Abstract
Background:
In patients with symptomatic vertebrobasilar intracranial atherosclerotic disease (ICAD), impaired distal flow predicts recurrent stroke, but limited data exist on the association between perfusion status and recurrent stroke in anterior circulation ICAD.
Methods:
This is a retrospective study of patients hospitalized for symptomatic ICAD with 50–99% stenosis of the intracranial carotid or middle cerebral artery. The primary outcome is recurrent symptomatic ischemic stroke in the territory of the artery with ≥50% stenosis within 90 days. The primary predictor is distal hypoperfusion on MR or CT perfusion, defined as a ≥15 mL volume of territory of the symptomatic artery with Tmax >6 seconds.
Results:
Fifty patients met inclusion criteria, including 15 (30%) with recurrent stroke and 15 (30%) with distal hypoperfusion. Distal hypoperfusion was present in 10/15 (66.7%) with recurrent stroke versus 5/35 (14.3%) without recurrent stroke. The hazard ratio for recurrent stroke in patients with distal hypoperfusion was 6.80 (95% CI 2.31–20.0).
Conclusion:
Distal hypoperfusion in acutely symptomatic ICAD with 50–99% stenosis is associated with stroke recurrence. Distal hypoperfusion could be used to enrich future trials of secondary stroke prevention in ICAD patients.
Keywords: acute ischemic hemorrhage, intracranial atherosclerosis, perfusion imaging, recurrent stroke
Introduction
Intracranial atherosclerotic disease (ICAD) is the cause of ischemic stroke in ~40% of Asians, 30% of African-Americans, and 10% of Caucasians, making it the most common cause of stroke in the world, with a high risk of early recurrence.1–3 ICAD causes stroke through different mechanisms, including atheroembolic, branch artery occlusion, thrombo-occlusive, and impaired distal perfusion.4 However, existing trials have not differentiated among these etiologies. Improved risk stratification among the various ICAD mechanisms could enrich future studies of ICAD stroke prevention. Our prior research has shown that impaired distal perfusion detected by noninvasive imaging can predict neurologic deterioration after ICAD stroke,5 but data on its association with recurrent stroke in patients with anterior circulation symptomatic ICAD is very limited.6 In addition, available data is from a small single center retrospective study, therefore lacking generalizability.
In this study, we aim to determine the association between perfusion delay and early recurrence in patients with acutely symptomatic proximal anterior circulation intracranial stenosis.
Methods
The study was approved by the Institutional Review Board in both centers. This is a retrospective study of patients hospitalized for symptomatic ICAD at two academic medical centers. We defined symptomatic ICAD as an intracranial internal carotid artery (ICA) or middle cerebral artery (MCA) M1 segment atherosclerotic lesion creating 50–99% stenosis that had caused ischemic stroke within the prior 48 hours. This was determined on CT angiography or MR angiography using previously defined criteria.7 Patients with tandem lesions (concomitant extra-cranial lesions causing 50–99% stenosis or total occlusion) were excluded. Causality of the stroke was determined by two board certified vascular neurologists (SY, AD). We excluded patients with bilateral hemispheric or posterior circulation stroke. Patients included were those with 70–99% stenosis and MRI perfusion (MRP) at study site 1 or 50–99% stenosis and CT perfusion (CTP) at study site 2. Perfusion imaging was obtained within 48 hours of stroke onset and processed using the RAPID software (IschemaView, Menlo Park, CA).
Our primary outcome was recurrent stroke due to recurrent infarct or infarct expansion in the territory of the stenotic artery within 90 days that met American Heart Association criteria for ischemic stroke.8 The primary predictor was distal hypoperfusion, with penumbra defined as a Tmax >6 seconds (Tmax6) and volume ≥15 mL of penumbra distal to the symptomatic ICAD, similar to prior studies.9 We fit Cox proportional hazards models to the outcome of recurrent stroke and report hazard ratios, both unadjusted (Model 1) and adjusted for three potential confounders in additional models, to limit overfitting: patient age, sex, prior stroke (Model 2); hypertension, diabetes, smoking (Model 3); and statin therapy, dual antiplatelet therapy, and symptomatic ICAD 50–69% vs 70–99% (Model 4). Stata 15.1 was used for statistical analysis and statistical significance was based on 0.05 level of significance.
Results
Fifty patents met inclusion criteria, 22 at study site 1 and 28 at study site 2. Baseline demographics are seen in Table 1, stratified by the primary outcome. Symptomatic ICAD was present in the intracranial ICA in 15 (30%) patients and in the MCA M1 segment in 35 (70%) patients. The index stroke was confirmed on MRI in 45 (90%) patients and on CT in the remaining 5 patients. At study site 1, all patients had 70–99% stenosis and an MRP study. At study site 2, 28 had 50–99% stenosis and a CTP study. In both sites combined, 20 (40%) of patients had 50–69% stenosis and 30 (60%) had 70–99%. The primary outcome of recurrent stroke was found in 15 (30%, 95% CI 17.9% – 44.6%) patients and distal hypoperfusion (Tmax6 ≥15 mL) in 15 (30%, 95% CI 17.9% – 44.6%) patients. Recurrent stroke occurred at a median of 7 days (range 1–77 days). Distal hypoperfusion was present in 10/15 (66.7%, 95% CI 38.4% – 88.2%) with recurrent stroke versus 5/35 (14.3%, 95% CI 4.8% – 30.3%) without recurrent stroke. The hazard ratio for recurrent stroke in patients with distal hypoperfusion was 6.80 (95% CI 2.31–20.0) (Figure 1), and similar hazard ratios were seen in the adjusted models (Table 2).
Table 1.
Baseline Demographics for the full cohort (n=50) and for patients with recurrent stroke (n=16) versus without recurrent stroke (n=34) with p value for difference.
| Full cohort (n=50) | Recurrent stroke (n=15) | No recurrent stroke (n=35) | p value | |
|---|---|---|---|---|
| Age | 68.1±14.6 | 71.3±14.1 | 66.8±14.1 | 0.316 |
| Male sex | 25, 50% | 7, 46.7% | 18, 51.4% | 0.758 |
| Prior stroke | 13, 26% | 4, 26.7% | 9, 25.7% | 0.994 |
| Hypertension | 40, 80% | 13, 86.7% | 27, 77.1% | 0.440 |
| Hyperlipidemia | 28, 56% | 9, 60% | 19, 54.3% | 0.709 |
| Diabetes Mellitus | 19, 38% | 7, 46.7% | 12, 34.3% | 0.409 |
| Atrial Fibrillation | 8, 16% | 4, 26.7% | 4, 11.4% | 0.178 |
| Current smoking | 10, 20% | 3, 20% | 7, 20.6% | 1.000 |
| Stenosis 70–99% | 30, 60% | 12, 80% | 18, 51.4% | 0.059 |
| Dual antiplatelet therapy | 26, 52% | 10, 66.7% | 16, 45.7% | 0.174 |
| Mono antiplatelet therapy | 16, 32% | 4, 26.7% | 12, 34.3% | 0.597 |
| Anticoagulation | 8, 16% | 2, 13.3% | 6, 17.1% | 0.736 |
| Statin therapy | 45, 90% | 13, 86.7% | 32, 91.4% | 0.607 |
| Index stroke core volume (mL) | 4.4±11.8 | 8.8±20.2 | 2.5±4.5 | 0.085 |
| Baseline Tmax6 hypoperfusion volume (mL) | 30.5±51.5 | 67.5±69.1 | 14.6±31.5 | <0.001 |
| Absolute mismatch volume (mL) | 26.1±48.0 | 58.7±65.4 | 12.1±29.7 | 0.001 |
Continuous variables presented as mean±SD, binary variables as n,%. Intergroup differences tested with Student’s t-test for continuous variables and the chi-squared for binary variables.
Figure 1.
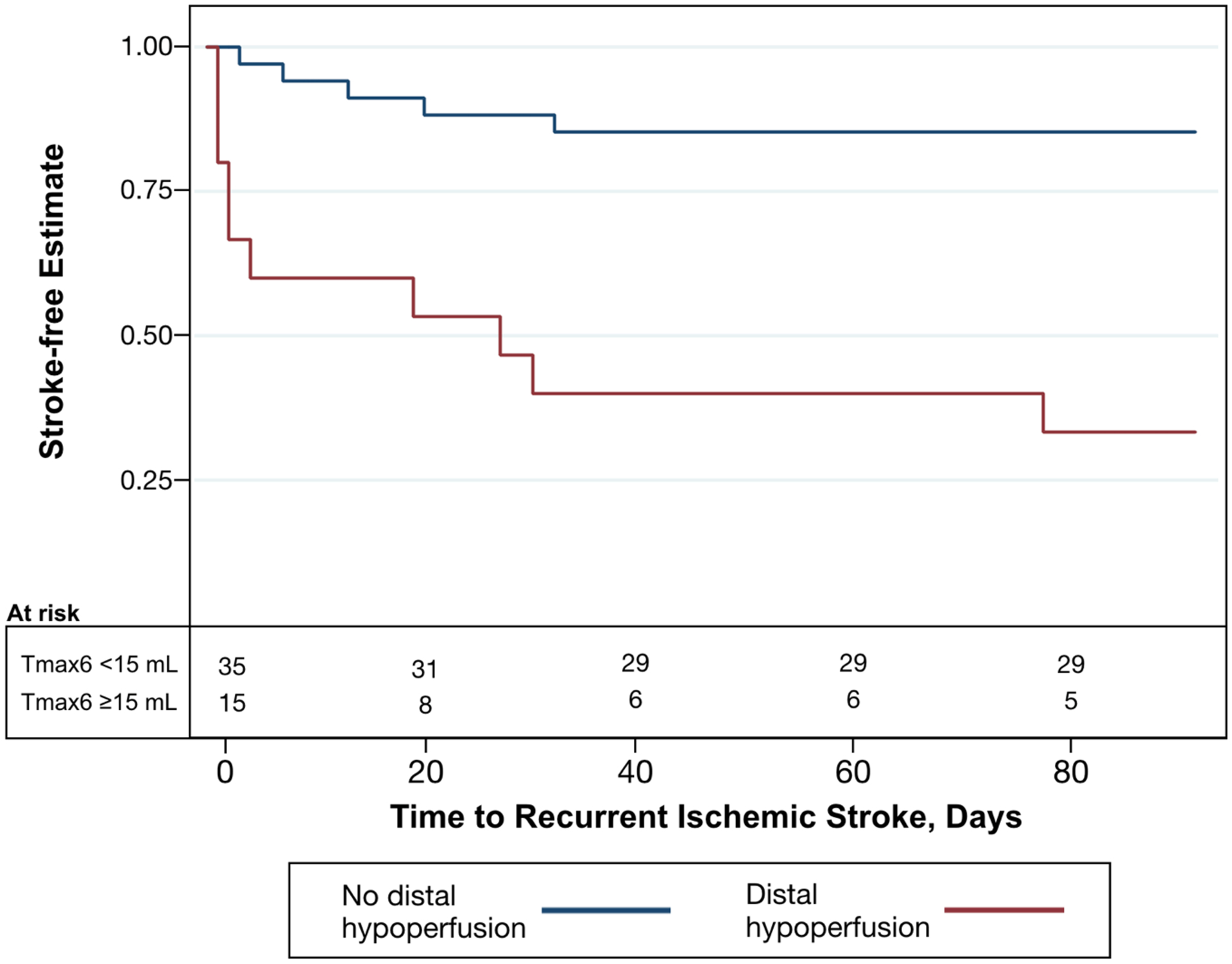
Kaplan-Meier curve showing estimates of stroke-free survival for patients with distal hypoperfusion (Tmax6 ≥15 mL, red line) versus without distal hypoperfusion (Tmax6 <15 mL, blue line).
Table 2.
Hazard ratios for recurrent stroke with the predictor of distal hypoperfusion.
| Hazard Ratio | 95% CI | p value | |
|---|---|---|---|
| Model 1 | 6.80 | 2.31–20.0 | 0.001 |
| Model 2 | 8.01 | 2.69–24.2 | <0.001 |
| Model 3 | 9.56 | 3.12–29.3 | <0.001 |
| Model 4 | 6.90 | 1.94–24.6 | 0.003 |
Model 1 is unadjusted; Model 2 is adjusted for patient age, sex, prior stroke; Model 3 is adjusted for hypertension, diabetes, smoking; and Model 4 is adjusted for statin therapy, dual antiplatelet therapy, and symptomatic ICAD ≥50–69% vs ≥70–99%
We found a similar effect of distal hypoperfusion on recurrent stroke risk at both study sites. At study site 1, which used CTP, the hazard ratio for recurrent stroke was 5.15 (95% CI 1.14–23.2) and at study site 2, which used MRP, the hazard ratio was 9.76 (95% CI 1.19–80.0).
Discussion
Our results suggest that hypoperfusion distal to recently symptomatic ICAD is associated with recurrent ischemic stroke within 90 days. The effect size was relatively large and the association persisted after adjustment for potential confounders and study site.
Mechanisms of Association
Aggressive medical treatment has been shown to be effective in stabilizing acutely symptomatic plaque,10,11 but it is questionable if it could significantly improve distal blood flow across a stenosis, especially in the acute setting,12 when the risk of neurological deterioration has been shown to be the highest.13 Therefore it is intuitive that patients with impaired distal blood flow are more likely to fail medical treatment.
Our findings can be compared to the Vertebrobasilar Flow Evaluation and Risk of Transient Ischemic Attack and Stroke (VERiTAS) study, which included patients with a symptomatic ≥50% stenosis or occlusion of a vertebral artery.14 In VERiTAS, distal low flow, measured with quantitative MR angiography, was also associated with recurrent stroke (HR 11.55, 95% CI 1.88–71.0), with a comparable effect size to our study (HR 6.80, 95% CI 2.31–20.0). However, patients in VERiTAS were enrolled at a median of >2 weeks from the qualifying stroke, while ours was 48 hours of stroke onset, allowing capture of more early stroke recurrence. Furthermore, studies have shown that in patients with anterior circulation ICAD, patients with borderzone infarcts (a marker of impaired distal perfusion) had a higher risk of recurrent stroke than those with non-borderzone infarct pattern.15, 16 Moreover, another study showed that in patients with symptomatic severe middle cerebral artery stenosis, severe hypoperfusion volume ratio (HVR) defined as HVR ≥ 50% is associated with recurrent stroke during 1 year follow up (OR 12.93 95% CI 1.57 – 106.24, p = 0.017).6
Therapeutic implications
Our study has several therapeutic implications. First, perfusion imaging may be a useful tool to help risk stratify patients with acutely symptomatic anterior circulation ICAD and identify those who are likely to fail aggressive medical treatment. Previous studies have suggested that impaired distal perfusion is associated with recurrent stroke in patients with acutely symptomatic proximal anterior circulation stenosis.5, 6, 17 That being said, due to the scarcity of evidence and major limitations of these studies, the optimal definition of impaired distal perfusion or flow in patients with acutely symptomatic proximal anterior circulation ICAD remains unknown and needs further study. Second, patients with symptomatic proximal anterior circulation ICAD and impaired distal perfusion may be a subgroup who may benefit from surgical intervention. In the Stenting and Aggressive Medical Management for Preventing Recurrent Stroke in Intracranial Stenosis (SAMMPRIS) trial, the 30-day risk of stroke or death in the stenting arm was 15% (95% CI 10% to 20%).18 In our study, the risk of recurrence was nearly 66.7% (95% CI 38.4% – 88.2%) in patients meeting our pre-specified definition for impaired distal perfusion, which is significantly higher than patients randomized to the stenting arm of SAMMPRIS. In fact, a post-hoc analysis of SAMMPRIS showed that patients with borderzone infarcts had fewer events with stenting as compared with maximal medical treatment (26.4% vs. 18.2%, p = 0.3), but this analysis was probably underpowered to achieve statistical significance. Therefore, larger studies are needed to identify the optimal definition of impaired distal perfusion in patients with acutely symptomatic proximal anterior circulation ICAD, aiming to identify a sub-group where the efficacy and safety of endovascular treatment can be studied in clinical trials.
Study limitations
Our study has several limitations, the most important being that it is a retrospective cohort from academic medical centers, and therefore susceptible to selection bias. For example, patients with perfusion imaging as part of their standard of care workup were presumably considered higher risk, compared to those who did not received perfusion imaging; this may account for the high rate of recurrent stroke in the study. We also do not have data on other possible confounders for our recurrent stroke outcome, such as blood pressure levels or medication compliance. Although the mix of MRP and CTP is a potential limitation, we used the same software for post-processing. Additionally, the small sample size did not allow for a fully adjusted model and reduced precision of our point estimates.
Conclusion
Distal hypoperfusion in recently symptomatic ICAD with 50–99% stenosis may be associated with stroke recurrence or neurological deterioration. Although the present study cannot definitely determine this as the mechanistic cause for the recurrent strokes, patients with Tmax6 volumes ≥15 may represent a high risk group for study in future trials of secondary stroke prevention in ICAD patients. Further prospective validation of these findings is needed.
Sources of Funding:
Dr. de Havenon is supported by NIH-NINDS K23NS105924.
References
- 1.Holmstedt CA, Turan TN, Chimowitz MI. Atherosclerotic intracranial arterial stenosis: Risk factors, diagnosis, and treatment. The Lancet. Neurology 2013;12:1106–1114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bang OY. Intracranial atherosclerosis: Current understanding and perspectives. Journal of stroke. 2014;16:27–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sangha RS, Naidech AM, Corado C, Ansari SA, Prabhakaran S. Challenges in the medical management of symptomatic intracranial stenosis in an urban setting. Stroke. 2017;48:2158–2163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kim JS, Nah HW, Park SM, Kim SK, Cho KH, Lee J, et al. Risk factors and stroke mechanisms in atherosclerotic stroke: Intracranial compared with extracranial and anterior compared with posterior circulation disease. Stroke. 2012;43:3313–3318 [DOI] [PubMed] [Google Scholar]
- 5.Yaghi S, Prabhakaran S, Khatri P, Liebeskind DS. Intracranial atherosclerotic disease. Stroke. 2019;50:1286–1293 [DOI] [PubMed] [Google Scholar]
- 6.Lyu J, Ma N, Tian C, Xu F, Shao H, Zhou X, et al. Perfusion and plaque evaluation to predict recurrent stroke in symptomatic middle cerebral artery stenosis. Stroke and vascular neurology. 2019;4:129–134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Samuels OB, Joseph GJ, Lynn MJ, Smith HA, Chimowitz MI. A standardized method for measuring intracranial arterial stenosis. AJNR. American journal of neuroradiology 2000;21:643–646 [PMC free article] [PubMed] [Google Scholar]
- 8.Sacco RL, Kasner SE, Broderick JP, Caplan LR, Connors JJ, Culebras A, et al. An updated definition of stroke for the 21st century: A statement for healthcare professionals from the american heart association/american stroke association. Stroke. 2013;44:2064–2089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Albers GW, Marks MP, Kemp S, Christensen S, Tsai JP, Ortega-Gutierrez S, et al. Thrombectomy for stroke at 6 to 16 hours with selection by perfusion imaging. The New England journal of medicine. 2018;378:708–718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wong KS, Chen C, Fu J, Chang HM, Suwanwela NC, Huang YN, et al. Clopidogrel plus aspirin versus aspirin alone for reducing embolisation in patients with acute symptomatic cerebral or carotid artery stenosis (clair study): A randomised, open-label, blinded-endpoint trial. The Lancet. Neurology 2010;9:489–497 [DOI] [PubMed] [Google Scholar]
- 11.Libby P, Aikawa M. Mechanisms of plaque stabilization with statins. The American journal of cardiology. 2003;91:4b–8b [DOI] [PubMed] [Google Scholar]
- 12.Dakay K, Yaghi S. Symptomatic intracranial atherosclerosis with impaired distal perfusion: A case study. Stroke. 2018;49:e10–e13 [DOI] [PubMed] [Google Scholar]
- 13.Yaghi S, Rostanski SK, Boehme AK, Martin-Schild S, Samai A, Silver B, et al. Imaging parameters and recurrent cerebrovascular events in patients with minor stroke or transient ischemic attack. JAMA neurology. 2016;73:572–578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Amin-Hanjani S, Pandey DK, Rose-Finnell L, Du X, Richardson D, Thulborn KR, et al. Effect of hemodynamics on stroke risk in symptomatic atherosclerotic vertebrobasilar occlusive disease. JAMA neurology. 2016;73:178–185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wabnitz AM, Derdeyn CP, Fiorella DJ, Lynn MJ, Cotsonis GA, Liebeskind DS, et al. Hemodynamic markers in the anterior circulation as predictors of recurrent stroke in patients with intracranial stenosis. Stroke. 2018:Strokeaha118020840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yaghi S, Grory BM, Prabhakaran S, Yeatts SD, Cutting S, Jayaraman M, et al. Infarct pattern, perfusion mismatch thresholds, and recurrent cerebrovascular events in symptomatic intracranial stenosis. Journal of neuroimaging : official journal of the American Society of Neuroimaging 2019;29:640–644 [DOI] [PubMed] [Google Scholar]
- 17.Yaghi S, Khatri P, Prabhakaran S, Yeatts SD, Cutting S, Jayaraman M, et al. What threshold defines penumbral brain tissue in patients with symptomatic anterior circulation intracranial stenosis: An exploratory analysis. Journal of neuroimaging : official journal of the American Society of Neuroimaging 2019;29:203–205 [DOI] [PubMed] [Google Scholar]
- 18.Chimowitz MI, Lynn MJ, Derdeyn CP, Turan TN, Fiorella D, Lane BF, et al. Stenting versus aggressive medical therapy for intracranial arterial stenosis. The New England journal of medicine. 2011;365:993–1003 [DOI] [PMC free article] [PubMed] [Google Scholar]


