Abstract
Sperm can be selected as a natural vector for the production of transgenic animals. Methyl-beta-cyclodextrin (MBCD) removes cholesterol from the phospholipid membrane of sperm and improves the efficiency of DNA uptake by sperm. In experiment 1, fresh sperm was treated with various concentrations of MBCD. The direct effects of MBCD on sperm parameters were monitored. In experiment 2, different concentrations of MBCD (0, 1, 2, and 4 mmol) were assessed for the transfection of genetically exogenous construction to rooster sperm. Washed semen was divided into 5 equal groups for the incubation and transfection with a pcDNA3.1+/hG-CSF vector (exogenous DNA) as follows; Treatment I—Control (washed semen without DNA); Treatment II—Control (washed semen with DNA); Treatment III—(washed semen incubated with DNA and 1 mmol MBCD); Treatment IV—(washed semen incubated with DNA and 2 mmol MBCD); and Treatment V—(washed semen incubated with DNA and 4 mmol MBCD). We demonstrated that rooster spermatozoa spontaneously can uptake exogenous DNA; this was assessed using exogenous DNA amplification (sperm genomic DNA used as a template for PCR reaction) after DNase I treatment. In addition, total motility (TM), progressive motility (PM), velocity parameters [curvilinear velocity (VCL), straight linear velocity (VSL), sperm track straightness (STR), linearity (LIN)], membrane integrity (MI), and membrane functionality were posttransfectionally evaluated. The concentrations of 1 and 2 mmol MBCD significantly (P < 0.05) improved the motion characteristics and membrane integrity of fresh sperm. The presence of hG-CSF in rooster sperm was detected by PCR and based on sperm analyses MBCD (1 mmol) improved the percentage of motility (98.9 ± 0.81), membrane functionality (64 ± 1.64), and MI (76.2 ± 1.65) after transfection when compared with the other groups (P < 0.05). For the production of transgenic chicken, hens were inseminated (AI) by transfected sperm treated with 1 and 0 mmol MBCD. A PCR analysis of the blood samples and dead embryo tissues of chicks did not reveal the transgene integration.
Key words: chicken, exogenous DNA, sperm, transfection
Introduction
The development of techniques that introduce DNA into animal cells is a model system for the basic and applied research leading to future clinical and industrial applications (Anzar and Buhr, 2006). Transgenic animals are valuable tools as bioreactors to produce therapeutic proteins developed in different animal systems such as cows, goats, and chickens (Montgomery, 2004; Houdebine, 2009). Recently, research has been focused on the development of transgenic chickens due to a number of biological and physiological advantages of hens such as shorter timescale for setup, potential for large-scale protein production, and lower costs associated with husbandry (Lillico et al., 2005). Moreover, high accurate glycosylation in the hen's egg encourages researchers to focus on the production of genetically modified chickens (Samoylov et al., 2013). Among transgenic methods, sperm-mediated gene transfer (SMGT) is based on autonomous capability of sperm cells to bind exogenous DNA molecules. This method uses the sperm as a natural vector to transfer transgenes into oocytes during the fertilization (Lavitrano et al., 1989; Pramod et al., 2016). This technique is postulated to be an efficient, simple, and cost-effective method for producing transgenic animals. However, the viability and motility of sperm after SMGT are not satisfactory (Smith and Spadafora, 2005; García-Vázquez et al., 2011; Zaniboni et al., 2013). Alternatively, the efficiency of this procedure depends on the sperm quality such as progressive motility, thereby reducing the number of fertilized oocytes occurring after SMGT. Therefore, the use of a protective agent for the transfection to facilitate the DNA absorption by sperm as well as preserving the quality of sperm seems to be an effective strategy (Harel-Markowitz et al., 2009; Yu et al., 2010). Methyl-beta-cyclodextrin (MBCD) is a water-soluble cyclic oligosaccharide with beneficial effects on sperm fertilization, embryo development, (Mao et al., 2005), and mouse sperm capacitation (Choi and Toyoda, 1998). MBCD is able to remove cholesterol from sperm plasma membrane (Mao et al., 2005) to increase DNA absorbance by sperm (Oddi et al., 2012).
Currently, there appears to be no published report concerning the applicability of MBCD for the SMGT in roosters. Therefore, the objective of this study was to use different concentrations of MBCD during the transfection of exogenous DNA into rooster sperm cells during the SMGT. It was purposed to use PCR and several sperm evaluation methods to determine the efficacy of MBCD on DNA absorption and quality of sperm after the transfection. At the end of study, artificial insemination (AI) was performed with posttransfected sperm for the production of transgenic chicks.
Materials and methods
Preparation of Gene Construct
In this study, a pcDNA3.1+ vector was used under the control of the CMV promoter as an exogenous DNA. The DNA fragment encoded by the hG-CSF gene was synthesized on the PBHA vector by Bioneer Korean Company. Afterward the gene was isolated from the PBHA vector by double digestion with XhoI and HindIII. It was then directly subcloned into the same restricted sites on the pcDNA3.1 plasmid to form the recombinant expressing vector pcDNA3.1-G/CSF. The length of recombinant expression vector was 6,061 bp. To confirm the construction, colony PCR reactions and DNA sequencing were performed. The recombinant plasmid was amplified in Escherichia coli DH5α cells. Plasmid extraction was performed using Qiagen High Pure Plasmid Isolation Kit and then was analyzed on a 1% agarose gel.
For linearization of plasmid, a restriction enzyme (pvuI, Invitrogen, Burlington, Canada) was used. The digestion product was confirmed by 1% agarose gel electrophoresis and purification was done with the High Pure PCR Product Purification Kit (Roche, Germany) according to the manufacturer's instructions.
Animal Groups and Semen Collection
All bird handling procedures were approved by the Tarbiat Modares University Animal Care and Use Committee. Six 28-week-old fertile White Leghorn layer breeder roosters were kept in individual cages with a photoperiod of 16 L:8 D. Semen samples were collected twice per week from roosters during a 5-week period (10 replicates) based on the method of Burrows and Quinn (1937). Five replicates were used in experiment 1 for the evaluation of the effects of MBCD on the fresh sperm parameters. Five replicates were used in experiment 2 for the transfection of exogenous DNA into the rooster sperm.
Semen Processing
Semen samples were placed in a thermal flask that contained water at a temperature between 38°C and 40°C and then transferred to the laboratory within 5 min after the collection for primary evaluations. Total motility and concentration were analyzed by computer-assisted sperm analyzer (CASA) and hemocytometer, respectively (Shahverdi et al., 2015). Ejaculates that met the following criteria were included in this study: volume of >0.2 mL, concentration of >3 × 109 sperm/mL, and total motility of >80%. To eliminate the individual differences, semen samples were pooled by day and then divided into equal parts according to the experimental designs of each experiment.
Experiment 1: Preliminary Study
A preliminary study was conducted on fresh semen to determine the direct effects of MBCD on the fresh rooster sperm. To this purpose, semen samples were divided into 4 aliquots to be incubated with various concentrations of MBCD (0, 1, 2, and 4 mmol). The suspension of sperm and MBCD were incubated for 1 h at 37°C and 5% CO2. Motion characteristics, membrane integrity (MI), and membrane functionality of postincubated sperm were assessed using a CASA, eosin-nigrosin staining, and hypoosmotic swelling test, respectively.
Experiment 2: Transfection of Exogenous DNA Into the Rooster Sperm
The purpose in experiment 2 was to assess the effects of the different concentrations of MBCD on the sperm-mediated gene transfer. For the transfection, pooled semen was immediately diluted by prewarmed (37 °C, 1:7) Lake Buffer (0.4 g/L D-fructose, 0.15 g/L polyvinylpyrrolidone, 0.96 g/L sodium glutamate, 0.25 g/L potassium citrate, 0/035 g/L magnesium acetate, and 0.187 g/L glycine; pH 7.1; 340 mOsm/kg). It was then subsequently washed twice by centrifuging (600 g for 15 min at 37°C) to remove the materials with DNase activity (Feyzi et al., 2018). The washed semen was divided into 5 equal groups for incubation and transfection with the pcDNA3.1+/hG-CSF vector (exogenous DNA) as follows: Treatment I—Control (washed semen without DNA); Treatment II—Control (washed semen with DNA); Treatment III—(washed semen incubated with DNA and 1 mmol MBCD); Treatment IV—(washed semen incubated with DNA and 2 mmol MBCD); and Treatment V—(washed semen incubated with DNA and 4 mmol MBCD). For the transfection, a suspension of approximately 109 sperm in 500 μL of Lake Buffer along with 1 mL of vector pcDNA3.1+/hG-CSF (10 μg) was used for 30 min at 37°C. The sperm incubated in the control groups (with and without DNA) were analyzed 30 min after the incubation (the same as other groups). After the transfection, the sperm samples were centrifuged (600 g at 37°C) and washed twice in Lake Buffer to remove any DNA not adhered to the sperm membrane. In order to separate sperm cells from nonincorporated exogenous DNA, reactions were incubated with 0.1 mg DNase I for 30 min, followed by washing twice with the Lake Buffer (Lanes et al., 2009).
Sperm DNA Extraction and Evaluation of DNA Uptake by PCR
A phenol: chloroform procedure was used to extract the genomic DNA from the whole sperm with a slight modification (Rola et al., 2003). To extract the genomic DNA from the transfected sperm cells, 2 mL of extraction solution (2% beta-mercaptoethanol, 10 mmol Tris (pH 8.0), 100 mmol NaCl, 0.5% SDS, 10 mmol EDTA) and 20 μL proteinase K (20 mg/mL) were added to a 500 μlLsperm suspension containing 109 sperm cells. The mixture was vortexed to ensure an adequate digestion and then incubated at 40°C for 24 h to complete protein digestion. Then, 500 μL of phenol:chloroform was added to the solution, which was vortexed and centrifuged at 13,500 g for 15 min. The supernatant was slowly transferred into a sterile test tube and isopropanol was added to the supernatant (ratio of 2:1), which was mixed thoroughly and centrifuged at 12,000 g (4°C) for 10 min. The precipitated DNA was rinsed with 70% ethanol and then dried and dissolved in 30 to 50 μL of double-distilled water. The extracted DNA was quantified with a NanoDrop spectrophotometer (Nanodrop 2000, Thermo Scientific), diluted to a final concentration of 100 ng/μL in H2O, and preserved at −20°C until use.
Suitable primers (forward: 5′-TAACTCGAGAAAAGAGAGGCTGA-3′ and reverse: 5′-CGCGAATTCTTACTCCTTCAT-3′) for the hG-CSF gene were manually designed using gene runner software to amplify a 633 bp fragment. One microliter of genomic DNA was used as the template in a 25 μL reaction solution (1 μL of MgCl2 (50 mmol), 2.5 μL of 10X Buffer, 0.8 μL of dNTP (10 mmol), 0.7 μL of hG-CSF primers (For and REV:10 pmol), 0.7 μL of Taq DNA Polymerase (5 U/μL), and 17.6 μL of dH2O) for the PCR reactions. Amplification was performed with the following cycling conditions: initial denaturation at 94°C for 2 min and 35 cycles at 94°C for 45 s; annealing at 64°C for 45 s and 72°C for 45 s; and a final extension at 72°C for 4 min. The PCR products were loaded on 1% agarose gel and stained with ethidium bromide.
Assessment of Sperm Quality Parameters
Motion characteristics of sperm were determined using a CASA (Version 5.1; Microptic, Barcelona, Spain). For this purpose, 5 μL of sperm suspension was loaded onto a prewarmed 20 μm chamber (Leja 4, Leja Products Luzernestraat B.V., Holland). A minimum of five fields per sample were evaluated (a minimum of 300 spermatozoa were counted for each sample). The following parameters were analyzed: motility (%), progressive motility (%),VCL: curvilinear velocity (μm/sec), VSL straight line velocity (μm/sec), VAP: average path velocity (μm/sec), ALH: amplitude of lateral head displacement (μm), and BCF: beat cross frequency (Hz) (Moghbeli et al., 2016).
Membrane functionality was evaluated by the hypoosmotic swelling (HOS) test (Ansari et al., 2017). Twenty microliters of diluted semen (20 × 106 sperm/mL) was mixed with 200 μL of 100 mOsm/kg hypoosmotic solution (9 g fructose plus 4.9 g sodium citrate/L per liter of distilled water) and then incubated at room temperature for 30 min. Afterward, 10 μL of the incubated samples was analyzed by an inverted light microscope. At least 200 spermatozoa with coiled tails and noncoiled tails were considered with membrane functionality and membrane dysfunctionality, respectively.
MI of the transfected sperm was assessed with eosin/nigrosin staining according to the method of Fattah et al. (2017). The eosin-nigrosin solution was prepared by dissolving 1.6 g eosin and 3.0 g nigrosin in 100 mL Beltsville extender. Then, 10 μL of semen was placed on a prewarmed slide and mixed with 10 μL of the eosin nigrosin to prepare a smear. This mixture was allowed to air-dry and then visualized under an oil immersion 400x objective by light microscopy (CKX41; Olympus, Tokyo, Japan). Then, the numbers of unstained sperms were counted as spermatozoa with membrane integrity.
Artificial Insemination of the Hen With Posttransfected Sperm
In order to produce the transgenic chicks, AI was performed according to the method of Lotfi et al. (2017) with a slight modification (Lotfi et al., 2017). The AI was conducted on the hens inseminated with the transfected sperm in the group that contained 1 mmol MBCD since the highest quality of sperm was obtained in this group. Also, a group of hens were inseminated with the transfected sperm without MBCD (a control group). A total of 28 fertile laying leghorn hens (14 hens/group) were housed in individual cages. AI was performed 3 times per week during 1 month. The volume of sperm for each insemination was a 200 μL suspension containing 1 × 109 sperm. Time of AI was between 4 and 5 pm. Afterward, eggs were set in a common incubator (Victoria, G. Galilei, 3-22,070, Guanzate, Como, Italy) for 18 d at 37.7°C. After the 18th day of incubation, the eggs were transferred into the hatcher for the remaining 3 d of incubation. On the 10th day of incubation, the unfertilized eggs were detected using candling.
Identification of DNA on Offspring
The genomic DNA was extracted from the whole blood of embryos in accordance with Bailes et al. (2007). In order to extract the genomic DNA from the muscle tissues of dead chicks, small pieces of the muscle tissue (1–2 mm) were separated by a sterile scalpel blade and then slowly grounded in a special mortar while the nitrogen was slowly pouring on the pieces. (Fan and Gulley, 2001). The rest of the process was carried out according to the section 2.6. The PCR reaction was performed using various amounts of genomic DNA (50 and 100 ng in 25 μL), which was extracted from the blood and tissue samples. PCR amplification was performed using specific primers for the hG-CSF gene. The pcDNA3.1+/hG-CSF plasmid was considered as positive control, and DNA from a nontransgenic chick was considered as negative control.
Statistical Analysis
Five replicates of semen were used for the evaluation of direct effects of MBCD on the fresh sperm parameters. For the evaluations of the effects of MBCD on the SMGT, 5 replicates were used. All data were checked for normal distribution by Shapiro–Wilk test and analyzed using Proc GLM of SAS 9.1 (SAS Institute, version 9.1, 2002, Cary, NC, USA). Statistical differences among various group means were determined by Tukey's test and P ≤ 0.05 was considered statistically significant. Results are presented as the mean ± SEM.
Results
Direct Effects of MBCD on Fresh Sperm Parameters
In Table 1 the results of the preliminary study describing the percentages of motion characteristics, membrane integrity, and membrane functionality of rooster sperm after the incubation with the different concentrations of MBCD are presented. Total motility, progressive motility, and membrane functionality were significantly higher (Table 1, P < 0.05) in groups treated with 1 and 2 mmol MBCD as compared with other groups. Among the velocity parameters, 1 and 2 mmol MBCD had the highest significant percentages for VSL in comparison to other groups. Membrane functionality, VCL, STR, and LIN were not significantly affected by the MBCD incubation (Table 1, P ≥ 0.05).
Table 1.
Motility, velocity parameters, membrane integrity, and membrane functionality of rooster sperm treated with 0, 1, 2, and 4 mmo concentrations of methyl-beta-cyclodextrin (MBCD) before transfection.
| Characteristics1 | Control | 1 mmol | 2 mmol | 4 Mm | SEM |
|---|---|---|---|---|---|
| TM (%) | 91.6b | 97.3a | 96.3a | 89.2b | 2.23 |
| PM (%) | 60.7b | 67.3a | 66.1a | 59.8b | 1.12 |
| VCL (μm/s) | 107.9 | 108 | 105.4 | 106 | 2.45 |
| VSL (μm/s) | 55.6b | 64.9a | 68.1a | 55b | 3.1 |
| STR (%) | 77.5 | 74.8 | 72.9 | 73 | 2.95 |
| LIN (%) | 60.7 | 62.9 | 65.8 | 61.3 | 1.7 |
| MI (%) | 93.2b | 98.4a | 96.9a | 88.2b | 3.03 |
| Membrane functionality (%) | 92.4 | 93.1 | 90.5 | 91.7 | 3.8 |
a-cMeans within rows with different superscripts are significantly different (P < 0.05).
TM: Total motility; PM: Progressive motility; VCL: Curvilinear velocity; VSL: Straight linear velocity; STR: Sperm track straightness; LIN: Linearity.
PCR Reaction to Confirm Exogenous DNA Uptake by Sperm Cells
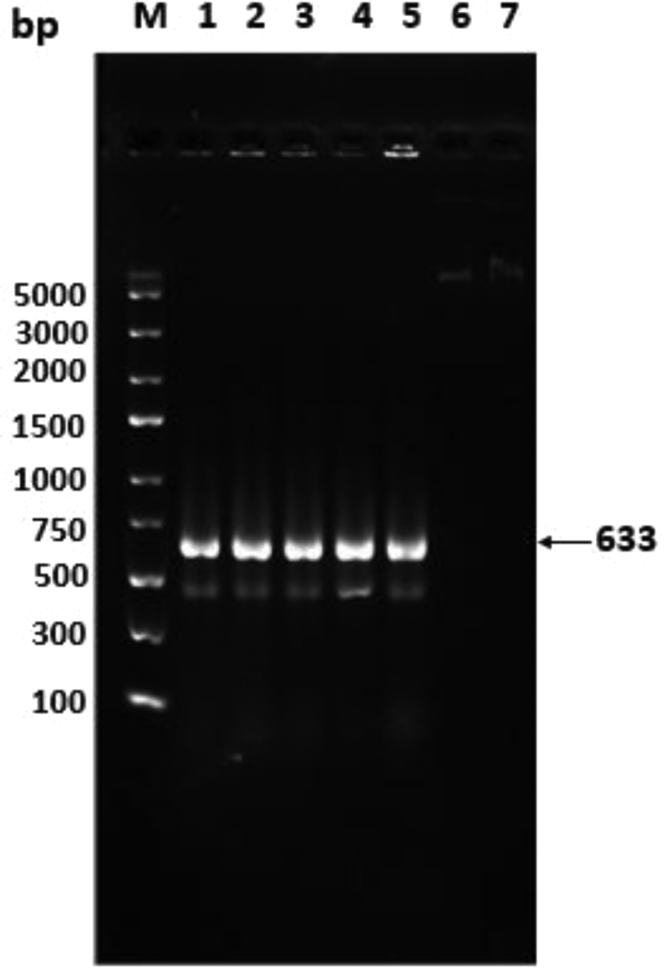
The PCR amplification detected the presence of the hG-CSF (633 base pairs) using the genomic DNA isolated from the transfected sperm. Figure 1 shows a clear band for the hG-CSF in sperm after being transfected in a medium contained 0, 1, 2, and 4 mmol MBCD. For PCR product, no difference was observed in transfected sperm in the experimental groups treated with various concentrations of MBCD. A higher frequency of DNA copies (sharp bands) was observed in groups treated with MBCD, whereas the group lacking MBCD had a lower frequency of PCR positive results. The PCR reaction consisted of a DNA template that contained the pcDNA3.1 + /hG-CSF plasmid as a positive control (P), a negative control (water addition), and DNA from nontransfected sperm cells as a blank control.
Figure 1.
PCR products on DNA extracted from transfected sperm cells. Lane M: Molecular marker DNA; lanes 1, 2, 3, and 4: transfected sperm in groups that contained 0, 1, 2, and 4 mmol methyl-beta-cyclodextrin (MBCD); lane 5: positive control; lane 6: negative water control; and lane 7: nontransfected sperm cells.
Motility and Velocity Characteristics of Sperm After Transfection
The percentages of motion characteristics of rooster sperm after being transfected in groups treated with 0, 1, 2, and 4 mmol MBCD are presented in Table 2. The transfection procedure in all groups significantly reduced the percentage of PM when compared with the group with no transfected sperm (control). No significant difference was observed in TM of sperm in the groups treated with 0 (81.6 ± 0.81), 1 (89.9 ± 0.81), and 2 (89 ± 0.81) mmol MBCD with respect to the control group with no transfected sperm (92.8 ± 0.81) (P ≥ 0.05). However, the lowest percentage of TM was detected in the group treated with 4 mmol MBCD (73 ± 0.81). The higher percentages of PM, VCL, and VSL were observed in groups that received 0 (62.5 ± 1.12, 108 ± 1.29, 53.9 ± 2.09, respectively), 1(50.1 ± 1.12, 108 ± 1.29, 54.3 ± 2.09, respectively), and 2 mmol (52.8 ± 1.12, 111 ± 1.29, 55.3 ± 2.09, respectively) MBCD as compared with the group treated with 4 mmol MBCD (40 ± 1.12, 78 ± 1.29, 38.7 ± 2.09). The group treated with 1 and 2 mmol MBCD produced a higher significant percentage of sperm with LIN after the transfection in comparison to the groups of 0 and 4 mmol MBCD (P < 0.05). No significant difference was observed among groups in terms of STR (P ≥ 0.05).
Table 2.
Motility and velocity parameters of rooster sperm treated with 0, 1, 2, and 4 mmol concentrations of methyl-beta-cyclodextrin (MBCD) after transfection.
| Characteristics1 | Control | 0 mmol | 1 mmol | 2 mmol | 4 Mm | SEM |
|---|---|---|---|---|---|---|
| TM (%) | 92.8a | 81.6a | 89.9a | 89a | 73b | 0.81 |
| PM (%) | 62.5a | 50.1b | 52.8b | 51.7b | 40c | 1.12 |
| VCL (μm/s) | 109a | 108a | 108a | 111a | 78b | 1.29 |
| VSL (μm/s) | 53.2a | 53.9a | 54.3a | 55.3a | 38.7b | 2.09 |
| STR (%) | 73.5 | 72.5 | 75.8 | 73.4 | 72.6 | 1.56 |
| LIN (%) | 58.2a | 50b | 60.2a | 60.9a | 49.3b | 0.97 |
a-cMeans within rows with different superscripts are significantly different (P < 0.05).
TM: Total motility; PM: Progressive motility; VCL: Curvilinear velocity; VSL: Straight linear velocity; STR: Sperm track straightness; LIN: Linearity.
Membrane Integrity and Functionality of Sperm
The percentages of membrane integrity and functionality of sperm after transfection in the groups that received various concentrations of MBCD are presented in Table 3. There was a significantly higher percentage of MI in transfected sperm in the group treated with 1 mmol (76.2 ± 1.65) as compared with the groups that received 0 (71 ± 1.65), 2 (69 ± 1.65), and 4 (68 ± 1.65) mmol MBCD. No significant difference was observed between the group that received 1 mmol MBCD (76.2 ± 1.65) and the control that was not transfected (79 ± 1.65). There was a significantly higher percentage of membrane functionality in the groups treated with 0 (63.2 ± 1.64) and 1 (64 ± 1.64) mmol MBCD when compared with the group that received 2 mmol (57.1 ± 1.64) and 4 mmol MBCD (52 ± 1.64). There was no significant difference for membrane functionality between the group that received 0 and 1 mmol MBCD (P ≥ 0.05).
Table 3.
Membrane functionality and membrane integrity of rooster sperm treated with 0, 1, 2, and 4 mmol concentrations of methyl-beta-cyclodextrin (MBCD) after transfection.
| Characteristics | Control | 0 mmol | 1 mmol | 2 mmol | 4 Mm | SEM |
|---|---|---|---|---|---|---|
| Membrane1 functionality (%) | 83a | 63.2b | 64b | 57.1c | 52c | 1.64 |
| MI2 (%) | 79a | 71b | 76.2a | 69b | 68b | 1.65 |
a-c eans within rows with different superscripts are significantly different (P < 0.05).
Membrane functionality.
MI: membrane integrity.
PCR Reaction to Confirm the Presence of hG-CSF in Chickens
After insemination of 28 leghorn breeder hens, 140 fertile eggs were transferred to an incubator (70 eggs per each group). After 21 d, there were 34 live chicks and 5 dead embryos in MBCD group. In the control group, 51 live chicks and 4 dead embryos were observed. The blood and tissue samples were collected from live chicks and dead embryos, respectively. The PCR reaction of the hG-CSF gene did not show any band for the hG-CSF in the live chicks.
Discussion
The chicken embryo has been a leading model system in developmental biology due to the availability of embryos, short incubation period, and the ease of experimental study (Kamihira et al., 2004). The most common methods for the production of transgenic chickens include pronuclear microinjection, somatic cell nuclear transfer, transduction using retroviruses, and sperm-mediated gene transfer. Microinjection is the most common method, but it is expensive and there are fewer documents that report use of this method in livestock species (Rubessa et al., 2019). SMGT is the method with a low-cost strategy based on the introduction of foreign DNA into the sperm before the fertilization process (Lavitrano et al., 1989; Smith and Spadafora, 2005; Collares et al., 2011). Rooster sperm can spontaneously uptake the exogenous DNA and subsequently transfer the gene construction to eggs during the fertilization (Collares et al., 2011). The mechanism in which the exogenous DNA penetrates into the sperm cells has been only partially understood; however, it seems the interplay of specific proteins is involved in this scenario. It has been reported that foreign DNA binds to two 30–35 kDa membrane proteins prior to internalization, likely via receptor-mediated endocytosis (Lavitrano et al., 1992; Zani et al., 1995). Others have reported that the membrane glycoprotein CD4, a member of the immunoglobulin superfamily, mediates the DNA internalization and aids its movement into the sperm nucleus (Lavitrano et al., 1997).
Although the method of SMGT is simple and low-cost, its efficiency is low and a dramatic reduction in fertility potential of sperm occurs after a gene transfection, which is a serious challenge for the optimization of this technique (Spadafora, 1998). The problem mainly pertains to the structural and biochemical changes of the plasma membrane during the penetration of exogenous DNA into the sperm cells (Bacci et al., 2009; Parrington et al., 2011). Cytoplasmic reactions and activation of endonucleases during penetration of gene to sperm are other potential factors affecting the sperm performance after the transfection process (Canovas et al., 2010; Pramod et al., 2016). As sperm cells are considered as hard cell to transfection, studies have done to increase its DNA uptake (Daneluz et al., 2020). It has been reported that the efficacy of the DNA binding to the sperm cells is increased using special chemicals or adjuvants in the transfection medium. Therefore, in the study herein, an effort to optimize this technique using a novel protective agent (MBCD) to improve the rate of DNA uptake as well as the sperm quality after the transfection is reported. In this study, supplementation of the medium with MBCD improved the frequency of DNA absorption to the sperm cells when compared with a medium lacking MBCD. It should be noted that absorption of exogenous DNA by the sperm cells is not a passive process. The mechanism of DNA absorption into the sperm cells has not been completely understood. In the study herein, treatment of rooster sperm with MBCD increased the absorption of DNA that was observed with the PCR results. This finding is in agreement with the report of Oddi et al. (2012), who reported that MBCD increases the uptake of DNA with swine sperm by alteration of cholesterol levels in sperm plasma membrane (Oddi et al., 2012). These researchers also suggested that the proteins responsible for the DNA attachment in the plasma membrane were influenced by the cholesterol. Therefore, the higher uptake of DNA by sperm reported in the study may be related to the depletion of cholesterol in the plasma membrane of sperm. Another reason for the efficiency of MBCD on the DNA uptake is increasing the membrane fluidity of sperm, which resulted in better penetration of foreign DNA into the sperm. This membrane fluidity is the result of partial exodus of cholesterol from the plasma membrane, thus increasing the membrane flexibility of the phospholipid bilayer, which, in turn, increases the possibility of the DNA binding to the sperm cells (Oddi et al., 2012).
Another purpose of this study was to use MBCD for the preservation of the sperm, particularly the plasma membrane during the transfection. The motility, MI, and membrane functionality of sperm were assessed as the main indices of sperm quality, which were reduced in all transfected groups as compared with the group lacking transfected sperm. This result is consistent with the findings of García-Vázquez et al. (2011), who reported that sperm performance was decreased after the transfection. The results of this study are comparable with other reports where either lipofection or electroporation was able to improve the rate of DNA binding to sperm cells. Moreover, the average percentage of the motility after the transfection in this study was 89.9 ± 0.81, which was the same as the results obtained in a study using DMSO (Collares et al., 2011).
Progressive motility after the incubation with 1 mmol MBCD was 62.5 ± 1.12, which was comparable with other research in which the sperm were treated with DMSO (Kang et al., 2008). In another study, the rate of sperm viability after the transfection was 76.2 ± 1.65, which partially supported the results of the present study, whereas nanotransfection had similar effects with MBCD employed in this experiment (Campos et al., 2011). Therefore, the treatment of rooster sperm with MBCD could be considered an alternative way in comparison to other commercial transfectants (Nakanishi and Iritani, 1993). Also, in the study herein, artificial insemination was performed using transfected sperm in the group that contained 1 mmol MBCD. Chicks were hatched after the incubation of fertilized eggs and DNA was extracted from 85 live chicks and 9 dead embryos. A PCR amplification was performed with the genomic DNA extracted from the blood of chicks and dead embryo tissues. In neither the presence of the exogenous plasmid was observed. These findings do not agree with findings by Collares et al. (2011), who obtained transgenic chickens after AI using the SMGT. However, these results are in agreement with Kang et al. (2008) and Garcia-Vazquez et al. (2011), who did not find transgenic offspring after AI with the SMGT procedure in porcine. This phenomenon may be linked to the unique structure of the hen reproductive system (Suarez and Pacey, 2006; Chaparian et al., 2016). Since the sperm may be damaged during the gene transfection, the hen reproductive system would possibly remove the low-quality or damaged sperm with respect to intact sperm (no transfected sperm) (Kang et al., 2008). Therefore, this phenomenon may be a reason for the lack of transfected sperm at the fertilization site. Suarez and Pacey (2006) suggested that positive sperm carrying the exogenous DNA and being exposed to contraceptive interaction with the genital tract of the hen do not reach the oocyte. This event is also verified by a direct injection of sperm (ICSI) (Umeyama et al., 2012) and IVF (Chandrashekran et al., 2014), which are more efficient in the production of transgenic animals. Deep AI may increase the chance of DNA-loaded sperm to participate in the fertilization procedure (Garcia-Vazquez et al., 2011).
Based on these results, rooster sperm can absorb the exogenous hG-CSF. The MBCD protected sperm during SMGT, improved the sperm parameters, and has beneficial effect on increasing the frequency of positive results in SMGT. However, this process did not result in the production of a transgenic animal.
Disclosures
The authors declare that they have no conflict of interest in this study.
References
- Ansari M., Zhandi M., Kohram H., Zaghari M., Sadeghi M., Sharafi M.J.T. Improvement of post-thawed sperm quality and fertility of Arian rooster by oral administration of d-aspartic acid. Theriogenology. 2017;92:69–74. doi: 10.1016/j.theriogenology.2017.01.014. [DOI] [PubMed] [Google Scholar]
- Anzar M., Buhr M. Spontaneous uptake of exogenous DNA by bull spermatozoa. Theriogenology. 2006;65:683–690. doi: 10.1016/j.theriogenology.2005.06.009. [DOI] [PubMed] [Google Scholar]
- Bacci M., Zannoni A., De Cecco M., Fantinati P., Bernardini C., Galeati G., Spinaci M., Giovannoni R., Lavitrano M., Seren E. Sperm-mediated gene transfer–treated spermatozoa maintain good quality parameters and in vitro fertilization ability in swine. Theriogenology. 2009;72:1163–1170. doi: 10.1016/j.theriogenology.2009.06.033. [DOI] [PubMed] [Google Scholar]
- Bailes S., Devers J., Kirby J., Rhoads D. An inexpensive, simple protocol for DNA isolation from blood for high-throughput genotyping by polymerase chain reaction or restriction endonuclease digestion. Poult. Sci. 2007;86:102–106. doi: 10.1093/ps/86.1.102. [DOI] [PubMed] [Google Scholar]
- Burrows W., Quinn J. The collection of spermatozoa from the domestic fowl and Turkey. Poult. Sci. 1937;16:19–24. [Google Scholar]
- Campos V., Komninou E., Urtiaga G., de Leon P., Seixas F., Dellagostin O., Deschamps J., Collares T. NanoSMGT: transfection of exogenous DNA on sex-sorted bovine sperm using nanopolymer. Theriogenology. 2011;75:1476–1481. doi: 10.1016/j.theriogenology.2011.01.009. [DOI] [PubMed] [Google Scholar]
- Canovas S., Gutierrez-Adan A., Gadea J. Effect of exogenous DNA on bovine sperm functionality using the sperm mediated gene transfer (SMGT) technique. Mol. Reprod. Dev. 2010;77:687–698. doi: 10.1002/mrd.21205. [DOI] [PubMed] [Google Scholar]
- Chandrashekran A., Sarkar R., Thrasher A., Fraser S.E., Dibb N., Casimir C., Winston R., Readhead C. Efficient generation of transgenic mice by lentivirus-mediated modification of spermatozoa. FASEB J. 2014;28:569–576. doi: 10.1096/fj.13-233999. [DOI] [PubMed] [Google Scholar]
- Chaparian S., Abdulahnejad A., Rashidi F., Toghyani M., Gheisari A., Eghbalsaied S. Is passive transmission of non-viral vectors through artificial insemination of sperm-DNA mixtures sufficient for chicken transgenesis? J. Reprod. Dev. 2016;62:265–270. doi: 10.1262/jrd.2015-176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi Y.-H., Toyoda Y. Cyclodextrin removes cholesterol from mouse sperm and induces capacitation in a protein-free medium. Biol. Reprod. 1998;59:1328–1333. doi: 10.1095/biolreprod59.6.1328. [DOI] [PubMed] [Google Scholar]
- Collares T., Campos V.F., de Leon P.M.M., Cavalcanti P.V., Amaral M.G., Dellagostin O.A., Deschamps J.C., Seixas F.K. Transgene transmission in chickens by sperm-mediated gene transfer after seminal plasma removal and exogenous DNA treated with dimethylsulfoxide or N, N-dimethylacetamide. J. Biosci. 2011;36:613–620. doi: 10.1007/s12038-011-9098-x. [DOI] [PubMed] [Google Scholar]
- Daneluz L.O., Acosta I.B., Nunes L.S., Blodorn E.B., Domingues W.B., Martins A.W., Dellagostin E.N., Rassier G.T., Corcini C.D., Fróes C.N., Komninou E.R., Varela A.S., Jr., Campos V.F. Efficiency and cell viability implications using tip type electroporation in zebrafish sperm cells. Mol. Biol. Rep. 2020;47:5879–5887. doi: 10.1007/s11033-020-05658-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan H., Gulley M.L. DNA extraction from fresh or frozen tissues. Mol. Pathol. Protoc. 2001;49:5–10. doi: 10.1385/1-59259-081-0:5. [DOI] [PubMed] [Google Scholar]
- Fattah A., Sharafi M., Masoudi R., Shahverdi A., Esmaeili V., Najafi A. L-Carnitine in rooster semen cryopreservation: Flow cytometric, biochemical and motion findings for frozen-thawed sperm. Cryobiology. 2017;74:148–153. doi: 10.1016/j.cryobiol.2016.10.009. [DOI] [PubMed] [Google Scholar]
- Feyzi S., Sharafi M., Rahimi S. Stress preconditioning of rooster semen before cryopreservation improves fertility potential of thawed sperm. Poult. Sci. 2018;97:2582–2590. doi: 10.3382/ps/pey067. [DOI] [PubMed] [Google Scholar]
- García-Vázquez F.A., Ruiz S., Grullón L.A., de Ondiz A., Gutiérrez-Adán A., Gadea J. Factors affecting porcine sperm mediated gene transfer. Res. Vet. Sci. 2011;91:446–453. doi: 10.1016/j.rvsc.2010.09.015. [DOI] [PubMed] [Google Scholar]
- Harel-Markowitz E., Gurevich M., Shore L.S., Katz A., Stram Y., Shemesh M. Use of sperm plasmid DNA lipofection combined with REMI (restriction enzyme-mediated insertion) for production of transgenic chickens expressing eGFP (enhanced green fluorescent protein) or human follicle-stimulating hormone. Biol. Reprod. 2009;80:1046–1052. doi: 10.1095/biolreprod.108.070375. [DOI] [PubMed] [Google Scholar]
- Houdebine L.M. Production of pharmaceutical proteins by transgenic animals. Comp. Immunology, Microbiology Infectious Diseases. 2009;32:107–121. doi: 10.1016/j.cimid.2007.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamihira M., Nishijima K., Iijima S. Transgenic birds for the production of recombinant proteins. Adv. Biochem. Eng. Biotechnol. 2004;91:171–189. doi: 10.1007/b94209. [DOI] [PubMed] [Google Scholar]
- Kang J.H., Hakimov H., Ruiz A., Friendship R.M., Buhr M., Golovan S.P. The negative effects of exogenous DNA binding on porcine spermatozoa are caused by removal of seminal fluid. Theriogenology. 2008;70:1288–1296. doi: 10.1016/j.theriogenology.2008.06.011. [DOI] [PubMed] [Google Scholar]
- Lanes C.F.C., Sampaio L., Marins L.J.T. Evaluation of DNase activity in seminal plasma and uptake of exogenous DNA by spermatozoa of the Brazilian Flounder Paralichthys Orbignyanus. Theriogenology. 2009;71:525–533. doi: 10.1016/j.theriogenology.2008.08.019. [DOI] [PubMed] [Google Scholar]
- Lavitrano M., Camaioni A., Fazio V.M., Dolci S., Farace M.G., Spadafora C. Sperm cells as vectors for introducing foreign DNA into eggs: genetic transformation of mice. Cell. 1989;57:717–723. doi: 10.1016/0092-8674(89)90787-3. [DOI] [PubMed] [Google Scholar]
- Lavitrano M., French D., Zani M., Frati L., Spadafora C. The interaction between exogenous DNA and sperm cells. Mol. Reprod. Development. 1992;31:161–169. doi: 10.1002/mrd.1080310302. [DOI] [PubMed] [Google Scholar]
- Lavitrano M., Maione B., Forte E., Francolini M., Sperandio S., Testi R., Spadafora C. The interaction of sperm cells with exogenous DNA: A role of CD4 and major histocompatibility complex class II molecules. Exp. Cell Res. 1997;233:56–62. doi: 10.1006/excr.1997.3534. [DOI] [PubMed] [Google Scholar]
- Lillico S.G., McGrew M.J., Sherman A., Sang H.M. Transgenic chickens as bioreactors for protein-based drugs. Drug Discov. Today. 2005;10:191–196. doi: 10.1016/S1359-6446(04)03317-3. [DOI] [PubMed] [Google Scholar]
- Lotfi S., Mehri M., Sharafi M., Masoudi R. Hyaluronic acid improves frozen-thawed sperm quality and fertility potential in rooster. Anim. Reprod. Sci. 2017;184:204–210. doi: 10.1016/j.anireprosci.2017.07.018. [DOI] [PubMed] [Google Scholar]
- Mao J., Wu G.-M., Prather R.S., Smith M.F., Cantley T., Rieke A., Didion B.A., Day B.N. Effect of methyl-β-cyclodextrin treatment of pig spermatozoa on in vitro fertilization and embryo development in the absence or presence of caffeine. Theriogenology. 2005;64:1913–1927. doi: 10.1016/j.theriogenology.2005.03.024. [DOI] [PubMed] [Google Scholar]
- Moghbeli M., Kohram H., Zare-Shahaneh A., Zhandi M., Sharafi M., Nabi M.M., Zahedi V., Sharideh H.J.C. Are the optimum levels of the catalase and vitamin E in rooster semen extender after freezing-thawing influenced by sperm concentration? Cryobiology. 2016;72:264–268. doi: 10.1016/j.cryobiol.2016.03.008. [DOI] [PubMed] [Google Scholar]
- Montgomery S.A. Transgenic animals: walking bioreactors. BioProcess Int. 2004;2:40–51. [Google Scholar]
- Nakanishi A., Iritani A. Gene transfer in the chicken by sperm-mediated methods. Mol. Reprod. Dev. 1993;36:258–261. doi: 10.1002/mrd.1080360225. [DOI] [PubMed] [Google Scholar]
- Oddi S., Bernabò N., Di Tommaso M., Angelucci C.B., Bisicchia E., Mattioli M., Maccarrone M. DNA uptake in swine sperm: effect of plasmid topology and methyl-beta-cyclodextrin-mediated cholesterol depletion. Mol. Reprod. Dev. 2012;79:853–860. doi: 10.1002/mrd.22124. [DOI] [PubMed] [Google Scholar]
- Parrington J., Coward K., Gadea J. Sperm and testis mediated DNA transfer as a means of gene therapy. Syst. Biol. Reprod. Med. 2011;57:35–42. doi: 10.3109/19396368.2010.514022. [DOI] [PubMed] [Google Scholar]
- Pramod R.K., Kumar R., Mitra A. Transgenic expression of green fluorescent protein in caprine embryos produced through electroporation-aided sperm-mediated gene Transfer. Gene. 2016;576:505–511. doi: 10.1016/j.gene.2015.10.066. [DOI] [PubMed] [Google Scholar]
- Rola J., Polak M.P., Zmudzinski J. Amplification of DNA of BHV1 isolated from semen of naturally infected bulls. Bulletin-Veterinary Inst. Pulawy. 2003;47:71–76. [Google Scholar]
- Rubessa M., Lotti S.N., Kandel M.E., Popescu G., Wheeler M.B. SLIM microscopy allows for visualization of DNA-containing liposomes designed for sperm-mediated gene transfer in cattle. Mol. Biol. Rep. 2019;46:695–703. doi: 10.1007/s11033-018-4525-9. [DOI] [PubMed] [Google Scholar]
- Samoylov A., Kesyan A., Suraeva N. Development of transgenic chicken with a gene of human granulocyte colony-stimulating factor using sperm-mediated gene transfer. Biol. Bull. 2013;40:419–422. [PubMed] [Google Scholar]
- Shahverdi A., Sharafi M., Gourabi H., Yekta A.A., Esmaeili V., Sharbatoghli M., Janzamin E., Hajnasrollahi M., Mostafayi F. Fertility and flow cytometric evaluations of frozen-thawed rooster semen in cryopreservation medium containing low-density lipoprotein. Theriogenology. 2015;83:78–85. doi: 10.1016/j.theriogenology.2014.07.044. [DOI] [PubMed] [Google Scholar]
- Smith K., Spadafora C. Sperm-mediated gene transfer: applications and implications. BioEssays. 2005;27:551–562. doi: 10.1002/bies.20211. [DOI] [PubMed] [Google Scholar]
- Spadafora C. Sperm cells and foreign DNA: a controversial relation. BioEssays : news and reviews in molecular. Cellular Dev. Biol. 1998;20:955–964. doi: 10.1002/(SICI)1521-1878(199811)20:11<955::AID-BIES11>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- Suarez S., Pacey A. Sperm transport in the female reproductive tract. Hum. Reprod. Update. 2006;12:23–37. doi: 10.1093/humupd/dmi047. [DOI] [PubMed] [Google Scholar]
- Umeyama K., Saito H., Kurome M., Matsunari H., Watanabe M., Nakauchi H., Nagashima H. Characterization of the ICSI-mediated gene transfer method in the production of transgenic pigs. Mol. Reprod. Dev. 2012;79:218–228. doi: 10.1002/mrd.22015. [DOI] [PubMed] [Google Scholar]
- Yu F., Ding L.J., Sun G.B., Sun P.X., He X.H., Ni L.G., Li B.C. Transgenic sperm produced by electrotransfection and allogeneic transplantation of chicken fetal spermatogonial stem cells. Mol. Reprod. Dev. 2010;77:340–347. doi: 10.1002/mrd.21147. [DOI] [PubMed] [Google Scholar]
- Zani M., Lavitrano M., French D., Lulli V., Maione B., Sperandio S., Spadafora C. The mechanism of binding of exogenous DNA to sperm cells: factors controlling the DNA uptake. Exp. Cell Res. 1995;217:57–64. doi: 10.1006/excr.1995.1063. [DOI] [PubMed] [Google Scholar]
- Zaniboni A., Merlo B., Zannoni A., Bernardini C., Lavitrano M., Forni M., Mari G., Bacci M.L. Expression of fluorescent reporter protein in equine embryos produced through intracytoplasmic sperm injection mediated gene transfer (ICSI-MGT) Anim. Reprod. Sci. 2013;137:53–61. doi: 10.1016/j.anireprosci.2012.12.010. [DOI] [PubMed] [Google Scholar]