Dear Editor,
Patients with severe Corona Virus Disease 2019 (COVID-19) may show profound pulmonary inflammation and require mechanical ventilation and Intensive Care Unit (ICU) admission. Despite adequate thromboprophylaxis the majority of patients are in a prothrombotic state. Reported incidence differs substantially between studies with a mean incidence of venous thromboembolism (VTE) of 40.3% when patients are screened for thrombotic disease and 9.5% without screening [1].
The pathogenesis of COVID-19-associated activation of coagulation is not fully understood and seems to differ from disseminated intravascular coagulation (DIC). Where DIC is initiated by tissue factor leading to consumption of platelets and coagulation factors with thrombocytopenia and prolonged PT and aPTT, COVID-19 coagulopathy shows, apart from high D-dimer levels, mostly normal platelets and coagulation tests suggesting a different mechanism of activation of coagulation [2,3]. Differences between these two groups have also been observed in Computed Tomography (CT) findings. In COVID-19 patients pulmonary embolism (PE) is frequently located in peripheral lung segments and less extensive compared to PE in non-COVID-19 patients, suggesting that COVID-19 associated PE has a different phenotype than ‘conventional’ PE [4].
PE is treated with anticoagulants, which in the ICU is often unfractionated heparin (UFH). As the pathophysiology of coagulation in COVID-patients is unknown, it is uncertain whether UFH -or anticoagulation in general- is effective in the attenuation of the procoagulant state. Since insufficient treatment of PE can be fatal, we aimed to study the effect of UFH on clinical, radiological and laboratory signs of PE in COVID-19 patients.
This prospective cohort study using routinely collected data was conducted in the ICU of the Leiden University Medical Center (LUMC) in the Netherlands and approved by the Institutional Review Board of the LUMC for COVID-19-studies. Adult patients on mechanical ventilation receiving treatment with UFH for proven PE documented by CT-scanning during proven COVID-19 disease by PCR sampling of nasal/oral airway swab were eligible to participate. Patients who received therapeutic doses of heparin within 48 h prior to the diagnosis of PE, patients who received treatment with reperfusion techniques including fibrinolytic drugs or patients with missing data on D-dimer levels prior to the start of UFH therapy were excluded.
A CT-scan was performed in case of suspected PE. Repeated CT-scanning was indicated if no pulmonary improvement was present after a minimum of 7 days following initiation of anticoagulant treatment. All scans were evaluated by a radiologist with >20 years of experience in chest-CT. The thrombus load within the pulmonary arteries was determined by using the Qanadli obstruction index and calculated as percentage vascular obstruction [5].
Data was collected for 21 days or until ICU discharge. From the medical records we extracted: age, sex, year of birth, body mass index (BMI), date of ICU admission, date of ICU discharge, reason for discharge, condition 28 days after admission, starting time of UFH therapy and available D-dimer levels which were measured as a part of routine care every day. Outcomes were defined as: D-dimer levels at day 0 versus day 2 (laboratory outcome), resolution of PE at follow-up CT-scan or discharged alive from ICU (clinical outcome) and Qanadli index at follow-up CT-scan versus CT-scan at diagnosis PE (radiologic outcome).
Normally distributed data are presented as means with standard deviation (SD); data outside normal distribution are presented as medians with interquartile range (IQR). Groups were compared using a t-test and Wilcoxon signed-rank test, as appropriate. A two-sided p < 0.05 was considered statistically significant.
In total, 90 confirmed COVID-19 patients were admitted to the ICU between 15th March and 1st May 2020. Twenty-six patients had CT-proven PE of which 7 patients were excluded due to the use of Low Molecular Weight Heparin (LMWH) instead of UFH (n = 2), therapeutic LMWH prior to diagnosis of PE (n = 4) or thrombolytic treatment (n = 1). The remaining 19 patients were eligible for analysis. Baseline and laboratory characteristics are listed in Table 1 .
Table 1.
Baseline characteristics, COVID-19 treatment and course of D-dimer levels.
| Mean age, year (SD) | 63 (6.6) |
| Male, n (%) | 16 (84) |
| Mean body mass index, kg/m2 (SD) | 27.5 (2.8) |
| Thrombosis prophylaxis when admitted at ICU | |
| - Prophylactic (2850 IU/day or 5700 IU/day if >90 kg), n (%) | 17 (89.5) |
| - Double prophylactic (5700 IU/day), n (%) | 2 (10.5) |
| COVID-19 treatment | |
| - Chloroquine, n (%) | 14 (73,7) |
| - Hydrochloroquine, n (%) | 4 (21) |
| - Corticosteroids, n (%) | 2 (10,5) |
| - Remdesivir, n (%) | 2 (10,5) |
| D-dimer levels | |
| D-dimer levels day −2 median + IQR | 5379 ng/mL (2460–10,604) |
| D-dimer levels day −1 median + IQR | 5555 ng/mL (4317–9769) |
| D-dimer levels day 0 median + IQR | 6197 ng/mL (4682–9360) |
| D-dimer levels day 1 median + IQR | 4766 ng/mL (3047–7773) |
| D-dimer levels day 2 median + IQR | 3665 ng/mL (2470–5437)⁎ |
p < 0.001 for difference between day 0 and day 2.
The mean Qanadli index of the CT-scans before start of UFH was 17.5% (SD 10.8%), with the segmental artery being the most frequent location of the thrombi (n = 16, 84.2%). Follow-up CT-scans were performed in 6 patients with a mean follow-up of 18 days. The Qanadli index decreased significantly from baseline to follow-up (p = 0.03 for difference with baseline CT-scan). The Qanadli index decreased to 0% in 5 patients and to 5% in the remaining patient.
Successful treatment defined as either no PE on follow-up CT or alive at ICU discharge was observed in 14 (74%) patients: in 6 (32%) patients there was no PE on follow-up CT, and 8 (42%) patients were discharged alive from the ICU. Amongst patients without successful treatment, one patient died in the ICU without follow-up CT-scan, and 4 (21%) patients were transferred to another ICU without follow-up. At 28 days after admission, 9 (47,4%) patients were still in the ICU (including 3 of the 4 patients that were eventually transferred to another ICU), 7 (36.8%) patients were discharged from the ICU to either the nursing ward (n = 5, 26.3%), a rehabilitation center (n = 1, 5.3%) or home (n = 1, 5.3%). Two (10.5%) patients had died (of which one had clinical improvement on the follow-up CT) in the ICU and one (5.3%) was transferred to another ICU. 6 patients (32%) suffered from bleeding complications, of which 2 (10.5%) were classified as major bleeding according to the International Society on Thrombosis and Haemostasis (ISTH) definition of bleeding in non-surgical patients. These severe bleedings were located in the lung (n = 1) and the lower gastrointestinal tract (n = 1). None of these bleedings resulted in death.
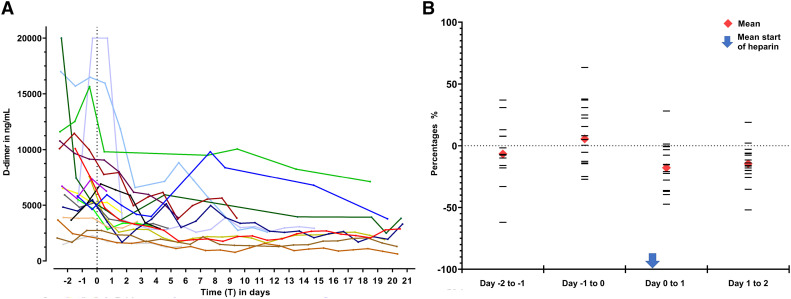
The course of D-dimer levels of all patients is shown in Table 1 and Fig. 1a. The percentage change of D-dimer levels per 24-hour period from 2 days before UFH to 2 days after start of UFH is shown in Fig. 1b. From day −2 to day −1, D-dimer levels decreased on average 6.7% (SD 26.9) after which, from day −1 to day 0, it increased 5.6% (SD 25.6). The first day after start of UFH, a mean drop of 17.9% (SD 19.4) and the second day a drop of 14.6% (SD 15.9) was seen.
Fig. 1.
a. Course of D-dimer levels before and after start of heparin. D-dimer levels from 2 days before the start of UFH until 21 days after start of UFH or until ICU discharge are shown. T = 0 represents the start of heparin for each individual patient, which is marked by the dotted line.
b. Percentage change of D-dimer levels for different time frames before and after start of heparin. All samples were taken at 6:00 A.M. For every patient T = 0 represents the time of blood sampling at 6:00 AM of the day that heparin was started. The horizontal lines represent the percentage change in that time frame for each individual patient. The arrow represents the mean actual time of start of heparin, on average 9 h (SD4.9) after blood sampling.
This is the first study to show that clinical and radiological signs of PE and plasma D-dimer levels decreased after administration of UFH in patients with COVID-19 and PE. Earlier, Tang and others studied the effect of LMWH in 449 COVID-19 patients [6]. They found an association between increasing D-dimer levels and higher mortality in non-LMWH treated patients. Also, a reduced mortality was seen in patients with coagulopathy who were treated with LMWH compared with patients with coagulopathy who were not treated with LMWH (40% vs 64.2%, p = 0.029). However, the effect of UFH on D-dimer levels or PE resolution was not reported.
Despite the fact that our results suggest that therapeutic UFH is an effective treatment of COVID-19 associated PE, thrombo-embolic complications are common despite prophylactic LMWH [1]. This may be explained by the route of administration or the dose. Subcutaneous LMWH prophylaxis in the ICU might lead to lower anti-Xa activity by concurrent use of vasoconstrictors [7]. Furthermore, prophylactic doses of LMWH are lower than therapeutic doses and result in lower anti-Xa activity, which may be insufficient to prevent PE. It is currently unknown whether increasing the dose of LMWH can be beneficial in preventing thrombotic complications in COVID-19 patients. Pesavento et al. reported an increase in relevant bleeding events when increasing the dose of anticoagulants to (sub)therapeutic [8].
There have been concerns about a high incidence of Chronic Thrombo Embolic Pulmonary Hypertension (CTEPH) after COVID-19 associated PE, in particular because inflammatory states have been associated with poor thrombus resolution [9,10]. Although our sample size is small, the rapid clot resolution observed in our study suggests that the incidence of CTEPH in COVID-19 associated PE survivors may not be notably increased. Even so, physicians should remain vigilant on the presence of CTEPH in patients treated for COVID-19 associated PE who have not been recovered after a 3-month follow-up period.
Our study has several limitations. The change in D-dimer levels may have been influenced by other factors than administration of UFH. In this uncontrolled observational study we cannot exclude confounding by factors modifying the severity of illness. Therefore, the decline in D-dimers that we found in the two days after start of UFH could also be caused by clinical improvement in general. Another limitation is the limited sample size of 19 patients with only 6 patients having had a follow-up CT-scan. Lastly, we could not compare with COVID-patients treated with LMWH nor with non-COVID-patients with PE treated with UFH because daily D-dimer levels were not available in these cohorts. Strengths of this study include the strict protocol in our ICU dictating repeated CT-scanning if no improvement of pulmonary status was present after one week of UFH treatment, and the meticulous comparison of index and follow-up CTPA scan images.
In conclusion, we show a considerable clinical and radiological improvement in COVID-19 patients and proven PE after starting UFH therapy. Standard anticoagulant treatment seems to be effective in this setting, supporting current guideline recommendations.
Ethical approval
This study was approved by the Institutional Review Board of the LUMC for COVID-19 studies. The need for consent was waived by the Institutional Review Board for COVID-19 studies.
CRediT authorship contribution statement
LIW collected the data and wrote the manuscript. EJ initiated and supervised this study. All authors contributed to the writing of the manuscript and approved the final version.
Declaration of competing interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: MV Huisman received grants from ZONMW, Bayer Health Care, Pfizer-BMS, Boehringer-Ingelheim, Leo Pharma, all outside the submitted work. J Eikenboom received research support from CSL Behring and he has been teacher on educational activities of Roche, all outside the submitted work. FA Klok reports research support from Bayer Health Care, Bristol-Myers Squibb, Boehringer-Ingelheim, MSD, Daiichi-Sankyo, Actelion, the Dutch thrombosis association, The Netherlands Organisation for Health Research and Development and the Dutch Heart foundation, all outside the submitted work. The authors declare that they have no competing interests.
Acknowledgements
JE, MVH, FAK and EJ are investigators of the Dutch COVID & Thrombosis Coalition. The Dutch COVID & Thrombosis Coalition received grant funding from The Netherlands Organisation for Health Research and Development (ZonMw) and the Dutch Thrombosis Association.
Contributor Information
Dutch COVID & Thrombosis Coalition (DCTC):
L.I. van der Wal, L.J.M. Kroft, L.F. van Dam, C.M. Cobbaert, J. Eikenboom, M.V. Huisman, H.J.F. Helmerhorst, F.A. Klok, and E. de Jonge
Appendix A. Authors
The author's full names are as follows: L.I. van der Wal1, L.J.M. Kroft2, L.F. van Dam3, C.M. Cobbaert4, J. Eikenboom3, M.V. Huisman3, H.J.F. Helmerhorst1,5, F.A. Klok3, E. de Jonge1.
The author's affiliations are as follows: 1 Department of Intensive Care Medicine, Leiden University Medical Center, Leiden, The Netherlands; 2 Department of Radiology, Leiden University Medical Center, Leiden, The Netherlands; 3 Department of Thrombosis and Hemostasis, Leiden University Medical Center, Leiden, The Netherlands; 4 Department of Clinical Chemistry and Laboratory Medicine, Leiden University Medical Center, Leiden, The Netherlands; 5 Department of Anesthesiology, Leiden University Medical Center, Leiden, The Netherlands.
References
- 1.Nopp S., Moik F., Jilma B., Pabinger I., Ay C. Risk of venous thromboembolism in patients with COVID-19: a systematic review and meta-analysis. Research and Practice in Thrombosis and Haemostasis. 2020;4(7):1178–1191. doi: 10.1002/rth2.12439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Levi M., De Jonge E., Van Der Poll T. New treatment strategies for disseminated intravascular coagulation based on current understanding of the pathophysiology. Ann. Med. 2004;36(1):41–49. doi: 10.1080/07853890310017251. [DOI] [PubMed] [Google Scholar]
- 3.Connors J.M., Levy J.H. COVID-19 and its implications for thrombosis and anticoagulation. Blood. 2020;135(23):2033–2040. doi: 10.1182/blood.2020006000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Van Dam L.F., Kroft L.J.M., Van Der Wal L.I., Cannegieter S.C., Eikenboom J., De Jonge E., et al. Clinical and computed tomography characteristics of COVID-19 associated acute pulmonary embolism: a different phenotype of thrombotic disease? Thromb. Res. 2020;193:86–89. doi: 10.1016/j.thromres.2020.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Qanadli S.D., El Hajjam M., Vieillard-Baron A., Joseph T., Mesurolle B., Oliva V.L., et al. New CT index to quantify arterial obstruction in pulmonary embolism: comparison with angiographic index and echocardiography. AJR Am. J. Roentgenol. 2001;176(6):1415–1420. doi: 10.2214/ajr.176.6.1761415. [DOI] [PubMed] [Google Scholar]
- 6.Tang N., Bai H., Chen X., Gong J., Li D., Sun Z. Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy. J. Thromb. Haemost. 2020;18(5):1094–1099. doi: 10.1111/jth.14817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dorffler-Melly J., de Jonge E., Pont A.C., Meijers J., Vroom M.B., Buller H.R., et al. Bioavailability of subcutaneous low-molecular-weight heparin to patients on vasopressors. Lancet. 2002;359(9309):849–850. doi: 10.1016/s0140-6736(02)07920-5. [DOI] [PubMed] [Google Scholar]
- 8.Pesavento R., Ceccato D., Pasquetto G., Monticelli J., Leone L., Frigo A., et al. The hazard of (sub)therapeutic doses of anticoagulants in non-critically ill patients with Covid-19: the Padua province experience. J. Thromb. Haemost. 2020;18(10):2629–2635. doi: 10.1111/jth.15022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huisman M.V., Barco S., Cannegieter S.C., Le Gal G., Konstantinides S.V., Reitsma P.H., et al. Pulmonary embolism. Nature Reviews Disease Primers. 2018;4:18028. doi: 10.1038/nrdp.2018.28. [DOI] [PubMed] [Google Scholar]
- 10.Ende-Verhaar Y.M., Cannegieter S.C., Vonk Noordegraaf A., Delcroix M., Pruszczyk P., Mairuhu A.T., et al. Incidence of chronic thromboembolic pulmonary hypertension after acute pulmonary embolism: a contemporary view of the published literature. Eur. Respir. J. 2017;49(2) doi: 10.1183/13993003.01792-2016. [DOI] [PubMed] [Google Scholar]