Abstract
Purpose
The current literature reports a wide range of diagnostic accuracy of non-contrast magnetic resonance coronary angiography (NC-MRCA) for the assessment of coronary artery disease (CAD). We aimed to compare the clinical effectiveness of NC-MRCA with that of invasive coronary angiography (ICA) in patients with suspected CAD using a systematic review and meta-analysis.
Methods
Two investigators independently extracted 36 published manuscripts between 2010 and 2019. Databases including Medline, Web of Knowledge, Google Scholar, Scopus, and Cochrane were searched using pre-established keywords. Analysis of the data followed the PRISMA statement for reporting systematic reviews and meta-analyses and primary analysis followed the Mantel-Hansel methodology. Correctness of classification for detecting coronary artery stenosis ≥50% (CAS) was measured using ICA as the gold standard.
Results
A total of five studies met inclusion criteria, with a total of 417 patients and 2883 coronary segments. The pooled per patient sensitivity and specificity of NC-MRCA for CAS in suspected patients was 90.3% (95% CI 85.6–95.1%) and 77.9% (95% CI 69.5–86.3%). Pooled per vessel assessment of NC- MRCA revealed a sensitivity of 83.7% (95%CI 79.7–87.8%) and specificity of 90.0% (95%CI 86.7–93.4%). Per-segment assessment of NC-MRCA showed a pooled sensitivity of 81.6% (95% CI 76.8–86.4) and specificity of 97.0% (95% CI 95.5–98.5). Mild to moderate heterogeneity was noted in most diagnostic parameters with larger heterogeneity noted in the per-segment analyses. There was less heterogeneity in sensitivity and NPV than specificity and PPV.
Conclusion
According to this meta-analysis, non-contrast coronary MRA resulted in adequate screening in patients with suspected CAD with high sensitivity and specificity. This result was true for per-patient, per-vessel, and per-segment assessment.
Keywords: Non-contrast magnetic resonance angiography, Coronary angiography, Coronary artery disease, Accuracy
non-contrast magnetic resonance angiography, coronary angiography, coronary artery disease, accuracy
1. Introduction
Magnetic resonance angiography (MRA) is a noninvasive and accurate tool commonly used for vascular assessment. The main advantages of this technique include detailed anatomic assessment without ionizing radiation, superior soft tissue characterization, high spatial resolution, and is less costly than catheter angiography [1, 2]. Non-contrast MRA (NC-MRA) is increasingly used to assess blood vessels, which allows for broader application, especially in patients with reduced kidney function or prior reaction to gadolinium, and also prevents potential contrast side effects such as nephrogenic systemic fibrosis [3].
Another benefit of NC-MRA is improved safety among pregnant women and children as well as decreased costs compared to contrast-based imaging [4, 5]. Challenges of NC-MRA include longer acquisition time, image quality limitations, and concerns about technique robustness [6].
Cardiovascular MRI is a commonly used imaging tool, offering one-stop quantitative structural and functional assessment, as well as luminal assessment of the coronary arteries [7, 8]. Non-contrast
MR coronary angiography (NC-MRCA) is particularly appealing, with potential applications for assessment of acquired coronary artery disease (CAD) as well as congenital coronary artery anomalies in a variety of patient groups [9, 10, 11, 12, 13]. As compared to digital subtraction angiography, NC-MRA using coronary imaging protocols demonstrates high sensitivity and specificity for the detection of hemodynamically significant peripheral vascular disease in the lower extremities, with sensitivity and specificity well over 90% and high interobserver agreement [14]. This level of accuracy remains constant even in high risk subgroups such as those with renal insufficiency or diabetics [15]. Hence, the application of NC-MRA has expanded to assess coronary, pulmonary, extracranial carotid, and intracranial arteries. However, with respect to coronary assessment, a wide range of diagnostic accuracy has been reported, suggesting the need for a comprehensive evaluation of its accuracy [16].
The purpose of this analysis was to systematically review and analyze the current literature to determine the diagnostic accuracy of NC-MRCA for detecting ≥50% coronary stenosis in patients with suspected coronary artery disease as compared to invasive coronary angiography (ICA).
2. Materials and methods
2.1. Search strategy
This study was performed according to established methods and in compliance with PRISMA (Preferred Reporting Items for Systematic review and Meta-Analysis) Protocols [17]. Two investigators searched the manuscript databases including Medline, Web of Knowledge, Google Scholar, Scopus, and Cochrane for all eligible studies in accordance with the considered keywords including: "non-contrast magnetic resonance angiography," "coronary angiography," "coronary artery disease," and "accuracy." The studies were restricted to the English language and were published between January 2010 and December 2019. Studies before 2010 were excluded in order to use the latest coronary MR protocols and devices. We also searched the references of retrieved articles. Inclusion criteria included: prospective study design; use of intra-arterial digital subtraction angiography as the reference standard; study included at least 10 patients with suspected or known CAD; application of homogenous imaging techniques for all the patients in each study including 1.5 vs 3 T, and the number of channels and slices; and sufficient data available to construct 2 x 2 or 3 x 3 contingency tables for each coronary segment. All studies assessing the diagnostic value of contrast-enhanced MRCA were excluded. All articles evaluating non-contrast magnetic resonance angiography of arteries other than the coronaries were excluded. Additional exclusion criteria included: 1) a lack of clear and reproducible results, 2) lack of access to the full text of the manuscript, and 3) case reports, cast series and review papers (Figure 1).
Figure 1.
PRISMA flow diagram of the search strategy.
2.2. Data abstraction and validity assessment
Data abstraction was independently performed by two un-blinded reviewers on structured collection forms without divergence in data collection. Any disagreements were resolved by consensus. Study quality was evaluated based on the following criteria: I) the systematic review and meta-analysis were based on the questions primarily described and formulated; 2) inclusion and exclusion criteria were pre defined in the studies as eligibility criteria; 3) searching the literature was performed on a systematic and comprehensive approach; 4) to minimize bias, the Poll texts of the articles were dually reviewed; 5) the quality of included studies were rated independently by the reviewers for appraising internal validity; 6) studies characteristics and findings were comprehensively listed; 7) the publication and risk of bias were listed; and 8) heterogeneity was assessed [18]. The primary endpoint of our study was pooled sensitivity and specificity of NC-MRCA to detect ≥50% coronary artery stenosis. Along with this endpoint, the year of publication, number of patients included, and method of design were also evaluated.
2.3. Statistical analysis
Contingency tables were collected from each of the analyzed articles. Pooled statistics for each parameter were generated using a random effects model by inverse variance method with untransformed proportions and Clopper-Pearson method for calculation of 95% confidence intervals. Cochrane's Q statistic was calculated for which a p-value is reported. Heterogeneity was principally reported as F. Publication bias was assessed using Egger's regression test for which a p-value is reported using the metafor package (Viechtbauer, W. (2010). Meta-analysis was conducted using R with the metafor package. Journal of Statistical Software, 36(3), 1–48. URL: https://www.jstatsoft.org/v36/i03/). Forest plots were created using R version 3.6.3 (R Core Team (2020): R: A language and environment for statistical computing. R foundation for Statistical Computing, Vienna, Austria. URL https://www.R-project.org/) using the meta package (Balduzzi S, Rucker G, Schwarzer G (2019), How to perform a meta-analysis with R: a practical tutorial, Evidence-Based Mental Health) and following the guidelines in Shim ct al [19]. Funnel plots were created using a random effects model with contour added at 95% and 99% confidence levels using the mctaviz package (Michael Kossmcicr, Ulrich S. Tran and Martin Voracek (2020). metaviz: Forest Plots, Funnel Plots, and Visual Funnel Plot Inference for Meta-Analysis. R package version 0.3.1. https://CRAN.R-project.org/package=metaviz).
3. Results
Initially, 36 studies met broad inclusion criteria based on matching keywords, and five met all inclusion criteria in the final analysis [9, 20, 21, 22, 23]. During the screening process, one study was excluded because it was in German. The five studies comprise 417 patients suspected of having CAD (mean age of 60 years, range 43–69 years, 286 men and 131 women) (Table 1). The prevalence of CAD in our pooled population who underwent coronary angiography was 55.8%. All patients were imaged using 1.5-T MR scanners with 5,8, and 32 coils except Mohammadzadeh et al study in which 3T MR was used.
Table 1.
Details of studies evaluated in our meta-analysis.
| Author, year | Number | Mean age | M/F | Target population | Technique type | Standard | NC-MRCA Time |
|---|---|---|---|---|---|---|---|
| Kato et al, 2010 [9] | 138 | 67 ± 9 | 95/43 | suspected CAD | 1.5-T MRI, free-breathing steady-state free precession whole MRCA |
Invasive coronary angiography | Mean imaging time of 9.5 ± 3.5 min |
| Hamdan et al, 2011 [17] | 120 | 65 ± 8 | 77/43 | suspected CAD | 3.0-T MRI with 32-channel and 64-slice CT angiography | Invasive coronary angiography | Mean imaging time of 17 ± 4.7 min |
| Nagata et al, 2011 [18] | 67 | 69 ± 13 | 49/18 | suspected CAD | 1.5-T MRI with 5, 32-channel coils | Invasive coronary angiography | Mean imaging time of 6.2min ±2.8 (range, 1.2–16.2) |
| Yonezawa et al, 2014 [19] | 62 | 69 ± 13 | 46/16 | suspected CAD | 1.5-T MRI with 32-channel coils | Invasive coronary angiography | Mean imaging time of 6.8min ±2.6 (range, 3.2–12.1) |
| Mohammadzadeh et al, 2017 [20] | 30 | 43 ± 10 | 19/11 | suspected CAD | 1.5 T MRI with 8-channel body coil | Invasive coronary angiography | Mean imaging time of 3.5–5 min |
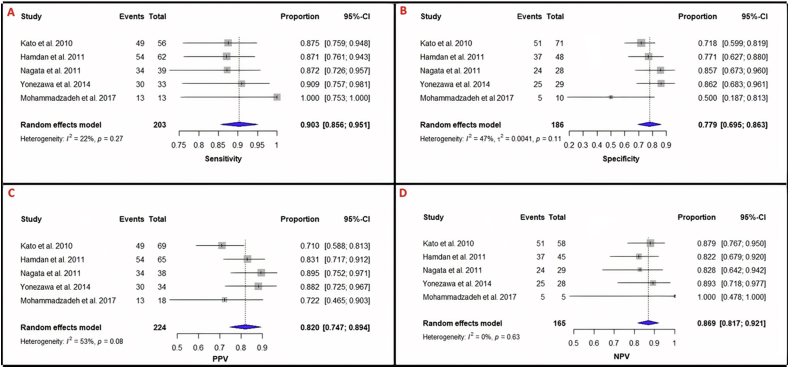
The per-patient and per-vessel diagnostic value of NC-MRCA as compared to invasive angiography in each study is summarized (Table 2). The pooled per-patient sensitivity, specificity, PPV, and NPV of NC-MRCA for detecting coronary artery stenosis ≥50% was 90.3% (95%CI: 85.6%–95.1%), 77.9% (95%CI: 69.5%–86.3%), 82.0% (95%CI: 74.7%–89.4%), and 86.9% (95%CI: 81.7%–92%), respectively (Table 3 and Figure 2).
Table 2.
Diagnostic value of MRCA as compared to invasive angiography in different papers.
| Author, year | Sensitivity | Specificity | PPV | NPV | Accuracy | AUC |
|---|---|---|---|---|---|---|
| Kato et al, 2010 | Per patient: 88% Per vessel: 83% |
Per patient: 72% Per vessel: 90% |
Per patient: 71% Per vessel: 67% |
Per patient: 88% Per vessel: 96% |
Per patient: 79% Per vessel: 89% |
0.91 |
| Hamdan et al, 2011 | Per patient: 87% Per vessel: 81% |
Per patient: 77% Per vessel: 84% |
Per patient: 83% Per vessel: 70% |
Per patient: 82% Per vessel: 90% |
Per patient: 83% Per vessel: 83% |
0.82 |
| Nagata et al, 2011 | Per patient: 87% Per vessel: 86% |
Per paient: 86% Per vessel: 93% |
Per patient: 89% Per vessel: 86% |
Per patient: 83% Per vessel: 93% |
Per patient: 87% Per vessel: 91% |
0.90 |
| Yonezawa et al, 2014 | Per patient: 91% Per vessel: 84% |
Per patient: 86% Per vessel: 93% |
Per patient: 88% Per vessel: 80% |
Per patient: 89% Per vessel: 94% |
Per patient: 88% Per vessel: 92% |
0.92 |
| Mohammadzadeh et al, 2017 | Per patient: 100% Per vessel: 89% |
Per patient: 50% Per vessel: 89% |
Per patient: 72% Per vessel: 78% |
Per patient: 100% Per vessel: 95% |
Per patient: 78% Per vessel: 84% |
--- |
Table 3.
Pooled statistics and heterogeneity of studies included in the meta-analysis. Pooled diagnostics were calculated using random effects models and confidence intervals generated using the Clopper-Pearson method. I2 represents the residual heterogeneity of the samples. Best-performing metrics are highlighted in bold. Per-patient analysis was most sensitive with highest PPV, while per-segment analysis was most specific with the highest NPV. There is a moderate amount of heterogeneity present within many of the diagnostic metrics.
| Sensitivity | (%) | Specificity | (%) | PPV | (%) | NPV | (%) | |
|---|---|---|---|---|---|---|---|---|
| Per-Patient | 0.903 (0.856–0.951) | 22 | 0.779 (0.695–0.863) | 47 | 0.820 (0.747–0.894) | 53 | 0.869 (0.817–0.921) | 0 |
| Per-Vessel | 0.837 (0.797–0.878) | 0 | 0.909 (0.867–0.934) | 63 | 0.761 (0.687–0.835) | 63 | 0.939 (0.920–0.958) | 21 |
| Per-Segment | 0.816 (0.768–0.864) | 0 | 0.970 (0.955–0.985) | 79 | 0.744 (0.669–0.818) | 53 | 0.981 (0.972–0.991) | 59 |
Figure 2.
Forest plots for analysis of per-patient detection of >50% stenosis. Heterogeneity is reported as I2 and Cochran's Q statistic, for which a p-value is reported (α = 0.05). 95% Confidence intervals were calculated using the Clopper-Pearson method for each study and the pooled statistics. A. Per-patient sensitivity = 0.903 (0.856–0.951); I2 = 22%, p = 0.27. B. Per-patient specificity = 0.779 (0.695–0.863); I2 = 47%, p = 0.11. C. Per-patient PPV = 0.820 (0.747–0.894); I2 = 53%, p = 0.08. D. Per-patient NPV = 0.869 (0.817–0.921); I2 = 0%, p = 0.63. Sensitivity, specificity, and PPV show moderate heterogeneity while NPV has little heterogeneity.
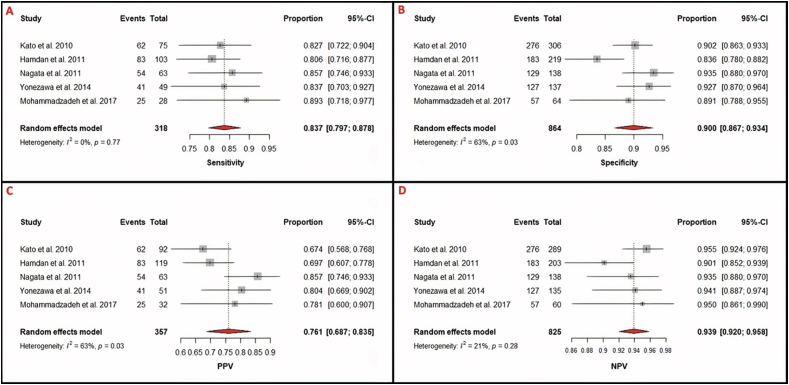
Per-vessel diagnostic performance of NC-MRCA revealed a pooled sensitivity of 83.7% (95%CI: 79.7%–87.8%), specificity of 90.9% (95%CI: 86.7%–93.4%), PPV of 76.1% (95%Cl: 68.7%–83.5%), and NPV of 93.9% (95%CI: 92.0%–95.8%) (Table 3 and Figure 3).
Figure 3.
Forest plots for analysis of per-vessel detection of >50% stenosis. Heterogeneity is reported as I2 and Cochran's Q statistic, for which a p-value is reported. 95% Confidence intervals were calculated using the Clopper-Pearson method for each study and the pooled statistics. A. Per-vessel sensitivity = 0.837 (0.797–0.878); I2 = 0%, p = 0.77. B. Per-vessel specificity = 0.900 (0.867–0.934); I2 = 63%, p = 0.03. C. Per-vessel PPV = 0.761 (0.687–0.835); I2 = 63%, p = 0.03. D. Per-vessel NPV = 0.939 (0.920–0.958); I2 = 21%, p = 0.28. Sensitivity and NPV show weak heterogeneity while specificity and PPV have significant heterogeneity.
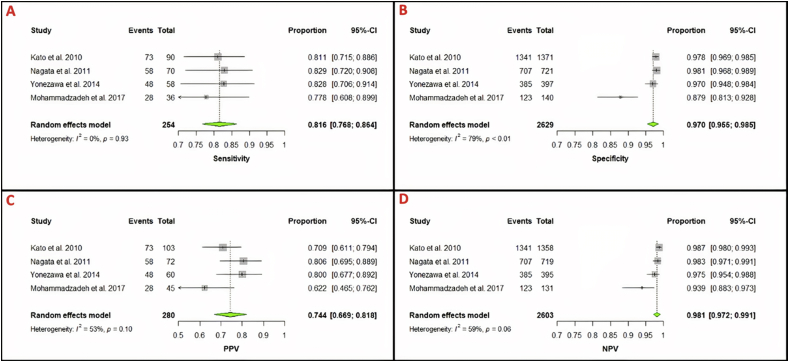
Per-segment diagnostic data of NC-MRCA was available for 4 out of the 5 available papers, with a pooled sensitivity of 81.6% (95%CI 76.8–86.4), specificity of 97.0% (95%CI 95.5–98.5), PPV of 74.4% (95%CI 66.9–81.8), and NPV of 98.1% (95%CI 97.2–99) (Figure 4).
Figure 4.
Forest plots for analysis of per-segment detection of >50%. Heterogeneity is reported as I2 and Cochran's Q statistic, for which a p-value is reported. 95% Confidence intervals were calculated using the Clopper-Pearson method for each study and the pooled statistics. A. Per-segment sensitivity = 0.816 (0.768–0.864); I2 = 0%, p = 0.93. B. Per-segment specificity = 0.970 (0.955–0.985); I2 = 79%, p < 0.01. C. Per-segment PPV = 0.744 (0.669–0.818); I2 = 53%, p = 0.10. D. Per-segment NPV = 0.981 (0.972–0.991); I2 = 59%, p = 0.06. Specificity shows weak heterogeneity while sensitivity, PPV, and NPV have significant heterogeneity.
Statistical heterogeneity was observed in assessing sensitivity in per-patient, per-vessel, and per-segment assessment (Table 3). Mild to moderate heterogeneity was noted in the majority of diagnostic parameters with larger heterogeneity noted in the per-segment analyses. There was less heterogeneity in sensitivity and NPV than specificity and PPV.
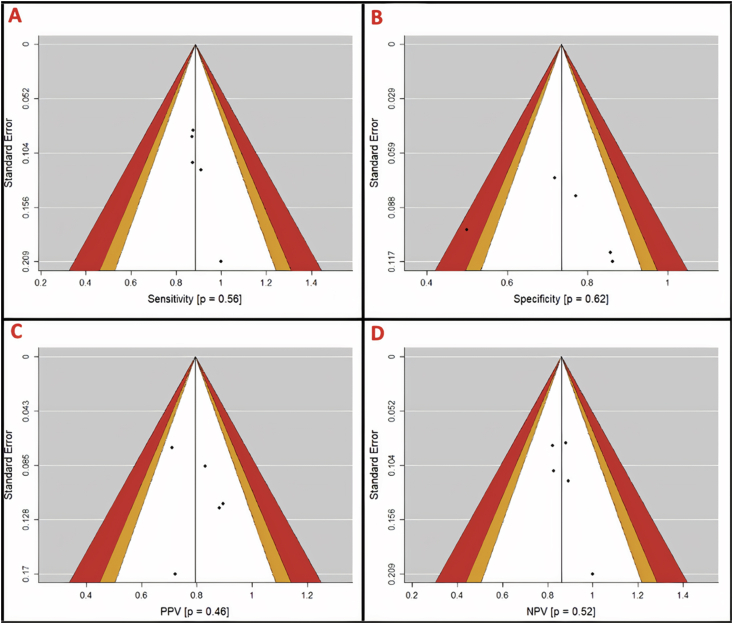
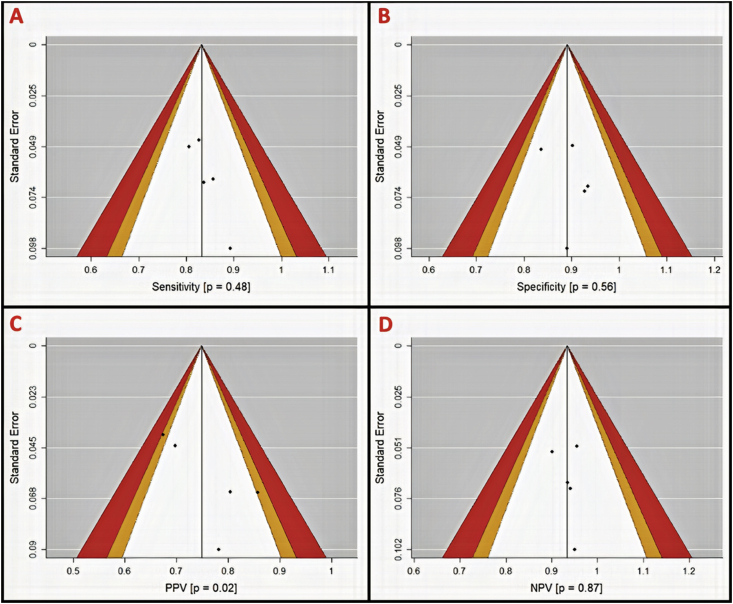
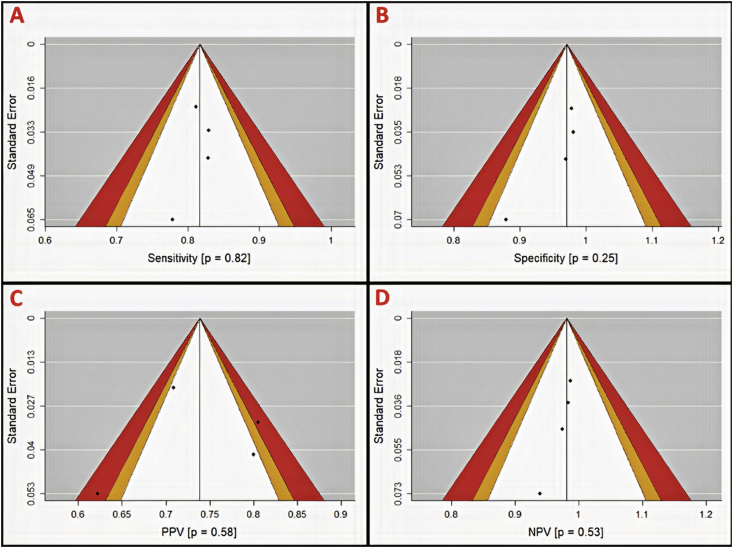
Funnel plots were generated for assessment of publication bias using Egger's regression test. Due to our study sample size our power for detecting publication bias was low. On per-patient analysis, more publication bias was observed in specificity and PPV (p = 0.62 and 0.46, respectively) (Figure 5). Per-vessel analysis again suggested more publication bias in specificity and PPV (p = 0.56 and 0.02, respectively) (Figure 6) while per-segment analysis suggested publication bias only in PPV (p = 0.58) (Figure 7). Summary p-values for the funnel plots are illustrated in Table 4.
Figure 5.
Per-patient funnel plots with contour for assessment of publication bias in diagnostic parameters (A, sensitivity; B, specificity; C, positive predictive value (PPV); and D, negative predictive value (NPV)). Areas in white, orange, and red represent <90%, 95–99%, and >99% confidence levels for publication bias. P-values are derived from Egger's regression (α = 0.05) and represent the likelihood of a study result at least this large given the null hypothesis to be true (H0 = No publication bias). Mohammadzadeh et al. is the lone study identified as likely to have publication bias.
Figure 6.
Per-vessel funnel plots with contour for assessment of publication bias in diagnostic parameters (A, sensitivity; B, specificity; C, positive predictive value (PPV); and D, negative predictive value (NPV)). Areas in white, orange, and red represent <90%, 95–99%, and >99% confidence levels for publication bias. P-values are derived from Egger's regression (α = 0.05) and represent the likelihood of a study result at least this large given the null hypothesis to be true (H0 = No publication bias). PPV is likely subject to publication bias (P = 0.02).
Figure 7.
Per-segment funnel plots with contour for assessment of publication bias in diagnostic parameters (A, sensitivity; B, specificity; C, positive predictive value (PPV); and D, negative predictive value (NPV)). Areas in white, orange, and red represent <90%, 95–99%, and >99% confidence levels for publication bias. P-values are derived from Egger's regression (α = 0.05) and represent the likelihood of a study result at least this large given the null hypothesis to be true (H0 = No publication bias). Hamdan et al. 2011 was omitted from analysis due to lack of contingency tables for per-segment analysis.
Table 4.
Egger's regression p-values for assessment of publication bias of diagnostic metrics. Per-vessel PPV is likely subject to publication bias (P = 0.02). Given the small sample size of available studies, a lack of publication bias cannot be asserted. Funnel-plot figures are highly suggestive that Mohammadzadeh et al. carries at least some publication bias into the meta-analysis. Hamdan et al. 2011 was omitted from per-segment analysis due to lack of contingency tables for segment data.
| Sensitivity | Specificity | PPV | NPV | |
|---|---|---|---|---|
| Per-Patient | 0.56 | 0.62 | 0.46 | 0.52 |
| Per-Vessel | 0.48 | 0.56 | 0.02 | 0.87 |
| Per-Segment | 0.82 | 0.25 | 0.58 | 0.53 |
Bold value indicates significant p-value.
4. Discussion
This systematic review and meta-analysis showed a high level of diagnostic accuracy of NCMRCA for detecting ≥50% stenosis in patients with suspected CAD in comparison to invasive coronary angiography. NC-MRCA was particularly useful in ruling out stenosis on a per-segment and per-vessel basis with negative predictive values of 98% and 90.4%, respectively. However, positive predictive values were only moderate using this method. This shows that false negative results are minimal in the application of NC-MRCA though the false positive results seem to be moderate.
4.1. Conventional methods
Conventional coronary angiography has been traditionally preferred for diagnosis due to a higher spatial resolution as well as a higher accuracy and specificity, especially for the assessment of distal coronary artery branches [24]. However, its invasive nature, potential for hospital admission, requirement of iodinated contrast, and overall increased cost make it a more expensive and risk-fraught undertaking. In addition, there remains controversy over the need for intervention in distal branch coronary artery stenosis.
Historically, the accuracy of CT angiography has been shown to be higher as compared to MRCA and thus CT angiography has been preferred over MRCA for streamlined CAD diagnosis [22, 23]. However, recent advancements in the technology have significantly improved MRCA image quality and decreased scan times [25, 26]. A previous meta-analysis study done by Di Leo and colleagues revealed a pooled sensitivity and specificity of 89% and 72%, respectively [27]. Their study differed from ours in that they didn't perform a per-vessel analysis, included contrast enhanced studies, and included older studies.
4.2. The benefits of non-contrast MR coronary angiography
Compared with CTA, MRCA allows a more global assessment of the heart in addition to detailed assessment of the coronary vessels and is effective for the assessment of coronary anomalies and aneurysms, interrogation of coronary bypass grafts, soft tissue characterization, viability assessment, coronary vein imaging, and identification of arterial wall abnormalities [28]. In previous studies, particularly in the assessment of the proximal segments of the coronary arteries, high agreement has been demonstrated between the results of MRCA and invasive angiography [29, 30]. However, some questions have remained unanswered such as the comparative performance of non-contrast or contrast-enhanced MRCA for CAD diagnosis or diagnostic accuracy of MRCA in patients who have contraindications for invasive coronary assessment. Furthermore, some studies have shown significantly low specificity of non-contrast MRCA in assessment of CAD in suspected patients [9].
As demonstrated in our meta-analysis, non-contrast coronary MRCA has a high sensitivity and specificity as well as a high negative predictive value for CAD in per-patient, per-vessel, and per-segment assessment. Per-patient analysis has high sensitivity and PPV, which is beneficial for detecting any evidence of coronary artery disease in a patient (90.3 sensitivity and 82.0% PPV). Overall, per-vessel analysis was intermediate between per-patient and per-segment parameters. However, the NPV of the per-vessel analysis was better than the per-patient NPV (98.1% versus 86.9%). The per-segment analysis had the highest specificity and NPV (97.0% and 98.1%, respectively), meaning this analysis was effective in ruling out stenosis of >50% in the specific segment. With the advent of new MRCA techniques, high-quality studies with excellent spatial resolution can be achieved in shorter scan times (around 10 min or less) without the need for contrast agents. Despite the higher likelihood of motion artifacts in NC-MRCA as compared to contrast-enhanced MRCA, the former may be more favorable due to lowering the risk for contrast-related complications [31, 32, 33].
Considering these advantages, along with its high per-patient and per-vessel accuracy revealed in our analysis, this tool may be considered for CAD diagnosis in a variety of clinical settings, potentially leading to a higher satisfaction level in both physicians and patients. Other benefits of NC-MRCA include avoidance of blooming artifact from heavily calcified coronary artery segments, lack of ionizing radiation, and opportunities for vascular flow imaging with phase contrast techniques, native T1 and T2 quantification and detailed cardiac functional assessment [34, 35]. Limits of NC-MRCA include sensitivity to field inhomogeneity, poor quality and long acquisition times in cases with irregular respiratory rate or heart rate, poor visualization of vessels <1.5mm diameter (same for CCTA) and artifacts from stents. These limitations can be minimized by optimization of NC-MRCA by utilization of new techniques being developed to reduce scanning times and improve vessel lumen resolution [26].
4.3. Publication bias
Publication bias in medical journals refers to the publication of more articles that contain positive conclusions or significant statistical results. Studies at the apex of the produced funnel plots have lower standard errors and contribute more to the assessment of publication bias. Overall, the rate of publication bias in these studies was low. Most publication bias stemmed from the study performed by Mohammadzadeh et al [23]. Factors contributing to this assessment include the low sample size of 23 patients, the high margin of error, and the high risk of selection bias with 48% of initial study enrollees dropping out.
Factors that may affect heterogeneity of per-patient, per-vessel, and per-segment sensitivity in this study could be explained by different MRI machines and coils (i.e. 3T vs 1.5T, number of channels, parallel imaging factor) as well as employing different sample sizes; however, there were not enough studies that could be included to control for these factors adequately.
4.4. Limitations
The principle limitation of this meta-analysis was the small sample size of noncontrast-MRCA studies that were eligible to be included. The use of different channel body coils, field strengths, MR scanners, and scan protocols may induce a confounding bias to the study though the promising results in advanced CMR imaging techniques [24] empowers the necessity of more adequately concrete studies to identify the differences in imaging acquisitions and magnet strengths. The inclusion of contrasted-MRCA would possibly offset the small sample size and the publication bias. However, such a study would negate the ability to assess the accuracy of NC-MRA for patients who are unable to receive gadolinium contrast for various masons. Lastly, our sample size is unpowered to detect all instances of publication bias; however, there are adequate patients and segments in the pooled analysis to make conclusions about diagnostic parameters.
5. Conclusion
This meta-analysis showed a high level of diagnostic accuracy of noncontrast coronary MRCA for detecting ≥50% stenosis in patients with suspected CAD in comparison to conventional coronary angiography. Though there is no evidence of the superiority of non-contrast CMR to CCTA in the detection of CAD it seems that it may be a helpful modality in particular cases of low to intermediate risk of ACS patients including those suspected with myocarditis and patients with ESRD. This imaging method is particularly useful in ruling out stenosis on a per-patient, per-vessel basis, and per-segment, but has only moderate positive predictive values.
Declarations
Author contribution statement
Aryan Zahergivar and Jeremy R Burt: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Madison R. Kocher and Jeffrey Waltz: Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Ismail Kabakus, and Jordan Chamberlin: Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
Selcuk Akkaya: Performed the experiments; Analyzed and interpreted the data.
Ali M Agha and U Joseph Schoepf: Analyzed and interpreted the data; Wrote the paper.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Data availability statement
Data included in article/supplementary material/referenced in article.
Declaration of interests statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
References
- 1.Hartung M.P., Grist T.M., François C.J. Magnetic resonance angiography: current status and future directions. J. Cardiovasc. Magn. Reson. 2011;13(1):19. doi: 10.1186/1532-429X-13-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kuo A.H., Nagpal P., Ghoshhajra B.B., Hedgire S.S. Vascular magnetic resonance angiography techniques. Cardiovasc. Diagn. Ther. 2019;9(Suppl 1):S28. doi: 10.21037/cdt.2019.06.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cavallo A.U., Koktzoglou I., Edelman R.R., Gilkeson R., Mihai G., Shin T., Rajagopalan S. Noncontrast magnetic resonance angiography for the diagnosis of peripheral vascular disease. Circulation: Cardiovasc. Imag. 2019;12(5) doi: 10.1161/CIRCIMAGING.118.008844. [DOI] [PubMed] [Google Scholar]
- 4.Lim R.P., Koktzoglou I. Noncontrast magnetic resonance angiography: concepts and clinical applications. Radiol. Clin. 2015;53(3):457–476. doi: 10.1016/j.rcl.2014.12.003. [DOI] [PubMed] [Google Scholar]
- 5.Lim R.P., Koktzoglou I. Noncontrast magnetic resonance angiography: concepts and clinical applications. Radiol. Clin. 2015;53(3):457–476. doi: 10.1016/j.rcl.2014.12.003. [DOI] [PubMed] [Google Scholar]
- 6.Ishii M., Sato Y., Matsumoto N., Kunimasa T., Tani S., Tachibana E., Hirayama A. Acute myocardial infarction in a patient with anomalous origin of the right coronary artery: depiction at whole-heart coronary magnetic resonance angiography and delayed-enhanced imaging. Int. J. Cardiol. 2008;131(1):e22–e24. doi: 10.1016/j.ijcard.2007.07.052. [DOI] [PubMed] [Google Scholar]
- 7.D’Angelo T., Grigoratos C., Mazziotti S., Bratis K., Pathan F., Blandino A., Nagel E. High-throughput gadobutrol-enhanced CMR: a time and dose optimization study. J. Cardiovasc. Magn. Reson. 2017;19(1):83. doi: 10.1186/s12968-017-0400-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Burt J.R., Zimmerman S.L., Kamel I.R., Halushka M., Bluemke D.A. Myocardial T1 mapping: techniques and potential applications. Radiographics. 2014;34(2):377–395. doi: 10.1148/rg.342125121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kato S., Kitagawa K., Ishida N., Ishida M., Nagata M., Ichikawa Y., Kobayashi Y. Assessment of coronary artery disease using magnetic resonance coronary angiography: a national multicenter trial. J. Am. Coll. Cardiol. 2010;56(12):983–991. doi: 10.1016/j.jacc.2010.01.071. [DOI] [PubMed] [Google Scholar]
- 10.François C.J., Tuite D., Deshpande V., Jerecic R., Weale P., Carr J.C. Unenhanced MR angiography of the thoracic aorta: initial clinical evaluation. Am. J. Roentgenol. 2008;190(4):902–906. doi: 10.2214/AJR.07.2997. [DOI] [PubMed] [Google Scholar]
- 11.Greil G.F., Stuber M., Botnar R.M., Kissinger K.V., Geva T., Newburger J.W., Manning W.J., Powell A.J. Coronary magnetic resonance angiography in adolescents and young adults with Kawasaki disease. Circulation. 2002 Feb 26;105(8):908–911. doi: 10.1161/hc0802.105563. [DOI] [PubMed] [Google Scholar]
- 12.Greil G.F., Seeger A., Miller S., Claussen C.D., Hofbeck M., Botnar R.M., Sieverding L. Coronary magnetic resonance angiography and vessel wall imaging in children with Kawasaki disease. Pediatr. Radiol. 2007 Jul;37(7):666–673. doi: 10.1007/s00247-007-0498-x. [DOI] [PubMed] [Google Scholar]
- 13.Prakken N.H., Cramer M.J., Olimulder M.A. Screening for proximal coronary artery anomalies with 3-dimensional MR coronary angiography. Int. J. Cardiovasc. Imag. 2010;26:701–710. doi: 10.1007/s10554-010-9617-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lim R.P., Hecht E.M., Xu J., Babb J.S., Oesingmann N., Wong S., Lee V.S. 3D nongadolinium-enhanced ECG-gated MRA of the distal lower extremities: preliminary clinical experience. J. Magn. Reson. Imag.: Offic. J. Int. Soc. Magn. Res. Med. 2008;28(1):181–189. doi: 10.1002/jmri.21416. [DOI] [PubMed] [Google Scholar]
- 15.Lim R.P., Hecht E.M., Xu J., Babb J.S., Oesingmann N., Wong S., Lee V.S. 3D nongadolinium-enhanced ECG-gated MRA of the distal lower extremities: preliminary clinical experience. J. Magn. Reson. Imag.: Offic. J. Int. Soc. Magn. Res. Med. 2008;28(1):181–189. doi: 10.1002/jmri.21416. [DOI] [PubMed] [Google Scholar]
- 16.Arendt C.T., Leithner D., Lenga L., Wichmann J.L., Albrecht M.H., Czwikla R., Nagel E. Multi-observer comparison study between unenhanced quiescent-interval single-shot magnetic resonance angiography and invasive carbon dioxide angiography in patients with peripheral arterial disease and chronic renal insufficiency. Eur. J. Radiol. 2018;108:140–146. doi: 10.1016/j.ejrad.2018.09.029. [DOI] [PubMed] [Google Scholar]
- 17.Shamseer L., Moher D., Clarke M. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: elaboration and explanation [published correction appears in BMJ. 2016 Jul 21;354:i4086. BMJ. 2015;350:g7647. doi: 10.1136/bmj.g7647. [DOI] [PubMed] [Google Scholar]
- 18.Zeng X., Zhang Y., Kwong J.S., Zhang C., Li S., Sun F., Du L. The methodological quality assessment tools for preclinical and clinical studies, systematic review and meta-analysis, and clinical practice guideline: a systematic review. J. Evid. Based Med. 2015;8(1):2–10. doi: 10.1111/jebm.12141. [DOI] [PubMed] [Google Scholar]
- 19.Shim S.R., Kim S.J., Lee J. Diagnostic test accuracy: application and practice using R software. Epidemiol. Health. 2019;41 doi: 10.4178/epih.e2019007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hamdan A., Asbach P., Wellnhofer E., Klein C., Gebker R., Kelle S., Fleck E. A prospective study for comparison of MR and CT imaging for detection of coronary artery stenosis. JACC: Cardiovasc. Imag. 2011;4(1):50–61. doi: 10.1016/j.jcmg.2010.10.007. [DOI] [PubMed] [Google Scholar]
- 21.Nagata M., Kato S., Kitagawa K., Ishida N., Nakajima H., Nakamori S., Sakuma H. Diagnostic accuracy of 1.5-T unenhanced whole-heart coronary MR angiography performed with 32-channel cardiac coils: initial single-center experience. Radiology. 2011;259(2):384–392. doi: 10.1148/radiol.11101323. [DOI] [PubMed] [Google Scholar]
- 22.Yonezawa M., Nagata M., Kitagawa K., Kato S., Yoon Y., Nakajima H., Honda H. Quantitative analysis of 1.5-T whole-heart coronary MR angiograms obtained with 32-channel cardiac coils: a comparison with conventional quantitative coronary angiography. Radiology. 2014;271(2):356–364. doi: 10.1148/radiol.13122491. [DOI] [PubMed] [Google Scholar]
- 23.Mohammadzadeh A., Faeghi F., Sahraee N., Pouraliakbar H., Kiani R., Mohammadzadeh V., Mohammadzadeh M. Diagnostic efficacy of coronary artery three-dimensional steady-state free precession magnetic resonance angiography in comparison with invasive coronary angiography for detecting coronary artery disease. Arch. Iran. Med. 2017;20(5):314–319. [PubMed] [Google Scholar]
- 24.Kim W.Y., Danias P.G., Stuber M., Flamm S.D., Plein S., Nagel E., Botnar R.M. Coronary magnetic resonance angiography for the detection of coronary stenoses. N. Engl. J. Med. 2001;345(26):1863–1869. doi: 10.1056/NEJMoa010866. [DOI] [PubMed] [Google Scholar]
- 25.De Cecco C.N., Muscogiuri G., Varga-Szemes A., Schoepf U.J. Cutting edge clinical applications in cardiovascular magnetic resonance. World J. Radiol. 2017;9(1):1. doi: 10.4329/wjr.v9.i1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nakamura M., Kido T., Kido T., Watanabe K., Schmidt M., Forman C., Mochizuki T. Non-contrast compressed sensing whole-heart coronary magnetic resonance angiography at 3T: a comparison with conventional imaging. Eur. J. Radiol. 2018 Jul 1;104:43–48. doi: 10.1016/j.ejrad.2018.04.025. [DOI] [PubMed] [Google Scholar]
- 27.Di Leo G., Fisci E., Secchi F., Alì M., Ambrogi F., Sconfienza L.M., Sardanelli F. Diagnostic accuracy of magnetic resonance angiography for detection of coronary artery disease: a systematic review and meta-analysis. Eur. Radiol. 2016;26(10):3706–3718. doi: 10.1007/s00330-015-4134-0. [DOI] [PubMed] [Google Scholar]
- 28.Writing Committee Members, Hundley W.G., Bluemke D.A., Finn J.P., Flamm S.D., Fogel M.A., Manning W.J. ACCF/ACR/AHA/NASCI/SCMR 2010 expert consensus document on cardiovascular magnetic resonance: a report of the American college of cardiology foundation task force on expert consensus documents. Circulation. 2010;121(22):2462–2508. doi: 10.1161/CIR.0b013e3181d44a8f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Scheidegger M.B., Müller R., Boesiger P. Magnetic resonance angiography: methods and its applications to the coronary arteries. Technol. Health Care. 1994;2(4):255–265. [PubMed] [Google Scholar]
- 30.Lin L., Wang L., Zhang X.N., Li X., Wang J., Shen Z.J., Chen W., Jin Z.Y., Wang Y.N. A clinical strategy to improve the diagnostic accuracy of 1.5-T non-contrast MR coronary angiography for detection of coronary artery disease: combination of whole-heart and volume-targeted imaging. Eur. Radiol. 2020 Sep 25 doi: 10.1007/s00330-020-07135-7. 1-1. [DOI] [PubMed] [Google Scholar]
- 31.Perazella M.A., Rodby R.A. Gadolinium-induced nephrogenic systemic fibrosis in patients with kidney disease. Am. J. Med. 2007;120(7):561–562. doi: 10.1016/j.amjmed.2007.01.032. [DOI] [PubMed] [Google Scholar]
- 32.De Cecco C.N., Muscogiuri G., Varga-Szemes A., Schoepf U.J. Cutting edge clinical applications in cardiovascular magnetic resonance. World J. Radiol. 2017;9(1):1. doi: 10.4329/wjr.v9.i1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sadowski E.A., Bennett L.K., Chan M.R., Wentland A.L., Garrett A.L., Garrett R.W., Djamali A. Nephrogenic systemic fibrosis: risk factors and incidence estimation. Radiology. 2007;243(1):148–157. doi: 10.1148/radiol.2431062144. [DOI] [PubMed] [Google Scholar]
- 34.Varga-Szemes A., Wichmann J.L., Schoepf U.J., Suranyi P., De Cecco C.N., Muscogiuri G., Duguay T.M. Accuracy of noncontrast quiescent-interval single-shot lower extremity MR angiography versus CT angiography for diagnosis of peripheral artery disease: comparison with digital subtraction angiography. JACC: Cardiovasc. Imag. 2017;10(10 Part A):1116–1124. doi: 10.1016/j.jcmg.2016.09.030. [DOI] [PubMed] [Google Scholar]
- 35.Korosec F.R., Frayne R., Grist T.M., Mistretta C.A. Time-resolved contrast-enhanced 3D MR angiography. Magn. Reson. Med. 1996;36(3):345–351. doi: 10.1002/mrm.1910360304. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data included in article/supplementary material/referenced in article.