Abstract
While Ewing sarcoma of bone is the second most common primary osseous malignancy in childhood where it typically involves the diaphysis or metadiaphyses of long bones of skeletally immature patients, primary epiphyseal involvement of the long bone in skeletally mature patients is rare with no cases reported in the literature to our knowledge, rendering this case the first of its kind.
We present the first case of primary Ewing Sarcoma of the epiphyses of the long bones in a skeletally mature 20-year-old male patient. The patient initially presented with left knee stiffness and pain that was empirically treated with non-steroidal anti-inflammatory medications. His pain progressed despite treatment. An x-ray of the left knee was obtained 5 months later demonstrating an irregular lucent lesion in the medial femoral condyle. A subsequent MRI revealed an enhancing lesion in the medial femoral condyle, and when biopsied it was consistent with Ewing sarcoma (positive for EWSR1gene rearrangement by fluorescence in situ hybridization). The lesion was resected surgically, and the patient underwent neoadjuvant chemotherapy with a good clinical outcome.
Keywords: Ewing sarcoma, Epiphysis, Bone tumors
Introduction
Ewing sarcoma (ES) of bone is a rare and highly malignant tumor. In the United States, incidence is estimated to be 3 per 1 million [1], [2], [3]. In children and adolescents, it is the second most common primary malignant bone tumor after osteosarcoma with incidence peaking in the second decade of life. More than 50% of cases are diagnosed between the ages of 10-20 years [2,4] and ES predominantly affects Caucasians [5], with a slight male predominance (M: F = 1.5:1) [2], [2].
Skeletal ES most frequently involves the metadiaphyses (44%-59%), diaphysis (33%-35%) and metaphysis (5%-15%) of long bones [4,[6], [7], [8]]. Lesions centered in the epiphysis are rare (0.5%-2%), although extension into the epiphysis is seen in up to 10% of cases [4,[6], [7], [8]]. Lower extremity involvement is more common than in the upper extremities. Other regions that are frequently involved include the pelvis followed by the ribs and spine [2,4,9]. The worst prognostic factor is metastatic disease. However, patients with isolated pulmonary metastases have a slightly better outcome than those with extrapulmonary metastases at initial diagnosis [2].
In 1984 the Intergroup Ewing's Sarcoma Study (IESS) described the typical radiographic features of ES as a medullary based lesion that tends to be poorly marginated, metadiaphyseal in location and having aggressive periosteal reaction and a large associated soft tissue mass [10]. This series of 7299 patients also noted that more than a quarter of patients with ES would have at least one uncommon or rare component to their lesion. Only 1 patient in this series had a lesion in the epiphysis. CT is less helpful in the evaluation of ES [1,8].
We report the first case of skeletal ES in the epiphysis of the long bone of a skeletally mature patient, thus skeletal ES is not usually considered in the differential diagnosis of an end-of-bone lesion in a skeletally mature patient.
Case Report
A 20-year-old college student in his usual state of health presented to his primary care doctor with mild left knee stiffness in December 2011. Physical exam revealed a painful left distal femur with limited range of motion. He was initially treated with non-steroidal anti-inflammatory medication, which had intermittently controlled his pain.
Over the next few months, his pain medication requirement gradually increased despite good control of the left knee stiffness. Three months later, he again experienced increasing pain and stiffness in his left knee, such that if he walked a fair distance, he would not be able to walk normally for a few days.
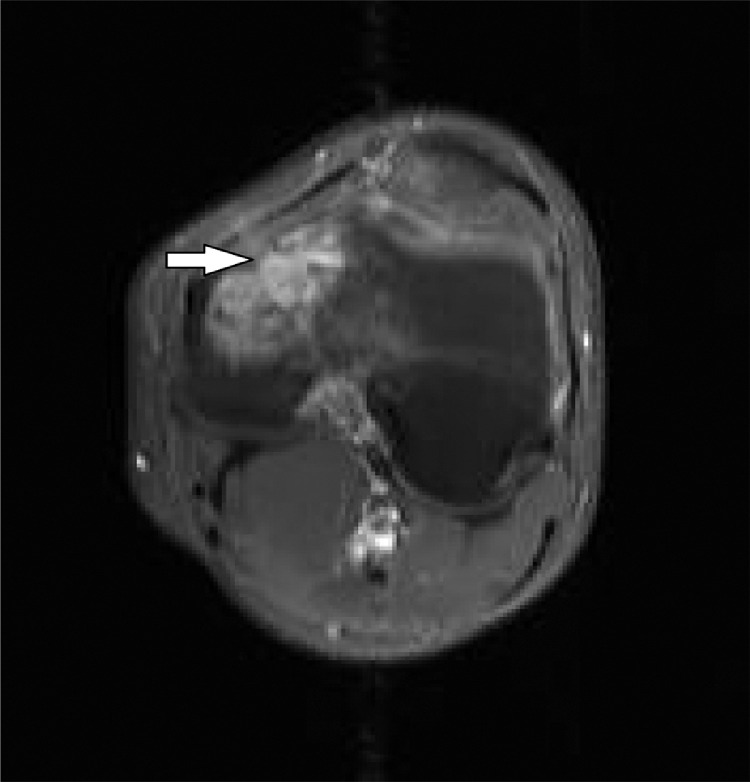
In May 2012, 5 months from his first presentation, a left knee radiograph was obtained showing a subtle irregular lucency in the left medial femoral condyle (Fig. 1). A subsequent MRI obtained that same month demonstrated a 2.9 × 4.0 × 3.9 cm intraosseous lesion within the left medial femoral condyle (Fig. 2). On T1 weighted images the lesion showed decreased signal intensity – hypointense with the surrounding muscle. On STIR the lesion appeared heterogeneously intermediate to high in signal intensity, which extended up along the medial aspect of the distal femur. There was no accompanying soft tissue mass. Following contrast administration, the lesion showed moderate enhancement (Fig. 3). The differential diagnosis at this point included chondroblastoma, giant cell tumor, osteomyelitis or a vascular lesion.
Fig. 1.
Initial radiographs of the left knee. Frontal (a) and lateral (b) projections demonstrate a subtle irregular lucency (arrow) within the medial femoral condyle.
Fig. 2.
Initial noncontrast MRI of the left femur. Coronal T1 (a) an STIR(b) images demonstrate intermediate T1 signal (arrow) within the medial femoral condyle corresponding to increased signal on STIR (arrow) consistent with bone marrow edema.
Fig. 3.
Initial contrast enhanced MRI of the left femur. Axial T1WI with fat saturation demonstrates moderate enhancement (arrow) of the lesion in the medial femoral condyle.
Pertinent labs obtained in June 2012 included Erythrocyte Sedimentation Rate (ESR), which was 9 (normal range 0-15 mm/hr). C-reactive protein (CRP) level was 0.6 (normal range 0-5 mg/L). White blood cell (WBC) count was 5.4 (normal range 4.5-11 × 103/uL). A lactate dehydrogenase (LDH) level in July 2012 was 163 (normal range 100-220 U/L).
An open surgical biopsy was performed in June 2012 for further evaluation and demonstrated a proliferation of atypical small round cells with clear cytoplasm, uniform round to ovoid nuclei with delicate chromatin and inconspicuous nucleoli (Fig. 4).
Fig. 4.
Microscopic pictures of the patient's tumor hematoxylin and eosin stain (A)x10 and (B) x40 magnifications demonstrating a proliferation of atypical small round cells with clear cytoplasm, uniform round to ovoid nuclei with delicate chromatin and inconspicuous nucleoli.
Immunohistochemical stains revealed positive membranous staining for CD99 (Fig. 5). Vimentin was diffusely positive. Stains for LCA, S-100 protein, HMB-45, PLAP, and pancytokeratins (AE1/AE3 and CAM 5.2) were negative.
Fig. 5.
Immunohistochemical stains reveal positive membranous staining for CD99 (x40 magnification).
EWSR1 gene rearrangement was identified by fluorescence in situ hybridization, thus confirming the diagnosis of ES.
Microbiology demonstrated no acid-fast bacilli or fungal elements, Gram stain was negative, and there was no growth in the cultures.
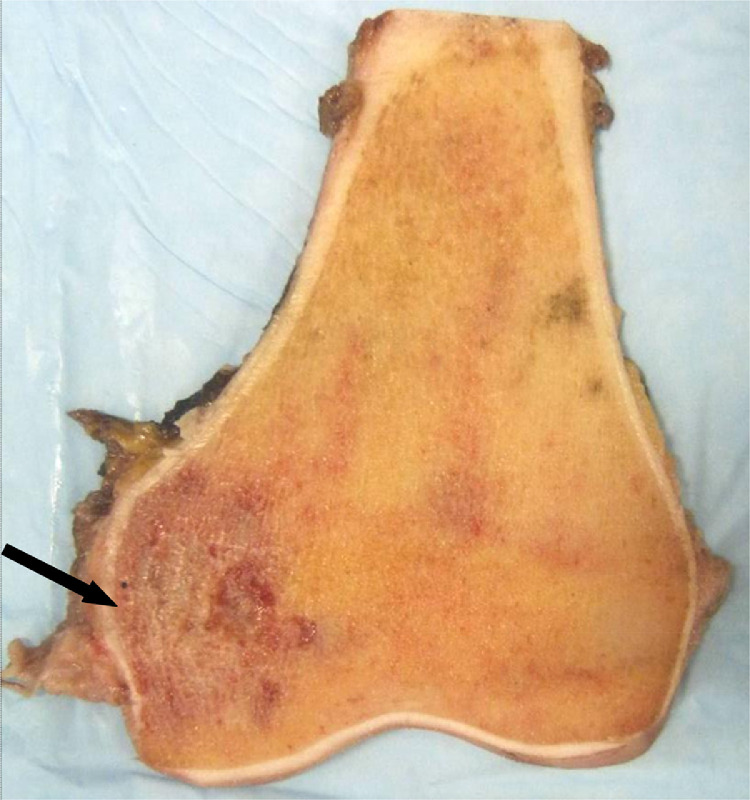
The patient was treated with six cycles of neoadjuvant chemotherapy starting in late July 2012 and ending in October 2012. A bone scan in October 2012 demonstrated localized uptake in the left distal femur with no evidence of skeletal metastases (Fig. 6). Later that month, he underwent radical resection of the left distal femur with reconstruction of the left knee using a modular arthroplasty. The surgery was soon followed by an additional 11 cycles of chemotherapy. Pathology of the resected distal femur demonstrated marrow fibrosis with no viable tumor cells, focal hematopoietic marrow and mild sclerosis of cancellous bone consistent with excellent chemotherapy response (Fig. 7, Fig. 8).
Fig. 6.
Whole body bone scan with technetium-99m HDP prior to left knee arthroplasty. Anterior (a) and posterior (b) planar images demonstrate increased radiotracer uptake (arrow) within the medial femoral condyle of the left femur with no evidence of skeletal metastases. Radiotracer accumulation is seen at the injection site in the right antecubital fossa (curved arrow).
Fig. 7.
Gross resection specimen post-chemotherapy. An irregular area of hyperemia (arrow) is seen in the epiphyseal medullary cavity close to the medial cortex.
Fig. 8.
Microscopic picture of treated tumor with excellent chemotherapy response showing hypo-cellular, loosely fibrotic marrow with few inflammatory cells (hematoxylin and eosin x5 magnification).
Discussion
The differential diagnosis for a primary bone tumor depends on clinical and imaging assessment. Our case is unusual in terms of imaging assessment but rather typical in terms of clinical assessment. Common clinical features of skeletal ES are nonspecific with 82%-88% of patients presenting with pain [8,11]. Patients often have symptoms for greater than 6 months prior to diagnosis, much like our patient. Occasionally, ES may be mistaken initially for infection or less commonly Langerhans cell histiocytosis (LCH) given the similar clinical features including bone pain, systemic symptoms, and elevated inflammatory markers [1,12]. However, our patient's normal white count and nonelevated inflammatory markers made these alternative diagnoses less likely [1,13,14].
Although radiographs are considered first line in the evaluation of ES, MRI is the most sensitive modality to evaluate marrow infiltration, a soft tissue component and a noncontiguous lesion [1,15]. ES is usually homogenous and intermediate on T1 weighted images and homogeneous and low to intermediate on T2 weighted images [8]. Larger lesions as well as those with hemorrhage and necrosis more commonly demonstrate heterogeneity and high signal on long TR images [8]. Contrast enhancement of ES is almost always present [1,8].
Our patient's MRI demonstrated STIR hyperintensity overlying the tumor and extending up along the medial aspect of the distal femur suggesting edema and periosteal reaction. This may represent a type of cortical penetration via the Haversian and Volkmann's canals, which frequently occur in small round cell tumors [8]. The tumor was hypointense to muscle on T1WI, while 95% of ES are typically intermediate in signal [8].
In 1921, Dr. James Ewing described the radiographic appearance of this entity as involving a “large portion of the whole of the shaft…but the ends are generally spared [16].” Lesions centered in the epiphysis are rare (0.5%-2%) [4,[6], [7], [8]]. While several cases of epiphyseal ES have been described, they have all been in skeletally immature patients [17], [18], [19], [20], [21], [22], apart from one case, where it involved the calcaneus in a young female adult patient [23]. The patient presented with heel pain that worsened over time, showing aggressive features on imaging. Consequently, the lesion has metastasized to the bony skeleton and the lung, thus the patient was started on chemotherapy.
Conversely, with regards to the epiphyseal ES in the skeletally immature patient, the lesions were located within the proximal and distal tibial epiphysis, distal femoral epiphysis, and proximal humeral epiphysis [17], [18], [19], [20], [21], [22]. The radiographic findings in these cases were varied but highlight some potential features of ES. Three lesions were well defined and lytic, which is seen in only 4% of all cases [6,8,10]. Most of these cases were at first thought to be chondroblastoma based on radiograph and MRI [19,21,22]. Another lesion was aggressive appearing with ill-defined margins, solid periosteal reaction, moth eaten appearance, a soft tissue component and homogeneous infiltrative marrow signal on MRI [17]. These are very common imaging features with a permeative pattern seen in 76%-82% of lesions and a wide zone of transition seen in 96% of lesions [4,6,8,10]. Two lesions were sclerotic on radiograph [18,20], with one case demonstrating cortical destruction on MRI in addition to heterogeneous signal on standard pre and post contrast sequences [18]. Sclerotic components are seen in 32%-40% of cases [8]. While the radiographic appearance of our case is typical for skeletal ES, the location and the skeletal maturity distinguish this case as exceedingly rare. To our knowledge, this is the only case of Epiphyseal ES in a long bone, described in a skeletally mature patient.
In children, malignant tumors such as osteosarcoma and ES rarely arise within the epiphysis [24]. The differential diagnosis of epiphyseal lesions in children is usually a benign etiology such as a chondroblastoma or infection. Since our patient was 20 years old at presentation, chondroblastoma, clear cell chondrosarcoma, and giant cell tumor are reasonable considerations.
Esiashvili et al demonstrated that younger age (<10 year) was associated with better outcome compared with older age (10-19 year). Additionally, there was no difference in survival by race and female patients had slightly better survival compared with males. Over a 30-year period, 10-year survival increased from 39% in the first study decade to 63% for localized disease and from 16% to 32% for metastatic ES. This series also demonstrated an unfavorable prognosis with tumor in the pelvis that may be attributable to the larger tumor volume typically found at that site. Our patient had no evidence of metastatic disease at presentation and has been disease-free at least 4 years following surgery [2].
In general, survival for patients with localized ES has increased over the last few decades, while those with metastatic disease demonstrate a less definitive improvement, as they appear to be chemo-resistant [2,23]. The presence of metastasis plays as the key prognostic element, rendering the 5-year survival 21% in comparison to 55% in patients with no metastasis, where lungs, bones and bone marrow are the commonest sites of metastasis in a decreasing order of frequency. A better prognosis was found in (1) cases with isolated metastases to the lung rather than those with extrapulmonary metastases (2) cases that showed complete tumor necrosis [23].
With regards to treatment of a localized disease, surgical resection, chemotherapy and radiotherapy take the lead, with neoadjuvant chemotherapy showing promising results. As ES is a radiosensitive tumor, radiotherapy could be used alone or in combination with chemotherapy in cases of unresectable disease, or it can be used post operatively in cases with positive surgical margins [23].
Although the area of gene therapy is still under development, some aspects are showing high potential by targeting the t(11; 22) (q24; q12) translocation found in 85% of cases of ES, or the EWS-FLI1 fusion protein itself [23,25].
Conclusion
We describe an unusual case of a 20-year-old man with epiphyseal ES. This case illustrates that epiphyseal ES may rarely manifest in skeletally mature patients. It is important not to disregard this entity in skeletally mature patients given the dire oncologic consequences. Given the nonspecific clinical presentation, other well described imaging features on radiograph and MRI should be utilized to narrow the differential diagnosis.
Patient Consent Statement
No consent was obtained nor required for the writing of this manuscript, as it is waived by our institution's IRB policy.
Footnotes
Conflicts of interest: The authors declare that they have no conflict of interest.
Acknowledgments: We would like to thank Dr. George Hermann, who passed away during the making of this manuscript. He was a member of the division of Musculoskeletal Radiology, Department of Radiology, Icahn School of Medicine at Mount Sinai, New York, New York, USA. He provided the inspiration behind this work and has served as an invaluable mentor for the contributing authors.
The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
References
- 1.Moore DD, Haydon RC. Ewing's sarcoma of bone. Cancer Treat Res. 2014;162:93–115. doi: 10.1007/978-3-319-07323-1_5. [DOI] [PubMed] [Google Scholar]
- 2.Esiashvili N, Goodman M, Marcus RB. Changes in incidence and survival of Ewing sarcoma patients over the past 3 decades: surveillance epidemiology and end results data. J Pediatr Hematol Oncol. 2008;30(6):425–430. doi: 10.1097/MPH.0b013e31816e22f3. [DOI] [PubMed] [Google Scholar]
- 3.Ozaki T. Diagnosis and treatment of Ewing sarcoma of the bone: a review article. J Orthop Sci. 2015 doi: 10.1007/s00776-014-0687-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dorfman HD, Czerniak B. Bone cancers. Cancer. 1995;75(1 Suppl):203–210. doi: 10.1002/1097-0142(19950101)75:1+<203::aid-cncr2820751308>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 5.Jawad MU, Cheung MC, Min ES, Schneiderbauer MM, Koniaris LG, Scully SP. Ewing sarcoma demonstrates racial disparities in incidence-related and sex-related differences in outcome: an analysis of 1631 cases from the SEER database, 1973-2005. Cancer. 2009;115(15):3526–3536. doi: 10.1002/cncr.24388. [DOI] [PubMed] [Google Scholar]
- 6.Resnick D KM, Greenway G. Saunders; Philadelphia, PA: 2002. Tumors and tumor-like lesions of bone: imaging and pathology of specific lesions. [Google Scholar]
- 7.Worch J MK, Neuhaus J, Goldsby R, Dubois SG. Ethnic and racial differences in patients with Ewing sarcoma. Cancer. 2010;116(4):983–988. doi: 10.1002/cncr.24865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Murphey MD, Senchak LT, Mambalam PK, Logie CI, Klassen-Fischer MK, Kransdorf MJ. From the radiologic pathology archives: ewing sarcoma family of tumors: radiologic-pathologic correlation. Radiographics. 2013;33(3):803–831. doi: 10.1148/rg.333135005. [DOI] [PubMed] [Google Scholar]
- 9.Choi EY, Gardner JM, Lucas DR, McHugh JB, Patel RM. Ewing sarcoma. Semin Diagn Pathol. 2014;31(1):39–47. doi: 10.1053/j.semdp.2014.01.002. [DOI] [PubMed] [Google Scholar]
- 10.Reinus WR, Gilula LA, Shirley SK, Askin FB, Siegal GP. Radiographic appearance of Ewing sarcoma of the hands and feet: report from the Intergroup Ewing Sarcoma Study. AJR Am J Roentgenol. 1985;144(2):331–336. doi: 10.2214/ajr.144.2.331. [DOI] [PubMed] [Google Scholar]
- 11.Widhe B, Widhe T. Initial symptoms and clinical features in osteosarcoma and Ewing sarcoma. J Bone Joint Surg Am. 2000;82(5):667–674. doi: 10.2106/00004623-200005000-00007. [DOI] [PubMed] [Google Scholar]
- 12.Henninger B, Glodny B, Rudisch A, Trieb T, Loizides A, Putzer D. Ewing sarcoma versus osteomyelitis: differential diagnosis with magnetic resonance imaging. Skeletal Radiol. 2013;42(8):1097–1104. doi: 10.1007/s00256-013-1632-5. [DOI] [PubMed] [Google Scholar]
- 13.Nakamura T, Grimer RJ, Gaston CL, Watanuki M, Sudo A, Jeys L. The prognostic value of the serum level of C-reactive protein for the survival of patients with a primary sarcoma of bone. Bone Joint J. 2013;95-B(3):411–418. doi: 10.1302/0301-620X.95B3.30344. [DOI] [PubMed] [Google Scholar]
- 14.Rutkowski P, Kamińska J, Kowalska M, Ruka W, Steffen J. Cytokine and cytokine receptor serum levels in adult bone sarcoma patients: correlations with local tumor extent and prognosis. J Surg Oncol. 2003;84(3):151–159. doi: 10.1002/jso.10305. [DOI] [PubMed] [Google Scholar]
- 15.Peersman B, Vanhoenacker FM, Heyman S, Van Herendael B, Stam M, Brys P. Ewing's sarcoma: imaging features. JBR-BTR. 2007;90(5):368–376. [PubMed] [Google Scholar]
- 16.Ewing J. Classics in oncology. Diffuse endothelioma of bone. James Ewing. Proceedings of the New York Pathological Society, 1921. CA Cancer J Clin. 1972;22(2):95–98. doi: 10.3322/canjclin.22.2.95. [DOI] [PubMed] [Google Scholar]
- 17.Pérez-González Y, García-Esparza E, Conde E, Azorín D. Epiphyseal ewing sarcoma: first reported case with molecular confirmation. Int J Surg Pathol. 2013;21(2):173–176. doi: 10.1177/1066896912453202. [DOI] [PubMed] [Google Scholar]
- 18.Bülbül M, Özger H, Bilgiç B, Eralp L. Primary epiphyseal Ewing sarcoma: a case report. Acta Orthop Traumatol Turc. 2012;46(6):460–463. [PubMed] [Google Scholar]
- 19.Esmaili HA, Niknejad MT, Mohajeri S. Ewing's Sarcoma of Proximal Humeral Epiphysis. Arch Iran Med. 2015;18(2):133–134. [PubMed] [Google Scholar]
- 20.Muscolo DL, Campaner G, Aponte-Tinao LA, Ayerza MA, Santini-Araujo E. Epiphyseal primary location for osteosarcoma and Ewing sarcoma in patients with open physis. J Pediatr Orthop. 2003;23(4):542–545. [PubMed] [Google Scholar]
- 21.Morris P, Dickman PS, Seidel MJ. Ewing's sarcoma/primitive neuroectodermal tumor of the proximal humeral epiphysis. Orthopedics. 2013;36(1):e113–e116. doi: 10.3928/01477447-20121217-29. [DOI] [PubMed] [Google Scholar]
- 22.Kowalczyk B, Lejman T, Drabik G, Zaleska-Czepko E. Primary epiphyseal localization of primitive neuroectodermal tumor in a child. Eur J Radiol Extra. 2011;78:e77–e80. [Google Scholar]
- 23.Guarnieri C. Case study of a young adult with ewing sarcoma. J Adv Practitioner Oncol. 2016;7(6):634. [PMC free article] [PubMed] [Google Scholar]
- 24.Hovy L. [Epiphyseal tumors] Z Orthop Ihre Grenzgeb. 1996;134(5):413–417. doi: 10.1055/s-2008-1037429. [DOI] [PubMed] [Google Scholar]
- 25.Rizk VT, Walko CM, Brohl AS. Precision medicine approaches for the management of Ewing sarcoma: current perspectives. Pharmacogenom Personalized Med. 2019;12:9. doi: 10.2147/PGPM.S170612. [DOI] [PMC free article] [PubMed] [Google Scholar]