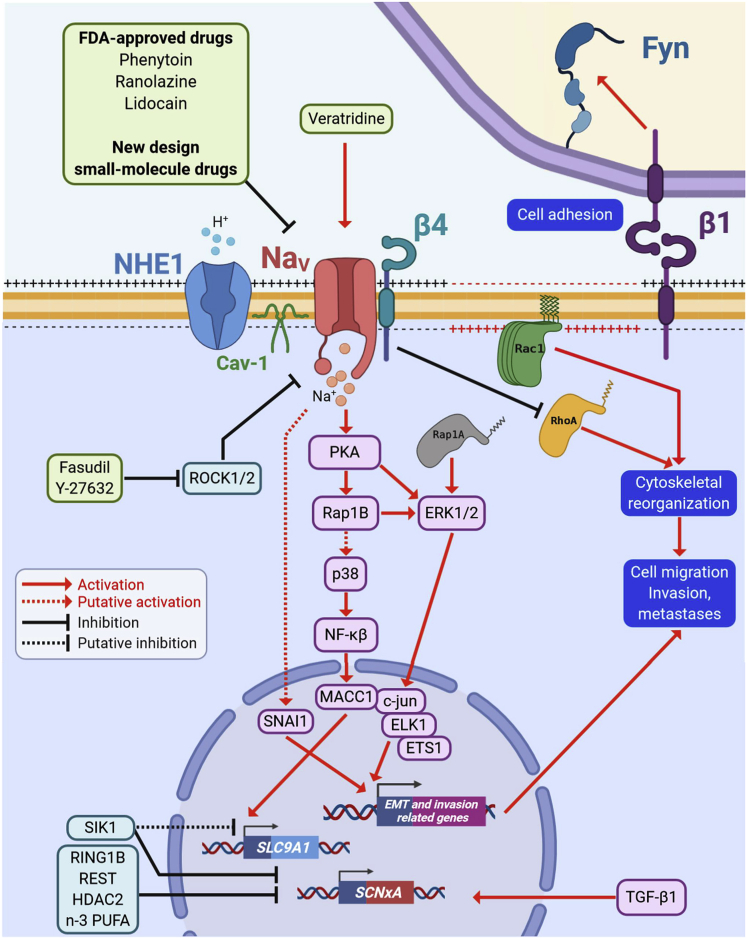
Figure 2.
Participation of NaVα and/or NaVβ in pro-metastatic signaling pathways
NaVα subunit overexpression and activity in cancer cells trigger biochemical or an electro-biochemical cascades, leading to the acquisition of a pro-invasive cell phenotype. NaV is co-localized with NHE1 in caveolin-1 (Cav-1)-containing lipid rafts and promotes the efflux of protons. NaV activity can be further stimulated by the use of pharmacological activators such as veratridine (inhibitor of the inactivation phase). Activity of NaVα subunits leads to a cAMP-independent activation of protein kinase A (PKA) that activates the cytosolic small GTPase Ras-related protein 1 (Rap1A/B) and the extracellular-signal-regulated kinases (ERK1/2). The transcription factor (TF) metastasis associated in colon cancer 1 (MACC1) is activated by the p38/NF-κβ signaling, whereas the TFs c-jun, ELK1, and ETS1 are activated by ERK1/2 and the zinc finger protein SNAI1 is activated through a NaVα-dependent mechanism regulating the expression of genes associated with cytoskeleton reorganization, cell motility, extracellular matrix degradation, and cell invasiveness. It has been demonstrated that MACC1 upregulates the expression of the SLC9A1 gene, encoding for NHE1, thus enhancing its activity at plasma membrane. On the other hand, the electro-biochemical triggering begins with a resting potential depolarization due to the activity of NaVα subunits promoting the activation and recruitment of the small GTPase Ras-related C3 botulinum toxin substrate 1 (Rac1) at the leading edge of migrating cells. Transforming growth factor β 1 (TGF-β1) increases the expression levels of NaV channels genes (SCNxA), whereas ring finger protein 1 (RING1B), RE1 silencing transcription factor (REST), histone deacetylase 2 (HDAC2) and salt inducible kinase 1 (SIK-1), as well as the n-3 polyunsaturated fatty acids n-3 (PUFA) repress their expression. SIK-1 also impairs the functioning of NHE1 exchanger. The “auxiliary subunit” NaVβ4 is expressed in normal epithelial cells but is importantly downregulated in invasive cells and high-grade metastatic tumors. The absence of this protein, but specifically the lack of the intracellular C-terminus domain, triggers the acquisition of an amoeboid-mesenchymal hybrid phenotype dependent of the small GTPase Ras homolog family member A (RhoA). NaVβ1 proteins have a dual role in cancer cells acting as cell adhesion molecules (CAMs), reducing cell migration and proliferation. However, it has also been demonstrated that NaVβ1 promotes tumor growth, metastasis, and vascularization via the proto-oncogene tyrosine-protein kinase Fyn. The Rho-associated protein kinases (ROCK1/2) negatively regulate the expression of NaVα subunits, therefore, silencing or inhibition of these repressors restore NaV channels activity promoting an aggressive cell phenotype. Pharmacological intervention with FDA-approved drugs or new-design small-molecule lead compounds against NaV channels represents a promising strategy to decrease sodium-channel-associated metastases.