Abstract
The efficiency of radiotherapy treatment regimes varies from tumour to tumour and from patient to patient but it is generally highly influenced by the tumour microenvironment (TME). The TME can be described as a heterogeneous composition of biological, biophysical, biomechanical and biochemical milieus that influence the tumour survival and its’ response to treatment. Preclinical research faces challenges in the replication of these in vivo milieus for predictable treatment response studies. 2D cell culture is a traditional, simplistic and cost-effective approach to culture cells in vitro, however, the nature of the system fails to recapitulate important features of the TME such as structure, cell-cell and cell-matrix interactions. At the same time, the traditional use of animals (Xenografts) in cancer research allows realistic in vivo architecture, however foreign physiology, limited heterogeneity and reduced tumour mutation rates impairs relevance to humans. Furthermore, animal research is very time consuming and costly. Tissue engineering is advancing as a promising biomimetic approach, producing 3D models that capture structural, biophysical, biochemical and biomechanical features, therefore, facilitating more realistic treatment response studies for further clinical application. However, currently, the application of 3D models for radiation response studies is an understudied area of research, especially for pancreatic ductal adenocarcinoma (PDAC), a cancer with a notoriously complex microenvironment. At the same time, specific novel and/or more enhanced radiotherapy tumour-targeting techniques such as MRI-guided radiotherapy and proton therapy are emerging to more effectively target pancreatic cancer cells. However, these emerging technologies may have different biological effectiveness as compared to established photon-based radiotherapy. For example, for MRI-guided radiotherapy, the novel use of static magnetic fields (SMF) during radiation delivery is understudied and not fully understood. Thus, reliable biomimetic platforms to test new radiation delivery strategies are required to more accurately predict in vivo responses. Here, we aim to collate current 3D models for radiation response studies of PDAC, identifying the state of the art and outlines knowledge gaps. Overall, this review paper highlights the need for further research on the use of 3D models for pre-clinical radiotherapy screening including (i) 3D (re)-modeling of the PDAC hypoxic TME to allow for late effects of ionising radiation (ii) the screening of novel radiotherapy approaches and their combinations as well as (iii) a universally accepted 3D-model image quantification method for evaluating TME components in situ that would facilitate accurate post-treatment(s) quantitative comparisons.
pancreatic cancer, the tumour microenvironment and current treatment strategies
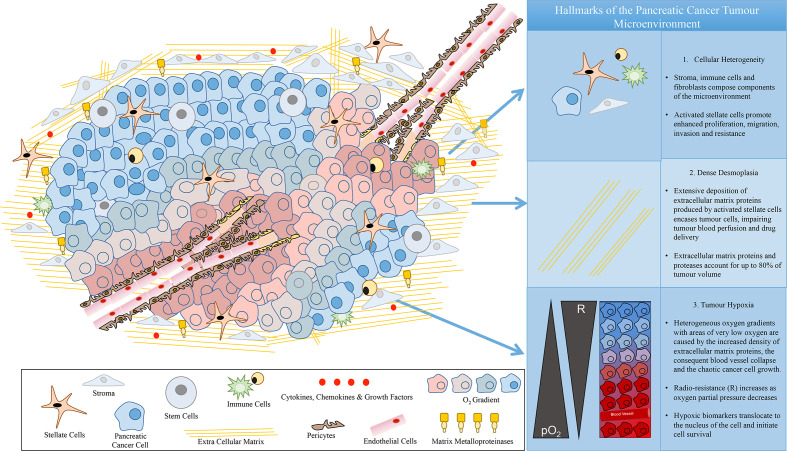
Pancreatic ductal adenocarcinoma (PDAC) is a cancer of unmet clinical need. More specifically, this disease is initially asymptomatic resulting in distant stage diagnosis, a 5-year survival rate of just 9%1 and a UK average life expectancy of 4–6 months.2 These figures have barely improved for over 50 years2 and with incidence rates increasing, predictions suggest that this cancer could rise to a main cause of cancer deaths by 2030.3 This disease is classified as multigene-based, with numerous biological fingerprints, including mutations in K-Ras, p53 and Smad4, influenced by environmental factors such as smoking.4,5 Epidemiology reveals a slight gender discrepancy, favouring males, with Central, Eastern and Western Europe displaying the highest national disease prevalence.6 Unique hallmarks of this cancer impair treatment success and challenge pre-clinical-to-clinical translation. These hallmarks include a complex concoction of cellular components that compose the tumour microenvironment (TME), which lead to the formation of dense desmoplasia/fibrosis and extensive heterogeneous hypoxia (Figure 1). More specifically, PDAC induces stellate cell activation that secrete very high amounts of extracellular matrix (ECM) proteins. Such accumulation of ECM proteins increases the stiffness around the cancer, this is known as extensive fibrosis/desmoplastic reaction surrounding the tumour.7 Consequently, stiffness increase causes blood vessel collapse which leads to (i) impaired drug delivery and (ii) the creation of heterogeneous expanses of low oxygen concentration (hypoxia).8 This ecosystem of diverse histological and behavioural hallmarks hosts a unique pancreatic cancer niche that actively promotes tumour cell survival, migration and resistance to current therapeutics7–13 (Figure 1). Thus, there is a clinical demand for advancing understanding, characterisation and therapeutics for this devastating disease.
Figure 1.
Hallmarks of the pancreatic cancer tumour microenvironment.
Current treatment strategies for pancreatic ductal adenocarcinoma (PDAC)
Surgery
It is reported that 50% of UK PDAC patients are diagnosed after presenting as an emergency hospital admission.2 Furthermore, 40% of UK patients visit a general practitioner three or more times prior to receiving a diagnosis.2 As a result of unassuming symptoms, late diagnosis elucidates that only 15% of patients are eligible for curative surgical intervention.2,14,15 Moreover, resection as a treatment option for locally advanced PDAC improves the 5-year survival rate by only 30%.2,16 Thereafter, resection and a combination of chemotherapy and radiotherapy are advised for consideration for borderline resectable PDAC and locally advanced PDAC. Furthermore, treatment regimes for metastasis of this disease remain poor, with a 1-year survival rate of just 20% post-treatment.15
Chemotherapy
Adjuvant chemotherapy following surgical resection is a widely recognised treatment option with significant survival benefits as compared to chemotherapy alone.17 Gemcitabine, an antimetabolite/nucleic acid synthesis inhibitor, is considered the first-line treatment for locally advanced or metastatic PDAC.18 Moreover, Capecitabine another antimetabolite investigated in the ESPAC-4 trial (2017) supported the adjuvant combination of Gemcitabine and Capecitabine (GemCap) for resected patients.19 The trial showed increased patient survival and tumour response in patients receiving gemcitabine plus capecitabine compared with gemcitabine alone, that is, 28 versus 25.5 months median survival (p = 0.032).19 FOLFIRINOX, a treatment regime consisting of a concoction of an antimetabolite, a topoisomerase inhibitor, a platinum-based drug and an antitoxin, for locally advanced or metastatic PDAC treatment is associated with increased patient survival and higher toxicity when compared to Gemcitabine as a monotherapeutic, that is, 11.1 versus 6.8 months median survival (p < 0.001).20 Moreover, combination treatment of Gemcitabine and nab-paclitaxel for metastatic PDAC has shown to improve overall survival as compared to Gemcitabine alone, that is, 8.5 versus 6.7 months median survival (p < 0.001); however, patient toxicity including neuropathy and myelosuppression was increased.18 Furthermore, a Phase-III trial revealed increased overall survival in patients treated with Gemcitabine in combination with erlotinib (HER1/EGFR tyrosine kinase inhibitor) as compared to Gemcitabine alone for patients with unresectable, locally advanced or metastatic PDAC suffering adverse side effects, that is, 6.24 versus 5.91 months median survival (p = 0.038).21
Radiotherapy
There are a limited number of studies investigating preoperative multi-functional treatment for borderline resectable and advanced PDAC. Therefore, preoperative treatment guidelines are based on small criteria; however, chemo-radiotherapy is generally advised for consideration for borderline resectable PDAC.16 The A021101 trial (2016) demonstrated the multimodality use of preoperative modified FOLFIRINOX and Capecitabine chemo-radiotherapy for borderline resectable PDAC, that is, 21.7 months median survival in all patients.22 These results are supported by Phase-II clinical trials investigating neoadjuvant therapy with FOLFIRINOX in combination with radiotherapy for borderline resectable and locally advanced PDAC, in which chemo-radiotherapy resulted in increased progression-free survival and overall survival.23,24 On the contrary, a clinical trial investigating neoadjuvant modified FOLFIRNOX and gemcitabine/nab-paclitaxel alone or in combination with chemo-radiotherapy after surgical resection revealed that resection and chemo-radiotherapy combined was associated with increased pathological treatment response but no significant difference in overall survival was identified.25
Generally, radiotherapy is advised for consideration as an adjuvant therapy for locally advanced and PDAC metastasis after a -month period of Gemcitabine.14,16,26 However, adjuvant chemo-radiotherapy data for PDAC are generally limited.2 Nonetheless, adjuvant chemo-radiotherapy for PDAC has been a treatment option in the US for a long time, especially after the GITSG 9173 trial (1985) revealed improved overall survival of patients treated with combined therapy as compared to no adjuvant treatment, that is, 20 versus 11 months median survival (p = 0.035).27 More recently, radiotherapy treatment for PDAC has proved controversial due to clinical trials failing to improve overall survival.17,19 More specifically, the European Study Group for Pancreatic Cancer 1 Trial (2004) showed a deleterious effect on survival in patients with resected pancreatic cancer treated with adjuvant chemo-radiotherapy as compared to chemotherapy alone, that is,15.9 versus 17.9 months median survival (p = 0.05).17 This outcome resulted in the limiting of radiotherapy use in Europe in contrast to international PDAC standard treatments. Moreover, the LAP07 Randomised Clinical Trial (2016) for locally advanced PDAC showed no difference in patient response and survival with chemotherapy alone as compared to chemo-radiotherapy after 4 months of Gemcitabine treatment, that is, 16.5 versus 15.2 months median survival (p = 0.83).28 On the contrary, a pooled analysis of 955 PDAC patients treated with adjuvant chemoradiotherapy combined with 5-FU or capecitabine chemotherapy showed an improved overall survival of patients receiving chemo-radiotherapy compared to chemotherapy alone, that is, 39.9 versus 24.8 months median survival (p < 0.001).29
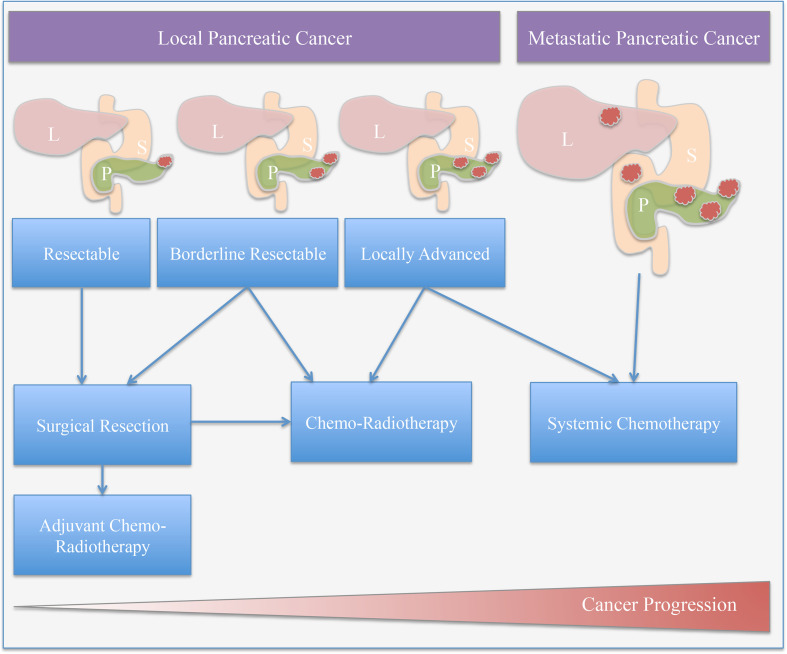
Overall, from the above studies, it is evident that the roles of neoadjuvant and adjuvant radiation, for PDAC, are still evolving and remain controversial. A general overview of the treatment regimes suggested for consideration for local PDAC are summarised in Figure 2.
Figure 2.
Treatment options for pancreatic cancer: Treatment plans are personalised and dependent on disease progression and the general health of the patient and can include multiple regimes (L: liver, P: pancreas, S: stomach).
PDAC resistance to radiotherapy
Generally, PDAC is described as an extremely radio-resistant cancer.30–32 The hallmarks of PDAC directly impact treatment response. More specifically, as described in section 1, the TME and cellular response to radiation are closely interconnected in vivo30,31 (Figure 1). Cell-matrix interactions in the cellular response to radiation are suggested as critical to treatment success.33 Furthermore, tumour models have shown increased radio-resistance in the presence of the other cells of the TME and/or the ECM components.34 More specifically, pancreatic stellate cells co-cultured with cancer cells reveal the promotion of radio-resistance.32 Moreover, Pickup et al. (2014) suggest that the ECM influences and enhances Hanahan and Weinberg’s famous hallmarks of cancer, namely, (i) sustained proliferation, (ii) evasion of growth suppressors, (iii) resistance to cell death, (iv) replicative immortality, (v) induction of angiogenesis and (vi) cell invasion and metastasis ability.7
Overall, the heterogeneous hypoxic TME hallmark reduces the radiotherapy efficiency.30 50 years of pre-clinical and clinical research describe the sensitivity of oxygenated cells to radiotherapy.35 More specifically, the oxygen enhancement ratio (OER) boosts radiation treatment by a factor of 2.5–3.36 The radiochemical rational for this phenomenon is widely known as the oxygen fixation hypothesis (OFH), in which DNA damage is irreversible in the presence of oxygen. In contrast, low oxygen can evade radiation-induced cell death by initiating cell quiescence, inhibiting cell senescence, apoptosis, p53 activity, autophagy and mitochondrial activity.37 Moreover, clinical trials including hyperbaric oxygen therapy, hyperthermia and carbogen breathing as well as vasodilators attempt to reduce hypoxia during radiotherapy increasing oxygen tension to improve treatment efficacy.38 Similarly, treatments to target the complex PDAC TME are emerging, including PARP inhibitors and gene therapy.5 Hypoxia-activated pro-drug (HAPs) and hypoxic nanocarriers are under development with the potential to enhance radiotherapy for hypoxic cancers such as PDAC.38,39 However, traditional pre-clinical testing via 2D cell-culture cannot accurately model hypoxia distribution due to the lack of structure, making the accuracy/biomimicry of hypoxia-induced radiation resistance in vitro studies challenging.
Recent advances in radiotherapy for PDAC
Advances in radiotherapy such as (i) intensity or volumetric modulated radiation therapy (IMRT or VMAT), (ii) stereotactic body radiation therapy (SBRT), and (iii) proton therapy are revealing promising results for the future of radiotherapy for PDAC.14,40–44 These modalities are evolving to specifically target the tumour tissue, whilst reducing adverse treatment side effects and could, therefore, help overcome the resistance shown in conventional radiotherapy. Moreover, this optimisation is essential for PDAC, due to location of the pancreas, situated deep within the internal cavity, surrounded by radiosensitive organs such as the stomach and duodenum.43 Furthermore, the path of an X-ray beam to the pancreas often meets the spinal cord, liver and kidneys demanding stringent dose optimisation to limit damage to these critical organs.43
IMRT or VMAT delivers treatment in a number of radiation beams that are adjusted for different levels of intensities, speeds and patterns allowing dose control and precise tumour targeting.45,46 IMRT has shown to improve toxicity effects in a study of 205 locally advanced pancreatic cancer patients (LAPC) as compared to 3D conformal radiation therapy.41 Consequently, IMRT in this study allowed a window for a better dose toleration and further dose escalation.41 Moreover, analysis of the American National Cancer Database (NCDB) showed that IMRT in combination with chemotherapy lead to an increased 1 year overall survival in comparison with classical 3D radiotherapy, that is, 45.6 and 38.7% 1 year overall survival, respectively.32 Overall due to its’ positive effects, the IMRT use for PDAC treatment in has increased substantially from the years 2003–2011 by 27–72% equivalently while conventional radiotherapy decreased by 73–28%.32
SBRT, employing either IMRT or VMAT, is a focused treatment that allows a number of radiation beams to precisely target a tumour from several different angles, allowing tighter dose distribution and higher doses.40 SBRT has shown high rates of local disease control leading to an improvement of the overall 1-year survival rates for borderline resectable pancreatic cancer and locally advanced pancreatic cancer patients (16.4 and 15 months 1 year overall survival).42 Furthermore, analysis of over 19 published clinical trials for the use of SBRT for inoperable PDAC, suggested advantages in treatment time, overall median survival and locoregional control.47
Proton therapy is another novel radiotherapy approach for the treatment of various types of cancer, including PDAC.44,48–50 Proton therapy exploits the use of charged particles as opposed to conventional photon therapy allowing for more effective dose deposition and a reduction of exit dose.44,48–50 Thus, the use of protons for PDAC treatment is showing advantages over conventional radiotherapy in terms of improved overall survival and toxicity toleration.43,44 More specifically, a study of 11 unresectable PDAC patients treated with proton therapy displayed a median survival of 18 months and 69% of the patients enrolled experienced 2-year freedom from local disease progression.43 Moreover, proton therapy was well-tolerated with no Grade 2 or higher gastrointestinal toxicities.43 Furthermore, analysis of 42 unresectable locally advanced PDAC patients treated with proton beam therapy in combination with chemotherapy with Gemcitabine a 2 year overall survival of 50.8% with a mean survival time of 25.6 months without severe toxicity events.44
Advances in MRI while delivering 3D radiation treatment for enhancing radiation planning allows for improved precision via better identification of the internal structures and their metabolism. The combined MR-Linac technology implies the presence of SMF during radiation, prompting the research question if this would affect the radiation efficacy and/or could be exploited to improve treatments. More specifically, some studies have been published related to the effect of SMF on radiation response.51–55 Although these studies are not conclusive, they suggest that SMF may affect biological endpoints of radiation particularly with regard to the altering of the cellular environment and repair processes involved with radiation response. 3D systems may further be affected by such mechanisms.
Overall, the above studies offer substantial improvements as compared to conventional radiotherapy outputs and data for PDAC; however, they are very limited in number and continue to be part of ongoing clinical trials. Furthermore, for faster delivery of novel therapies from bench to bedside, it is of high importance to have appropriate platforms for radiotherapy screening, that can capture the structural, biological and chemical complexity of the tissue microenvironment.
Current preclinical treatment screening tools for PDAC
The overall failure of PDAC treatments at a clinical level can be associated with misrepresentative pre-clinical testing systems. Traditional 2D cell culture is a fast low cost-conventional approach to study PDAC treatments in vitro. However, it fails to represent cell-cell, cell-matrix interactions, microarchitecture and environmental gradients of the in vivo TME that are imperative to treatment response.5,10–13 Alternatively, the traditional use of animals (Xenografts) in cancer research allows realistic in vivo architecture; however, foreign physiology, limited heterogeneity, and reduced tumour mutation rates impair relevance to humans.5,10–13 Thus, translational discrepancies emerge when applying relevance to pre-clinical treatment screening and resistance profiling. Tissue engineering is emerging to produce more clinically relevant biomimicry of complex cellular milieus responsible for treatment resistance.33,34,56,57 3D (re)-modeling of the TME has the potential to improve resistance profiling for cancers with high radio-resistance and poor patient survival, such as PDAC. Currently 3D (re)-modeling for PDAC for radiation treatment screening is an understudied area of research. The following sections describe different available pre-clinical models for PDAC radiotherapy screening.
2D cell culture
Traditionally PDAC radiotherapy research in 2D cell culture investigates radiosensitisers and chemo-radiotherapy screening. DNA damage and cytotoxic effects of treatments are easily quantified. More specifically, Tuli et al. (2014) report DNA damage and dose-dependent decreases in PDAC cell viability in radiation fractions of 0–10 Gy in 2D monolayers.58 Moreover, Weiss et al. (2003) report on the effect of irradiation alone compared to chemo-radiotherapy in BxPC-3 pancreatic cancer cells, demonstrating significant increases in DNA damage and cell death 24 h after combined treatment in 2D cell culture.59 Furthermore, Cordes et al. (2007) investigated the sensitisation of pancreatic cancer cells to radiotherapy.60 More specifically, the transmembrane protein Caveolin-1, which acts in cellular adhesion of integrins, cytoskeleton proteins and signaling molecules, was downregulated in pancreatic cell lines PATU8902, MiaPaCa2 and Panc-1 after 24 h of 0, two or 6 Gy radiation treatment.60 Thereafter, caveolin-1 expression knock down resulted in an increase of radiosensitisation of the pancreatic cancer cells in 2D culture.60 Similarly, Giagkousiklidis et al. (2007) utilised 2D cell culture to identify increased sensitivity to fractions of 10 Gy and 20 Gy, 96 h post-radiation, when X-linked inhibitor of apoptosis (XIAP) was inhibited.61 More recently, Moertl et al., (2019) verified that pancreatic cancer cell (SU.86.86, MiaPaCa2, T3M-4) sensitivity to radiotherapy increases in a 2D culture system when inhibiting histone deacetylase inhibitors (HDACi).62,63
Despite the simplicity in use and the cost-efficiency of 2D cell-culture systems, realistic tissue structure, cell-cell and cell-ECM interactions as well as integrin-mediated signaling and morphology-related cellular function that have been associated with radio-resistance in vitro cannot be recapitulated.34,64 Moreover, enriching this 2D platform with TME features such as the co-culture of ECM proteins and cancer cells provides variations in treatment resistance, as previously mentioned. More specifically, Cordes and Meineke (2003) demonstrate improved post-radiation cell survival in pancreas carcinoma as well as glioblastoma, lung carcinoma, melanoma human skin and lung fibroblasts and human keratinocytes grown in 2D with fibronectin, a phenomenon known as cell adhesion-mediated radio-resistance.64 Furthermore, co-culture with microenvironment components such as pancreatic stellate cells can promote radio-protection in 2D (Mantoni et al., 2011).65 Nonetheless, despite the addition of proteins and/or non-cancerous cells of the TME, 2D culture systems lack structure and robust spatial organisation, resulting in random cell or protein distribution and lack of a realistic in vivo architecture.
Xenografts
Traditional cancer research techniques for screening pre-clinical treatments also include the xenotransplantation of human cells into nude/immunosuppressed mouse models (xenografts). Unlike 2D cell-culture systems, radiotherapy studies for pancreatic cancer in xenografts are much more accurate as they allow for a more realistic recapitulation of the TME, including architecture, environmental gradients and cellular interactions as well as realistic time frames of treatment and post-treatment survival. Consequently, such studies result in more relevant clinical translation for radiation response studies. For example, Mantoni et al. (2011) utilised xenograft models to verify pancreatic stellate cell-induced radio-protection in vivo.32 Similarly, Al-Assar et al. (2014) describe co-injection of stellate cells (PSC) and PANC-1 to improve radio-resistance in xenografts as compared to 2D cell culture.66 Furthermore, Mukubou et al. (2010) investigate chemotherapy- and radiotherapy-induced autophagy in xengraft models, comparing 7 vs 40 days post treatment analysis, suggesting that autophagy suppresses pancreatic cancer.67 Tuli et al., (2014) developed a PDAC xenograft via an orthotopic implant that allowed 39 days post-treatment analysis, supporting a clinically relevant timeframe than 2D cell culture.58 More recently, murine models have been utilised as a platform to test tripartite treatments for PDAC, such as hyperthermia, radiation therapy and immunotherapy. The tripartite treatment regime lead to a decrease in tumour growth and to improved survival rates when compared to monotherapy.68
Despite the facilitation of realistic in vivo milieus, the xenotransplantation of human cells into mouse models has disadvantages. More specifically, foreign mouse physiology and size, variations in genetic sequence and alternative immune responses can impair clinical relevance to humans.5,10–13 Moreover, these models express limited heterogeneity and reduced tumour mutation rates,5 resulting in translational inaccuracies and high clinical trial failure rate.5 Additionally, animal studies are time-consuming and expensive, especially for radiotherapy research, there are a very limited number of radiation facilities supporting animal experimentation.12,51 Furthermore, animal research is met with ethical complications, the three R’s known as, refine, reduce and replace calling for reliable animal-free treatment screening alternatives.69
Tissue engineering: An emerging approach for in vitro PDAC radiotherapy screening
As previously mentioned, the TME acts as a complex ecosystem, hosting an abundance of different cells and cellular dynamics. Considering the role that the TME plays in the evolution of PDAC and its’ response to treatment, it is of vital importance to be able to perform treatment screening studies in platforms that can mimic as accurately as possible of the TME. Such platform would improve our understanding of this complex ecosystem along with our ability to create relevant treatment options. Tissue engineering is emerging as an alternative preclinical approach, supporting more advanced TME biomimicry. More specifically, tissue engineering allows for better spatial and structural organisation, 3D configuration of ECM components and co-culture of different cells of the TME, therefore offering more realistic cellular spatial orientations and interactions, better architecture and the formation of physiological environmental gradients.10,11,70–74 PDAC tumour development and metastasis is largely shaped by cell-ECM cross-talk.75 Moreover, enriching a 3D environment with cells of the PDAC TME that are associated with disease progression and treatment resistance, such as stellate cells and immune cells, to remodel the ECM, facilitates more realistic treatment platforms. The possibility of tailoring the biochemical and biomechanical features of 3D tissue engineering constructs enables the incorporation of different cell types of the TME.76 Currently, there are a limited number of 3D tissue engineering models to support radiation treatment and resistance profiling for PDAC, herein, we analyse those 3D models.
Spheroids
Spheroids are simple 3D cell aggregates in suspension. Some spheroids contain a cocktail of natural peptides such as collagen and/or matrigel to support better cell-cell interaction and adhesion.10,77–79 Spheroid systems enable the development of 3D histological and physiological tumour features including spatial organisation and genetic expression.10,77–81 Moreover, spheroid models are able to lead to the formation of hypoxic regions, pH and metabolic gradient unlike 2D cell-culture systems.,10,77–81 As a result, this model offers a more realistic platform to study tumour cell treatment responses as compared to 2D cell-culture systems. More specifically, 3D spheroid modeling results in higher treatment resistance in both chemotherapeutics and radiotherapy, when directly comparing to simplistic monolayer 2D cell cultures.
An overview of spheroid radiation research for PDAC is summarized in Table 1. More specifically, Wen et al., (2013) reported that the pancreatic cancer cells lines MIAPaCa-2 and PANC-1, revealed higher levels of drug resistance to gemcitabine and 5-fluorouracil in spheroid models when directly compared to a 2D cell-culture system.78 Furthermore, Longati et al. (2013) identified changes in metabolic activity, ECM protein production and chemotherapy and radiotherapy resistance in spheroid models of PANC-1 as compared to 2D cell-culture systems.82 More specifically, spheroid analysis revealed increased cell-cell interaction, aggregation, lactate accumulation, HIF-1α protein stability, collagen and fibronectin expression and resistance specific gene up-regulation, suggesting that this matrix rich culture method is more advantageous than 2D cell-culture systems for biomimetic treatment screening.82 Al-Ramadan et al. (2017) showed a radiation dose dependent sensitivity, that is, for a dose range of 0–6 Gy, of the pancreatic neuroendocrine cell line BON-1 in spheroids seven days post-treatment detected via apoptosis induction.83
Table 1.
Spheroid and Scaffold models for radiation response studies
| Platform | Author | Date | Cancer Type/ Cell Line | Key Findings |
|---|---|---|---|---|
| Spheroid Model | Hehlgans et al57 | 2009 | Pancreatic Cancer: MiaPacCa2 | Identified increased sensitivity to radiation with membrane protein (Caveolin-1) knock down in MiaPacCa2. |
| Spheroid Model | Hehlgans et al84 | 2009 | Pancreatic Cancer: MiaPaCa-1 PANC-1 Head and Neck: HNSCC Lung Cancer Cells: A549 Colorectal Cancer: DLD-1, HCT-116 |
Radio-sensitivity of pancreatic cancer cell lines human head and neck, lung, and colorectal cell lines by the focal adhesion kinase inhibitor (TAE226) |
| Spheroid Model | Longati et al82 | 2013 | Pancreatic cancer PDAC | Identified chemo-radio resistance in PDAC spheroids when compared to 2D cell-culture systems |
| Co-culture Spheres & Xenografts |
Al-Assar et al66 | 2014 | Pancreatic Cancer: PANC-1 |
Co-culture of stellate cells (PSC) and PANC-1 were more radio-resistant in spheres and mouse models. |
| Spheroid Model | Al-Ramadan et al83 | 2018 | Pancreatic Cancer: BON-1 | 7-day post-radiation treatment revealed dose-dependent increase in apoptosis in BON-1 |
| Polyurethane Scaffold | Gupta et al12 | 2019 | Pancreatic Cancer: PANC-1 | PU scaffolds can be utilised as a radiation response platform. Variations in post-treatment response after short-term (24 h) and long-term (17 days) PANC-1 culture. |
Spheroid models are also utilised to identify potential radio-sensitizers for PDAC. More specifically, Hehlgans et al. (2009) identified Cav-1 (Caveolin-1: a membrane protein) as a potential radio-sensitising target in the pancreatic cancer cell line MiaPaCa2, identifying increased radio-sensitivity in knock-down spheroid models.57 Furthermore, this research group investigated the radio-sensitisation of pancreatic cancer, head and neck, lung, and colorectal cancer cell spheroids when exposed to the focal adhesion kinase inhibitor (TAE226) (Hehlgans et al., 2009).84
Recent spheroid developments include co-culture of multiple cells of the TME such as cancer cells, fibroblasts and endothelial cells, offering a biologically improved 3D environment that more readily replicates the desmoplastic reaction of the PDAC TME.85 More specifically, Lazzari et al., (2018) developed a multicellular spheroid facilitating the growth of pancreatic cancer cells (PANC-1), fibroblasts (MRC-5) and endothelial cells (HUVEC) showing increased resistance to chemotherapy (gemcitabine and doxorubicin) as compared to mono-type PANC-1 spheroids. Moreover, Al-Assar et al. (2014) developed a co-culture of stellate cells (hPSC) with pancreatic cancer cell lines (PANC-1, PSN-1 and MiaPaCa-2) in spheroid configuration enriched with matrigel for a period of 10 days. An increase in (i) epithelial to mesenchymal transition (EMT), (ii) stem cell phenotype, that is, enhanced stem-cell marker expression, (iii) resistance to radiotherapy treatment (enhanced clonogenic survival) was observed in spheroid co-cultures as compared to mono-cultures.66
However, despite the fact that spheroid systems offer more realistic cellular interactions and the ability to spatially add multicellular components as compared to 2D culture systems, they lack robust porosity and mechanical stability consequently limiting the accuracy of the TME biomechanical and structural re-modeling.11,12,72,74 More specifically, these platforms are unable to sustain long-term cell-culture studies due to necrotic core formation, which is a result of the lack of porosity and structure. Consequently, it is challenging to perform long-term radiotherapy studies including fractionated treatment and to study long-term post-treatment effects in such 3D configurations.12
Hydrogels
Hydrogels are emerging as platforms to allow 3D mimetic microstructure and mechanical properties for PDAC research. Hydrogels are cross-linked polymeric networks, which retain very high levels of water and are able to support 3D growth and more realistic tumour properties in terms of porosity, structure, ECM composition and spatial nutrient and oxygen diffusion gradient mimicry.10,11,74,77,86 Consequently, they are valuable models for cancer research in vitro. More specifically, Ki et al. (2014) developed a photo-curable and bio-orthogonal thiol-ene hydrogel (fabricated from a multi-arm poly(ethylene glycol)-norbornene cross-linked with protease-sensitive peptide) to allow matrix modeling, for the pancreatic cell line COLO-357. An increase in cell growth, cell invasion and chemotherapy resistance was observed in the hydrogels, as compared to a simple 2D culture system.86 Furthermore, Chiellini et al. (2016) developed a hydrogel of the pancreatic cancer cell line BxPC-3. The hydrogel was made of either chitosan (mSC) or polyelectrolyte complex (mPEC) cross-linked with CS and poly(g-glutamic acid) (g-PGA).77 Pancreatic cancer cells remained live and proliferating, forming dense cell aggregates for 28 days in this hydrogel system.77 Despite these, substantial advantages, challenges of this model include the lack of uniform cellular distribution and difficulty in handling.10–12,72 To the best of our knowledge, there are currently no hydrogel structures reporting radiotherapy treatment screening for PDAC found in the literature.
Polymeric scaffolds
Polymeric scaffolds are mechanically robust, porous and interconnected structures made mainly from synthetic biocompatible polymers. Examples of synthetic polymers include polyurethane (PU), polylactide, polyglycolide and co-polymers.10–13,72,74 Such scaffolds can lead to the development of a variety of different porous of fibrous micro- and macro-structures, offering a good and variable mimicry of real tissue architecture. Furthermore, such robust and tunable internal structural configurations facilitate good control of cell and ECM spatial distribution and the formation of realistic environmental gradients. Moreover, these models are low cost and reproducible in comparison animal models.10–13,72,74 As a result, scaffold models are evolving as pre-clinical testing platforms. For example, Ricci et al. (2014) report on the spatial arrangement of primary PDAC cells in three biocompatible polymeric scaffolds, i.e. poly(vinyl alcohol)/gelatin, poly(ethylene oxide terephthalate)/poly(butylene terephthalate (PEOT/PBT) sponge and PEOT/PBT mesh.72 More specifically, all three structures supported PDAC cell viability for 9 days and expressed tumour-specific markers. Moreover, this research showed pore size, topography and polymer chemistry affected PDAC spatial organisation.72 Furthermore, Totti et al., (2018) established PU scaffolds to support and sustain long-term growth of dense PDAC cell masses, that is, 4 weeks, where cancer cells produced substantial amount of ECM (collagen-1) and formed realistic environmental gradients, that is, hypoxic regions.11 The work of Gupta et al. (2019) is the first to report PDAC radiotherapy treatment screening and long-term post-radiation treatment analysis in a 3D scaffold system.12 More specifically, chemotherapy (with Gemcitabine) (10, 50 and 100 µM), radiotherapy (250 kV X-ray) (2, 6 and 8 Gy) and their combination (10 µM Gemcitabine for 48 h followed by 6 Gy radiation), was performed in a 4-week-old PU scaffold-assisted PDAC model. Thereafter, post-treatment monitoring of the cancer cell viability and apoptosis was monitored for up to 17-day pos- treatment. To the best of our knowledge, this is the longest culture of PDAC cells in a 3D scaffold and the first post-radiation analysis.12 The time frame presented is aligned with the time frame used in radiotherapy treatments in animal models and clinical trials, providing an animal free, promising alternative to pre-clinical radiotherapy treatment screening.12 The advantages and disadvantages of polymeric scaffolds for PDAC radiation response studies are discussed in Figure 3. An overview of scaffold radiation research for PDAC is summarised in Table 1.
Figure 3.
Advantages and disadvantages of spheroids and polymeric scaffolds as radiation research models for PDAC.
The flexibility and versatility of the synthetic scaffold platforms enable the further addition of biological complexity via co-culturing multiple cells of the TME. More specifically, Gupta et al. (2020) report for the first time a hybrid, multicellular (tri-culture) PDAC scaffold, incorporating cancer cells (PANC-1 cell line), endothelial cells (HMEC cells) and pancreatic stellate cells PS-1 cells).13 Protein coating of the scaffold was bespoke to maximise growth of different cellular compartments of the TME, that is, a fibronectin rich PU centre seeded with cancer cells surrounded by a collagen rich PU area seeded with endothelial cells and stellate cells. Such a bi-structure facilitated a realistic zonal distribution of the PDAC TME, which enabled the realistic long-term mimicry of fibrosis/desmoplsaia and, therefore, shows promise as a robust model for future treatment screening.13
Further to PDAC, there is some limited research on radiotherapy screening on polymeric scaffolds for other cancer types. For example, Gomez-Roman et al. (2016) demonstrate a 3D-mediated radio-resistance in patient derived glioblastoma cells (E2, R10 and G7) within a polystyrene scaffold as compared to a 2D culture system as evaluated via cell extraction from scaffolds and conduction of subsequent clonogenic survival assays at 21 and 0.5% oxygen.87 Hamdi et al. (2015) developed a 3D collagen sponge scaffold for hadron therapy for the chondrosarcoma cell line SW1353.88 More specifically, hadron-therapy (50 MeV/a O ions) (2 Gy) was compared to conventional radiotherapy (225 kV X-ray) (2 Gy). Thereafter, cell viability, proliferation and DNA damage were assessed via clonogenic assay, Ki67 and gamma-H2AX presence revealed lower proliferation profiles and higher DNA damage after hadron therapy as compared to X-rays.88
Re-evaluating approaches for radiotherapy-induced quantification of cell death in 3d cancer models
As described in the previous sections, 3D models are advancing to support in vitro cancer research including pre-clinical radiotherapy screening. Despite the many advantages of 3D models for radiation response studies, the ability to extract the cells for post-treatment analysis is challenging and can be described as a drawback of this technique to achieve accurate cell death quantification.10–12,89,90
Traditionally, the radiobiological definition of cell death is determined by the cells loss of reproductive integrity.91–93 Thus, a cell is regarded as being killed by radiation not by the cellular ability to physically survive in the population but by its reproductive integrity.91,92 As a result, the clonogenic assay is a valued and reliable method used to quantify cell death after radiation treatment.92 This assay measures the ability of cells to produce colonies; this method is simple, cost-effective and very well known as a gold standard approach for radiotherapy evaluation for several decades.91,92 However, there is no established methodology for estimating the fraction of cells killed by radiation in 3D systems. More specifically, there is no simple strategy for collecting the cells from various scaffolds to allow the performance of the traditionally used clonogenic assays.
Analysis of cells in situ in 3D models via image quantification is, therefore, a promising approach. Such imaging analysis includes appropriate staining and fluorescence imaging via confocal microscopy (CLSM) and/or observations of structural damages at cell level with scanning electron microscopy (SEM), with the latter being more qualitative rather than quantitative. Hamdi et al. (2015) describe adaptive experimental strategies to quantify the cellular treatment effect, as the inability to extract chondrosarcoma cells from 3D scaffolds via trypsinisation subsequently hindered the possibility to perform clonogenic assays. More specifically, radiation toxicity in their 3D scaffolds was monitored with (i) a viable cell fraction determination, that is, the in situ cytotoxic assay Toxilight, (ii) a proliferation index, that is, via scaffold sectioning for mapping the Ki-67 proliferation marker secretion, (iii) a comparison of protein secretion from treated and untreated samples.88 Similarly, Gupta et al. (2019) performed in situ analysis of the cell survival in the scaffolds post-radiotherapy. More specifically in situ viability was monitored with (i) the cell viability assay Alamar Blue, (ii) sectioning staining and imaging multiple scaffold sections. Image analysis of the live/dead cell ratios and distribution in each image enabled the provision of quantitative results on post-radiotherapy cell viability and proliferation in 3D.12 In contrast, Gomez-Roman et al. (2016) successfully extracted glioblastoma cells (E2 (R10 and G7) from polystyrene scaffolds via trypsinisation, allowing for the identification of radio-resistance in 3D models via the traditional clonogenic survival assay.87
From the above limited studies in 3D, it is evident that cellular extraction and, therefore, quantification of radiotherapy induced cell death is scaffold dependent. The structural complexity of scaffolds can contribute to such variations. Therefore, with the very recent advancements in radiotherapy screening in biochemically and structurally complex 3D models, there is a need for the re-evaluation of traditional approaches along with the development of alternative and novel protocols for post-radiotherapy quantitative cell death evaluation. Overall, 3D models are able to act as building blocks to add features of the unique and complex TME, proving a more realistic approach to mapping treatment profiling in vitro. Studies are emerging to harness this technology, however clear and universal quantification methods are required.
Concluding Remarks
Preclinical research and clinical data indicate that current radiotherapy regimes are not efficient to treat pancreatic cancer. At the same time, novel radiotherapy approaches such as MRI-linacs or proton therapy are being developed and could be promising alternative approaches for PDAC treatment. To better screen current and novel radiotherapy treatment for PDAC appropriate, simple, low-cost yet accurate pre-clinical models are needed and have started to emerge. Such models allow the evaluation of different biological endpoints, the addition of radio-resistant parameters, and can facilitate long-term post-radiation analysis for more realistic treatment response studies. At the same time, there are challenges associated with characterisation and quantification of post-radiotherapy effects in such 3D models. Overall, this review paper highlights the need for further research on the use of 3D models for pre-clinical radiotherapy screening including (i) 3D (re)-modeling of the PDAC hypoxic TME to allow for late effects of ionising radiation (ii) screening of novel radiotherapy approaches and their combinations as well as (iii) a universally accepted 3D model image quantification method for evaluating TME components in situ. This review aims to act as a reference point for ongoing tissue engineering research into the development of 3D models for advancing radiotherapy for pancreatic cancer.
Footnotes
Acknowledgment: This work was supported by the Doctoral College of the University of Surrey, the National Physical Laboratory and the Department of Chemical & Process Engineering at the University of Surrey. P.G. and E.V. received funding from the Commonwealth Rutherford Fund (Post-Doctoral Fellowship to P.G.) and the 3Dbionet network. E.V. is grateful to the Royal Academy of Engineering for an Industrial Fellowship.
Contributor Information
Gabrielle Wishart, Email: g.wishart@surrey.ac.uk.
Priyanka Gupta, Email: priyanka.gupta@surrey.ac.uk.
Giuseppe Schettino, Email: giuseppe.schettino@surrey.ac.uk.
Andrew Nisbet, Email: andrew.nisbet@ucl.ac.uk.
Eirini Velliou, Email: e.velliou@ucl.ac.uk.
REFERENCES
- 1.American Cancer Society Cancer facts and figures 2019. 2019;.
- 2.O’Reilly D, et al. Diagnosis and management of pancreatic cancer in adults: a summary of guidelines from the UK National Institute for health and care excellence. Pancreatology 2018;: 1–9.29389525 [Google Scholar]
- 3.Cascinu S, Falconi M, Valentini V, Jelic S. Pancreatic cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 21, 2010;: v55–8. [DOI] [PubMed] [Google Scholar]
- 4.Sarnecka AK, Zagozda M, Durlik M. An overview of genetic changes and risk of pancreatic ductal adenocarcinoma. J Cancer 2016; 7: 2045–51. doi: 10.7150/jca.15323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bailey KL, Carlson MA. Porcine models of pancreatic cancer. Front. Oncol 2019; 9: 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rawla P, Sunkara T, Gaduputi V. Epidemiology of pancreatic cancer: global trends, etiology and risk factors. World J Oncol 2019; 10: 10–27. doi: 10.14740/wjon1166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pickup MW, Mouw JK, Weaver VM. The extracellular matrix modulates the hallmarks of cancer. EMBO Rep 2014; 15: 1243–53. doi: 10.15252/embr.201439246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xie D, Xie K. Pancreatic cancer stromal biology and therapy. Genes Dis 2015; 2: 133–43. doi: 10.1016/j.gendis.2015.01.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Melstrom LG, Salazar MD, Diamond DJ. The pancreatic cancer microenvironment: a true double agent. J Surg Oncol 2017; 116: 7–15. doi: 10.1002/jso.24643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Totti S, Vernardis SI, Meira L, Pérez-Mancera PA, Costello E, Greenhalf W, et al. Designing a bio-inspired biomimetic in vitro system for the optimization of ex vivo studies of pancreatic cancer. Drug Discov Today 2017; 22: 690–701. doi: 10.1016/j.drudis.2017.01.012 [DOI] [PubMed] [Google Scholar]
- 11.Totti S, Allenby MC, Dos Santos SB, Mantalaris A. Velliou, E. G. A 3D bioinspired highly porous polymeric scaffolding system for in vitro simulation of pancreatic ductal adenocarcinoma. RSC Adv 2018; 8: 20928–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gupta P, et al. Chemoradiotherapy screening in a novel biomimetic polymer based pancreatic cancer model. RSC Adv 2019; 9: 41649–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gupta P, et al. A novel Scaffold-Based hybrid multicellular model for pancreatic ductal Adenocarcinoma—Toward a better mimicry of the in vivo tumor microenvironment. Front. Bioeng. Biotechnol 2020; 8: 290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Adriana Blakaj1, Stacey M. Stein2, Sajid A. Khan3, K. L. J., Stein, S. M., Khan, S. A. & Johung, K. L. Review and current state of radiation therapy for locally advanced pancreatic adenocarcinoma. J. Gastrointest. Oncol 2018; 9: 1027–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sarantis P, Koustas E, Papadimitropoulou A, Papavassiliou AG, Karamouzis MV. Pancreatic ductal adenocarcinoma: treatment hurdles, tumor microenvironment and immunotherapy. World J Gastrointest Oncol 2020; , 12: 173-18112. doi: 10.4251/wjgo.v12.i2.173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kamisawa T, Wood LD, Itoi T, Takaori K. Pancreatic cancer. Lancet 2016; 388: 73–85. doi: 10.1016/S0140-6736(16)00141-0 [DOI] [PubMed] [Google Scholar]
- 17.Neoptolemos JP, Stocken DD, Friess H, Bassi C, Dunn JA, Hickey H, et al. A randomized trial of chemoradiotherapy and chemotherapy after resection of pancreatic cancer. N Engl J Med 2004; 350: 1200–10. doi: 10.1056/NEJMoa032295 [DOI] [PubMed] [Google Scholar]
- 18.Von Hoff DD, Ervin T, Arena FP, Chiorean EG, Infante J, Moore M, et al. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med 2013; 369: 1691–703. doi: 10.1056/NEJMoa1304369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Neoptolemos JP, Palmer DH, Ghaneh P, Psarelli EE, Valle JW, Halloran CM, et al. Comparison of adjuvant gemcitabine and capecitabine with gemcitabine monotherapy in patients with resected pancreatic cancer (ESPAC-4): a multicentre, open-label, randomised, phase 3 trial. Lancet 2017; 389: 1011–24. doi: 10.1016/S0140-6736(16)32409-6 [DOI] [PubMed] [Google Scholar]
- 20.Conroy T, Desseigne F, Ychou M, Bouché O, Guimbaud R, Bécouarn Y, et al. Folfirinox versus gemcitabine for metastatic pancreatic cancer. N Engl J Med 2011; 364: 1817–25. doi: 10.1056/NEJMoa1011923 [DOI] [PubMed] [Google Scholar]
- 21.Moore MJ, et al. Erlotinib plus gemcitabine compared with gemcitabine alone in patients with advanced pancreatic cancer: a phase III trial of the National cancer Institute of Canada clinical Trials Group. 2007;. [DOI] [PubMed]
- 22.Katz MHG, Shi Q, Ahmad SA, Herman JM, Marsh RdeW, Collisson E, et al. Preoperative modified Folfirinox treatment followed by capecitabine-based chemoradiation for borderline resectable pancreatic cancer: alliance for clinical trials in oncology trial A021101. JAMA Surg 2016; 151: e161137. doi: 10.1001/jamasurg.2016.1137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Murphy JE, Wo JY, Ryan DP, Jiang W, Yeap BY, Drapek LC, et al. Total neoadjuvant therapy with Folfirinox followed by individualized chemoradiotherapy for borderline resectable pancreatic adenocarcinoma: a phase 2 clinical trial. JAMA Oncol 2018; 4: 963. doi: 10.1001/jamaoncol.2018.0329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Murphy JE, Wo JY, Ryan DP, Clark JW, Jiang W, Yeap BY, et al. Total neoadjuvant therapy with Folfirinox in combination with losartan followed by chemoradiotherapy for locally advanced pancreatic cancer: a phase 2 clinical trial. JAMA Oncol 2019; 5: 1020–7. doi: 10.1001/jamaoncol.2019.0892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wolfe AR, Prabhakar D, Yildiz VO, Cloyd JM, Dillhoff M, Abushahin L, et al. Neoadjuvant-modified Folfirinox vs nab-paclitaxel plus gemcitabine for borderline resectable or locally advanced pancreatic cancer patients who achieved surgical resection. Cancer Med 2020; 9: 4711–23. doi: 10.1002/cam4.3075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Balaban EP, Mangu PB, Khorana AA, Shah MA, Mukherjee S, Crane CH, et al. Locally advanced, unresectable pancreatic cancer: American Society of clinical oncology clinical practice guideline. J Clin Oncol 2016; 34: 2654–68. doi: 10.1200/JCO.2016.67.5561 [DOI] [PubMed] [Google Scholar]
- 27.Kalser MH, Ellenberg SS. Pancreatic cancer. adjuvant combined radiation and chemotherapy following curative resection. Arch Surg 1985; 120: 899–903. doi: 10.1001/archsurg.1985.01390320023003 [DOI] [PubMed] [Google Scholar]
- 28.Hammel P, Huguet F, van Laethem J-L, Goldstein D, Glimelius B, Artru P, et al. Effect of chemoradiotherapy vs chemotherapy on survival in patients with locally advanced pancreatic cancer controlled after 4 months of gemcitabine with or without erlotinib: the LAP07 randomized clinical trial. JAMA 2016; 315: 1844. doi: 10.1001/jama.2016.4324 [DOI] [PubMed] [Google Scholar]
- 29.Morganti AG, Falconi M, van Stiphout RGPM, Mattiucci G-C, Alfieri S, Calvo FA, et al. Multi-Institutional pooled analysis on adjuvant chemoradiation in pancreatic cancer. Int J Radiat Oncol Biol Phys 2014; 90: 911–7. doi: 10.1016/j.ijrobp.2014.07.024 [DOI] [PubMed] [Google Scholar]
- 30.Yoshimura M, Itasaka S, Harada H, Hiraoka M. Microenvironment and radiation therapy. Biomed Res Int 2013; 2013: 68530813 (2013).. doi: 10.1155/2013/685308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Menon H, Ramapriyan R, Cushman TR, Verma V, Kim HH, Schoenhals JE, et al. Role of radiation therapy in modulation of the tumor stroma and microenvironment. Front Immunol 2019; 10: 193. doi: 10.3389/fimmu.2019.00193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mantoni TS, Lunardi S, Al-Assar O, Masamune A, Brunner TB. Pancreatic stellate cells Radioprotect pancreatic cancer cells through 1-integrin signaling. Cancer Res 2011; 71: 3453–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hehlgans S, Eke I, Deuse Y, Cordes N. Integrin-Linked kinase: dispensable for radiation survival of three-dimensionally cultured fibroblasts. Radiother Oncol 2008; 86: 329–35. doi: 10.1016/j.radonc.2007.09.007 [DOI] [PubMed] [Google Scholar]
- 34.Eke I, Cordes N. Radiobiology goes 3D: how ECM and cell morphology impact on cell survival after irradiation. Radiother Oncol 2011; 99: 271–8. doi: 10.1016/j.radonc.2011.06.007 [DOI] [PubMed] [Google Scholar]
- 35.Gray LH, Conger AD, Ebert M, Hornsey S, Scott OC. The concentration of oxygen dissolved in tissues at the time of irradiation as a factor in radiotherapy. Br J Radiol 1953; 26: 638–48. doi: 10.1259/0007-1285-26-312-638 [DOI] [PubMed] [Google Scholar]
- 36.Grimes DR, Partridge M. A mechanistic investigation of the oxygen fixation hypothesis and oxygen enhancement ratio. Biomed Phys Eng Express 2015; 1: 045209. doi: 10.1088/2057-1976/1/4/045209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Muz B, de la Puente P, Azab F, Azab AK. The role of hypoxia in cancer progression, angiogenesis, metastasis, and resistance to therapy. Hypoxia 2015; 3: 83–92. doi: 10.2147/HP.S93413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Spiegelberg L, Houben R, Niemans R, de Ruysscher D, Yaromina A, Theys J, et al. Hypoxia-Activated prodrugs and (lack of) clinical progress: the need for hypoxia-based biomarker patient selection in phase III clinical trials. Clin Transl Radiat Oncol 2019; 15: 62-69. doi: 10.1016/j.ctro.2019.01.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhou M, Xie Y, Xu S, Xin J, Wang J, Han T, et al. Hypoxia-Activated nanomedicines for effective cancer therapy. Eur J Med Chem 2020; 195: 112274. doi: 10.1016/j.ejmech.2020.112274 [DOI] [PubMed] [Google Scholar]
- 40.Xie C, Duffy AG, Brar G, Fioravanti S, Mabry-Hrones D, Walker M, et al. Immune checkpoint blockade in combination with stereotactic body radiotherapy in patients with metastatic pancreatic ductal adenocarcinoma. Clin Cancer Res 2020; 26: 2318–26. doi: 10.1158/1078-0432.CCR-19-3624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Prasad S, Cambridge L, Huguet F, Chou JF, Zhang Z, Wu AJ, et al. Intensity modulated radiation therapy reduces gastrointestinal toxicity in locally advanced pancreas cancer. Pract Radiat Oncol 2016; 6: 78–85. doi: 10.1016/j.prro.2015.09.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chuong MD, Springett GM, Freilich JM, Park CK, Weber JM, Mellon EA, et al. Stereotactic body radiation therapy for locally advanced and borderline resectable pancreatic cancer is effective and well tolerated. Int J Radiat Oncol Biol Phys 2013; 86: 516–22. doi: 10.1016/j.ijrobp.2013.02.022 [DOI] [PubMed] [Google Scholar]
- 43.Sachsman S, et al. Proton therapy and concomitant capecitabine for non-metastatic unresectable pancreatic adenocarcinoma. Int. J. Part. Ther 2014; 1: 692–701. [Google Scholar]
- 44.Hiroshima Y, Fukumitsu N, Saito T, Numajiri H, Murofushi KN, Ohnishi K, et al. Concurrent chemoradiotherapy using proton beams for unresectable locally advanced pancreatic cancer. Radiother Oncol 2019; 136: 37–43. doi: 10.1016/j.radonc.2019.03.012 [DOI] [PubMed] [Google Scholar]
- 45.McGrath A, et al. Radiotherapy in locally advanced pancreatic cancer. Cancer Forum 2016; 40: 46–53. [Google Scholar]
- 46.Hazard L, Hazard L. The role of radiation therapy in pancreas cancer. Gastrointest Cancer Res 2009; 3: 20–8. [PMC free article] [PubMed] [Google Scholar]
- 47.Petrelli F, Comito T, Ghidini A, Torri V, Scorsetti M, Barni S. Stereotactic body radiation therapy for locally advanced pancreatic cancer: a systematic review and pooled analysis of 19 trials. Int J Radiat Oncol Biol Phys 2017; 97: 313–22. doi: 10.1016/j.ijrobp.2016.10.030 [DOI] [PubMed] [Google Scholar]
- 48.Bussiere R. M. & Adams, A, J. Treatment Planning for Conformal Proton Radiation Therapy. Technol. Cancer Res. Treat 2003; 2. [DOI] [PubMed] [Google Scholar]
- 49.Badiyan SN, Hallemeier CL, Lin SH, Hall MD, Chuong MD. Proton beam therapy for gastrointestinal cancers: past, present, and future. J Gastrointest Oncol 2018; 9: 962–71. doi: 10.21037/jgo.2017.11.07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dell'Oro M, Short M, Wilson P, Bezak E. Clinical limitations of photon, proton and carbon ion therapy for pancreatic cancer. Cancers 2020; 12: E16309 01 2020. doi: 10.3390/cancers12010163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mohajer JK, Nisbet A, Velliou E, Ajaz M, Schettino G. Biological effects of static magnetic field exposure in the context of MR-guided radiotherapy. Br J Radiol 2019; 92: 20180484. doi: 10.1259/bjr.20180484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rubinstein AE, Gay S, Peterson CB, Kingsley CV, Tailor RC, Pollard-Larkin JM, et al. Radiation-Induced lung toxicity in mice irradiated in a strong magnetic field. PLoS One 2018; 13: e0205803. doi: 10.1371/journal.pone.0205803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wordsworth OJ. Comparative long-term effects of liver damage in the rat after (a) localized x-irradiation and (B) localized x-irradiation in the presence of a strong homogeneous magnetic field. Radiat Res 1974; 57: 442. [PubMed] [Google Scholar]
- 54.Feng J, Sheng H, Zhu C, Jiang H, Ma S. Effect of adjuvant magnetic fields in radiotherapy on non-small-cell lung cancer cells in vitro. Biomed Res Int (2013; 2013: 6572592013, 657259. doi: 10.1155/2013/657259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wang L, Hoogcarspel SJ, Wen Z, van Vulpen M, Molkentine DP, Kok J, et al. Biological responses of human solid tumor cells to X-ray irradiation within a 1.5-Tesla magnetic field generated by a magnetic resonance imaging-linear accelerator. Bioelectromagnetics 2016; 37: 471–80. doi: 10.1002/bem.21991 [DOI] [PubMed] [Google Scholar]
- 56.Xue G, Ren Z, Grabham PW, Chen Y, Zhu J, Du Y, et al. Reprogramming mediated radio-resistance of 3D-grown cancer cells. J Radiat Res 2015; 56: 656–62. doi: 10.1093/jrr/rrv018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hehlgans S, Eke I, Storch K, Haase M, Baretton GB, Cordes N. Caveolin-1 mediated radioresistance of 3D grown pancreatic cancer cells. Radiother Oncol 2009; 92: 362–70. doi: 10.1016/j.radonc.2009.07.004 [DOI] [PubMed] [Google Scholar]
- 58.Tuli R, Surmak AJ, Reyes J, Armour M, Hacker-Prietz A, Wong J, et al. Radiosensitization of pancreatic cancer cells in vitro and in vivo through poly (ADP-ribose) polymerase inhibition with ABT-888. Transl Oncol 2014; 713 05 2014. doi: 10.1016/j.tranon.2014.04.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Weiss C, Grabenbauer GG, Sauer R, Distel L. Significant increase in residual DNA damage as a possible mechanism of radiosensitization by gemcitabine. Strahlenther Onkol 2003; 179: 93–8. doi: 10.1007/s00066-003-1046-8 [DOI] [PubMed] [Google Scholar]
- 60.Cordes N, Frick S, Brunner TB, Pilarsky C, Grützmann R, Sipos B, et al. Human pancreatic tumor cells are sensitized to ionizing radiation by knockdown of caveolin-1. Oncogene 2007; 26: 6851–62. doi: 10.1038/sj.onc.1210498 [DOI] [PubMed] [Google Scholar]
- 61.Giagkousiklidis S, Vellanki SH, Debatin K-M, Fulda S. Sensitization of pancreatic carcinoma cells for gamma-irradiation-induced apoptosis by XIAP inhibition. Oncogene 2007; 26: 7006–16. doi: 10.1038/sj.onc.1210502 [DOI] [PubMed] [Google Scholar]
- 62.Moertl S, Payer S, Kell R, Winkler K, Anastasov N, Atkinson MJ. Comparison of radiosensitization by HDAC inhibitors CUDc-101 and SAHA in pancreatic cancer cells. Int J Mol Sci 2019; 20: 3259. doi: 10.3390/ijms20133259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wu Z, Jing S, Li Y, Gao Y, Yu S, Li Z, et al. The effects of SAHA on radiosensitivity in pancreatic cancer cells by inducing apoptosis and targeting Rad51. Biomed Pharmacother 2017; 89: 705–10. doi: 10.1016/j.biopha.2017.02.067 [DOI] [PubMed] [Google Scholar]
- 64.Cordes N, Meineke V. Cell adhesion-mediated radioresistance (CAM-RR). extracellular matrix-dependent improvement of cell survival in human tumor and normal cells in vitro. Strahlenther Onkol 2003; 179: 337–44. doi: 10.1007/s00066-003-1074-4 [DOI] [PubMed] [Google Scholar]
- 65.Mantoni TS, Lunardi S, Al-Assar O, Masamune A, Brunner TB, Thomas B. Pancreatic stellate cells radioprotect pancreatic cancer cells through β1-integrin signaling. Cancer Res 2011; 71: 3453–8. doi: 10.1158/0008-5472.CAN-10-1633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Al-Assar O, Demiciorglu F, Lunardi S, Gaspar-Carvalho MM, McKenna WG, Muschel RM, et al. Contextual regulation of pancreatic cancer stem cell phenotype and radioresistance by pancreatic stellate cells. Radiother Oncol 2014; 111: 243–51. doi: 10.1016/j.radonc.2014.03.014 [DOI] [PubMed] [Google Scholar]
- 67.Mukubou H, Tsujimura T, Sasaki R, Ku Y. The role of autophagy in the treatment of pancreatic cancer with gemcitabine and ionizing radiation. Int J Oncol 2010; 37: 821–8. doi: 10.3892/ijo_00000732 [DOI] [PubMed] [Google Scholar]
- 68.Mahmood J, Alexander AA, Samanta S, Kamlapurkar S, Singh P, Saeed A, et al. A combination of radiotherapy, hyperthermia, and immunotherapy inhibits pancreatic tumor growth and prolongs the survival of mice. Cancers 2020; 12: 1015. doi: 10.3390/cancers12041015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Tannenbaum J, Bennett BT. Russell and Burch's 3Rs then and now: the need for clarity in definition and purpose. J Am Assoc Lab Anim Sci 2015; 54: 120–32. [PMC free article] [PubMed] [Google Scholar]
- 70.Blanco TM, Mantalaris A, Bismarck A, Panoskaltsis N. The development of a three-dimensional scaffold for ex vivo biomimicry of human acute myeloid leukaemia. Biomaterials 2010; 31: 2243–51. doi: 10.1016/j.biomaterials.2009.11.094 [DOI] [PubMed] [Google Scholar]
- 71.Nyga A, Cheema U, Loizidou M. 3D tumour models: novel in vitro approaches to cancer studies. J Cell Commun Signal 2011; 5: 239–48. doi: 10.1007/s12079-011-0132-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ricci C, Mota C, Moscato S, D'Alessandro D, Ugel S, Sartoris S, et al. Interfacing polymeric scaffolds with primary pancreatic ductal adenocarcinoma cells to develop 3D cancer models. Biomatter 2014; 4: e955386. doi: 10.4161/21592527.2014.955386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Velliou EG, Dos Santos SB, Papathanasiou MM, Fuentes-Gari M, Misener R, Panoskaltsis N, et al. Towards unravelling the kinetics of an acute myeloid leukaemia model system under oxidative and starvation stress: a comparison between two- and three-dimensional cultures. Bioprocess Biosyst Eng 2015; 38: 1589–600. doi: 10.1007/s00449-015-1401-z [DOI] [PubMed] [Google Scholar]
- 74.Velliou E, Gupta P, Ricci C, Danti S. Biomaterial based in vitro models for pancreatic cancer. In: Kundu S, Reis R. L, eds.Biomaterials for 3D tumour modelling, Elsevier (ISBN: 9780128181287; 2020. [Google Scholar]
- 75.Winkler J, Abisoye-Ogunniyan A, Metcalf KJ, Werb Z. Concepts of extracellular matrix remodelling in tumour progression and metastasis. Nat Commun 2020; 11: 5120. doi: 10.1038/s41467-020-18794-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kuen J, Darowski D, Kluge T, Majety M. Pancreatic cancer cell/fibroblast co-culture induces M2 like macrophages that influence therapeutic response in a 3D model. 2017;. [DOI] [PMC free article] [PubMed]
- 77.Chiellini F, et al. Modelling of pancreatic ductal adenocarcinoma in vitro with three-dimensional microstructured hydrogels. 2016;.
- 78.Wen Z, Liao Q, Hu Y, You L, Zhou L, Zhao Y. A spheroid-based 3-D culture model for pancreatic cancer drug testing, using the acid phosphatase assay. Braz J Med Biol Res 2013; 46: 634–42. doi: 10.1590/1414-431X20132647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ricci C, Danti S. 3D Models of Pancreatic Ductal Adenocarcinoma via Tissue Engineering. in Pancreatic Cancer: Methods in Molecular Biology. 2019; 1882: 81–95. [DOI] [PubMed] [Google Scholar]
- 80.Katt ME, Placone AL, Wong AD, Xu ZS, Searson PC. In vitro tumor models: advantages, disadvantages, variables, and selecting the right platform. Front Bioeng Biotechnol 2016; 4: 12. doi: 10.3389/fbioe.2016.00012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Senavirathna LK, Fernando R, Maples D, Zheng Y, Polf JC, Ranjan A. Tumor spheroids as an in vitro model for determining the therapeutic response to proton beam radiotherapy and thermally sensitive nanocarriers. Theranostics 2013; 3: 687–91. doi: 10.7150/thno.6381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Longati P, Jia X, Eimer J, Wagman A, Witt M-R, Rehnmark S, et al. 3D pancreatic carcinoma spheroids induce a matrix-rich, chemoresistant phenotype offering a better model for drug testing. BMC Cancer 2013; 13: 95. doi: 10.1186/1471-2407-13-95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Al-Ramadan A, Mortensen AC, Carlsson J, Nestor MV. Analysis of radiation effects in two irradiated tumor spheroid models. Oncol Lett 2018; 15: 3008–16. doi: 10.3892/ol.2017.7716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hehlgans S, Lange I, Eke I, Cordes N. 3D cell cultures of human head and neck squamous cell carcinoma cells are radiosensitized by the focal adhesion kinase inhibitor TAE226. Radiother Oncol 2009; 92: 371–8. doi: 10.1016/j.radonc.2009.08.001 [DOI] [PubMed] [Google Scholar]
- 85.Lazzari G, Nicolas V, Matsusaki M, Akashi M, Couvreur P, Mura S. Multicellular spheroid based on a triple co-culture: a novel 3D model to mimic pancreatic tumor complexity. Acta Biomater 2018; 78: 296–307. doi: 10.1016/j.actbio.2018.08.008 [DOI] [PubMed] [Google Scholar]
- 86.Ki CS, Lin T-Y, Korc M, Lin C-C. Thiol-Ene hydrogels as desmoplasia-mimetic matrices for modeling pancreatic cancer cell growth, invasion, and drug resistance. Biomaterials 2014; 35: 9668–77. doi: 10.1016/j.biomaterials.2014.08.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Gomoz-Roman N, Stevenson K, Gilmour L, Hamilon G. Chalmers, a. A novel 3D human glioblastoma cell culture system for modeling drug and radiation responses. Neuro. Oncol 2016; 19: 229–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hamdi DH, et al. In vitro engineering of human 3D chondrosarcoma: a preclinical model relevant for investigations of radiation quality impact. BMC Cancer 2015; 15: 579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Gupta P, Pérez-Mancera PA, Kocher H, Nisbet A, Schettino G, Velliou EG. A Novel Scaffold-Based Hybrid Multicellular Model for Pancreatic Ductal Adenocarcinoma-Toward a Better Mimicry of the in vivo Tumor Microenvironment. Front Bioeng Biotechnol 2020; 8: 290. doi: 10.3389/fbioe.2020.00290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hamdi DH, Barbieri S, Chevalier F, Groetz J-E, Legendre F, Demoor M, et al. In vitro engineering of human 3D chondrosarcoma: a preclinical model relevant for investigations of radiation quality impact. BMC Cancer 2015; 15: 579. doi: 10.1186/s12885-015-1590-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.D Malkinson, F. C. Some principles of radiobiology: a selective review. 76. [DOI] [PubMed] [Google Scholar]
- 92.Whitmore GF, Till JE. Quantitation of cellular radiobiological RESPONSES1.2.. [DOI] [PubMed]
- 93.Little JB. Principal cellular and tissue effects of radiation. 2003;.