This systematic review and meta-analysis investigates visual attention functioning during the first 2 years of life in infants born preterm versus full term.
Key Points
Question
Is preterm birth associated with visual attention impairments in early life, and if so, in which attention functions?
Findings
This systematic review and meta-analysis of 53 studies including 2047 preterm-born and 1951 full-term–born neonates and infants found that preterm birth was significantly associated with impairments in visual attention functioning. Despite a short-term advantage in visual-following in preterm infants, deficits cascaded from basic orienting responses to focused attention during the first 2 years of life.
Meaning
The findings suggest that preterm birth is associated with challenges in the development of visual attention beginning in the early stages of life.
Abstract
Importance
Preterm birth is associated with an increased risk for long-lasting attention deficits. Early-life markers of attention abnormalities have not been established to date but could provide insights into the pathogenesis of attention abnormalities and could help identify susceptible individuals.
Objective
To examine whether preterm birth is associated with visual attention impairments in early life, and if so, in which attention functions and at which developmental period during the first 2 years of life.
Data Sources
PubMed and PsycINFO were searched on November 17, 2019, to identify studies involving visual attention outcomes in infants born preterm vs full term.
Study Selection
Peer-reviewed studies from the past 50 years met the eligibility criteria if they directly assessed visual attention outcomes until the age of 2 years in generally healthy infants born preterm or full term. The selection process was conducted by 2 independent reviewers.
Data Extraction and Synthesis
The Meta-analysis of Observational Studies in Epidemiology (MOOSE) reporting guideline was followed. Random-effects models were used to determine standardized mean differences. The risk of bias was assessed both within and between studies.
Main Outcomes and Measures
Five nascent indices of visual attention were analyzed, including very basic functions—namely, the abilities to follow and fixate on visual targets—and more complex functions, such as visual processing (ie, habituation), recognition memory (ie, novelty preference), and the ability to effortfully focus attention for learning.
Results
A total of 53 studies were included, with 69 effect sizes and assessing a total of 3998 infants (2047 born preterm and 1951 born full term; of the 3376 for whom sex was reported, 1693 [50.1%] were girls). Preterm birth was associated with impairments in various attention indices, including visual-following in infancy (Cohen d, −0.77; 95% CI, −1.23 to −0.31), latency to fixate (Cohen d, −0.18; 95% CI, −0.33 to −0.02), novelty preference (Cohen d, −0.20; 95% CI, −0.32 to −0.08), and focused attention (Cohen d, −0.28; 95% CI, −0.45 to −0.11). In the neonatal period, preterm birth was associated with superior visual-following (Cohen d, 0.22; 95% CI, 0.03 to 0.40), possibly owing to the additional extrauterine exposure to sensory stimulation. However, this early association waned rapidly in infancy (Cohen d, –0.77; 95% CI, –1.23 to –0.31).
Conclusions and Relevance
The findings suggest that preterm birth is associated with impingements to visual attention development in early life, as manifested in basic and then complex forms of attention. Advancements in neonatal care may underlie improvements found in the current era and accentuate several early protective factors.
Introduction
Preterm birth, a live birth before gestational age (GA) of 37 weeks,1 is associated with substantial developmental challenges. Advancements in obstetrics and neonatal care have been associated with an increase in preterm births worldwide,2 which account for 10.6% of live births.3 Despite the positive aspects of better survival and care, preterm birth has implications for learning,4 cognitive performance,4,5,6 and attention5; deficits in these areas may underlie the association of preterm birth with poorer quality of life7 and substantial economic costs.8,9
Susceptibility to these long-term deficits may manifest during the first 2 years of life,10,11 which is a pivotal period for attention development involving the progression from basic abilities that are highly reactive and dependent on external cues12 to an initial exertion of volitionally channeled attention.13 It is important to understand how a congenital vulnerability (eg, preterm birth) affects this expected developmental course. Neurodevelopmental frameworks advocate a model that traces the long-term cognitive sequelae of preterm birth to the cascading implications of early dysfunctions in regulatory and attentional facets.14,15,16 This approach accentuates the idea that the emerging operations of higher cortical loci, which are later to materialize from both the phylogenetic and the ontogenetic perspectives, are influenced by earlier dysregulation of midbrain structures,17,18 thus compromising the distributed attentional networks further as the child matures.19,20,21 The stage-specific behavioral-attention expressions of this cascade are not yet fully mapped.
The third trimester of pregnancy is a period of rapid neural growth for the fetus.22 Younger GA at birth is associated with diminished myelinogenesis,23 synaptogenesis, and dendritic sprouting.24 Extrauterine exposure to sensory stimulation in premature neonates exposes the underdeveloped neural network to stressors, resulting in augmented sensitivity25,26; this marks the neonatal period as a supersensitive period for attention development. Thus, reviewing the formation of notable attention functions in the early life of infants born preterm may reveal factors needing consideration.
Basic attention faculties, typically available in the neonatal period, include the ability to fixate on salient cues in the periphery of the visual field27 and follow salient visual stimuli.28 These faculties further develop during the first year of life.28,29 After the transition from reliance on brainstem-basilar–mediated pathways to the increasing involvement of cortical structures in the second year of life, infants exert more endogenous direction over visuospatial attention.13 This is expressed by 2 notable abilities that predict intellectual performance in later childhood in both the typically developing and the preterm-born populations10,11: first, novelty preference (ie, the ability to preferentially attend to a novel stimulus),12 and then focused attention (ie, the ability to effortfully sustain attention to explore objects).30
To date, it remains unclear whether preterm birth triggers a negative cascading effect on attention development and how it affects each essential attention function in early life. Based on the cascade assertion, it is expected that deficits in more basic functions such as following and latency to fixate on visual stimuli will be evident in early developmental stages and might ebb in later stages; regarding endogenous attention functions such as novelty preference and focused attention, it is expected that earlier neurophysiological dysregulation will lead to long-lasting deficits.13,15,20,21 Vis-à-vis the implications of early extrauterine exposure to sensory stimulation, it is possible that during the first weeks of life, preterm infants will nevertheless benefit in precocial attention abilities resulting from the additional exercise of the visual system.29 However, it is conjectured that this early advantage at term age will rapidly wane, because the burden of premature stimulation and activation of attention networks will be associated with durable deficits in attention tasks from early infancy onward.29 To assess these hypotheses, a concise review of the literature is required.
During the past 50 years, a myriad of studies assessed the association between preterm birth and attention development in early life, but only a few reviews attempted to synthesize and generalize the findings.29,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82 Two notable reviews83,84 from previous decades suggested that infants born preterm show less-optimal attention performance. However, because both reviews were nonsystematic and did not attempt a statistical synthesis, there was a necessity for a methodical review to revisit the important claims of the previous studies while assigning distinct emphasis to each of the focal-attention faculties.
We expected that the developmental course would be significantly associated with preterm birth in ways that cascade from basic to more endogenous forms of attention. The aim of this study was to examine which attention faculties might benefit from early extrauterine exposure to stimuli and which might be compromised as the preterm-born infant experiences increasing attentional demands. In addition, we aimed to assess whether the substantial advancements in neonatal care were also associated with ameliorations in attention development.
Methods
Systematic Review Protocol
In this systematic review and meta-analysis, we reviewed the literature to identify studies involving visual attention outcomes in infants born preterm vs full term. This study followed the Meta-analysis of Observational Studies in Epidemiology (MOOSE) reporting guideline.85
The eligibility criteria for included studies were (1) publication in a peer-reviewed scientific journal or book, (2) publication during the past 50 years, (3) inclusion of both a preterm (ie, GA<37 weeks at birth) and a full-term group, (4) inclusion of infants aged 2 years or younger, (5) inclusion of healthy participants (indicating that at least 75% of the preterm-born sample was reported to be without chronic neurological, genetic, or medical impairment at the time of assessment), and (6) reporting of at least 1 visual attention measure that had been directly attained from testing the participant (ie, questionnaires and indirect report indices were not eligible).
Information Sources and Search Strategy
The literature review was conducted November 17, 2019, in the PubMed and PsycINFO databases using combinations of the following keywords: [preterm OR premature OR pre-term OR prematurity] AND attention AND [(infant OR infants OR infancy) OR (neonate OR neonates OR neonatal) OR (toddler OR toddlers OR toddlerhood)]. Further records were identified by manual review of studies’ references.
Study Selection
Records were integrated into the Colandr platform,86 and duplicates were removed. Subsequently, titles and abstracts were screened. Full-text articles were assessed for eligibility according to the inclusion criteria. Reasons for exclusion were stored in the system. The entire selection process was conducted by 2 independent reviewers (O.B. and Z.Z.); conflicts (less than 10% of the cases) were resolved by dialogue.
Data Collection
Data were collected and maintained using a sheet generated ad hoc. Apart from the outcome statistics, crucial covariates were extracted. The specific method and operational definition of the attention measures were documented (eTable 1 in the Supplement). The quality of the studies was screened using a modified version of the Newcastle-Ottawa Scale87 (eAppendix 1 in the Supplement).
Statistical Analysis
Data were analyzed using the metafor package in R, version 3.6.1 (R Project for Statistical Computing).88 Because various studies used different scales to measure the same constructs, group differences were expressed as standardized mean differences with the Cohen d index.89 Random-effects models were used based on the DerSimonian-Laird method.90
Heterogeneity between studies was assessed using Cochrane Q and I2 statistics.91 For the Q statistic, 2-sided P < .10 was considered significant.91 For the I2 statistic, previously established guidelines were followed.91
Screening for potential outliers was conducted through inspection of the externalized studentized residuals.92 The risk of bias between studies was assessed by inspection of the degree of asymmetry of the funnel plot using Egger regression93 and by the trim-and-fill method.94 These assessments are presented in eFigure 1 in the Supplement.
The contribution of important demographic and medical factors was assessed using moderation analyses. Only significant moderators are reported (further details are provided in eAppendix 2 in the Supplement).
Results
Overall Characteristics of Studies
The selection process yielded 53 eligible studies,29,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82 providing a total of 69 effect sizes distributed over 5 meta-analyses. The overall sample included 3998 neonates and infants (of the 3376 for whom sex was reported, 1693 [50.1%] were girls); 2047 were born preterm, and 1951 were full-term control individuals. The mean (SD) birthweight and GA at birth of the preterm populations were 1514 (458) g and 31.4 (2.5) weeks, respectively. The infants’ mean (SD) age at testing was 29.3 (22.8) weeks. The visual attention outcomes included (1) visual-following, (2) latency to fixate, (3) habituation, (4) novelty preference, and (5) focused attention. A flow diagram depicting the selection process is shown in eFigure 2 in the Supplement. A comprehensive description of the characteristics of the studies included in this meta-analysis are publicly available through an open data repository.95
Visual-Following
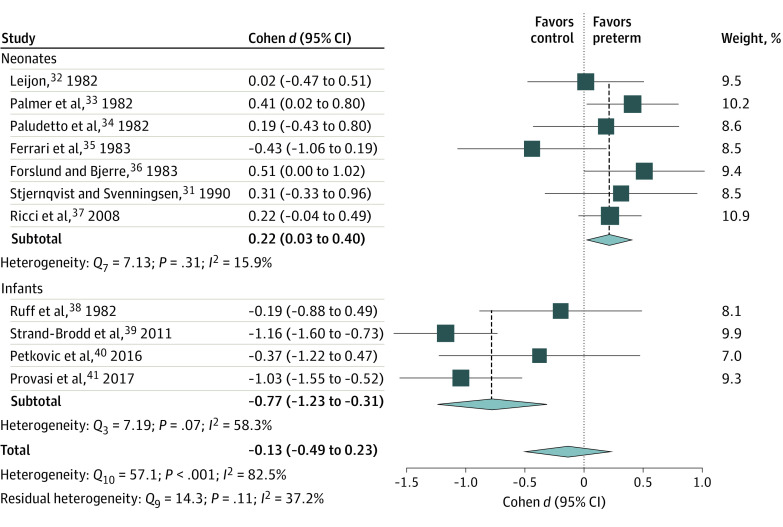
Eleven eligible studies31,32,33,34,35,36,37,38,39,40,41 with 11 effect sizes on measures of visual following of an inanimate object were included (Figure 1). No differences were found between the full-term and preterm groups in visual-following (Cohen d, −0.13; 95% CI, −0.49 to 0.23). However, there was evidence of heterogeneity in effect sizes between studies (Q10 [subscript indicates degrees of freedom {df}], 57.08; P < .001), suggesting that a portion of the variance was explained by heterogeneity rather than chance (I2, 82.5%). Subgroup analysis indicated that the source of heterogeneity was associated with the infants’ age at the time of the test (Qmoderator [M], 23.14; df, 1; P < .001). A qualitative interaction was detected, suggesting that individuals born preterm were more likely to manifest superior performance during the neonatal period (Cohen d, 0.22; 95% CI, 0.03- 0.40) but inferior performance in early infancy (Cohen d, −0.77; 95% CI, −1.23 to −0.31).
Figure 1. Forest Plot for the Differences in Visual-Following Between Infants and Neonates Born Preterm and Full-term.
Squares represent point estimates, with the marker size indicating weight; horizontal lines represent 95% CIs. Diamonds represent the pooled point estimate, with the points indicating the 95% CI.
No evidence of heterogeneity was found in the studies of neonates, but an indication of heterogeneity was found in the studies of infants (Q3, 7.19 [P = .06]; I2, 58.3%). A difference in effect sizes was found (QM, 6.95; df, 1; P = .008) between studies that used real-time observers’ coding of gaze (Cohen d, –0.26; 95% CI, −0.80 to 0.27) and those that used computerized gaze tracking (Cohen d, –1.11; 95% CI, −1.44 to −0.77). These findings suggest a quantitative interaction, with a more robust difference between the groups when computerized equipment was used (plausibly owing to increased tracking sensitivity).
Taken together, the studies showed that neonates born preterm had a greater likelihood for advantage in following salient cues. However, soon after birth, this likelihood for advantage waned.
Latency to Fixate
Ten eligible studies29,42,43,44,45,46,47,48,49,50 with 10 effect sizes on measures of latency to fixate on peripheral or salient stimuli were included (Figure 2). Preterm birth was associated with delayed latency to fixate (Cohen d, −0.18; 95% CI, −0.33 to −0.02; z, −2.15; P = .03). There was no evidence of heterogeneity. However, a moderation analysis indicated that birth era was associated with the differences (QM, 6.38; df, 2; P = .041), suggesting an increased risk for deficits in preterm cohorts of infants born before 1990 and who are now at least 30 years of age (eAppendix 3 in the Supplement).
Figure 2. Forest Plot for the Differences in Latency to Fixate Between Infants Born Preterm and Full-term.
Squares represent point estimates, with the marker size indicating weight; horizontal lines represent 95% CIs. Diamonds represent the pooled point estimate, with the points indicating the 95% CI.
Habituation and Novelty Preference
The constructs of habituation (the gradual decline in visual processing as a result of forming a mental representation of a stimulus) and novelty preference (the tendency to prefer exploring a novel stimulus rather than a familiar [ie, habituated] one) are theoretically intertwined because both reflect gaining familiarity with the stimulus through inspection. Both constructs were, therefore, considered in this review, and the association between them was analyzed (Figure 3).
Figure 3. Forest Plot for the Differences in Habituation and Novelty Preference Between Infants Born Preterm and Full-term.
Squares represent point estimates, with the marker size indicating weight; horizontal lines represent 95% CIs. Diamonds represent the pooled point estimate, with the points indicating the 95% CI.
For the meta-analysis of habituation, 12 eligible studies41,42,48,51,52,53,54,55,56,57,58,59 with 13 effect sizes were included. No differences were found between the groups in habituation (Cohen d, −0.10; 95% CI, −0.22 to 0.03). There was no evidence of heterogeneity. However, a moderation analysis indicated that birth era was associated with the differences (QM, 4.77; df, 1; P = .03), suggesting an increased risk for deficits in preterm infants born before the year 2000 and who are now in their 20s (eAppendix 4 and eFigure 4 in the Supplement).
For the meta-analysis of novelty preference, 15 eligible studies48,51,52,53,54,56,57,58,59,60,61,62,63,64,65 with 17 effect sizes were included. Preterm birth was associated with diminished novelty preference (Cohen d, −0.20; 95% CI, −0.32 to −0.08; z, −3.21; P = .001). There was no evidence of heterogeneity.
To assess whether differences in habituation are associated with the extent of differences in novelty preference, we explored the 10 populations for whom both measures were reported. The analysis showed no association (β, −0.40; 95% CI, –1.20 to 0.39; P = .32).
Taken together, the findings showed that infants born prematurely were more likely to experience impairment in visual recognition memory as reflected in novelty preference, a difference that was also noticeable using the habituation paradigm in participants born before the year 2000.
Focused Attention
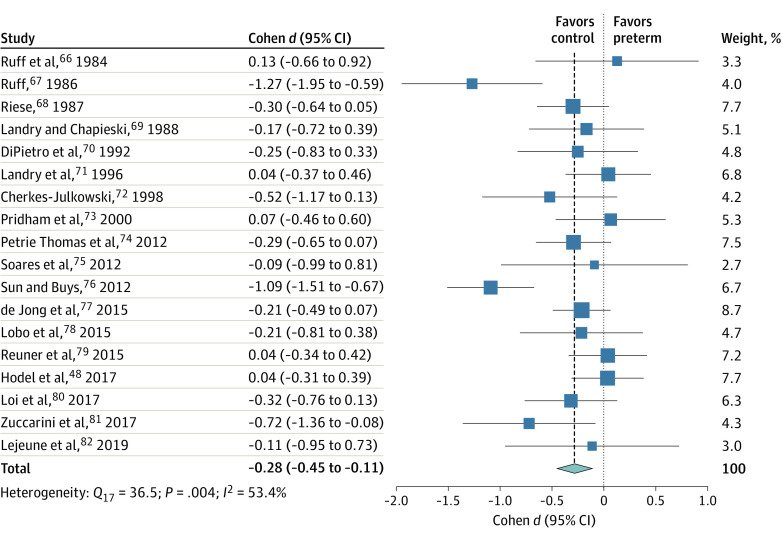
Eighteen eligible studies48,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82 with 18 effect sizes on measures of intensified or deliberate orienting to an object were included (Figure 4). Preterm birth was associated with diminished focused attention (Cohen d, −0.28; 95% CI, −0.45 to −0.11; z, −3.25; P = .001). There was evidence of heterogeneity (Q17, 36.46; P = .004), indicating that a moderate portion of the variance was explained by heterogeneity rather than chance (I2, 53.4%). The heterogeneity was explained by 2 outlier findings; their removal attenuated the effect size but did not annul it (eAppendix 5 in the Supplement). A moderation analysis indicated an interaction between birth era and GA group (QM, 11.65; df, 5; P = .04), suggesting that the increased risk for focused-attention deficits after extremely preterm birth was attenuated in cohorts born after 2000 but did not completely ebb (eAppendix 5 and eFigure 5 in the Supplement).
Figure 4. Forest Plot for the Differences in Focused Attention Between Infants Born Preterm and Full-term.
Squares represent point estimates, with the marker size indicating weight; horizontal lines represent 95% CIs. Diamonds represent the pooled point estimate, with the points indicating the 95% CI.
Taken together, the findings showed that in the multifaceted ability of focused attention, infants born preterm (especially extremely preterm) were more likely to experience difficulties compared with typically developing infants born at full term.
Discussion
To our knowledge, this is the first systematic review and meta-analysis of preterm birth and the development of visual attention during infancy. Considering findings from 53 studies including 3998 infants, this review revealed that individuals born preterm had greater likelihood of attention difficulties as early as during the first 2 years of life. Increased likelihood for impairments cascaded from more reflexive functions—namely, visual-following (in early infancy but not during the neonatal period) and latency to fixate—to more endogenous forms of attention, such as visual recognition memory (as expressed by novelty preference but not by habituation), and was most apparent in focused attention. The current findings support findings from previous studies suggesting that the antecedents for the increased risk for diagnosis of attention deficit hyperactivity disorder and learning disabilities in individuals who were born at term96 and at preterm4,5 might already be discernible in infancy.
These differences were compatible with the hypothesized precocial exposure effect.29,97 Neonates born preterm were more likely to show an advantage in visual-following. It has been suggested that exposure to sensory stimulation after preterm birth might prime basic visual attention abilities.29 This claim is corroborated by the findings of this analysis. Preterm birth was associated with superior following even in a study of a population of extremely premature neonates with birthweight less than 901 g.31 An additional analysis (eFigure 3 in the Supplement) suggests that even though preterm birth was associated with superior visual-following to neutral stimuli, the visual system of preterm neonates was less primed to track human figures. An advantage in visual-following among neonates born preterm may not signal typical attention development; the current results suggest that this early advantage is likely to change in early infancy.
In measures of visual-following in infancy and latency to fixate, the meta-analyses showed that preterm infants had increased risk for deficits. Rudimentary ability to fixate on or follow salient visual targets is exogenous; retinal stimulation elicits activation of visual perception via the geniculate nucleus and its axonal oscillation of the primary visual cortex and oculomotor reaction via the superior colliculus.98 The findings from the current review and those of previous reports on children99,100 and adolescents101 concerning exogenous orienting suggest a neonatal advantage at term age, followed by a decline in infancy and then, putatively, recovery in exogenous orienting in childhood.
The recovery in exogenous orienting to stimuli is not corroborated by typical volitional orienting of attention. Preterm birth was associated with diminished performance in the reviewed early indices of endogenous attention (ie, novelty preference and focused attention). This finding complements the notion that preterm birth is associated with impeded development of endogenous attention throughout adolescence and adulthood.102,103,104 Focused attention—the hallmark of endogenous attention explored in the current meta-analysis—is contingent on executive substrates105 that undergo a maturational neural growth spurt at the approximate age of 10 months106 to facilitate the coordination of attention orienting and parasympathetic activation.107 Similarly, novelty preference relies on the dorsolateral prefrontal and anterior cingulate cortices that sustain attention and modulate arousal but also involves the hippocampus and parahippocampal cortex for encoding and decoding.108 Increased risk for alterations in the development of these regions in preterm-born populations109,110 further explains the difficulties in novelty preference. The risk for deficiencies in endogenous attention in infants born preterm that was observed in the current study thus implies the involvement of a widely distributed neural network that mostly involves the salience network.111 Unlike more reflexive attention abilities, deficits in endogenous or focused attention appear as a tenacious sequela of preterm birth104 that possibly initially manifest in the abilities to effortfully explore, process, and map the environment during infancy.
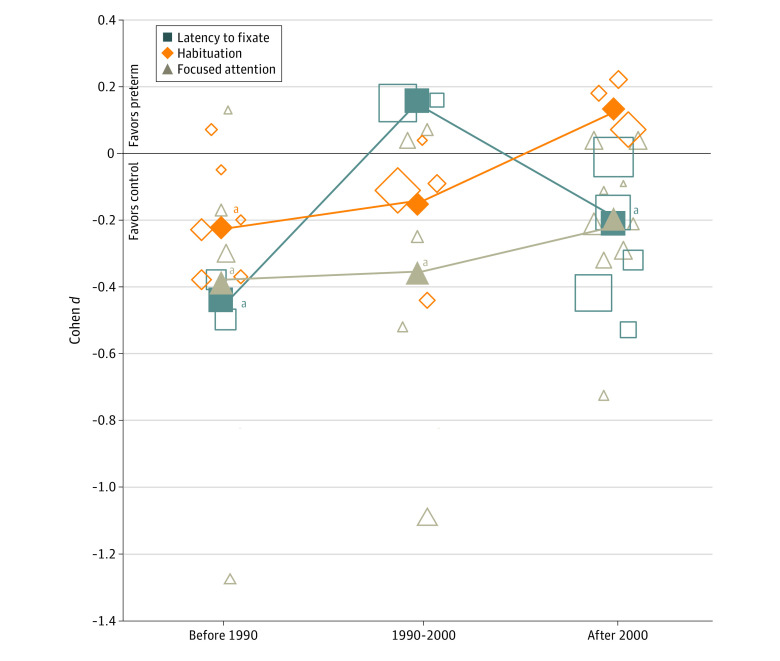
The most promising finding from the present review in this regard involved signs that improvements in care and age-sensitive exposure were associated with attention outcomes. The current findings revealed a partial amelioration in focused-attention difficulties as a function of birth era and the accompanied revisions in care protocols (Figure 5). Two major revolutions have taken place in obstetrics and neonatal care in the past semi-century. The first was a medical revolution that occurred in approximately 1990112 and included the implementation of antenatal corticosteroid and postnatal surfactant treatments to diminish the risks for respiratory distress, brain damage, and mortality. The second was related to tailored sensorimotor stimulation, nurturance, and physio-emotional and social care during the neonatal stay and is manifested in excitation reduction, physical proximity to caregivers, and breastfeeding facilitation techniques.113,114 This analysis suggests that attention development during infancy may be sensitive to better-regulated oxygenation and well-titrated stimulation and that advancements in neonatal care may underlie the associated improvements in attention development by preventing overstimulation of posterior orienting and thereafter providing a stronger basis for the development of anterior executive attention networks.115 However, despite the positive trajectory, increased risk for early-life impairments in focused attention after extremely preterm birth still exists in the current era, suggesting that advancements in neonatal care may have enabled better prospects but that there is still more work to be done.
Figure 5. Differences in Visual Attention Development Between Infants Born Preterm and Full-term According to Birth Eras.
Outlined shapes represent effect sizes of distinct studies in the meta-analyses. Studies are separated according to the cohorts’ birth era. Filled shapes represent the standardized total mean difference between the groups in each birth era. The size of each faded shape is proportional to the study’s weight in the distinct meta-analysis.
aSignificant difference (2-sided P < .05) between the full-term and preterm groups in a specific era.
The findings support the notion that early development of attention skills may be sensitive to stimulation and care in ways that might merit future research: (1) extrauterine exposure to sensory stimulation may account for the short-term association with the precocial establishment of visual-following at term age, and (2) refinement of neonatal and pediatric care in the past 5 decades has improved remarkably in providing individually tailored medical, pharmacological, sensorimotor, emotional, thermal-physical, and social care. The findings of this review suggest that increased risk for deficits in volitional control over visual attention is associated with prematurity (and more pronouncedly with extreme prematurity). Advancements in care, mostly in the current era, seem to underlie a partial remission. Future developments in care during early infancy may support the ability of infants born preterm to focus attention to learn and fulfill their potential.
Limitations
This study has limitations. First, a demographic bias resulting from overrepresentation of studies from Western Europe and North America (49 of the 53 included studies [92.3%]) curtailed the ecological validity of the findings vis-à-vis other nonrepresented populations. Second, some studies were missing information regarding central covariates (eg, 17.3% of the included studies were missing data on participants’ sex, 38.5% were missing information on participants’ age at the time of testing, and 79.0% were missing data on participants’ socioeconomic status). Reporting an adequate set of important background, demographic, and medical characteristics will likely improve the validity of future systematic reviews and deepen the understanding of the mechanisms involved.116
A third limitation was a lack of consistent reporting guidelines and the inaccessibility of data. When conducting an extensive literature review, attempts to reach authors in cases of insufficient data for calculation of effect sizes are not always successful. Most of the lost evidence in this review was from studies conducted in the 1970s, when there was less consensus on reporting guidelines.117 Adherence to acknowledged reporting guidelines may constrain this limitation in the future.118,119
Conclusions
This systematic review and meta-analysis found that infants born preterm had increased risk for deficits in visual attention, cascading from basic reflexive functions (namely visual-following and latency to fixate) to difficulties in early operations of endogenous attention, such as novelty preference and focused attention, which are vital for learning about the world. The deficits were more pronounced in infants born extremely preterm. Advancements in neonatal care may underlie improvements found in the current era and accentuate several early protective factors.
eFigure 1. Funnel Plots of Effect Sizes and Standard Errors Used for the Trim and Fill Analyses for Assessing Possible Publication Biases
eFigure 2. A Flow Diagram of Studies’ Selection Process
eFigure 3. Forest and Funnel Plots for the Meta-Analysis on the Differences in Visual Following (Animate Stimuli) Between Preterm and Full-Term Born Neonates
eTable 1. Characteristics of the Studies Included in the Meta-Analyses
eTable 2. Preterm Birth and the Development of Visual Attention During the First Two Years of Life: A Systematic Review and Meta-analysis
eAppendix 1. Modified Newcastle-Ottawa Scale for Assessment of Studies Quality and Risk of Bias
eAppendix 2. Description of the Moderation Analyses
eAppendix 3. Moderation Analysis in the Latency to Fixate Meta-analysis
eAppendix 4. Moderation Analysis in the Habituation Meta-analysis
eFigure 4. Graphical Depiction of the Moderation of the Differences Between the Preterm and Full-term Groups in Habituation by Birth Era
eAppendix 5. Additional Analyses in the Focused Attention Meta-analysis
eFigure 5. Graphical Depiction of the Moderation of the Differences Between the Preterm and Full-term Groups in Focused Attention by Birth Era and GA Group
eReferences
References
- 1.World Health Organization . Born Too Soon: The Global Action Report on Preterm Birth. 2012. Accessed February 17, 2021. http://whqlibdoc.who.int/publications/2012/9789241503433_eng.pdf
- 2.Blencowe H, Cousens S, Oestergaard MZ, et al. National, regional, and worldwide estimates of preterm birth rates in the year 2010 with time trends since 1990 for selected countries: a systematic analysis and implications. Lancet. 2012;379(9832):2162-2172. doi: 10.1016/S0140-6736(12)60820-4 [DOI] [PubMed] [Google Scholar]
- 3.Chawanpaiboon S, Vogel JP, Moller AB, et al. Global, regional, and national estimates of levels of preterm birth in 2014: a systematic review and modelling analysis. Lancet Glob Health. 2019;7(1):e37-e46. doi: 10.1016/S2214-109X(18)30451-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Twilhaar ES, de Kieviet JF, Aarnoudse-Moens CSH, van Elburg RM, Oosterlaan J. Academic performance of children born preterm: a meta-analysis and meta-regression. Arch Dis Child Fetal Neonatal Ed. 2018;103(4):F322-F330. doi: 10.1136/archdischild-2017-312916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Allotey J, Zamora J, Cheong-See F, et al. Cognitive, motor, behavioural and academic performances of children born preterm: a meta-analysis and systematic review involving 64 061 children. BJOG. 2018;125(1):16-25. doi: 10.1111/1471-0528.14832 [DOI] [PubMed] [Google Scholar]
- 6.Twilhaar ES, Wade RM, de Kieviet JF, van Goudoever JB, van Elburg RM, Oosterlaan J. Cognitive outcomes of children born extremely or very preterm since the 1990s and associated risk factors: a meta-analysis and meta-regression. JAMA Pediatr. 2018;172(4):361-367. doi: 10.1001/jamapediatrics.2017.5323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baumann N, Bartmann P, Wolke D. Health-related quality of life into adulthood after very preterm birth. Pediatrics. 2016;137(4):e20153148. doi: 10.1542/peds.2015-3148 [DOI] [PubMed] [Google Scholar]
- 8.Mangham LJ, Petrou S, Doyle LW, Draper ES, Marlow N. The cost of preterm birth throughout childhood in England and Wales. Pediatrics. 2009;123(2):e312-e327. doi: 10.1542/peds.2008-1827 [DOI] [PubMed] [Google Scholar]
- 9.Korvenranta E, Linna M, Rautava L, et al. ; Performance, Effectiveness, and Cost of Treatment Episodes (PERFECT) Preterm Infant Study Group . Hospital costs and quality of life during 4 years after very preterm birth. Arch Pediatr Adolesc Med. 2010;164(7):657-663. doi: 10.1001/archpediatrics.2010.99 [DOI] [PubMed] [Google Scholar]
- 10.Lawson KR, Ruff HA. Early focused attention predicts outcome for children born prematurely. J Dev Behav Pediatr. 2004;25(6):399-406. doi: 10.1097/00004703-200412000-00003 [DOI] [PubMed] [Google Scholar]
- 11.Kavšek M. Predicting later IQ from infant visual habituation and dishabituation: a meta-analysis. J Appl Dev Psychol. 2004;25(3):369-393. [Google Scholar]
- 12.Geva R, Gardner JM, Karmel BZ. Feeding-based arousal effects on visual recognition memory in early infancy. Dev Psychol. 1999;35(3):640-650. doi: 10.1037/0012-1649.35.3.640 [DOI] [PubMed] [Google Scholar]
- 13.Ruff HA, Rothbart MK. Attention in Early Development: Themes and Variations. Oxford University Press; 2001. doi: 10.1093/acprof:oso/9780195136326.001.0001 [DOI] [Google Scholar]
- 14.Rose SA, Feldman JF, Jankowski JJ. Modeling a cascade of effects: the role of speed and executive functioning in preterm/full-term differences in academic achievement. Dev Sci. 2011;14(5):1161-1175. doi: 10.1111/j.1467-7687.2011.01068.x [DOI] [PubMed] [Google Scholar]
- 15.Geva R, Feldman R. A neurobiological model for the effects of early brainstem functioning on the development of behavior and emotion regulation in infants: implications for prenatal and perinatal risk. J Child Psychol Psychiatry. 2008;49(10):1031-1041. doi: 10.1111/j.1469-7610.2008.01918.x [DOI] [PubMed] [Google Scholar]
- 16.Tucker DM, Derryberry D, Luu P. Anatomy and physiology of human emotion: vertical integration of brainstem, limbic, and cortical systems. In: Borod JC, ed. The Neuropsychology of Emotion. Oxford University Press; 2000: 56-79. [Google Scholar]
- 17.Jackson JH. On some implications of dissolution of nervous system. Med Press Circ. 1882;2:411-433. [Google Scholar]
- 18.Gillett G, Franz E. John Hughlings Jackson: bridging theory and clinical observation. Lancet. 2013;381(9866):528-529. doi: 10.1016/S0140-6736(13)60268-8 [DOI] [PubMed] [Google Scholar]
- 19.Jiang ZD, Zhou Y, Ping LL, Wilkinson AR. Brainstem auditory response findings in late preterm infants in neonatal intensive care unit. Acta Paediatr. 2011;100(8):e51-e54. doi: 10.1111/j.1651-2227.2011.02232.x [DOI] [PubMed] [Google Scholar]
- 20.Geva R, Dital A, Ramon D, Yarmolovsky J, Gidron M, Kuint J. Brainstem as a developmental gateway to social attention. J Child Psychol Psychiatry. 2017;58(12):1351-1359. doi: 10.1111/jcpp.12746 [DOI] [PubMed] [Google Scholar]
- 21.Geva R, Schreiber J, Segal-Caspi L, Markus-Shiffman M. Neonatal brainstem dysfunction after preterm birth predicts behavioral inhibition. J Child Psychol Psychiatry. 2014;55(7):802-810. doi: 10.1111/jcpp.12188 [DOI] [PubMed] [Google Scholar]
- 22.de Graaf-Peters VB, Hadders-Algra M. Ontogeny of the human central nervous system: what is happening when? Early Hum Dev. 2006;82(4):257-266. doi: 10.1016/j.earlhumdev.2005.10.013 [DOI] [PubMed] [Google Scholar]
- 23.Hüppi PS, Warfield S, Kikinis R, et al. Quantitative magnetic resonance imaging of brain development in premature and mature newborns. Ann Neurol. 1998;43(2):224-235. doi: 10.1002/ana.410430213 [DOI] [PubMed] [Google Scholar]
- 24.Folkerth RD, Kinney HC. Disorders of the perinatal period. In: Love S, Louis DN, Ellison DW, eds. Greenfield’s Neuropathology. 8th ed. CRC Press; 2008:241-334. [Google Scholar]
- 25.Wachman EM, Lahav A. The effects of noise on preterm infants in the NICU. Arch Dis Child Fetal Neonatal Ed. 2011;96(4):F305-F309. doi: 10.1136/adc.2009.182014 [DOI] [PubMed] [Google Scholar]
- 26.Soubasi V, Mitsakis K, Nakas CT, et al. The influence of extrauterine life on the aEEG maturation in normal preterm infants. Early Hum Dev. 2009;85(12):761-765. doi: 10.1016/j.earlhumdev.2009.10.004 [DOI] [PubMed] [Google Scholar]
- 27.Farroni T, Massaccesi S, Pividori D, Johnson MH. Gaze following in newborns. Infancy. 2004;5(1):39-60. doi: 10.1207/s15327078in0501_2 [DOI] [Google Scholar]
- 28.Lengyel D, Weinacht S, Charlier J, Gottlob I. The development of visual pursuit during the first months of life. Graefes Arch Clin Exp Ophthalmol. 1998;236(6):440-444. doi: 10.1007/s004170050103 [DOI] [PubMed] [Google Scholar]
- 29.Hunnius S, Geuze RH, Zweens MJ, Bos AF. Effects of preterm experience on the developing visual system: a longitudinal study of shifts of attention and gaze in early infancy. Dev Neuropsychol. 2008;33(4):521-535. doi: 10.1080/87565640802101508 [DOI] [PubMed] [Google Scholar]
- 30.Ruff HA, Saltarelli LM, Capozzoli M, Dubiner K. The differentiation of activity in infants’ exploration of objects. Dev Psychol. 1992;28(5):851-861. doi: 10.1037/0012-1649.28.5.851 [DOI] [Google Scholar]
- 31.Stjernqvist K, Svenningsen NW. Neurobehavioural development at term of extremely low-birthweight infants (less than 901g). Dev Med Child Neurol. 1990;32(8):679-688. doi: 10.1111/j.1469-8749.1990.tb08428.x [DOI] [PubMed] [Google Scholar]
- 32.Leijon I. Assessment of behaviour on the Brazelton scale in healthy preterm infants from 32 conceptional weeks until full-term age. Early Hum Dev. 1982;7(2):109-118. doi: 10.1016/0378-3782(82)90130-X [DOI] [PubMed] [Google Scholar]
- 33.Palmer PG, Dubowitz LMS, Verghote M, Dubowitz V. Neurological and neurobehavioural differences between preterm infants at term and full-term newborn infants. Neuropediatrics. 1982;13(4):183-189. doi: 10.1055/s-2008-1059620 [DOI] [PubMed] [Google Scholar]
- 34.Paludetto R, Mansi G, Rinaldi P, et al. Behaviour of preterm newborns reaching term without any serious disorder. Early Hum Dev. 1982;6(4):357-363. doi: 10.1016/0378-3782(82)90073-1 [DOI] [PubMed] [Google Scholar]
- 35.Ferrari F, Grosoli MV, Fontana G, Cavazzuti GB. Neurobehavioural comparison of low-risk preterm and fullterm infants at term conceptional age. Dev Med Child Neurol. 1983;25(4):450-458. doi: 10.1111/j.1469-8749.1983.tb13789.x [DOI] [PubMed] [Google Scholar]
- 36.Forslund M, Bjerre I. Neurological assessment of preterm infants at term conceptional age in comparison with normal full-term infants. Early Hum Dev. 1983;8(3-4):195-208. doi: 10.1016/0378-3782(83)90002-6 [DOI] [PubMed] [Google Scholar]
- 37.Ricci D, Cesarini L, Romeo DMM, et al. Visual function at 35 and 40 weeks’ postmenstrual age in low-risk preterm infants. Pediatrics. 2008;122(6):e1193-e1198. doi: 10.1542/peds.2008-1888 [DOI] [PubMed] [Google Scholar]
- 38.Ruff HA, Lawson KR, Kurtzberg D, McCarton-Daum C, Vaughan HG Jr. Visual following of moving objects by full-term and preterm infants. J Pediatr Psychol. 1982;7(4):375-386. doi: 10.1093/jpepsy/7.4.375 [DOI] [PubMed] [Google Scholar]
- 39.Strand-Brodd K, Ewald U, Grönqvist H, et al. Development of smooth pursuit eye movements in very preterm infants: 1. General aspects. Acta Paediatr. 2011;100(7):983-991. doi: 10.1111/j.1651-2227.2011.02218.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Petkovic M, Chokron S, Fagard J. Visuo-manual coordination in preterm infants without neurological impairments. Res Dev Disabil. 2016;51-52:76-88. doi: 10.1016/j.ridd.2016.01.010 [DOI] [PubMed] [Google Scholar]
- 41.Provasi J, Lemoine-Lardennois C, Orriols E, Morange-Majoux F.. Do preterm infants perceive temporal synchrony? an analysis with the eye-tracking system. Timing Time Percept. 2017;5(2):190-209. doi: 10.1163/22134468-00002089 [DOI] [Google Scholar]
- 42.Friedman SL, Jacobs BS, Werthmann MWJ. Sensory processing in pre- and full-term infants in the neonatal period. In: Friedman SL, Sigman M, eds. Preterm Birth and Psychological Development. Academic Press; 1981:159-178. [Google Scholar]
- 43.Foreman N, Fielder A, Price D, Bowler V. Tonic and phasic orientation in full-term and preterm infants. J Exp Child Psychol. 1991;51(3):407-422. doi: 10.1016/0022-0965(91)90085-7 [DOI] [PubMed] [Google Scholar]
- 44.Butcher PR, Kalverboer AF, Geuze RH, Stremmelaar EF. A longitudinal study of the development of shifts of gaze to a peripheral stimulus in preterm infants with transient periventricular echogenicity. J Exp Child Psychol. 2002;82(2):116-140. doi: 10.1016/S0022-0965(02)00006-1 [DOI] [PubMed] [Google Scholar]
- 45.Rose SA, Feldman JF, Jankowski JJ, Caro DM. A longitudinal study of visual expectation and reaction time in the first year of life. Child Dev. 2002;73(1):47-61. doi: 10.1111/1467-8624.00391 [DOI] [PubMed] [Google Scholar]
- 46.De Schuymer L, De Groote I, Desoete A, Roeyers H. Gaze aversion during social interaction in preterm infants: a function of attention skills? Infant Behav Dev. 2012;35(1):129-139. doi: 10.1016/j.infbeh.2011.08.002 [DOI] [PubMed] [Google Scholar]
- 47.Pel JJM, Dudink J, Vonk M, Plaisier A, Reiss IKM, van der Steen J. Early identification of cerebral visual impairments in infants born extremely preterm. Dev Med Child Neurol. 2016;58(10):1030-1035. doi: 10.1111/dmcn.13115 [DOI] [PubMed] [Google Scholar]
- 48.Hodel AS, Senich KL, Jokinen C, Sasson O, Morris AR, Thomas KM. Early executive function differences in infants born moderate-to-late preterm. Early Hum Dev. 2017;113:23-30. doi: 10.1016/j.earlhumdev.2017.07.007 [DOI] [PubMed] [Google Scholar]
- 49.Ross-Sheehy S, Perone S, Macek KL, Eschman B. Visual orienting and attention deficits in 5- and 10-month-old preterm infants. Infant Behav Dev. 2017;46:80-90. doi: 10.1016/j.infbeh.2016.12.004 [DOI] [PubMed] [Google Scholar]
- 50.Kooiker MJG, Swarte RMC, Smit LS, Reiss IKM. Perinatal risk factors for visuospatial attention and processing dysfunctions at 1 year of age in children born between 26 and 32 weeks. Early Hum Dev. 2019;130:71-79. doi: 10.1016/j.earlhumdev.2019.01.015 [DOI] [PubMed] [Google Scholar]
- 51.Rose SA, Gottfried AW, Bridger WH. Effects of haptic cues on visual recognition memory in fullterm and preterm infants. Infant Behav Dev. 1979;2:55-67. doi: 10.1016/S0163-6383(79)80008-9 [DOI] [Google Scholar]
- 52.Rose SA. Enhancing visual recognition memory in preterm infants. Dev Psychol. 1980;16(2):85-92. doi: 10.1037/0012-1649.16.2.85 [DOI] [Google Scholar]
- 53.Rose SA, Feldman JF, Wallace IF. Individual differences in infants’ information processing: reliability, stability, and prediction. Child Dev. 1988;59(5):1177-1197. doi: 10.2307/1130482 [DOI] [PubMed] [Google Scholar]
- 54.Ross G, Tesman J, Auld PA, Nass R. Effects of subependymal and mild intraventricular lesions on visual attention and memory in premature infants. Dev Psychol. 1992;28(6):1067-1074. doi: 10.1037/0012-1649.28.6.1067 [DOI] [Google Scholar]
- 55.Wilcox T, Nadel L, Rosser R. Location memory in healthy preterm and full-term infants. Infant Behav Dev. 1996;19(3):309-323. doi: 10.1016/S0163-6383(96)90031-4 [DOI] [Google Scholar]
- 56.Bonin M, Pomerleau A, Malcuit G.. A longitudinal study of visual attention and psychomotor development in preterm and full-term infants during the first six months of life. Infant Behav Dev. 1998;21(1):103-118. doi: 10.1016/S0163-6383(98)90057-1 [DOI] [Google Scholar]
- 57.Rose SA, Feldman JF, Jankowski JJ. Attention and recognition memory in the 1st year of life: a longitudinal study of preterm and full-term infants. Dev Psychol. 2001;37(1):135-151. doi: 10.1037/0012-1649.37.1.135 [DOI] [PubMed] [Google Scholar]
- 58.Ortiz-Mantilla S, Choudhury N, Leevers H, Benasich AA. Understanding language and cognitive deficits in very low birth weight children. Dev Psychobiol. 2008;50(2):107-126. doi: 10.1002/dev.20278 [DOI] [PubMed] [Google Scholar]
- 59.Harel H, Gordon I, Geva R, Feldman R. Gaze behaviors of preterm and full-term infants in nonsocial and social contexts of increasing dynamics: visual recognition, attention regulation, and gaze synchrony. Infancy. 2011;16(1):69-90. doi: 10.1111/j.1532-7078.2010.00037.x [DOI] [PubMed] [Google Scholar]
- 60.Rose SA, Gottfried AW, Bridger WH. Cross-modal transfer in infants: relationship to prematurity and socioeconomic background. Dev Psychol. 1978;14(6):643-652. doi: 10.1037/0012-1649.14.6.643 [DOI] [Google Scholar]
- 61.Rose SA. Differential rates of visual information processing in full-term and preterm infants. Child Dev. 1983;54(5):1189-1198. doi: 10.2307/1129674 [DOI] [PubMed] [Google Scholar]
- 62.Lawson KR, Ruff HA, McCarton-Daum C, Kurtzberg D, Vaughan Jr. HG. Auditory–visual responsiveness in full-term and preterm infants. Dev Psychol. 1984;20(1):120-127. doi: 10.1037/0012-1649.20.1.120 [DOI] [Google Scholar]
- 63.Landry SH, Leslie NA, Fletcher JM, Francis DJ. Visual attention skills of premature infants with and without intraventricular hemorrhage. Infant Behav Dev. 1985;8(3):309-321. doi: 10.1016/0163-6383(85)90020-7 [DOI] [Google Scholar]
- 64.Spungen LB, Kurtzberg D, Vaughan HG Jr. Patterns of looking behavior in full-term and low birth weight infants at 40 weeks post-conceptional age. J Dev Behav Pediatr. 1985;6(5):287-294. doi: 10.1097/00004703-198510000-00011 [DOI] [PubMed] [Google Scholar]
- 65.Mash C, Quinn PC, Dobson V, Narter DB. Global influences on the development of spatial and object perceptual categorization abilities: evidence from preterm infants. Dev Sci. 1998;1(1):84-102. doi: 10.1111/1467-7687.00017 [DOI] [Google Scholar]
- 66.Ruff HA, McCarton C, Kurtzberg D, Vaughan HG Jr. Preterm infants’ manipulative exploration of objects. Child Dev. 1984;55(4):1166-1173. doi: 10.2307/1129985 [DOI] [PubMed] [Google Scholar]
- 67.Ruff HA. Attention and organization of behavior in high-risk infants. J Dev Behav Pediatr. 1986;7(5):298-301. doi: 10.1097/00004703-198610000-00004 [DOI] [PubMed] [Google Scholar]
- 68.Riese ML. Longitudinal assessment of temperament from birth to 2 years: a comparison of full-term and preterm infants. Infant Behav Dev. 1987;10(3):347-363. doi: 10.1016/0163-6383(87)90022-1 [DOI] [Google Scholar]
- 69.Landry SH, Chapieski ML. Visual attention during toy exploration in preterm infants: effects of medical risk and maternal interactions. Infant Behav Dev. 1988;11(2):187-204. doi: 10.1016/S0163-6383(88)80005-5 [DOI] [Google Scholar]
- 70.DiPietro JA, Porges SW, Uhly B. Reactivity and developmental competence in preterm and full-term infants. Dev Psychol. 1992;28(5):831-841. doi: 10.1037/0012-1649.28.5.831 [DOI] [Google Scholar]
- 71.Landry SH, Garner PW, Swank PR, Baldwin CD. Effects of maternal scaffolding during joint toy play with preterm and full-term infants. Merrill Palmer Q. 1996;42(2):177-199. [Google Scholar]
- 72.Cherkes-Julkowski M. Learning disability, attention-deficit disorder, and language impairment as outcomes of prematurity: a longitudinal descriptive study. J Learn Disabil. 1998;31(3):294-306. doi: 10.1177/002221949803100309 [DOI] [PubMed] [Google Scholar]
- 73.Pridham K, Becker P, Brown R. Effects of infant and caregiving conditions on an infant’s focused exploration of toys. J Adv Nurs. 2000;31(6):1439-1448. doi: 10.1046/j.1365-2648.2000.01448.x [DOI] [PubMed] [Google Scholar]
- 74.Petrie Thomas JH, Whitfield MF, Oberlander TF, Synnes AR, Grunau RE. Focused attention, heart rate deceleration, and cognitive development in preterm and full-term infants. Dev Psychobiol. 2012;54(4):383-400. doi: 10.1002/dev.20597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Soares D de A, von Hofsten C, Tudella E. Development of exploratory behavior in late preterm infants. Infant Behav Dev. 2012;35(4):912-915. doi: 10.1016/j.infbeh.2012.09.002 [DOI] [PubMed] [Google Scholar]
- 76.Sun J, Buys N. A comparison of sustained attention in very preterm and term infants. Intl J Child Adolesc Health. 2012;5(3):291-300. doi: 10.1515/ijamh.2012.042 [DOI] [Google Scholar]
- 77.de Jong M, Verhoeven M, van Baar AL. Attention capacities of preterm and term born toddlers: a multi-method approach. Early Hum Dev. 2015;91(12):761-768. doi: 10.1016/j.earlhumdev.2015.08.015 [DOI] [PubMed] [Google Scholar]
- 78.Lobo MA, Kokkoni E, Cunha AB, Galloway JC. Infants born preterm demonstrate impaired object exploration behaviors throughout infancy and toddlerhood. Phys Ther. 2015;95(1):51-64. doi: 10.2522/ptj.20130584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Reuner G, Weinschenk A, Pauen S, Pietz J. Cognitive development in 7- to 24-month-old extremely/very-to-moderately/late preterm and full-term born infants: the mediating role of focused attention. Child Neuropsychol. 2015;21(3):314-330. doi: 10.1080/09297049.2014.899571 [DOI] [PubMed] [Google Scholar]
- 80.Loi EC, Vaca KEC, Ashland MD, Marchman VA, Fernald A, Feldman HM. Quality of caregiver-child play interactions with toddlers born preterm and full term: antecedents and language outcome. Early Hum Dev. 2017;115:110-117. doi: 10.1016/j.earlhumdev.2017.10.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zuccarini M, Guarini A, Savini S, et al. Object exploration in extremely preterm infants between 6 and 9 months and relation to cognitive and language development at 24 months. Res Dev Disabil. 2017;68:140-152. doi: 10.1016/j.ridd.2017.06.002 [DOI] [PubMed] [Google Scholar]
- 82.Lejeune F, Lordier L, Pittet MP, et al. Effects of an early postnatal music intervention on cognitive and emotional development in preterm children at 12 and 24 months: preliminary findings. Front Psychol. 2019;10:494. doi: 10.3389/fpsyg.2019.00494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.van de Weijer-Bergsma E, Wijnroks L, Jongmans MJ. Attention development in infants and preschool children born preterm: a review. Infant Behav Dev. 2008;31(3):333-351. doi: 10.1016/j.infbeh.2007.12.003 [DOI] [PubMed] [Google Scholar]
- 84.Kavšek M, Bornstein MH. Visual habituation and dishabituation in preterm infants: a review and meta-analysis. Res Dev Disabil. 2010;31(5):951-975. doi: 10.1016/j.ridd.2010.04.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Stroup DF, Berlin JA, Morton SC, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. JAMA. 2000;283(15):2008-2012. doi: 10.1001/jama.283.15.2008 [DOI] [PubMed] [Google Scholar]
- 86.Cheng SH, Augustin C, Bethel A, et al. Using machine learning to advance synthesis and use of conservation and environmental evidence. Conserv Biol. 2018;32(4):762-764. doi: 10.1111/cobi.13117 [DOI] [PubMed] [Google Scholar]
- 87.Wells G, Shea B, O’Connell D, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. 2012. Accessed February 17, 2021. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp
- 88.Viechtbauer W. Conducting meta-analyses in R with the metaphor package. J Stat Softw. 2010;36(3). doi: 10.18637/jss.v036.i03 [DOI] [Google Scholar]
- 89.Cohen J. Statistical Power Analysis for the Behavioral Sciences. Academic Press; 1977. [Google Scholar]
- 90.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177-188. doi: 10.1016/0197-2456(86)90046-2 [DOI] [PubMed] [Google Scholar]
- 91.Higgins JPT, Thomas J, Chandler J, et al. , eds. Cochrane Handbook for Systematic Reviews of Interventions. Cochrane; 2019. doi: 10.1002/9781119536604 [DOI] [Google Scholar]
- 92.Viechtbauer W, Cheung MW-L. Outlier and influence diagnostics for meta-analysis. Res Synth Methods. 2010;1(2):112-125. doi: 10.1002/jrsm.11 [DOI] [PubMed] [Google Scholar]
- 93.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629-634. doi: 10.1136/bmj.315.7109.629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Duval S, Tweedie R. Trim and fill: a simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics. 2000;56(2):455-463. doi: 10.1111/j.0006-341X.2000.00455.x [DOI] [PubMed] [Google Scholar]
- 95.Burstein O, Zevin Z, Geva R. Preterm birth and the development of visual attention during the first two years of life: a systematic review and meta-analysis (data set). Version 2. Mendeley Data. February 2, 2021. doi: 10.17632/y6y85b5pty.2 [DOI] [PMC free article] [PubMed]
- 96.Gurevitz M, Geva R, Varon M, Leitner Y. Early markers in infants and toddlers for development of ADHD. J Atten Disord. 2014;18(1):14-22. doi: 10.1177/1087054712447858 [DOI] [PubMed] [Google Scholar]
- 97.Geva R, Feldman R. Circadian sleep-wake rhythms in preterm infants. In: Salvenmoser O, Meklau B, eds. Biological Clocks: Effects On Behavior, Health and Outlook. NOVA Science Publishers Inc; 2008:101-120. [Google Scholar]
- 98.Munoz DP, Everling S. Look away: the anti-saccade task and the voluntary control of eye movement. Nat Rev Neurosci. 2004;5(3):218-228. doi: 10.1038/nrn1345 [DOI] [PubMed] [Google Scholar]
- 99.Geldof CJA, de Kieviet JF, Dik M, Kok JH, van Wassenaer-Leemhuis AG, Oosterlaan J. Visual search and attention in five-year-old very preterm/very low birth weight children. Early Hum Dev. 2013;89(12):983-988. doi: 10.1016/j.earlhumdev.2013.08.021 [DOI] [PubMed] [Google Scholar]
- 100.Pizzo R, Urben S, Van Der Linden M, et al. Attentional networks efficiency in preterm children. J Int Neuropsychol Soc. 2010;16(1):130-137. doi: 10.1017/S1355617709991032 [DOI] [PubMed] [Google Scholar]
- 101.Twilhaar ES, Belopolsky AV, de Kieviet JF, van Elburg RM, Oosterlaan J. Voluntary and involuntary control of attention in adolescents born very preterm: a study of eye movements. Child Dev. 2020;91(4):1272-1283. doi: 10.1111/cdev.13310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Nosarti C, Giouroukou E, Micali N, Rifkin L, Morris RG, Murray RM. Impaired executive functioning in young adults born very preterm. J Int Neuropsychol Soc. 2007;13(4):571-581. doi: 10.1017/S1355617707070725 [DOI] [PubMed] [Google Scholar]
- 103.Mulder H, Pitchford NJ, Hagger MS, Marlow N. Development of executive function and attention in preterm children: a systematic review. Dev Neuropsychol. 2009;34(4):393-421. doi: 10.1080/87565640902964524 [DOI] [PubMed] [Google Scholar]
- 104.Breeman LD, Jaekel J, Baumann N, Bartmann P, Wolke D. Attention problems in very preterm children from childhood to adulthood: the Bavarian Longitudinal Study. J Child Psychol Psychiatry. 2016;57(2):132-140. doi: 10.1111/jcpp.12456 [DOI] [PubMed] [Google Scholar]
- 105.Manna A, Raffone A, Perrucci MG, et al. Neural correlates of focused attention and cognitive monitoring in meditation. Brain Res Bull. 2010;82(1-2):46-56. doi: 10.1016/j.brainresbull.2010.03.001 [DOI] [PubMed] [Google Scholar]
- 106.Xie W, Mallin BM, Richards JE. Development of infant sustained attention and its relation to EEG oscillations: an EEG and cortical source analysis study. Dev Sci. 2018;21(3):e12562. doi: 10.1111/desc.12562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Lansink JM, Mintz S, Richards JE. The distribution of infant attention during object examination. Dev Sci. 2000;3(2):163-170. doi: 10.1111/1467-7687.00109 [DOI] [Google Scholar]
- 108.Reynolds GD. Infant visual attention and object recognition. Behav Brain Res. 2015;285:34-43. doi: 10.1016/j.bbr.2015.01.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.de Kieviet JF, Zoetebier L, van Elburg RM, Vermeulen RJ, Oosterlaan J. Brain development of very preterm and very low-birthweight children in childhood and adolescence: a meta-analysis. Dev Med Child Neurol. 2012;54(4):313-323. doi: 10.1111/j.1469-8749.2011.04216.x [DOI] [PubMed] [Google Scholar]
- 110.Nosarti C, Froudist-Walsh S. Alterations in development of hippocampal and cortical memory mechanisms following very preterm birth. Dev Med Child Neurol. 2016;58(suppl 4):35-45. doi: 10.1111/dmcn.13042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Zhou L, Zhao Y, Liu X, et al. Brain gray and white matter abnormalities in preterm-born adolescents: A meta-analysis of voxel-based morphometry studies. PLoS One. 2018;13(10):e0203498. doi: 10.1371/journal.pone.0203498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Fanaroff AA, Hack M, Walsh MC. The NICHD neonatal research network: changes in practice and outcomes during the first 15 years. Semin Perinatol. 2003;27(4):281-287. doi: 10.1016/S0146-0005(03)00055-7 [DOI] [PubMed] [Google Scholar]
- 113.Roué JM, Kuhn P, Lopez Maestro M, et al. Eight principles for patient-centred and family-centred care for newborns in the neonatal intensive care unit. Arch Dis Child Fetal Neonatal Ed. 2017;102(4):F364-F368. doi: 10.1136/archdischild-2016-312180 [DOI] [PubMed] [Google Scholar]
- 114.Suberi M, Morag I, Strauss T, Geva R. Feeding imprinting: the extreme test case of premature infants born with very low birth weight. Child Dev. 2018;89(5):1553-1566. doi: 10.1111/cdev.12923 [DOI] [PubMed] [Google Scholar]
- 115.Berndt M, Bäuml JG, Menegaux A, et al. Impaired structural connectivity between dorsal attention network and pulvinar mediates the impact of premature birth on adult visual-spatial abilities. Hum Brain Mapp. 2019;40(14):4058-4071. doi: 10.1002/hbm.24685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Sentenac M, Boutron I, Draper ES, et al. Defining very preterm populations for systematic reviews with meta-analyses. JAMA Pediatr. 2020;174(10):997-999. doi: 10.1001/jamapediatrics.2020.0956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Altman DG. Making research articles fit for purpose: structured reporting of key methods and findings. Trials. 2015;16:53. doi: 10.1186/s13063-015-0575-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Appelbaum M, Cooper H, Kline RB, Mayo-Wilson E, Nezu AM, Rao SM. Journal article reporting standards for quantitative research in psychology: the APA Publications and Communications Board task force report. Am Psychol. 2018;73(1):3-25. doi: 10.1037/amp0000191 [DOI] [PubMed] [Google Scholar]
- 119.von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP; STROBE Initiative . The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Ann Intern Med. 2007;147(8):573-577. doi: 10.7326/0003-4819-147-8-200710160-00010 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Burstein O, Zevin Z, Geva R. Preterm birth and the development of visual attention during the first two years of life: a systematic review and meta-analysis (data set). Version 2. Mendeley Data. February 2, 2021. doi: 10.17632/y6y85b5pty.2 [DOI] [PMC free article] [PubMed]
Supplementary Materials
eFigure 1. Funnel Plots of Effect Sizes and Standard Errors Used for the Trim and Fill Analyses for Assessing Possible Publication Biases
eFigure 2. A Flow Diagram of Studies’ Selection Process
eFigure 3. Forest and Funnel Plots for the Meta-Analysis on the Differences in Visual Following (Animate Stimuli) Between Preterm and Full-Term Born Neonates
eTable 1. Characteristics of the Studies Included in the Meta-Analyses
eTable 2. Preterm Birth and the Development of Visual Attention During the First Two Years of Life: A Systematic Review and Meta-analysis
eAppendix 1. Modified Newcastle-Ottawa Scale for Assessment of Studies Quality and Risk of Bias
eAppendix 2. Description of the Moderation Analyses
eAppendix 3. Moderation Analysis in the Latency to Fixate Meta-analysis
eAppendix 4. Moderation Analysis in the Habituation Meta-analysis
eFigure 4. Graphical Depiction of the Moderation of the Differences Between the Preterm and Full-term Groups in Habituation by Birth Era
eAppendix 5. Additional Analyses in the Focused Attention Meta-analysis
eFigure 5. Graphical Depiction of the Moderation of the Differences Between the Preterm and Full-term Groups in Focused Attention by Birth Era and GA Group
eReferences