients with COVID-19 effect on COVID-19 outcomes. The purpose of this systematic review was to identify the prevalence of malnutrition and levels of nutrients associated with outcomes of the older patients with COVID-19.
Materials and Methods
A literature search was performed using PubMed, Science direct and Google scholar database using specific keywords related to the aims. All related articles published on COVID-19 during 2020 were retrieved. PRISMA Statement was followed. The quality of the study was assessed using the quality assessment tools of the National Heart, Lung, and Blood Institute of the National Institutes of Health.
Results
Of the 2979 studies found, a total of eight studies were included in this review. Of these studies, three provided data on nutritional status and outcomes of COVID-19 among older patients with COVID-19. The prevalence of malnutrition among older patients with COVID-19 was high and it was associated with negative outcomes including hospital deaths and transfer to intensive care units. Five studies provided data on nutrients and outcomes of COVID-19. Low albumin, vitamin D, magnesium ,vitamin B12, Se status were associated with malnutrition, oxygen therapy and/or intensive care support of the patients, survival of COVID -19.
Conclusions
Extra care should be provided to older patients with COVID-19 to minimize the prevalence of malnutrition and negative outcomes of COVID-19.
Keywords: older people, malnutrition, nutrients, COVID-19
1. Introduction
Coronavirus disease (COVID-19) has become a public health crisis resulting in a great variety of challenges to the world (Chen, 2020). It is caused by the novel coronavirus SARS-CoV-2 and represents a significant threat to healthcare worldwide(Lidoriki et al., 2020). All people are affected by the pandemic. However, older people are more severely affected by the disease (Butler & Barrientos, 2020). In addition to the classical symptoms of COVID-19, older patients with COVID-19 display geriatric frailty symptoms such as confusion, walking impairments and high mortality (Karlsson et al., 2020).
Older patients with COVID-19 disease are at risk of malnutrition or co-malnutrition. Huang et al. (2020) found that SARS-CoV-2 attacks mucosal epithelium and causes gastrointestinal symptoms, worsening the nutritional status of older patients. Many identified risk factors related to viral infections and deaths from COVID-19 have a causal relationship with nutritional status and specific essential nutrients. It is well known that essential nutrients play a major role in maintaining the normal functions of the immune system (Richardson & Lovegrove, 2020). In addition, malnutrition negatively affects the outcomes of older people including changes in cardiorespiratory, gastrointestinal and musculoskeletal systems and poor quality of life (Bencivenga et al., 2020; Damayanthi et al., 2018).Evidence suggests that several indices of nutritional status could be used in the prognosis of morbidity and mortality of the older people diagnosed with COVID-19 infection (Lidoriki et al., 2020).
An optimal immune response is very crucial in fighting the infection. An adequate diet and nutrition plays a major role in order to prevent infections. Sufficient protein intake is needed for the optimal antibody production. Acute inflammatory response of the infection consumes the protein that made up body muscles. The synthesis of acute-phase proteins such as C-reactive protein, ferritin, tumor necrosis factor alpha, interleukin family factors require the consumption of albumin and muscle protein (Jia, 2016). Further, inflammatory response caused by the SARS-CoV-2 virus and use of glucocorticoids in treatments relate to pathogenesis (Rehman et al., 2020). Numerous micronutrients have well-established immunomodulatory effects. Key dietary components such as vitamins A, C, D, E, B6, B12, and folate, iron, magnesium and trace elements including zinc, selenium and copper and omega 3 fatty acids have a potential role in the management of COVID-19 (Shakoor et al., 2021) . Besides the under nutrition, in terms of over nutrition, obesity and being overweight were represented as unfavourable factors for infection of novel coronavirus among older patients. The higher BMI of the older patients relate to negative outcomes of COVID-19 (de Siqueira et al., 2020). The high consumption of diets rich in saturated fats, sugars, and refined carbohydrates contribute to the prevalence of obesity among this population leading them at an increased risk of severe COVID-19 pathology and mortality (Butler & Barrientos, 2020).
A variety of nutritional status related negative outcomes are associated with older patients with COVID-19. Scholars use multiple and different screening tools and techniques to evaluate nutritional status as well as levels of micronutrients of older patients with COVID-19. Various outcomes of the older patients with COVID-19 have been investigated. This difference is relatively large and needs further investigation. The purpose of this systematic review was to identify the prevalence of malnutrition and levels of nutrients associated with outcomes of the older patients with COVID-19.
2. Materials and methods
2.1. Data sources and search strategy
A systematic review of literature published during 2020 was performed. Three electronic databases (PubMed, Science direct and Google scholar) were searched using the key words: (“malnutrition” OR “nutritional status” OR “under nutrition”) AND (“elderly” OR “older” AND (“COVID 19” OR “corona virus disease”). The search was done without study location limits to capture all possible relevant titles. In order to identify additional studies, references, related articles and citations of papers were used.
This systematic review is carried out as per the guidelines of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement (Moher et al., 2009). Due to the heterogeneity of methodologies employed in the selected studies, the data were not appropriate for a meta-analysis. This decision was applied for both prevalence and nutrient data.
2.2. Study selection
All relevant articles were merged into a single file. EndNote X7 was used for the removal of duplicates. Then, the titles and abstracts of all the remaining articles were screened for relevance independently by two reviewers (HDWTD and KIPP). Full papers were retrieved when the relevance could not be discovered from the abstract. . The literature review was conducted from January 2020.
Evaluation of full text of potential relevant studies was done independently. Any disagreement was resolved through consensus.
2.3. Inclusion and exclusion criteria
Inclusion criteria: We included original full-text articles that met the following predefined criteria.
Setting: Community-dwelling or institutionalized or hospitalized patients who were aged 60 years or older were included. As a condition, all patients should be diagnosed as COVID-19 using positive Real-time reverse transcription polymerase chain reaction (RT-PCR) or evocative computed tomography scan (CT-scan) lesions.
Outcomes: Only studies that examined the associations of nutritional status, micronutrient and/or macronutrient status and/or a clear operational definition/measurement of nutritional status with COVID-19 outcomes were included. We focused the search on undernutrition and malnutrition and any research on over nutrition or obesity was not included. Body Mass Index (BMI) was used to define over nutrition or obesity.
Language: Only full-text articles published in English were included.
Exclusion criteria: Systematic reviews, articles that did not contain outcomes related to COID-19, studies with exclusively non older people were excluded.
2.4. Data extraction
Data extraction of the selected studies was done using a predefined data extraction form. Authors and year, study design, country and sample characteristics (age and sex), setting, diagnosis method of COVID-19, nutritional measurement tools, prevalence nutritional status, outcomes and main findings of searched studies were included in the data extraction form. The extracted outcomes were transferred to intensive care unit, hospital deaths, 14-day mortality, ratio of arterial oxygen partial pressure (PaO2)/ fractional inspired oxygen (FiO2), malnutrition, Oxygen therapy and/or intensive care support, mechanical ventilation, time to discharge or death, and Ordinal Scale for Clinical Improvement (OSCI) score in acute phase.
2.5. Assessment of study quality
The included studies were evaluated using the study quality assessment tools of the National Heart, Lung, and Blood Institute of the National Institutes of Health (NHLBI) .(NIH National Heart, Lung and Blood Institute) Reviewers used separate quality tools under NHLBI for different studies. Each tool contains 14 items and each item is rated as ‘yes’, ‘no’ or ‘other’ (cannot determine, not applicable or not reported). The reviewers systematically appraised the most critical factors that affect the internal and external validity of each study design. The tool related to quality assessment of observational, cohort and cross-sectional studies includes the following items. 1) Was the research question or objective in this paper clearly stated?, 2) Was the study population clearly specified and defined?, 3) Was the participation rate of eligible persons at least 50%?, 4) Were all the subjects selected or recruited from the same or similar populations (including the same time period)? Were inclusion and exclusion criteria for being in the study pre specified and applied uniformly to all participants?, 5) Was a sample size justification, power description, or variance and effect estimates provided?, 6) For the analyses in this paper, were the exposure(s) of interest measured prior to the outcome(s) being measured?, 7) Was the timeframe sufficient so that one could reasonably expect to see an association between exposure and outcome if it existed?, 9) For exposures that can vary in amount or level, did the study examine different levels of the exposure as related to the outcome (e.g., categories of exposure, or exposure measured as continuous variable)?, 10) Were the exposure measures (independent variables) clearly defined, valid, reliable, and implemented consistently across all study participants?, 11) Was the exposure(s) assessed more than once over time?, 12) Were the outcome measures (dependent variables) clearly defined, valid, reliable, and implemented consistently across all study participants?, 13) Were the outcome assessors blinded to the exposure status of participants?, 14) Was loss to follow-up after baseline 20% or less? Were key potential confounding variables measured and adjusted statistically for their impact on the relationship between exposure(s) and outcome(s)?. All the quasi experimental studies were assessed using the tool designed for quality assessment of quasi experimental studies and consists of these items. 1) Was the study described as randomized, a randomized trial, a randomized clinical trial, or an RCT?, 2) Was the method of randomization adequate (i.e., use of randomly generated assignment)?, 3) Was the treatment allocation concealed (so that assignments could not be predicted)?, 4) Were study participants and providers blinded to treatment group assignment?, 5)Were the people assessing the outcomes blinded to the participants' group assignments?, 6) Were the groups similar at baseline on important characteristics that could affect outcomes (e.g., demographics, risk factors, co-morbid conditions)?, 7) Was the overall drop-out rate from the study at endpoint 20% or lower of the number allocated to treatment?, 8) Was the differential drop-out rate (between treatment groups) at endpoint 15 percentage points or lower?, 9) Was there high adherence to the intervention protocols for each treatment group?, 10) Were other interventions avoided or similar in the groups (e.g., similar background treatments)?, 11) Were outcomes assessed using valid and reliable measures, implemented consistently across all study participants?, 12) Did the authors report that the sample size was sufficiently large to be able to detect a difference in the main outcome between groups with at least 80% power?, 13) Were outcomes reported or subgroups analysed prespecified (i.e., identified before analyses were conducted)?, and 14) Were all randomized participants analysed in the group to which they were originally assigned, i.e., did they use an intention-to-treat analysis?
2.6. Data analysis
An assessment of data and analysis of the included studies was performed to conclude nutritional status, macro/micro nutrient status and outcomes related to COVID-19. Extracted data was evaluated to examine the relationship between prevalence of malnutrition, various nutrients and COVID-19 outcomes. Overall, demographics of older people diagnosed with COVID-19, prevalence of malnutrition, nutrient status and any association between COVID-19 outcomes were reviewed. Quantitative analysis was done based on available data of the selected studies. Association between nutritional status and COVID-19 outcomes were presented as odds ratios (OR) and hazard ratio (HR) with 95% confidence interval (CI). P value was considered as < 0.05.
3. Results
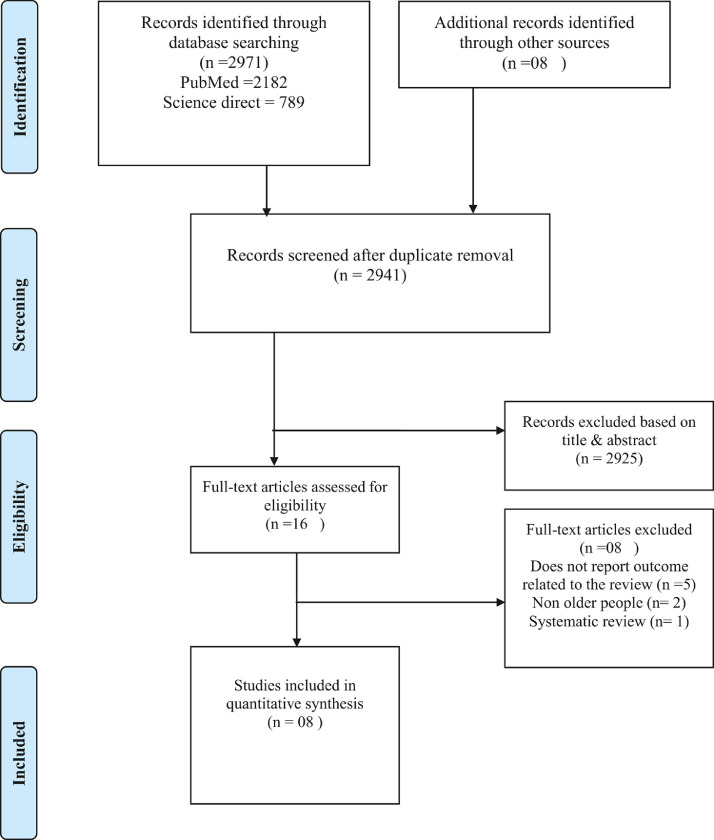
The details of the review process is depicted in Fig. 1 . A total of 2979 records were identified. After the removal of duplicates, 2941 records remained and were screened for pertinent content. From these studies, 2925 were excluded based on the title and abstract, leaving 16 study to be assessed for eligibility resulting in the exclusion of eight studies. Reasons for exclusion were: does not report outcome related to the review (n =5), non-older people (n= 2) and systematic review (n=1). Finally, eight studies met the criteria and were included in this review.
Fig. 1.
Flow diagram of the study selection process.
3.1. Participants and study characteristics
The included articles encompassed a sample of 1070 older adults with 50.65% of females (extracted whenever possible). A total of 66 participants were patients in nursing homes. The majority of the studies presented the mean age of participants. The range of the mean age of the studies was from 58.4 (±7) to 88 (±5) years. All the others were from hospital settings. The range of the sample sizes of the selected studies was 17 to 446. Five studies were conducted in Europe ( Annweiler et al., 2020; Annweiler et al., 2020; Bedock et al., 2020; Moghaddam et al., 2020; Recinella et al., 2020), and three in Asia (Rehman et al., 2020; Tan et al., 2020; Zuo et al., 2020). The diagnosis of older people for COVID-19 was done using two techniques: real-time PCR test (Annweiler et al., 2020; Annweiler et al., 2020; Bedock et al., 2020; Moghaddam et al., 2020; Recinella et al., 2020; Rehman et al., 2020; Tan et al., 2020; Zuo et al., 2020) and chest CT scans Annweiler et al., 2020; Bedock et al., 2020).
Of a total of eight included studies, three were cross sectional studies, one observational longitudinal study, one cohort observational study, one retrospective cohort study design, and two quasi-experimental studies. Table 1 shows the summary of the essential characteristics of the included studies.
Table 1.
Key characteristics of studies included in the systematic review.
| No | Authors and Year | Study Design | Mean age/Age range (years) | Country | Setting | Sample (n) | Diagnosis method- COVID-19 |
|---|---|---|---|---|---|---|---|
| 1 | Tan et al. (2020) | Cohort observational study | DBM group 58.4 (7.0) Control group 64.1 (7.9) |
Singapore | Hospital | DBM group n= 17 Male-64.7% control group n= 26 Male-57.7% |
A positive SARS-CoV-2 PCR from nasopharyngeal or throat swab |
| 2 | Zuo et al. (2020) | Retrospective cohort study design | 72.95 ±6.39 | China | Hospital | n= 446 Male 55.41% |
A positive RT-PCR from throat swab |
| 3 | Bedock et al. (2020) | Observational longitudinal study | 59.9 ±15.9 | France | Hospital | n = 114 Male 60.5% |
A positive PCR from nasopharyngeal or throat swab and/or evocative CT-scan lesions |
| 4 | Li et al. (2020) | Cross-sectional study, | 68.5 ± 8.8 | China | Hospital | n= 182 Male = 65 |
A positive PCR / next-generation sequencing |
| 5 | Recinella et al. (2020) | Cross sectional study | 83 /(76–91.5) | Italy | Hospital | 109 Male= 54 |
A positive RT-PCR from nasopharyngeal or throat swab |
| 6 | Moghaddam et al. (2020) | Cross-sectional study | Median age 77 | Germany | Hospital | n = 166 enrolled n = 33 for analysis |
A positive RT-PCR |
| 7 | Annweiler et al. (2020) | Quasi-experimental study | Intervention - 87.7±9.3 Comparator - 87.4±7.2 |
France | Nursing homes | Intervention - n=57 Women - 79% Comparator n=9 Women – 67% |
A positive RT-PCR |
| 8 | Annweiler et al. (2020) | Quasi-experimental study | 88 ± 5/ (78−100) | France | Hospital | 77 Women -49.4% |
A positive RT-PCR and/or chest CT-scan |
DMB: vitamin D/magnesium/vitamin B12; RT-PCR: Real-time reverse transcription polymerase chain reaction; CT-scan: computed tomography scan.
3.2. Quality of study assessment
Studies with various study designs were included in this review and assessed accordingly with the quality assessment tools of the National Heart, Lung, and Blood Institute of the National Institutes of Health (Table 2 ). The majority of the studies ( Annweiler et al., 2020; Annweiler et al., 2020; Bedock et al., 2020; Li et al., 2020; Moghaddam et al., 2020; Tan et al., 2020; Zuo et al., 2020) were rated ‘fair’. Only two studies (Recinella et al., 2020) rated ‘good’ in the quality assessment.
Table 2.
Quality appraisal of the included studies.
| Study | Quality appraisal criteria | Quality rating | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | ||
| Quality Assessment of Observational, Cohort and Cross-Sectional Studies | |||||||||||||||
| Tan et al. (2020) | * | * | * | * | ⁎⁎ | * | * | ⁎⁎⁎ | * | * | * | ⁎⁎ | ⁎⁎⁎ | * | Fair |
| Zuo et al. (2020) | * | * | * | * | ⁎⁎ | * | * | * | * | ⁎⁎ | * | ⁎⁎⁎ | ⁎⁎ | * | Fair |
| Bedock et al. (2020) | * | * | * | * | ⁎⁎ | ⁎⁎⁎ | * | * | * | * | * | ⁎⁎⁎ | * | * | Good |
| Li et al. (2020) | * | * | ⁎⁎⁎ | * | ⁎⁎ | ⁎⁎⁎ | ⁎⁎⁎ | * | * | ⁎⁎⁎ | * | ⁎⁎⁎ | ⁎⁎⁎ | * | Fair |
| Recinella et al. (2020) | * | * | ⁎⁎⁎ | * | ⁎⁎ | * | * | * | * | ⁎⁎ | * | ⁎⁎⁎ | * | * | Good |
| Moghaddam et al. (2020) | * | * | ⁎⁎⁎ | ⁎⁎⁎ | ⁎⁎ | * | * | ⁎⁎ | * | * | * | ⁎⁎⁎ | ⁎⁎ | ⁎⁎ | Fair |
| Quality Assessment of quasi experimental Studies | |||||||||||||||
| Annweiler et al. (2020) | ⁎⁎ | ⁎⁎ | ⁎⁎ | ⁎⁎⁎ | ⁎⁎⁎ | * | * | * | * | ⁎⁎ | * | ⁎⁎ | * | * | Fair |
| Annweiler et al. (2020) | ⁎⁎ | ⁎⁎ | ⁎⁎ | ⁎⁎⁎ | ⁎⁎⁎ | * | * | * | * | ⁎⁎ | * | ⁎⁎ | * | * | Fair |
Yes
No
Not applicable/ not stated.
3.3. Prevalence of nutritional status
Three studies showed the prevalence of malnutrition among older patients with COVID-19 (Bedock et al., 2020; Recinella et al., 2020; Rehman et al., 2020). Among these three studies, one study used Global Leadership Initiative on Malnutrition (GLIM) to screen the patients for malnutrition (Bedock et al., 2020). Another study used Mini Nutritional Assessment (MNA) (Rehman et al., 2020). Geriatric Nutritional Risk Index (GNRI) was used by Recinella et al. (2020) to assess the prevalence of malnutrition. Based on GLIM, the prevalence of malnutrition, moderate malnutrition and severe malnutrition of older patients in Europe was 42.1%, 23.7%, and18.4% respectively (Bedock et al., 2020).According to the MNA, the prevalence of malnutrition and risk of malnutrition among older patients were 52.7% and 27.5% in the study of .Li et al. (2020) assessed nutritional status using GNRI in both surviving and death group of COVID-19 older patients in China separately. In the surviving group, the prevalence of low, low risk and moderate-severe risk of GNRI was 26%, 12% and 28% correspondently whereas that of death group was 4%, 0% and 39% respectively (Recinella et al., 2020). Specific nutritional measurement tools used in these studies are described in Table 3 .
Table 3.
Nutritional status and COVID-19 outcomes.
| Authors and Year | Nutritional Measurement Tools | COVID-19 Outcome | Main Findings |
|---|---|---|---|
| Bedock et al. (2020) | Serum albumin Anthropometric measurements (Height, weight) Global Leadership Initiative on Malnutrition (GLIM) |
Transfer to intensive care unit (ICU) or death. | Prevalence of malnutrition = 42.1% Moderate= 23.7% Severe=18.4% Lower albumin levels were associated with a higher risk of transfer to ICU (OR 0.31; 95% CI 0.1; 0.7, p < 0.01) There was a trend for a higher risk of mortality in patients with weight loss above 5% of initial weight (OR: 3.7 95% CI 1.0; 26.5, p = 0.09). Nutritional status was not associated with the risk of transfer to ICU or death. |
| Li et al. (2020) | Mini Nutritional Assessment (MNA) Albumin and blood count (hemoglobin and lymphocyte count) |
Malnutrition | Prevalence of malnutrition= 52.7% Risk of malnutrition = 27.5% Diabetes (OR 2.12; 95% CI 1.92–3.21) Low calf circumference (OR 2.42; 95% CI 2.29–3.53) Low albumin (OR 2.98; 95% CI 2.43–5.19) were independent risk factors for malnutrition |
| Recinella et al. (2020) | Geriatric Nutritional Risk Index (GNRI) |
Hospital deaths PaO2/ FiO2 ratio |
Percentage of deaths = 39.4% Surviving group: Prevalence of GNRI low = 26%, Low risk=12% Moderate–severe risk = 28% Death group: Prevalence of GNRI low = 4%, Low risk=0% Moderate–severe risk = 39% |
ICU: Intensive care units; OR: odds ratio; CI: confidence interval.
3.4. COVID-19 outcomes
Various different COVID-19 related outcomes were identified in the selected studies. Transfer to intensive care unit (ICU) or death (Bedock et al., 2020), malnutrition itself (Rehman et al., 2020), hospital deaths, PaO2/FiO2 ratio (ratio of arterial oxygen partial pressure (PaO2 in mmHg) to fractional inspired oxygen) (Recinella et al., 2020), oxygen therapy and/or intensive care support (Tan et al., 2020), ICU admission and mechanical ventilation (Zuo et al., 2020), time to discharge or death (Moghaddam et al., 2020), COVID-19 mortality and Ordinal Scale for Clinical Improvement (OSCI) score in acute phase (Annweiler et al., 2020), and 14-day mortality and highest (worst) score OSCI measured during COVID-19 acute phase ( Annweiler et al., 2020). The details of these outcomes are presented in Tables 3 and 4 .
Table 4.
Macro/Micro nutrient status and COVID-19 outcomes.
| Authors and Year | Measured nutrients | Intervention | Outcome | Main Findings |
|---|---|---|---|---|
| Tan et al. (2020) | Vitamin D Magnesium Vitamin B12 exposure |
Administered Oral vitamin D3 1000 IU OD, Magnesium 150mg OD and Vitamin B12 500mcg OD |
Oxygen therapy and/or intensive care support | Exposure of Vitamin D, Magnesium and Vitamin B12 was associated with oxygen therapy and/or intensive care support (OR: 0.20; 95% CI 0.04;0.93) |
| Zuo et al. (2020) | Prealbumin level | ICU admission Mechanical ventilation |
The incidence of all-cause death, ICU admission and mechanical ventilation were significantly decreased across prealbumin tertiles All-cause death - 35.14% vs. 7.43% vs. 2.01% for tertile 1 vs. tertile 2 vs. tertile 3. ICU admission - 37.16% vs. 6.08% vs. 3.33% for tertile 1 vs. tertile 2 vs. tertile 3. Mechanical ventilation - 42.57% vs. 13.15% vs. 8.05% for tertile 1 vs. tertile 2 vs. tertile 3. Borderline significant association between BMI and risk of mechanical ventilation (OR:1.17; 95% CI, 1.0; 1.37; p =0.0446) |
|
| Moghaddam et al. (2020) | Se status (Se, SELENOP) |
Time to discharge or death | Se status was significantly higher in samples from surviving COVID patients as compared with non-survivors (Se; 53.3 _ 16.2 vs. 40.8 _ 8.1 _g/L, SELENOP; 3.3 _ 1.3 vs. 2.1 _ 0.9 mg/L) Percentage of deaths = 18.18% |
|
| Annweiler et al. (2020) | Vitamin D3 | Intervention group - Received bolus vitamin D3 supplementation during COVID-19 or in the preceding month Comparator group – All other COVID-19 residents who did not receive any recent vitamin D supplementation |
COVID-19 mortality Ordinal Scale for Clinical Improvement (OSCI) score in acute phase |
82.5% of participants in the Intervention group survived COVID-19, compared to only 44.4% in the Comparator group (p=0.023) Vitamin D3 supplementation was inversely associated with OSCI score for COVID-19 (β=- 3.84;95%CI:-6.07;-1.62, p=0.001) Percentage of deaths = 22.72% |
| Annweiler et al. (2020) | Vitamin D | Intervention groups – Group 1 Regularly supplemented with vitamin D over the preceding year Group 2 Supplemented with vitamin D after COVID-19 diagnosis Group 3/comparator group Received no vitamin D Supplements |
14-day mortality Highest (worst) score on OSCI |
Experiencing onset of severe COVID-19 was lower in Group 1 (10.3%) compared to Group 3 (31.3%, p = 0.047) 14-day mortality was lower in Group 1 (6.9%) compared to Group 3 (31.3%, p = 0.02) No outcome differences between Groups 1 and 2 (p = 0.23 for the onset of severe COVID-19, and p = 0.33 for 14-day mortality) No outcome differences between Group 2 and 3 (p = 0.75 for the onset of severe COVID-19, and p = 0.50 for 14-day mortality) Group 1 (reference Group 3) - Inverse association between regular vitamin D supplementation and 14-day mortality (HR= 0.07; 95%CI: 0.01; 0.61, p=0.03) Group 2 (reference Group 3) –No association between regular vitamin D supplementation and 14-day mortality (HR= 0.37; 95%CI: 0.06; 2.21, p=0.28) Shorter survival time in Group 3 than those in Group 1 (log-rank p = 0.015) Shorter survival time - No difference between Groups 2 and 3 (log-rank p = 0.32) and between Groups 1 and 2 (log-rank p = 0.22) Group 1 - Regular vitamin D supplementation was associated with a lower proportion of participants with severe COVID-19 in acute phase/ lower risk of OSCI score (OR = 0.08; 95% CI: 0.01; 0.81, p = 0.033) compared to Group 3 Group 2 was not associated with any beneficial effect compared to Group 3 (OR = 0.46; 95% CI: 0.07; 2.85, p = 0.40) Percentage of deaths = 19.48% |
Se- Selenium; SELENOP -Selenoprotein P; OSCI - Ordinal Scale for Clinical Improvement; OR: odds ratio; CI: confidence interval; HR: hazard ratio
3.5. Nutritional status and COVID-19 outcomes
Three studies provided data on nutritional status and COVID-19 outcomes (Tan et al. (2020), Zuo et al. (2020), Moghaddam et al. (2020). Among the three studies that considered nutritional status, Recinella et al. (2020) assessed nutritional status by GNRI. In univariate analysis, GNRI moderate–severe risk category was a risk factor for in-hospital death (HR: 8.571; 95% CI 1.096–67.031). PaO2/FiO2 ratio (HR: 0.996; 95% CI 0.993–0.999) and body mass index (HR: 0.875; 95% CI 0.782–0.979) were protective factors for in-hospital death. Further, they found that higher survival in patients without GNRI moderate or severe risk category (p = 0.0013). At multivariate analysis, PaO2/FiO2 ratio (HR: 0.993; 5% 9CI 0.987–0.999, p = 0.046) and GNRI moderate–severe risk category (HR: 9.285; 95% CI 1.183–72.879, p = 0.034) were independently and significantly associated with in-hospital death.
The cross sectional study conducted by Li et al. (2020) identified malnutrition as a COVID-19 outcome. They found that diabetes (OR 2.12; 95% CI 1.92–3.21), low calf circumference (OR 2.42; 95% CI 2.29–3.53), and low albumin (OR 2.98; 95% CI 2.43–5.19) were as independent risk factors for malnutrition. Bedock et al. (2020) found a trend for a higher risk of mortality in patients with weight loss above 5% of initial weight (OR: 3.7; 95% CI 1.0; 26.5, p = 0.09). In the same study, nutritional status was not associated with the risk of transfer to ICU or death (Table 3).
3.6. Macro/micro nutrients and COVID-19 outcomes
Six studies reported data on the macro/micro nutrients and COVID-19 outcomes (Bedock et al. (2020), Tan et al. (2020), Zuo et al. (2020), Moghaddam et al. (2020), Annweiler et al. (2020), Annweiler et al. (2020) (Table 4). Measuring of prealbumin/albumin was based on laboratory data on admission to the hospital settings (Bedock et al., 2020; Li et al., 2020; Recinella et al., 2020; Zuo et al., 2020). Se status of the patients was measured after confirming the COVID-19 diagnosis (Moghaddam et al., 2020).
Two of the studies found that either albumin levels (Bedock et al. (2020), or prealbumin level (Zuo et al. (2020) was associated with a higher risk of transfer to ICU (OR 0.31; 95% CI 0.1; 0.7, p < 0.01) and all cause death, ICU admission and mechanical ventilation respectively (All-cause death - 35.14% vs. 7.43% vs. 2.01% for tertile 1 vs. tertile 2 vs. tertile 3, ICU admission - 37.16% vs. 6.08% vs. 3.33% for tertile 1 vs. tertile 2 vs. tertile 3 and Mechanical ventilation - 42.57% vs. 13.15% vs. 8.05% for tertile 1 vs. tertile 2 vs. tertile 3). Zuo et al. (2020) further found borderline significant association between BMI and risk of mechanical ventilation (OR:1.17; 95% CI, 1.0; 1.37; p =0.0446).
Selenium is another micro nutrient associated with survival of COVID-19. Moghaddam et al. (2020) found that Se status was significantly higher in samples from surviving COVID patients as compared with non-survivors (Se; 53.3 _ 16.2 vs. 40.8 _ 8.1 _g/L, SELENOP; 3.3 _ 1.3 vs. 2.1 _ 0.9 mg/L). Tan et al. (2020) reported that exposure of Vitamin D, Magnesium and Vitamin B12 was associated with oxygen therapy and/or intensive care support (OR: 0.20; 95% CI 0.04; 0.93). Further, Annweiler et al. (2020) conducted a in a quasi-experimental study among group of patients in nursing homes. In this study group 1 was received bolus vitamin D3 supplementation during COVID-19 or in the preceding month and group/comparator group did not receive any recent vitamin D supplementation. They reported that Vitamin D3 supplementation was inversely associated with OSCI score for COVID-19 (β=- 3.84;95%CI:-6.07;-1.62, p=0.001).
Another quasi-experimental study was conducted among hospitalized patients by Annweiler et al. (2020). There were three study groups in this particular study. Group 1 was regularly supplemented with vitamin D over the preceding year, group 2 was supplemented with vitamin D after COVID-19 diagnosis and group 3/comparator group did not receive vitamin D supplementation. Supplements compared to group 1 and 3, experiencing onset of severe COVID-19 was lower in group 1 (10.3%) than that of group 3 (31.3%, p = 0.047). 14-day mortality also was lower in group 1 (6.9%) compared to group 3 (31.3%, p = 0.02). No differences in onset of severe COVID-19 and 14-day mortality was observed between groups 1 and 2 (p = 0.23 for the onset of severe COVID-19, and p = 0.33 for 14-day mortality). Similar results were found between group 2 and 3 (p = 0.75 for the onset of severe COVID-19, and p = 0.50 for 14-day mortality). In group 1 compared to group 3, an inverse association between regular vitamin D supplementation and 14-day mortality was reported (HR= 0.07; 95%CI: 0.01; 0.61, p=0.03). No association was found between regular vitamin D supplementation and 14-day mortality (HR= 0.37; 95%CI: 0.06; 2.21, p=0.28) compared to group 1 and 2. Survival time was shorter in group 1 than those in group 3 (log-rank p = 0.015). No difference of survival time was observed between Groups 2 and 3 (log-rank p = 0.32) and between Groups 1 and 2 (log-rank p = 0.22). In the same study, regular vitamin D supplementation in group 1 was associated with a low severe COVID-19 in acute phase/ lower risk of OSCI score (OR = 0.08; 95% CI: 0.01; 0.81, p = 0.033) compared to Group 3. Finally, the study reported no association of Group 2 who received vitamin D supplementation after COVID-19 diagnosis which was not associated with any beneficial effect compared to Group 3 (OR = 0.46; 95% CI: 0.07; 2.85, p = 0.40).
4. Discussion
This review included eight studies in different countries. The studies focused on effects of malnutrition and nutrient deficiencies in older patients with COVID-19 on disease outcomes.
The quality of included studies ranges from fair to good.
Three of the articles studied the prevalence of malnutrition among older COVID-19 patients (Bedock et al., 2020; de Siqueira et al., 2020; Rehman et al., 2020). The prevalence of malnutrition among older patients with COVID-19 is high in these studies. The high prevalence of malnutrition could be related to fear of the illness, worrying about isolation, lack of social contacts during illness, high level of anxiety, anorexia secondary to infection, dyspnea, dysosmia, dysgeusia, stress, confinement, and organizational problems. These factors may reduce the appetite and food intake leading patients to be more malnourished (Lambrinakou et al., 2017; Thibault, Seguin, et al., 2020). In addition, life style, physical activity and social support might have associations with poor nutritional status of this vulnerable population (Rehman et al., 2020). Of those studies assessing nutritional status utilized heterogeneous methodologies such as GLIM, MNA and GNRI. Lack of standardized methods in nutritional assessment has led to unharmonized results which are difficult to compare (Hallström et al., 2018). Further, malnutrition among older people is considered as an underrecognized and undertreated condition leading the the underestimation of the aforementioned prevalence values (Bencivenga et al., 2020).
Among three studies which studied nutritional status among patients, one study (Li et al., 2020) did not clearly mention about the COVID-19 outcomes. It mainly focused on malnutrition as an outcome among older patients with COVID-19. It is an example for the bidirectional relationship between nutritional status and COVID-19 outcomes. COVID-19 infection can potentially lead to malnutrition and malnutrition may negatively affect the prognosis of the infected patients with COVID-19 (Bedock et al., 2020).
Transfer to ICU, death and PaO2/FiO2 ratio are interrelated outcomes. These outcomes are associated with low nutritional status in the reviewed studies (Bedock et al., 2020; Recinella et al., 2020). Generally, 5% to 10% of the patients with COVID-19 are affected with an acute respiratory distress syndrome (ARDS). All of them require urgent respiratory and hemodynamic support in the intensive care units (Thibault, Seguin, et al., 2020). Mortality rates of COVID-19 were strongly associated with older age (Yanez et al., 2020). Impairment in PaO2/FiO2 was independently associated with mortality of COVID-19 patients (Santus et al., 2020). These outcomes may be due to various reasons. First, COVID-19 inflamatory responses lead to reduced food intake of patients. Secondly, it increases the muscle catabolism resulting in patients at high risk of being malnoursihed (Thibault, Coëffier, et al., 2020). Therefore, a implementing a nutritional assessment for all patients with COVID-19 is essential. The European Society for Clinical Nutrition and Metabolism (ESPEN) has recommended to integrate early nutritional care management of COVID-19 patients into the overall therapeutic strategy of COVID-19 (Thibault, Seguin, et al., 2020).
Six studies showed the relationship between macro and micronutrients and COVID-19 outcomes. Lower albumin levels/ pre-albumin (Bedock et al., 2020; de Siqueira et al., 2020; Recinella et al., 2020; Zuo et al., 2020), Vitamin D, vitamin B12 and magnesium (Annweiler et al., 2020; Annweiler et al., 2020; Tan et al., 2020), Se status (Moghaddam et al., 2020) of older patients with COVID-19 had negative outcomes of the infection. All these nutrients have well-established immunomodulatory effects, with benefits in infectious disease (Shakoor et al., 2021). It is well known that, SARS-CoV-2 virus affects the immunity systems of the patients. The low levels of these nutrients might be the result of the imbalance of the functions of the immune system of the patients.
The important strength of this systematic review is that this is the first study that examines the nutritional status, micro and macro nutrients and their association with outcomes of older patients with COVID-19. However, our study has a few limitations. The results presented in this study cannot be generalized as the inclusion of very limited number of studies. No research study was found to see the association of other nutrients with COVID-19 outcomes. Therefore, future research should consider the level of other nutrients and their effects on the outcome of the older patients with COVID-19.
5. Conclusion
This review is done using eight related studies. It analyses recent evidence that nutritional status and macro/micro nutrient intake is associated with COVID-19 related outcomes. This review found that malnutrition among older patients with COVID-19 was relatively high. Further, low levels of macro and micro nutrients affect the negative outcomes of older patients with COVID-19. Future studies are warranted to explore the effects of other important nutrients on COVID-19 outcomes of this vulnerable group of patients.
Funding sources
No funding was received from funding agencies in the public, commercial, or not-for-profit sectors for the preparation of this review.
Credit Author Statement
HDWT Damayanthi led the study and developed the protocol with KIP Prabani. Both authors undertook the search, screening and data extraction. Damayanthi wrote the first draft; both authors contributed to subsequent drafts and have read and approved the final draft.
Declaration of Competing Interest
The authors declare that they have no conflict of interest.
References
- Annweiler C., Hanotte B., Grandin de l'Eprevier C., Sabatier J.-M., Lafaie L., Célarier T. Vitamin D and survival in COVID-19 patients: A quasi-experimental study. The Journal of Steroid Biochemistry and Molecular Biology. 2020;204 doi: 10.1016/j.jsbmb.2020.105771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Annweiler G., Corvaisier M., Gautier J., Dubée V., Legrand E., Sacco G., Annweiler C. Vitamin D Supplementation Associated to Better Survival in Hospitalized Frail Elderly COVID-19 Patients: The GERIA-COVID Quasi-Experimental Study. Nutrients. 2020;12(11) doi: 10.3390/nu12113377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedock D., Bel Lassen P., Mathian A., Moreau P., Couffignal J., Ciangura C., Poitou-Bernert C., Jeannin A.-C., Mosbah H., Fadlallah J., Amoura Z., Oppert J.-M., Faucher P. Prevalence and severity of malnutrition in hospitalized COVID-19 patients. Clinical Nutrition ESPEN. 2020;40:214–219. doi: 10.1016/j.clnesp.2020.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bencivenga L., Rengo G., Varricchi G. Elderly at time of COronaVIrus disease 2019 (COVID-19): possible role of immunosenescence and malnutrition. Geroscience. 2020;42(4):1089–1092. doi: 10.1007/s11357-020-00218-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler M.J., Barrientos R.M. The impact of nutrition on COVID-19 susceptibility and long-term consequences. Brain Behav Immun. 2020;87:53–54. doi: 10.1016/j.bbi.2020.04.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L.-K. Older adults and COVID-19 pandemic: Resilience matters. Arch Gerontol Geriatr. 2020;89 doi: 10.1016/j.archger.2020.104124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damayanthi H.D.W.T., Moy F.M., Abdullah K.L., Dharmaratne S.D. Health related quality of life and its associated factors among community-dwelling older people in Sri Lanka: A cross-sectional study. Arch Gerontol Geriatr. 2018;76:215–220. doi: 10.1016/j.archger.2018.03.009. [DOI] [PubMed] [Google Scholar]
- de Siqueira J.V.V., Almeida L.G., Zica B.O., Brum I.B., Barceló A., de Siqueira Galil A.G. Impact of obesity on hospitalizations and mortality, due to COVID-19: A systematic review. Obes Res Clin Pract. 2020;14(5):398–403. doi: 10.1016/j.orcp.2020.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallström E., Davis J., Woodhouse A., Sonesson U. Using dietary quality scores to assess sustainability of food products and human diets: A systematic review. Ecological Indicators. 2018;93:219–230. doi: 10.1016/j.ecolind.2018.04.071. [DOI] [Google Scholar]
- Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., Zhang L., Fan G., Xu J., Gu X., Cheng Z., Yu T., Xia J., Wei Y., Wu W., Xie X., Yin W., Li H., Liu M., Xiao Y., Gao H., Guo L., Xie J., Wang G., Jiang R., Gao Z., Jin Q., Wang J., Cao B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. The Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia H. Pulmonary Angiotensin-Converting Enzyme 2 (ACE2) and Inflammatory Lung Disease. Shock. 2016;46(3):239–248. doi: 10.1097/shk.0000000000000633. [DOI] [PubMed] [Google Scholar]
- Karlsson L.K., Jakobsen L.H., Hollensberg L., Ryg J., Midttun M., Frederiksen H., Glenthøj A., Kodahl A.R., Secher-Johnsen J., Nielsen L.K., Bofill N.G., Knudtzen F.C., Lund C.M. Clinical presentation and mortality in hospitalized patients aged 80+ years with COVID-19 – a retrospective cohort study. Arch Gerontol Geriatr. 2020 doi: 10.1016/j.archger.2020.104335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambrinakou S., Katsa M.E., Zyga S., Ioannidis A., Sachlas A., Panoutsopoulos G., Pistikou A.M., Magana M., Kougioumtzi Dimoligianni D.E., Kolovos P., Rojas Gil A.P. Correlations Between Nutrition Habits, Anxiety and Metabolic Parameters in Greek Healthy Adults. Adv Exp Med Biol. 2017;987:23–34. doi: 10.1007/978-3-319-57379-3_3. [DOI] [PubMed] [Google Scholar]
- Li T., Zhang Y., Gong C., Wang J., Liu B., Shi L., Duan J. Prevalence of malnutrition and analysis of related factors in elderly patients with COVID-19 in Wuhan, China. Eur J Clin Nutr. 2020;74(6):871–875. doi: 10.1038/s41430-020-0642-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lidoriki I., Frountzas M., Schizas D. Could nutritional and functional status serve as prognostic factors for COVID-19 in the elderly? Med Hypotheses. 2020;144 doi: 10.1016/j.mehy.2020.109946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moghaddam A., Heller R.A., Sun Q., Seelig J., Cherkezov A., Seibert L., Hackler J., Seemann P., Diegmann J., Pilz M., Bachmann M., Minich W.B., Schomburg L. Selenium Deficiency Is Associated with Mortality Risk from COVID-19. Nutrients. 2020;12(7) doi: 10.3390/nu12072098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moher D., Liberati A., Tetzlaff J., Altman D.G. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7) doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Recinella G., Marasco G., Serafini G., Maestri L., Bianchi G., Forti P., Zoli M. Prognostic role of nutritional status in elderly patients hospitalized for COVID-19: a monocentric study. Aging Clin Exp Res. 2020;32(12):2695–2701. doi: 10.1007/s40520-020-01727-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehman T., Josephson G., Sunbuli M., Chadaga A.R. Spontaneous Pneumothorax in an Elderly Patient With Coronavirus Disease (COVID-19) Pneumonia. Ochsner J. 2020;20(3):343–345. doi: 10.31486/toj.20.0072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson D.P., Lovegrove J.A. Nutritional status of micronutrients as a possible and modifiable risk factor for COVID-19: a UK perspective. Br J Nutr. 2020:1–7. doi: 10.1017/s000711452000330x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santus P., Radovanovic D., Saderi L., Marino P., Cogliati C., De Filippis G., Rizzi M., Franceschi E., Pini S., Giuliani F., Del Medico M., Nucera G., Valenti V., Tursi F., Sotgiu G. Severity of respiratory failure at admission and in-hospital mortality in patients with COVID-19: a prospective observational multicentre study. BMJ Open. 2020;10(10) doi: 10.1136/bmjopen-2020-043651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shakoor H., Feehan J., Al Dhaheri A.S., Ali H.I., Platat C., Ismail L.C., Apostolopoulos V., Stojanovska L. Immune-boosting role of vitamins D, C, E, zinc, selenium and omega-3 fatty acids: Could they help against COVID-19? Maturitas. 2021;143:1–9. doi: 10.1016/j.maturitas.2020.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan C.W., Ho L.P., Kalimuddin S., Cherng B.P.Z., Teh Y.E., Thien S.Y., Wong H.M., Tern P.J.W., Chandran M., Chay J.W.M., Nagarajan C., Sultana R., Low J.G.H., Ng H.J. Cohort study to evaluate the effect of vitamin D, magnesium, and vitamin B(12) in combination on progression to severe outcomes in older patients with coronavirus (COVID-19) Nutrition. 2020;111017:79–80. doi: 10.1016/j.nut.2020.111017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thibault R., Coëffier M., Joly F., Bohé J., Schneider S.M., Déchelotte P. How the Covid-19 epidemic is challenging our practice in clinical nutrition—feedback from the field. Eur J Clin Nutr. 2020 doi: 10.1038/s41430-020-00757-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thibault R., Seguin P., Tamion F., Pichard C., Singer P. Nutrition of the COVID-19 patient in the intensive care unit (ICU): a practical guidance. Critical Care. 2020;24(1):447. doi: 10.1186/s13054-020-03159-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanez N.D., Weiss N.S., Romand J.-A., Treggiari M.M. COVID-19 mortality risk for older men and women. BMC Public Health. 2020;20(1):1742. doi: 10.1186/s12889-020-09826-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuo P., Tong S., Yan Q., Cheng L., Li Y., Song K., Chen Y., Dai Y., Gao H., Zhang C. Decreased prealbumin level is associated with increased risk for mortality in elderly hospitalized patients with COVID-19. Nutrition. 2020;78 doi: 10.1016/j.nut.2020.110930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- https://www.nhlbi.nih.gov/health-pro/guidelines/in-develop/cardiovascular-risk-reduction/tools/cohort