Abstract
Background
Epoxyeicosatrienoic acids (EETs) are metabolites of arachidonic acid with multiple biological functions. Rodent experiments suggest EETs play a role in insulin sensitivity and diabetes, but evidence in humans is limited. To address this knowledge gap, we conducted a case-cohort study in the Strong Heart Family Study, a prospective cohort among American Indians.
Methods
We measured 4 EET species and 4 species of corresponding downstream metabolites, dihydroxyeicosatrieonic acids (DHETs), in plasma samples from 1161 participants, including 310 with type 2 diabetes. We estimated the associations of total (esterified and free) EETs and DHETs with incident diabetes risk, adjusting for known risk factors. We also examined cross-sectional associations with plasma fasting insulin and glucose in the case-cohort and in 271 participants without diabetes from the older Strong Heart Study cohort, and meta-analyzed the results from the 2 cohorts.
Findings
We observed no significant association of total EET or DHET levels with incident diabetes. In addition, plasma EETs were not associated with plasma insulin or plasma glucose. However, higher plasma 14,15-DHET was associated with lower plasma insulin and lower plasma glucose.
Interpretation
In this first prospective study of EETs and diabetes, we found no evidence for a role of total plasma EETs in diabetes. The novel associations of 14,15-DHET with insulin and glucose warrant replication and exploration of possible mechanisms.
Funding
US National Institutes of Health
Keywords: Diabetes, Epidemiology, Epoxyeicosatrienoic acids, Dihydroxyeicosatrieonic acids, Insulin, Glucose
Research in context.
Evidence before this study
Animal studies have suggested that free EETs play a role in insulin sensitivity and glucose metabolism, which are important factors in the development of diabetes. EETs are converted to DHETs that have been assumed to have less biological activity than EETs. The role of EETs and DHETs in the development of diabetes in humans has been largely underexplored.
Added value of this study
We showed total plasma EETs (esterified and free) were not associated with incident diabetes, insulin or glucose in a large prospective study. However, the EET downstream metabolite, 14,15-DHET, was associated with lower plasma insulin and lower plasma glucose.
Implications of all the available evidence
We found little evidence for a role of total plasma EETs in diabetes development in humans suggesting interventions targeting total circulating EETs may have limited ability to prevent diabetes among people at risk. In contrast, 14,15-DHET may have biological activities independent of its EET precursor, and low total plasma levels may be an early marker of impaired glucose metabolism.
Alt-text: Unlabelled box
1. Introduction
Type 2 diabetes has reached epidemic proportions world-wide [1]. An estimated 7.2% of US population had diagnosed diabetes in 2015 and the prevalence of type 2 diabetes is particularly high in American Indians and Alaska Natives [2]. With diabetes being a leading cause of coronary heart disease, heart failure and stroke, [3,4] novel biomarker and prevention measures are critical.
Epoxyeicosatrienoic acids (EETs) are metabolites of arachidonic acid produced by cytochrome P450 enzymes. Multiple cytochrome P450 enzymes produce epoxy fatty acids from a variety of unsaturated fatty acids including n-3 fatty acids. In humans, CYP2C and CYP2J2 generate four EET species (5,6-EET, 8,9-EET, 11,12-EET and 14,15-EET) from arachidonic acid which is usually esterified in the membrane and is released by phospholipase A2 in response to stimuli [5]. EETs act as paracrines or autorines and are rapidly converted to dihydroxyeicosatrieonic acids (DHETs) by soluble epoxide hydrolases (sEH) [6]. DHETs are generally considered less active than EETs, and sEH inhibitors are routinely used to increase EETs levels in animal studies [7].
EETs exhibit multiple biological activities including anti-inflammatory, vasodilatory and electrophysiologic effects [8], [9], [10]. In addition, experiments in rodents suggest EETs play a role in insulin sensitivity and diabetes. For example, overexpression of CYP2J3 increases EET levels, reduces homeostasis model assessment insulin resistance (HOMA-IR) in db/db mice, and prevents fructose-induced decrease in insulin receptor signaling in rats [11]. Knockout or inhibition of sEH also increases EET levels, reduces plasma glucose in mice fed a high fat diet, [12] improves glucose tolerance and decreases islet cell apoptosis in streptozotocin treated mice [13]. Informed by these animal data, we hypothesized that higher plasma EETs were associated with lower risk of incident diabetes.
Evidence for a role of EETs in diabetes in humans is limited to a handful of genetic studies [14], [15], [16]. A functional variant (287Gln) of sEH, encoded by EPHX2, is associated with greater insulin sensitivity in a study of 85 subjects without diabetes however, the genetic variant was not associated with plasma EETs [14]. In a Japanese study of 294 patients with type 2 diabetes and 205 controls, the genotype distribution of 287Gln in EPHX2 did not differ between the two groups; and in contrast to the previous study, there was an association of EPHX2 287Gln with reduced glucose infusion rate, a marker of insulin resistance among those with diabetes [15]. In a Chinese study of 1747 patients with type 2 diabetes and 994 controls, genotype distribution of a functional polymorphism of CYP2J2 did not differ between the two groups. However, the variant was associated with younger age at diagnosis among the patients with diabetes [16]. There are no prospective studies of plasma EETs and incident diabetes.
To address our study hypothesis and bring much needed additional evidence in human subjects, we measured total (esterified and free) plasma EETs and total DHETs and examined the association of plasma EETs and DHETs with risk of incident diabetes, and with fasting plasma insulin and glucose in the Strong Heart Study (SHS), a large prospective cohort of American Indians.
2. Methods
2.1. Study design and setting
The SHS is a population-based longitudinal study of risk factors for cardiovascular disease in 12 American Indian communities in Arizona, North Dakota, South Dakota, and Oklahoma [17]. The original cohort included 4,549 participants between the ages of 45–74 years who were seen at a baseline exam conducted in 1989–1991, and two follow-up exams conducted in 1993–1995 and 1998–1999. In 2001–2003, 1st, 2nd, and 3rd degree relatives of original SHS participants were recruited into a new study, called the Strong Heart Family Study (SHFS) [18]. In total, the SHFS comprised 2768 participants between the ages of 14–93 years from 92 families. The SHFS participants completed two examinations over an eleven-year period, in 2001–2003 and 2007–2009. Subsequent to the study examinations, surveillance for major cardio-metabolic outcomes, including diabetes, continued for all participants through December 2017 using a single phone interview and medical record review. Details of the study designs, survey methods and laboratory techniques have been reported previously [17,18]. The institutional review board from each Indian Health Service region and all communities approved the study, and written informed consent was obtained from all participants at each exam.
The primary study design was a case-cohort among the SHFS participants. We used samples that were collected at the SHFS baseline exam (2001-2003) from participants who attended the baseline visit, who had follow-up data and did not have diabetes at baseline; we selected all those who developed diabetes during follow-up (N = 339, including 214 diabetes cases identified at the 2007-2009 follow-up exam and 125 diabetes cases identified afterwards) and a random sample of 900 from those who did not. Among these sampled participants, 8 diabetes cases and 30 non cases did not have any available plasma samples; we measured EETs and DHETs in 331 diabetes cases and 870 non cases. Among these, 310 of the samples from diabetes cases and 851 of the samples from non cases passed laboratory QC and had no missing values of key covariates (education and waist circumference) and comprised the analytical sample. In addition to the case-cohort sample, we also measured EETs and DHETs in 1993-1995 plasma samples from 271 participants from the original SHS cohort, without prevalent diabetes at the 1993-1995 exam, that had been randomly selected for a previous study [19].
2.2. Data collection
Each exam included a standardized personal interview, physical examination, and laboratory work-up. Blood samples were collected into EDTA tubes after a 12 h overnight fast and plasma was stored at -80 °C. Except for physical activity, the data collection procedures were identical at all exams and have been described in detail [18,20]. In the SHFS, usual levels of ambulatory activity (i.e., average steps per day over one week) were estimated using Accusplit AE120 pedometers (Yamax, Japan), which were worn for up to 7 days [21,22]. In the SHS, participation in leisure-time and occupational physical activities was assessed using a questionnaire designed specifically for American Indians [23,24]. Both the physical activity questionnaire and pedometers have been shown to be reliable and valid in free-living conditions [25,26].
2.3. Diabetes ascertainment
Incident diabetes was identified at the SHFS 2007-2009 follow-up exam based on fasting plasma glucose ≥ 126 mg/dl or use of insulin or oral anti-diabetic medications or evidence of a physician-diagnosis of diabetes from annual medical record surveillance and review [27]. After the 2007–2009 exam and through December 2017, cases of diabetes in the SHFS were ascertained through telephone interview and confirmed by review of medical records, which provided a diagnosis date. Since type 1 diabetes is rare in American Indian populations, [28,29] we assumed all new occurrences of diabetes were type 2.
2.4. Glucose and insulin ascertainment
Plasma glucose and insulin were measured at the baseline exams of each cohort and at the SHFS follow-up exam using enzymatic methods and a modified version of the Morgan and Lazarow radioimmunoassay, respectively [17]. Impaired fasting glucose (IFG) was classified as a fasting plasma glucose level of between 100 and 125 mg/dL.
2.5. EETs measurements
EETs were measured in EDTA-plasma using a high throughput method that we developed for the measurement of hydroxy and epoxy metabolites of arachidonic acid [30]. Briefly, total lipids were extracted using a modified Bligh and Dyer method; [31] phospholipids were hydrolyzed by saponification to release fatty acids; the fatty acids were extracted by solid phase extraction and derivatized by a modified Bollinger method; [32] eicosanoids were then separated and quantitated by ultra performance liquid chromatograph tandem mass spectrometry. With this method, we quantitated total levels of 4 EET species (14,15-EET, 11,12-EET, 8,9-EET and 5,6-EET) with the epoxide in the cis configuration and 4 DHETs (14,15-DHET, 11,12-DHET, 8,9-DHET and 5,6-DHET). The numbering refers to the position of the epoxide bond.
EETs and DHETs were measured in 96-well plates that each included a calibration curve made of commercial standards [30]. Within each plate, values of each EET and DHET were normalized using the calibration curve of the appropriate standard. Due to skewed distributions, species values were log-transformed. In addition, because of plate-to-plate variability, species concentrations were centered to the plate mean and scaled to plate standard deviation (SD).
2.6. Statistical analyses
In univariate analyses, we examined the distribution of participant characteristics in quartiles of log 14,15-EET, and log 14,15-DHET in the SHFS case-cohort, and in the independent sample of SH participants. In the case-cohort, inverse probability weighting was used to account for sampling, with incident diabetes cases assigned a weight of 1, and non-cases assigned a weight of 1.83 (851 non-cases out of a possible 1557).
Incident Diabetes Analyses: Parametric survival models with a Weibull distribution [33] were used to examine the associations of each EET and DHET with risk of incident diabetes in the case-cohort. Parametric-Weibull models were selected for the analysis to incorporate both interval-censored (prior to the SHFS 2007-2009 follow-up exam) and right-censored (after the follow-up exam) diabetes event data using the SurvRegCensCov R package. The R implementation also accommodates clustering of outcomes among family members with robust standard errors based on a within-family exchangeable working correlation matrix in the sandwich variance, which is important because the SHFS comprised extended families. We used inverse probability weighting to account for the sampling of the case-cohort, as described above [34,35]. We fit 2 models: Model 1 (a minimal model) adjusted for age, sex, and site, and Model 2 (multivariate model) additionally adjusted for a priori confounders including education (years), smoking (never, former, current), physical activity (steps per day), BMI (log-transformed), waist circumference and LDL cholesterol. We present hazard ratios of incident diabetes associated with one SD higher log species concentration and Wald's tests based on these estimates and standard errors.
Insulin and glucose: The outcome of plasma insulin showed a skewed distribution and was log-transformed for the analyses. Plasma glucose had a symmetric distribution and was used without transformation. In the SHFS case-cohort, associations of each EET and DHET species with log plasma insulin and plasma glucose were assessed using Wald's tests based on generalized estimating equations (GEE) with sampling weights and a robust sandwich variance as described above to account for familial aggregation. Cross-sectional analyses of log insulin and glucose were restricted to participants without prevalent diabetes at baseline. Analyses of EETs and DHETs with insulin and glucose measured at the follow-up exam in SHFS were restricted to those without prevalent diabetes at both baseline and follow-up exam. Models were fitted with the same adjustment covariates as the SHFS diabetes analysis.
In the samples from the SHS cohort, we used Wald's tests based on linear regression with robust sandwich variance to investigate cross-sectional associations of each EET and DHET species with log plasma insulin and plasma glucose concentrations. Models were fitted with the same adjustment covariates as the SHFS analysis.
In the SHFS and SHS datasets, multiple imputation by chained equations were used to replace missing values of physical activity (SHFS: n = 86; SHS: n = 14) and LDL (SHFS: n = 4; SHS: n = 10), using information on age, sex, site, BMI, waist circumference, insulin, glucose and incident diabetes. Imputations (n=20) were executed using the Fully Conditional Expectation method with predictive mean matching methods, as described previously [36], [37], [38].
Meta-analyses of the cross-sectional insulin and glucose results were performed using inverse-variance-weighted fixed effects methods. Inverse-variance weighted fixed-effects meta-analysis results approximate those that would be obtained in a joint analysis of the data from the two studies, adjusted for study [39]. Heterogeneity between the SHFS and SHS results was assessed using the I2 index derived from the Cochran Q statistic [40].
We examined whether age, sex, or BMI modified the associations of 14,15-EET and 14,15-DHET with incident diabetes, insulin and glucose, by including a multiplicative term in the primary adjustment model (model 2). Wald tests like those described above for main effects were used to test the statistical significance of the interaction terms.
We used a Bonferroni correction to adjust for multiple comparisons; a significance threshold of 0.0063 (0.05/8 species) was used for the associations with outcomes. Associations with p-values <0.05 but ≥ 0.0063 were considered “borderline”. A threshold of 0.0083 (0.05/6 test of interactions) was used for the interaction tests. Analyses of incident diabetes were conducted using R (version 3.4.0); all other analyses were conducted using STATA 14.2 (Stata Corporation, College Station, TX).
2.7. Role of the funding source
The study sponsors did not have any role in the study design; the collection, analysis, or interpretation of data; the writing of the report; or the decision to submit the paper for publication. RNL had full access to all the data in the study and had final responsibility for the decision to submit for publication.
3. Results
Study mean baseline age was 37 years (SD: 16 years, range: 14-86 years) with 60% women among the 1161 SFHS participants and it was 57 years (SD: 7 years, range: 48-78 years) with 66% women among the 271 SHS participants.
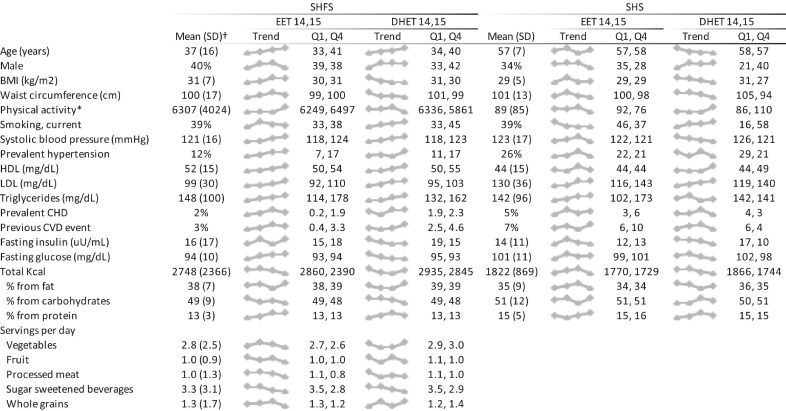
Plasma concentration of the four species of EETs were highly correlated with each other with Pearson correlations from 0.89 to 0.95 (Fig. S1). Correlations among the DHET species were 0.51 to 0.68. The distributions of participants characteristics in the SHFS case-cohort and the participants from the SHS cohort are shown overall and in quartiles of 14,15-EET and 14,15-DHET in Fig. 1. Higher plasma concentration of 14,15-EET was associated with older age in the SHFS cohort but not among the older SH participants. 14,15-EET was associated with higher LDL and triglycerides in both cohorts. In the SHFS case-cohort, 14,15-EET was also associated with higher HDL, higher blood pressure and hypertension. 14,15-DHET generally showed the same associations as 14,15-EET but in addition, higher concentration of 14,15-DHET was associated with male sex, lower BMI, lower waist circumference and current smoking. In the SHFS case-cohort, higher concentrations of both 14,15-EET and 14,15-DHET were associated with lower consumption of sugar-sweetened beverages.
Fig. 1.
Title: Distribution of participant characteristics in quartiles of 14,15-EET and 14,15-DHET in 1161 participants in the Strong Heart Family Study and 271 participants in the Strong Heart Study. Footnotes: The figure shows overall mean of each characteristics in the two cohorts, a small plot of values of each characteristic across quartiles of each metabolite (Trend), and mean values of each characteristic in quartile 1 (Q1) and quartile 4 (Q4). Food servings were only available in the SHFS cohort. *Physical activity was assessed as steps/day in SHFS, and MET hours per week in SHS. †For the SHFS we present weighted means in order to take into account the sampling weights in the case-cohort.
3.1. Diabetes risk
We did not observe significant associations of individual plasma EETs with incident diabetes (Table 1). For example, the estimated relative risk of diabetes associated with one SD higher 14,15-EET was 1.02 (95% CI: 0.93, 1.12) in models adjusted for age, sex, site, education, smoking, physical activity, BMI, waist circumference and LDL levels. Adjustment for HDL or exclusion of participants with impaired fasting glucose at baseline did not change the results (not shown). We found no significant interaction of EETs with age, sex or BMI (Table S1) with the lowest observed p value for interaction of 0.06. Plasma levels of DHET species showed no association with diabetes either at the specified significance threshold of 0.0063 (Table 1).
Table 1.
Association of total plasma EETs and DHETs with incident diabetes among 1161 participants in the Strong Heart Family Study.
| Model 1* |
Model 2* |
|||||
|---|---|---|---|---|---|---|
| RR† | 95% CI | P^ | RR† | 95% CI | P^ | |
| 14,15-EET | 1.05 | (0.95, 1.16) | 0.30 | 1.02 | (0.93, 1.12) | 0.69 |
| 11,12-EET | 1.04 | (0.93, 1.16) | 0.50 | 1.00 | (0.90, 1.12) | 1.00 |
| 8,9-EET | 1.10 | (1.00, 1.20) | 0.06 | 1.07 | (0.98, 1.18) | 0.14 |
| 5,6-EET | 1.06 | (0.96, 1.18) | 0.22 | 1.03 | (0.94, 1.13) | 0.54 |
| 14,15-DHET | 0.90 | (0.80, 1.01) | 0.07 | 0.94 | (0.85, 1.05) | 0.30 |
| 11,12-DHET | 0.98 | (0.86, 1.11) | 0.71 | 1.01 | (0.89, 1.14) | 0.89 |
| 8,9-DHET | 1.01 | (0.88, 1.17) | 0.87 | 1.08 | (0.95, 1.22) | 0.25 |
| 5,6-DHET | 1.03 | (0.91, 1.18) | 0.62 | 1.14 | (1.00, 1.30) | 0.04 |
Abbreviations: EET=Epoxyeicosatrienoic Acid; DHET= Dihydroxyeicosatrieonic acid
Model 1 included adjustments for age, sex, site; Model 2 included age, sex, site, education, smoking, physical activity, BMI, waist circumference and LDL levels.
RR: Relative risk of incident diabetes associated with one SD higher log EET/DHET species
Inverse-probability-of-being-sampled weighted Wald's test with robust sandwich variance and within-family-cluster exchangeable working correlation matrix under a Weibull time-to-event model with interval censoring (evidence from 20 imputations combined using Rubin's rules).
3.2. Baseline fasting plasma insulin and glucose
In cross-sectional analyses in the SHFS, each plasma EET species showed borderline associations with elevated fasting insulin concentrations (all p-values: 0.01-0.03; Table 2). However, there was no association of EETs with insulin in the sample from the SHS cohort and no significant association in the meta-analysis of SHFS and SHS results (all p-values :≥0.05). Plasma EETs were not associated with fasting plasma glucose either (all p-values: :≥0.19; Table 3).
Table 2.
Cross-sectional associations of total plasma EETs and DHETs with fasting plasma insulin.
| ———–SHFS ———– | ———–SHS ———– | ———Meta-analysis——— | |||||||
|---|---|---|---|---|---|---|---|---|---|
| GMR* | 95% CI | P^ | GMR* | 95% CI | P^^ | GMR* | 95% CI | P^^^ | |
| 14,15-EET | 1.04 | (1.01, 1.08) | 0.02 | 0.99 | (0.93, 1.06) | 0.85 | 1.03 | (1.00, 1.06) | 0.05 |
| 11,12-EET | 1.05 | (1.01, 1.09) | 0.01 | 0.98 | (0.92, 1.04) | 0.51 | 1.03 | (1.00, 1.06) | 0.07 |
| 8,9-EET | 1.04 | (1.01, 1.08) | 0.02 | 0.97 | (0.91, 1.03) | 0.30 | 1.03 | (1.00, 1.06) | 0.11 |
| 5,6-EET | 1.04 | (1.00, 1.08) | 0.03 | 0.95 | (0.89, 1.02) | 0.19 | 1.02 | (0.99, 1.05) | 0.18 |
| 14,15-DHET | 0.94 | (0.90, 0.97) | 0.001 | 0.92 | (0.86, 0.99) | 0.03 | 0.93 | (0.90, 0.97) | 2.0×10−05 |
| 11,12-DHET | 0.97 | (0.93, 1.00) | 0.08 | 0.95 | (0.87, 1.04) | 0.26 | 0.97 | (0.93, 1.00) | 0.04 |
| 8,9-DHET | 1.03 | (0.99, 1.07) | 0.14 | 0.96 | (0.89, 1.04) | 0.29 | 1.02 | (0.98, 1.05) | 0.37 |
| 5,6-DHET | 1.01 | (0.95, 1.07) | 0.74 | 0.92 | (0.85, 1.00) | 0.04 | 0.98 | (0.93, 1.02) | 0.32 |
Abbreviations: EET=Epoxyeicosatrienoic Acid; DHET= Dihydroxyeicosatrieonic acid; SHFS= Strong Heart Family Study; SHS= Strong Heart Study
The analyses were conducted in 1161 participants in the SHFS and 271 participants in the SHS and included adjustments for age, sex, site, education, smoking, physical activity, BMI (Kg/m2), waist circumference and LDL levels. Results from the two cohorts were combined in meta-analyses.
GMR: Geometric mean ratios of plasma insulin associated with one SD higher log EET/DHET species.
Inverse probability-of-being-sampled weighted Generalized Estimating Equations (GEE) regression Wald test with robust sandwich variance-covariance under a family-cluster-specific exchangeable working correlation structure, and results from multiple imputations combined using Rubin's rules
Linear regression Wald test with robust standard errors and results from multiple imputations combined using Rubin's rules.
Inverse-variance weighted fixed-effects meta analysis.
Table 3.
Cross-sectional associations of total plasma EETs and DHETs with fasting plasma glucose.
| —————-SHFS—————- | —————–SHS—————– | ———-Meta-analysis———- | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean diff.* | 95% CI | P^ | Mean diff.* | 95% CI | P^^ | Mean diff.* | 95% CI | P^^^ | ||
| 14,15-EET | -0.25 | (-0.77, 0.26) | 0.33 | 0.05 | (-1.08, 1.17) | 0.94 | -0.20 | (-0.67, 0.26) | 0.39 | |
| 11,12-EET | -0.28 | (-0.82, 0.27) | 0.32 | 0.01 | (-1.13, 1.15) | 0.98 | -0.22 | (-0.71, 0.27) | 0.37 | |
| 8,9-EET | -0.39 | (-0.97, 0.20) | 0.19 | 0.30 | (-0.90, 1.50) | 0.63 | -0.26 | (-0.78, 0.27) | 0.34 | |
| 5,6-EET | -0.21 | (-0.77, 0.34) | 0.45 | 0.13 | (-1.16, 1.42) | 0.85 | -0.16 | (-0.67, 0.35) | 0.54 | |
| 14,15-DHET | -0.93 | (-1.53, -0.33) | 0.002 | -0.20 | (-1.41, 1.01) | 0.75 | -0.79 | (-1.32, -0.25) | 0.004 | |
| 11,12-DHET | -0.82 | (-1.31, -0.33) | 0.001 | 0.45 | (-0.66, 1.57) | 0.43 | -0.62 | (-1.07, -0.17) | 0.007 | |
| 8,9-DHET | -0.74 | (-1.39, -0.09) | 0.02 | 0.18 | (-1.09, 1.46) | 0.78 | -0.55 | (-1.13, 0.03) | 0.06 | |
| 5,6-DHET | -0.53 | (-1.26, 0.20) | 0.16 | -0.91 | (-2.23, 0.41) | 0.18 | -0.62 | (-1.26, 0.02) | 0.06 | |
Abbreviations: EET=Epoxyeicosatrienoic Acid; DHET= Dihydroxyeicosatrieonic acid; SHFS= Strong Heart Family Study; SHS= Strong Heart Study
The analyses were conducted in 1161 participants in the SHFS and 271 participants in the SHS and included adjustments for age, sex, site, education, smoking, physical activity, BMI (Kg/m2), waist circumference and LDL levels. Results from the two cohorts were combined in meta-analyses.
Mean diff.: mean difference in mg/dL plasma glucose associated with one SD higher log EET/DHET species.
Inverse probability-of-being-sampled weighted Generalized Estimating Equations (GEE) regression Wald test with robust sandwich variance-covariance under a family cluster-specific exchangeable working correlation structure, and results from multiple imputations combined using Rubin's rules
Linear regression Wald test with robust standard errors and results from multiple imputations combined using Rubin's rules.
Inverse-variance weighted fixed-effects meta analysis.
Among the DHET species, 14,15-DHET was cross-sectionally associated with lower fasting insulin in the case-cohort (p = 0.001). This association was nominally replicated in the SHS controls (p = 0.03) and significant in the meta-analysis (p = 2×10−5, Table 2). Higher levels of 11,12-DHET were associated with lower plasma glucose in the SHFS cohort and in the meta-analysis of SHFS and SHS results (p = 0.004, Table 3), while the association of 14,15 DHET with glucose in the meta-analysis was borderline (p = 0.007).
We investigated possible interaction of 14,15-EET and 14,15-DHET with age, sex and BMI on plasma glucose and insulin (Tables S2-S5). While accounting for multiple comparisons, we observed an interaction of 14,15-EET with BMI on plasma glucose (p for interaction=0.001) such that higher plasma concentration of 14,15-EET was associated with lower plasma glucose at BMIs lower than 30 but higher glucose at higher BMIs (Table 4). We did not find an interaction of 14,15-DHET with BMI on the outcome of glucose but we observed an interaction on fasting insulin (p for interaction < 0.001). In this case too, the association of 14,15-DHET with lower plasma insulin was observed at lower BMIs only (Table 5).
Table 4.
Associations of total plasma 14,15-EET with fasting glucose according to BMI.
| ————–SHFS————— | —————–SHS———— | ———-Meta-analysis——— | ||||
|---|---|---|---|---|---|---|
| BMI (Kg/m2) | Mean diff.* | 95% CI | Mean diff.* | 95% CI | Mean diff.* | 95% CI |
| 20 | -1.55 | (-2.49, -0.61) | -1.54 | (-3.86, 0.77) | -1.55 | (-2.42, -0.68) |
| 25 | -0.83 | (-1.42, -0.23) | -0.29 | (-1.54, 0.97) | -0.73 | (-1.27, -0.19) |
| 30 | -0.24 | (-0.73, 0.26) | 0.74 | (-0.66, 2.13) | -0.13 | (-0.59, 0.34) |
| 35 | 0.26 | (-0.36, 0.88) | 1.60 | (-0.56, 3.77) | 0.37 | (-0.23, 0.96) |
| 40 | 0.70 | (-0.13, 1.52) | 2.35 | (-0.62, 5.33) | 0.81 | (0.02, 1.61) |
| 45 | 1.08 | (0.04, 2.11) | 3.01 | (-0.71, 6.74) | 1.22 | (0.22, 2.21) |
| 50 | 1.42 | (0.18, 2.66) | 3.61 | (-0.81, 8.03) | 1.58 | (0.39, 2.77) |
Abbreviations: EET=Epoxyeicosatrienoic Acid; SHFS= Strong Heart Family Study; SHS= Strong Heart Study
The analyses were conducted in 1161 participants in the SHFS and 271 participants in the SHS and included adjustments for age, sex, site, education, smoking, physical activity, BMI (Kg/m2), waist circumference and LDL levels. Results from the two cohorts were combined in meta-analyses.
The p-value^ for the 14,15-EET x BMI interaction coefficient in the SHFS analysis was 0.003; the p-value^^ in the SHS analysis was 0.11; and the p-value^^^ in the meta-analysis was 0.001.
^Inverse probability-of-being-sampled weighted Generalized Estimating Equations (GEE) regression Wald test with robust sandwich variance-covariance under a family cluster-specific exchangeable working correlation structure, and results from multiple imputations combined using Rubin's rules
^^Linear regression Wald test with robust standard errors and results from multiple imputations combined using Rubin's rules.
^^^Inverse-variance weighted fixed-effects meta analysis.
Mean diff.: mean difference in mg/dL glucose associated with one SD higher log 14,15-EET.
Table 5.
Associations of total plasma 14,15-DHET with fasting insulin according to BMI.
| ——–SHFS ——– | ———SHS——- | —Meta-analysis—- | ||||
|---|---|---|---|---|---|---|
| BMI (Kg/m2) | GMR* | 95% CI | GMR* | 95% CI | GMR* | 95% CI |
| 20 | 0.84 | (0.78, 0.91) | 0.83 | (0.70, 0.97) | 0.84 | (0.78, 0.90) |
| 25 | 0.89 | (0.85, 0.93) | 0.89 | (0.81, 0.97) | 0.89 | (0.85, 0.93) |
| 30 | 0.94 | (0.90, 0.97) | 0.94 | (0.87, 1.01) | 0.94 | (0.91, 0.97) |
| 35 | 0.98 | (0.93, 1.02) | 0.98 | (0.89, 1.09) | 0.98 | (0.94, 1.02) |
| 40 | 1.01 | (0.95, 1.08) | 1.02 | (0.89, 1.18) | 1.01 | (0.96, 1.07) |
| 45 | 1.05 | (0.97, 1.13) | 1.06 | (0.88, 1.28) | 1.05 | (0.97, 1.13) |
| 50 | 1.08 | (0.98, 1.18) | 1.10 | (0.88, 1.37) | 1.08 | (0.99, 1.18) |
Abbreviations: SHFS= Strong Heart Family Study; SHS= Strong Heart Study; DHET= Dihydroxyeicosatrieonic acid
The analyses were conducted in 1161 participants in the SHFS and 271 participants in the SHS and included adjustments for age, sex, site, education, smoking, physical activity, BMI (Kg/m2), waist circumference and LDL levels. Results from the two cohorts were combined in meta-analyses.
The p-value^ for the 14,15-DHET x BMI interaction coefficient in the SHFS analysis was 0.002; the p-value^^ in the SHS analysis was 0.11; and the p-value^^^ in the meta-analysis was <0.001.
^Inverse probability-of-being-sampled weighted Generalized Estimating Equations (GEE) regression Wald test with robust sandwich variance-covariance under a family cluster-specific exchangeable working correlation structure, and results from multiple imputations combined using Rubin's rules
^^Linear regression Wald test with robust standard errors and results from multiple imputations combined using Rubin's rules.
^^^Inverse-variance weighted fixed-effects meta analysis.
GMR: Geometric mean ratios of insulin associated with one SD higher log 14,15-DHET.
We found no evidence of heterogeneity between the SHFS and SHS cohorts in any of the meta-analyses of EETs and DHETs with insulin and glucose (main effects and interactions).
3.3. Follow-up fasting insulin and glucose
In the SHFS case-cohort, baseline plasma levels of EETs or DHETS were not associated with plasma levels of insulin or glucose measured an average of 5.4 years (range 2.8–8.5 years) after baseline (Tables 6 and 7).
Table 6.
Association of total plasma EETs and DHETs with fasting plasma insulin measured on average 5.4 years later in the Strong Heart Family Study.
| GMR* | 95% CI | p^ | |
|---|---|---|---|
| 14,15-EET | 0.99 | (0.95, 1.04) | 0.76 |
| 11,12-EET | 1.00 | (0.95, 1.05) | 0.95 |
| 8,9-EET | 0.99 | (0.95, 1.04) | 0.73 |
| 5,6-EET | 0.99 | (0.95, 1.04) | 0.70 |
| 14,15-DHET | 0.98 | (0.94, 1.03) | 0.42 |
| 11,12-DHET | 0.99 | (0.94, 1.03) | 0.55 |
| 8,9-DHET | 0.99 | (0.94, 1.05) | 0.84 |
| 5,6-DHET | 1.00 | (0.94, 1.06) | 0.90 |
Abbreviations: EET=Epoxyeicosatrienoic Acid; DHET= Dihydroxyeicosatrieonic acid
The analyses included adjustments for age, sex, site, education, smoking, physical activity, BMI (Kg/m2), waist circumference and LDL levels.
GMR: Geometric mean ratios of plasma insulin associated with one SD higher log EET/DHET species.
Inverse probability-of-being-sampled weighted Generalized Estimating Equations (GEE) regression Wald test with robust sandwich variance-covariance under a family cluster-specific exchangeable working correlation structure, and results from multiple imputations combined using Rubin's rules
Table 7.
Association of total plasma EETs and DHETs with fasting plasma glucose measured on average 5.4 years later in the Strong Heart Family Study.
| Mean diff.* | 95% CI | P^ | |
|---|---|---|---|
| 14,15-EET | 0.03 | (-0.62, 0.67) | 0.94 |
| 11,12-EET | -0.01 | (-0.68, 0.65) | 0.97 |
| 8,9-EET | 0.18 | (-0.45, 0.81) | 0.58 |
| 5,6-EET | 0.20 | (-0.44, 0.85) | 0.54 |
| 14,15-DHET | -0.11 | (-0.77, 0.55) | 0.75 |
| 11,12-DHET | 0.12 | (-0.42, 0.67) | 0.65 |
| 8,9-DHET | -0.12 | (-0.83, 0.58) | 0.73 |
| 5,6-DHET | -0.28 | (-0.96, 0.40) | 0.41 |
Abbreviations: EET=Epoxyeicosatrienoic Acid; DHET= Dihydroxyeicosatrieonic acid
The analyses included adjustments for age, sex, site, education, smoking, physical activity, BMI (Kg/m2), waist circumference and LDL levels.
Mean diff.: mean difference in mg/dL plasma glucose associated with one SD higher log EET/DHET species.
Inverse probability-of-being-sampled weighted Generalized Estimating Equations (GEE) regression Wald test with robust sandwich variance-covariance under a family cluster-specific exchangeable working correlation structure, and results from multiple imputations combined using Rubin's rules
4. Discussion
In this large prospective study, we found no overall support for our a priori hypothesis that circulating EETs are associated with lower risk of incident diabetes. In this study of participants free of diabetes at baseline, we observed no association of any of the EET species with diabetes risk, and no overall associations of EETs with fasting plasma levels of insulin and glucose.
The findings contrast with a large body of evidence from animal studies suggesting higher levels of EETs protect against diabetes. In genetically or diet-induced rodent models of insulin resistance, increasing EET generation by overexpression of CYP2J3 or inhibition of sEH reduces HOMAIR and plasma glucose [11,12]. While the animal studies may inform the effect of EETs in patients with established diabetes, the present study does not support an involvement of total EETs measured in plasma in the future development of type 2 diabetes in healthy participants. However, the study did not address the possibility that plasma levels of free EETs, a minor component of total plasma EETs, could be associated with diabetes risk.
We observed an interaction of 14,15-EET with BMI such that high levels of plasma 14,15-EET were associated with decreased plasma glucose in participants with BMI up to about 30, and increased plasma glucose in those with higher BMIs. Obesity results in broad metabolic changes [41] and it is hypothetically conceivable that EET metabolism and/or biological activities differ in obese and non-obese individuals. However, this sub-group finding from cross-sectional analysis should be interpreted cautiously until replicated.
As with EETs, total plasma DHETs were not significantly associated with lower risk of diabetes. The borderline significant association of higher concentration of 5,6-DHET with increased diabetes risk, observed only in one of the cohorts, may be a chance finding. In contrast, we observed cross-sectional associations of high concentrations of 14,15-DHET with decreased fasting insulin and decreased glucose, suggesting the possibility that elevated 14,15-DHET might be protective during early phases of diabetes. Interestingly, similar with 14,15-EET, we also observed an interaction of 14,15-DHET with BMI, such that the inverse association of 14,15-DHET with fasting insulin was only in participants with BMI ≤ 30.
DHETs are generally accepted to have less biological activity than EETs. However, the lack of overall association of 14,15-EET with insulin and glucose in the present study, contrasting with the inverse associations of 14,15-DHET with circulating fasting insulin and glucose, suggests 14,15-DHET may exhibit biological activities independent of its EET precursor. Interestingly, 14,15-DHET has been shown to strongly bind to the peroxisome proliferator-activated receptor (PPAR)-alpha in vitro and to increase the expression of a PPAR-alpha responsive gene, prompting the hypothesis that 14,15-DHET may function as an endogenous activator of PPAR-alpha [42]. Activation of PPAR-alpha would lead to fatty acid oxidation that in turn could lead to decreased BMI and reduced insulin resistance. While this mechanism is speculative, we observed a univariate, cross-sectional inverse association of 14,15-DHET with BMI in agreement with this hypothesis. Further exploration of 14,15-DHET biological activities is warranted.
EETs are generated by several cytochrome P450 enzymes from arachidonic acid but can also be hydrolyzed from membrane phospholipids and plasma phospholipids where they appear to be stored in high levels possibly as a sequestered pool [43,44]. In the present study, we measured total EETs, both free and esterified in plasma phospholipids, and total DHETs. DHETs are converted from free EETs by action of sEH. The effects of free EETs are generally accepted to be limited by the hydrolysis to DHETs [45] and genetic and pharmacological inhibition of sEH is used to boost free EETs levels in animal studies. In our study, we observed positive correlations between plasma levels of total EET species and the corresponding DHET species, suggesting that at least in patients without diabetes, higher plasma esterified DHETs are not a proxy for lower esterified EETs. During the extraction process of EETs and DHETs from plasma, all precautions were taken to prevent the in vitro hydrolysis of EETs to DHETs as detailed in Zeigler et al.; [30] the positive correlation observed in plasma also indicates the DHETs observed were not due to hydrolysis of EETs during processing.
Strengths of the study include the setting in a population at high risk of diabetes, the use of two cohorts with different age distributions, and detailed risk factors assessment. The study is the first to measure plasma EETs and DHETs in a large prospective cohort. The study also has limitations. To reduce variability in the laboratory measurement, we centered EET and DHET levels to each plate mean, which may have reduced the power to find associations. The measurement did not provide estimates of absolute levels of EETs and DHETs. The stability of free EETs and free DHETs in plasma stored at -80 °C over long periods of time is not known; however, we measured total EETs and total DHETs, which are mostly esterified to phospholipids and esterified fatty acids are routinely used in association studies. Cross-sectional associations do not allow assessment of temporality. Finally, the study was observational and causality inferences cannot be made.
5. Conclusions
In conclusion, the study did not provide evidence that total plasma levels of EETs and DHETs are associated with incident diabetes risk in a population at high risk of diabetes. The novel associations of total plasma 14,15-DHET with lower plasma insulin and lower glucose warrant replication and extension to other populations and exploration of possible mechanisms.
5.1. Contributors
R.N.L. contributed to the conception and design of the study, the acquisition of data, and drafted the manuscript. P.N.J. conducted the statistical analysis and critically reviewed the manuscript. MZ and JD performed biospecimen measurements and critically reviewed the manuscript. A.M.F., C.M.S., and B.McK. contributed to the data analysis and critically reviewed the manuscript. JGU, BVH, BMP and RAT contributed to the acquisition of data and critically reviewed the manuscript. SAG., I.B.K., D.S.S., and N.S. contributed to the interpretation of results and critically reviewed the manuscript. All authors have read and approved the final version of the manuscript.
Declaration of Competing Interest
Drs Lemaitre, Jensen, Fretts, McKnight and Sitlani report grants from the National Institutes of Health during the conduct of the study. Dr. Psaty reports serving on the Steering Committee of the Yale Open Data Access Project funded by Johnson & Johnson. The other authors have nothing to disclose.
Acknowledgments
Acknowledgments
This research was supported by R01HL130880 and R01HL128709 from the National Heart Lung Blood Institute (NHLBI). The Strong Heart Study has been funded in whole or in part with federal funds from the National Heart, Lung, and Blood Institute, NIH, Department of Health and Human Services, under contract numbers 75N92019D00027, 75N92019D00028, 75N92019D00029, and 75N92019D00030. The study was previously supported by research grants: R01HL109315, R01HL109301, R01HL109284, R01HL109282, and R01HL109319 and by cooperative agreements: U01HL41642, U01HL41652, U01HL41654, U01HL65520, and U01HL65521. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or the Indian Health Service (IHS).
Data Sharing Statement
Due to privacy agreements with the tribal communities involved in this study, access to study data is restricted. Further information can be found at https://strongheartstudy.org/.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.ebiom.2021.103279.
Appendix. Supplementary materials
REFERENCES
- 1.Collaboration NCDRF Worldwide trends in diabetes since 1980: a pooled analysis of 751 population-based studies with 4.4 million participants. Lancet. 2016;387(10027):1513–1530. doi: 10.1016/S0140-6736(16)00618-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Prevention CfDCa. National diabetes statistics report, 2017: estimates of diabetes and its burden in the United States. In: US Department of Health and Human Services CfDCaP, editor. Atlanta, GA2017.
- 3.Low Wang CC, Hess CN, Hiatt WR, Goldfine AB. Clinical update: cardiovascular disease in diabetes mellitus: atherosclerotic cardiovascular disease and heart failure in type 2 diabetes mellitus - mechanisms, management, and clinical considerations. Circulation. 2016;133(24):2459–2502. doi: 10.1161/CIRCULATIONAHA.116.022194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dal Canto E, Ceriello A, Ryden L, Ferrini M, Hansen TB, Schnell O. Diabetes as a cardiovascular risk factor: an overview of global trends of macro and micro vascular complications. Eur J Prev Cardiol. 2019;26:25–32. doi: 10.1177/2047487319878371. 2_suppl. [DOI] [PubMed] [Google Scholar]
- 5.Capdevila JH, Falck JR, Harris RC. Cytochrome P450 and arachidonic acid bioactivation. Molecular and functional properties of the arachidonate monooxygenase. J Lipid Res. 2000;41(2):163–181. [PubMed] [Google Scholar]
- 6.Zeldin DC, Wei S, Falck JR, Hammock BD, Snapper JR, Capdevila JH. Metabolism of epoxyeicosatrienoic acids by cytosolic epoxide hydrolase: substrate structural determinants of asymmetric catalysis. Arch Biochem Biophys. 1995;316(1):443–451. doi: 10.1006/abbi.1995.1059. [DOI] [PubMed] [Google Scholar]
- 7.Huang H, Weng J, Wang MH. EETs/sEH in diabetes and obesity-induced cardiovascular diseases. Prostaglandins Other Lipid Mediators. 2016;125:80–89. doi: 10.1016/j.prostaglandins.2016.05.004. [DOI] [PubMed] [Google Scholar]
- 8.Kaspera R, Totah RA. Epoxyeicosatrienoic acids: formation, metabolism and potential role in tissue physiology and pathophysiology. Expert Opin Drug Metab Toxicol. 2009;5(7):757–771. doi: 10.1517/17425250902932923. [DOI] [PubMed] [Google Scholar]
- 9.Spector AA. Arachidonic acid cytochrome P450 epoxygenase pathway. J Lipid Res. 2009:S52–S56. doi: 10.1194/jlr.R800038-JLR200. 50 Suppl. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aliwarga T, Evangelista EA, Sotoodehnia N, Lemaitre RN, Totah RA. Regulation of CYP2J2 and EET levels in cardiac disease and diabetes. Int J Mol Sci. 2018;19(7):1916. doi: 10.3390/ijms19071916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xu X, Zhao CX, Wang L, Tu L, Fang X, Zheng C. Increased CYP2J3 expression reduces insulin resistance in fructose-treated rats and db/db mice. Diabetes. 2010;59(4):997–1005. doi: 10.2337/db09-1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Luria A, Bettaieb A, Xi Y, Shieh GJ, Liu HC, Inoue H. Proceedings of the national academy of sciences of the United States of America. Vol. 108. 2011. Soluble epoxide hydrolase deficiency alters pancreatic islet size and improves glucose homeostasis in a model of insulin resistance; pp. 9038–9043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Luo P, Chang HH, Zhou Y, Zhang S, Hwang SH, Morisseau C. Inhibition or deletion of soluble epoxide hydrolase prevents hyperglycemia, promotes insulin secretion, and reduces islet apoptosis. J Pharmacol Exper Ther. 2010;334(2):430–438. doi: 10.1124/jpet.110.167544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ramirez CE, Shuey MM, Milne GL, Gilbert K, Hui N, Yu C. Arg287Gln variant of EPHX2 and epoxyeicosatrienoic acids are associated with insulin sensitivity in humans. Prostaglandins Other Lipid Mediators. 2014;113-115:38–44. doi: 10.1016/j.prostaglandins.2014.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ohtoshi K, Kaneto H, Node K, Nakamura Y, Shiraiwa T, Matsuhisa M. Association of soluble epoxide hydrolase gene polymorphism with insulin resistance in type 2 diabetic patients. Biochem Biophys Res Commun. 2005;331(1):347–350. doi: 10.1016/j.bbrc.2005.03.171. [DOI] [PubMed] [Google Scholar]
- 16.Wang CP, Hung WC, Yu TH, Chiu CA, Lu LF, Chung FM. Genetic variation in the G-50T polymorphism of the cytochrome P450 epoxygenase CYP2J2 gene and the risk of younger onset type 2 diabetes among Chinese population: potential interaction with body mass index and family history. Exper Clin Endocrinol Diabetes. 2010;118(6):346–352. doi: 10.1055/s-0029-1243604. German Society of Endocrinology [and] German Diabetes Association. [DOI] [PubMed] [Google Scholar]
- 17.Lee ET, Welty TK, Fabsitz R, Cowan LD, Le NA, Oopik AJ. The strong heart study. A study of cardiovascular disease in American Indians: design and methods. Am J Epidemiol. 1990;132(6):1141–1155. doi: 10.1093/oxfordjournals.aje.a115757. [DOI] [PubMed] [Google Scholar]
- 18.North KE, Howard BV, Welty TK, Best LG, Lee ET, Yeh JL. Genetic and environmental contributions to cardiovascular disease risk in American Indians: the strong heart family study. Am J Epidemiol. 2003;157(4):303–314. doi: 10.1093/aje/kwf208. [DOI] [PubMed] [Google Scholar]
- 19.Fretts AM, Jensen PN, Hoofnagle A, McKnight B, Howard BV, Umans J. Plasma Ceramide Species Are Associated with Diabetes Risk in Participants of the Strong Heart Study. J Nutr. 2020;150(5):1214–1222. doi: 10.1093/jn/nxz259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee E, Welty T, Fabsitz R, Cowan L, Ngoc A, Oopik A. The strong heart study: a study of cardiovascular disease in american indians: design and methods. Am J Epidemiol. 1990;132:1141–1155. doi: 10.1093/oxfordjournals.aje.a115757. [DOI] [PubMed] [Google Scholar]
- 21.Marsh A, Vance R, Fredrick T, Hesselmann S, Rejeski J. Objective assessment of activity in older adults at risk for mobility disability. Med Sci Sports Exerc. 2007;39(6):1020–1026. doi: 10.1249/mss.0b013e3180423ac3. [DOI] [PubMed] [Google Scholar]
- 22.Fretts AM, Howard BV, McKnight B, Duncan GE, Beresford SA, Calhoun D. Modest levels of physical activity are associated with a lower incidence of diabetes in a population with a high rate of obesity: the strong heart family study. Diabetes Care. 2012;35(8):1743–1745. doi: 10.2337/dc11-2321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kriska A, Saremi A, Hanson R, Bennett P, Kobes S, Williams D. Physical activity, obesity, and incident type 2 diabetes in a high-risk population. Am J Epidemiol. 2003;158(7):669–675. doi: 10.1093/aje/kwg191. [DOI] [PubMed] [Google Scholar]
- 24.Fretts AM, Howard BV, Kriska AM, Smith NL, Lumley T, Lee ET. Physical activity and incident diabetes in American Indians: the strong heart study. Am J Epidemiol. 2009;170(5):632–639. doi: 10.1093/aje/kwp181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tudor-Locke C, Ainsworth BE, Thompson RW, Matthews CE. Comparison of pedometer and accelerometer measures of free-living physical activity. Med Sci Sports Exerc. 2002;34(12):2045–2051. doi: 10.1097/00005768-200212000-00027. [DOI] [PubMed] [Google Scholar]
- 26.Kriska AM, Knowler WC, LaPorte RE, Drash AL, Wing RR, Blair SN. Development of questionnaire to examine relationship of physical activity and diabetes in Pima Indians. Diabetes Care. 1990;13(4):401–411. doi: 10.2337/diacare.13.4.401. [DOI] [PubMed] [Google Scholar]
- 27.World Health Organization; Geneva: 1999. Definition, diagnosis, and classification of diabetes mellitus and its complications; pp. 1–59. [Google Scholar]
- 28.Sievers M, Fisher J. Diabetes in America. U.S. Department of Health and Human Services; Bethesda, MD: 1985. Diabetes in North American Indians; pp. 1–20. NIH Publication No. 85-1468 ed. [Google Scholar]
- 29.Venkat Narayan K. Diabetes mellitus in native Americans: the problem and its implications. In: Council NR, editor. Changing numbers, changing needs: American Indian demography and public health. The National Academies Press; Washington, DC: 1996. pp. 262–314. editor. [PubMed] [Google Scholar]
- 30.Zeigler M, Whittington D, Sotoodehnia N, Lemaitre RN, Totah RA. A sensitive and improved throughput UPLC-MS/MS quantitation method of total cytochrome P450 mediated arachidonic acid metabolites that can separate regio-isomers and cis/trans-EETs from human plasma. Chem Phys Lipids. 2018;216:162–170. doi: 10.1016/j.chemphyslip.2018.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bligh EG, Dyer WJ. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959;37(8):911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- 32.Bollinger JG, Thompson W, Lai Y, Oslund RC, Hallstrand TS, Sadilek M. Improved sensitivity mass spectrometric detection of eicosanoids by charge reversal derivatization. Anal Chem. 2010;82(16):6790–6796. doi: 10.1021/ac100720p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kalbfleisch J, Prentice R. John Wiley & Sons; 2002. The statistical analysis of failure time data. [Google Scholar]
- 34.Binder DA. Fitting Cox's proportional hazards models from survey data. Biometrika. 1992;79(1):139–147. [Google Scholar]
- 35.Therneau T, Grambsch P. Springer; New York: 2000. Modeling survival data: extending the cox model. [Google Scholar]
- 36.Lemaitre RN, Yu C, Hoofnagle A, Hari N, Jensen PN, Fretts AM. Circulating sphingolipids, insulin, HOMA-IR, and HOMA-B: the strong heart family study. Diabetes. 2018;67(8):1663–1672. doi: 10.2337/db17-1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schafer JL. Multiple imputation: a primer. Stat Methods Med Res. 1999;8(1):3–15. doi: 10.1177/096228029900800102. [DOI] [PubMed] [Google Scholar]
- 38.Van Buuren S, Groothuis-Oudshoorn K. MICE: multivariate imputation by chained equations in R. J Stat Softw. 2011:45. [Google Scholar]
- 39.Lin DY, Zeng D. Meta-analysis of genome-wide association studies: no efficiency gain in using individual participant data. Genet Epidemiol. 2010;34(1):60–66. doi: 10.1002/gepi.20435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. Brit Med J. 2003;327(7414):557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Heymsfield SB, Wadden TA. Mechanisms, pathophysiology, and management of obesity. N Engl J Med. 2017;376(3):254–266. doi: 10.1056/NEJMra1514009. [DOI] [PubMed] [Google Scholar]
- 42.Fang X, Hu S, Xu B, Snyder GD, Harmon S, Yao J. 14,15-Dihydroxyeicosatrienoic acid activates peroxisome proliferator-activated receptor-alpha. Am J Physiol Heart Circulatory Physiol. 2006;290(1):H55–H63. doi: 10.1152/ajpheart.00427.2005. [DOI] [PubMed] [Google Scholar]
- 43.Klett EL, Chen S, Edin ML, Li LO, Ilkayeva O, Zeldin DC. Diminished acyl-CoA synthetase isoform 4 activity in INS 832/13 cells reduces cellular epoxyeicosatrienoic acid levels and results in impaired glucose-stimulated insulin secretion. J Biol Chem. 2013;288(30):21618–21629. doi: 10.1074/jbc.M113.481077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shearer GC, Newman JW. Lipoprotein lipase releases esterified oxylipins from very low-density lipoproteins. Prostaglandins Leukot Essent Fatty Acids. 2008;79(6):215–222. doi: 10.1016/j.plefa.2008.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Luther JM, Brown NJ. Epoxyeicosatrienoic acids and glucose homeostasis in mice and men. Prostaglandins Other Lipid Mediat. 2016;125:2–7. doi: 10.1016/j.prostaglandins.2016.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.