Highlights
-
•
There is a high prevalence of cardiovascular disease in South African older adults.
-
•
HIV-negative adults were at greater risk of high CVD risk than HIV-positive adults.
-
•
Older men relative to women, and increasing with age, were similarly at greater risk of CVD disease.
-
•
Alcohol and high triglycerides were other factors significantly associated with high CVD risk.
-
•
Regular monitoring of CVD risk using prediction tools is needed for appropriate interventions.
Keywords: Framingham risk score, Cardiovascular risk, Older adults, HIV, South Africa
Abstract
The relationship between HIV and cardiovascular diseases (CVDs) remains complex. The aim of this study was to estimate the 10-year CVD risk among HIV-positive and HIV-negative people. The validated Framingham Risk Score (FRS) based on the Framingham Heart study was used to predict the CVD risk. Data for this analysis came from a 2016 cross-sectional study of South African community-dwelling older adults (≥50 years). Logistic regression models were constructed to assess the association between CVD risk and HIV. 403 respondents with a mean age 60 (SD = 6.7) years were enrolled, of whom 70% were female, 75% black African, 21.9% smokers, 77.2% never did any vigorous physical activity, and 17% were HIV-positive. The average 10-year CVD risk was 17%; significantly higher in men than women (23.2 vs 14.3%, p < 0.001). Overall, 33% had low CVD risk (FRS < 10%), 39% intermediate (FRS 10–19%) and 28% high risk (FRS ≥ 20%). Furthermore, participants who were HIV-positive were less likely than HIV-negative participants to have high CVD risk (aOR 0.27, 95% CI 0.11–0.66, p = 0.004). These findings of HIV-positive respondents having lower CVD risk than HIV-negative respondents could be due to three issues i) HIV-positive people having lesser cardio-metabolic disease risk factors; ii) possibly higher health care utilization by HIV-positive people; and/or iii) the neglect of HIV-negative people in HIV focused health systems. Periodic cardiovascular disease monitoring using tools like the Framingham Risk Scores is needed. Furthermore, studies with more robust designs are needed to further elucidate the relationship between HIV and CVD risks in HIV endemic sub-Saharan Africa.
1. Background
Globally, the leading causes of death are cardiovascular diseases (CVDs) (Danaei, 2014, Mensah et al., 2014). In 2016, CVDs§ accounted for approximately a third of the global deaths or 18 million lives, of whom over three quarters were among people in low- and middle-income countries (LMICs) (WHO, 2020). As the global population continues to age, more so in LMICs, the risk of CVDs will continue to grow (Giannarelli et al., 2011). In South Africa, CVDs such as atherosclerotic disease and heart failure are increasing in particular among the black African population (Lloyd-Sherlock et al., 2014, van Heerden et al., 2017). South Africa as a country with a rapidly ageing population and a large proportion of people living with HIV, is experiencing a quadruple burden of disease (Econex, 2009, Nojilana et al., 2016, Mayosi et al., 2009, Msemburi et al., 2019). The more people live longer with HIV due to successful treatment, the greater the risk of HIV infected people developing cardio-metabolic conditions (Bor et al., 2013, Clark et al., 2015). Studies assessing the risk of cardio-metabolic diseases in HIV infected people suggest that longer duration since HIV diagnosis and time since uptake of ART are associated with a higher likelihood of CVD morbidity and mortality (Todowede et al., 2019, Melo et al., 2020, Esser et al., 2013). This has been attributed to among other reasons, HIV induced dyslipidemia and inflammation (Esser et al., 2013, de Gaetano et al., 2010, Rasheed et al., 2008). Some antiretroviral regimens have also been shown to increase the risk of CVD in HIV infected persons. For example, protease inhibitors (PIs) compared to non-nucleoside reverse transcriptase inhibitors (NNRTI) have been found to induce an increase in total cholesterol, low density lipoprotein cholesterol, triglycerides, as well as an increase in insulin resistance, and hence a reduced glucose tolerance (Oh and Hegele, 2007, Pinto and da Silva, 2018). However, as noted in a review article, the relationship between HIV and CVD risk remains complex (de Gaetano et al., 2010).
Further, many of the studies suggesting an association between HIV infected people and increased risk of cardio-metabolic diseases were conducted in health facility-based HIV-positive patients (de Gaetano et al., 2010). These studies are thus limited by a lack of a suitable HIV-negative comparison group as well as not being population-based. Thus, there is still a lot of uncertainty on the effect of HIV and HIV treatment on the CVD risks of older adults in sub-Saharan countries. Furthermore, in an HIV focused health system significant amounts of resources are directed towards the care and management of individuals with known or current diseases (Benatar et al., 2018). With limited use of community health workers and a large segment of the population only visiting health facilities when they get very sick or their pain worsens (Benatar et al., 2018, Puoane et al., 2017), HIV uninfected people may tend to be left behind which is believed to have contributed to inequalities in health status between HIV infected and uninfected adults in South Africa (Negin et al., 2013).
It is imperative to have an accurate estimation of CVD risks not only in HIV infected but in HIV uninfected people also because now that HIV has turned into a chronic and manageable condition, the risks for CVDs may be similar in the two populations (de Gaetano et al., 2010). Individualized CVD risk prediction tools are essential for developing appropriate plans to better manage the growing number of people at risk of CVDs such as older people in HIV endemic settings (Melo et al., 2020, Rosolova and Nussbaumerova, 2011). The Framingham Risk Score (FRS) is one such prediction tool widely used that can be applied to relatively healthy individuals to estimate their probability of fatal or non-fatal cardiovascular events within the next ten years (de Ruijter et al., 2009, D’Agostino et al., 2008).
Therefore, the aim of this study was to estimate the 10-year risk of cardiovascular diseases using the Framingham risk scores in a sample of community-dwelling HIV-positive and HIV-negative older adults from South Africa. We further assessed the relationship between high CVD risk and HIV, adjusting for known confounding factors. This study will contribute to the dearth of information on CVD risks in HIV infected compared to HIV uninfected older adults from an African setting.
2. Methods
2.1. Study setting and design
Data for this analysis came from a cross-sectional study titled: The Sexual health, HIV infection and comorbidity with non-communicable diseases among Older Persons (SHIOP) conducted in 2016. In the SHIOP, 435 older men and women aged 50 years and older were recruited (median age 61, interquartile range 12) from semi-urban (Botha’s Hill) and urban (Chatsworth) communities of Durban, KwaZulu-Natal, South Africa. Details about the study design and methods have been published elsewhere (Abbai et al., 2018, Nyirenda et al., 2018). Briefly, well-trained and experienced community engagement officers and field recruiters went to the households within the Botha’s Hill and Chatsworth areas where they identified households with members aged ≥ 50 years. These individuals were then invited to the research sites located within the respective areas for further screening and enrolment into the study. A standardized case record form was used to collect socio-demographic information (age, sex, marital status, population group), self-reported lifestyle factors (smoking, alcohol consumption, physical activity), self-reported health status (diagnosis and treatment for hypertension, heart disease, stroke, diabetes, arthritis), sexual behaviour (current sexual activity, lifetime partners, condom use), and knowledge and attitudes towards HIV. In addition, anthropometrical (weight, height) and clinical measurements (blood pressure, glycated haemoglobin (HbA1c), total cholesterol (TC), low-density lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol (HDL-C), triglycerides, and HIV testing) were done for each participant.
Written informed consent was obtained from all participants and participant privacy was observed throughout the study. The study was approved by the South African Medical Research Council Ethics committee (EC030-9-2015).
2.2. Clinical measurements
Blood pressure was measured using a European Society of Hypertension (ESH) validated Healthease digital blood pressure (BP) instrument. Three readings of systolic and diastolic blood pressure were taken with a participant in a sitting position and at least two minutes rest interval between each reading. The average of the three readings was used to classify participants as normal (systolic BP < 120 mm Hg or diastolic BP < 80 mm Hg), pre-hypertensive (systolic BP 120–139 mm Hg or diastolic BP 80–89 mm Hg), or hypertensive (systolic BP ≥ 140 mm Hg or diastolic blood pressure of ≥ 90 mm Hg).
HIV testing: All participants in the study provided a venous collected blood specimen which was used for HIV testing using two parallel HIV kits - UnigoldTM Recombigen® and Determine HIV-1/2 (Abbott Laboratories, Japan) as per manufacturer instructions. All HIV discordant results were to be checked using an ELISA test, but none were recorded in this study.
Glycated haemoglobin (HbA1c): HbA1c was measured using standard laboratory approach from the venous blood specimen. Lipid profile for each participant was also measured from the venous blood specimen.
Body mass index (BMI) for each participant was calculated from the weight and height measurements as weight in kilograms divided by height in square meters. In the variable ‘obesity’, BMI was then categorised as: Normal (BMI ≤ 24.9), overweight (BMI 25–29.9), and obese (BMI ≥ 30). Further details on the clinical measurements and how they were tested have been published elsewhere (Abbai et al., 2018).
2.3. Statistical analysis
Descriptive bivariate analyses were used to describe the data. Data are presented in frequencies and percent for categorical variables, and using means and standard deviations for continuous variables as appropriate. Multiple logistic regression models were constructed to examine the relationship between the binary dependant variable high CVD risk (FRS ≥ 20%) and HIV, adjusted for population group, education attainment, physical activity, alcohol use, obesity, and triglycerides (independent variables). These factors are known from the literature to be commonly associated with cardio-metabolic morbidity. All factors that are part of the FRS outcome variable like age, hypertension and total cholesterol were, however, excluded from the models. Where we report p-values, a two-tailed p-value of < 0.05 was used to determine statistical significance. All analyses were conducted in STATA 14.2 (StataCorp LP, 2014).
2.3.1. Description of independent factors
Age was collected in single years as at last birthday from interview date. This was then categorised into 5-year and 10-year age groups. Classification into male or female was based on the assigned sex as per national identity document. Population group was categorised as black African or non-African based on South Africa’s population group classifications. The latter group were predominantly South Africans of Asian origin, except for one white participant. Regarding education, participants were asked for their highest level of education attained. This was categorised into: none, primary, and secondary or higher. The variable physical activity was based on the question “Do you do any vigorous sports, fitness or recreational (leisure) activities like running, swimming, bowling, playing football, or cricket for at least 10 min that cause large increases in breathing or heart rate?” Responses were on a Likert scale: Always, Often, Sometimes/Rarely, Never, Don’t Know. The variable alcohol is included in the model as a dichotomous factor (Yes/No), although in the survey participants were asked if they ever consumed alcohol with responses on a Likert scale (Yes, daily; Yes, but not daily; No, quit; Never). Obesity was a categorical variable in the logistic regression model based on the BMI measurements. Likewise, we used the dichotomised triglycerides variable in the logistic regression models (Normal ≤ 2.25 mmol/L vs High > 2.5 mmol/L).
2.3.2. Description of outcome variable
Framingham Risk Score (FRS) predicts a general 10-year risk of developing a cardiovascular event based on a Cox model developed from the Framingham Heart Study (D’Agostino et al., 2008, D'Agostino et al., 2013). We computed the FRS using information on age, sex, smoking, total cholesterol, high density lipoprotein (HDL-c) cholesterol, diabetes, systolic blood pressure and treatment for hypertension based on validated predictors and coefficients developed by Linden (Framingham, 2015). As done by others (Selvarajah et al., 2014, Boateng et al., 2018, Wekesah et al., 2020, Mashinya et al., 2015), FRS was stratified into low, intermediate and high cardiovascular risk. Where low risk was defined as FRS < 10%, FRS 10–19% as intermediate risk and FRS ≥ 20% as high risk. Although the survey was among participants aged ≥ 50 years (range 50–94 years), our analysis was restricted to individuals aged 50–75 years as the Framingham Heart Study on which the FRS was developed only enrolled participants between age 30 and 75 years. The model has not been validated in persons beyond age 75 years.
3. Results
3.1. Descriptive characteristics of study respondents
A total of 403 participants with a mean age 60.3 (SD = 6.7) met the age inclusion criterion for the Framingham Risk Score (FRS), of whom 70.0% were female, 74.7% black African, 21.9% smokers, 77.2% never did any vigorous physical activity, and 17.4% were HIV-positive (Table 1). Participants had a high mean BMI of 32.1 (SD 22.5). Participants were also characterised by a high mean systolic (135.4 mmHg, SD 21.1) and diastolic (82.6 mmHg, SD 12.6) blood pressure as well as high mean cholesterol and triglycerides. Over half of the participants self-reported to have hypertension (58.1%), of whom 86.8% self-reported to be on hypertension treatment. About a quarter of participants self-reported being diabetic (24.6%), with 89.9% of them reporting to be on treatment for diabetes. Less than one in ten self-reported ever having had a heart attack event (4.5%), and currently taking aspirin or statins to prevent a heart attack (8.7%).
Table 1.
Baseline characteristics of the included participants aged 50–75 years.
| Participants | N = 403 |
|---|---|
| Age in years, mean (SD) | 60.3 (6.7) |
| Female, n (%) | 282 (70.0) |
| African, n (%) | 301 (74.7) |
| Smoking status, n (%) | |
| Never | 281 (69.7) |
| Current | 87 (21.9) |
| Quit | 35 (8.7) |
| Physical activity, n (%) | |
| Never | 311 (77.2) |
| Always | 31 (7.7) |
| Rarely | 35 (8.7) |
| Don’t know | 26 (6.5) |
| BMI, mean (SD) | 32.1 (22.5) |
| Obesity | |
| Normal | 121 (30.1) |
| Overweight | 99 (24.6) |
| Obese | 182 (45.3) |
| Blood Pressure | |
| Systolic blood pressure, mean (SD), mmHg | 135.4 (21.1) |
| Diastolic blood pressure, mean (SD), mmHg | 82.6 (12.6) |
| Cholesterol | |
| Total Cholesterol, mean (SD), mg/dl | 173.5 (37.8) |
| HDL Cholesterol, mean (SD), mg/dl | 50.5 (15.7) |
| LDL Cholesterol, mean (SD), mg/dl | 89.4 (35.3) |
| Triglycerides, mean (SD), mg/dl | 174.0 (112.8) |
| Self-reported chronic morbidities, n (%) | |
| Heart disease | 22 (5.5) |
| Arthritis | 147 (36.5) |
| Hypertension | 234 (58.1) |
| Diabetes | 99 (24.6) |
| Self-reported treatment for chronic morbidities¥, n (%) | |
| Heart disease | 16 (72.7) |
| Arthritis | 114 (77.6) |
| Hypertension | 203 (86.8) |
| Diabetes | 89 (89.9) |
| Heart Attack | |
| Ever had a heart attack, n (%) | 18 (4.5) |
| Currently taking Aspirin or statins, n (%) | 35 (8.7) |
| HIV, n (%) | |
| Negative | 376 (82.6) |
| Positive | 70 (17.4) |
Note: ¥ Represents proportion on treatment among those self-reporting the chronic morbidity. For example, for Heart disease (16/22*100 = 72.7%); Arthritis (114/147*100 = 77.6%); Hypertension (203/234*100 = 86.8%); Diabetes (89/99*100 = 89.9%).
3.2. Prevalence of cardiovascular disease risk using Framingham risk scores (FRS)
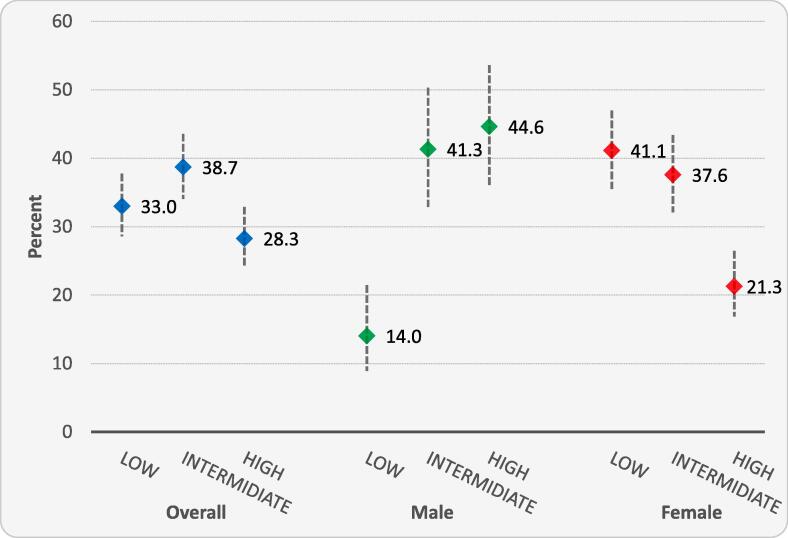
The average 10-year cardiovascular disease event risk in this sample was 17.0%, with the average significantly higher in men than women (23.2 vs 14.3%, p < 0.001). Fig. 1 displays the point estimates and 95% confidence intervals for the 10-year cardiovascular disease risk classified as low (FRS < 10%), intermediate (FRS 10–19%) or high (FRS ≥ 20%) stratified by sex. Overall, 33.0% of participants had low 10-year CVD risk; 38.7% intermediate and 28.3% were at high risk. Among women, the highest proportion (41.1%) were at low risk and only 21.3% were at high risk. The opposite was the case among men, where the highest proportion were high risk (44.6%) and only 14.0% were at low risk.
Fig. 1.
displays the point estimate and its 95% confidence interval for the proportion of participants classified as low (FRS < 10%), intermediate (FRS 10–19%) or high (FRS ≥ 20%) CVD risk stratified by sex.
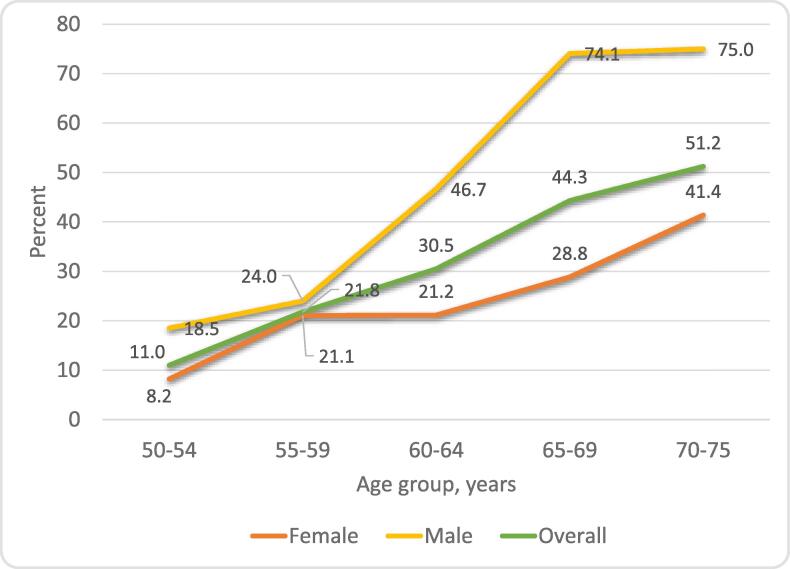
Trends in high CVD risk by age and sex are shown in Fig. 2. The 10-year high CVD risk increased with increasing age, with 51.2% of those aged 70–75 at high risk for the overall sample. Although the patterns were similar, there was a huge difference between men and women in the level of CVD risk. The proportion at high CVD risk increased exponentially from around 20% by age 59 to a high of 75% in the age group 70–75 for men compared to 41% for women.
Fig. 2.
Percentage of participants with estimated high cardiovascular disease risk by age group and sexNote: OR = Unadjusted odds ratios; aOR = Adjusted odds ratios; adjusted for: HIV, population group, educational attainment, alcohol drinking, physical exercise, obesity and triglycerides.
3.3. Factors associated with high risk of cardiovascular disease
Table 2 shows factors associated with a high CVD risk obtained using logistic regression models. HIV, currently drinking or having quit alcohol, and high triglycerides were significantly associated with a high CVD risk in unadjusted and adjusted analyses. Black African relative to non-African participants were significantly less likely to be at high CVD risk only in the unadjusted analysis. Education attainment, physical activity and obesity were all not significantly associated with high CVD risk.
Table 2.
Logistic regression factors associated with high risk of cardiovascular disease – modified.
| OR (95% CI) | P > z | aOR (95% CI) | P > z | |
|---|---|---|---|---|
| HIV status | ||||
| HIV Negative (referent) | 1.00 | 1.00 | ||
| HIV Positive | 0.28 (0.13–0.60) | 0.001 | 0.27 (0.11 – 0.66) | 0.004 |
| Population group | ||||
| Non-African (referent) | 1.00 | 1.00 | ||
| African | 0.48 (0.30 – 0.77) | 0.002 | 0.60 (0.34 – 1.07) | 0.086 |
| Education attained | ||||
| None (referent) | 1.00 | 1.00 | ||
| Primary | 1.08 (0.57 – 2.04) | 0.821 | 1.07 (0.54 – 2.12) | 0.852 |
| Secondary or higher | 0.83 (0.42 – 1.64) | 0.593 | 0.64 (0.30 – 1.36) | 0.245 |
| Drink alcohol | ||||
| No (referent) | 1.00 | 1.00 | ||
| Yes | 1.49 (0.90 – 2.45) | 0.119 | 1.95 (1.07 – 3.54) | 0.029 |
| Quit | 1.92 (0.99 – 3.66) | 0.054 | 2.31 (1.12 – 4.76) | 0.023 |
| Physical exercise | ||||
| Never (referent) | 1.00 | 1.00 | ||
| Always | 0.73 (0.30 – 1.75) | 0.477 | 0.85 (0.34 – 2.16) | 0.734 |
| Rarely | 1.14 (0.54 – 2.43) | 0.728 | 1.54 (0.66 – 3.58) | 0.313 |
| Don’t know | 0.92 (0.37 – 2.26) | 0.854 | 1.43 (0.54 – 3.82) | 0.470 |
| Obesityα | ||||
| Normal | 1.00 | 1.00 | ||
| Overweight (referent) | 1.33 (0.75 – 2.38) | 0.330 | 1.06 (0.54 – 2.07) | 0.874 |
| Obese | 0.93 (0.55 – 1.56) | 0.779 | 0.88 (0.46 – 1.68) | 0.705 |
| Triglyceridesβ | ||||
| Normal (referent) | 1.00 | 1.00 | ||
| High | 3.13 (1.98 – 4.93) | <0.001 | 3.21 (1.95 – 5.29) | <0.001 |
Notes:OR = Odds Ratios; aOR = Adjusted Odds Ratios
Body Mass Index (BMI) measurements categorised as: Normal (BMI ≤ 24.9), overweight (25–29.9), & obese (≥30)
Triglycerides measurements categorized as: Normal ≤ 2.25 mmol/L or High > 2.5 mmol/L.
3.4. Effect of HIV on high cardiovascular disease risk
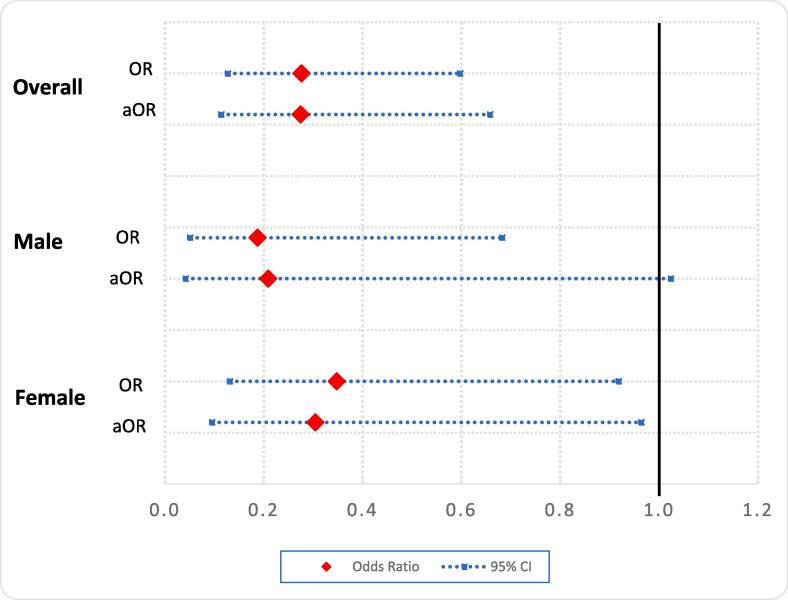
We further explored the risk of high CVD risk by HIV status and sex. Fig. 3 displays the relationship between high CVD risk and HIV for men and women separately, and for the overall sample. Where high CDV risk was defined as Framingham Risk Score (FRS) ≥ 20%. The relationship between HIV and high CVD risk was adjusted for: population group, educational attainment, alcohol drinking, physical activity, obesity and triglycerides. Model results showed that participants who were HIV-positive were less likely to have high CVD risk among men in unadjusted analyses (odds ratios (OR) 0.19, 95% confidence interval (CI) 0.05–0.68, p-value = 0.011), but not in the adjusted analyses (aOR) 0.21, 95% CI 0.04–1.02, p-value = 0.054). For women the association was significant in both the unadjusted (OR 0.35, 95% CI 0.13–0.92, p-value = 0.033) and adjusted analyses (aOR 0.30, 95% CI 0.10–0.96, p-value = 0.043). Likewise for the overall sample, being HIV-positive was associated with statistically significant lower odds of high CVD risk in both the unadjusted (OR 0.28, 95% CI 0.13–0.60, p-value = 0.001) and adjusted analyses (aOR 0.27, 95% CI 0.11–0.66, p-value = 0.004).
Fig. 3.
The chart displays the unadjusted and adjusted odds ratios in HIV-positive relative to HIV-negative participants for high CVD risk. The modelled relationship between high CVD and HIV was adjusted for population group, educational attainment, alcohol drinking, physical exercise, obesity and triglycerides. Where high CDV risk was defined as Framingham Risk Score (FRS) ≥ 20%.
4. Discussion
In this study using the Framingham risk scores (FRS) for cardiovascular (CVD) risk, our main goal was to examine the 10-year CVD risk in HIV-positive relative to HIV-negative older adults aged ≥ 50 years. We identified a high CVD risk in our analysis and found that those who were HIV-negative were associated with greater likelihood of high CVD risk than HIV-positive people. Our findings are consistent with those from a study within a longitudinal surveillance system in South Africa. In that study which enrolled only HIV infected participants they reported a better CVD risk profile compared to previous analyses from the same surveillance population that had included HIV uninfected and ART naïve individuals (Mashinya et al., 2015). Findings that HIV-positive participants had lower 10-year CVD risk than HIV-negative participants could possibly be attributed to HIV-positive participants having lower cardio-metabolic disease risk factors than their HIV-negative counterparts, as shown by Rooyen et al. (2014) who found that in HIV infected Black South Africans, HIV-infected participants had significantly lower total cholesterol and HDL cholesterol levels. This is said to be due to ART which has led to a general decrease in opportunistic infections associated with HIV and increases in life expectancy (Bor et al., 2013, Melo et al., 2020). In addition, HIV infected people also tend to have higher utilization of health facilities increasing the probability of early detection and proper management of chronic infections (Negin et al., 2013). This is compounded by reports of limited utilisation of community health care workers and a large part of the population only seeking health care when they become very sick or the pain worsens (Benatar et al., 2018, Puoane et al., 2017). As such there may be a neglect of HIV-negative people in HIV focused health systems as much resources tend to be channelled towards HIV care and management (Benatar et al., 2018).
Generally, there was a high cardiovascular disease risk in this sample. The prevalence of participants with a high and intermediate CVD risk was 28.3% and 44.6%, respectively. Those with low CVD risk was only 21.3%. The prevalence of high CVD risk we report in this study is higher than what has been reported in other studies (Todowede et al., 2019, Mashinya et al., 2015, Policarpo et al., 2019). However, our CVD risk prevalence findings are consistent with studies from similar settings (Wu et al., 2019, Pinto Neto et al., 2017). We further found that the 10-year CVD risk was higher in men than women. Our findings on men being at greater CVD risk are in line with what was recently reported by Melo et al. (2020), in a cross-sectional study that evaluated the CVD risks in persons living with HIV using the FRS. It is not clear from our study why men had higher CVD risks than women but previous studies have attributed it to smoking and alcohol, which tend to be prevalent in men than women, risk factors that have been shown to be significantly associated with CVD events (de Gaetano et al., 2010, Wu et al., 2019). Differences between what we report and what has been found by others, however, could be a result of our sample being much older (mean age 60 compared to around 45 years in other studies), as well as our sample having included HIV negative participants (Todowede et al., 2019, Mashinya et al., 2015, Policarpo et al., 2019).
Contributory traditional risk factors to the rising CVD cases are age, hypertension, diabetes mellitus, smoking and obesity (de Gaetano et al., 2010, Ramsay et al., 2016, Danaei et al., 2013). Of these factors, it was shown in a study among HIV-positive adults in Taiwan that a smoking cessation intervention could potentially result in 20 to 35% reduction in the 10-year risk of CVD (Wu et al., 2019). In South Africa, a key driver of the increasing cardio-metabolic disorders is obesity (Mensah, 2013). However, it is yet to be determined what the effect of an obesity reduction intervention would be on cardiovascular morbidity and mortality in the country. A study to this effect is welcome. HIV has also been identified as contributing to increasing cardio-metabolic conditions (Policarpo et al., 2019). If risk factors are not routinely monitored and well controlled, the risk of CVDs may increase with the ageing process, and HIV-induced CVD risk including the longer duration on treatment. Studies suggest HIV infected people are at increased risk of CVD, especially the longer since HIV diagnosis and longer exposure to antiretroviral treatment (ART) (Todowede et al., 2019, Melo et al., 2020, Aboud et al., 2010). It is understood that some ARVs may trigger hyperlipidaemia and a reduced glucose tolerance, which may increase the risk of CVDs in HIV positive individuals (Esser et al., 2013, de Gaetano et al., 2010). Policarpo et. al. (2019) further identified high triglycerides and low HDL cholesterol as key factors in this increased CVD risk among HIV positive individuals. However, a major limitation of the studies that report HIV positive people to be associated with increased CVD risk is the lack of a suitable HIV negative control group (Todowede et al., 2019, de Gaetano et al., 2010).
Data for this analysis came from a cross-sectional study using a convenient sampling approach. As such the study is limited in making causal inferences in our outcomes or extrapolating our findings to the general population. There is also the inherent desirability bias in self-reported measures such as being on treatment for hypertension and diabetes as well as smoking status, which are inputs to the Framingham risk score computation. Hence our results may be biased to the extent to which desirability bias influenced participants in self-reported responses. Another potential limitation of our study relates to the limitations inherent in the FRS measure. The FRS is among a vast array of CVD-risk estimation tools (Tunstall-Pedoe, 2011, Truett et al., 1967) that one can use. Other estimation tools include: the Pooled Cohort Equations (PCE) (Muntner et al., 2014), QRISK (Hippisley-Cox et al., 2017) and the ACC/AHA (Arnett Donna et al., 2019). Choice of which CVD-risk estimation tool to use is a matter of judgment of robustness and appropriateness for the context at hand (D’Agostino et al., 2008, Tunstall-Pedoe, 2011, Gaziano et al., 2013). There are conflicting results on the performance of the FRS with some studies suggesting the FRS tends to underestimate CVD risk (Boateng et al., 2018, Mashinya et al., 2015), while others report an overestimation of risk by the Framingham (Moreira Guimaraes et al., 2010). It is believed that the inconsistency in findings of underestimation or overestimation in CVD risk by the FRS could be attributed to the score not accounting for HIV and ART associated CVD risk (Wu et al., 2019, Law et al., 2006, Krikke et al., 2016, Triant et al., 2018). We applied the FRS to a sample of HIV-positive and HIV-negative participants despite this uncertainty. Evaluation of the validity of the FRS for this population was beyond the scope of the study. The decision to use the FRS was informed by it being a widely used robust tool in similar settings to ours (Melo et al., 2020, de Ruijter et al., 2009, Wekesah et al., 2020, Mashinya et al., 2015).
In conclusion, our findings are first to suggest HIV-negative older adults may be at higher risk of CVDs than their HIV-positive counterparts. At present it is not clear why this could be so, but can be hypothesized to be related to improved lifestyles, greater health care utilization and recovery of health due to HIV treatment, as previously reported (Bor et al., 2013, Melo et al., 2020, Negin et al., 2013, Mashinya et al., 2015). It could also be indicative of the neglect of the health of HIV-negative people in HIV focused health systems. The prevalence of high CVD risk we report is higher than some previously reported studies but consistent with those from similar settings. This study therefore suggests that despite the challenges in CVD diagnosis and risk prediction, there is a need for periodic cardiovascular disease monitoring using tools like Framingham scores not only in HIV infected older adults, but in HIV uninfected older adults as well who may be left behind or neglected in HIV dominated health systems. Further studies with more robust designs and possibly prospective follow-up are needed to further elucidate the relationship between HIV and CVD risks in HIV endemic sub-Saharan Africa settings.
CRediT authorship contribution statement
Makandwe Nyirenda: Conceptualization, Methodology, Formal analysis, Data curation, Writing - original draft, Writing - review & editing, Project administration, Funding acquisition.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgements
We acknowledge the contribution of all participants for the SHIOP study for making this study possible. Special mention also goes to all SAMRC project members and support staff and personnel at local clinics to where participants were referred for further care. The anonymous peer reviewers and journal editors are acknowledged for their contributions to this paper.
Funding
This work was supported by the South African Medical Research Council (SAMRC). The funder had no role in the design of the study, data collection, analysis, interpretation of the data, writing of this manuscript or decision to submit the article for publication.
Footnotes
CVDs consist of disorders relating to the heart and blood vessels including: coronary, rheumatic and congenital heart diseases; cerebrovascular disease; peripheral disease; deep vein thrombosis and pulmonary embolism. WHO. Cardiovascular diseases (CVDs). World Health Organization. Accessed 29 July 2020, https://www.who.int/news-room/fact-sheets/detail/cardiovascular-diseases-(cvds).
References
- Abbai N.S., Nyirenda M., Reddy T., Ramjee G. Good correlation between the Afinion AS100 analyser and the ABX Pentra 400 analyser for the measurement of glycosylated haemoglobin and lipid levels in older adults in Durban, South Africa. SAMJ S. Afr. Med. J. 2018;108:50–55. doi: 10.7196/SAMJ.2017.v108i1.12548. [DOI] [PubMed] [Google Scholar]
- Aboud M., Elgalib A., Pomeroy L. Cardiovascular risk evaluation and antiretroviral therapy effects in an HIV cohort: implications for clinical management: the CREATE 1 study. Int. J. Clin. Pract. 2010;64(9):1252–1259. doi: 10.1111/j.1742-1241.2010.02424.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnett Donna K., Blumenthal Roger S., Albert Michelle A. 2019 ACC/AHA Guideline on the Primary Prevention of Cardiovascular Disease: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019;140(11):e563–e595. doi: 10.1161/CIR.0000000000000677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benatar S., Sullivan T., Brown A. Why equity in health and in access to health care are elusive: Insights from Canada and South Africa. Global Public Health. 2018;13(11):1533–1557. doi: 10.1080/17441692.2017.1407813. [DOI] [PubMed] [Google Scholar]
- Boateng D., Agyemang C., Beune E. Cardiovascular disease risk prediction in sub-Saharan African populations — Comparative analysis of risk algorithms in the RODAM study. Int. J. Cardiol. 2018;254:310–315. doi: 10.1016/j.ijcard.2017.11.082. [DOI] [PubMed] [Google Scholar]
- Bor J., Herbst A.J., Newell M.L., Barnighausen T. Increases in adult life expectancy in rural South Africa: valuing the scale-up of HIV treatment. Science. 2013;339(6122):961–965. doi: 10.1126/science.1230413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark S.J., Gomez-Olive F.X., Houle B. Cardiometabolic disease risk and HIV status in rural South Africa: establishing a baseline. BMC Public Health. 2015;15:135. doi: 10.1186/s12889-015-1467-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Agostino, R.B., Vasan, R.S., Pencina, M.J., et al., 2008. General Cardiovascular Risk Profile for Use in Primary Care. The Framingham Heart Study. Circulation. 11710.1161/circulationaha.107.699579. [DOI] [PubMed]
- D'Agostino R.B., Sr., Pencina M.J., Massaro J.M., Coady S. Cardiovascular disease risk assessment: insights from Framingham. Glob. Heart. 2013;8(1):11–23. doi: 10.1016/j.gheart.2013.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danaei, G., Singh, G.M., Paciorek, C.J., et al., 2013. The global cardiovascular risk transition: associations of four metabolic risk factors with national income, urbanization, and Western diet in 1980 and 2008. Circulation. 12710.1161/circulationaha.113.001470. [DOI] [PMC free article] [PubMed]
- Danaei, G., Collaborators, 2014. Cardiovascular disease, chronic kidney disease, and diabetes mortality burden of cardiometabolic risk factors from 1980 to 2010: a comparative risk assessment. The lancet Diabetes & endocrinology.2(8):634-647. 10.1016/S2213-8587(14)70102-0. [DOI] [PMC free article] [PubMed]
- de Gaetano Donati K., Cauda R., Iacoviello L. HIV Infection, Antiretroviral Therapy and Cardiovascular Risk. Mediterr. J. Hematol. Infect Dis. 2010;2(3) doi: 10.4084/mjhid.2010.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Ruijter W., Westendorp R.G.J., Assendelft W.J.J. Use of Framingham risk score and new biomarkers to predict cardiovascular mortality in older people: population based observational cohort study. Article. BMJ: British Medical Journal (Overseas & Retired Doctors Edition). 2009:219–222. doi: 10.1136/bmj.a3083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Econex. South Africa's Burden of Disease. 2009. https://econex.co.za/wp-content/uploads/2015/07/ECONEX_NHInote_2.pdf.
- Esser S., Gelbrich G., Brockmeyer N. Prevalence of cardiovascular diseases in HIV-infected outpatients: results from a prospective, multicenter cohort study. Clin Res Cardiol. 2013;102(3):203–213. doi: 10.1007/s00392-012-0519-0. [DOI] [PubMed] [Google Scholar]
- Framingham: Stata module for calculating the Framingham 10-year Cardiovascular Disease Risk Prediction. 2015.
- Gaziano, T.A., Pandya, A., Steyn, K., et al., 2013. Comparative assessment of absolute cardiovascular disease risk characterization from non-laboratory-based risk assessment in South African populations. BMC Med. 11:170. 10.1186/1741-7015-11-170. [DOI] [PMC free article] [PubMed]
- Giannarelli C., Klein R.S., Badimon J.J. Cardiovascular implications of HIV-induced dyslipidemia. Atherosclerosis. 2011;219(2):384–389. doi: 10.1016/j.atherosclerosis.2011.06.003. [DOI] [PubMed] [Google Scholar]
- Hippisley-Cox J., Coupland C., Brindle P. Development and validation of QRISK3 risk prediction algorithms to estimate future risk of cardiovascular disease: prospective cohort study. BMJ. 2017;357 doi: 10.1136/bmj.j2099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krikke M., Hoogeveen R.C., Hoepelman A.I., Visseren F.L., Arends J.E. Cardiovascular risk prediction in HIV-infected patients: comparing the Framingham, atherosclerotic cardiovascular disease risk score (ASCVD), Systematic Coronary Risk Evaluation for the Netherlands (SCORE-NL) and Data Collection on Adverse Events of Anti-HIV Drugs (D:A:D) risk prediction models. HIV Med. 2016;17(4):289–297. doi: 10.1111/hiv.12300. [DOI] [PubMed] [Google Scholar]
- Law M.G., Friis-Moller N., El-Sadr W.M. The use of the Framingham equation to predict myocardial infarctions in HIV-infected patients: comparison with observed events in the D:A: D Study. HIV Med. 2006;7(4):218–230. doi: 10.1111/j.1468-1293.2006.00362.x. [DOI] [PubMed] [Google Scholar]
- Lloyd-Sherlock, P., Beard, J., Minicuci, N., Ebrahim, S., Chatterji, S., 2014. Hypertension among older adults in low- and middle-income countries: prevalence, awareness and control. Int. J. Epidemiol. February 6, 2014;10.1093/ije/dyt215. [DOI] [PMC free article] [PubMed]
- Mashinya F., Alberts M., Van Geertruyden J.P., Colebunders R. Assessment of cardiovascular risk factors in people with HIV infection treated with ART in rural South Africa: a cross sectional study. AIDS Res Ther. 2015;12:42. doi: 10.1186/s12981-015-0083-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayosi B.M., Flisher A.J., Lalloo U.G., Sitas F., Tollman S.M., Bradshaw D. The burden of non-communicable diseases in South Africa. Lancet. 2009;374(9693):934–947. doi: 10.1016/S0140-6736(09)61087-4. [DOI] [PubMed] [Google Scholar]
- Melo E.S., Antonini M., Costa C.R.B., Sorensen W., Gir E., Reis R.K. Evaluation of cardiovascular risk factors in people living with HIV in Sao Paulo, Brazil. J. Infect. Dev. Ctries. 2020;14(1):89–96. doi: 10.3855/jidc.11326. [DOI] [PubMed] [Google Scholar]
- Mensah G.A. Descriptive epidemiology of cardiovascular risk factors and diabetes in sub-Saharan Africa. Prog. Cardiovasc. Dis. 2013;56(3):240–250. doi: 10.1016/j.pcad.2013.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mensah G.A., Moran A.E., Roth G.A., Narula J. The global burden of cardiovascular diseases, 1990–2010. Glob Heart. 2014;9(1):183–184. doi: 10.1016/j.gheart.2014.01.008. [DOI] [PubMed] [Google Scholar]
- Moreira Guimaraes, M.M., Bartolomeu Greco, D., Ingles Garces, A.H., de Oliveira, A.R., Jr., Bastos Foscolo, R., de Campos Machado, L.J., 2010. Coronary heart disease risk assessment in HIV-infected patients: a comparison of Framingham, PROCAM and SCORE risk assessment functions. Int. J. Clin. Pract. 64(6):739-45. 10.1111/j.1742-1241.2009.02248.x. [DOI] [PubMed]
- Msemburi, W., Pillay-van Wyk, V., Dorrington, R.E., et al., 2019. Second national burden of disease study for South Africa: Cause-of-death profile for South Africa,1997–2012. . 2016. http://www.samrc.ac.za/sites/default/files/files/2016-12-08/SouthAfrica2012.pdf. [DOI] [PubMed]
- Muntner P., Colantonio L.D., Cushman M. Validation of the atherosclerotic cardiovascular disease Pooled Cohort risk equations. JAMA. 2014;311(14):1406–1415. doi: 10.1001/jama.2014.2630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negin J., Nyirenda M., Seeley J., Mutevedzi P. Inequality in health status among older adults in Africa: the surprising impact of anti-retroviral treatment. J. Cross Cult. Gerontol. 2013;28(4):491–493. doi: 10.1007/s10823-013-9215-4. [DOI] [PubMed] [Google Scholar]
- Nojilana B., Bradshaw D., Pillay-van Wyk V. Emerging trends in non-communicable disease mortality in South. Africa. 2016;106(2016):1997–2010. doi: 10.7196/SAMJ.2016.v106i5.10674. [DOI] [PubMed] [Google Scholar]
- Nyirenda, M., Abbai, N.S., Naidoo, J., Ramjee, G., 2018. Sexual activity and healthcare-seeking behaviour: A cross-sectional study of older adults in an HIV- endemic South African setting. journal article. Southern African J. Demography. 18(1):7-57. 10: 1682-4482.
- Oh J., Hegele R.A. HIV-associated dyslipidaemia: pathogenesis and treatment. Lancet Infect Dis. 2007;7(12):787–796. doi: 10.1016/s1473-3099(07)70287-6. [DOI] [PubMed] [Google Scholar]
- Pinto D.S.M., da Silva M.J.L.V. Cardiovascular Disease in the Setting of Human Immunodeficiency Virus Infection. Curr. Cardiol. Rev. 2018;14(1):25–41. doi: 10.2174/1573403X13666171129170046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinto Neto L., Dias F.R., Bressan F.F., Santos C.R.O. Comparison of the ACC/AHA and Framingham algorithms to assess cardiovascular risk in HIV-infected patients. Braz. J. Infect. Dis. 2017;21(6):577–580. doi: 10.1016/j.bjid.2017.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Policarpo S., Rodrigues T., Moreira A.C., Valadas E. Cardiovascular risk in HIV-infected individuals: A comparison of three risk prediction algorithms. Rev Port Cardiol. 2019;38(7):463–470. doi: 10.1016/j.repc.2019.08.002. [DOI] [PubMed] [Google Scholar]
- Puoane T., Abrahams-Gessel S., Gaziano T.A., Levitt N. Training community health workers to screen for cardiovascular disease risk in the community: experiences from Cape Town, South Africa. Cardiovasc J. Afr. 2017;28(3):170–175. doi: 10.5830/CVJA-2016-077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsay M., Crowther N., Tambo E. H3Africa AWI-Gen Collaborative Centre: a resource to study the interplay between genomic and environmental risk factors for cardiometabolic diseases in four sub-Saharan African countries. Glob. Health Epidemiol. Genom. 2016;1 doi: 10.1017/gheg.2016.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasheed S., Yan J.S., Lau A., Chan A.S. HIV Replication Enhances Production of Free Fatty Acids, Low Density Lipoproteins and Many Key Proteins Involved in Lipid Metabolism: A Proteomics Study. PLoS ONE. 2008;3(8) doi: 10.1371/journal.pone.0003003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosolova H., Nussbaumerova B. Cardio-metabolic risk prediction should be superior to cardiovascular risk assessment in primary prevention of cardiovascular diseases. EPMA J. 2011;2(1):15–26. doi: 10.1007/s13167-011-0066-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selvarajah, S., Kaur, G., Haniff, J., et al., 2014. Comparison of the Framingham Risk Score, SCORE and WHO/ISH cardiovascular risk prediction models in an Asian population. Int. J. Cardiol. 176(1):211-218. http://dx.doi.org/10.1016/j.ijcard.2014.07.066. [DOI] [PubMed]
- Stata: Release 14. Statistical Software. Version 13.1. StataCorp LP, 2014.
- Todowede O.O., Sartorius B., Magula N., Schutte A.E. Association of predicted 10 years cardiovascular mortality risk with duration of HIV infection and antiretroviral therapy among HIV-infected individuals in Durban, South Africa. Diabetology & Metabolic Syndrome. 2019;11(1):105. doi: 10.1186/s13098-019-0502-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Triant V.A., Perez J., Regan S. Cardiovascular Risk Prediction Functions Underestimate Risk in HIV Infection. Circulation. 2018;137(21):2203–2214. doi: 10.1161/CIRCULATIONAHA.117.028975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Truett J., Cornfield J., Kannel W. A multivariate analysis of the risk of coronary heart disease in Framingham. J. Chronic. Dis. 1967;20(7):511–524. doi: 10.1016/0021-9681(67)90082-3. [DOI] [PubMed] [Google Scholar]
- Tunstall-Pedoe H. Cardiovascular Risk and Risk Scores: ASSIGN, Framingham, QRISK and others: how to choose. Heart. 2011;97(6):442–444. doi: 10.1136/hrt.2010.214858. [DOI] [PubMed] [Google Scholar]
- van Heerden, A., Barnabas, R.V., Norris, S.A., Micklesfield, L.K., van Rooyen, H., Celum, C., 2017. High prevalence of HIV and non-communicable disease (NCD) risk factors in rural KwaZulu-Natal, South Africa. J Int AIDS Soc. Oct 20(2)10.1002/jia2.25012. [DOI] [PMC free article] [PubMed]
- van Rooyen J.M., Fourie C.M., Steyn H.S. Cardiometabolic markers to identify cardiovascular disease risk in HIV-infected black South Africans. S. Afr. Med. J. 2014;104(3):195–199. doi: 10.7196/samj.7739. [DOI] [PubMed] [Google Scholar]
- Wekesah F.M., Mutua M.K., Boateng D. Comparative performance of pooled cohort equations and Framingham risk scores in cardiovascular disease risk classification in a slum setting in Nairobi Kenya. IJC Heart & Vasculature. 2020;28:100521. doi: 10.1016/j.ijcha.2020.100521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO. Cardiovascular diseases (CVDs). World Health Organization. Accessed 29 July 2020, https://www.who.int/news-room/fact-sheets/detail/cardiovascular-diseases-(cvds).
- Wu P.Y., Chen M.Y., Sheng W.H. Estimated risk of cardiovascular disease among the HIV-positive patients aged 40 years or older in Taiwan. J. Microbiol. Immunol. Infect. 2019;52(4):549–555. doi: 10.1016/j.jmii.2019.03.006. [DOI] [PubMed] [Google Scholar]