Abstract
Healthcare workers (HCWs) due to their job profile are at utmost risk of contracting severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) infection. Serological survey is an useful tool for vulnerability mapping in an infectious disease pandemic. The aim of the current study was to assess seroprevalence of IgG against SARS-CoV-2 and its determinants among HCWs of a tertiary healthcare facility of India. It was an observational study, cross-sectional in design conducted among 919 HCWs of All India Institute of Medical Sciences, Patna, Bihar, India during September, 2020. In results, IgG seroprevalence for SARS-CoV-2 among the study subjects was 13.3% [95% confidence interval (CI): 11.2-15.6%]. In univariate logistic regression analysis; gender, occupation, place of posting, use of full personal protective equipment (PPE), prior corona virus disease (COVID)-19 infection, influenza like illness (ILI), use of steam inhalation, consumption of azithromycin, zinc and vitamin C were the significant attributes which affected the IgG seropositivity for SARS-CoV-2. In the multivariable logistic regression model; occupation, place of posting, prior COVID-19 infection and ILI were significant determinants of IgG seropositivity for SARS-CoV-2. To conclude, majority of the HCWs were found to be IgG seronegative for SARS-CoV-2. Till availability of effective vaccine all of the HCWs should abide by infection prevention and control (IPC) measures to keep themselves and their contacts protected from SARS-CoV-2.
Keywords: COVID-19, immunoglobulin G, seroepidemiologic studies, health personnel, epidemiologic factors
Introduction
Since its emergence in late 2019 corona virus disease-19 (COVID-19) emerged as global public health emergency of the century affected about ninety million and claimed about one and two million lives till date [1,2]. India reported its first case of COVID-19 in January, 2020 and currently it is second in terms of total number of reported cases following USA with over ten million cases and over hundred and fifty thousand reported deaths [1,3].
Serum antibody response (IgM and IgG) to SARS-CoV-2 is detectable in between 10-21 days of infection in most of the cases with median seroconversion time of 11 and 14 days after symptom onset for IgM and IgG respectively [4]. The level of antibody production is proportional to severity of symptoms and may reduce or even disappear after three months after the disease onset [4,5]. The aim of a serological survey is to measure proportion of people in a community or group have detectable and moreover protective level of antibodies (especially IgG) against a particular disease of interest. It not only helps to track progress of an infectious disease pandemic like COVID-19 in a certain community or group moreover it also helps to quantify risk of the members of that particular community or group to subsequent infection [6-8].
Healthcare workers (HCWs) are backbone of any health care system more so during times of a global pandemic like COVID-19. During the ongoing COVID-19 pandemic, health-care workers are at a substantially increased risk of contracting SARS-CoV-2. This is because of their more frequent contact with a confirmed or suspect COVID-19 case or their body fluids during discharging their duties [9,10]. Some of the COVID-19 cases among HCWs may gone undetected due to absence of symptoms or decision of not undergoing antigen test for the disease [11]. As per existing literature seroprevalence of IgG for SARS-CoV-2 among the HCWs varies between 0.8-13.6%. Previous studies have also reported that age, gender, place of posting, comorbidity status, personal protective equipment (PPE) use, level of exposure, prior SARS-CoV-2 infection and influenza like illness (ILI) significantly determines IgG seropositivity for SARS-CoV-2 among HCWs [5,12-19]. Although there may be several other factors which might influence immunogenesis against SARS-CoV-2 like diet preference, steam inhalation, consumption of zinc, azithromycin and multivitamins [20-23]. Most of the prior studies in this regard were conducted in western countries like Belgium, Germany, Spain, Italy and USA [5,13,14,16,18,19]. Among the Indian studies most were reported from western part of the country [12,15,17]. With this background and to bring about better understanding on the issue the current research was envisioned to assess IgG seropositivity for SARS-CoV-2 and its determinants among HCWs of a COVID-19 dedicated tertiary care health facility of India. The study has taken into account all the prior reported and postulated factors which could influence SARS-CoV-2 IgG seropositivity among the HCWs.
Methods
Study type and design
It was a monocentric, observational study, cross-sectional in design conducted among HCWs of All India Institute of Medical Sciences (AIIMS), Patna, Bihar, India during the month of September 2020.
Study setting
AIIMS-Patna is one of the centres of excellence in terms of medical education and patient care in India. Since the emergence of the pandemic in the state of Bihar the institute has provided best possible care and treatment for the attending COVID-19 patients from not only Bihar but some adjoining states too. On 10th July 2020, the institute was designated as COVID-19 dedicated hospital by the Government of Bihar. Currently the institute has about 460 general and 60 intensive care unit (ICU) beds for the attending COVID-19 patients manned by approximately 3150 HCWs.
Sample size, sampling and enrolment
Assuming that at least 11.1% (IgG seroprevalence among HCWs reported by a prior Indian study by Kumar et al. [17]) of the subjects in the study population will be IgG seropositive for SARS-CoV-2, adjusting for finite population size (3150), 20% relative precision (~2.2% absolute precision) and 95% confidence, the final minimum sample size for the study was calculated to be 624. The sample size was calculated using ‘statulator’, an online sample size calculator. The study was envisioned for HCWs of AIIMS-Patna only. Thus, any HCW who was working in AIIMS-Patna during the study period were included while those who were not working in the institute and unwilling to participate were excluded. For enrolment in the study, email and short message service (SMS) invitation to all the working staff in the institute during the study period was sent which contained their scheduled date and venue of blood sample collection for antibody testing. Before 5 ml blood sample collection for SARS-CoV-2 IgG testing they were self-administered a structured schedule along with a consent form to obtain their background characteristics and consent for the study respectively. The serum IgG report of the study subjects were made available in health management information system of AIIMS-Patna and a designated report dispensing counter in outpatient department (OPD) within 24 hours of blood sample collection. In total 967 study subjects participated in the study which is about 30% of our total workforce. Out of these 919 study subjects met the eligibility criteria. Data for all the variables were available in case of 689 study subjects. The details of recruitment process of the study subjects is depicted in Figure 1.
Figure 1.
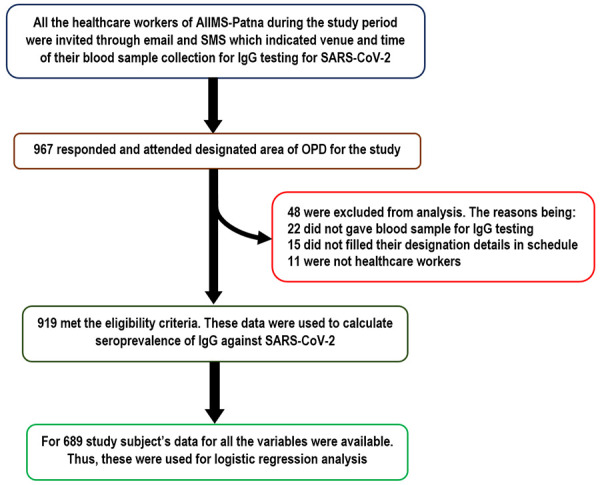
Flowchart showing recruitment of the study subjects.
Study variables
The structured schedule comprised of their sociodemographic details [age in completed years, gender (male/female)], occupational characteristics [occupation (doctor/nurse/technician/account staff/attendant/sanitary staff/others), place of posting (triage area/wards/ICUs/laboratories/others), whether exposed to confirmed COVID-19 case or their body fluids during duty, if yes average duration of exposure per duty shift (in hours), personal protective equipment (PPE) used during duty], personal history [currently smokes (yes/no), consumes alcohol (yes/no), known chronic co-morbidity status (yes/no), If yes name of co-morbidity suffering from, diet preference (vegetarian/non-vegetarian), history of prior COVID infection detected by reverse-transcriptase-polymerase chain reaction (RTPCR) or rapid antigen test for SARS-CoV-2 (yes/no), history of ILI in last 8 months (yes/no)] and practices related to the disease in last 8 months [used masks other than workplace (yes/no), sanitiser other than workplace (yes/no), steam inhalation (yes/no), hot beverages like hot water, tea and coffee (yes/no), consumed hydroxychloroquine (HCQ) (yes/no), azithromycin (yes/no), zinc (yes/no), multivitamin (yes/no), vitamin C (yes/no), vitamin E (yes/no)].
Some operational definitions used in the study were as following
Full PPE
Those who reported to use goggles, N-95 mask, gown covering the whole body except hand, foot and front of face, double layer gloves and shoe cover were considered to be using full PPE.
Serum IgG level for SARS-CoV-2
It was estimated using chemiluminescent immunoassay (CLIA) named ‘ADVIA Centaur COV2G’ which is a qualitative and semi-quantitative assay with excellent reported sensitivity (100.0%) and specificity (99.8%) by the manufacturer [24].
IgG seropositive for SARS-CoV-2
Those with serum IgG level of 1.00 or higher was considered as IgG seropositive for SARS-CoV-2 [24].
Ethical Issues
Ethical clearance of the Institutional Ethics Committee (IEC) of AIIMS-Patna (Ref. No. -AIIMS/Pat/IEC/2020/575) was taken before conducting the research. Informed written consent of each study subject was obtained before their enrolment in the study. The data analysis and manuscript drafting were done ensuring anonymity of the study participants. The study was designed, conducted and reported abiding by declaration of Helsinki.
Statistical analysis
IBM statistical package for social sciences (SPSS) (Chicago, USA) (version 22) was used for analysis of the data. At first, descriptive analysis using number, percentage and 95% confidence interval (CI) was performed. This has shown distribution of the study subjects as per their background characteristics and IgG seropositivity for SARS-CoV-2. Then to find out univariate and multivariable determinants of IgG seropositivity among the study subjects logistic regression analysis was performed. Attributes which were found to be significant (P<0.05) in univariate logistic regression were only entered in multivariable logistic regression model using forced entry method. The strength of association was reported in terms of odds ratio (OR). Insignificant Hosmer-Lemeshow test (P≥0.05) indicated multivariable logistic regression model fit. For all the analysis minimum acceptable confidence level was α=0.95.
Results
Background characteristics and seroprevalence
The median age of the study subjects was 29 years with interquartile range (IQR) of 26-32 years (range: 20-56 years). There was almost equal representation of both the sexes. Majority of the study subjects (72.8%) reported direct exposure to confirmed COVID-19 cases or their body fluids during performance of their duties with median duration of exposure per duty of 7 hours with IQR (6-9 hours) (range: 1-12 hours). Considering comorbidities, 4% of the HCWs reported to have it with hypothyroidism (2.0%) being most common co-morbidity reported followed by diabetes (0.7%) and hypertension (0.5%). Out of 919 HCWs, 13.3% (11.2-15.6%) were found to be IgG seropositive for SARS-CoV-2. The seropositivity was almost double in males (17.6%) in comparison to females (8.7%). Considering occupation attendants were most likely (31.4%) to be IgG seropositive followed by account staffs (30.0%), sanitary staffs (25.8%) and technicians (24.4%). Intensive care unit (ICU) staffs were least likely (5.2%) and laboratory staffs were most likely (28.6%) to be IgG seropositive (Table 1).
Table 1.
Distribution of the healthcare workers as per their background characteristics and seropositivity for IgGn=919
| Variable | Total | IgG seropositive against SARS-CoV-2 | ||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| N | % | 95% CI | N | % | 95% CI | |
| Age in years | ||||||
| <30 (median 29 years) | 515 | 56.0 | 52.8-59.2 | 67 | 13.0 | 10.4-16.2 |
| ≥30 | 404 | 44.0 | 40.8-47.2 | 55 | 13.6 | 10.6-17.3 |
| Gender | ||||||
| Male | 471 | 51.3 | 48.0-54.5 | 83 | 17.6 | 14.4-21.3 |
| Female | 448 | 48.7 | 45.3-52.0 | 39 | 8.7 | 6.4-11.7 |
| Occupation | ||||||
| Doctor | 124 | 13.5 | 11.4-15.8 | 16 | 12.9 | 8.1-19.9 |
| Nurse | 523 | 56.9 | 53.7-60.1 | 31 | 5.9 | 4.2-8.3 |
| Technician | 41 | 4.5 | 3.3-6.0 | 10 | 24.4 | 13.8-39.3 |
| Account staff | 20 | 2.2 | 1.5-3.5 | 6 | 30.0 | 14.5-51.9 |
| Attendant | 118 | 12.8 | 10.8-15.2 | 37 | 31.4 | 23.7-40.2 |
| Sanitary staff | 31 | 3.4 | 2.4-4.8 | 8 | 25.8 | 13.7-43.2 |
| Others | 62 | 6.7 | 5.3-8.5 | 14 | 22.6 | 14.0-34.4 |
| Place of posting: (n=839) | ||||||
| Triage | 58 | 6.9 | 5.4-8.8 | 7 | 12.1 | 6.0-22.9 |
| Wards | 397 | 47.3 | 44.0-50.7 | 56 | 14.1 | 11.0-17.9 |
| ICUs | 249 | 29.7 | 26.7-32.9 | 13 | 5.2 | 3.1-8.7 |
| Laboratories | 42 | 5.0 | 3.7-6.7 | 12 | 28.6 | 17.2-43.6 |
| Others | 93 | 11.1 | 9.1-13.4 | 23 | 24.7 | 17.1-34.4 |
| Exposure to confirmed COVID-19 cases or their body fluids during duty: (Yes) | 669 | 72.8 | 69.8-75.6 | 74 | 11.1 | 8.9-13.7 |
| PPE use: (n=840) | ||||||
| Full PPE | 610 | 72.6 | 69.5-75.5 | 68 | 11.1 | 8.9-13.9 |
| Both N-95 and surgical mask with gloves | 46 | 5.5 | 4.1-7.2 | 14 | 30.4 | 19.1-44.8 |
| Both N-95 and surgical mask | 41 | 4.9 | 3.6-6.5 | 12 | 29.3 | 17.6-44.5 |
| N-95 mask and gloves | 37 | 4.4 | 3.2-6.0 | 5 | 13.5 | 5.9-27.9 |
| N-95 mask only | 47 | 5.6 | 4.2-7.4 | 5 | 10.6 | 4.6-22.6 |
| Others | 59 | 7.0 | 5.5-8.9 | 5 | 8.5 | 3.7-18.3 |
| Had prior COVID-19 infection: (Yes) | 79 | 8.6 | 6.9-10.6 | 40 | 50.6 | 39.8-61.4 |
| Had ILI in past few months: (Yes) | 69 | 7.5 | 6.0-9.4 | 21 | 30.4 | 20.8-42.1 |
| Had co-morbidity: (No) | 882 | 96.0 | 94.5-97.1 | 113 | 12.8 | 10.8-15.2 |
| Used to smoking: (Yes) | 33 | 3.6 | 2.6-5.0 | 1 | 3.0 | 0.5-15.3 |
| Used to alcohol drinking: (Yes) | 31 | 3.4 | 2.4-4.7 | 4 | 12.9 | 5.1-28.8 |
| Used to drink hot beverages: (Yes) | 808 | 87.9 | 85.7-89.9 | 108 | 13.4 | 11.2-15.9 |
| Used to take steam inhalation: (Yes) | 112 | 12.2 | 10.2-14.5 | 27 | 24.1 | 17.1-32.8 |
| Used mask other than workplace: (Yes) | 899 | 97.8 | 96.7-98.6 | 118 | 13.1 | 11.1-15.5 |
| Used sanitiser other than workplace: (Yes) | 903 | 98.3 | 97.2-98.9 | 117 | 13.0 | 10.9-15.3 |
| Diet preference: (n=808) | ||||||
| Vegetarian | 244 | 30.2 | 27.1-33.5 | 28 | 11.5 | 8.1-16.1 |
| Non-vegetarian | 564 | 69.8 | 66.5-72.9 | 83 | 14.7 | 12.0-17.9 |
| Have consumed HCQ: (Yes) | 106 | 11.5 | 9.6-13.8 | 13 | 12.3 | 7.3-19.9 |
| Have consumed Azithromycin: (Yes) | 138 | 15.0 | 12.8-17.5 | 40 | 29.0 | 22.1-37.0 |
| Have consumed Zinc: (Yes) | 49 | 5.3 | 4.1-7.0 | 15 | 30.6 | 19.5-44.5 |
| Have consumed Multivitamin: (Yes) | 96 | 10.4 | 8.6-12.6 | 21 | 21.9 | 14.8-31.1 |
| Have consumed Vitamin C: (Yes) | 182 | 19.8 | 17.3-22.5 | 45 | 24.7 | 19.0-31.5 |
| Have consumed Vitamin E: (Yes) | 43 | 4.7 | 3.5-6.2 | 10 | 23.3 | 13.1-37.7 |
ICU: intensive care unit, PPE: personal protective equipment, ILI: influenza like illness, HCQ: hydroxychloroquine, CI: confidence interval.
Predictors of seroprevalence
In univariate logistic regression analysis; gender, occupation, place of posting, use of full PPE, prior COVID-19 infection, ILI, use of steam inhalation, consumption of azithromycin, zinc and vitamin C were the significant attributes affecting IgG seropositivity for SARS-CoV-2. In multivariable logistic regression analysis; occupation, place of posting, prior COVID infection and ILI were significant determinants of IgG seropositivity for SARS-CoV-2. Overall, the independent variables in the multivariable logistic regression model predicted 30.8% variability of the SARS-CoV-2 IgG seropositivity of the HCWs with high predictive accuracy rate (PAR) (88.1%) (Table 2).
Table 2.
Univariate and multivariable logistic regression analysis showing determinants of serum IgG status of the healthcare workers n=689*
| Variable | Total | IgG seropositive against SARS-CoV-2 | ||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| N | % | N | % | COR (95% CI) | AOR (95% CI) | |
| Age in years | ||||||
| <30 (median 29 years) | 381 | 55.3 | 52 | 13.6 | 1.0 (0.7-1.6) | - |
| ≥30 | 308 | 44.7 | 41 | 13.3 | Ref. | |
| Gender | ||||||
| Male | 358 | 52.0 | 62 | 17.3 | 2.0 (1.3-3.2) | 1.1 (0.6-1.9) |
| Female | 331 | 48.0 | 31 | 9.4 | Ref. | |
| Occupation | ||||||
| Nurse | 388 | 56.3 | 23 | 5.9 | Ref. | Ref. |
| Doctor | 105 | 15.2 | 11 | 10.5 | 1.8 (0.9-3.9) | 1.6 (0.7-3.8) |
| Technician | 29 | 4.2 | 9 | 31.0 | 7.1 (2.9-17.4) | 3.9 (1.3-12.4) |
| Account staff | 15 | 2.2 | 5 | 33.3 | 7.9 (2.5-25.1) | 9.3 (2.1-40.8) |
| Attendant | 91 | 13.2 | 31 | 34.1 | 8.2 (4.5-15.0) | 9.6 (4.4-20.9) |
| Sanitary staff | 14 | 2.0 | 4 | 28.6 | 6.3 (1.8-21.8) | 10.8 (2.8-41.0) |
| Others | 47 | 6.8 | 10 | 21.3 | 4.3 (1.9-9.7) | 6.3 (2.0-19.5) |
| Place of posting | ||||||
| ICUs | 197 | 28.6 | 8 | 4.1 | Ref. | Ref. |
| Triage | 48 | 7.0 | 6 | 12.5 | 3.4 (1.1-10.2) | 1.7 (0.5-5.8) |
| Wards | 338 | 49.1 | 50 | 14.8 | 4.1 (1.9-8.8) | 1.9 (0.8-4.5) |
| Laboratories | 30 | 4.4 | 10 | 33.3 | 11.8 (4.2-33.3) | 6.0 (1.8-20.5) |
| Others | 76 | 11.0 | 19 | 25.0 | 7.9 (3.3-18.9) | 2.1 (0.7-6.7) |
| Exposure to confirmed COVID-19 cases or their body fluids during duty: (Yes) | 507 | 73.6 | 61 | 12.0 | 1.6 (0.9-2.5) | - |
| Used full PPE: (Yes) | 493 | 71.6 | 58 | 11.8 | 0.6 (0.4-0.9) | 0.6 (0.3-1.2) |
| Had prior COVID-19 infection: (Yes) | 60 | 8.7 | 28 | 46.7 | 7.6 (4.3-13.4) | 6.9 (2.9-16.5) |
| Had ILI in past few months: (Yes) | 45 | 6.5 | 14 | 31.1 | 3.2 (1.6-6.3) | 2.6 (1.2-5.8) |
| Had co-morbidity: (No) | 659 | 95.6 | 86 | 13.1 | 0.5 (0.2-1.2) | - |
| Used to smoking: (Yes) | 25 | 3.6 | 0 | 0.0 | - | - |
| Used to alcohol drinking: (Yes) | 24 | 3.5 | 3 | 12.5 | 0.9 (0.3-3.1) | - |
| Used to drink hot beverages: (Yes) | 612 | 88.8 | 82 | 13.4 | 0.9 (0.5-1.8) | - |
| Used to take steam inhalation: (Yes) | 94 | 13.6 | 22 | 23.4 | 2.2 (1.3-3.9) | 1.0 (0.5-2.2) |
| Used mask other than workplace: (Yes) | 677 | 98.3 | 92 | 13.6 | 1.7 (0.2-13.5) | - |
| Used sanitiser other than workplace: (Yes) | 680 | 98.7 | 92 | 13.5 | 1.2 (0.2-10.1) | - |
| Diet preference | ||||||
| Vegetarian | 206 | 29.9 | 23 | 11.2 | Ref. | |
| Non-vegetarian | 483 | 70.1 | 70 | 14.5 | 1.3 (0.8-2.2) | |
| Have consumed HCQ: (Yes) | 89 | 12.9 | 9 | 10.1 | 0.7 (0.3-1.4) | - |
| Have consumed Azithromycin: (Yes) | 104 | 15.1 | 30 | 28.8 | 3.3 (2.0-5.5) | 1.5 (0.7-3.3) |
| Have consumed Zinc: (Yes) | 38 | 5.5 | 10 | 26.3 | 2.4 (1.1-5.2) | 0.6 (0.2-1.6) |
| Have consumed Multivitamin: (Yes) | 73 | 10.6 | 14 | 19.2 | 1.6 (0.9-3.0) | - |
| Have consumed Vitamin C: (Yes) | 140 | 20.3 | 34 | 24.3 | 2.7 (1.7-4.3) | 1.3 (0.6-2.7) |
| Have consumed Vitamin E: (Yes) | 28 | 4.1 | 7 | 25.0 | 2.2 (0.9-5.4) | - |
| Negelkerke R2 | - | - | - | - | - | .308 |
| Hosmer Lemeshow test p-value | - | - | - | - | - | .919 |
| Predictive accuracy rate (PAR) | - | - | - | - | - | 88.1 |
data for all the variables were available for 689 study subjects thus it was used for performing logistic regression analysis;
ICU: intensive care unit, PPE: personal protective equipment, ILI: influenza like illness, HCQ: hydroxychloroquine, COR: crude odds ratio, AOR: adjusted odds ratio, CI: confidence interval.
Discussion
The study was aimed to assess IgG seropositivity for SARS-CoV-2 and its determinants among HCWs of a COVID dedicated tertiary healthcare facility of India.
We found that 13.3% of our HCWs were seropositive for SARS-CoV-2. It was similar with the findings of two Indian studies by Prakash et al. [12] (13.6%) and Kumar et al. [17] and a study conducted in Belgium by Martin et al. [25] (11.0%). Although an prior Indian study by Baveja et al. [15] (6.9%), three European country studies (1 Germany, 2 Italy, 1 Spain) by Schmidt et al. [19] (2.9%), Amendola et al. [14] (5.1%), Sotgiu et al. [26] (7.4%), Garcia-Basteiro et al. [16] (7.6%) and an American study by Mughal et al. [18] (0.8%) reported it to be less compared to us. The variability of the finding may be attributed to many factors. Such as variation in study subject selection (Martin et al. [25] recruited only staffs working in COVID-19 units, Prakash et al. [12] and Amendola et al. [14] recruited both HCWs and non-HCWs, Sotgiu et al. [26] recruited apparently healthy HCWs and Mughal et al. [18] recruited only ICU staff which was unlike us); different techniques used for serum IgG for SARS-CoV-2 estimation (Prakash et al. [12], Kumar et al. [17], Martin et al. [25], Schmidt et al. [19], Amendola et al. [14] and Garcia-Basteiro et al. [16] used enzyme linked immunosorbent assay (ELISA) while Baveja et al. [15], Sotgiu et al. [26] and Mughal et al. [18] used rapid immunochromatography test which was unlike us), socio-cultural differences and moreover due to variation in immune responses which is likely to be influenced by genetic, ethnic and climatic factors [27,28].
In the present study we found no association between age and SARS-CoV-2 IgG seropositivity. This was in line with the findings of Martin et al. [13] and Kumar et al. [17]. We found that males were more likely to be IgG seropositive compared to females. This was in line with the findings of Amendola et al. [14] and Kumar et al. [17] which reported similar observations. This might be because Indian men due to their various outdoor activities (i.e. shopping of household goods) and high mobility in comparison to their female counterparts are at more risk of contracting SARS-CoV-2 infection. Moreover, Indian women are twice more likely to be anaemic in comparison to their male counterparts irrespective of their socio-economic status. Anaemia is a known influencer of immune response to any infectious agent [29,30].
In our study occupation of the study subjects emerged as a significant influencer of IgG seropositivity to SARS-CoV-2. We found that staffs other than doctors and nurses were more likely to be IgG seropositive for the disease. Here educational level of the study subjects might have played a role as nurses and doctors by virtue of their professional training likely to be more aware of infection prevention and control (IPC) measures to be taken for contagious disease like COVID-19. Thus, they might have taken more precaution in comparison to the other staffs to get themselves protected from SARS-CoV-2 infection. Similarly, we found place of posting as significant attribute affecting IgG seropositivity among the study subjects as in comparison to those who were posted in ICUs all the other staffs were more likely to be IgG seropositive for the disease. This may be because those who were working in ICUs might have more served patients with severe form of the disease (i.e. multi organ failure, death) unlike other staffs. Moreover, HCWs deployed in ICUs oftenly conducts high-risk procedures (i.e. intubation, cardiopulmonary resuscitation) which are known to increase risk of the disease transmission. All these might have increased their perceived risk of contracting SARS-CoV-2 infection and enforced them to practice more stringent IPC measures compared to others. This was similar with the findings of Baveja et al. [15] which reported that working in COVID area as protective for IgG seroprevalence. Although Amendola et al. [14] reported that those who were posted in paediatric intensive care and surgery were more likely to be IgG seropositive against the disease. The variation of findings could be due to overall lower IgG seroprevalence was reported by Amendola et al. [9] (5.1%) and additionally the study has included non-HCWs in addition to HCWs in it which were unlike us. In our study, those who used full PPE during their duty were 40% less likely to be IgG seropositive for SARS-CoV-2 which was in line with the findings of Baveja et al. [15]. This re-establishes the importance of use of proper personal protective measures to reduce the risk of infection.
We found that those who had prior COVID infection were 6.9 times more likely to be IgG seropositive against the disease. This was in concordance with the findings of Garcia-Basteiro et al. [16] and Kumar et al. [17]. This was an obvious finding as acquiring infection of an infectious disease agent is the only way to develop immunity against that particular disease in absence of an effective vaccine. Similarly, those who had ILI symptoms in previous 8 months had 2.6 times higher odds for IgG seropositivity. Garcia-Basteiro et al. [16] and Kumar et al. [17] reported similar observations. The persons with ILI symptoms are the major focus of COVID testing strategy of India since very early stage of the pandemic due to higher probability of these persons to be SARS-CoV-2 positive [31]. These study subjects with history of ILI symptoms and seropositive excepting those who undergone testing might have acquired mild form of SARS-CoV-2 infection and developed immunity against it. These study subjects remained undiagnosed as they did not opt for testing for COVID-19. Some other factors like use of steam inhalation, consumption of azithromycin, zinc and vitamin-C although have shown significant association with IgG seropositivity in univariate analysis got neutralised in multivariable model which signifies their limited role in immunity development against SARS-CoV-2. Thus, these should be continued to use as supportive measure. Therapeutic role of these attributes in immunity development against SARS-CoV-2 is subject to further investigation.
Limitations
Self-reporting by the HCWs were the source of most of the study data thus there may be social desirability and reporting biases which were inevitable. Secondly, as we invited all the HCWs of our institute for the study so there might be chances that those with current or prior ILI symptoms, SARS-CoV-2 infection and working in more high-risk areas (i.e. ICUs) might have participated more to know their immunity status against the disease. So, there might be response bias which limited the generalisability of the study findings to other healthcare settings.
Conclusion
Majority of the HCWs were found to be IgG seronegative for SARS-CoV-2. Occupation, place of posting, prior SARS-CoV-2 infection and ILI were found to be significant multivariable determinants of IgG seropositivity for SARS-CoV-2 in the study subjects. Till availability of effective vaccine all of the HCWs should abide by infection prevention and control (IPC) measures to keep themselves and their contacts protected from SARS-CoV-2 as most them were found to be lacking protective antibody level against the disease. Serum IgG antibody surveillance for SARS-CoV-2 may be a useful strategy to track the progress of the COVID-19 pandemic by assessment of immunity level for the disease among population at increased risk such as HCWs.
Acknowledgements
We would like to acknowledge the sincere efforts of the laboratory staffs and other staffs of All India Institute of Medical Sciences, Patna involved in the study. Without their unconditional support the study would not have been possible. We received no additional fund for the study. The institutional fund as part of healthcare workers health policy was utilised for this study.
Disclosure of conflict of interest
None.
References
- 1.Coronavirus Update (Live): 88,618,548 Cases and 1,908,982 Deaths from COVID-19 Virus Pandemic-Worldometer [Internet]. [cited 2021 Jan 8] Available from: https://www.worldometers.info/coronavirus/
- 2.Gates B. Responding to Covid-19 - a once-in-a-century pandemic? N Engl J Med. 2020;382:1677–1679. doi: 10.1056/NEJMp2003762. [DOI] [PubMed] [Google Scholar]
- 3.Andrews MA, Areekal B, Rajesh KR, Krishnan J, Suryakala R, Krishnan B, Muraly CP, Santhosh PV. First confirmed case of COVID-19 infection in India: a case report. Indian J Med Res. 2020;151:490–492. doi: 10.4103/ijmr.IJMR_2131_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Immune responses and immunity to SARS-CoV-2 [Internet]. Eur. Cent. Dis. Prev. Control. [cited 2020 Jan 8] Available from: https://www.ecdc.europa.eu/en/covid-19/latest-evidence/immune-responses.
- 5.Patel MM, Thornburg NJ, Stubblefield WB, Talbot HK, Coughlin MM, Feldstein LR, Self WH. Change in antibodies to SARS-CoV-2 over 60 days among health care personnel in nashville, tennessee. JAMA. 2020;324:1781–1782. doi: 10.1001/jama.2020.18796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Coronavirus disease (COVID-19): Serology, antibodies and immunity [Internet]. [cited 2021 Jan 8] Available from: https://www.who.int/news-room/q-a-detail/coronavirus-disease-covid-19-serology.
- 7.Metcalf CJ, Farrar J, Cutts FT, Basta NE, Graham AL, Lessler J, Ferguson NM, Burke DS, Grenfell BT. Use of serological surveys to generate key insights into the changing global landscape of infectious disease. Lancet Lond Engl. 2016;388:728–730. doi: 10.1016/S0140-6736(16)30164-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Serology in the context of COVID-19 [Internet]. [cited 2021 Jan 9] Available from: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/serology-in-the-context-of-covid-19.
- 9.Nguyen LH, Drew DA, Graham MS, Joshi AD, Guo CG, Ma W, Mehta RS, Warner ET, Sikavi DR, Lo CH, Kwon S. Risk of COVID-19 among front-line health-care workers and the general community: a prospective cohort study. Lancet Public Health. 2020;5:e475–e483. doi: 10.1016/S2468-2667(20)30164-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ng K, Poon BH, Kiat Puar TH, Shan Quah JL, Loh WJ, Wong YJ, Tan TY, Raghuram J. COVID-19 and the risk to health care workers: a case report. Ann Intern Med. 2020;172:766–767. doi: 10.7326/L20-0175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhao D, Wang M, Wang M, Zhao Y, Zheng Z, Li X, Zhang Y, Wang T, Zeng S, Hu W, Yu W, Hu K. Asymptomatic infection by SARS-CoV-2 in healthcare workers: a study in a large teaching hospital in Wuhan, China. Int J Infect Dis. 2020;99:219–225. doi: 10.1016/j.ijid.2020.07.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Prakash O, Solanki B, Sheth JK, Joshi B, Kadam M, Vyas S, Shukla A, Tiwari H, Rathod S, Rajput A, Trivedi T. Assessing seropositivity for IgG antibodies against SARS-CoV-2 in Ahmedabad city of India: a cross-sectional study. BMJ Open. 2021;11:e044101. doi: 10.1136/bmjopen-2020-044101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Martin C, Montesinos I, Dauby N, Gilles C, Dahma H, Van Den Wijngaert S, De Wit S, Delforge M, Clumeck N, Vandenberg O. Dynamics of SARS-CoV-2 RT-PCR positivity and seroprevalence among high-risk healthcare workers and hospital staff. J Hosp Infect. 2020;106:102–106. doi: 10.1016/j.jhin.2020.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Amendola A, Tanzi E, Folgori L, Barcellini L, Bianchi S, Gori M, Cammi G, Albani E, Zuccotti GV. Low seroprevalence of SARS-CoV-2 infection among healthcare workers of the largest children hospital in Milan during the pandemic wave. Infect Control Hosp Epidemiol. 2020;41:1468–1469. doi: 10.1017/ice.2020.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Baveja S, Karnik N, Natraj G, Natkar M, Bakshi A, Krishnan A. Rapid volunteer-based SARS-Cov-2 antibody screening among health care workers of a hospital in Mumbai, India. Indian J Med Sci. 2020;72:148–154. [Google Scholar]
- 16.Garcia-Basteiro AL, Moncunill G, Tortajada M, Vidal M, Guinovart C, Jiménez A, Santano R, Sanz S, Méndez S, Llupià A, Aguilar R, Alonso S, Barrios D, Carolis C, Cisteró P, Chóliz E, Cruz A, Fochs S, Jairoce C, Hecht J, Lamoglia M, Martínez MJ, Mitchell RA, Ortega N, Pey N, Puyol L, Ribes M, Rosell N, Sotomayor P, Torres S, Williams S, Barroso S, Vilella A, Muñoz J, Trilla A, Varela P, Mayor A, Dobaño C. Seroprevalence of antibodies against SARS-CoV-2 among health care workers in a large Spanish reference hospital. Nat Commun. 2020;11:3500. doi: 10.1038/s41467-020-17318-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kumar N, Bhartiya S, Desai S, Mutha A, Beldar A, Singh T. Seroprevalence of antibodies against SARS-CoV-2 among health care workers in Mumbai, India. Asia Pac J Public Health. 2020:1010539520977307. doi: 10.1177/1010539520977307. [DOI] [PubMed] [Google Scholar]
- 18.Mughal MS, Kaur IP, Patton CD, Mikhail NH, Vareechon C, Granet KM. The prevalence of severe acute respiratory coronavirus virus 2 (SARS-CoV-2) IgG antibodies in intensive care unit (ICU) healthcare personnel (HCP) and its implications-a single-center, prospective, pilot study. Infect Control Hosp Epidemiol. 2020:1–2. doi: 10.1017/ice.2020.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schmidt SB, Grüter L, Boltzmann M, Rollnik JD. Prevalence of serum IgG antibodies against SARS-CoV-2 among clinic staff. PLoS One. 2020;15:e0235417. doi: 10.1371/journal.pone.0235417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bleyzac N, Goutelle S, Bourguignon L, Tod M. Azithromycin for COVID-19: more than just an antimicrobial? Clin Drug Investig. 2020;40:683–686. doi: 10.1007/s40261-020-00933-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wessels I, Rolles B, Rink L. The potential impact of zinc supplementation on COVID-19 pathogenesis. Front Immunol. 2020;11:1712. doi: 10.3389/fimmu.2020.01712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Craddock JC, Neale EP, Peoples GE, Probst YC. Vegetarian-based dietary patterns and their relation with inflammatory and immune biomarkers: a systematic review and meta-analysis. Adv Nutr. 2019;10:433–451. doi: 10.1093/advances/nmy103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dowell AC, Turner N. Closing evidence to practice gaps: an end to an attack of the vapours? Br J Gen Pract. 2016;66:118–119. doi: 10.3399/bjgp16X683893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.SARS-CoV-2 IgG Assay [Internet]. [cited 2020 Jan 8] Available from: https://www.siemens-healthineers.com/en-in/laboratory-diagnostics/assays-by-diseases-conditions/infectious-disease-assays/cov2g-assay.
- 25.Martin C, Montesinos I, Dauby N, Gilles C, Dahma H, Van Den Wijngaert S, De Wit S, Delforge M, Clumeck N, Vandenberg O. Dynamics of SARS-CoV-2 RT-PCR positivity and seroprevalence among high-risk healthcare workers and hospital staff. J Hosp Infect. 2020;106:102–106. doi: 10.1016/j.jhin.2020.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sotgiu G, Barassi A, Miozzo M, Saderi L, Piana A, Orfeo N, Colosio C, Felisati G, Davì M, Gerli AG, Centanni S. SARS-CoV-2 specific serological pattern in healthcare workers of an Italian COVID-19 forefront hospital. BMC Pulm Med. 2020;20:203. doi: 10.1186/s12890-020-01237-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Khalil I, Barma P. Sub-continental atmosphere and inherent immune system may have impact on novel corona virus’ 2019 (nCovid-19) prevalence in South East Asia. Mymensingh Med J. 2020;29:473–480. [PubMed] [Google Scholar]
- 28.Yamamoto N, Bauer G. Apparent difference in fatalities between Central Europe and East Asia due to SARS-COV-2 and COVID-19: four hypotheses for possible explanation. Med Hypotheses. 2020;144:110160. doi: 10.1016/j.mehy.2020.110160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Didzun O, De Neve JW, Awasthi A, Dubey M, Theilmann M, Bärnighausen T, Vollmer S, Geldsetzer P. Anaemia among men in India: a nationally representative cross-sectional study. Lancet Glob Health. 2019;7:e1685–e1694. doi: 10.1016/S2214-109X(19)30440-1. [DOI] [PubMed] [Google Scholar]
- 30.Jonker FA, Boele van Hensbroek M. Anaemia, iron deficiency and susceptibility to infections. J Infect. 2014;69(Suppl 1):S23–7. doi: 10.1016/j.jinf.2014.08.007. [DOI] [PubMed] [Google Scholar]
- 31.Testing Strategy [Internet]. [cited 2020 Jan 9] Available from: https://www.icmr.gov.in/cteststrat.html.


