Abstract
The widespread of viral airborne diseases is becoming a critical problem for human health and safety, not only for the common cold and flu, but also considering more serious infection as the current pandemic COVID-19. Even if the current heating, ventilating and air conditioning (HVAC) systems limit the disease transmission by air, the air filters are susceptible to microbial colonization. In addition, viruses spread via droplets (aerosol) produced by direct or indirect contact with infected people. In this context, the necessity of an efficient HVAC system, able to capture and inactivate viruses- and bacteria-rich aerosols, thus preserving a safe indoor air environment and protecting people, is of enormous importance. The aim of this work is the assessment of the antiviral properties of a silver nanoclusters/silica composite coating deposited via co-sputtering technique on glass, on metallic fibre-based air filters as well as on cotton textiles. The selected human respiratory viruses are: respiratory syncytial virus (RSV), the human rhinovirus (HRV) and the influenza virus type A (FluVA). The coated air filters show that the nanostructured coating develops a strong virucidal activity against RSV and FluVA, but not against the HRV.
Keywords: Air filter, Antiviral, Sputtered coatings, Nano-silver, Respiratory viruses
Graphical abstract
1. Introduction
Air-transmitted pathogens as bacteria, fungi or viruses are the cause of several airborne diseases, from common colds or influenza to even more acute respiratory illnesses, like the Severe Acute Respiratory Syndrome (SARS) and the current pandemic of COVID-19, caused by the coronavirus SARS-CoV-2. The widespread of airborne diseases becomes a crucial problem, especially, for indoor and crowded places such as public transport, kindergartens, offices, gyms and sports facilities, or in hospital where the risk of infectious disease transmission for patients, workers, and visitors is further increased. An exposure to airborne microorganisms for a long time together with a poor air quality reduces people comfort and productivity [1,2] and increases the risk of adverse health effects development [3,4]. For this, a clean indoor air environment has to be preserved considering that in the world, people spend most of their time indoor, on average about 90% [5,6].
Current Heating, Ventilating and Air Conditioning (HVAC) systems are able to handle and mitigate the air-transmission of disease. HVAC system reduces the circulation of microorganisms in the air, as they capture and retain both particulates and microorganisms in the air filters [7]. However, air filter is predisposed to microbial colonization, stimulating also by dirt accumulation in the long term, inadequate filter maintenance and the presence of cooling coil condensate or other moisture sources [7,8]. The respiratory viruses spread efficiently among humans through the air, via droplets (aerosol) or via direct or indirect contact with contaminated people, or via contaminated hands and objects, causing outbreaks that are difficult to control [9].
Besides its well-known antibacterial and antifungal broad-spectrum effect [[10], [11], [12]], silver recently demonstrated also antiviral properties [13]. Studies, performed in vitro, proved that silver nanoparticles are able to directly inactivate and prevent the infection of adenovirus type 3 [14] and have efficient inhibitory activity on H1N1 influenza A virus [15]. Few studies are more specific to antiviral air filters. In particular, silica particles decorated with silver nanoparticles, deposited on air filter media, demonstrated effective antiviral behaviour, towards aerosolized bacteriophage MS2 in a continuous airflow condition, without altering filtration performance [16]. Only silver nanoparticles, deposited on air filter, demonstrated similar results even if the dust particles loaded on the filtration media reduced the antimicrobial effect of the metallic agent [17].
The aim of this work is the evaluation of the antiviral effect of a silver nanocluster/silica composite coating deposited on fibre-based air filters via co-sputtering through a patented procedure [31]. Over the past ten years, authors developed this nanostructured composite coating [[19], [20], [21]] for several applications, from biomedical implants [22,23], to mobile phones [24], natural and technical textiles [25,26], aerospace structures [27,28], and also glass-fibre air filters [29], but evaluating only antibacterial and antifungal effect. In a recent short communication, authors' preliminary demonstrated the virucidal effect of the silver nanoclusters/silica composite coating deposited on disposable facemask towards SARS-CoV-2 [30].
In this study, the nanostructured coating was tested against representative members of the respiratory virus category of human origin, instead of bacteriophage as in the previously mentioned study [16]. Specifically, the respiratory syncytial virus (RSV) is the most frequent cause of paediatric bronchiolitis and pneumonia [31]; the human rhinovirus (HRV) is the major etiologic agent of the common cold [32]; influenza virus type A (FluVA) is the cause of the well-known respiratory disease that annually results in about 3 to 5 million cases of severe illness [33].
2. Materials and methods
2.1. Coating deposition and characterization
A nanostructured composite coating was deposited on several air filters by means of radio frequency (RF) co-sputtering technique. The nanostructured coating was a composite thin layer (thickness of 300 nm) of silica matrix which homogeneously embedded silver nanoclusters [20,21]. The selected substrates were glass-fibre and stainless-steel fibre-based air filters, supplied by Sagicofim Spa and GV Filtri Spa, respectively. A cotton fabric, supplied by Tessitura Fratelli Ballesio Srl, was used as fibre-based substrate for other kinds of application as the personnel protective clothes. The deposition process was patented by authors [18,19]. The coatings were deposited using the sputtering equipment (Microcoat™ MS450) with two cathodes, one of silver target (Sigma-Aldrich™ 99.99% purity) and one of silica target (Franco Corradi S.r.l.TM 99.9% purity). The deposition was performed in pure argon atmosphere for 80 min, operating in RF mode for silica (power 200 W) and in DC mode for silver (power 1 W) under a pressure of 5.5 dPa (corresponding to 5.5 × 10−3 mbar). The water-cooled targets have different diameters, 6 in. for silica and 1 for silver. The distance between targets and substrates was 14 cm. The co-sputtering process parameters were optimized as reported in the previous studies for cotton substrate [25], other textile materials [26,28] and glass-fibre air filter [29].
Morphology of the coated and uncoated filters was observed by means of scanning electron microscopy (Field Emission Scanning Electron Microscope FESEM, QUANTA INSPECT 200, Zeiss SUPRATM 40™) equipped with Energy Dispersive Spectroscopy (EDS, EDAX PV 9900™), used for detecting the composition of all the samples. EDS analysis was performed on three different areas at low magnification (100×) for a statistical analysis.
Silver release tests quantified silver ions released into water from the coated filters up to 14 days. For this test, coated filters with dimension of about 1cm2 of area were dipped into 25 ml of MilliQ water. Silver ion content was analysed after 3 h, 24 h, and 3, 7 and 14 days of immersion using silver photometer (Hanna Instruments™). Each measurement was done in triplicate.
2.2. Cells and viruses
Madin-Darby Canine Kidney cells (MDCK) (ATCC® CCL-34), human epithelial cells (Hep-2) (ATCC® CCL-23) and human epithelial adenocarcinoma cells (HeLa) (ATCC® CCL-2) were cultured in Dulbecco's modified Eagle's medium (DMEM; Sigma, St. Louis, MO), supplemented with heat-inactivated 10% (v/v) fetal bovine serum (FBS) (Sigma). All media were supplemented with 1% (v/v) penicillin/streptomycin solution (Euroclone, Milan, Italy) and cells were grown at 37 °C in an atmosphere of 5% of CO2. The A/H3N2 strain A/Christchurch/28/03 (FluVA) (Italian National Institute of Health) was propagated in MDCK cells by using MEM containing 1 μg/ml of IX type porcine pancreatic trypsin (Sigma, St. Louis, Mo) [34] and titrated by means of the plaque assay. The A2 (ATCC VR-1540) respiratory syncytial virus (RSV) strain was propagated in Hep-2 and titrated by means of the indirect immunoperoxidase staining procedure. Human rhinovirus (HRV) 1A (ATCC®VR-1559™) was produced in HeLa cells, at 33 °C, in a humidified 5% CO2 incubator and titrated by means of plaque assay.
2.3. Virus titration by plaque assay
To evaluate the HRV titre, HeLa cells were seeded in 96-well plates, reaching 60%–70% confluence at the time of infection. The HRV suspension was serially diluted in DMEM supplemented with 2% FBS and inoculated on cells at 33 °C for 1 h. After this time, cells were washed with medium and overlaid with a 1:1 combination of 1.6% SeaPlaque Agarose (BioWhittaker Molecular Applications) and 2 × DMEM. After an incubation at 33 °C for 3 days, the plates were fixed and stained with 0.1% of crystal violet solution for 30 min to allow the plaque count. To evaluate the FluVA titre, subconfluent MDCK cells in 96 well plates were inoculated with increasing dilutions of virus prepared in cold DMEM with 2% of FBS. After 2 h of adsorption at 37 °C, the virus inoculum was removed, cells overlaid with 1.6% SeaPlaque Agarose solution supplemented with 2 μg/ml of trypsin and incubated at 37 °C for 72 h. Cells were then fixed and coloured with 0.1% of crystal violet solution. The RSV titration was performed on Hep-2 cells in 96 well plates. Cells, seeded at a density of 9 × 103 cells/well, were inoculated with serial dilutions of RSV suspension for 3 h at 37 °C. Subsequently, the inoculum was removed and cells were overlaid with 1.2% methylcellulose and incubated at 37 °C. After 72 h, cells were fixed with acetone-methanol (50:50). The number of syncytia were determined by indirect immunostaining by using an RSV monoclonal antibody (Ab35958; Abcam, Cambridge, the United Kingdom) and a secondary antibody peroxidase-conjugated AffiniPure F(ab')2 Fragment Goat Anti-Mouse IgG (H + L) (Jackson ImmunoResearch Laboratories Inc., 872 W. Baltimore Pike, West Grove, PA 19390) [35].
Virus titration was performed in duplicate for each dilution and the virus titre was estimated as plaque forming units per ml (PFU/ml) by counting the number of plaques at an appropriate dilution.
2.4. Antiviral tests
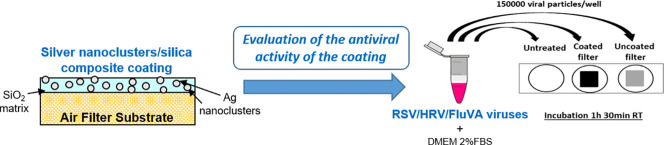
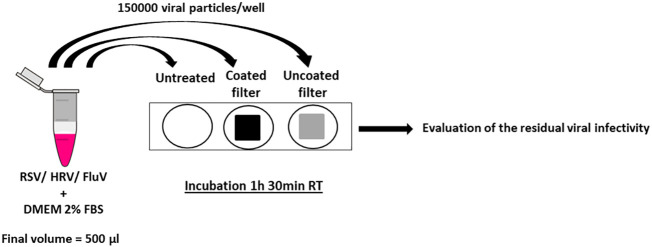
Approximately 1.5 × 105 viral particles (RSV, FluVA, HRV) were suspended in a final volume of 500 μl of DMEM supplemented with 2% of FBS. The mixture was incubated with coated or uncoated filters (cut into pieces of 1 cm2 and sterilized at 170 °C for 1 h) with a gentle oscillation for 1 h 30 min RT. As control (referred to untreated in the text), 1.5 × 105 viral particles were incubated under the same conditions of time and temperature, but without any filter. After this incubation, the mixture was collected and the residual viral infectivity was calculated by means of plaque assay and compared with the untreated control. The results are presented as the mean values from three independent experiments (mean ± SEM). One-way ANOVA, followed by Bonferroni test, was used to assess the statistical significance of the differences between treated and untreated samples, where appropriate (significance was set at P < 0.05). Fig. 1 reports the schematic representation of the procedure.
Fig. 1.
Schematic representation of the antiviral test procedure. Viral particles were incubated with coated or uncoated cotton or filters (metallic or glass-fibre filter) or left untreated. After 1 h 30 min at room temperature the residual viral infectivity was calculated and compared with the untreated control.
3. Results and discussion
3.1. Coating deposition and characterization
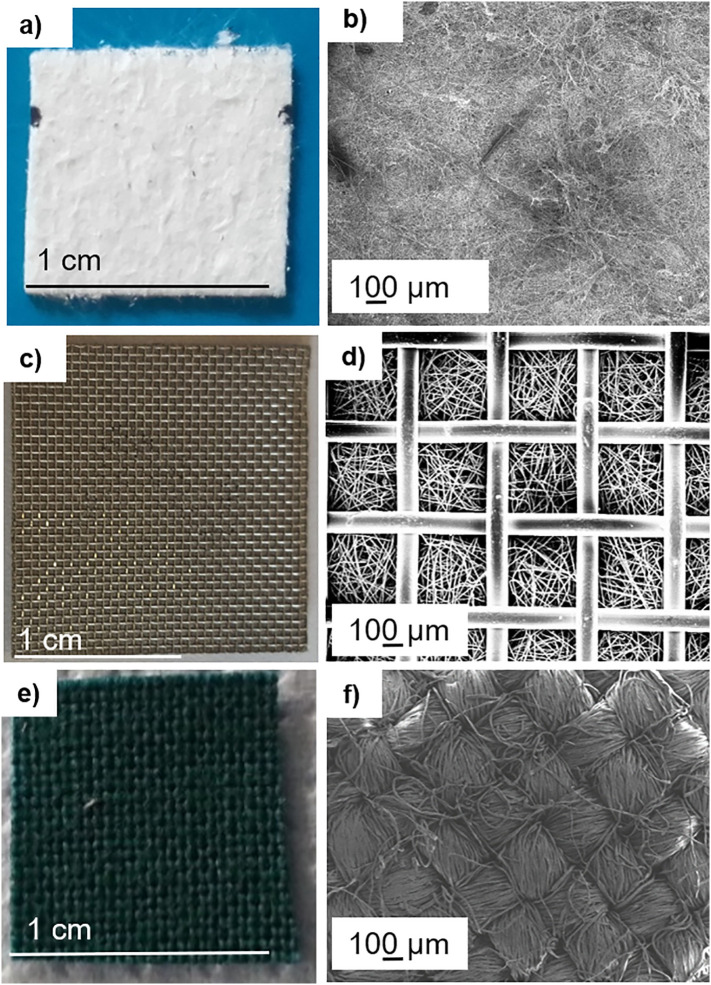
The glass and metallic fibre-based substrates were selected as materials used for air filters whereas cotton is used for other type of applications as an example the realization of personnel protective devices. Fig. 2 reports the photographs and the relative surface morphology observed at FESEM of the uncoated substrates. Glass-fibre air filter (Fig. 2a, b) was white coloured (Fig. 2a) and characterized by randomly oriented glass-fibres as observed in FESEM images (Fig. 2b). Higher magnified-micrograph relative to the fibres orientation was reported in the Fig. 2b of previous work [29]. Metallic air filter (Fig. 2c, d) is composed of a regular metal grid over randomly arranged metallic fibres. Lastly, woven cotton fabric (Fig. 2e, f) is green dyed with warp and weft fibres.
Fig. 2.
Photographs of substrates and the relative FESEM images for observing morphology at low magnification: (a), (b) glass-fibre filter; (c), (d) metallic filter; (e), (f) cotton.
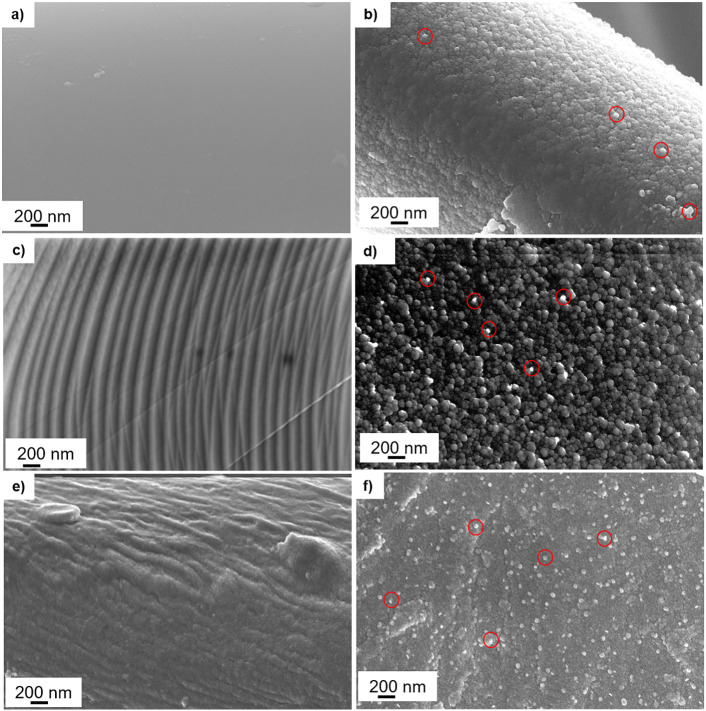
Fig. 3 reports the comparison of the fibres surface morphology before and after coating deposition. The silver nanocluster/silica composite coating, deposited by means of co-sputtering technique, visibly changed the surface morphology of the fibres of all the used substrates. The smooth uncoated glass fibres (Fig. 3a) became rougher with a globular morphology after the coating deposition (Fig. 3b). This surface microstructure was already observed for the substrates treated in the previous papers [25,29]. The same modification occurred in the case of metallic air filter and cotton. In the first, the circular bands visible on the uncoated fibre (Fig. 3c) completely disappeared with the presence of silver nanocluster/silica composite coating (Fig. 3d). In the second, the uniform composite coating (Fig. 3f) uniformly covered the uncoated cotton (Fig. 3e) and the stripes were not visible anymore. Silver nanoclusters appear as bright particles (dimensions less than 50 nm) dispersed into the silica matrix. They were well noticeable (red-circled in Fig. 3b,d,f) on the surfaces of both coated metallic air filter and cotton and less visible on the glass fibre filter as they were surrounded by silica matrix. As already observed for the glass fibre filter and cotton in the previous works [25,29], the silver nanocluster/composite coating covered the fibre of all the here studied substrates without altering filtration performance, and the filters porosity remained unchanged (no closed or obstructed pores).
Fig. 3.
FESEM images relative to the substrates fibres before and after the deposition of silver nanoclusters/silica composite coating: (a) uncoated and (b) coated glass-fibre filter; (c) uncoated and (d) coated metallic filter; (e) uncoated and (f) coated cotton. Silver nanoclusters in red circles.
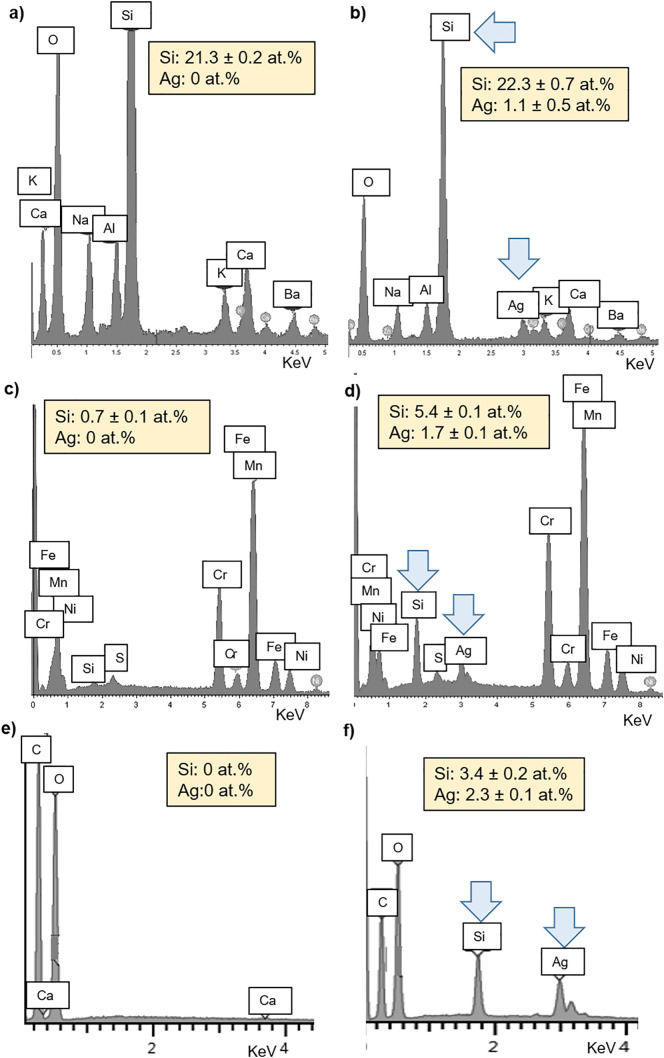
Fig. 4 shows the compositional analysis, comparing the EDS graphs between uncoated and coated substrates. The analysis confirmed the presence of silver nanocluster/silica composite coating through the detection of the two main elements of the deposited layer, Si and Ag as reported in the previous works relative to the substrate of cotton and glass-fibre filter [25,29]. The original composition of uncoated glass-fibre filter is mainly based on Si, Na, Ca, Al and Ba (Fig. 4a). After coating deposition, the amount of Si slightly increased of about 1 at.% due to the silica matrix and Ag peaks appears (Fig. 4b). Ag amount is about 2.3 at.%. The metallic air filter is composed of stainless steel and the main detected elements are Fe, Cr, Mn, Ni with trace of S and Si (Fig. 4c). Si peak intensity and, so, the relative atomic percentage increased from 0.7 to 5.4 at.% and Ag peaks (amount about 1.7 at.%) were identified on the surface of the coated sample (Fig. 4d). In the case of cotton, naturally composed of C and O with an external contamination of Ca (Fig. 3e), Si and Ag peaks appeared in the coated textile (Fig. 4f), with an amount of about 3.4 and 2.3 at.%, respectively.
Fig. 4.
EDS analysis relative to the substrates fibres before and after the deposition of silver nanoclusters/silica composite coating: (a) uncoated and (b) coated glass-fibre filter; (c) uncoated and (d) coated metallic filter; (e) uncoated and (f) coated cotton.
Fig. 5 reports the curve of the silver ion release from all the coated substrates in distilled water at RT. The acquisition of the data relative to the amount of silver ions was done up to 14 days. The trend of the silver ions release is progressive and very similar for both the air filter samples, reaching the maximum quantity at about 0.25 and 0.3 ppm for metallic filter and glass-fibre filter, respectively. The curve relative to cotton used as model substrate is characterized by an abrupt release of ions after 3 h, a decrement after 1 day and a further increment after 3 days, achieving higher value (about 0.55 ppm) then the data reported for the two air filters. This is probably due to the water absorption by cotton, differently from glass-fibre and metallic filter. For all substrates, the maximum quantity of ions detected into water after 14 days remained in the range of antimicrobial behaviour without reaching the toxic limit for human cells (10 ppm) [36,37]. Even if the curves did not reach a plateau after 14 days, it can be supposed that the amount of ion release is not going to exceed the toxicity threshold (10 ppm). Moreover, it must be underlined that HVAC system and in general air filters did not work immersed in water or any other aqueous solution, but in a moisture environment, so the silver release test into water up to 14 days could be considered a very harsh analysis.
Fig. 5.
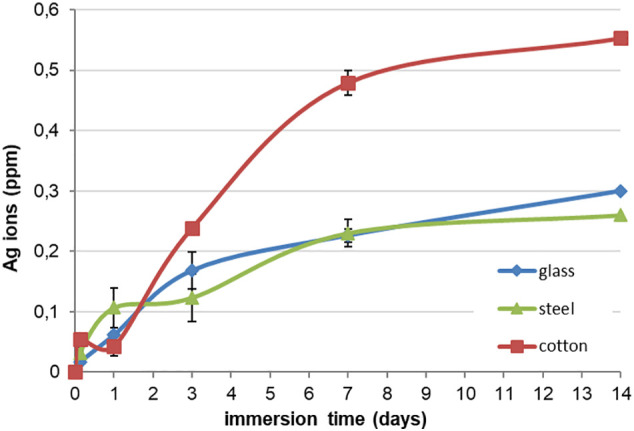
Silver ion release up to 14 days in water at RT of the coated substrates: glass-fibre air filter (glass); metallic air filter (steel) and cotton textile (cotton).
3.2. Antiviral tests
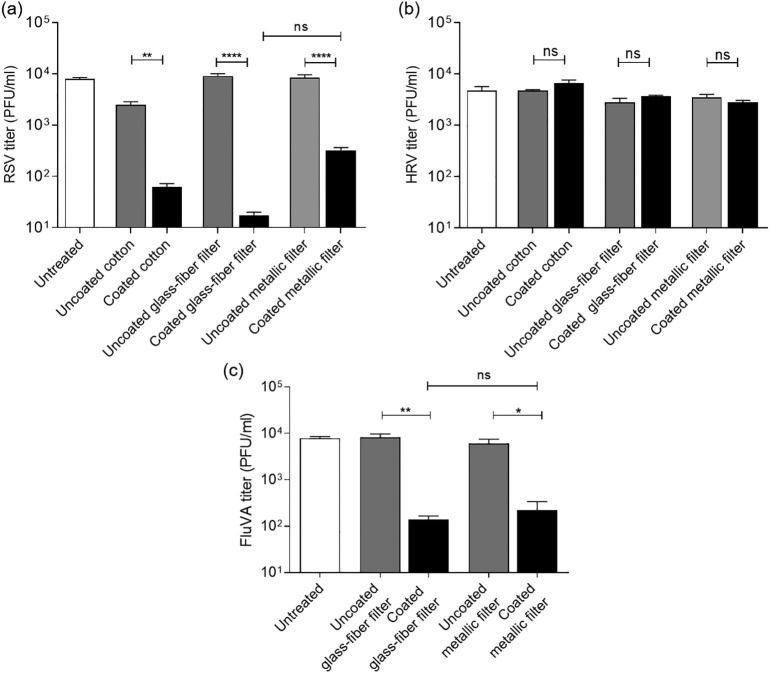
The antiviral assays were performed in order to investigate the virucidal activity of the silver nanoclusters/silica composite coating by simulating the passage of viral particles through the filters. Possible dust contamination of the air filters was not considered in this test as dirt accumulation usually occurs after long period of exposition. The results obtained from the experiments with RSV, HRV and FluVA are reported in Table 1 and schematically presented in Fig. 6(a, b and c respectively), comparing all the substrates. The first set of experiments was carried out with the cotton as model substrate, to setup the procedure and to verify the antiviral action of the nanostructured composite coating against only two of the respiratory viruses, the respiratory syncytial virus (RSV) and the rhinovirus (HRV) (Fig. 6a, b). As reported in Fig. 6(a), the coated cotton significantly reduces the number of infectious RSV particles if compared with the uncoated cotton and the untreated (which refers to the viral particles incubated under the same conditions of temperature and time but without testing any samples). The reduction of RSV titre visible between both the cotton samples and the control is probably due to an aspecific absorption of the medium containing viral particles to the cotton substrate (Untreated: 7954 ± 511 PFU/ml; Uncoated cotton: 2482 ± 377 PFU/ml; Coated cotton: 62 ± 10 PFU/ml). On the contrary, any virucidal activity against HRV was observed. In fact, as shown in Fig. 6(b), the coated cotton does not reduce the HRV titre if compared to the uncoated cotton treated sample (Untreated: 4731 ± 939 PFU/ml; Uncoated cotton: 4680 ± 256 PFU/ml; Coated cotton: 6640 ± 989 PFU/ml). This result could be attributed to the different structural conformation of these two viruses. In general, the protein capsid of naked viruses (as HRV) is less susceptible to environmental conditions (desiccation, detergents, pH, temperature…) than the lipid bilayer wrapping enveloped viruses (as RSV).
Table 1.
Data relative to the plaque forming unit per ml (PFU/ml) for the untreated, the cotton textile, the glass-fibre filter and the metallic filter (coated and uncoated), against RSV, HRV and FluVA.
| Samples | Plaque forming unit per ml (PFU/ml) |
|||
|---|---|---|---|---|
| RSV | HRV | FluVa | ||
| Untreated | / | 7954 ± 511 | 4731 ± 939 | 7819 ± 754 |
| Cotton textile | Uncoated | 2482 ± 377 | 4680 ± 256 | / |
| Coated | 62 ± 10 | 6640 ± 989 | / | |
| Glass fibre filter | Uncoated | 9013 ± 988 | 2800 ± 560 | 8241 ± 1513 |
| Coated | 18 ± 3 | 3680 ± 160 | 140 ± 26 | |
| Metallic filter | Uncoated | 8400 ± 1200 | 3467 ± 525 | 6054 ± 1430 |
| Coated | 323 ± 41 | 2773 ± 282 | 220 ± 116 | |
Fig. 6.
Antiviral tests performed with the cotton textile, the glass-fibre filter and the metallic filter against (a) RSV, (b) HRV and (c) FluVA. The coated and the uncoated samples are compared. The untreated refers to the viral particles incubated under the same conditions of temperature and time but without testing any samples. The viral titre is reported on the Y-axis and expressed as plaque forming unit per ml (PFU/ml). On the X-axis the type of treatment is reported. ****pANOVA<0.0001; **pANOVA<0.01; *pANOVA<0.05; ns: not significant.
Having verified the feasibility of the antiviral procedure and the antiviral action of the nanostructured silver nanocluster/silica composite coating, the antiviral tests were performed with the metallic and the glass-fibre filters, adding also the FluVA virus, in order to conduct a significant screening against respiratory viruses. As reported in Fig. 6(a) and as expected, the nanostructured silver nanocluster/silica composite coating exerts a virucidal effect against RSV regardless of the type of filter (metallic or glass-fibre filter) on which it is deposed. There was indeed a significant reduction of RSV titre between both the coated samples and the uncoated one but no significant difference was observed comparing the antiviral effect of the coated samples. As shown in Fig. 6(b) and in accordance with the results obtained with the cotton substrate, any significant inhibition of HRV titre was observed. On the contrary, the antiviral tests performed with the FluVA (Fig. 6c) showed that the silver nanocluster/silica composite coating significantly inhibits influenza virus infectivity. Also, in this case, the nature of the substrate did not influence the antiviral effect of the coating. These results are in line with previous papers demonstrating the antiviral activity of silver nanoparticles (Ag NPs) against these two human pathogens [[38], [39], [40], [41]]. Despite the antiviral mechanism of AgNPs is still unclear, some hypotheses have been put forward. AgNPs have been proposed to interfere with viral replication by binding viral surface glycoproteins and therefore preventing the attachment and entry of the virus into the host cell or by crossing the cell membrane and actively blocking cellular factors necessary for viral replication. The experimental settings, here presented, allowed excluding the second mechanism of action, because cells and Ag nanoclusters did not come in contact. In this case, it is possible to assume that the virus is structurally altered or inactivated in such way that cell infection is no more possible, after interacting with the Ag nanoclusters embedded into the composite coating. Considering that the test was performed with samples immersed into a liquid solution, a second hypothesis could be ascribable also to the antiviral effect of silver ions, released from the nanoclusters. In fact, according to the data reported in Fig. 5, it was demonstrated that the ion release in water began in the first 3 h with a trend of progressive increment. In addition, skin test, performed in [25], demonstrated that silica matrix completely trapped silver nanoclusters, avoiding in this way the release of them into the surrounding environment. In addition, the porous morphology of silica allowed Ag ion release also from the nanoclusters disposed into the innermost matrix areas. In this way, the homogenous distribution of the nanoclusters inside the matrix increased ions amount. Here, no antiviral action of the silver nanoclusters/silica composite coating against HRV was reported. This phenomenon could be attributed to the different structural conformation of this virus with respect to the other two viruses. In general, the protein capsid of naked viruses (as HRV) is less susceptible to environmental conditions (desiccation, detergents, pH, temperature…) than the lipid bilayer wrapping enveloped viruses (as RSV and FluVA). To the best of our knowledge, no reports are available against the antiviral effect of silver nanoparticles against the human rhinovirus. Thanks to the premise about the promising results on virucidal effect of the silver nanoclusters/silica composite coating towards SARS-CoV-2 on disposable facemask [30], the coated filtering substrates, described in this paper, will be shortly investigated in details their antiviral activity against viruses belonging to Coronaviridae family.
4. Conclusions
In conclusion, the nanostructured silver nanoclusters/silica composite coating effectively coated glass-fibre and metallic air filters and the cotton textile. The filtration performance was not reduced as the coating was conformal deposited only on the fibres, maintaining unaltered the filter porosity. Even if the air filter will find the final application in a moisture surrounding environment, the release test in water demonstrate that the silver ions were released without reaching the toxicity threshold after 14 days. The results obtained from the antiviral tests, simulating a close contact between infectious viral aerosol and air filters, demonstrated that the silver nanoclusters/silica composite coating is characterized by a strong virucidal activity against RSV and FluVA. The reduction of RSV titre resulted of almost three orders of magnitude between coated and uncoated glass-fibre filters and more than one order of magnitude between coated and uncoated metallic filters. Instead, the nanostructured coating is able to reduce FluVA titre of about two orders of magnitude, independently of the filters used as substrate, compared to the uncoated filters or the control. Here, no antiviral action of the silver nanoclusters/silica composite coating against HRV was reported.
In this study, the antiviral action of the nanostructured coating was tested, performing the experiments in a liquid environment with an incubation time of hour and half. In future tests, it will be interesting to assess the ability of silver nanocluster/silica composite coating to inactivate viral aerosols in a shorter time and to inactivate infectious viruses after their aerosolization. On the other hand, tests could be performed in order to evaluate the possible effect of dust accumulation on the air filter on the antiviral efficacy. The silver nanocluster/silica coating deposited on the air filters (and masks), can lead to an important contribution to the antiviral biological risk restraint, especially but not only in the health sector. In fact, it can improve the current prevention and safety procedures, also in crowded places or in the public transport, kindergartens, offices, gyms and sports facilities. In addition, staff and workers operating in critical conditions such as hospitals, conflict areas or regions affected by natural disasters or pandemics, could benefit of the antiviral air filters. Furthermore, the antiviral coating can confer an add value to the protective clothing such as gowns, masks or gloves.
CRediT authorship contribution statement
C. Balagna: Funding acquisition, Project administration, Investigation, Conceptualization, Writing – original draft, Writing – review & editing. R. Francese: Investigation, Methodology, Writing – original draft, Writing – review & editing. S. Perero: Methodology, Writing- review & editing. D. Lembo: Supervision, Writing- review & editing. M. Ferraris: Supervision, Funding acquisition, Project administration, Writing- review & editing.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgment
This work was supported by means of Proof of Concept project funded by Politecnico di Torino and Compagnia di San Paolo and by means of “BIO-kILLER – Anti-BIOpollutant coatIng for reusabLe fiLtER” Manunet III Transnational Call (2018), MNET18/OTHR3507.
References
- 1.Tham K.W. Indoor air quality and its effects on humans - a review of challenges and developments in the last 30 years. Energy Build. 2016;130:637–650. doi: 10.1016/j.enbuild.2016.08.071. [DOI] [Google Scholar]
- 2.Liu G., Xiao M., Zhang X., Gal C., Chen X., Liu L., Pan S., Wu J., Tang L., Clements-Croome D. A review of air filtration technologies for sustainable and healthy building ventilation. Sustain. Cities Soc. 2017;32:375–396. doi: 10.1016/j.scs.2017.04.011. [DOI] [Google Scholar]
- 3.Kim K., Kabir E., Jahan S.A. Airborne bioaerosols and their impact on human health. J. Environ. Sci. 2018;67:23–35. doi: 10.1016/j.jes.2017.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Douwes J., Thorne P., Pearce N., Heederiki D. Bioaerosol health effects and exposure assessment: progress and prospects. Ann. Occup. Hyg. 2003;47:187–200. doi: 10.1093/annhyg/meg032. [DOI] [PubMed] [Google Scholar]
- 5.Kepleis N.E., Nelson W.C., Ott W.R., Robinson J.P., Tsang A.M., Switzer P., Behar J.V., Hern S.C., Engelmann W.H. The national human activity pattern survey (NHAPS): a resource for assessing exposure to environmental pollutants. J. Expo. Anal. Environ. Epidemiol. 2001;11:231–252. doi: 10.1038/sj.jea.7500165. [DOI] [PubMed] [Google Scholar]
- 6.European Commission Press Release Database Indoor air pollution: new EU research reveals higher risks than previously thought, IP/03/1278. 2003. https://europa.eu/rapid/press-release_IP-03-1278_en.htm (accessed 22 September 2003)
- 7.Möritz M., Peters H., Nipko B., Rüden H. Capability of air filters to retain airborne bacteria and molds in heating, ventilating and air-conditioning (HVAC) systems. Int. J. Hyg. Environ. Health. 2001;203:401–409. doi: 10.1078/1438-4639-00054. [DOI] [PubMed] [Google Scholar]
- 8.Flemming H.C. Biofilms and environmental protection. Wat Sci. Tech. 1993;27:1–10. doi: 10.2166/wst.1993.0528. [DOI] [Google Scholar]
- 9.Kutter J.S., Spronken M.I., Fraaij P.L., Fouchier R.A.M., Herfst S. Transmission routes of respiratory viruses among humans. Curr. Opin. Virol. 2018;28:142–151. doi: 10.1016/j.coviro.2018.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Deshmukh S.P., Patil S.M., Mullani S.B., Delekar S.D. Silver nanoparticles as an effective disinfectant: a review. Mater. Sci. Eng. C. 2019;97:954–965. doi: 10.1016/j.msec.2018.12.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rai M., Yadav A., Gade A. Silver nanoparticles as a new generation ofantimicrobials. Biotechnol. Adv. 2009;27:76–83. doi: 10.1016/j.biotechadv.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 12.Dakal T.C., Kumar A., Majumdar R.S., Yadav V. Mechanistic basis of antimicrobial actions of silver nanoparticles. Front. Microbiol. 2016;7:1831. doi: 10.3389/fmicb.2016.01831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ciriminna R., Albo Y., Pagliaro M. New antivirals and antibacterials based on silver nanoparticles. Chem. Med. Chem. 2020;15:1619–1623. doi: 10.1002/cmdc.202000390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen N., Zheng Y., Yin J., Li X., Zheng C. Inhibitory effects of silver nanoparticles against adenovirus type 3 in vitro. J. Virol. Methods. 2013;193:470–477. doi: 10.1016/j.jviromet.2013.07.020. [DOI] [PubMed] [Google Scholar]
- 15.Xiang D., Chen Q., Pang L., Zheng C. Inhibitory effects of silver nanoparticles on H1N1 influenza A virus in vitro. J.Virol. Methods. 2011;178:137–142. doi: 10.1016/j.jviromet.2011.09.003. [DOI] [PubMed] [Google Scholar]
- 16.Joe Y.H., Woo K., Hwang J. Fabrication of an anti-viral air filter with SiO2–Ag nanoparticles and performance evaluation in a continuous airflow condition. J. Hazard. Mater. 2014;280:356–363. doi: 10.1016/j.jhazmat.2014.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Joe Y.H., Park D.H., Hwang J. Evaluation of Ag nanoparticle coated air filter against aerosolizedvirus: anti-viral efficiency with dust loading. J. Hazard. Mater. 2016;301:547–553. doi: 10.1016/j.jhazmat.2015.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ferraris M., Balagna C., Perero S. 2019. Method for the Application of an Antiviral Coating to a Substrate and Relative Coating. (WO2019/082001) [Google Scholar]
- 19.Ferraris M., Chiaretta D., Fokine M., Miola M., Verné E. 2008. Pellicole antibatteriche ottenute da sputtering e procedimento per conferire proprietà antibatteriche ad un substrato TO2008A000098. [Google Scholar]
- 20.Ferraris M., Perero S., Miola M., Ferraris S., Verné E., Morgiel J. Silver nanocluster– silica composite coatings with antibacterial properties. Mater. Chem. Phys. 2010;120:123–126. doi: 10.1016/j.matchemphys.2009.10.034. [DOI] [Google Scholar]
- 21.Ferraris M., Perero S., Miola M., Ferraris S., Gautier G., Maina G., Fucale G., Verne E. Chemical, mechanical, and antibacterial properties of silver nanocluster– silica composite coatings obtained by sputtering. Adv. Eng. Mater. 2010;12:B276–B282. doi: 10.1002/adem.200980076. [DOI] [Google Scholar]
- 22.Muzio G., Perero S., Miola M., Oraldi M., Ferraris S., Vernè E., Festa F., Canuto R.A., Festa V., Ferraris M. Biocompatibility versus peritoneal mesothelial cells of polypropylene prostheses for hernia repair, coated with a thin silica/silver layer. J Biomed Mater Res B Appl Biomater. 2016;105:1586–1593. doi: 10.1002/jbm.b.33697. [DOI] [PubMed] [Google Scholar]
- 23.Baino F., Ferraris S., Miola M., Perero S., Verné E., Coggiola A., Dolcino D., Ferraris M. Novel antibacterial ocular prostheses: proof of concept and physico-chemical characterization. Mater. Sci. Eng. 2016;60:467–474. doi: 10.1016/j.msec.2015.11.075. [DOI] [PubMed] [Google Scholar]
- 24.Miola M., Perero S., Ferraris S., Battiato A., Manfredotti C., Vittone E., Del Vento D., Vada S., Fucale G., Ferraris M. Silver nanocluster-silica composite antibacterial coatings for materials to be used in mobile telephones. Appl. Surf. Sci. 2014;313:107–115. doi: 10.1016/j.apsusc.2014.05.151. [DOI] [Google Scholar]
- 25.Irfan M., Perero S., Miola M., Maina G., Ferri A., Ferraris M., Balagna C. Antimicrobial functionalization of cotton fabric with silver nanoclusters/silica composite coating via RF co-sputtering technique. Cellulose. 2017;24:2331–2345. doi: 10.1007/s10570-017-1232-y. [DOI] [Google Scholar]
- 26.Balagna C., Irfan M., Perero S., Miola M., Maina G., Santella D., Simone A. Characterization of antibacterial silver nanocluster/silica composite coating on high performance Kevlar® textile. Surf. Coat. Technol. 2017;321:438–447. doi: 10.1016/j.surfcoat.2017.05.009. [DOI] [Google Scholar]
- 27.Balagna C., Perero S., Ferraris S., Miola M., Fucale G., Manfredotti C., Battiato A., Santella D., Verné E., Vittone E., Ferraris M. Antibacterial coating on polymer for space application. Mater. Chem. Phys. 2012;135:714–722. doi: 10.1016/j.matchemphys.2012.05.049. [DOI] [Google Scholar]
- 28.Balagna C., Irfan M., Perero S., Miola M., Maina G., Crosera M., Santella D., Simone A., Ferraris M. Antibacterial nanostructured composite coating on high performance Vectran™ fabric for aerospace structures. Surf. Coat. Technol. 2019;373:47–55. doi: 10.1016/j.surfcoat.2019.05.076. [DOI] [Google Scholar]
- 29.Balagna C., Perero S., Bosco F., Mollea C., Irfan M., Ferraris M. Antipathogen nanostructured coating for air filters. Appl. Surf. Science. 2020;508 doi: 10.1016/j.apsusc.2020.145283. [DOI] [Google Scholar]
- 30.Balagna C., Perero S., Percivalle E., Vecchio Nepita E., Ferraris M. Virucidal effect against coronavirus SARS-CoV-2 of a silver nanocluster/silica composite sputtered coating. Open Ceramics. 2020;1:100006. doi: 10.1016/j.oceram.2020.100006. [DOI] [Google Scholar]
- 31.Piedimonte G., Perez M.K. Respiratory syncytial virus infection and bronchiolitis. Pediatr. Rev. 2014;35:519–530. doi: 10.1542/pir.35-12-519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Turner R., Lee W. In: Clinical Virology. Richman D., Whitley R., Hayden F., editors. ASM Press; Washington DC: 2009. Rhinovirus; pp. 1063–1082. [Google Scholar]
- 33.World Health Organization Influenza (Seasonal) 2018. https://www.who.int/news-room/fact-sheets/detail/influenza-(seasonal) (accessed 06 November 2018)
- 34.Szretter K.J., Balish A.L., Katz J.M. Influenza: propagation, quantification, and storage. Curr. Protoc. Microbiol. 2006;15:15G.1. doi: 10.1002/0471729256.mc15g01s3. [DOI] [PubMed] [Google Scholar]
- 35.Donalisio M., Rittà M., Francese R., Civra A., Tonetto P., Coscia A. High temperature—short time pasteurization has a lower impact on the antiviral properties of human milk than holder pasteurization. Front. Pediatr. 2018;6:304. doi: 10.3389/fped.2018.00304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen W., Liu Y., Courtney H.S., Bettenga M., Agrawal C.M., Bumgardner J.D., Ong J.L. In vitro anti-bacterial and biological properties of magnetron co-sputtered silver-containing hydroxyapatite coating. Biomaterials. 2006;27:5512–5517. doi: 10.1016/j.biomaterials.2006.07.003. [DOI] [PubMed] [Google Scholar]
- 37.Jamuna-Thevi K., Bakar S.A., Ibrahim S., Shahab N., Toff M.R.M. Quantification of silver ion release, in vitro cytotoxicity and antibacterial properties of nanostuctured Ag doped TiO2 coatings on stainless steel deposited by RF magnetron sputtering. Vacuum. 2011;86:235–241. doi: 10.1016/j.vacuum.2011.06.011. [DOI] [Google Scholar]
- 38.Nakamura S., Sato M., Sato Y., Ando N., Takayama T., Fujita M., Ishihara M. Synthesis and application of silver nanoparticles (Ag NPs) for the prevention of infection in healthcare workers. Int. J. Mol. Sci. 2019;20:3620. doi: 10.3390/ijms20153620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lara H.H., Garza-Treviño E.N., Ixtepan-Turrent L., Singh D.K. Silver nanoparticles are broad-spectrum bactericidal and virucidal compounds. J. Nanobiotechnol. 2011;3:9–30. doi: 10.1186/1477-3155-9-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yang X.X., Li C.M., Huang C.Z. Curcumin modified silver nanoparticles for highly efficient inhibition of respiratory syncytial virus infection. Nanoscale. 2016;8:3040. doi: 10.1039/c5nr07918g. [DOI] [PubMed] [Google Scholar]
- 41.Morris D., Ansar M., Speshock J., Ivanciuc T., Qu Y., Casola A., Garofalo R. Antiviral and immunomodulatory activity of silver nanoparticles in experimental RSV infection. Viruses. 2019;11:732. doi: 10.3390/v11080732. [DOI] [PMC free article] [PubMed] [Google Scholar]