Abstract
Background
Surfactant protein D (SP-D) is a promising biomarker proposed for the prediction of community-acquired pneumonia (CAP) severity. Therefore, we aimed to assess the role of SP-D in the prediction of CAP severity in pediatric patients.
Methods
A prospective cohort study was carried out at the Pediatric Intensive Care Unit (PICU) and wards of Menoufia University Hospital. We recruited 112 children admitted into wards with pneumonia (simple pneumonia) and 68 children admitted into PICU with severe pneumonia (PICU admitted). World Health Organization (WHO) classification and mortality predictive scores were calculated to determine the severity of pneumonia for the two groups, including the Pediatric Respiratory Severity Score (PRESS) and the Predisposition, Insult, Response, and Organ dysfunction modified Score (PIROm). SP-D was measured at admission.
Results
The SP-D level was significantly lower in patients with simple pneumonia than in patients with severe pneumonia (P < 0.001). SP-D was significantly higher among children with severe pneumonia, as determined by WHO, PRESS, and PIROm (P = 0.001). SP-D was significantly higher among children with mechanical ventilation, shock, hypoxia, sepsis, and mortality. Receiver operating characteristic curve analysis for SP-D showed that the area under the curve was 0.741 (P value < 0.001), with a sensitivity of 85.3% and a specificity of 44.6%.
Conclusions
Serum SP-D level has a predictive value for the detection of community-acquired pneumonia severity in children.
Impact
SP-D is a good predictor for the detection of CAP severity in hospitalized children. SP-D was correlated with severity scores and was associated with indicators of CAP severity, including mechanical ventilation, shock, hypoxia, sepsis, and mortality.
Introduction
Community-acquired pneumonia (CAP) is defined as symptoms and signs of lung parenchyma infection in a previously healthy child who acquired the infection in the community.1 CAP is one of the leading causes of mortality in children under the age of 5 years in developing countries.2
According the World Health Organization (WHO), 156 million children <5 years old are diagnosed with pneumonia each year; 20 million of these children are admitted to the hospital, and >2 million die.3
Surfactant protein D (SP-D) is a protein found in surfactant that is produced by type II alveolar epithelial cells. Its expression is upregulated in response to lung injury or infection.4
SP-D is important for lung stability, and its composition and functional state are changed during pneumonia and acute respiratory distress syndrome.5 Elevated SP-D is a specific indicator of lung injury and is accompanied by both acute and chronic lung disease in adults and respiratory distress syndrome in premature infants.4
SP-D plays a vital role in host defense by recognizing the carbohydrate components of bacteria and viruses.6 It is important in aggregation, neutralization, and opsonization during phagocytosis, resulting in direct lysis of Gram-negative bacterial cell membranes and inhibiting the growth of bacteria and fungi in macrophages.7 SP-D and other collectins, such as conglutinin, mannose-binding lectin, and CL43, inhibit influenza A virus by binding to glycans on the viral envelope proteins. Binding of these collectins to high mannose oligosaccharides on hemagglutinin appears to be important for viral neutralization.8–10
SP-D is increased in inflammatory lung diseases, such as lung infections and pneumonitis, due to damage to alveolar cells.11 The aim of the present study was to assess the role of serum SP-D in the prediction of CAP severity in pediatric patients.
Patients and methods
Patients
This study was conducted on two groups of children admitted with CAP where Group (simple pneumonia) was admitted into wards with simple pneumonia and Group (PICU admitted) was admitted into the PICU with severe pneumonia. All patients were admitted to the pediatric department of Menoufia University Hospital, Egypt, during the period of 1 May 2017 to 30 April 2018. All parents of the included children gave their written consent to participate in the study. The study was approved by the Menoufia University Ethics Committees.
Criteria for inclusion in the study included (1) age beyond 2 months up to 59 months, according to the 2014 revised WHO guidelines, in which treatment is performed only for patients aged from 2 months up to 59 months, (2) parental consent, and (3) any patient admitted with CAP, defined as the presence of signs and symptoms suggesting lower respiratory tract infection in a previously healthy child that was acquired outside the hospital. This was confirmed by the radiological finding of a consolidation (British Thoracic Society guidelines).12
Criteria for exclusion included age under 2 months or older than 59 months, underlying chronic respiratory disease, persistent asthma, suspected tuberculosis, neurologic disease, immunodeficiency, immunosuppressive treatment, congenital heart disease, patients with a diagnosis of bronchiolitis, and coexistence of another infection with CAP, e.g., gastroenteritis and antibiotic treatment in the last 48 h.
All patients were classified according to the revised WHO guidelines13: the presence of tachypnea and/or chest indrawing indicated a diagnosis of pneumonia (simple pneumonia group). The presence of general danger signs (persistent vomiting, lethargy or unconsciousness, seizures, severe malnutrition, stridor in a calm child, or not able to drink) indicates a diagnosis of severe pneumonia (PICU admitted group).
We used three severity scores, namely, the Pediatric Respiratory Severity Score (PRESS),14 the Respiratory Index of Severity Score (RISC)15 and the Predisposition, Insult, Response, Organ dysfunction modified (PIROm).16 First, the PRESS score was validated in a prospective study composed of pediatric patients in a Japanese hospital, and pneumonia is considered severe if the score is 4–5. Second, the RISC score was validated in pediatric populations of South Africa, and a score of 3 or more indicates a poor prognosis. Finally, the PIROm score was validated in Paraguay in a retrospective study and a score of 5–6 indicates severe pneumonia, while a score of 7–10 indicates very severe pneumonia.
Criteria for Pediatric Intensive Care Unit (PICU) admission included (1) the need for invasive or noninvasive mechanical ventilation, (2) impending respiratory failure, (3) oxygen saturation (SPO2) <92% on inspired oxygen ≥50%, (4) signs of shock, and (5) altered mental status.1
The primary outcome measure was development of any indicator of pneumonia severity, such as hypoxia, shock, sepsis, or death, within 30 days. Patients who experienced hypoxia, shock, and sepsis were consequent with the development of CAP.
Methodology
A complete workup was performed, including history taking and physical examinations, for all patients. For each patient, the vital signs and oxygen saturation were monitored. Systemic inflammatory response syndrome (SIRS) and sepsis were defined according to the criteria established by the international pediatric sepsis consensus conference.17 A patient was considered as having sepsis if there was evidence of SIRS in the presence of or as a result of suspected or proven infection. Hypoxia was defined as a sustained peripheral SPO2 <94%.18 Shock is defined as a condition in which peripheral tissues and end organs do not receive adequate oxygen and nutrients. The body compensates for shock through various mechanisms, most commonly through increased heart rate to increase cardiac output.19,20
Furthermore, mortality predictive scores were calculated to determine the severity of pneumonia for the two groups, including the PRESS to evaluate the severity of respiratory tract infections, the RISC in children for respiratory infections, and the PIROm; this score evaluates clinical criteria and radiological and laboratory data collected from clinical data.
The laboratory workup for all participants included collection of 1 ml of blood into an EDTA-containing tube for complete blood count by a Sysmex XN-1000 (Japan; 19723), and another 2 ml of blood was evacuated into a plain tube and left to clot and then centrifuged for 10 min at 4000 R.P.M. The obtained sera were utilized for estimating the SP-D level, which was accomplished by a sandwich enzyme immunoassay technique using the Quantikine Kit for Human SP-D Immunoassay, catalog number DSFPD0, USA & Canada, R&D Systems, Inc.16 This kit has a sensitivity of 0.11 ng/ml for SP-D detection, with an intra-assay precision of coefficient of variation (CV) <10% and an interassay precision of CV <10%. A single SP-D measurement was performed for all patients in the separated sera from blood samples collected on admission. The sera were preserved at −20 °C, and repeated freeze–thaw was avoided as indicated to ensure the stability of SP-D. The measurements were performed within a maximum of 2 weeks from the time of sampling. Every sample was run in duplicate, and the mean of both readings was estimated to ensure the precision of results. C-reactive protein (CRP) was measured by enzyme-linked immunosorbent assay (ELISA) using a Sun Red Elisa Kit, China (catalog no. 201-12-1799). The laboratory investigators were blinded to clinical data. Additionally, serum electrolytes (Na and K), arterial blood gases, liver enzymes (alanine transaminase and aspartate aminotransferase), and serum creatinine were estimated as part of usual clinical care. Other laboratory tests or radiological investigations were performed when appropriate.
Statistical analysis
Data analysis was performed using the IBM SPSS software version 20.0 (SPSS, Inc., Chicago, IL, USA). Descriptive analyses, e.g., percentage (%), mean, standard deviation (SD), median and interquartile range, t test, and Mann–Whitney U test, were used to compare quantitative data, while the Chi-square test was used for qualitative data. Spearman correlation was also performed. The diagnostic power of the SP-D was evaluated by the receiver operating characteristic (ROC) curve with the Youden index to select the optimal cut-off values. The independent risk factors for PICU admission were evaluated using binary logistic regression analysis. A two-sided P value <0.05 was considered statistically significant.
Results
The studied patients consisted of 180 children with CAP, including 112 (62.2%) with simple pneumonia and 68 (37.8%) with severe pneumonia. Their main demographic and clinical characteristics are shown in Table 1 and were recorded on admission. Pathogenic bacteria were isolated from blood culture in only 15 patients (22.1%). The organisms were Streptococcus pneumoniae (11patients), Staphylococcus aureus (2 patients), and group A beta hemolytic Streptococci (2 patients). Clinical indicators of pneumonia severity, such as the need for mechanical ventilation, hypoxia, shock, and sepsis, presented in the PICU admitted group as 38.2, 70.6, 35.3, and 33.8%, respectively. All patients of the PICU admitted group and 48.2% of patients of the simple pneumonia group had SIRS criteria. These clinical indicators were secondary to CAP.
Table 1.
Demographic and clinical data of the studied groups.
| Studied variables | Studied groups | P value | |
|---|---|---|---|
| Simple pneumonia N = 112 | PICU admitted N = 68 | ||
| Age/months | |||
| Median | 31 | 51 | 0.013 |
| IQR | 20–41 | 35–55 | |
| Sex, N (%) | |||
| Male | 56 (50.0) | 47 (69.1) | 0.012 |
| Female | 56 (50.0) | 21 (30.9) | |
| Weight (kg) | |||
| Median | 10 | 7 | 0.001 |
| IQR | 7–14 | 5–11 | |
| Hospital stay/days | |||
| Median | 8.5 | 18 | 0.001 |
| IQR | 7–17 | 10–32 | |
| Need for MV, N (%) | |||
| Ventilated | 0 (0.00) | 26 (38.2) | 0.001 |
| Non-ventilated | 112 (100) | 42 (61.8) | |
| Hypoxia, N (%) | 0 (0 %) | 48 (70.6%) | <0.001 |
| Shock, N (%) | 0 (0 %) | 24 (35.3) % | <0.001 |
| SIRS, N (%) | 54 (48.2%) | 68 (100%) | <0.001 |
| Sepsis, N (%) | 0 (0 %) | 23 (33.8%) | <0.001 |
| Blood culture, N (%) | |||
| Positive | 0 (0 %) | 15 (22.1%) | 0.002 |
| Negative | 112 (100%) | 53 (77.9%) | |
MV mechanical ventilation, IQR interquartile range.
Serum SP-D was significantly elevated among PICU admitted compared with simple pneumonia [259.65 (199.94–313.79 ng/mL)] versus [87.257 (69.19–189.21 ng/ml)] P = 0.001)]. Acidosis was significantly higher in PICU admitted than in simple pneumonia [Table 2].
Table 2.
Laboratory and radiological characteristics of the studied groups.
| Studied variables | Studied groups | P value | |
|---|---|---|---|
| Simple pneumonia N = 112 | PICU admitted N = 68 | ||
| WBCs (1000/ml) | |||
| Median | 12.2 | 12.3 | 0.434 |
| IQR | 9.50–15.3 | 9.0–16.5 | |
| Platelets count (1000/ml) | |||
| Median | 342 | 300 | 0.030 |
| IQR | 181–401 | 106–360 | |
| CRP (mg/l) | |||
| Median | 24 | 24 | |
| IQR | 14–41 | 19–48 | 0.938 |
| PH in the arterial blood | |||
| Mean ± SD | 7.35 ± 0.06 | 7.31 ± 0.03 | <0.001 |
| Range | 7.29–7.46 | 7.28–7.45 | |
| Surfactant protein D level (ng/ml) | |||
| Median | 87.275 | 259.65 | 0.001 |
| IQR | 69.19–189.21 | 199.94–313.79 | |
| Radiological finding: N (%) | |||
| Lobar pneumonia | 70 (62.5) | 38 (55.9) | 0.380 |
| Patchy consolidation | 42 (37.5) | 30 (44.1) | |
WBC white blood cell, CRP C-reactive protein, IQR interquartile range.
SP-D was significantly higher in patients with severe pneumonia complicated with hypoxia, shock, SIRS criteria, and sepsis and was also higher among those needing mechanical ventilation and who did not survive [Table 3].
Table 3.
Surfactant protein D in relation to patients’ complications and outcome.
| Studied variables | No. | Surfactant protein D level (ng/ml), median (IQR) | P value |
|---|---|---|---|
| Hypoxia | |||
| Yes | 80 | 91.5 (65–118) | 0.032 |
| No | 100 | 51.2 (43–59) | |
| Mechanical ventilation | |||
| Ventilated | 26 | 88 (62–172) | 0.003 |
| Non-ventilated | 154 | 55 (41–62) | |
| Shock | |||
| Yes | 24 | 59 (40–120) | 0.007 |
| No | 156 | 39 (35–54) | |
| Fulfilled SIRS criteria | |||
| SIRS | 122 | 45.2 (41.3–99.2) | 0.04 |
| NO SIRS | 58 | 38.1 (32.5–48.7) | |
| Complicated with sepsis | |||
| Septic | 39 | 79 (59–133) | 0.004 |
| Non-septic | 141 | 49 (40.6–61.3) | |
| Mortality | |||
| Survivor | 169 | 48.2 (41–66.2) | 0.001 |
| Non-survivor | 11 | 133 (88–166) | |
SIRS systemic inflammatory response syndrome, IQR interquartile range.
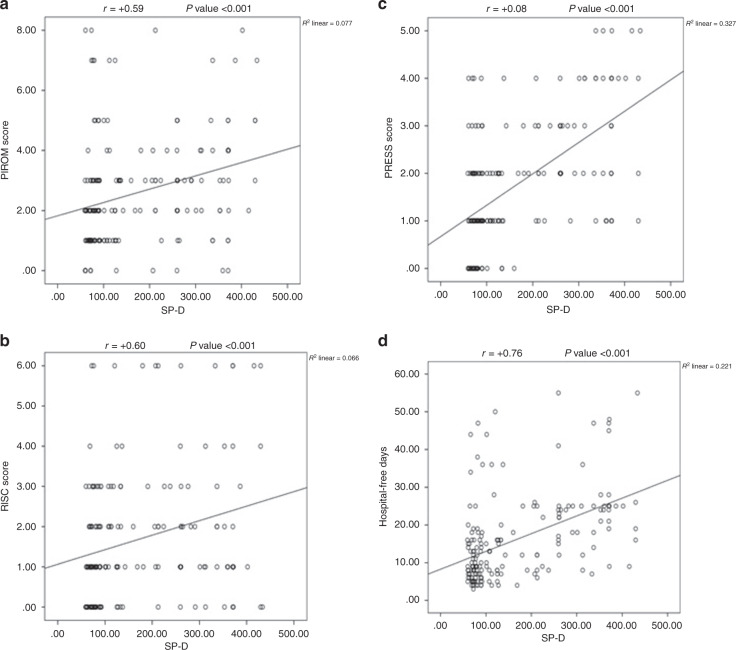
There are significant positive correlations between SP-D and RISC score, PRESS score, PIROm score, and duration of hospital stay [Fig. 1].
Fig. 1. Correlation between SP-D level and different measured sepsis scores and duration of hospital stay.
a Correlation between SP-D level and PIROm score. b Correlation between SP-D level and RISC score. c Correlation between SP-D level and PRESS score. d Correlation between SP-D level and duration of hospital stay.
In addition, no significant correlation was detected between SP-D and CRP, and no significant difference in CRP level was detected between the patient group with severe pneumonia and the other patient group.
Multivariate regression analysis revealed that serum SP-D level, hospital-free days, mechanical ventilation need, hypoxia, shock, and sepsis were independent risk factors for the occurrence of severe pneumonia with odds ratios (ORs) 1.9, 2.1, 1.77, 0.91, 0.99, and 1.07, respectively [Table 4].
Table 4.
Multivariate regression analysis of risk factors for pneumonia severity.
| P value | OR | 95% CI (lower–upper) | Hosmer–Lemeshow | P value | |
|---|---|---|---|---|---|
| Age/months | 0.58 | 0.77 | 0.09–7.87 | ||
| Sex/N (%) | 0.34 | 0.88 | 0.11–10.76 | ||
| Weight (kg) | 0.11 | 0.56 | 0.23–5.67 | 4.22 | 0.675 |
| Platelets count (1000/ml) | 0.18 | 1.18 | 0.44–8.53 | ||
| Serum surfactant protein D level (ng/ml) | 0.04 | 1.9 | 1.6–6.43 | ||
| Hospital-free days | 0.03 | 2.1 | 1.6–10.79 | ||
| Need for MV | 0.045 | 1.77 | 0.43–5.57 | ||
| Hypoxia | 0.04 | 0.91 | 0.33–4.65 | ||
| Shock | 0.03 | 0.99 | 0.21–3.76 | ||
| Sepsis | 0.007 | 1.07 | 0.77–7.65 |
MV mechanical ventilation, OR odds ratio, CI confidence interval.
The ROC curve analysis for SP-D level as a predictor of pneumonia severity showed that the area under the curve (AUC) was 0.741 (P value 0.001, 95% confidence interval (CI) 0.66–0.82); at a cut-off point (79.77), the sensitivity was 85.3%, the specificity was 44.6%, and the accuracy of the test was 60% [Fig. 2].
Fig. 2.
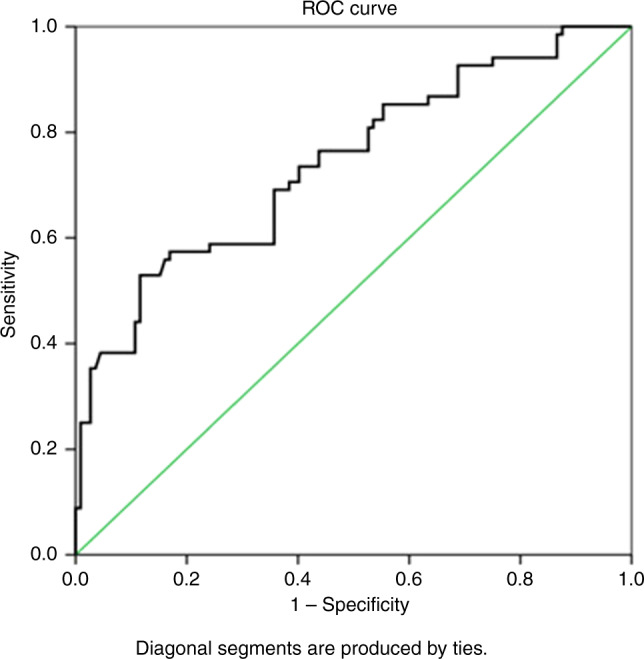
ROC curve analysis of SP-D level for the prediction of pneumonia severity.
Discussion
The protein component is important for surfactant function. Surfactant proteins A (SP-A) and D (SP-D) belong to the collectin protein family, which is directly related to the innate immunity of the lung and the elimination of pathogens.21 SP-D is mainly secreted into the alveoli; thus, an increase in serum concentrations may reflect protein translocation caused by the abnormal increase in alveolar–capillary membrane permeability, possibly due to the loss of its structural integrity.22,23
Surfactant proteins SP-A and SP-D stimulate many aspects of immune cell behavior by enhancing phagocytosis, stimulation of chemotaxis, and reactive oxidant generation, particularly in macrophages. In some types of lung disease or after exposures, the balance between these inhibitory and stimulatory effects may be disrupted and result in inflammatory injury.24 Thus, in the present study, SP-D appeared to be a good predictor of CAP severity in hospitalized children.
SP-D level was found to be significantly higher among children with severe pneumonia compared with those with non-severe pneumonia regarding the severity classification system used (WHO, PRESS, or RISC). Experimental studies have shown that specific pulmonary bio-indicators that are secreted from deteriorating lung tissue, such as SP-A, SP-D, Krebs von den lungren-6, and Clara cell protein (CC-16), are effective in determining the severity of lung deterioration.25 Similarly, previous studies of pediatric CAP detected significantly higher SP-D among patients with severe pneumonia.25–27 Studies of CAP in adults had similar results.28
In addition, elevated serum SP-D, which was associated with respiratory distress syndrome in neonates, has been reported by finding high SP-D capillary levels in infants in need of respiratory support, such as oxygen, continuous positive airway pressure or ventilation.29 Furthermore, plasma SP-D is elevated in children with acute respiratory distress syndrome (ARDS) (n = 18; P < 0.05).30 Dahmer et al. reported a significant increase in plasma SP-D among children with pneumonia more than those with asthma or bronchiolitis.4
In the present study, all patients in the PICU admitted group and 48.2% of patients in the simple pneumonia group fulfilled the SIRS criteria. The high prevalence of SIRS was explained by the fact that fever and tachypnea are common manifestations in pneumonia and are among the SIRS criteria. All patients with clinical indicators of pneumonia severity (such as shock, sepsis, and mechanical ventilation need) had SIRS.
However, almost all patients with SIRS did not show manifestations of disease severity. In fact, the utility of SIRS has been challenged and was eventually removed from the definition of sepsis in adults (Sepsis-3)31 since many patients with SIRS never develop adverse clinical outcomes, and many patients with organ dysfunction do not fulfill SIRS criteria. SP-D is an inflammatory mediator; its level was significantly higher in patients with SIRS, but patients with clinical indicators of pneumonia severity tended to have SP-D levels higher than the median levels of the SIRS subgroup.
Clinical indicators of pneumonia severity, such as need for mechanical ventilation, hypoxia, shock, and sepsis, presented in PICU admitted as 38.2, 70.6, 35.3, and 33.8%, respectively. Patients who experienced these clinical indicators were consequent to the development of CAP, which is the most common cause of sepsis in many studies.32,33 Approximately 40–50% of patients with sepsis present respiratory sources of infection.34 In addition, a large adult study suggested that severe sepsis is a common feature in CAP (48% of hospitalized patients), with 4.5% of patients developing septic shock.35
There are significant positive correlations between SP-D and RISC score, PRESS score, PIROM score, and duration of hospital stay. There are strong positive correlations with PRESS and moderate positive correlations with both RISC and PIROm scores. These findings support the use of SP-D in the assessment of pediatric CAP severity and are consistent with a prior study that demonstrated correlations between SP-D and pneumonia severity scores, such as the pneumonia severity index (PSI).27 Furthermore, the ROC curve analysis for SP-D as a predictor of pneumonia severity showed that the AUC was 0.741 (P value < 0.001, 95% CI 0.66–0.82), its validity at a cut-off point was 79.770, the sensitivity was 85.3%, the specificity was 44.6%, and the accuracy of the test was 60%. To our knowledge, no prior studies have evaluated SP-D in relation to the other pneumonia severity scores in pediatric patients. Further analysis of our data will be required to determine the relationship of SP-D to pneumonia severity scores.
SP-D was significantly elevated among patients with shock, hypoxia, or needing mechanical ventilation and in patients complicated with sepsis. In addition, SP-D showed a strong positive correlation with the length of hospital stay. A study by Ichiyasu et al.25 showed that serum SP-D levels were higher in severe cases requiring intensive care. Mosbah et al.26 determined that serum SP-D levels were higher in patients who required mechanical ventilation support and oxygen supplementation. These two studies indicate that the serum SP-D level increases in severe pulmonary infection. Another study demonstrated a significantly higher SP-D among children with mycoplasma pneumonia who had severe disease.36
Additionally, there was a significant correlation between serum SP-D level and hospitalization duration in patients with bacteremia.27 Dahmer et al.4 showed that elevated SP-D levels were associated with duration of mechanical ventilation (P = 0.012), length of PICU stay (P = 0.019), highest oxygenation index (P = 0.040), and death (OR 1.02; CI = 1.01–1.04; P = 0.004).
However, no significant correlation was detected between SP-D and CRP, and no significant difference in CRP level was detected between the patient group with severe pneumonia and the other patient group. Acıkgoz et al.27 noted that there were no significant correlations between serum SP-D level and CRP, total white blood cell, or neutrophil count (r = 0.064, P = 0.773; r = 0.221, P = 0.310; and r = 0.339, P = 0.114, respectively). A previous adult study showed no differences in CRP levels between severe and non-severe CAP patients, and no significant correlation was found between PSI and CRP concentration.37 Pertseva et al.38 reported no correlation between SP-D and CRP in patients with CAP before and after antibacterial therapy (R = 0.095, P = 0.589; R = 0.159, P = 0.363, respectively). This can be explained by the fact that the serum CRP level may take 6 h to rise, and the peak is reached at approximately 48 h,39 which leads to a lack of correlation of CRP with pneumonia severity and SP-D levels.
Regarding SP-D as a potential prognostic biomarker for pneumonia outcome, we showed that the highest SP-D level was found among the patients who died. Carlos et al.40 noted that higher circulating levels of SP-D are associated with higher mortality risk in patients with pneumonia due to the A/H1N1 virus.
Different studies have demonstrated that SP-A and SP-D are important biomarkers of interstitial pulmonary fibrosis, where high levels of these surfactant proteins are predictors of mortality.41 Spoorenberg et al.42 found that patients with high SP-D levels and PSI classes 4–5 were significantly associated with 30-day, 1-year, and long-term mortality.
SP-D was also used to differentiate groups of patients at highest risk for adverse clinical outcomes, as a change in SP-D level over the first 3 days among patients with acute lung injury/ARDS is associated with adverse clinical outcomes.43
Future studies are needed to investigate whether these agents are useful for the amelioration of pneumonia severity. There are some limitations of our study. First, the sample size was small; therefore, our main conclusions were about the relationship between SP-D and pneumonia severity classifications and other clinical parameters of severity rather than mortality itself. Second, we did not have any values for procalcitonin or lung ultrasound score. Third, the SP-D level was not measured serially over the disease course to determine exactly when it started to rise, when it reached a peak, and when it began to decline with effective treatment. In addition, the utility of SP-D for differentiating viral from bacterial infections was not evaluated by our study because that goal was not included in the aim of our study; rather, we aimed to use SD-P as an effective bio-indicator for determining the clinical severity of CAP in children.
Conclusion
SP-D seems to be a good predictor for the detection of CAP severity in hospitalized children. SP-D was correlated with PRESS score, RISC score, and PIROm score. It was also associated with indicators of CAP severity, including PICU admission, mechanical ventilation, shock, hypoxia, sepsis, and mortality. Future larger studies are needed to confirm these findings and to directly evaluate the association of SP-D with mortality risk in CAP.
Author contributions
N.Y.S.: participated in study design, data analysis, data interpretation, drafting, revision of the manuscript, and correspondence. R.A.L.I.: participated in data analysis and responsible for statistical part in the study and revision of manuscript. A.A.h.S. and S.E.s.S.: participated in study design and responsible for practical part in the study and revision of manuscript. A.A.S.M.: participated in study design, data analysis, data interpretation, drafting, and revision of the manuscript.
Competing interests
The authors declare no competing interests.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Bradley JS, et al. The management of community-acquired Pneumonia in infants and children older than 3 months of age: clinical practice guidelines by the Pediatric Infectious Diseases Society and the Infectious Diseases Society of America. Clin. Infect. Dis. 2011;53:617–630. doi: 10.1093/cid/cir625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Liu L, et al. Global, regional, and national causes of child mortality in 2000-13, with projections to inform post-2015 priorities: an updated systematic analysis. Lancet. 2015;385:430–440. doi: 10.1016/S0140-6736(14)61698-6. [DOI] [PubMed] [Google Scholar]
- 3.Esposito S, Principi N. Pneumococcal vaccines and the prevention of community-acquired pneumonia. Pulm. Pharmacol. Ther. 2014;1094:31–35. doi: 10.1016/j.pupt.2014.02.003. [DOI] [PubMed] [Google Scholar]
- 4.Dahmer, M. K. et al. Surfactant protein D is associated with severe pediatric acute respiratory distress syndrome, prolonged ventilation, and death in children with acute respiratory failure. Chest158, 1027–1035 (2020). [DOI] [PMC free article] [PubMed]
- 5.Kuroki Y, Takahashi M, Nishitani C. Pulmonary collectins in innate immunity of the lung. Cell. Microbiol. 2007;9:1871–1879. doi: 10.1111/j.1462-5822.2007.00953.x. [DOI] [PubMed] [Google Scholar]
- 6.Kotecha, S. et al. Functional heterogeneity of pulmonary surfactant protein-D in cystic fibrosis. Biochim. Biophys. Acta1832, 2391–2400 (2013). [DOI] [PubMed]
- 7.Sorensen GL, Husby S, Holmskov U. Surfactant protein A and surfactant protein D variation in pulmonary disease. Immunobiology. 2007;212:381–416. doi: 10.1016/j.imbio.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 8.Hartshorn K, et al. Mechanism of binding of surfactant surfactant protein D to influenza A viruses: importance of binding to hem-agglutinin to antiviral activity. Biochem. J. 2000;351:449–458. doi: 10.1042/bj3510449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hartshorn KL, et al. Distinctive anti-influenza properties of recombinant collectin 43. Biochem. J. 2002;366:87–96. doi: 10.1042/bj20011868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hartshorn KL, et al. Human mannose-binding protein functions as an opsonin for influenza A viruses. J. Clin. Investig. 1993;91:1414–1420. doi: 10.1172/JCI116345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guzel A, Kater M, Guzel A. tective effect of curcumin on acute lung injury induced by intestinal ischaemia/reperfusion. Health. 2013;29:969. doi: 10.1177/0748233711430984. [DOI] [PubMed] [Google Scholar]
- 12.Harris M, et al. British Thoracic Society guidelines for the management of community-acquired pneumonia in children: update 2011. Thorax. 2011;356:1–23. doi: 10.1136/thoraxjnl-2011-200598. [DOI] [PubMed] [Google Scholar]
- 13.World Health Organization. Revised WHO Classification and Treatment of pneumonia in Children at Health Facilities: evidence summaries. http://apps.Who.int/iris/bitstream/10665/137319/1/9789241507813_eng.pdf (2014). [PubMed]
- 14.Miyaji Y, et al. Pediatric respiratory severity score (PRESS) for respiratory tract infections in children. Austin Virol Retrovirol. 2015;2:1009. [Google Scholar]
- 15.Reed C, et al. Developments of the respiratory index of severity in children (RISC) score among young children with respiratory infections in South Africa. PLoS ONE. 2012;7:e27793. doi: 10.1371/journal.pone.0027793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Araya S, et al. Application of a prognostic scale to estimate the mortality of children hospitalized with community-acquired pneumonia. Pediatr. Infect. Dis. J. 2016;35:369–373. doi: 10.1097/INF.0000000000001018. [DOI] [PubMed] [Google Scholar]
- 17.Goldstein, B., Giroir, B., Randolph, A. & International Consensus Conference on Pediatric Sepsis. International pediatric sepsis consensus conference: definitions for sepsis and organ dysfunction in pediatrics. Pediatr. Crit. Care Med. 6, 2–8 (2005). [DOI] [PubMed]
- 18.World Health Organization. Hypoxemia and Hypoxia in “Oxygen Therapy for Children: A Manual for Health Workers” (WHO, 2016).
- 19.Monica EK, et al. 2010 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Part 14: Pediatric advanced life support. Circulation. 2010;122:S876–S908. doi: 10.1161/CIRCULATIONAHA.109.915165. [DOI] [PubMed] [Google Scholar]
- 20.Matalon S, et al. Modification of surfactant protein D by reactive oxygen-nitrogen intermediates is accompanied by loss of aggregating activity in vitro and in vivo. FASEB J. 2009;23:1415–1430. doi: 10.1096/fj.08-120568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sandra Orgeig, et al. Recent advances in alveolar biology: evolution and function of alveolar proteins. Respir. Physiol. Neurobiol. 2010;173(Aug):S43–S54. doi: 10.1016/j.resp.2010.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Winkler C, et al. Comprehensive characterisation of pulmonary and serum surfactant protein D in COPD. Respir. Res. 2011;12:29. doi: 10.1186/1465-9921-12-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang L, et al. Neither SP-A nor NH2-terminal domains of SP-A can substitute for SP-D in regulation of alveolar homeostasis. Am. J. Physiol. Lung Cell. Mol. Physiol. 2006;291:L181–L190. doi: 10.1152/ajplung.00015.2006. [DOI] [PubMed] [Google Scholar]
- 24.Phelps, D. S. Surfactant regulation of host defense function in the lung: a question of balance. Pediatr. Pathol. Mol. Med. 20, 269–292 (2001). [PubMed]
- 25.Ichiyasu H, et al. Pneumocyte biomarkers KL-6 and surfactant protein D reflect the distinct findings of high-resolution computed tomography in nonspecific interstitial pneumonia. Respiration. 2012;83:190–197. doi: 10.1159/000326924. [DOI] [PubMed] [Google Scholar]
- 26.Mosbah AA, Abdellatif NA, Sorour EI, Awadallah MF, Serum SP- D levels as a biomarker of lung injury in children suffering of bronchopneumonia. J. Egypt Soc. Parasitol. 2012;42:25–32. doi: 10.12816/0006291. [DOI] [PubMed] [Google Scholar]
- 27.Acıkgoz M, Guzel A, Şişman B. Can serum surfactant protein D levels be used as an effective factor instead of clinical severity scores of pneumonia in pediatric emergency departments? Eurasian J. Emerg. Med. 2016;15:1–6. doi: 10.5152/eajem.2016.24119. [DOI] [Google Scholar]
- 28.Leth-Larsen R, et al. Surfactant protein D (SP-D) serum levels in patients with community-acquired pneumonia. Clin. Immunol. 2003;108:29–37. doi: 10.1016/S1521-6616(03)00042-1. [DOI] [PubMed] [Google Scholar]
- 29.Dahl M, Holmskov U, Husby S, Juvonen PO. Surfactant protein D levels in umbilical cord blood and capillary blood of premature infants: the influence of perinatal factors. Pediatr. Res. 2006;59:806–810. doi: 10.1203/01.pdr.0000219122.81734.03. [DOI] [PubMed] [Google Scholar]
- 30.Todd DA, et al. Surfactant phospholipids, surfactant proteins, and inflammatory markers during acute lung injury in children. Pediatr. Crit. Care Med. 2010;11:82–91. doi: 10.1097/PCC.0b013e3181ae5a4c. [DOI] [PubMed] [Google Scholar]
- 31.Singer M, et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3) JAMA. 2016;315:801–810. doi: 10.1001/jama.2016.0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Angus DC, et al. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit. Care Med. 2001;29:1303–1310. doi: 10.1097/00003246-200107000-00002. [DOI] [PubMed] [Google Scholar]
- 33.Alberti C, et al. Systemic inflammatory response and progression to severe sepsis in critically ill infected patients. Am. J. Respir. Crit. Care Med. 2005;171:461–468. doi: 10.1164/rccm.200403-324OC. [DOI] [PubMed] [Google Scholar]
- 34.Ceccato, A. & Torres, A. Sepsis and community-acquired pneumonia. Ann. Res. Hosp. 2, 7 (2018).
- 35.Dremsizov T, et al. Severe sepsis in community-acquired pneumonia: when does it happen, and do systemic inflammatory response syndrome criteria help predict course? Chest. 2006;129:968–978. doi: 10.1378/chest.129.4.968. [DOI] [PubMed] [Google Scholar]
- 36.Shu LH, et al. Changes to surfactant proteins in the bronchoalveolar lavage fluid and serum of children with Mycoplasma pneumoniae pneumonia. Zhongguo Dang Dai Er Ke Za Zhi. 2012;14:928–932. [PubMed] [Google Scholar]
- 37.Paats MS, et al. Local and systemic cytokine profiles in non-severe and severe community-acquired pneumonia. Eur. Respir. J. 2013;41:1378–1385. doi: 10.1183/09031936.00060112. [DOI] [PubMed] [Google Scholar]
- 38.Pertseva T, Kireyeva T, Shtepa O. Surfactant protein D (SPD) and C-reactive protein (CRP) levels in patients with community acquired pneumonia (CAP) during the treatment program. Eur. Respir. J. 2014;44:P3645. [Google Scholar]
- 39.Bottazzi B, et al. The long pentraxin PTX3 as a prototypic humoral pattern recognition receptor: interplay with cellular innate immunity. Immunol. Rev. 2009;227:9–18. doi: 10.1111/j.1600-065X.2008.00719.x. [DOI] [PubMed] [Google Scholar]
- 40.Carlos D, et al. Serum surfactant protein D (SP-D) is a prognostic marker of poor outcome in patients with A/H1N1 virus infection. Lung. 2015;193:25–30. doi: 10.1007/s00408-014-9669-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Barlo NP, et al. Surfactant protein-D predicts survival in patients with idiopathic pulmonary fibrosis. Sarcoidosis Vasc. Diffuse Lung Dis. 2009;26:155–161. [PubMed] [Google Scholar]
- 42.Spoorenberg SM, et al. YKL-40, CCL18 and SP-D predict mortality in patients hospitalized with community-acquired pneumonia. Respirology. 2017;22:542–550. doi: 10.1111/resp.12924. [DOI] [PubMed] [Google Scholar]
- 43.Lorraine B. Prognostic determinants of acute respiratory distress syndrome in adults: impact on clinical trial design. Crit. Care Med. 2005;33:S217–S222. doi: 10.1097/01.CCM.0000155788.39101.7E. [DOI] [PubMed] [Google Scholar]